ABSTRACT
Guidelines for surgical prophylactic dosing of cefazolin in bariatric surgery vary in terms of recommended dose. This study aimed to describe the plasma and interstitial fluid (ISF) cefazolin pharmacokinetics in patients undergoing bariatric surgery and to determine an optimum dosing regimen. Abdominal subcutaneous ISF concentrations (measured using microdialysis) and plasma samples were collected at regular time points after administration of cefazolin 2 g intravenously. Total and unbound cefazolin concentrations were assayed and then modeled using Pmetrics. Monte Carlo dosing simulations (n = 5,000) were used to define cefazolin dosing regimens able to achieve a fractional target attainment (FTA) of >95% in the ISF suitable for the MIC for Staphylococcus aureus in isolates of ≤2 mg · L−1 and for a surgical duration of 4 h. Fourteen patients were included, with a mean (standard deviation [SD]) bodyweight of 148 (35) kg and body mass index (BMI) of 48 kg · m−2. Cefazolin protein binding ranged from 14 to 36% with variable penetration into ISF of 58% ± 56%. Cefazolin was best described as a four-compartment model including nonlinear protein binding. The mean central volume of distribution in the final model was 18.2 (SD 3.31) L, and the mean clearance was 32.4 (SD 20.2) L · h−1. A standard 2-g dose achieved an FTA of >95% for all patients with BMIs ranging from 36 to 69 kg · m−2. A 2-g prophylactic cefazolin dose achieves appropriate unbound plasma and ISF concentrations in obese and morbidly obese bariatric surgery patients.
KEYWORDS: antibiotic, bariatric, cefazolin, population pharmacokinetics, surgery
INTRODUCTION
The reported incidence of surgical site infection in bariatric surgery varies widely, from 1.9 to 15% (1–6). Obesity has repeatedly been demonstrated to be a risk factor for surgical site infection in both bariatric and nonbariatric abdominal surgery (2, 5, 7–9). Therefore, optimizing strategies to prevent surgical site infection in patients undergoing bariatric surgery is important to reduce patient morbidity and mortality and to reduce health care costs.
Cefazolin is recommended to be administered to patients undergoing bariatric surgery. Prior to April 2019, Australian guidelines recommended use of a 2-g intravenous cefazolin dose administered prior to incision (10). Updated Australian guidelines (11) and American guidelines (12) recommend a cefazolin dose of 3 g be administered if total body weight is greater than 120 kg. French guidelines recommend that 4 g be administered if the body mass index (BMI) exceeds 35 kg · m−2 (13). Efficacy is considered reliant on achieving therapeutic unbound concentrations in the plasma and the interstitial fluid (ISF) of adipose tissue, the surgical site of potential infection, throughout the duration of surgery.
Methicillin-susceptible Staphylococcus aureus (MSSA) is the most common pathogen in bariatric surgical site infection (14). A cefazolin concentration of 2 mg · L−1 is the MIC90 for MSSA (15) and can be considered a therapeutic unbound concentration.
There is little evidence that the recommended dosing regimens achieve therapeutic ISF concentrations. Most data are derived from studies measuring total (protein-bound and unbound) cefazolin concentrations in plasma and homogenized adipose tissue, the latter being a suboptimal matrix mixing interstitial and intracellular fluid (16–22). Microdialysis is an in vivo sampling technique and the gold standard for measuring tissue ISF cefazolin concentrations (23, 24). Two studies have utilized this technique but provided conflicting dosing recommendations, suggesting that 2 g (25) or 3 g (26) is required to meet therapeutic target concentrations for patients undergoing bariatric surgery. As such, there remains uncertainty in optimal dosing requirements for these patients.
This study aimed to describe the unbound plasma and ISF population pharmacokinetics of cefazolin at 2 g administered as antibiotic prophylaxis for bariatric surgery and subsequently to perform dosing simulations to recommend optimal dosing regimens for patients undergoing bariatric surgery.
We hypothesized that pharmacokinetic changes associated with obesity and surgery would lead to subtherapeutic ISF cefazolin concentrations in the bariatric surgery population when using a 2-g dose.
RESULTS
Fourteen patients were included in the study. Thirteen patients received a 2-g dose of cefazolin, according to local guidelines (10), and one patient initially received a 2-g dose of cefazolin that was followed by a 1-g dose of cefazolin 3 h after the initial dose, according to the preference of the anesthetist. The pharmacokinetic profile of this patient fit the model and therefore was included in our analysis. ISF samples were not available for one patient due to microdialysis sampling failure. One patient had prolonged surgery and consequently an additional 8-h plasma sample and 5-, 6.5-, and 8-h ISF samples. None of the patients developed a surgical site infection. Clinical patient characteristics are reported in Table 1.
TABLE 1.
Characteristics of obese adult patients (n = 14) presenting for elective bariatric surgery
| Characteristic | Mean | Median | SD | Range |
|---|---|---|---|---|
| Sex (male/female) | 6/8 (42.9%) | |||
| Age (yrs) | 41 | 43 | 26–56 | |
| Ht (cm) | 172 | 173 | 7.4 | 160–182 |
| Wt (kg) | 148 | 154 | 34.6 | 98.8–198 |
| BMI (kg · m−2) | 50 | 47 | 11.2 | 36–69 |
| Duration of surgery (min) | 92.5 | 70–175 | ||
| Plasma albumin (g · L−1) | 36 | 38 | 4.5 | 28–42 |
| Serum creatinine (μmol · L−1) | 63 | 67 | 13.6 | 42–80 |
| Creatinine clearance (mL · min−1) | 180 | 178 | 62 | 118–239a |
Interquartile range, calculated using the Cockcroft-Gault formula and lean body weight (36).
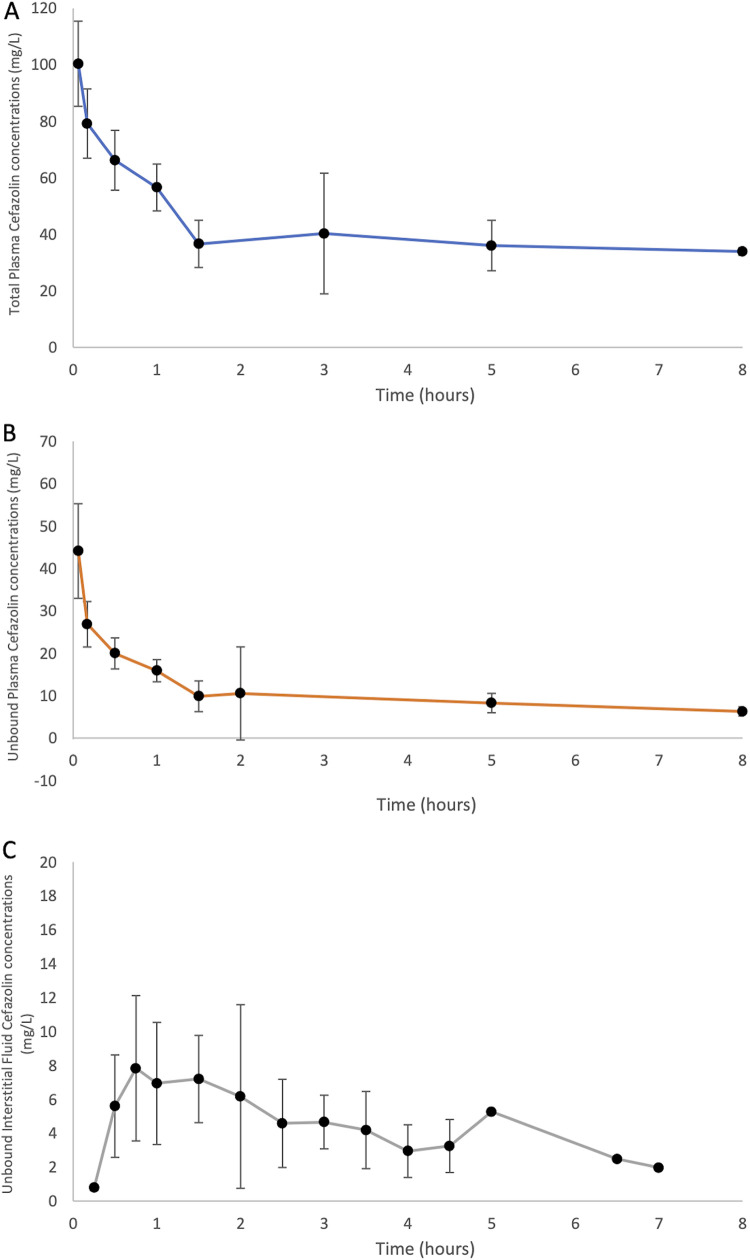
All total and unbound plasma concentrations of patients were above the lower limit of quantification of 1 mg · L−1, with all microdialysate concentrations above 0.1 mg · L −1. The percentage cefazolin recovery was calculated based on pre- and postperfusate cefalothin concentrations from all samples obtained from five patients and was then used for the measured cefazolin concentrations, which was 19%. The mean ± standard deviation (SD) interstitial fluid penetration was 58% ± 56%. The protein binding for the 14 patients ranged from 14% to 36%. The median (SD) concentration-time profiles for unbound plasma, bound plasma, and ISF are presented below in Fig. 1. The data presented contain total and unbound plasma concentrations from 14 patients and unbound interstitial fluid concentrations in 13 subjects, because one subject experienced microdialysis sampling failure.
FIG 1.
Median (with standard deviations [error bars]) concentration-time pharmacokinetic profile for cefazolin in obese patients undergoing bariatric surgery. (A) Total plasma (n = 14); (B) unbound plasma (n = 14); (C) unbound interstitial fluid (n = 13). One subject had a modified sampling time due to prolonged surgery; therefore, the 8-h plasma sample and 5-, 6.5-, and 7-h ISF samples were from one subject only.
Pharmacokinetic model.
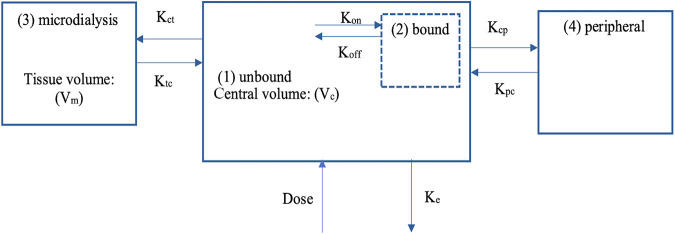
A four-compartment model, incorporating an additive error, adequately described the total and unbound plasma as well as microdialysate cefazolin concentrations (see Fig. 2 for a schematic of our model). Maximum binding (Bmax) was incorporated within the model using differential equations to describe the maximum binding amount of cefazolin per unit time (dx/dt, where x is the amount of cefazolin [in milligrams] and t is the unit time [in hours]). Individual patient values for Bmax were estimated in the final model and are reported as an amount rather than the conventional concentration (see Table S3 in the supplemental material). Plasma albumin concentrations were included empirically to describe the protein binding relationship. The fourth compartment was an additional binding compartment [“(2) bound” in Fig. 2] that was supported for inclusion based on a decrease in the −2 log-likelihood (−2*LL) of 59 and an Akaike information criterion (AIC) of 55. BMI was supported for inclusion as a covariate in the model on both central volume (Vc), with −2*LL and AIC decreased by 4 and 4, respectively, and peripheral volume (Vp), with −2*LL and AIC decreased by 7 and 7, respectively, as well as the bias and imprecision of the observed-predicted plot (see Fig. S1 in the supplemental material), when tested separately against the base model. Sex was not supported for inclusion in the model. The diagnostic plots to confirm the goodness of fit of the model were considered acceptable (see Fig. S1). The pharmacokinetic data are summarized in Table 2.
FIG 2.
Schematic of pharmacokinetic model compartments for an intravenous infusion of cefazolin in obese adult patients undergoing bariatric surgery. Vc, central volume; Koff, first-order dissociation rate constant to albumin; Kon, second-order association rate constant to albumin; Kct, rate constant for unbound cefazolin distribution from the central to the tissue compartment; Ktc, rate constant for unbound cefazolin distribution from the tissue to the unbound central compartment; Vm, volume of distribution of cefazolin in the interstitial microdialysis compartment; Kcp, rate constant for unbound cefazolin distribution from the central to peripheral compartment; Kpc, rate constant for cefazolin distribution from the peripheral to the central compartment; Ke, rate of elimination from the unbound compartment.
TABLE 2.
Population pharmacokinetic model data of cefazolin concentrations of obese patients (n = 14) undergoing bariatric surgery
| PK parametera | Units | Mean | SD | CV% | Median | Shrink% |
|---|---|---|---|---|---|---|
| CL | L · h−1 | 32.4 | 20.2 | 62.2 | 37.4 | 0.115 |
| Vc | L | 18.2 | 3.31 | 18.2 | 17.7 | 0.629 |
| Vm | L | 5.21 | 0.948 | 18.2 | 4.50 | 0.268 |
| K ct | h−1 | 0.164 | 0.079 | 48.0 | 0.182 | 1.97 |
| K tc | h−1 | 0.941 | 0.370 | 39.4 | 1.03 | 3.18 |
| K cp | h−1 | 5.93 | 4.01 | 67.6 | 4.23 | 1.24 |
| K pc | h−1 | 7.21 | 5.14 | 71.3 | 9.71 | 0.101 |
| K on | L · mg−1 · h−1 | 0.389 | 0.176 | 45.3 | 0.335 | 0.080 |
| K off | h−1 | 16.3 | 4.96 | 30.4 | 14.4 | 0.251 |
| B max | mg | 2,708 | 584 | 21.6 | 2,672 | NA |
PK, pharmacokinetic; CL, clearance of unbound cefazolin; Vc, central volume of distribution of cefazolin; Vm, volume of distribution of cefazolin in the intersitial fluid microdialysis compartment; Kct, rate of transfer from the unbound compartment to a tissue compartment; Ktc, rate of transfer from a tissue compartment to the unbound compartment; Kcp, rate of transfer from the unbound compartment to a peripheral compartment; Kpc, rate of transfer from a peripheral compartment to the unbound compartment; Kon, rate of association binding to albumin (second-order constant); Koff, rate of dissociation binding from albumin (first-order constant); Bmax, maximum binding amount of cefazolin; CV%, coefficient of variation; Shrink%, model shrinkage; NA, not applicable, calculated based on individual parameter estimates from the final model.
Dosing simulations.
Monte Carlo dosing simulations were performed using the model with a time to redosing of 4 h, and from these simulations estimates of the probability of target attainment (PTA) and fractional target attainment (FTA) were determined. The PTA of unbound cefazolin concentrations in plasma and ISF for dose regimens simulated for a typically obese patient (BMI of 48.2 kg · m−2, based on the mean total body weight from our sample) undergoing bariatric surgery are presented in the Fig. S2 in the supplemental material.
The FTA results, based on ISF concentrations for various cefazolin dosing regimens and body weights, for a MIC distribution for MSSA are presented in Table 3.
TABLE 3.
Fractional target attainmenta
| BMI (kg m−2) | FTA (%) for cefazolin dose and timing of surgery |
||||
|---|---|---|---|---|---|
| 2 g, T0.5 | 2 g, T1 | 3 g, T0 | 3 g, T0.5 | 3 g, T1 | |
| 36 | 98.2 | 97.8 | 99.7 | 99.4 | 99.1 |
| 40 | 98.2 | 97.8 | 99.6 | 99.5 | 99.1 |
| 50 | 98.4 | 98.1 | 99.5 | 99.6 | 99.4 |
| 60 | 98.5 | 98.2 | 99.4 | 99.6 | 99.5 |
| 69 | 98.5 | 98.2 | 99.2 | 99.6 | 99.5 |
The fractional target attainment for various cefazolin doses and preoperative weights, calculated based on the MIC (EUCAST) for Staphylococcus aureus (15). The FTA assessed whether simulated patients maintained cefazolin concentrations in the ISF above the MIC for S. aureus isolates, ≤2 mg · L−1, the MIC90 of cefazolin for a surgical duration of 4 h. Doses (as 3-min infusions) and preoperative BMI of 50 achieving the a priori target PTA against >=95% of isolates are indicated in table. T indicates time (in hours) after antibiotic administration that surgery commenced, i.e., T0 indicates surgery commenced immediately after antibiotic administration. The total duration of surgery was 4 h post-antibiotic administration.
DISCUSSION
We demonstrated that a 2-g bolus of cefazolin given 30 to 60 min prior to skin incision resulted in an ISF FTA of >95% in the studied patients (BMI, 36 to 69 kg · m−2) undergoing bariatric surgery with normal renal function. This suggests that there is no need to further adjust the dose of cefazolin based on body size for antimicrobial prophylaxis in this scenario.
Median ISF cefazolin concentrations were above the target concentration of 2 mg · L−1, which is consistent with previous studies (25–27). One previous study (25) demonstrated that a 2-g bolus of cefazolin resulted in a reduced tissue penetration of cefazolin and reduced probability of achieving a concentration in ISF above 2 mg · L−1 for up to 4 h in an obese cohort compared to the nonobese cohort. This previous paper reported similar achievement of target exposures to our model. However, a stated limitation of this model by Brill et al. was an overestimation of lower ISF cefazolin concentrations. Our model improves upon that of Brill et al., and indeed this may account for the lower calculated PTAs in our study. For example, the PTA for an ISF of >2 mg · L−1 for 4 h in obese patients following a 2-g cefazolin bolus 30 min prior to skin incision in our study was 75% (see Fig. S2b), versus 95% from the Brill et al. study.
Another recent study, by Palma et al., recommended a 3-g dose for female bariatric surgery patients (26). The PTA for a target of 2 mg · L−1 at 4 h in that study was 89%. The discrepancy in the recommendations may be accounted for by the chosen pharmacodynamic target. If instead of PTA the FTA is used, which considers the PTA and the specific population of S. aureus, a target of 95% may be reached with a 2-g bolus.
Previous studies have measured tissue concentrations using homogenized tissue samples (16–21). The largest study using this technique (22) demonstrated that following cefazolin at 4 g, the mean adipose tissue cefazolin concentrations at the end of 117 laparoscopic sleeve gastrectomies was greater than 2 μg · g−1. Monte Carlo dosing simulations demonstrated that the probability of achieving a target tissue concentration of 2 μg · g−1 for 4 h was optimized if a 3-g bolus followed by a 1-g · h−1 infusion of cefazolin was administered (28). Measuring tissue concentrations using homogenized samples can underestimate interstitial fluid concentration, because the homogenized sample contains intracellular fluid, which will not contain cefazolin, and this may account for the higher dose requirement reported in studies using homogenized tissue samples.
We developed a four-compartment model that included a binding compartment to describe the pharmacokinetics of cefazolin in this population. We observed significant correlations between the central and peripheral volumes of distribution and the BMI, rather than total body weight, which has been previously reported (25). Recent pharmacokinetic modeling (26) based on female-only data did not support the inclusion of BMI or weight in the model. Nonobese patients were not included in the current study. Further studies, including patients from a larger range of body sizes, are required to further investigate the most relevant body size descriptor to guide dosing.
Despite the influence of BMI on the central and peripheral compartment volumes, there was no statistically significant relationship between BMI and cefazolin distribution into the ISF or the pharmacodynamic measure of FTA in the studied bariatric surgery population. This is evidenced in Table 3, as there was only a minimal change in FTA across the studied BMI values. These data suggest that for patients with a BMI between 36 and 69 kg · m−2, a graduated size-dependent dose is not required.
There are some limitations to the study we wish to declare. The FTA considers only a susceptible MSSA distribution. Other pathogens implicated less frequently for causing surgical site infection, such as Escherichia coli, have higher MICs, and therefore the FTA achieved with a 2-g cefazolin bolus would be lower. Indeed, some prior studies have used higher target concentrations (22, 25).
Although the sample size of 14 appears small, when combined with intensive multicompartment sampling this sample size allows the development of a robust pharmacokinetic model (25, 29). The visual predictive check (see Fig. S4 in the supplemental material) demonstrates that 96% of the observed concentrations were within the 5th and 95th simulated percentiles. The population predicted model does not predict higher concentrations (>10 mg · L−1) as accurately, but this represents a small number (n = 5) of the concentrations in the model.
The collection of samples using microdialysis can be procedurally challenging, with blockages of the transport of the analyte across the membrane commonly encountered. The result of this is that we were only able to reliably measure pre- and postperfusate microdialysis cefalothin concentrations in samples from 5 patients, and these have been used to calculate the recovery. The calculated recovery ratio of cefazolin was 19%, which is lower than previously reported (25). Inclusion of more subjects’ results in the recovery ratio calculation may have resulted in a different ratio. Changes to the calculated recovery ratio will affect ISF concentration accuracy and consequently the pharmacokinetic analysis. Although microdialysis remains the gold standard in measuring interstitial fluid concentrations, this reflects a disadvantage of the technique.
Our study design only included obese patients with a BMI between 36 and 69 kg · m−2. However, extrapolations to dosing recommendations outside this BMI range cannot be made. Additionally, limiting the body size range may have resulted in the lack of covariates supported for inclusion, such as tissue penetration, which has been supported for inclusion in previous models (25).
Additionally, this study was not designed to examine the optimal dose and timing of a second antibiotic dose in prolonged surgeries. Most bariatric surgeries would be expected to last less than 4 h. Further studies in prolonged surgeries are suggested to confirm time to redosing.
In conclusion, this study suggests that a 2-g cefazolin bolus for patients undergoing bariatric surgery of up to a 4-h duration provides therapeutic ISF cefazolin concentrations to adequately protect against MSSA surgical site infection.
MATERIALS AND METHODS
Study design and setting.
This study is a prospective open-labeled observational pharmacokinetic study conducted between April and October 2016 at the Royal Brisbane and Women’s Hospital, Australia. The study was approved by the Royal Brisbane and Women’s Hospital Ethics Committee (HREC/15/QRBQ/551) and The University of Queensland (2016001428). Written informed consent was obtained from all subjects. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (30) (see Table S1 in the supplemental material) was followed. Intensive multicompartment sampling allows for the development of a robust pharmacokinetic model with a sample size of 14, as demonstrated in previous studies (29).
Patient selection.
Patients undergoing uncomplicated elective laparoscopic sleeve gastrectomy or laparoscopic gastric bypass who had a BMI of >35 kg · m−2 (class II or III obesity) were considered for inclusion. Patients were excluded if they had been administered cefazolin within the previous 72 h before surgery, had an allergy to cefazolin, were pregnant, or had an estimated glomerular filtration rate (eGFR) of <60 mL · min−1 · 1.73m−2.
Study protocol.
Two grams of cefazolin (Cephazolin; DBL, Sydney, Australia) in 10 mL 0.9% sodium chloride was administered as an intravenous bolus, 0 to 60 min prior to skin incision. This was the dose recommended in the Australian Therapeutic Guidelines (10), regardless of body weight, at the time the study was conducted. General anesthesia and fluid management were administered as per the treating anaesthetist. Sex, age, height, and weight on the day of surgery as well as clinical data including surgical duration and surgical subtype, serum albumin, creatinine, and eGFR were recorded. Creatinine clearance was calculated using the Cockcroft-Gault equation with the lean body weight metric.
Sample collection, storage, and management.
Blood samples were drawn from an intra-arterial line at 0, 30, 60, 90, 180, and 240 min after cefazolin administration or until the patients left the perioperative area. Samples were stored on ice prior to centrifugation at 3,000 rpm for 10 min. The resulting plasma was stored at −80°C until analysis.
Microdialysis catheters (CMA 60; Microdialysis AB, Stockholm, Sweden) were inserted into subcutaneous tissue in an upper quadrant of the abdomen outside the anticipated sterile surgical field to measure ISF cefazolin concentration. The perfusate, cefalothin (10 mg · L−1) in normal saline, was used as a microdialysis internal standard to aid determination of the antibiotic ISF concentration. A 30-min baseline perfusion period occurred prior to cefazolin administration. The recovery of cefazolin in the microdialysate solution was interpolated from the loss of cefalothin across the microdialysis membrane (31): % cefazolin recovery = [(Cin − mean Cout)/Cin] × 100, where Cin = 10 mg · L−1 cefalothin and Cout is the measured cefalothin concentration in the microdialysate.
ISF samples were taken from the microdialysis catheters at baseline, then at 15-min intervals after cefazolin administration for the first hour, and then at 30-min intervals up to 240 min or until the patient was discharged from the perioperative area. Microdialysis samples were initially stored on ice and then at −80°C until analysis.
Measurement of total and unbound cefazolin concentrations.
Total and unbound concentrations of cefazolin (1 to 500 mg · L−1) in serum and concentrations of unbound cefazolin (0.1 to 20 mg · L−1) and cefalothin (1 to 100 mg · L−1) in microdialysate and perfusate were measured by a validated high-pressure liquid chromatography mass spectrometry methodology at The University of Queensland Centre for Clinical Research (32). The assay method was validated using the U.S. Food and Drug Administration criteria for bioanalysis (33). Measured total and unbound cefazolin concentrations were used to calculate protein binding: % cefazolin protein binding = (Cunbound/Ctotal) × 100.
Population pharmacokinetic modeling.
Pharmacokinetic modeling was performed using PMetrics 1.5.0 with RStudio 0.99.902 and digital compiler Gfortran 5.2. The pharmacokinetic analysis protocol was approved by us prior to analyses. For the population pharmacokinetic analysis, two- and three-compartment models, which each included an additional protein binding compartment, were fitted using plasma (total and unbound) and microdialysis cefazolin concentration data and nonparametric adaptive grid subroutines from the PMetrics package for R (Los Angeles, CA, USA). Primary pharmacokinetic parameters of clearance (CL) and volume of distribution of cefazolin in the central compartment (Vc) and ISF microdialysis compartment (Vm) were calculated (Fig. 1 provides a schematic of the model). Elimination from the central compartment and intercompartmental distribution were modeled as first-order processes.
Unbound cefazolin concentrations (Cunbound) were related to total cefazolin concentrations, with the assumption that albumin is the sole binding site for cefazolin in plasma and taking into account protein binding (34), as follows. The Cunbound was calculated as follows: Ctotal − [(Bmax × Cunbound)/(Kd + Cunbound)], with Bmax calculated as ALB × [(N × MWcef × 1,000)/MWALB] and Kd is calculated as Koff/Kon.
For these calculations, saturable protein binding was assumed (35). In these equations, Cunbound is the unbound cefazolin concentration (in milligrams per liter), Ctotal is the total cefazolin concentration (in milligrams per liter), Bmax is the maximum binding concentration of cefazolin (in milligrams per liter), Kd is the equilibrium dissociation constant (in milligrams per liter) for cefazolin binding to albumin, ALB is the serum albumin concentration (in grams per liter), N is the number of binding sites, with 0.6 binding sites for cefazolin per molecule of albumin, MWcef is the molecular weight of cefazolin (454.5 g · mol−1), MWalb is the molecular weight of albumin (66,500 g · mol−1), Koff is the first-order dissociation rate constant (per hour), and Kon is the second-order association rate constant (in liters per milligram per hour). It should be noted that Bmax is calculated as an amount (in milligrams) for each patient within the model, as a function of the calculated volume of the central compartment and measured albumin (see Table S2 in the supplemental material for the model file).
Intercompartmental distribution was described as the rate of transfer from the unbound compartment to a tissue compartment (Kct), the rate of transfer from a tissue compartment to the unbound compartment (Ktc), the rate of transfer from the unbound compartment to a peripheral compartment (Kcp), the rate of transfer from a peripheral compartment to the unbound compartment (Kpc), and the rate of elimination from the unbound compartment (Ke) (see Table S2 for the model file).
Model diagnostics.
We selected the final model based on minimizing the Akaike information criterion and likelihood of the model (−2*LL), penalized by the number of parameters in the model. We also factored bias (mean weighted predicted-observed error) and imprecision (bias-adjusted, mean weighted squared predicted-observed error) into the selection of the final model.
Covariate screening.
Covariate model building was performed using sequential assessment of biologically plausible clinical characteristics. Covariates were tested individually, and inclusion was based upon a statistically significant improvement in the AIC and −2*LL. The covariates evaluated against pharmacokinetic parameters were sex, age, height, weight, BMI, lean body weight (36), creatinine, creatinine clearance calculated using the Cockcroft-Gault equation (using total and lean body weight) and plasma albumin concentration.
Probability of target attainment.
Monte Carlo dosing simulations (n = 5,000) were performed using the final covariate model in Pmetrics to determine the probability of target attainment (PTA) with the pharmacokinetic/pharmacodynamic (PK/PD) target of 100% time for unbound drug plasma concentration above the MIC and ISF cefazolin concentrations for clinically relevant MICs (0.125 to 32 mg · L−1) during bariatric surgery of up to 4 h for a typical obese patient, with a preoperative weight of 145 kg, based on the mean weight of our patient group (BMI of 50 kg · m−2). Loading and maintenance doses were tested as 3-min infusions (unless continuous infusion was used), as follows: (i) 2 g at 0 h with surgery commencing at 0 h; (ii) 2 g at 0 h with surgery commencing at 0.5 h; (iii) 2 g at 0 h with surgery commencing at 1 h; (iv) 3 g at 0 h with surgery commencing at 0 h; (v) 3 g at 0 h with surgery commencing at 0.5 h; (vi) 3 g at 0 h with surgery commencing at 1 h.
Fractional target attainment.
MSSA MIC data from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) database (15) were used to determine the fractional target attainment (FTA). The FTA identifies the likely success of treatment by comparing the pharmacodynamic exposure, the PTA, against a MIC distribution. MSSA isolates with a MIC ≤2 mg · L−1, the MIC90 of cefazolin for S. aureus (15), were included. In this analysis, the FTA was calculated using various doses to achieve an a priori target of 95% in the ISF for a surgical duration of 4 h. Simulated doses for the FTA were as described above. Five levels of preoperative BMI were tested (36, 40, 50, 60, and 69 kg · m−2), which reflected the distribution of values in the study cohort.
ACKNOWLEDGMENTS
We acknowledge the work of Anesthesia Research Nurses C. Town and L. Dalgleish, Department of Anesthesia and Perioperative Medicine, Royal Brisbane and Women’s Hospital, Brisbane, for their assistance in participant recruitment and data collection.
This work was supported by the Australia and New Zealand College of Anaesthetists Research Foundation (Melbourne, Australia) grant number 17/037; J. A. Roberts acknowledges funding from the Australian National Health and Medical Research Council (NHMRC) for a Centre of Research Excellence (APP2007007) and an Investigator Grant (APP2009736) as well as an Advancing Queensland Clinical Fellowship. S. L. Parker acknowledges funding from the NHMRC for an Early Career Fellowship (APP1142757). R. L. Ryan acknowledges funding from the Avant Doctor in Training Research Scholarship Scheme and an Advancing Queensland Clinical Fellowship. These study sponsors had no influence on any aspect of the study.
A. A. J. Van Zundert is a board member of the Research Grant Committee of the Australian and New Zealand College of Anaesthetists. J. Lipman has received honararia for lectures for Pfizer and MSD. R. L. Ryan, D. Jackson, G. Hopkins, V. Eley, R. Christensen, S. C. Wallis, S. L. Parker, and J. A. Roberts have no relevant conflicts to declare with this work.
R. L. Ryan helped with protocol design, ethics application, data interpretation, and writing of the manuscript. D. Jackson helped with protocol design, ethics application, data collection, and writing of the manuscript. G. Hopkins helped with data collection and writing of the manuscript. V. Eley helped with protocol design, ethics application, and writing of the manuscript. R. Christensen helped with protocol design, ethics application, data collection, and writing of the manuscript. A. A. J. Van Zundert helped with protocol design and writing of the manuscript. S. C. Wallis helped with data analysis and interpretation and writing of the manuscript. S. L. Parker helped with data analysis, interpretation of results, and writing of the manuscript. J. Lipman helped with protocol design and writing of the manuscript. J. A. Roberts helped with protocol design, data analysis, interpretation of results, and writing of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Nguyen NT, Goldman C, Rosenquist J, Arango A, Cole CJ. 2001. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life and costs. Ann Surg 3:279–291. 10.1097/00000658-200109000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz-Tovar J, Oller I, Llavero C, Arroyo A, Munoz JL, Calero A, Diez M, Zubiaga L, Calpena R. 2013. Pre-operative and early post-operative factors associated with surgical site infection after laparoscopic sleeve gastrectomy. Surg Infect (Larchmt) 14:369–373. 10.1089/sur.2012.114. [DOI] [PubMed] [Google Scholar]
- 3.Arias E, Martinez PR, Ka Ming Li V, Szomstein S, Rosenthal RJ. 2009. Mid-term follow-up after sleeve gastrectomy as a final approach for morbid obesity. Obes Surg 19:544–548. 10.1007/s11695-009-9818-6. [DOI] [PubMed] [Google Scholar]
- 4.Corcelles R, Boules M, Froylich D, Daigle CR, Hag A, Schauer PR, Rogula T. 2016. Laparoscopic three-port sleeve gastrectomy: a single institution case series. J Laparoendosc Adv Surg Tech A 26:361–365. 10.1089/lap.2015.0532. [DOI] [PubMed] [Google Scholar]
- 5.Topaloglu S, Avsar FM, Ozel H, Babacan M, Berkem H, Yildiz Y, Hengirmen S. 2005. Comparison of bariatric and non-bariatric elective operations in morbidly obese patients on the basis of wound infection. Obes Surg 15:1271–1276. 10.1381/096089205774512465. [DOI] [PubMed] [Google Scholar]
- 6.Lidor AO, Moran-Atkin E, Stem M, Magnuson TH, Steele KE, Feinberg R, Schweitzer MA. 2014. Hospital-acquired conditions after bariatric surgery: we can predict, but can we prevent? Surg Endosc 28:3285–3292. 10.1007/s00464-014-3602-y. [DOI] [PubMed] [Google Scholar]
- 7.Warren DK, Nickel KB, Wallace AE, Mines D, Tian F, Symons WJ, Fraser VJ, Olsen MA. 2017. Risk factors for surgical site infection after cholecystectomy. Open Forum Infect Dis 4:ofx036. https://academic.oup.com/ofid/article/4/2/ofx036/3044173?login=false. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano SA, Garvey PB, Baumann DP, Liu J, Butler CE. 2017. The impact of body mass index on abdominal wall reconstruction outcomes: a comparative study. Plast Reconstr Surg 139:1234–1244. 10.1097/PRS.0000000000003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thelwall S, Harrington P, Sheridan E, Lamagni T. 2015. Impact of obesity on the risk of wound infection following surgery: results from a nationwide prospective multicentre cohort study in England. Clin Microbiol Infect 21:1008.e1. 10.1016/j.cmi.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 10.SA Health, Government of South Australia. 2018. Surgical prophylaxis for abdominal surgery (revised November 2014). Therapeutic Guidelines Limited. https://www.sahealth.sa.gov.au. [Google Scholar]
- 11.SA Health, Government of South Australia. 2019. Surgical antibiotic prophylaxis for obese patients. Therapeutic Guidelines Limited. https://www.sahealth.sa.gov.au. [Google Scholar]
- 12.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA, American Society of Health-System Pharmacists, Infectious Disease Society of America, Surgical Infection Society, Society for Healthcare Epidemiology of America . 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 13.Martin C, Auboyer C, Dupont H, Gauzit R, Kitzis M, Lepape A, Mimoz O, Montravers P, Pourriat J. 2011. Antibioprophylaxie en chirurgie et médecine interventionnelle (patients adultes). Actualisation 2010. Ann Françaises D'Anesthésie Réanimation 30:168–190. (In French.) [DOI] [PubMed] [Google Scholar]
- 14.Christou NV, Jarand J, Sylvestre J-L, McLean APH. 2004. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg 14:16–22. 10.1381/096089204772787239. [DOI] [PubMed] [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing. Antimicrobial wild type distributions of microorganisms. https://mic.eucast.org/search/. Accessed 17 March 2022.
- 16.Anlicoara R, Ferraz AA, da PCK, de Lima Filho JL, Siqueira LT, de Araujo JG, Jr, Campos JM, Ferraz EM. 2014. Antibiotic prophylaxis in bariatric surgery with continuous infusion of cefazolin: determination of concentration in adipose tissue. Obes Surg 24:1487–1491. 10.1007/s11695-014-1231-0. [DOI] [PubMed] [Google Scholar]
- 17.Ho VP, Nicolau DP, Dakin GF, Pomp A, Rich BS, Towe CW, Barie PS. 2012. Cefazolin dosing for surgical prophylaxis in morbidly obese patients. Surg Infect 13:33–37. 10.1089/sur.2010.097. [DOI] [PubMed] [Google Scholar]
- 18.van Kralingen S, Taks M, Diepstraten J, van de Garde EM, van Dongen EP, Wiezer MJ, van Ramshorst B, Vlaminckx B, Deneer VH, Knibbe CA. 2011. Pharmacokinetics and protein binding of cefazolin in morbidly obese patients. Eur J Clin Pharmacol 67:985–992. 10.1007/s00228-011-1048-x. [DOI] [PubMed] [Google Scholar]
- 19.Edmiston CE, Krepel C, Kelly H, Larson J, Andris D, Hennen C, Nakeeb A, Wallace JR. 2004. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery 136:738–747. 10.1016/j.surg.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Brathwaite CE, Barkan A, Hall K, Chu G, Cherasard P, Wang S, Nicolau DP, Islam S, Cunha BA. 2017. Optimal cefazolin prophylactic dosing for bariatric surgery: no need for higher doses or intraoperative redosing. Obes Surg 27:626–629. 10.1007/s11695-016-2331-9. [DOI] [PubMed] [Google Scholar]
- 21.Hites M, Deprez G, Wolff F, Ickx B, Verleije A, Closset J, Loi P, Prevost J, Taccone FS, Racape J, Cotton F, Jacobs F. 2016. Evaluation of total body weight and body mass index cut-offs for increased cefazolin dose for surgical prophylaxis. Int J Antimicrob Agents 48:633–640. 10.1016/j.ijantimicag.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Cinotti R, Dumont R, Ronchi L, Roquilly A, Atthar V, Gregoire M, Planche L, Letessier E, Dailly E, Asehnoune K. 2018. Cefazolin tissue concentrations with prophylactic dose administered before sleeve gastrectomy in obese patients: a single centre study in 116 patients. Br J Anaesthesia 120:1202–1208. 10.1016/j.bja.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange ECM, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL, Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BWY, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. 2007. AAPS-FDA Workshop white paper. Microdialysis principles, application and regulatory perspectives. Pharm Res 24:1014–1025. 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 24.Marchand S, Chauzy A, Dahyot-Fizelier C, Couet W. 2016. Microdialysis as a way to measure antibiotics concentrations in tissues. Pharmacol Res 111:201–207. 10.1016/j.phrs.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Brill MJ, Houwink AP, Schmidt S, Van Dongen EP, Hazebroek EJ, van Ramshorst B, Deneer VH, Mouton JW, Knibbe CA. 2014. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother 69:715–723. 10.1093/jac/dkt444. [DOI] [PubMed] [Google Scholar]
- 26.Palma EC, Meinhardt NG, Stein AT, Heineck I, Fischer MI, de Araujo B, Dalla Costa T. 2018. Efficacious cefazolin prophylactic dose for morbidly obese women undergoing bariatric surgery based on evidence from subcutaneous microdialysis and populational pharmacokinetic modeling. Pharm Res 35:116. 10.1007/s11095-018-2394-5. [DOI] [PubMed] [Google Scholar]
- 27.Dorn C, Petroff D, Stoelzel M, Kees MG, Kratzer A, Dietrich A, Kloft C, Zeitlinger M, Kees F, Wrigge H, Simon P. 2021. Perioperative administration of cefazolin and metronidazole in obese and non-obese patients: a pharmacokinetic study in plasma and interstitial fluid. J Antimicrob Chemother 76:2114–2120. 10.1093/jac/dkab143. [DOI] [PubMed] [Google Scholar]
- 28.Gregoire M, Dumont R, Ronchi L, Woillard JB, Atthar V, Letessier E, Cinotti R, Roquilly A, Deslandes G, Jolliet P, Asehnoune K, Dailly E. 2018. Prophylactic cefazolin concentrations in morbidly obese patients undergoing sleeve gastrectomy: do we achieve targets? Int J Antimicrob Agents 52:28–34. 10.1016/j.ijantimicag.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Douglas A, Udy AA, Wallis SE, Jarrett P, Stuart J, Lassig-Smith M, Deans R, Roberts MS, Taraporewalla K, Jenkins J, Medley G, Lipman J, Roberts JA. 2011. Plasma and tissue pharmacokinetics of cefazolin in patients undergoing elective and semi-elective abdominal aortic aneurysm repair surgery. Antimicrob Agents Chemother 55:5238–5242. 10.1128/AAC.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative . 2008. The Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349. 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wong SL, Sawchuk RJ. 1993. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res 10:1411–1419. 10.1023/A:1018906821725. [DOI] [PubMed] [Google Scholar]
- 32.Parker SL, Guerra Valero YC, Roberts DM, Lipman J, Roberts JA, Wallis SC. 2016. Determination of cefalothin and cefazolin in human plasma, urine and peritoneal dialysate by UHPL-MS/MS: application to a pilot pharmacokinetic study in humans. Biomed Chromatogr 30:872–879. 10.1002/bmc.3622. [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration. 2018. Guidance for industry: bioanalytical method validation. FDA, Silver Spring, MD. [Google Scholar]
- 34.Byrne CJ, Parton T, McWhinney B, Fennell JP, O'Byrne P, Deasy E, Egan S, Enright H, Desmond R, Ryder SA, D'Arcy DM, McHugh J, Roberts JA. 2018. Population pharmacokinetics of total and unbound teicoplanin concentrations and dosing simulations in patients with haematological malignancy. J Antimicrob Chemother 73:995–1003. 10.1093/jac/dkx473. [DOI] [PubMed] [Google Scholar]
- 35.Vella-Brincat JWA, Begg EJ, Kirkpatrick CMJ, Zhang M, Chambers ST, Gallagher K. 2007. Protein binding of cefazolin is saturable in vivo both between and within patients. Br J Clin Pharmacol 63:753–757. 10.1111/j.1365-2125.2006.02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean body weight. Clin Pharmacokinet 44:1051–1065. 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 to S6. Download aac.00419-22-s0001.pdf, PDF file, 0.9 MB (974.8KB, pdf)