Abstract
Acute pulmonary embolism (APE) is a life‐threatening disease with nonspecific clinical signs and symptoms. Rapid and accurate diagnosis is crucial for the clinical management of patients with acute pulmonary embolism. A recommended echocardiography view may be of further help in the diagnosis and evaluation of the change in thrombosis and treatment. We reported a case of a 74‐year‐old man with a 12‐day history of decreased exercise capacity and dyspnea. The patient was diagnosed with intermediate‐risk APE as several pulmonary emboli in pulmonary artery were seen in multidetector computed tomographic pulmonary angiography with normal blood pressure and echocardiographic right ventricular overload. And we found a pulmonary artery clot in the right pulmonary artery through transthoracic echocardiography. After 11‐days anticoagulation, the patient underwent a reassessment, showed a decrease in RV diameter and pulmonary artery thrombus. This case highlights the significant role that echocardiography played in a patient who presented pulmonary embolism with a stable hemodynamic situation and normal blood pressure. The modified echocardiographic view could provide correct diagnosis by identifying the clot size and location visually. Knowledge of the echocardiography results of APE would aid the diagnosis.
Keywords: acute pulmonary embolism, modified echocardiographic view, pulmonary artery clot
We reported a patient diagnosed intermediate‐risk APE. A pulmonary artery clot in the right pulmonary artery was found through a modified TTE view. And the view could provide correct diagnosis by identifying the clot size and location visually.
1. INTRODUCTION
Venous thromboembolism clinically manifested as deep vein thrombosis or pulmonary embolism. 1 , 2 It is globally the third major cause of acute cardiovascular death 2 as a major health problem. Rapid and accurate diagnosis is crucial for the clinical management of patients with acute pulmonary embolism (APE). However, APE encompasses a wide clinical spectrum of severity, 3 , 4 ranging from asymptomatic disease to hemodynamic instability and shock. Due to the nonspecific clinical signs and symptoms, a diagnosis of APE is frequently extremely challenging. 3
In patients with suspected PE, multidetector computed tomographic pulmonary angiography (CTPA) is the preferred method for imaging the pulmonary vasculature. 1 Echocardiography examination is not a routine diagnostic procedure in hemodynamic stable APE patients, 5 but might detect an increase in right ventricular wall thickness or a jet velocity of tricuspid insufficiency beyond values compatible with acute right ventricular pressure overload. 6 In addition to these indirect signs, emboli in the pulmonary artery may occasionally be found in certain nonstandard echocardiographic views. 1 Herein, we report 1 case of intermediate‐risk APE patient with visualized pulmonary arterial thrombus by transthoracic echocardiography (TTE).
2. CASE PRESENTATION
A 74‐year‐old man was admitted to our hospital with a 12‐day history of decreased exercise capacity and dyspnea. At presentation, he was afebrile with a blood pressure of 130/110 mmHg, heart rate of 80/min, and respiratory rate of 20/min. The physical examination was within normal limits. Electrocardiography showed sinus rhythm with first‐degree atrioventricular block, S wave in lead I, q wave and inverted T wave in lead III (Figure 1). Laboratory evaluation was significant for elevated D‐dimer of 4450.08 ng/ml and brain natriuretic peptide level of 4398 ng/L. Complete blood count and coagulation studies were within normal limits. TTE was performed, and right ventricular (RV) dysfunction was defined as dilatation of the right ventricle (right/left ventricle diameter ratio 1.2 in the apical four‐chamber view) combined with elevated systolic gradient through the tricuspid valve (53 mmHg), lack of relevant left ventricular (LV) dysfunction or valvular heart disease. Further evaluation with TTE revealed a pulmonary artery clot of about 19 × 32 mm seen at the right pulmonary artery, 21 mm from the bifurcation of the pulmonary artery (Figure 2A,B, Video S1, S2). Meanwhile, venous Doppler studies of bilateral lower extremities showed right popliteal vein thrombosis. Computed tomography pulmonary angiography (CTPA) revealed several pulmonary emboli in right pulmonary artery (Figure 2C). The patient was diagnosed with intermediate‐risk APE as systemic systolic blood pressure on admission ≥90 mmHg with echocardiographic right ventricular overload and elevated serum levels of BNP. The patient was started on anticoagulation with low molecular weight heparin and oral warfarin. TTE was re‐evaluated after 11 days, the RV diameter was decreased combined with pulmonary artery thrombus decreased to 10 ×13 mm (Figure 2D,E, Video S3, S4). The systolic gradient through the tricuspid valve was 43 mmHg. The patient showed significant clinical improvement and was discharged home after being transitioned to oral warfarin.
FIGURE 1.
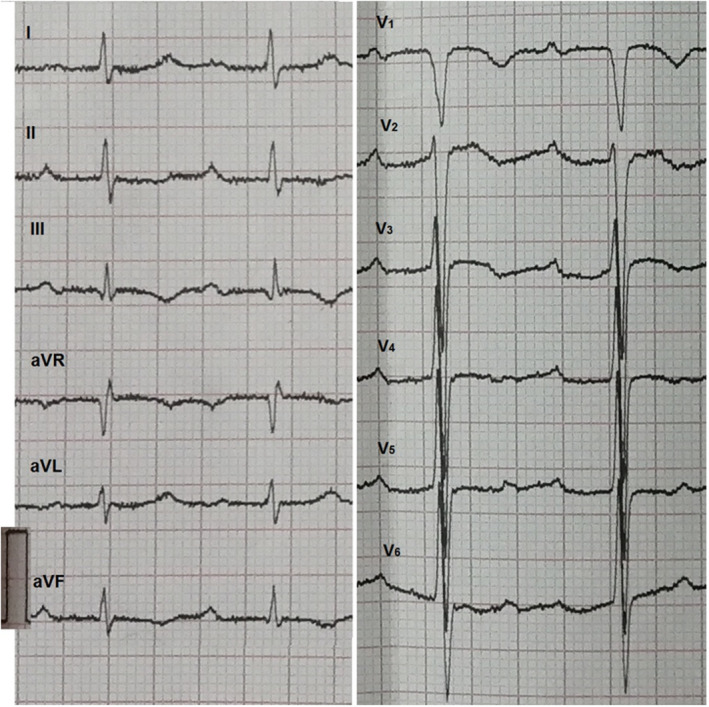
Electrocardiography of the patient. Shows sinus rhythm with first‐degree atrioventricular block, S wave in lead I, q wave and inverted T wave in lead III
FIGURE 2.
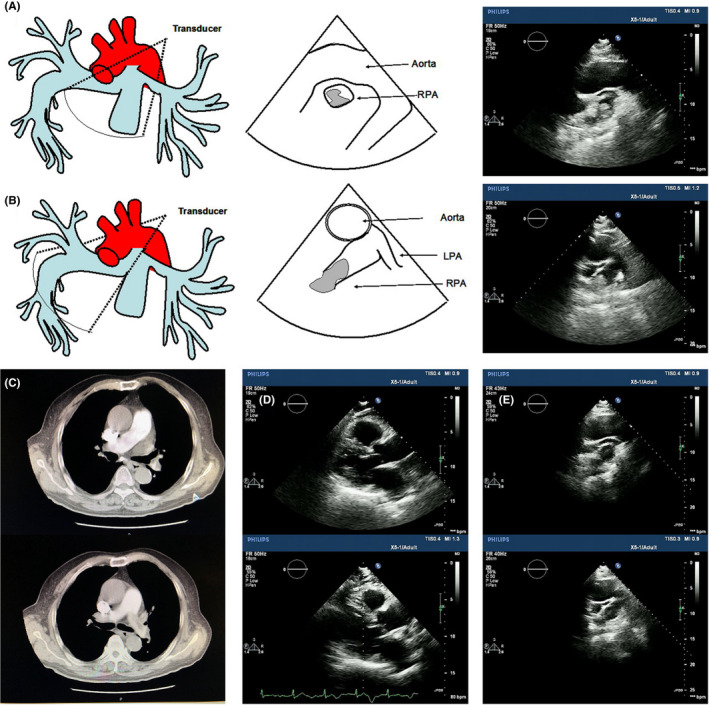
Imaging examinations of the patient. (A) Two‐dimensional transthoracic echocardiographic image in an off‐axis four‐chamber view demonstrates a venous clot in transit (arrow) in the right atrium. Video S1 corresponds to (A). (B) Two‐dimensional TTE at the level of pulmonary artery shows embolus (arrow) in the right PA (RPA). Video S2 corresponds to (B). (C) CTPA shows pulmonary emboli in right pulmonary artery from the transverse plane. (D) and (E) show decrease in RV diameter and pulmonary artery thrombus after 11‐days treatment. Video S3 corresponds to (D). Video S4 corresponds to (E). Ao, Ascending aorta
3. DISCUSSION
Direct and effective observation of pulmonary arterial thrombus in echocardiography is helpful for immediate diagnosis and appropriate treatment, especially when CTPA is not available rapidly and in pregnant patients or other situations warranting avoidance of radiation. It is very unusual to visualize a pulmonary artery thrombus in TTE. Therefore, we expect to improve the current TTE diagnosis of APE through the modified view mentioned in this case report.
3.1. Role of echocardiography in evaluation of PE
Echocardiography is not a diagnostic method for PE 5 but is used for risk stratification. 1 , 7 TTE provides noninvasive assessment of RV and LV size, systolic function, regional wall motion, valvular abnormalities, and hemodynamic assessment of filling pressure and right‐heart pressures.
Some studies have confirmed that echocardiography has an effective application in the diagnosis of hemodynamic instability PE patients. 5 , 7 , 8 Meanwhile, it plays a very limited role in diagnosing middle‐ or low‐risk PE. 9 This may be related to the size and location of thrombus. Larger thrombi or those located in the center or saddle are more likely to be detected and cause other changes in right heart. 10 , 11
3.2. The modified echocardiography view
TTE views recommended in current guidelines include parasternal long‐axis view, four‐chamber view, parasternal short axis view, and subcostal view. 1 These views can clearly evaluate the right ventricular pressure overload and dysfunction caused by APE, but the detection of thrombus with farther pulmonary artery bifurcation is insufficient.
In this case report, we recommend a modified right parasternal view, which allows real visualization of the thrombosis in pulmonary arteries. We demonstrate how TTE permits to obtaining the view, this requires tilting the patient into a steep left lateral decubitus position. First, two‐dimensional echocardiograms of the parasternal long‐axis view were obtained. Then, right pulmonary artery view was obtained by placing the transducer inclination 30–45 degrees to the chest wall at the second intercostal space immediately to the right of the sternum (Figure 3).
FIGURE 3.
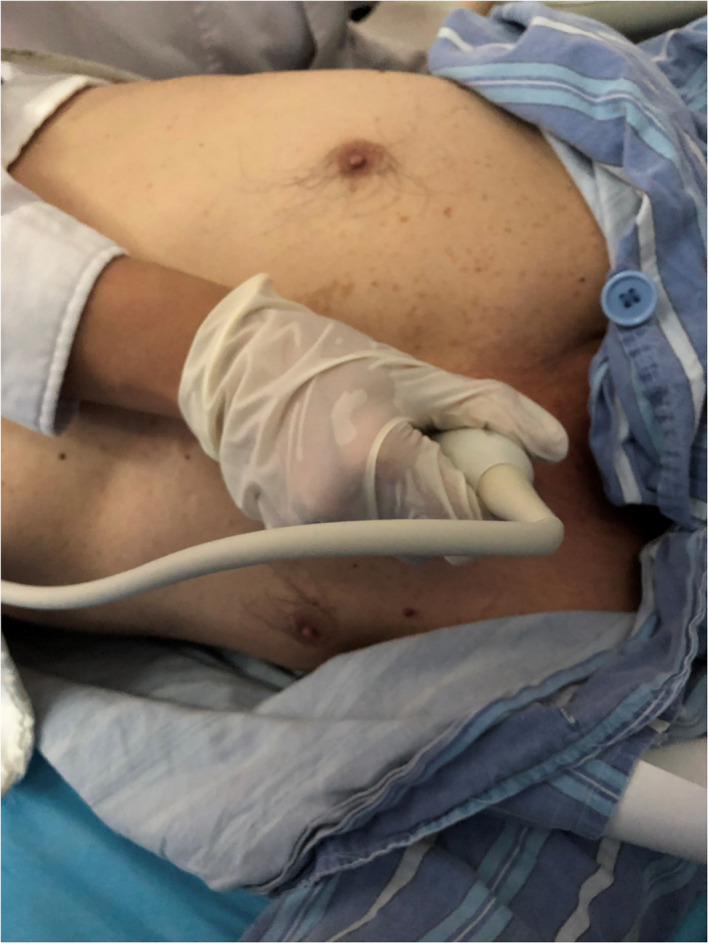
Acquirement of the modified view. Shows the transducer inclination 30–45 degrees to the chest wall at the second intercostal space immediately to the right of the sternum.
The modified view provides the unique opportunity to view the ascending aorta and distal right pulmonary artery by placing the transducer inclination 30–45 degrees to the chest wall at the second intercostal space immediately to the right of the sternum. It can directly observe the pulmonary artery farther away, increasing the possibility of thrombosis being found. Thus, the view could be included in the routine TTE of APE or in echocardiographic screening of suspected PE such as acute chest pain patients. This may provide assistance for detection and formulation of treatment strategies, especially when CTPA cannot be performed immediately.
4. CONCLUSION
A modified TTE view could provide correct diagnosis by identifying the clot size and location visually. Knowledge of the echocardiography results of APE would aid the diagnosis.
AUTHOR CONTRIBUTIONS
GM and HF conceived of the case report and its design. FL, BZ, and XC worked on acquisition of data and analysis. GM and HF drafted the manuscript. HF and GL revised the manuscript critically. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Ethical Approval
This case report was approved by the Clinical Research Ethics Committee of the Second Hospital of Tianjin Medical University.
CONSENT
All the data in this case report, including any individual details, images, or videos have consent for publication from the patient.
Supporting information
Video S1
Video S2
Video S3
Video S4
ACKNOWLEDGEMENTS
None.
Mu G, Li F, Chen X, Zhao B, Li G, Fu H. A visualized pulmonary arterial thrombus by using a modified echocardiographic view in an intermediate‐risk acute pulmonary embolism patient: A case report. Clin Case Rep. 2022;10:e06105. doi: 10.1002/ccr3.6105
DATA AVAILABILITY STATEMENT
All the data in this case report, including any individual details, images or videos have consent for publication from the patient.
REFERENCES
- 1. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019;40:3453‐3455.31697840 [Google Scholar]
- 2. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363‐2371. [DOI] [PubMed] [Google Scholar]
- 3. van der Hulle T, Dronkers CE, Klok FA, Huisman MV. Recent developments in the diagnosis and treatment of pulmonary embolism. J Intern Med. 2016;279(1):16‐29. [DOI] [PubMed] [Google Scholar]
- 4. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roy PM, Colombet I, Durieux P, Chatellier G, Sors H, Meyer G. Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism. BMJ. 2005;331:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014;112(3):598‐605. [DOI] [PubMed] [Google Scholar]
- 7. Kurnicka K, Lichodziejewska B, Goliszek S, et al. Echocardiographic pattern of acute pulmonary embolism: analysis of 511 consecutive patients. J Am Soc Echocardiogr. 2016;29:907‐913. [DOI] [PubMed] [Google Scholar]
- 8. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients‐part II: cardiac ultrasonography. Crit Care Med. 2016;44:1206‐1227. [DOI] [PubMed] [Google Scholar]
- 9. Bing R, Chow V, Lau JK, Thomas L, Kritharides L, Ng AC. Prevalence of echocardiography use in patients hospitalized with confirmed acute pulmonary embolism: a real‐world observational multicenter study. PLoS One. 2016;11:e0168554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariharan P, Dudzinski DM, Rosovsky R, et al. Relation among clot burden, right‐sided heart strain, and adverse events after acute pulmonary embolism. Am J Cardiol. 2016;118:1568‐1573. [DOI] [PubMed] [Google Scholar]
- 11. Jain CC, Chang Y, Kabrhel C, et al. Impact of pulmonary arterial clot location on pulmonary embolism treatment and outcomes (90 days). Am J Cardiol. 2017;119:802‐807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Video S3
Video S4
Data Availability Statement
All the data in this case report, including any individual details, images or videos have consent for publication from the patient.


