Abstract
Background
The aim of this paper is to estimate the economic burden of children with congenital Zika Syndrome (CZS) in Brazil over 5–10 years.
Methods
We conducted a modelling study based on data collected in a case–control study in Brazil, including children with CZS (cases) and typically developing children (controls), born in 2015 and 2016. In total, 484 participants were recruited in two sites, Recife and Rio de Janeiro. Social and economic information was collected in a survey from the carers of cases and controls, and detailed healthcare utilisation was recorded for each child in the Rio de Janeiro cohort prospectively in a database. We used this information to estimate the cost per child with severe, moderate and no CZS and incremental cost per child with severe and moderate versus no CZS from a disaggregated societal perspective. These estimates were incorporated into an economic burden model to estimate the incremental burden of the CZS epidemic in Brazil over 5 years and 10 years.
Findings
The societal cost per child with severe CZS was US$50 523 to 10 years of age (born in 2015 and 2016), substantially higher than the costs for moderate CZS (US$29 283) and without CZS (US$12 331). The incremental economic burden of severe versus no CZS in Brazil over 10 years was US$69.4 million from the household and US$129.0 million from the government perspective. For moderate CZS, these figures amounted to US$204.1 million and US$86.6 million. Over 10 years, 97% of the total societal economic cost of severe CZS is borne by the government, but only 46% for moderate CZS.
Interpretation
The economic burden of CZS is high at the household, provider and government levels. The compensatory government payments helped to alleviate some of the additional costs incurred by families with a child qualifying for the disability benefits, and could be scaled to include the children with moderate CZS.
Keywords: child health, health economics, epidemiology, health services research, public health
WHAT IS ALREADY KNOWN ON THIS TOPIC
Some studies have published modelled estimates of the economic burden of congenital Zika Syndrome (CZS) in the Latin American region; however, none of them based their model on locally collected data of children with CZS but used extrapolation of proxy measures such as the lifetime cost of intellectual impairment in the USA.
WHAT THIS STUDY ADDS
Our study will be the first to describe in detail the economic burden to family, government and society of having a baby with CZS by modelling data collected from babies with CZS as well as controls and their caretakers in two sites in Brazil.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study shows the high economic burden to the household, provider and government; however, it also shows that the compensatory government payments helped to alleviate some of the additional costs incurred by families with a child qualifying for the disability benefit in Brazil, which could be scaled to include a broader range of children with CZS, but also other disabilities.
Introduction
Since 2009, WHO has declared a total of six Public Health Emergency of International Concern, all of which have been caused by infectious diseases and have, therefore, brought the threat of widespread global transmission leading to death, illness and adverse social and economic consequences. In 2015, the world’s attention was drawn to Brazil when the mosquito-transmitted Zika virus (ZIKV) infection during pregnancy was linked to the birth of thousands of babies born with microcephaly.1–3 Microcephaly is a condition where a baby is either born with a small head or the head stops growing normally after birth.4
Between late 2015 and end of 2017, a total of 18 282 suspected cases of microcephaly were recorded in Brazil, of which 3474 (19%) were confirmed as cases by December 2019 with the true number likely to be much higher.5 But microcephaly is only one of the possible complications found in neonates exposed to ZIKV during pregnancy. Evidence has emerged that ZIKV infection during pregnancy is also linked to a broader range of conditions in the child, including visual, hearing, cognitive and musculoskeletal abnormalities, collectively called ‘congenital Zika Syndrome’ (CZS), which is the term we will use in this paper.6–9 CZS shares similarities with cerebral palsy (CP),10 as both are neurodevelopment disorders associated with a broad range of impairments. Indeed, Marques et al showed that in their study sample, 90% of 39 children with CZS met the criteria for the diagnosis of CP.11 A clear case definition of CZS is currently still lacking, as more evidence of the syndrome is emerging over time.
The immediate emphasis in response to the ZIKV epidemic was on the prevention of infection, development of diagnosis and treatment, and avoidance of pregnancy. Mitigating the social and economic impacts of CZS received less attention, although these are likely to be substantial, for both families and the government. Carers of children with disabilities experience increased costs, both direct costs (eg, drugs) and indirect costs (eg, foregone wages of carer),12 often leading to financial difficulties.13 These financial pressures further increase the risk of anxiety and depression, which are already commonly experienced by parents of disabled children.14–16 Purely model-based estimates suggest that the lifetime costs per microcephaly case are around $191 102 and $28 818 ($ refer to US$ unless is otherwise specified) for Latin America and the Caribbean, respectively. These estimates were calculated without collecting data on resource use or cost and were conservatively based on the extrapolation of the lifetime costs of a case of intellectual impairment in the USA.17 Another study conducted by UNDP assessing the socioeconomic impact of Zika used the same modelled cost data in their model.18 A third study conducted in Puerto Rico evaluating the cost effectiveness of contraception during the Zika outbreak used the MarketScan Commercial Database based on US residents covered by employer-sponsored insurance to estimate a lifetime cost of $3 788 843 ($2 243 124–$5 545 652) per severe case of CZS19 and was based on a sample size of four children with CZS. While the Brazilian unified health system (Sístema único de Saúde) is providing universal access to healthcare services for its population, including children with CZS,20 there is likely a gap between what the health system provides and what services families access. A better understanding of the true social and economic burden experienced by the families and their support needs is paramount for governments to be able to tailor services and budget adequately.
The aim of this paper is to estimate the economic burden of children affected by CZS to the government, household and society in Brazil, measured over the medium term, taken here to represent up to 5–10 years of age. The term ‘burden’ is used to be consistent with the economic literature, but not to suggest that children with CZS are themselves a burden. This paper builds on our previous work estimating the incremental cost of having a baby with CZS measured over the first 2 years of life21 (incremental cost is the additional cost experienced by one group vs another in this case of a baby with vs a baby without CZS). The lessons learnt in this paper could be applicable to future epidemics, where an infection during pregnancy causes congenital brain abnormalities. Furthermore, to the best of our knowledge, this paper is the first describing in detail the economic burden to both family and government of having a baby with a severe congenital disability by using a mixture of bottom-up and reference costing methods.
Methods
Overview and setting
We conducted a modelling study based on data collected in a case–control study, including children with microcephaly or other CZS manifestation (cases) and children without microcephaly or other CZS manifestation (controls), born from late 2015 through 2016.10 (To clarify, the term modelling the costs refers to simulating the costs based on a model over a longer than measured time period.) The case–control study was undertaken in two different sites in Brazil: Recife in Pernambuco State, which was the epicentre of the epidemic, and Rio de Janeiro, which was less affected. Social and economic information was collected in a survey from the carers of cases and controls in both sites, and detailed healthcare utilisation was recorded for each child in the Rio de Janeiro Cohort prospectively in a database over time.10 The survey information was used to estimate the household costs and the healthcare utilisation information to estimate the health provider cost. This information allowed us to estimate the cost per child with severe, moderate and no CZS as well as the incremental (=additional) costs of a child with (1) severe and (2) moderate CZS versus no CZS from the health provider, government, family and societal perspective over 5 years and 10 years. These estimates were incorporated into an economic burden model to estimate the total economic burden of the CZS epidemic in Brazil to 5 years and 10 years of age by calculating the total and incremental cost for all estimated cases of severe and moderate versus no CZS in Brazil.
Recruitment and data collection
In Pernambuco, cases and controls were selected from two different studies. Most cases came from an existing case–control study and some from an ongoing cohort of children with microcephaly. Cases were children born with a head circumference by at least two SD smaller than the mean for their sex and gestational age on the Fenton growth chart (case control) or Intergrowth standard (cohort).22 A very similar definition was used by the Ministry of Health in Brazil for all cases of suspected microcephaly that were being included for further clinical investigation, although their approach has changed 3× since 2015 (table 1).23 In Pernambuco, cases were classified as ‘severe’ if the head circumference was more than 3 SDs below the mean and as ‘mild/moderate’ if head circumference was between 2 SDs and 3 SDs smaller. Controls had a head circumference above −2 SDs, did not have any neurological or other health problems, were born in the same hospitals and were matched to cases by expected date of delivery and maternal residence. During the follow-up period, the Denver II Developmental Screening Test was conducted and if this identified developmental delays in a child in the control group, this child was excluded from the study and referred for further investigation (see the online supplemental appendix, p3 for more detail).
Table 1.
Definition of cases and controls used in study and by Ministry of Health in Brazil
| CZS/microcephaly cases | Controls/not microcephaly cases | Other disabilities included in controls | |
| Ministry of Health, Brazil | In the beginning of the microcephaly epidemic, the Brazilian Ministry of Health defined a more sensitive parameter (HC ≤33 cm, for both sex), but it changed in December 2015 (HC ≤32 cm), to reduce the number of false positive cases. In March of 2016, under WHO recommendation, it was modified to 31.5 cm for girls and 31.9 cm for boys, for full-term newborn. From August 2021, the Intergrowth standard was adopted, being even more specific, as the HC 30.24 cm for girls and 30.54 cm for boys, for those born at 37 weeks or more. Suspected microcephaly cases sent for investigation:
If <−3 SD classified as severe microcephaly |
See changing case definition over time | n/a |
| Case–control study in Pernambuco | Severe CZS included children with head circumference at least −3 SD than the mean for sex and gestational age on the Fenton growth chart and mild/moderate if at least −2 SD. All cases had mothers with a laboratory confirmed ZIKV infection during pregnancy |
Above −2 SD HC than the mean for sex and gestational age on the Fenton growth chart for children from the case–control study and compared with the Intergrowth standard in the cohort study | Excluded based on Denver II Developmental Screening Test conducted with caregivers of controls |
| Cohort study in Rio de Janeiro | Born to mothers that were ZIKV positive during pregnancy with microcephaly or significant developmental delays. Microcephaly was assessed as above, defining an HC of −3 SD as severe and of −2 SD as moderate (using Intergrowth standard) and other children were assessed using the Bayley Scale of Infant Development25) and/or by assessment by two paediatricians Severe: Bayley score of <70 Moderate: Bayley score of 70–<85 |
A head circumference of above -2SD on the Intergrowth standard and a Bayley score of ≥85 between 6 months and 36 months of age | Included N=3, prevalence of 1.6% in control group. As comparison, the prevalence of at least one disability in the 0–9 year age group in the National Health Survey in 2013 was 1.6%28 |
CZS, congenital Zika Syndrome; HC, head circumference; SD, Standard Deviation; ZIKV, Zika virus.
bmjgh-2022-008784supp001.pdf (1.3MB, pdf)
In Rio de Janeiro, cases and controls were recruited from an existing cohort study (ClinicalTrials.gov) set up to study the impact of CZS.24 Cases were born to mothers known to be ZIKV positive with either (1) microcephaly or significant developmental delay and/or other clinical conditions consistent with CZS (‘severe CZS’) or (2) less severe developmental delay (‘mild/moderate CZS’). Control subjects were born to mothers without a history of symptoms and without developmental delay. Assessment of both cases and controls without microcephaly was based on the Bayley Scale of Infant Development25 following the recommended guidelines and/or assessment by two paediatricians based on the child’s medical records (see table 1 for an overview and online supplemental appendix, p3 for more detail).
According to case and control definitions, children in both settings were categorised into three groups: (1) children with severe CZS (microcephaly or with serious developmental delay), (2) children with mild/moderate CZS and (3) children not affected by CZS.
In both sites, caregivers were interviewed by trained female interviewers either in their home, health facility or occasionally their workplace between May 2017 and January 2018. The questionnaire collected information on healthcare utilisation, direct and indirect costs, socioeconomic status of caregivers, and other parental indicators (eg, mental health indicators and social support). An electronic medical records database recorded detailed healthcare utilisation for each child in the Rio de Janeiro cohort prospectively between August 2015 and 31 May 2018. Hospital data from electronic medical records that included hospitalisations, tests and exams were not available for Pernambuco. Costs for each procedure or resource were taken from the Brazilian cost reference table (SIGTAP), and when not available they were calculated using an ingredients approach.26 27 A small number of additional parameters (eg, to calculate the cost of a wheelchair) were based on expert opinion. Details can be found in table 2 and on p4 in the online supplemental appendix.
Table 2.
Other parameters used in model
| Estimate | Distribution | Reference/source | |
| Additional cost parameters and assumptions | |||
| Parameters to estimate cost of wheelchair to both household and provider | |||
| % of children needing a wheelchair: severe CZS | 81.9% | Point estimate | Expert opinion |
| % of children needing a wheelchair: moderate CZS | 5.0% | Point estimate | Expert opinion |
| % of children needing a wheelchair: no CZS | 0.1% | Point estimate | Expert opinion |
| Wheelchair cost to the health provider (R$962.5) US$, 2017 | 301.4 | Point estimate | 27 |
| Wheelchair cost (incl. adaptation) to the household (R$4000) US$, 2017 | 1252.7 | Point estimate | Expert opinion |
| Replacement wheelchair at age (years) | 3,4,5,6,7 and 10 | Point estimate | Expert opinion |
| Other costs to the government (education and disability benefit) | |||
| Special creche from age 3 years for CZS (federal payment per pupil R$4420.7) US$, 2017 | 1384.5 | Gamma* | 45 |
| Primary education from age 4 (federal payment per pupil: R$4080.7) US$, 2017 | 1278.0 | Gamma* | 37 |
| Ratio cost special needs education/cost primary education | 1.2 | Lognormal | Expert opinion |
| Disability benefit per year (monthly min. wage: R$937) US$, 2017 | 3521.5 | Point estimate | 46 |
| Parameters to model costs and outcomes | |||
| General | |||
| Discount rate costs | 5% | Point estimate | 35 |
| Discount rate outcomes | 5% | Point estimate | 35 |
| Model length (years) | 5 and 10 | Assumption | |
| Exchange rate Brazilian Real to US$, 2017 | 0.31 | Point estimate | 31 |
| Average annual inflation rate Brazil 2008–2017 (mean, SE) | 6.1%, 1.9% | Beta | 36 |
| Modelling clinical burden | |||
| Mortality severe CZS year 1 (%, alpha, beta) | 4.9%, 6, 116 | Beta | Estimated from Rio de Janeiro Cohort |
| Mortality severe CZS year 2 (%, alpha, beta) | 2.6%, 3, 113 | Beta | Estimated from Rio de Janeiro Cohort |
| Mortality severe CZS year 3 (%, alpha, beta) | 0.9%, 1, 112 | Beta | Estimated from Rio de Janeiro Cohort |
| Mortality severe CZS year 4 (%, alpha, beta) | 0.9%, 1, 111 | Beta | Assumed to be the same as year 3 |
| Mortality severe CZS year 5 (%, alpha, beta) | 0.9%, 1, 110 | Beta | Assumed to be the same as year 3 |
| Mortality severe CZS year 6–10 per year (%) | 0.3% | Beta | Assumed to be 1/3 of mortality in years 3–5 |
| Mortality Brazil (moderate CZS and controls) year 1 (%, alpha, beta) | 1.30%, 13.0, 987.0 | Beta | 47 |
| Mortality Brazil (moderate CZS and controls) year 2 (%, alpha, beta) | 0.10%, 1.0, 999.0 | Beta | Estimate based on infant and <5-year mortality47 |
| Mortality Brazil (moderate CZS and controls) year 3 (%, alpha, beta) | 0.03%, 0.3, 999.7 | Beta | Estimate based on infant and <5-year mortality47 |
| Mortality Brazil (moderate CZS and controls) year 4 (%, alpha, beta) | 0.03%, 0.3, 999.7 | Beta | Estimate based on infant and <5-year mortality47 |
| Mortality Brazil (moderate CZS and controls) year 5 (%, alpha, beta) | 0.03%, 0.3, 999.7 | Beta | Estimate based on infant and <5-year mortality47 |
| Mortality Brazil (moderate CZS and controls) year 6–10 per year (%, alpha, beta) | 0.01% | Beta | Assumed to be 1/3 of mortality in years 3–5 |
| Cases of CZS Brazil confirmed | 3474 | Point Estimate | 5 |
| Cases of CZS Brazil under investigation | 2659 | point estimate | 5 |
| Cases of CZS Brazil probable | 743 | Point estimate | 5 |
| Cases of CZS Brazil deceased (fetal death, stillbirth, infant death and child death) | 505 | Point estimate | 5 |
| Disability weight severe cerebral palsy for severe CZS | 0.82 | Lognormal * | 33 |
| Disability weight mild/moderate cerebral palsy for mild/moderate CZS | 0.36 | Lognormal * | 33 |
| Length disability=time horizon of model (years) | 5 and 10 | Point estimate | Decided by study team |
Table 2 shows parameters used in the economic burden model, that were not estimated in the costing analysis. This includes parameters to model the cost of a wheelchair as well as other costs to the government, general model parameters such as discount rate and also parameters to model the epidemiological burden of CZS.
*Standard error assumed to be 10% of mean.
CZS, congenital Zika Syndrome.
Data analysis
Costs to the health provider, government, household and society as well as health burden were estimated separately for three groups: (1) ‘severe CZS’, (2) ‘mild/moderate CZS’ and (3) controls= ‘no CZS’. Costs were subsequently modelled over 5 years and 10 years. First, the total cost per child for each group and the incremental cost per child of severe CZS as well as mild/moderate CZS compared with ‘no CZS’ was calculated. Thereafter, the cost per child and the estimated number of cases of CZS in Brazil were used to model for the whole of Brazil the total economic burden for each group as well as the incremental economic burden of severe CZS and mild/moderate CZS compared with ‘no CZS’.
Children with a ZIKV-unrelated disability in the control group in Rio (not in Pernambuco) were included in the analysis (N=3, equals 1.6% prevalence in the control group; conditions were dwarfism, osteogenesis imperfecta and hypoxic ischaemic lesions) to ensure the control group and its estimated cost is relatively representative of the general population in Brazil. For comparison, the prevalence of at least one disability in the 0–9 year age group was 1.6% in the National Health Survey 2013 (95% CI 1.4% to 1.9%).28
Health provider costs
Health provider costs were calculated from health utilisation data recorded prospectively from 280 children in Rio de Janeiro, of which 95 had severe CZS, 19 moderate CZS and 166 no CZS. The health provider costs were calculated by multiplying the number of consumed resources (eg, specialist or non-specialist outpatient visits) from the medical records database from Rio de Janeiro times the cost for each procedure/resource as indicated on SIGTAP.27 Costs on SIGTAP have been shown to underestimate the true cost to the health provider and hence we followed the national cost-effectiveness literature, which suggests to adjust costs indicated on the database by a factor of 3.51.29 30 Provider costs were split into four cost categories: visits (specialised and non-specialised), hospitalisations, drugs/tests and other. The ‘other’ group included special interventions such as orthopaedic surgery or prothesis. Costs were estimated per group (severe, moderate and no CZS) and per year (table 3) up to the first 3 years of age, depending on the date of birth of the child, with data from 280, 277 and 109 children in the first year, second year and third year, respectively (for more detail on sample size per group and year and extrapolation of costs, please see online supplemental appendix table S1 and p4).
Table 3.
Input estimates—cost estimates measured in study
| Severe CZS | Moderate CZS | No CZS | |
| Health provider costs per year (Rio de Janeiro)*† | |||
| Cost of specialist/non-specialist visits to the health provider | Mean/SE | Mean/SE | Mean/SE |
| Year 1 US$, 2017 (mean, SE) | 258.4, 19.7 | 151.3, 19.0 | 128.3, 8.0 |
| Year 2 US$, 2017 (mean, SE) | 275.4, 16.0 | 135.7, 18.5 | 78.9, 6.8 |
| Year 3 US$, 2017 (mean, SE) | 326.1, 122.2 | 126.6, 85.4 | 14.0, 5.1 |
| Cost of hospitalisation to the health provider | |||
| Year 1 US$, 2017 (mean, SE) | 1098.6, 250.1 | 494.9, 360.9 | 189.1, 67.7 |
| Year 2 US$, 2017 (mean, SE) | 3.3, 1.1 | 0.0, 0.0 | 48.6, 35.5 |
| Year 3 US$, 2017 (mean, SE) | 1282.7, 816.6 | 346.9, 245.1 | 351.0, 217.9 |
| Cost of other services to the health provider (eg, orthotics and prosthetics) | |||
| Year 1 US$, 2017 (mean, SE) | 272.9, 67.7 | 0.0, 0.0 | 0.0, 0.0 |
| Year 2 US$, 2017 (mean, SE) | 256.0, 70.8 | 0.0, 0.0 | 0.0, 0.0 |
| Year 3 US$, 2017 (mean, SE) | 2798.7, 135.9 | 124.6, 12.5 | 0.0, 0.0 |
| Cost of diagnostic tests, physical examinations and drugs to the health provider | |||
| Year 1 US$, 2017 (mean, SE) | 638.7, 54.4 | 277.7, 51.1 | 304.5, 25.6 |
| Year 2 US$, 2017 (mean, SE) | 342.8, 42.5 | 325.6, 55.3 | 194.2, 21.4 |
| Year 3 US$, 2017 (mean, SE) | 181.1, 88.4 | 4.2, 4.2 | 50.5, 32.0 |
| Out of pocket costs to the household per year (Rio de Janeiro, Recife)*‡ | |||
| Irregular costs to the household | |||
| Cost of moving house/relocation due to disability of child | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 10.1, 5.7 | 4.7, 4.7 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 2.3, 4.8 | 22.2, 20.3 | 0.0, 0.0 |
| Cost of altering house due to disability of child | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 37.8, 17.7 | 0.0, 0.0 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 69.1, 15.5 | 35.4, 24.9 | 0.0, 0.0 |
| Cost of coping§ with change due to disability of child | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 261.4, 138.7 | 117.6, 92.3 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 179.1, 51.0 | 119.8, 72.5 | 0.0, 0.0 |
| Cost of special food for child (mostly special formula milk) | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 475.6, 79.0 | 124.1, 95.5 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 563.6, 67.7 | 261.1, 105.4 | 0.0, 0.0 |
| Regular costs to the household (to be modelled beyond 2 years) | |||
| Cost of visits (includes transport, fuel, etc) | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 1087.7, 207.0 | 1202.8, 349.0 | 186.2, 40.3 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 787.9, 157.0 | 384.5, 175.4 | 193.2, 33.8 |
| Cost of hospitalisation | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 85.7, 29.3 | 31.3, 31.3 | 241.5, 229.3 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 148.0, 68.9 | 29.6, 16.8 | 10.1, 4.6 |
| Cost of healthcare plan (average of both years to be modelled) | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 218.3, 53.5 | 94.0, 94.0 | 80.3, 29.2 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 172.4, 33.4 | 364.0, 112.7 | 155.1, 28.3 |
| Cost of drugs and vitamins (mostly epilepsy drugs) | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 374.7, 73.7 | 205.0, 86.3 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 595.2, 77.6 | 208.4, 58.5 | 0.0, 0.0 |
| Cost of visual aids for child (modelled for years 1–5, year 7 and year 9) | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 55.5, 10.9 | 0.0, 0.0 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 76.9, 12.5 | 9.1, 6.4 | 0.0, 0.0 |
| Cost of tests | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 13.7, 6.7 | 0.0, 0.0 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 12.2, 4.6 | 0.0, 0.0 | 0.0, 0.0 |
| Other direct costs to the household | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 36.5, 18.8 | 0.0, 0.0 | 0.0, 0.0 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 73.0, 21.0 | 0.0, 0.0 | 0.0, 0.0 |
| Indirect cost of lost household income due to visits, appointments | |||
| Year 1 (age 0–1 years) US$, 2017 (mean, SE) | 722.2, 263.9 | 766.5, 513.8 | 222.2, 62.5 |
| Year 2 (age 1–2 years) US$, 2017 (mean, SE) | 132.5, 60.6 | 239.5, 129.3 | 163.9, 43.2 |
Table 3 shows cost parameters to the health provider and household estimated in the costing analysis. Costs are shown for each cost category (eg, cost of hospitalisation) by year (year 1–3 for health provider costs and year 1–2 for household costs) and group (severe CZS, moderate CZS and no CZS). The first value represents the mean and the second represents the SE.
*Distribution used in the model for all provider and household costs was the gamma distribution.
†Source for all provider costs was healthcare utilisation data: Rio de Janeiro.27
‡Source for all household costs were the cross-sectional surveys conducted in Rio de Janeiro and Recife.
§The cost of coping includes selling of assets and borrowing money to cope with the additional costs incurred because of having child with CZS.
CZS, congenital Zika Syndrome; SE, Standard error.
Household costs
First year and second year of life out of pocket payments and indirect costs (ie, lost household income due to caregiving) were estimated using the survey data stratified by children’s age (≤1 year and >1 year). In total, 484 caregivers, 271 from Rio de Janeiro and 213 from Recife were interviewed. The average cost per year and per child was calculated for the first year and second year of life and presented in 12 cost subcategories grouped into irregular and regular (ie, recurring costs) to facilitate subsequent modelling. The irregular costs were not modelled beyond the measured years due to too much uncertainty (see table 3).
Data analysis
Data analysis was conducted in SPSS 25, STATA SE 16, Microsoft Excel 2013 and R. Cost results per child stratified by group (severe CZS, moderate/mild CZS and control) were valued in 2017 US$ using an exchange rate of R$/US$3.19.31
Epidemiological parameters
Estimates of the number of severe CZS cases in Brazil were obtained from the Ministry of Health up to December 2019: 3474 cases were confirmed from a total of 18 282 suspected cases, with a case definition consistent with severe CZS. In addition, 743 cases were probable, 615 inconclusive and 2659 under investigation.5 The base analysis (minimum) used the confirmed cases only. The number of children with mild/moderate CZS in Brazil is unknown, but a cohort study from Rio de Janeiro conducted with children exposed to ZIKV infection in pregnancy provides insights for the model. Out of a total of 216 children at 7–32 months of age, 2.7% had microcephaly (3.7%, but in two cases microcephaly resolved during follow-up), 31.5% scored below average in neurodevelopment and/or abnormal eye or hearing assessments with 12% scoring below −2 SDs in at least one domain.32 Based on these findings and discussion with researchers and physicians working with the children with CZS in Brazil, the number of cases of mild/moderate CZS in Brazil in our model were conservatively assumed to be 5× the number of cases of severe CZS. Specific annual mortality rates were applied to each group at the end of each year in the model, with annual mortality rates as observed in the Rio de Janeiro cohort used for severe CZS and national mortality rates for mild/moderate CZS and controls (table 2). Disability adjusted life years (DALYs) were calculated for each group using disability weights from a report on the economic impact of CP in Australia, verified by study physicians and researchers working with the children in Recife and Rio de Janeiro33 (table 2).
Economic burden model
A model to estimate the economic burden of CZS in Brazil was built in Excel 2013 and analysed from the health provider, government, household and societal perspectives of Brazil. Due to the uncertain life expectancy of children with CZS, we use two time horizons: 5 years and 10 years. The provider perspective includes the cost of healthcare and the government perspective additionally includes the disability benefits and education. The household perspective includes the direct and indirect costs incurred by a household. Finally, the societal perspective combines the costs incurred by the government and households. For children with severe CZS receiving disability benefits to prevent double counting, the disability benefit was deducted from the household costs to estimate ‘net household’ and ‘net societal costs’.
Costs beyond the third year of life (or second year for household costs) for all three groups (severe CZS, moderate/mild CZS, no CZS) were modelled using relative cost ratios between age groups taken from a Danish national study on the lifetime cost of CP versus no CP34—CP being considered by experts the best proxy for CZS (for detail see online supplemental appendix figure S1 and p5). Both costs and DALYs were discounted annually by 5%, as recommended in the Brazilian guidelines, starting from year 3 (2018) as the year of analysis was 2017.35 Costs were expressed in US$ in 2017, using annual historical exchange rates and the average annual inflation rate were used to convert costs to US$ in 2017.31 36 A detailed overview of all cost estimates for years 1–10 (estimated and modelled), the baseline cost for modelling, as well as the relative cost ratios (derived from the Danish national study on the lifetime cost of CP), which were used when modelling the costs beyond year 2 for household costs and year 3 for provider costs can be found in the online supplemental appendix table S1.
For each perspective (health provider/government/household/societal) and group (severe CZS/moderate CZS/controls), annual costs were calculated for years 1–10 and the overall cost burden to 5–10 years of age was obtained by multiplying those costs by yearly case number in each group. The incremental economic burden at country-level was estimated as the difference in total economic cost of either severe or moderate CZS versus controls estimated for each perspective to 5 years or 10 years of age.
Results are presented as the total (severe CZS, moderate CZS and no CZS) and incremental cost (=additional cost of a child with (1) severe CZS or (2) moderate CZS versus no CZS) per child by perspective to 5 years and 10 years of age in US$, while the economic burden in Brazil is presented as the incremental burden in US$ million by perspective to 5 years and 10 years of age of either severe CZS or moderate CZS compared with no CZS. For the latter, the online supplemental appendix contains in addition the total (not incremental) cost for each group in US$ million.
The robustness of our results were tested using a probabilistic and a deterministic sensitivity analysis. The probabilistic sensitivity analysis included 10 000 iterations, which were used to estimate the mean, median and a 95% CI (based on percentiles) of key outcomes. The deterministic sensitivity analysis consisted in a scenario analysis, where the estimated case numbers of severe CZS were varied to include additional numbers of cases compared with the conservative estimate of confirmed cases only (N=3474) used in the base case analysis (=confirmed): (1) likely (N=5175)—using confirmed cases plus 50% of the cases classified as probable and under investigation and, lastly, (2) maximum (N=6876)—using the sum of confirmed, probable and under investigation cases. The number of mild/moderate CZS cases were kept at 5× the confirmed number of severe CZS cases.
Ethical considerations
Ethical approval for the study was received from London School of Hygiene and Tropical Medicine (LSHTM) and the Fiocruz ethics committee (CAAE 60682516.2.1001.5269). All interviewees were adults and provided written informed consent, as outlined previously.10
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Patient and public involvement
Carers of babies were involved in the design of the questionnaire and assisted us in developing the most appropriate questions. We have disseminated the main results at several occasions to patients as well as general public.
Results
The costs of healthcare visits remained relatively similar across the 3 years for both children with severe CZS and moderate CZS but showed a decreasing trend for children without CZS. For all three groups, the cost of hospitalisation was much higher in year 1 and year 3 compared with year 2. For children with severe CZS, hospitalisation ($1099, $3 and $1283 in years 1–3) and in year 3 other services ($2799) were the highest cost contributors. The cost of other services was zero for children without CZS and comparatively low ($125 in year 3) for children with moderate CZS. A decreasing trend in the costs of tests and drugs was observed over the 3 years across all groups.
The largest contributors to the household costs for children with severe CZS were the costs of visits (including transport and fuel) and the indirect cost of income lost, followed by the cost of special food. According to the caregivers, the latter was mainly due to the cost of special thickened formula milk that these children required.
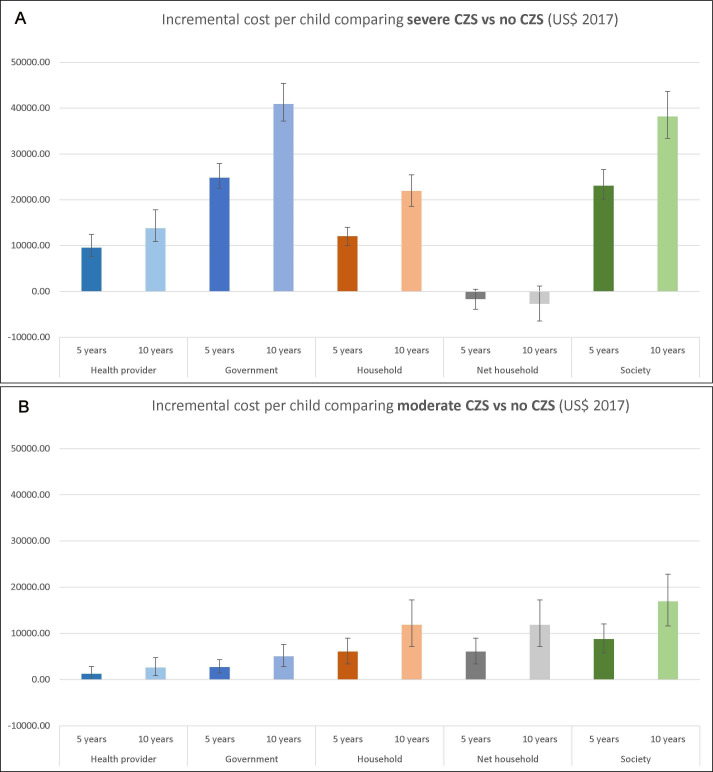
The disaggregated and total cost per child modelled over 5 years or 10 years and presented by cost bearer were estimated for children with severe CZS, moderate CZS and no CZS (table 4, online supplemental appendix figure S1 for base case results and online supplemental appendix table S2 for probabilistic sensitivity analysis results). The incremental cost per child of severe CZS and moderate CZS versus no CZS is shown by perspective in figure 1 and online supplemental appendix table S3. Our analysis demonstrates that, regardless of perspective, a child with severe CZS incurred the highest costs, followed by moderate CZS and no CZS. The societal cost per child with severe CZS was US$28 664 (95% CI 26 059 to 32 047) to 5 years and US$50 523 (95% CI 45 982 to 56 020) to 10 years, substantially higher than the costs for moderate CZS (US$14 383 (95% CI 11 799 to 17 608) and US$29 283 (95% CI 24 303 to 35 364), respectively) and without CZS (US$5554 (95% CI 4559 to 7043) and US$12 331 (95% CI 10 511 to 14 614)). For children with severe CZS, the largest share of the costs is born by the government with (US$27 703 (95% CI 25 494 to 30 764) and US$49 250 (95% CI 45 449 to 53 864) to 5 years and 10 years). Costs to households were also high (US$14 723 (95% CI 13 157 to 16 451) and US$25 932 (95% CI 22 972 to 29 254)). Interestingly, when concerning the household’s total and net cost per child (net cost meaning the disability benefits paid to households with children with severe CZS was deducted from the total household cost of these families), families of children with moderate CZS suffered a far higher cost (US$8753 (95% CI 6471 to 11 549) and US$15 917 (95% CI 11 608 to 21 213) to 5 years and 10 years) than families of children with severe CZS (US$961 (95% CI −859 to 2924) and US$1273 (95% CI −2103 to 4952)) or even no CZS (US$2677 (95% CI 1961 to 2994) and US$4021 (95% CI 3004 to 5747)) (table 4 and online supplemental appendix figure S2). This is best highlighted by looking at the incremental costs per child as shown in figure 1 (also see online supplemental appendix table S3). For families caring for a child with severe CZS compared with no CZS, the incremental net household cost to 5 years is −$1715 (95% CI −$3886 to $438) and to 10 years −$2743 (95% CI −$6476 to $1157). This is contrasted by an incremental net household cost of moderate CZS versus no CZS of $6071.1 (95% CI $3426 to $9002) to 5 years and $11 892 (95% CI $7209 to $17 276) to 10 years. These results show that the compensatory government payments cover to some extend the additional costs incurred by families with a child qualifying for the disability benefits, a policy specific to children with microcephaly. However, families with a child with moderate CZS (or a child with severe CZS not passing the disability benefit eligibility criteria) do not receive sufficient support and bear a significant cost burden.
Table 4.
Costs (US$, 2017) per child modelled to 5 years and 10 years of age (base case)
| Severe CZS | Moderate CZS | No CZS | |
| Costs per child to the health provider/government | Cost per child (US$, 2017) | Cost per child (US$, 2017) | Cost per child (US$, 2017) |
| Cost of specialist/non-specialist visits to the health provider | |||
| Modelled to 5 years of age | 1421.59 | 685.21 | 324.07 |
| Modelled to 10 years of age | 2859.77 | 1376.96 | 480.98 |
| Cost of hospitalisation to the health provider | |||
| Modelled to 5 years of age | 3260.21 | 1156.03 | 801.57 |
| Modelled to 10 years of age | 4132.43 | 1463.95 | 1032.43 |
| Cost of other services to the health provider (eg, orthotics and prosthetics) | |||
| Modelled to 5 years of age | 4501.51 | 167.66 | 0.00 |
| Modelled to 10 years of age | 5718.67 | 213.25 | 0.00 |
| Cost of diagnostic tests, physical examinations and drugs to the health provider | |||
| Modelled to 5 years of age | 1504.64 | 999.51 | 646.06 |
| Modelled to 10 years of age | 2327.93 | 1920.17 | 911.44 |
| Cost of wheelchair to the health provider | |||
| Modelled to 5 years of age | 672.43 | 41.04 | 0.82 |
| Modelled to 10 years of age | 1236.17 | 75.46 | 1.51 |
| Cost of education to the government | |||
| Modelled to 5 years of age | 2580.58 | 2580.58 | 1103.99 |
| Modelled to 10 years of age | 8316.22 | 8316.22 | 5883.69 |
| Cost of disability allowance to the government | |||
| Modelled to 5 years of age | 13 762.09 | n/a | n/a |
| Modelled to 10 years of age | 24 659.20 | n/a | n/a |
| Total costs per child to the health provider/government | |||
| Total cost per child to the health provider | |||
| Modelled to 5 years of age | 11 360.38 | 3049.45 | 1772.52 |
| Modelled to 10 years of age | 16 274.97 | 5049.78 | 2426.36 |
| Total cost per child to the government (incl. disability allowance if applicable and education) | |||
| Modelled to 5 years of age | 27 703.05 | 5630.03 | 2876.51 |
| Modelled to 10 years of age | 49 250.39 | 13 366.00 | 8310.05 |
| Costs per child to the household (detail) | |||
| Out of pocket costs of visits (and other) | |||
| Modelled to 5 years of age | 4929.32 | 3941.46 | 826.62 |
| Modelled to 10 years of age | 9909.54 | 7923.64 | 1230.41 |
| Out of pocket costs of hospitalisation | |||
| Modelled to 5 years of age | 482.71 | 125.82 | 518.65 |
| Modelled to 10 years of age | 610.94 | 159.24 | 666.66 |
| Out of pocket costs of drugs/vitamins | |||
| Modelled to 5 years of age | 2370.77 | 1010.46 | 0.00 |
| Modelled to 10 years of age | 4575.33 | 1950.08 | 0.00 |
| Out of pocket costs of tests | |||
| Modelled to 5 years of age | 63.31 | 0.00 | 0.00 |
| Modelled to 10 years of age | 122.19 | 0.00 | 0.00 |
| Out of pocket cost of healthcare plan | |||
| Modelled to 5 years of age | 922.76 | 1081.82 | 555.82 |
| Modelled to 10 years of age | 1653.42 | 1938.43 | 995.92 |
| Out of pocket cost of moving and altering house as well as cost of coping | |||
| Modelled to 5 years of age | 559.73 | 299.73 | 0.00 |
| Modelled to 10 years of age | 559.73 | 299.73 | 0.00 |
| Out of pocket cost of wheelchair (adaptation) and visual aids | |||
| Modelled to 5 years of age | 3107.27 | 192.07 | 3.41 |
| Modelled to 10 years of age | 5549.02 | 341.87 | 6.27 |
| Out of pocket cost of special food | |||
| Modelled to 5 years of age | 1039.17 | 385.28 | 0.00 |
| Modelled to 10 years of age | 1039.17 | 385.28 | 0.00 |
| Cost of income forgone | |||
| Modelled to 5 years of age | 1247.89 | 1716.57 | 772.61 |
| Modelled to 10 years of age | 1912.88 | 2918.48 | 1121.56 |
| Total costs per child to the household | |||
| Total cost per child to the household | |||
| Modelled to 5 years of age | 14 722.92 | 8753.21 | 2677.11 |
| Modelled to 10 years of age | 25 932.23 | 15 916.74 | 4020.83 |
| Total net cost per child to the household (disability benefit deducted) | |||
| Modelled to 5 years of age | 960.83 | 8753.21 | 2677.11 |
| Modelled to 10 years of age | 1273.03 | 15 916.74 | 4020.83 |
| Total cost per child to the household excluding income forgone | |||
| Modelled to 5 years of age | 13 475.04 | 7036.64 | 1904.50 |
| Modelled to 10 years of age | 24 019.35 | 12 998.27 | 2899.27 |
| Total costs per child to the society | |||
| Total net cost per child to society | |||
| Modelled to 5 years of age | 28 663.88 | 14 383.24 | 5553.62 |
| Modelled to 10 years of age | 50 523.42 | 29 282.75 | 12 330.88 |
The costs per child (US$, 2017) for severe CZS, moderate CZS and no CZS by time horizon (to 5 years and 10 years of age) are shown in this table using the results from the base case analysis. Detailed cost per child by cost category as well as total cost by perspective (health provider, government, household and societal) are shown. The net cost to the household means that the disability benefit provided by the government to families of children with severe CZS was deducted from the household cost. This net cost to the household was used when estimating the societal cost to avoid double counting. It only applies to children with severe CZS; for moderate CZS and no CZS children the net cost to the household is the same as the cost to the household, as they do not receive a disability benefit. The same results from the probabilistic sensitivity analysis can be found in the online supplemental appendix table S2.
CZS, congenital Zika Syndrome.
Figure 1.
Incremental cost per child comparing severe and moderate CZS versus no CZS. The incremental costs per child (US$, 2017) comparing severe CZS (A) and moderate CZS (B) with no CZS modelled to 5 years and 10 years of age for each perspective. The results shown here are from the probabilistic sensitivity analysis using 10 000 iterations with the bar representing the mean and the interval lines representing the 95% CI based on percentiles. Incremental costs by perspective (health provider, government, household and societal) are shown. The net household cost means that the disability benefit provided by the government to families of children with severe CZS was deducted from the household cost. This net cost to the household was used when estimating the societal cost to avoid double counting. It only applies to children with severe CZS, since for moderate CZS and no CZS children the net cost to the household is the same as the cost to the household, as they do not receive a disability benefit. CZS, congenital Zika Syndrome.
Moving on to the economic burden of Brazil as a whole (figure 2), from the health provider and governmental perspective, the incremental economic burden of severe versus no CZS in Brazil over 10 years was estimated to be US$44.0 million (95% CI 34.2 to 56.9) and US$129.0 million (95% CI 114.8 to 144.9), respectively. The incremental net household burden of severe versus no CZS was −US$9.6 million (95% CI −21.7 to 3.0), indicating that the payment made by the government helped this group to cope with the financial burden they face due to having a child with severe CZS (the negative number is because the net cost of a child with severe CZS is smaller than of a child with no CZS and hence the incremental cost becomes negative). For moderate CZS (using five times the confirmed cases of severe CZS), the incremental economic burden of moderate versus no CZS amounted to US$204.0 million (95% CI 123.7 to 296.6) from the household and US$86.5 million (95% CI 48.3 to 131.0) from the government perspective. Over 10 years, 97% of the total societal economic cost of severe CZS is borne by the government (157.5 million government vs 161.6 million societal cost). For moderate CZS, the government share is only 46% (229.0 million government vs 502.0 million societal cost), while 54% is incurred by the household (for details see figure 2 and online supplemental appendix table S4).
Figure 2.
Incremental cost burden for Brazil comparing severe CZS and moderate CZS versus no CZS. Figure 2 shows the incremental economic burden of the whole of Brazil (US$, 2017) comparing severe CZS (A) and moderate (B) CZS vesus no CZS by time horizon (to 5 years and 10 years of age) and perspective (health provider, government, household and society). The results shown here are from the probabilistic sensitivity analysis using 10 000 iterations with the bar representing the mean and the interval lines representing the 95% CI based on percentiles. The incremental net burden to the household means that the disability benefit provided by the government to families of children with severe CZS was deducted from the household cost when calculating their economic burden. This incremental net burden to the household was used when estimating the societal burden to avoid double counting. It only applies to severe CZS, since for moderate CZS and no CZS the net burden to the household is the same as the cost to the household, as they do not receive a disability benefit. The number of confirmed cases of severe CZS was N=3474 and the number of moderate CZS was assumed to be 5× this number. CZS, congenital Zika Syndrome.
The scenario analysis where number of cases of CZS were varied is presented in online supplemental appendix table S5 and figure S2. It shows total economic burden of severe, moderate and no CZS as well as incremental economic burden of severe and moderate CZS vs no CZS by perspective, time horizon and it varies the case number using three different scenarios (called confirmed, likely, maximum).In summary, if up to the maximum number of cases of severe CZS were confirmed, the incremental economic burden for severe CZS could be up to double the amount we estimated.
The incremental DALY burden for severe CZS versus no CZS varied between 23 622 (using confirmed cases) and 46 755 (maximum cases) DALYs with years of lives lost contributing only 9% to the incremental number of DALYs (more detail on the DALY burden can be found in the online supplemental appendix table S6 and on p6).
For severe CZS, the economic and DALY burden are driven mainly by the severity of the condition, while for moderate CZS by estimated case numbers.
Discussion
This study found high societal costs incurred for the care of children with severe CZS (cost per child US$28 664 to 5 years and US$50 523 to 10 years) compared with children with moderate CZS (US$14 383 and US$29 283) or without CZS (US$5554 and US$12 331). The greatest costs are incurred by the government through provision of healthcare, education and disability benefits. Strikingly, families of children with moderate CZS suffered higher costs than families of children more severely affected as they received less financial support through disability benefits. Over the course of 10 years, the estimated net household cost—which is the cost the household incurs minus the disability benefit received from the government—per child with moderate CZS was US$15 917, which was US$14 644 higher on average compared with a household with a child with severe CZS.
At a national level, the estimated incremental economic burden from the societal perspective over 10 years was around US$178 million for severe CZS and US$433 million for moderate CZS. The incremental DALY burden over 10 years was substantial, ranging between 24 000 and 47 000 DALYs for severe CZS and between 50 000 and 100 000 DALYs for moderate CZS. Both costs and DALY estimates are likely conservative as the number of children affected is thought to be greatly underestimated.
It is well recognised that the care of a child with CZS can have extensive social and economic impacts on the family.37 However, data on economic burden are lacking, particularly for low-income and middle-income settings. Other studies estimating the economic burden have either used secondary cost data from the USA or conducted their cost data collection in the USA, which is greatly different from costs in Brazil.17–19 Evidence on economic burden is more abundant for other types of childhood disability.38–41 Most relevant to this paper, a 2018 systematic review included 22 studies exploring the economic impact of CP and found a strong positive relationship between severity and expenditure.38 Significant costs were incurred by families and the welfare system to facilitate school and community engagement.
There are key strengths to this paper. It fills an important knowledge gap by presenting a robust economic burden analysis and modelling using cost data collected from the health provider and government in Brazil. The modelling methods and parameters are transparent and comprehensive. However, a number of limitations needs to be taken into account when interpreting the results. It is important to note that the different case definitions used in the two study sites (table 1) might have led to the inclusion of children with different levels of disability in the two CZS groups from the two sites but also compared with the children with microcephaly classified to receive disability benefits by the Ministry of Health. As noted in the introduction, the absence of microcephaly at birth does not exclude the presence of CZS, sometimes microcephaly develops after birth and in some cases microcephaly can even resolve over time without any long-term implications. This shows that using head circumference at birth has to some extend limitations for predicting long-term disabilities. But CZS is a new syndrome, which still needs to be understood better and what its implications in the long run are. Hence, the analysis presented here used available data to the best of our current understanding of the condition and situation, scrutinising parameters and assumptions vigorously. A further limitation is that the economic costs provide a narrow view of the impact, and the analysis does not take account of other dimensions, such as intangible cost, including the emotional pain of experiencing a fetal or neonatal death due to CZS or of having a child with disabilities. Moreover, the paper uses the term ‘burden’, meant from an economic perspective, but there are also many positive aspects of caring for a child with disabilities not captured in this study. Our model is based on data of healthcare utilisation and does not account for healthcare needs, which are more difficult to measure. Accounting for healthcare needs, which are likely substantially higher, would increase the economic burden from all perspectives considerably.
There were evidence gaps that constrained the development of the model. There was a relatively small sample of children with moderate CZS in both study sites, and the number of children examined in year 3 was limited across all three groups. There is little data available on the mortality rate of children with CZS, let alone by severity of CZS, and so a range of assumptions were made. We estimated that the mortality rate would be 10.2% over the first 5 years, in comparison to the government recorded 14.7% up until 2019.5 However, the government figure included stillbirth and miscarriage, and so our estimates appear realistic. A number of costs were not included, such as the costs of physical and psychological healthcare among the family and caregivers. Other costs were likely to be underestimated, such as estimates of the costs for altering the home, and coping costs (includes selling of assets and borrowing money to cope with the additional costs incurred because of having child with CZS), which are likely to have been disproportionately accrued at older ages but were not modelled beyond the first 2 years. Moreover, the estimate of income foregone appears to be low, considering the likely impacts of a lifetime of caring for a child with disabilities on employment opportunities, particularly for women. Throughout, when having to make choices to inform our model, we consistently opted for the conservative option. Consequently, the actual economic burden is likely higher than our estimate. Basing our modelling of costs over time on cost ratios estimated in a Danish study could introduce bias into our estimates as health system, prices and access to services differ substantially between the two countries. However, this was the only sufficiently detailed data available on the costs of a comparable condition, in this case CP, over different years and reporting by different cost categories such as visits and hospitalisation. In addition, we considered applying relative cost ratios for different cost categories from one setting to another as less prone to bias.
Policy, programmatic and research implications
The evidence indicates that the disability benefit protected the families of children with severe CZS from economic repercussions, while the families of children with moderate CZS were more economically vulnerable. Other studies have shown that greater financial stability may reduce other negative consequences of caring for a child with disabilities, such as mental health impacts.37 42 Consideration should, therefore, be given to protecting and extending the disability benefit to include a wider range of children with disabilities. The impacts of CZS extend beyond economic, however, and families and children affected report many challenges and unmet needs (eg, emotional support, negative attitudes, barriers to educational inclusion and healthcare access and contraception).43 Consequently, family support and other protection are required in addition to economic support.44 In terms of further research, data collection focused on the first 2 years of life (up to 3 years for provider costs) and extending the time-frame of data collection would generate valuable information to support programmatic and policy plans.
In conclusion, this paper reports detailed estimates of provider, household, government and societal costs per child and at the country level for severe CZS and moderate CZS over 5 years or 10 years. Our study is the first economic burden study based on actual cost data collected in Brazil and, therefore, fills an important gap in the literature of CZS. It also complements the scarce literature on the costs of childhood disabilities. Lastly, we show that for families with moderate CZS, the net economic burden is highest, as they lack the disability benefit received by families of children with severe CZS. Broadening benefit support, possibly following a staggered approach, to include a wider range of families of children with disabilities should be introduced by policy-makers to prevent some of the most vulnerable families in society from descending further into poverty leading to numerous negative ramifications for the family, potentially impacting the mental and physical health of the caregivers even more.
bmjgh-2022-008784supp002.pdf (72.2KB, pdf)
Acknowledgments
First of all, we would like to thank the mothers of children who were willing to give up their precious time to take part in this study. We are very grateful to the field team, who conducted the interviews in a sensitive and professional manner. Lastly, we would like to thank Dr Marie Kruse from the Danish Centre for Health Economics for sharing the detailed report of the lifetime cost of cerebral palsy with us.
Footnotes
Handling editor: Lei Si
Contributors: MELM, TVBdA, TML and HK conceived and designed the study. LB oversaw statistical aspects of the study. MP, SF and MJ-B contributed to the design of the economic aspects of the study, and MP led its implementation. MP trained field workers and supervised the field work of the economic study with support from MELP, TVBdA, TML, SV and SF. MP, LB and SF analysed the cost data. SF designed and lead the economic burden analysis with input from MJ-B. The manuscript was written by SF and HK and revised by MP, LB, MELM, TVBdA, TML, SV and MJ-B. All authors approved the final draft of the manuscript. MELM, LB, MP and SF had full access to the economic and epidemiological data required for this paper. SF and HK had final responsibility for the decision to submit for publication. HK acts as the guarantor of the study, accepting full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was supported by the Wellcome Trust and the Department for International Development (grant number: 206016/Z/17/Z). This study was also supported by a supplementary grant from the European Union’s Horizon 2020 research and innovation programme, under Zika-PLAN (grant agreement number: 734584).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The reflexivity statement for this paper is linked as an online supplemental file 2.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data requests to be made to silke.fernandes@lshtm.ac.uk.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
Ethics approval
This study was approved by the ethics committees of the London School of Hygiene and Tropical Medicine and the Fiocruz (CAAE 60682516.2.1001.5269).
References
- 1.PAHO . Zika-Epidemiological report, 2017. [Google Scholar]
- 2.de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 2016;16:1356–63. 10.1016/S1473-3099(16)30318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Who Director-General summarizes the outcome of the emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome, 2016. Available: https://www.who.int/en/news-room/detail/01-02-2016-who-director-general-summarizes-the-outcome-of-the-emergency-committee-regarding-clusters-of-microcephaly-and-guillain-barr%C3%A9-syndrome
- 4.World Health Organization . Fact sheets: microcephaly, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/microcephaly
- 5.Secretaria de Vigilância em Saúde − Ministério da Saúde Brazil . Monitoramento integrado de alterações no crescimento e desenvolvimento relacionadas infecção pelo vírus Zika e outras etiologias infecciosas, Boletim Epidemiológica - final report Boletim Epidemiológico. Numero Especial 2019.
- 6.Chan JFW, Choi GKY, Yip CCY, et al. Zika fever and congenital Zika syndrome: an unexpected emerging arboviral disease. J Infect 2016;72:507–24. 10.1016/j.jinf.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa F, Sarno M, Khouri R, et al. Emergence of congenital Zika syndrome: viewpoint from the front lines. Ann Intern Med 2016;164:689–91. 10.7326/M16-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda-Filho DdeB, Martelli CMT, Ximenes RAdeA, et al. Initial description of the presumed congenital Zika syndrome. Am J Public Health 2016;106:598–600. 10.2105/AJPH.2016.303115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen SA, Jamieson DJ, Honein MA, et al. Zika virus and birth defects-reviewing the evidence for causality. N Engl J Med 2016;374:1981–7. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- 10.Kuper H, Lyra TM, Moreira MEL, et al. Social and economic impacts of congenital Zika syndrome in Brazil: study protocol and rationale for a mixed-methods study. Wellcome Open Res 2018;3:127. 10.12688/wellcomeopenres.14838.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques FJP, Teixeira MCS, Barra RR, et al. Children born with congenital Zika syndrome display atypical gross motor development and a higher risk for cerebral palsy. J Child Neurol 2019;34:81–5. 10.1177/0883073818811234 [DOI] [PubMed] [Google Scholar]
- 12.Stabile M, Allin S. The economic costs of childhood disability. Future Child 2012;22:65–96. 10.1353/foc.2012.0008 [DOI] [PubMed] [Google Scholar]
- 13.DiGiacomo M, Green A, Delaney P, et al. Experiences and needs of carers of Aboriginal children with a disability: a qualitative study. BMC Fam Pract 2017;18:96. 10.1186/s12875-017-0668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos Oliveira SJG, Dos Reis CL, Cipolotti R, et al. Anxiety, depression, and quality of life in mothers of newborns with microcephaly and presumed congenital Zika virus infection: a follow-up study during the first year after birth. Arch Womens Ment Health 2017;20:473–5. 10.1007/s00737-017-0724-y [DOI] [PubMed] [Google Scholar]
- 15.Unsal-Delialioglu S, Kaya K, Ozel S, et al. Depression in mothers of children with cerebral palsy and related factors in Turkey: a controlled study. Int J Rehabil Res 2009;32:199–204. 10.1097/MRR.0b013e32832607b6 [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz H, Erkin G, Nalbant L. Depression and anxiety levels in mothers of children with cerebral palsy: a controlled study. Eur J Phys Rehabil Med 2013;49:823–7. [PubMed] [Google Scholar]
- 17.Alfaro-Murillo JA, Parpia AS, Fitzpatrick MC, et al. A cost-effectiveness tool for informing policies on Zika virus control. PLoS Negl Trop Dis 2016;10:e0004743. 10.1371/journal.pntd.0004743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United Nations Development Programme . A socio-economic impact assessment of the Zika virus in Latin America and the Caribbean: with a focus on Brazil, Colombia and Suriname 2017.
- 19.Li R, Simmons KB, Bertolli J, et al. Cost-effectiveness of increasing access to contraception during the Zika virus outbreak, Puerto Rico, 2016. Emerg Infect Dis 2017;23:74–82. 10.3201/eid2301.161322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro MC, Massuda A, Almeida G, et al. Brazil’s unified health system: the first 30 years and prospects for the future. Lancet 2019;394:345–56. 10.1016/S0140-6736(19)31243-7 [DOI] [PubMed] [Google Scholar]
- 21.Pinto M, Fernandes S, Barros L, et al. Estimating the cost of congenital Zika syndrome to families and healthcare providers in Rio de Janeiro and Pernambuco, Brazil: results of a case-control study. Wellcome Open Res 2021;6:78. 10.12688/wellcomeopenres.16623.1 [DOI] [Google Scholar]
- 22.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet 2014;384:857–68. 10.1016/S0140-6736(14)60932-6 [DOI] [PubMed] [Google Scholar]
- 23.França GVA, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 2016;388:891–7. 10.1016/S0140-6736(16)30902-3 [DOI] [PubMed] [Google Scholar]
- 24.Moreira M, Vasconcelos Z. Vertical exposure to Zika virus and its consequences for child neurodevelopment: cohort study in Fiocruz/IFF. Nat Lib Med. [Google Scholar]
- 25.Bayley N. Bayley scales of infant and toddler development. 3rd edn. SAn Antonio, TX: Pearson, 2006. [Google Scholar]
- 26.Conteh L, Walker D. Cost and unit cost calculations using step-down accounting. Health Policy Plan 2004;19:127–35. 10.1093/heapol/czh015 [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health Brazil . SIGTAP - Sistema de Gerenciamento da Tabela de Procedimentos, Medicamentos e OPM do SUS.
- 28.Malta DC, Stopa SR, Canuto R, et al. Self-reported prevalence of disability in Brazil, according to the National health survey, 2013. Cien Saude Colet 2016;21:3253–64. 10.1590/1413-812320152110.17512016 [DOI] [PubMed] [Google Scholar]
- 29.Steffen RE, Caetano R, Pinto M, et al. Cost-effectiveness of quantiferon-TB gold-in-tube versus tuberculin skin testing for contact screening and treatment of latent tuberculosis infection in Brazil. PLoS One 2013;8:e59546. 10.1371/journal.pone.0059546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Titinger DP, Lisboa LAF, Matrangolo BLR, et al. Cardiac surgery costs according to the preoperative risk in the Brazilian public health system. Arq Bras Cardiol 2015;105:130–8. 10.5935/abc.20150068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FXTOP . Historical exchange rates. Available: http://fxtop.com/en/historical-exchange-rates.php
- 32.Nielsen-Saines K, Brasil P, Kerin T, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019;25:1213–7. 10.1038/s41591-019-0496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Access Economics . The economic impact of cerebral palsy in Australia in 2007, 2008. [Google Scholar]
- 34.Kruse M, Michelsen SI, Flachs EM. Livstidsomkostminger ved cerebral Parese, 2007. [Google Scholar]
- 35.Brasil. Ministério da Saúde.Secretaria de Ciência TeIE . Diretrizes metodológicas: Diretriz de Avaliação Econômica. 2rd edn. Brasília: Ministério da Saúde, 2014. [Google Scholar]
- 36.International Monetary Fund . World economic outlook database. Consumer price index by country for 2014. Available: http://www.imf.org/external/pubs/ft/weo/2014/02/weodata/index.aspx
- 37.Scherer N, Verhey I, Kuper H. Depression and anxiety in parents of children with intellectual and developmental disabilities: a systematic review and meta-analysis. PLoS One 2019;14:e0219888. 10.1371/journal.pone.0219888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tonmukayakul U, Shih STF, Bourke-Taylor H, et al. Systematic review of the economic impact of cerebral palsy. Res Dev Disabil 2018;80:93–101. 10.1016/j.ridd.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Fejes M, Varga B, Hollódy K. [Epidemiology, cost and economic impact of cerebral palsy in Hungary]. Ideggyogy Sz 2019;72:115–22. 10.18071/isz.72.0115 [DOI] [PubMed] [Google Scholar]
- 40.Retzler J, Hex N, Bartlett C, et al. Economic cost of congenital CMV in the UK. Arch Dis Child 2019;104:559–63. 10.1136/archdischild-2018-316010 [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Chen Y, Zhang J, et al. A preliminary study into the economic burden of cerebral palsy in China. Health Policy 2008;87:223–34. 10.1016/j.healthpol.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 42.Emerson E, Hatton C, Llewellyn G, et al. Socio-economic position, household composition, health status and indicators of the well-being of mothers of children with and without intellectual disabilities. J Intellect Disabil Res 2006;50:862–73. 10.1111/j.1365-2788.2006.00900.x [DOI] [PubMed] [Google Scholar]
- 43.Ambrogi IG, Brito L, Diniz D. The vulnerabilities of lives: Zika, women and children in Alagoas State, Brazil. Cad Saude Publica 2021;36:e00032020. 10.1590/0102-311x00032020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duttine A, Smythe T, Ribiero Calheiro de Sá M, et al. Congenital Zika Syndrome-assessing the need for a family support programme in Brazil. Int J Environ Res Public Health 2020;17:17103559. 10.3390/ijerph17103559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ministry of Education Brazil . PORTARIA INTERMINISTERIAL N° 10, DE 28 DE DEZEMBRO DE 2017 2017.
- 46.Government of Brazil . Benefício de Prestação Continuada, 2019. Available: http://www.antigo.previdencia.gov.br/wp-content/uploads/2019/05/Relatorio-Avaliacao-BPC-Fasico_31_05_2019.pdf
- 47.UNICEF . Infant and under 5 mortality. Available: https://data.unicef.org/country/bra/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-008784supp001.pdf (1.3MB, pdf)
bmjgh-2022-008784supp002.pdf (72.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data requests to be made to silke.fernandes@lshtm.ac.uk.