Summary:
A method is described for the solid-phase synthesis of oligonucleotides containing the DNA oxidation damage product, 5-(hydroxymethyl)-2′-deoxyuridine (HMdU) at selected sites using a phosphoramidite synthon regiospecifically protected on the 5-(hydroxymethyl) group.
5-(Hydroxymethyl)-2′-deoxyuridine (HMdU) is a damaged nucleoside formed by hydroxyl radical attack on thymidine residues in DNA. HMdU has been identified in the DNA of cells exposed to oxidizing chemicals and radiation.1–4 When administered to cells in culture, HMdU is both mutagenic5,6 and cytotoxic,7,8 presumably due in part to incorporation into cellular DNA. Attempts to further investigate the deleterious effects of HMdU in DNA using biochemical and physical methods have been hampered by the absence of suitable methods for the preparation of HMdU-containing oligonucleotides. We report here an efficient method for the solid-phase synthesis of oligonucleotides containing HMdU residues at selected sites.
Selective tritylation9 of the 5′-hydroxyl of HMdU was deemed problematic because the 5- and 5′-(hydroxymethyl) groups are of similar nucleophilicity. Previously, Prusoff and co-workers10 reacted HMdU with 1 equiv of p-toluenesulfonyl chloride and obtained the two monosulfonates, the disulfonate and parent compound. The 5′-tosylated derivative was isolated with difficulty in 12% yield. When following this scheme, however, one is still left with the difficult task of selectively generating base-labile protection for the 5-(hydroxymethyl) group and subsequent preparation of the 3′ phosphoramidite.
Recently, Levy and Teebor11 described the preparation of oligodeoxynucleotides containing HMdU using DNA polymerase and the triphosphate derivative of HMdU. These investigators utilized a biosynthetic approach after encountering severe difficulties during attempts to synthesize a suitably protected phosphoramidite derivative. It was reported that the triphosphate of HMdU is prepared in only 1–4% yield.11 Furthermore, the polymerase method11,12 would be inadequate for the preparation of the quantities of oligonucleotides containing HMdU at selected sites that would be required for physical studies.
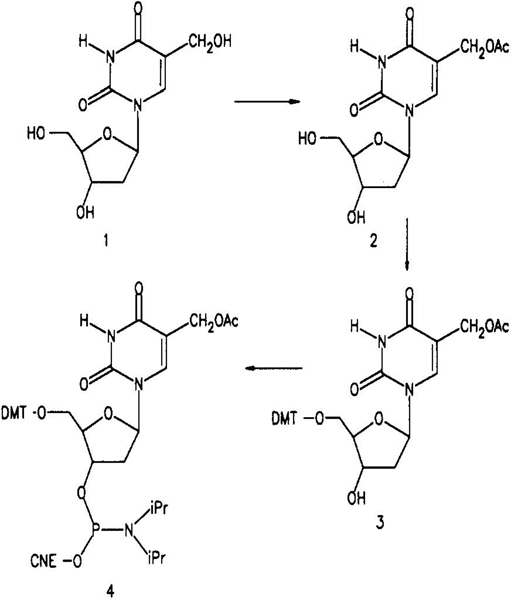
Alternatively, it is known that 5-(hydroxymethyl)uracil and derivatives will condense with both alcohols and carboxylic acids in the presence of protic acids.13,14 Scheit14 proposed the presence of a resonance-stabilized methylene cation to account for the difference in reactivity of the 5′-and 5-(hydroxymethyl) groups of 5-(hydroxymethyl) nucleosides under acidic conditions. We sought to exploit this selective reactivity to acetylate the 5-(hydroxymethyl) group of 5-(hydroxymethyl)-2′-deoxyuridine (HMdU). HMdU 1 (Scheme I), prepared by reaction of deoxyuridine and paraformaldehyde in aqueous KOH,10 was refluxed for 30 min in acetic acid containing HC1. While significant conversion occurred, glycosidic bond cleavage was also observed. Trifluoroacetic acid was substituted for the HC1, and the reaction was repeated.15 Analysis of the reaction components by silica gel thin-layer chromatography indicated the conversion of HMdU to a single product with little glycosidic bond cleavage. Under identical conditions, neither deoxyuridine nor thymidine reacted, consistent with the expected selectivity of the reaction.
Scheme 1.
Following the reaction of HMdU in acetic acid/trifluoroacetic acid, solvent was evaporated under reduced pressure. The product 2 was isolated as a white solid following silica gel chromatography in 84% yield. Mass spectral analysis of the product 2 indicated that the derivative was monoacetylated, with a mass of 30016 (M − 1 = 299, negative ion mode FAB). The composition of 2 was confirmed by elemental analysis. Upon aminolysis (60 °C, 12 h), the acetylated derivative 2 quantitatively regenerated the parent compound, HMdU 1.
The proton NMR spectrum of 2 confirms the site of acetylation. Previously, Frenkel et al.2 compared the proton NMR spectra of HMdU and the triacetylated derivative formed by reaction of HMdU with acetic anhydride in pyridine. The 5, 5′, and 3′ protons are well resolved for both HMdU and the triacetylated derivative. It was shown that acetylation shifts proton resonances for the 5, 5′, and 3′ protons downfield by 0.51,0.52, and 0.87 ppm, respectively. For the monoacetylated derivative 2, only the 5-methylene proton resonance was observed to shift downfield significantly (0.49 ppm) relative to parent compound 1.
Once acetylated, the HMdU derivative can be treated essentially as thymidine. The 5′-O-(4,4′-dimethoxytrityl) 3 and 3′-O-(2-cyanoethyl)-N,N-diisopropylphosphoramidite 4 derivatives were prepared by standard methods.9 A seven-base oligodeoxynucleotide of sequence 3′d(CGGH-GAC) was then prepared by manual solid-phase methods at the 15 μmol scale. All coupling yields, including that with the HMdU synthon, were >98% as indicated by release of trityl cation. Following the synthesis, the oligo was deprotected in aqueous ammonia at 60 °C, 12 h. The tritylated oligo was first purified by HPLC with a PRP column, followed by purification of the detritylated oligo by reversed-phase HPLC eluted with aqueous triethyl-ammonium acetate and acetonitrile.
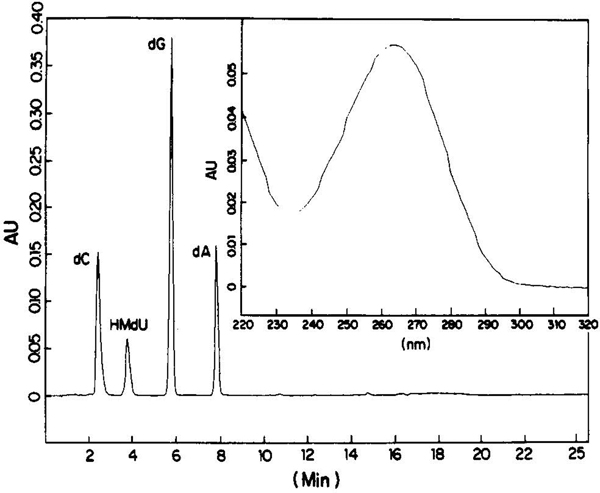
The purified seven-base oligo was homogeneous by HPLC. A fraction was enzymatically hydrolyzed with nuclease P1, followed by bacterial alkaline phosphatase.17 The oligo was digested to completion, indicating the correct formation of 3′−5′ internucleotide phosphate linkages. The resulting deoxynucleosides were analyzed by reversed-phase HPLC. As shown in Figure 1, peaks corresponding to the four bases present in the oligo were observed in the correct ratios based upon integration of the chromatogram at 260 nm (Figure 1). The UV spectrum of the HMdU peak eluting at 3.72 min (Figure 1, inset) was consistent with published data.18 Neither the acetylated derivative nor unknown peaks were observed.
Figure 1.
HPLC analysis of deoxynucleosides derived from the enzymatic hydrolysis of a seven base oligonucleotide 3′d-(CGGHGAC) containing HMdU. The labels indicate the identity of the peaks as determined by coincident retention times with authentic samples. Inset: UV spectrum of the HMdU peak (3.72 min); abs max, 264 nm; abs min 234 nm.
In this context, the quantitation of HMdU in DNA exposed to oxidizing conditions has been established by HPLC methods19 since GC/MS methods were unsatisfactory for this purpose.20 Deoxynucleosides for HPLC analysis are generated by enzymatic hydrolysis. However, for the GC/MS method, DNA is hydrolyzed with formic acid prior to trimethylsilylation. Reaction of HMdU under acidic conditions, which has been exploited here for the selective protection of HMdU, may likely explain the failure to detect (hydroxymethyl)uracil by the GC/MS method. The reactivity of HMdU derivatives under acidic conditions further suggests possible strategies for the selective derivatization of HMdU prior to either HPLC or GC/MS analysis.
The synthesis reported here provides a facile method for preparing oligonucleotides containing HMdU at specific positions suitable for biochemical and physical studies. This protection strategy also provides a method for generating additional nucleoside analogues and oligonucleotides containing modifications to the 5-position, including the radiolabeling of derivatives for quantitative analysis.
Supplementary Material
Acknowledgment.
This work was supported, in part, by NIH grants GM41336 and CA 42300 and funding from the City of Hope Cancer Center.
Footnotes
Supplementary Material Available: Experimental procedures (5 pages). This material is contained in libraries on microfiche, immediately follows this article in the microfilm version of the journal, and can be ordered from the ACS; see any current masthead page for ordering information.
References
- (1).Teebor GW; Frenkel K; Goldstein MS Proc. Natl. Acad. Sci. U.S.A. 1984, 81, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Frenkel K; Cummings A; Solomon J; Cadet J; Steinberg JJ; Teebor GW Biochemistry 1985, 24, 4527. [DOI] [PubMed] [Google Scholar]
- (3).Téoule R Int. J. Radiat. Biol. 1987, 51, 573. [DOI] [PubMed] [Google Scholar]
- (4).Cattley RC; Dietze SR; Richardson FC; Popp JA Nucleosides Nucleotides 1990, 9, 179. [Google Scholar]
- (5).Bilimoria H; Gupta SV Mutation Res. 1986, 169, 123. [DOI] [PubMed] [Google Scholar]
- (6).Shirnamé-Moré L; Rossman TG; Troll W; Teebor GW; Frenkel K Mutation Res. 1987, 178, 177. [DOI] [PubMed] [Google Scholar]
- (7).Kahilainen L; Bergstrom D; Kangas L; Vilpo JA Biochemical Pharmacology 1986, 35, 4211. [DOI] [PubMed] [Google Scholar]
- (8).Boorstein RJ; Teebor GW Cancer Res. 1989, 49, 1509. [PubMed] [Google Scholar]
- (9).Gait MJ, Ed. Oligonucleotide Synthesis, a practical approach; IRL Press: Washington D.C., 1984. [Google Scholar]
- (10).Shiau GT; Schinazi RF; Chen MS; Prusoff WH J. Med. Chem. 1980, 23, 127. [DOI] [PubMed] [Google Scholar]
- (11).Levy DD; Teebor GW Nucleic Acids Res. 1991,19, 3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yehle CO; Ganesan AT J. Biol. Chem. 1973, 248, 7456. [PubMed] [Google Scholar]
- (13).Cline RE; Fink RM; Fink KJ Am. Chem. Soc. 1959, 81,2521. [Google Scholar]
- (14).Scheit KH Chem. Ber. 1966, 99, 3884. [Google Scholar]
- (15).HMdU 1 was selectively acetylated on the 5-(hydroxymethyl) group by reflux in acetic acid containing catalytic trifluoroacetic acid. The acetylated derivative was isolated by silica gel chromatography in 84% yield.
- (16).The composition of 2 was verified by mass spectrometry, elemental analysis, and proton NMR spectroscopy.
- (17).Kasai H; Crain PF; Kuchino Y; Nishimura S; Ootsuyama A; Tanooka H Carcinogenesis 1986, 7, 1849. [DOI] [PubMed] [Google Scholar]
- (18).BSrwolff S; Langen P In Nucleic Acid Chemistry; Townsend LB, Tipson RS, Eds.; Wiley Interscience: New York, 1978; Part 1, p 359. [Google Scholar]
- (19).Frenkel K; Zhong Z; Wei H; Karkoszka J; Patel U; Rashid K; Georgescu M; Solomon JJ Anal. Biochem. 1991, 196, 126. [DOI] [PubMed] [Google Scholar]
- (20).Gajewski; Rao G; Nackerdien Z; Dizdaroglu M Biochemistry 1990, 29, 7876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.