Abstract
Takotsubo cardiomyopathy (TCM) causes QT interval prolongation, potentially leading to a fatal arrhythmia. We report the first case of TCM associated with licorice‐induced pseudoaldosteronism causing fatal arrhythmia in an older patient on polypharmacy including yokukansan (TJ‐54) and galantamine. Polypharmacy should be resolved to prevent unexpected adverse events in older patients.
Keywords: glycyrrhiza, kampo drugs, licorice, polypharmacy, pseudoaldosteronism, Takotsubo cardiomyopathy, torsade de pointes
Licorice can cause pseudoaldosteronism, which might be potentially associated with Takotsubo cardiomyopathy (TCM) onset. Furthermore, older patients on polypharmacy who have new onset of TCM might have a potential risk of fatal arrhythmia.
![]()
1. INTRODUCTION
Takotsubo cardiomyopathy (TCM) is an acute reversible ventricular dysfunction that often causes QT interval prolongation, which can lead to a fatal arrhythmia. 1 Glycyrrhiza uralensis (licorice in English; kanzo in Japanese) is one of the components of Kampo drugs, which are often used for the treatment of various diseases in older patients (Figure 1A–1C ). Long‐term use of licorice can initiate pseudoaldosteronism, mainly manifesting as severe hypokalemia leading to QT interval prolongation. 2 Yokukansan (TJ‐54), which consists of seven dried herbs, including licorice, is reportedly effective for dementia, insomnia, and schizophrenia (Figure 1D ). 3 , 4 Moreover, polypharmacy, an unresolved issue in the aging population, is associated with drug side effects, including fatal arrhythmia due to QT interval prolongation. 5 We herein report a cautionary case of licorice‐induced pseudoaldosteronism that precipitated the onset of TCM and subsequent fatal arrhythmia in an octogenarian patient on polypharmacy.
FIGURE 1.

Licorice and yokukansan (TJ‐54). An image of (A) Glycyrrhiza uralensis and (B, C) Kanzo. (D) Yokukansan (TJ‐54) consists of seven dried herbs, including Poria cocos Wolf., Atractylodes lancea DC., Angelica acutiloba L., Uncaria rhynchophylla Schreb., Cnidium officinale Makino, Bupleurum falcatum L., and Glycyrrhiza uralensis, in a ratio of 4:4:3:3:3:2:1.5, respectively. Images of (A) and (C) are provided by TSUMURA & CO. (©2022 TSUMURA & CO. All Rights Reserved)
2. CASE REPORT
An 84‐year‐old woman with a history of old cerebral infarction and Alzheimer‐type dementia was brought to our emergency department because of anorexia and body weakness that persisted for 1 week, progressively worsening dyspnea at rest, and subsequent somnolence. The patient had a history of long‐term use of several medications for her comorbidities: aspirin (100 mg/day) for old cerebral infarction, vonoprazan (10 mg/day) for chronic gastritis, galantamine (8 mg/day), memantine (10 mg/day), ramelteon (8 mg/day), and yokukansan (containing 1.5 g of licorice) for Alzheimer‐type dementia and its related symptoms. The patient had been taking memantine, galantamine, and yokukansan for 4 years, 9 years, and more than 10 years, respectively. In contrast, she had been taking other medications for at least more than 3 years. On arrival at the emergency department, her blood pressure was 154/80 mmHg, heart rate was 100 bpm, and percutaneous oxygen saturation was 98% under an oxygen supplementation of 5 L/min. The patient had orthopnea, and physical examinations showed bilateral inspiratory crackles and pedal edema, suggesting acute decompensated heart failure (HF) (New York Heart Association functional class IV). Laboratory tests revealed mild respiratory acidosis with metabolic alkalosis (pH, 7.35; pCO2, 65.8 mmHg; pO2, 110.0 mmHg; HCO3 ‐, 37.3 mmol/L), severe hypokalemia (potassium, 1.4 mmol/L; normal reference, 3.5–5.0 mmol/L), hypomagnesemia (magnesium, 0.49 mmol/L; normal reference, 0.74–1.07 mmol/L), and elevated troponin‐T levels (0.205 ng/mL; normal reference, <0.014 ng/mL). Chest radiography revealed bilateral pleural effusions and cardiomegaly (Figure 2A ). Electrocardiography (ECG) showed sinus rhythm and corrected QT interval prolongation (QTc, 464 ms; reference interval in females, 300–450 ms) with ST‐segment slight elevation in leads V1‐V3 (Figure 2B ). Transthoracic echocardiography showed severe hypokinesis in the apical left ventricle and hyperkinesis in the basal left ventricle (Figure 2C ). To investigate the cause of myocardial dysfunction, emergency coronary angiography (CAG) was performed; however, no significant stenosis was observed in the coronary arteries. Left ventriculography confirmed severe hypokinesis in the left apical ventricle (left ventricular apical ballooning). Accordingly, these findings are consistent with a diagnosis of TCM, although she had no history of emotional stress prior to her symptoms (Figure 2D,E ). Right heart catheterization revealed a low cardiac index of 1.3 L/min/m2 and elevated pulmonary capillary wedge pressure of 22 mmHg, which confirmed HF due to TCM‐induced low cardiac output. Additional laboratory tests for investigating causes of severe hypokalemia showed significant suppression of plasma renin activity (<0.5 ug/L/h) and plasma aldosterone concentration (<69.35 pmol/L). Considering a history of long‐term intake of licorice‐containing Kampo drugs, pseudoaldosteronism caused by licorice was believed to be the main cause of severe hypokalemia. Therefore, these clinical scenarios suggest that licorice‐induced pseudoaldosteronism progressively developed and subsequently caused TCM‐related acute decompensated HF.
FIGURE 2.
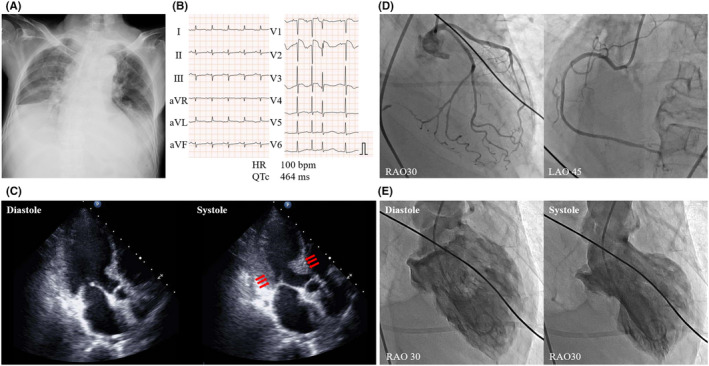
Acute decompensated heart failure due to Takotsubo cardiomyopathy. (A) Chest radiography showing bilateral pleural effusions and cardiomegaly. (B) Electrocardiogram on admission showing QT interval prolongation with ST‐segment elevation in leads V1‐V3. (C) Transthoracic echocardiography shows hypokinesis in the left apical ventricle and hyperkinesis in the basal left ventricle (red arrows). (D) Coronary angiography shows no significant stenosis in coronary arteries. (E) Left ventriculography showing severe hypokinesis in the left apical ventricle, suggestive of Takotsubo cardiomyopathy
As QT interval prolongation and concomitant premature ventricular contractions (PVCs) had already been observed since her admission, intravenous potassium and magnesium were immediately administered to prevent fatal arrhythmia (Figure 3A ). Although PVCs improved, ST‐segment elevation with QT interval prolongation progressively worsened (Figure 4 ). Subsequently, polymorphic ventricular tachycardia (VT) with torsade de pointes (TdP) suddenly occurred, followed by cardiogenic shock. Although supportive therapy using noradrenaline was administered, it was difficult to treat the fulminant TCM with medication alone (Figure 3B ). Further interventions, including mechanical circulatory supports (MCSs), such as percutaneous left ventricular assist device (Impella) and veno‐arterial extracorporeal membrane oxygenation, were not performed. This is because the patient had given a directive beforehand (prior to the progression of dementia) that she did not want to receive any life‐prolonging treatment, which her family agreed with. The patient died of TCM‐related fatal arrhythmia of intractable VT, 24 h after admission.
FIGURE 3.
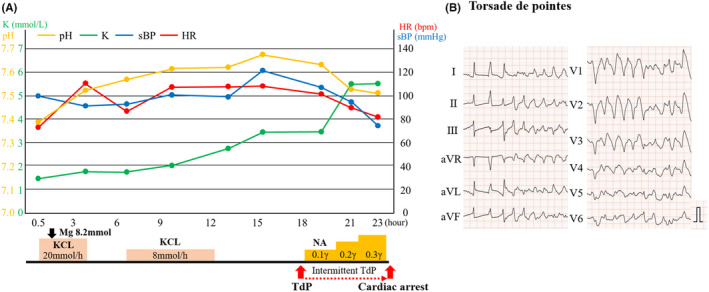
Patient's clinical time course. (A) Clinical time course. Severe hypokalemia was treated by intravenous potassium administration to stabilize the potassium level to approximately 3.0–3.5 mmol/L; however, Torsade de pointes intermittently occurred 18 h after hospitalization, and subsequent cardiogenic shock developed. The potassium level gradually elevated as cardiogenic shock developed. (B) Electrocardiogram showing TdP. pH, potential of hydrogen; K, serum potassium level; HR, heart rate; sBP, systolic blood pressure; TdP, torsade de pointes
FIGURE 4.
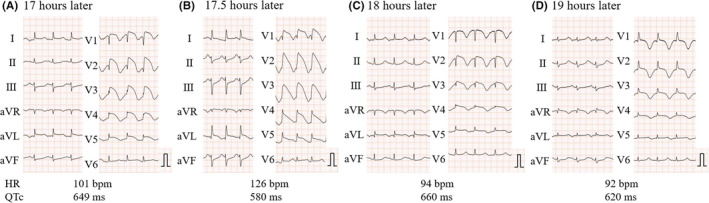
Serial electrocardiogram findings. (A)–(D) Electrocardiogram (ECG) before and after the onset of arrhythmic events. ECG performed (A) 1 h before the onset of TdP (17 h after admission), (B) 30 min before the onset of TdP (17.5 h after admission) (C), just after Tdp onset, and (D) 1 h after the onset of TdP (19 h after admission). Serial ECG shows the occurrence of drastic ST‐segment elevation, subsequent giant T wave inversion, and right bundle branch block. QTc, corrected QT interval prolongation; TdP, torsade de pointes
3. DISCUSSION
TCM is characterized by stress‐induced transient ventricular dysfunction caused by various physical and emotional triggers. Many studies have shown that TCM precipitated by either physical or unidentified triggers has poorer short‐ and long‐term cardiovascular outcomes than TCM precipitated by emotional triggers. 6 Furthermore, recent studies have shown that endocrine disorders, such as hyperthyroidism, pheochromocytoma, and primary aldosteronism, are associated with TCM onset. 7 The patient had been taking yokukansan, a licorice‐containing (1.5 g/day) Kampo drug, for over 10 years. According to the Japanese Adverse Drug Reports Database, yokukansan is associated with a low incidence of side effects. 2 Pseudoaldosteronism is a significant side effect of licorice‐containing Kampo drugs, which mimics primary hyperaldosteronism. 2 Although yokukansan is an effective Kampo drug for behavioral and psychological symptoms of dementia (BPSD), it has a higher risk of causing pseudoaldosteronism than other licorice‐containing Kampo drugs. Moreover, the risk factors for yokukansan‐related pseudoaldosteronism are as follows: high licorice dose, older age (age > 70 years), dementia, and low body weight (<50 kg). 2 In the present case, the patient had been taking yokukansan for BPSD for more than 10 years, and the condition had been well controlled; however, the patient eventually developed all the abovementioned risk factors for yokukansan‐related pseudoaldosteronism. The mechanism underlying pseudoaldosteronism is that excessive intake of licorice can result in excess cortisol by inhibiting 11β‐hydroxysteroid dehydrogenase type 2 (11βHSD2), an enzyme that inactivates cortisol. 8 Moreover, the accumulation of activated cortisol subsequently triggers the activation of mineralocorticoid receptors (MRs) and glucocorticoid receptors, potentially leading to cardiovascular events even in patients with normal or low serum aldosterone levels. 9 , 10 , 11 As TCM is reportedly caused by myocardial damage via activation of MRs that lead to sympathetic nerve activation, we believe that an apparent activation of MRs caused by the inhibition of 11βHSD2 in pseudoaldosteronism may be associated with the onset of TCM. 12 As the patient had no history of emotional stress before admission in this case, these findings led us to speculate that TCM might be triggered by an activation of MRs or physical stress caused by licorice‐induced pseudoaldosteronism. Although several cases demonstrating a potential association between pseudoaldosteronism and reversible secondary cardiomyopathy have been reported, to the best of our knowledge, this is the first case showing a potential association between licorice‐induced pseudoaldosteronism and TCM onset. 13 , 14 Therefore, further investigations regarding the relationship between pseudoaldosteronism and TCM are warranted.
Fatal arrhythmia caused by QT interval prolongation is also a prognostic factor for TCM. 1 In this case, QT interval prolongation had already been observed in the early TCM stage. Although it was difficult to determine the precise causes of QT interval prolongation, several potential causes, except for TCM, such as pseudoaldosteronism‐related severe hypokalemia and galantamine side effects, could be considered. Firstly, although potassium and magnesium levels were adequately adjusted, drastic changes in ECG abnormalities were observed. Thus, careful monitoring of ECG abnormalities related to QT interval prolongation should be conducted during the adjustment of electrolyte abnormality. Secondly, acetylcholinesterase inhibitors, such as galantamine and donepezil, are often used for the treatment of Alzheimer‐type dementia, and these medications can induce QT interval prolongation and TdP due to excessive drug efficacy under various conditions. 15 , 16 Since continuous drug intake without sufficient food intake potentially triggers excessive drug efficacy, one‐week persistent anorexia and subsequent acute renal insufficiency might have contributed to elevated galantamine concentration, leading to acquired QT interval prolongation in this case. Thus, more attention should be paid to resolving the issue of polypharmacy because severe cardiac events could be provoked in TCM cases with acquired multifocal QT interval prolongation.
In fulminant TCM cases that result in cardiogenic shock, MCSs, including a percutaneous left ventricular assist device (Impella) or veno‐arterial extracorporeal membrane oxygenation, are reportedly effective. 17 However, invasive treatments with MCSs are difficult or potentially unacceptable in older patients because of their generally reduced physiologic reserves and comorbidities, as shown in the present case. Thus, TCM cases with concomitant multifactorial QT interval prolongation should be carefully managed to prevent fatal arrhythmia.
AUTHOR CONTRIBUTIONS
CY, HY, and TT mainly conducted the study, analyzed the data, and wrote the initial draft of the manuscript. TI, MI, AS, and HK contributed to engaging technical support and supervised the study.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
CONSENT
Informed consent was obtained from the patient's family for the publication of this case report.
ACKNOWLEDGMENT
None.
Yoshida C, Yamamoto H, Inoue T, et al. Torsade de pointes in an older patient with Takotsubo cardiomyopathy caused by licorice‐induced pseudoaldosteronism: A case report. Clin Case Rep. 2022;10:e06104. doi: 10.1002/ccr3.6104
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Pant S, Deshmukh A, Mehta K, et al. Burden of arrhythmias in patients with takotsubo cardiomyopathy (apical ballooning syndrome). Int J Cardiol. 2013;170(1):64‐68. doi: 10.1016/j.ijcard.2013.10.041 [DOI] [PubMed] [Google Scholar]
- 2. Ishida T, Kawada K, Morisawa S, Jobu K, Morita Y, Miyamura M. Risk factors for pseudoaldosteronism with yokukansan use: analysis using the Japanese adverse drug report (JADER) database. Biol Pharm Bull. 2020;43(10):1570‐1576. doi: 10.1248/bpb.b20-00424 [DOI] [PubMed] [Google Scholar]
- 3. Iwasaki K, Satoh‐Nakagawa T, Maruyama M, et al. A randomized, observer‐blind, controlled trial of the traditional Chinese medicine Yi‐Gan san for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry. 2005;66(2):248‐252. doi: 10.4088/jcp.v66n0214 [DOI] [PubMed] [Google Scholar]
- 4. Yu CH, Ishii R, Yu SC, Takeda M. Yokukansan and its ingredients as possible treatment options for schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1629‐1634. doi: 10.2147/NDT.S67607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zwietering NA, Westra D, Winkens B, Cremers H, van der Kuy PHM, Hurkens KP. Medication in older patients reviewed multiple ways (MORE) study. Int J Clin Pharmacol. 2019;41(5):1262‐1271. doi: 10.1007/s11096-019-00879-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okura H. Update of takotsubo syndrome in the era of COVID‐19. J Cardiol. 2021;77(4):361‐369. doi: 10.1016/j.jjcc.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalra S, Lakhani OJ, Chaudhary S. Takotsubo endocrinopathy. Eur Endocrinol. 2020;16(2):97‐99. doi: 10.17925/EE.2020.16.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimada Y. Adverse effects of Kampo medicines. Intern Med. 2022;61(1):29‐35. [Epub ahead of print]. doi: 10.2169/internalmedicine.6292-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White PC, Mune T, Rogerson FM, Kayes KM, Agarwal AK. Molecular analysis of 11 beta‐hydroxysteroid dehydrogenase and its role in the syndrome of apparent mineralocorticoid excess. Steroids. 1997;62(1):83‐88. doi: 10.1016/s0039-128x(96)00164-x [DOI] [PubMed] [Google Scholar]
- 10. Frey FJ, Odermatt A, Frey BM. Glucocorticoid‐mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. 2004;13(4):451‐458. doi: 10.1097/01.mnh.0000133976.32559.b0 [DOI] [PubMed] [Google Scholar]
- 11. Funder JW. Mineralocorticoid receptors and cardiovascular damage: it's not just aldosterone. Hypertension. 2006;47(4):634‐635. doi: 10.1161/01.HYP.0000203732.03784.3b [DOI] [PubMed] [Google Scholar]
- 12. Zoltowska DM, Agrawal Y, Kalavakunta JK. Can aldosterone break your heart? takotsubo cardiomyopathy in a patient with newly diagnosed primary aldosteronism. BMJ Case Rep. 2018;2018. doi: 10.1136/bcr-2017-223472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamberlain TJ. Licorice poisoning, pseudoaldosteronism, and heart failure. Jama. 1970;213(8):1343. doi: 10.1001/jama.1970.03170340065018 [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa J, Suyama Y, Kinugawa T, Morisawa T, Kishimoto Y. Echocardiographic findings of the heart resembling dilated cardiomyopathy during hypokalemic myopathy due to licorice‐induced pseudoaldosteronism. Cardiovasc Drugs Ther. 1998;12(6):599‐600. doi: 10.1023/a:1007708025567 [DOI] [PubMed] [Google Scholar]
- 15. Nelson MW, Buchanan RW. Galantamine‐induced QTc prolongation. J Clin Psychiatry. 2006;67(1):166‐167. doi: 10.4088/jcp.v67n0123f [DOI] [PubMed] [Google Scholar]
- 16. Takaya T, Okamoto M, Yodoi K, et al. Torsades de pointes with QT prolongation related to donepezil use. J Cardiol. 2009;54(3):507‐511. doi: 10.1016/j.jjcc.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 17. Mariani S, Richter J, Pappalardo F, et al. Mechanical circulatory support for takotsubo syndrome: a systematic review and meta‐analysis. Int J Cardiol. 2020;316:31‐39. doi: 10.1016/j.ijcard.2020.05.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


