Significance
Improving access to family planning may promote contraceptive use after childbirth and reduce the likelihood of closely spaced high-risk births; however, empirical evidence for these claims is limited. We present findings from a field experiment that examines the causal impact of a broad-based family-planning intervention on postpartum contraceptive use and fertility over a 2-y period, which allows us to assess impact on high-risk short pregnancy intervals. Our findings demonstrate that the benefits of family planning extend beyond contraceptive use to improve fertility and healthy birth spacing. Evidence from this study will contribute to the design of effective family-planning programs and to informing the ongoing policy debate about how such programs impact longer-term health and development more broadly.
Keywords: family planning, contraceptive use, birth spacing, randomized controlled trial, Malawi
Abstract
Studies have suggested that improving access to family planning (FP) may improve contraceptive use and reduce fertility. However, high-quality evidence, particularly from randomized implementation trials, of the effect of FP programs and interventions on longer-term fertility and birth spacing is lacking. We conduct a nonblinded, randomized, controlled trial to assess the causal impact of improved access to FP on contraceptive use and pregnancy spacing in Lilongwe, Malawi. A total of 2,143 married women aged 18 to 35 who were either pregnant or had recently given birth were recruited through home visits between September 2016 and January 2017 and were randomly assigned to an intervention arm or a control arm. The intervention arm received four services over a 2-y period: 1) up to six FP counseling sessions; 2) free transportation to an FP clinic; 3) free FP services at the clinic or financial reimbursement for FP services obtained elsewhere; and 4) treatment for contraceptive-related side effects. Contraceptive use after 2 y of intervention exposure increased by 5.9 percentage points, mainly through an increased use of contraceptive implants. The intervention group’s hazard of pregnancy was 43.5% lower 24 mo after the index birth. Our results highlight the positive impact of increased access to FP on a woman’s contraceptive use. In addition, we show that exposure to the FP intervention led to a prolongation of birth intervals among intervention women relative to control women and increased her control over birth spacing and postpartum fertility, which, in turn, may contribute to her longer-term health and well-being.
Short pregnancy and birth intervals can adversely affect maternal and child health (MCH) (1, 2) and are associated with high levels of infant mortality and low birth weight, particularly in Sub-Saharan Africa (3, 4). For these reasons, the World Health Organization (WHO) recommends that women wait at least 24 mo after a live birth before attempting to become pregnant again (5). However, family planning (FP) use in the postpartum period is low, and many women in low- and middle-income countries become pregnant within this 2-y window (6). Improving access to postpartum FP has the potential to reduce these high-risk short birth intervals (7, 8), yet unmet need for FP in the postpartum period and the risk of short birth intervals remain high, particularly in Sub-Saharan Africa (9, 10).
Although there is a large empirical literature on FP, recent reviews of the impact of FP programs and interventions have found few high-quality studies that assessed intervention impact in the short term (within a year of intervention exposure) and even fewer studies that assessed outcomes beyond contraceptive use, such as pregnancy and births (11–14). In Egypt, an intervention that provided women using lactation amenorrhea with emergency contraception in advance, as a backup contraceptive method, was found to reduce pregnancies after 6 mo (15). In another study that was also conducted in Egypt, women who received an immediate postpartum insertion of an intrauterine device (IUD) were found to have lower pregnancy rates 1 y after insertion compared to women who received an insertion 6 wk after delivery (16).
Other randomized controlled trials of FP services have found equally mixed results. Findings from the Navrongo community-level experiment in Ghana found program impacts on both contraceptive use and longer-term fertility; however, balance across treatment and control communities was not achieved, and recent studies have found that effects of the intervention have attenuated over time (17–19). Two program evaluations in Ethiopia and Kenya that assessed FP services that were integrated into microcredit and HIV programs, respectively, found no intervention effects (20, 21). Finally, studies of the well-known Matlab MCH-FP program in Bangladesh have shown significant and long-standing reductions to fertility (22, 23) and increased birth intervals among women in program areas (24). However, findings from the Matlab program have been extensively debated, with critics noting that the bundling of FP with other MCH services makes it difficult to disentangle the independent impacts of FP (13, 25). In addition, the potential nonrandom selection of intervention and comparison areas has sparked questions about the extent to which causal inferences can be made from the program (24).
More recently, a cluster randomized controlled trial of a postpartum FP intervention in Burkina Faso found a significant effect on modern contraceptive use after 1 y of intervention exposure (26), but no effect after 2 y of exposure (27). A randomized trial of a similar intervention in the Democratic Republic of Congo found no effect on overall contraceptive use, but there was an observed shift in the method mix toward contraceptive implants after a year of exposure to the intervention (28). A cluster randomized controlled trial in Nepal, which improved women’s access to FP counseling during pregnancy and provided women with the option to receive IUD insertions in the immediate postpartum period, found positive effects on modern contraceptive use 1 y after exposure to the intervention. However, these findings were not sustained after 2 y of intervention exposure, with effects only being observed on the contraceptive method mix, mainly through an increase in IUDs and a reduction in other methods (29). Notably, these three randomized trials reported effects only on contraceptive use, but not on pregnancy or birth spacing. A recent evaluation of an integrated postpartum FP intervention in Bangladesh, which had intervention and comparison areas, but was not randomized, did find a significantly lower risk of short birth intervals in the intervention areas over the first 36 mo of exposure to the intervention (30).
We conduct a randomized controlled trial in Malawi to assess the causal impact of improved access to FP on women’s postpartum contraceptive use and pregnancy spacing. Details of the Malawi Family Planning Program are given in SI Appendix, Appendix S2.1. The 2015–16 Malawi Demographic and Health Survey reported a contraceptive prevalence rate of 59.2% among married women of reproductive age, while 18.7% of women had an unmet need for FP (31). Short birth intervals are common, with 11.5% of nonfirst births occurring within 2 y and 37.9% occurring within 3 y of a previous birth. In order to design the intervention, we reviewed the literature on the reasons for the nonuse of FP among fecund women in developing countries and found evidence for lack of knowledge, lack of access, opposition to use, and, particularly, fear of side effects to be key barriers to access and use (32). A recent qualitative study on access to FP in Malawi cited side effects, distance and transport costs, and lack of method choice, due to stockouts and provider preferences, as barriers to uptake, despite services being free through public-sector providers (33). An additional deterrent was the requirement to provide a blood sample for HIV and pregnancy testing, which would entail returning to the facility for results, before accessing public-sector FP services (34). We subsequently designed an intervention that aimed to address these key barriers. Our study follows the Consolidated Standards of Reporting Trials 2010 standard for reporting randomized trials (35).
Results
Study Design and Participants.
We conduct a pragmatic two-arm, randomized, multi-intervention trial with married women of reproductive age in Lilongwe, Malawi; the protocol for the trial has been published elsewhere (36). We selected neighborhoods of Lilongwe and screened households within neighborhoods for eligible women who, at the time of the baseline survey: 1) were married; 2) were either currently pregnant or had given birth within 6 mo; 3) were between the ages of 18 and 35; and 4) lived in the city of Lilongwe. Eligible women were informed about the study and recruited after giving informed consent. At most, one woman was recruited from each household. Screening, recruitment, and implementation of the baseline survey were conducted from September 2016 to January 2017. The intervention began in November 2016, and women assigned to the intervention arm received services for a 2-y period, until February 2019. Details on the uptake of intervention services are provided in SI Appendix, Appendix S2.2. Two annual follow-up surveys were conducted, 1 y and 2 y after the baseline survey, respectively. Data collection for the first follow-up survey began in August 2017 and was completed in February 2018, and data collection for the second follow-up survey began in August 2018 and was completed in February 2019.
Randomization.
Following the baseline survey, women were allocated to strata based on their number of living children, ever use of FP, age of sexual debut, educational attainment, work status, and neighborhood of residence at baseline. Each month, individuals within each stratum were randomized to either the treatment or control group by a computer-generated random-allocation sequence. For strata with only one individual, allocation to the intervention or control arm was based on a random draw with equal probability of treatment. Randomization within strata reduces the likelihood of imbalance in random assignment, which increases our statistical power to detect outcomes (37). Randomization of recruited women to treatment and control arms was conducted in monthly batches in order to avoid any delays to rolling out the intervention to women who had already given birth. The study was not blinded. Additional details of the randomization protocol are presented in SI Appendix, Appendix S1.
Intervention.
The design of the components of the intervention was informed by a literature review of the key barriers to FP (32); preliminary scoping assessments of the FP and reproductive health environment by researchers in Lilongwe; and discussions with key stakeholders at the Malawi Ministry of Health, the Reproductive Health Directorate, public- and private-sector service providers, and local communities at our study sites in Lilongwe. A woman who was randomly assigned to the intervention arm was presented with the following services:
-
1.
An FP information package and up to six private counseling visits at or near her home with FP counselors, who were trained by the Ministry of Health’s Reproductive Health Directorate;
-
2.
A free transportation (taxi) service to a designated high-quality FP clinic with low waiting times;
-
3.
Free FP services at the designated clinic or financial reimbursement for any FP services received at other clinics; and
-
4.
Free over-the-phone consultations with a doctor and referral services, along with reimbursement for treatment costs in the event that the woman experienced any contraindications or side effects related to her use of FP.
Additional details on each of these intervention components are available in the published protocol (36) and in SI Appendix, Appendix S2. Women who were assigned to the control arm received information about their nearest FP clinic at the time of the baseline interview (which was also given to the intervention group) and were only recontacted at follow-up.
The intervention was designed to reduce key information and cost barriers that were identified prior to the study launch, increase accessibility to FP services, and provide counseling on and treatment for any contraceptive-related side effects for women in urban Malawi. While women were counseled on how to engage their husband/male partner through counseling and communication, the design of the study did not directly address social norms related to community or partner views on FP. Evidence on the effect of male involvement in FP decisions in Sub-Saharan Africa is mixed (38); in our study, it was the woman’s choice to decide the extent to which she wanted to involve her husband/partner in any part of counseling or the intervention. Addressing social norms in FP more rigorously would require a larger, cluster randomized, multiarm trial, which is beyond the scope of this study.
Outcomes.
We focus on three primary outcomes of the study: contraceptive use, method mix, and short pregnancy intervals (becoming pregnant again within 24 mo of the index birth) at the second-year follow-up. If women reported using a contraceptive method, they were then asked about the type of method they had used. In addition, women who reported they had become pregnant again since the index birth were asked the month in which they became pregnant. In addition to overall contraceptive use, we also report the effect of the intervention on contraceptive method mix and the use of long-acting contraceptive methods (sterilization, implants, and IUDs), and we specifically explore the intervention’s impact on the relative uptake of injectables and implants, two commonly used long-acting reversible methods in Malawi (31).
Finally, our study protocol also lists the downstream effect of induced changes to fertility on female labor supply, health and education of children, income, and well-being as secondary outcomes. These outcomes will be analyzed in a structural equation framework, using the first-stage effects of the intervention on parity and spacing, to estimate the effects of fertility and birth spacing on these downstream outcomes; refer to figure 3 in the research protocol (36) and the preanalysis plan that is included with the trial registration. The analysis of these additional outcomes will be presented in future studies.
Estimation Strategy.
We conduct an intention-to-treat analysis on contraceptive use and pregnancy since the index birth, which were measured at the 2-y follow-up, as set out in our preanalysis plan (36). All women who were followed up are included, irrespective of adherence to the study protocol. We conduct an unadjusted analysis, regressing the outcome on a constant and an indicator variable for treatment. The treatment effect therefore identifies the difference in the contraceptive prevalence rate, or pregnancy rate, between the treatment and control groups. We also report adjusted effects, where we include a number of woman-level controls that were measured at baseline, including age (in three age groups), age of sexual debut, the total number of children who were alive at baseline, a woman’s ever use of FP, a woman’s work status, educational attainment (primary or less versus secondary and higher), religion (Christian versus other), and ethnicity (Chewa versus other). We also include neighborhood-level fixed effects to control for unobserved heterogeneity at the community level. In addition to these adjustments for all outcomes, we include additional controls depending on the outcome. We did not measure and are unable to study possible heterogeneity of effects by HIV status. Finally, some women in our sample had given birth and were already using a contraceptive method at the time of the baseline interview. We control for this use (or the specific contraceptive method in question for the secondary outcomes related to the contraceptive method mix). For the outcome on pregnancy since the index birth, we control for pregnancy status at the time of the baseline interview and for the duration between the index birth and the second-year follow-up interview, in months. We report Eicker–Huber–White heteroskedasticity-robust CIs for all analyses.
The intent-to-treat analysis of the subsequent pregnancy since the index birth at the time of the second-year follow-up follows our prespecified analysis plan. However, the time between the index birth and the second-year follow-up varies across women. The index birth date differs from the date of the baseline interview because we recruited women who were either pregnant or had given birth within the last 6 mo at the time of the baseline interview. There is an issue that some of the reported pregnancies may occur after the 2-y spacing interval that is recommended by the WHO, while data for other women may be censored if their follow-up interview is conducted within 24 mo of their index birth. Given the variation in the duration of follow-up relative to the date of the index birth, a more appropriate estimation strategy is to conduct a survival analysis that examines the time from the index birth to 1) a subsequent pregnancy, 2) a loss to follow-up, or 3) to the end of the 24-mo period when a new pregnancy is considered to be high risk. In our survival analysis, we also include women who participated in the first-year follow-up, but who were subsequently lost to follow-up in the second year. Observations in this analysis are censored after the last interview date. Finally, women who were initially pregnant at baseline, but who subsequently failed to carry the child to term or have a live index birth, were excluded from the intent-to-treat pregnancy and survival analyses in light of the WHO recommendation that women who experience a termination of their pregnancy need only wait 6 mo, rather than 24 mo, before becoming pregnant again (5).
Our survival-analysis approach allows us to limit the analysis to the high-risk period in the 24 mo after the index birth, while efficiently using data from women for whom we have less than 24 mo of follow-up. This analysis also allows for the underlying incidence rate of pregnancy since the index birth to be time-varying. We report the results of a survival model as an ancillary analysis since this approach was not originally specified in our preanalysis plan. However, it should be noted that while the survival analysis may be more appropriate for the outcome of interest, the Cox proportional hazards model imposes a number of assumptions on the functional form of the intervention effect over and above those that are made in our prespecified intention-to-treat analysis (39).
For our survival analysis, we report Kaplan–Meier curves of the survival time to the next pregnancy for both the treatment and control groups. We also estimate the effect of the intervention on the hazard of pregnancy using a Cox proportional hazards model; we report both unadjusted and adjusted estimates. The adjusted estimates control for the same covariates as our intention-to-treat analysis for pregnancy, except for time between the index birth and the last follow-up interview, since both of these controls are adjusted for directly in the survival model.
Analytic Sample and Randomization.
A total of 12,590 households were approached during the recruitment period, from September 2016 to January 2017, and 2,490 women in these households met the eligibility criteria. Of these women, 2,175 women consented to participate and were enrolled in the study, and 2,143 women (86.1% of eligible women) consented to participate, completed the baseline survey, and were randomized into the intervention or control groups.
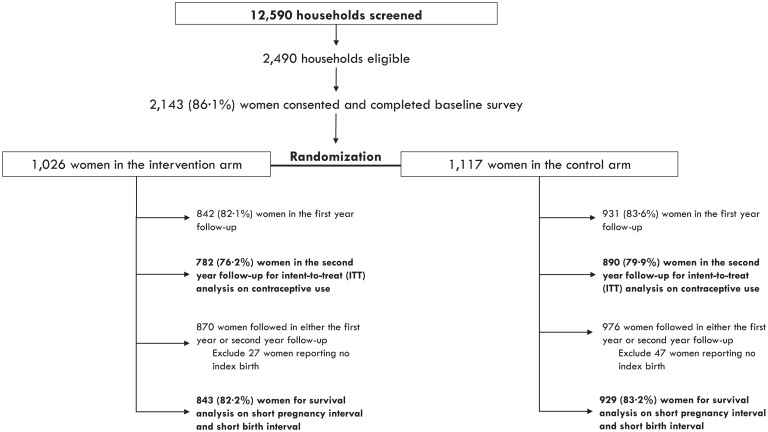
Of the 2,143 women who competed the baseline survey, 1,026 women were randomly assigned to the intervention group, while the remaining 1,117 women were randomly assigned to the control group. SI Appendix, Table S1 presents additional descriptive statistics of the sample of women in the study, while SI Appendix, Table S2 presents a balance table of outcomes at baseline and endline and covariates at baseline by treatment group; an abbreviated version is presented in Table 1. Fig. 1 describes the screening, recruitment, and randomization process, as well as the follow-up rates at 1 and 2 y. Follow-up rates relative to the baseline sample were 82.7% in the first wave and 78.0% in the second wave and were similar across treatment (76.2%) and control (79.9%) groups, as shown in Fig. 1. Attrition was somewhat higher in the treatment group than the control group; however, we do not find any observable evidence of differential attrition between intervention and control groups. SI Appendix, Table S5 presents our comparisons of women who were lost to follow-up by treatment assignment.
Table 1.
Balance table of outcomes and covariates by treatment group
| Full | Treatment | Control | Difference | |
|---|---|---|---|---|
| Baseline values | ||||
| Current use of FP (1 = yes) | 0.237 | 0.239 | 0.235 | 0.003 |
| Currently pregnant (1 = yes) | 0.516 | 0.516 | 0.516 | 0.000 |
| Ever use of FP (1 = yes) | 0.755 | 0.775 | 0.736 | 0.039** |
| Long-acting method use (1 = yes) | 0.034 | 0.034 | 0.033 | 0.001 |
| Injectable use (1 = yes) | 0.187 | 0.189 | 0.185 | 0.004 |
| Implant use (1 = yes) | 0.031 | 0.031 | 0.031 | 0.000 |
| Observations | 2,139 | 1,026 | 1,133 | |
| Endline outcomes (year 2 follow-up survey) | ||||
| Current use of FP (1 = yes) | 0.745 | 0.775 | 0.718 | 0.057*** |
| Long-acting method use (1 = yes) | 0.257 | 0.286 | 0.231 | 0.055** |
| Injectable use (1 = yes) | 0.403 | 0.399 | 0.406 | 0.007 |
| Implant use (1 = yes) | 0.219 | 0.240 | 0.200 | 0.040** |
| Pregnant since index birth (1 = yes) | 0.074 | 0.053 | 0.093 | 0.040*** |
| Birth since index birth (1 = yes) | 0.040 | 0.029 | 0.051 | 0.022** |
| Observations | 1,672 | 782 | 890 |
**P <0.05; ***P < 0.01.
Fig. 1.
Participant flowchart.
Intent-to-Treat Results.
Estimates of the effect of the intervention on outcomes at the second-year follow-up are reported in Table 2. Sample size for the adjusted analysis (1,667) is smaller than the unadjusted (1,672) due to missing data on covariates. Panel A of Table 2 reports intention-to-treat results for contraceptive use in the second year.
Table 2.
The effect of the intervention on FP use and pregnancy at second-year follow-up
| Model | Unadjusted | Adjusted |
|---|---|---|
| Panel A: Current use of FP | ||
| Treatment | 0.057** | 0.059** |
| [0.022, 0.092] | [0.024, 0.094] | |
| Panel B: Long-acting method use | ||
| Treatment | 0.055** | 0.054** |
| [0.020, 0.090] | [0.020, 0.089] | |
| Panel C: Injectable use | ||
| Treatment | –0.0066 | 0.00088 |
| [–0.046,0.033] | [–0.039,0.040] | |
| Panel D: Implant use | ||
| Treatment | 0.040** | 0.043** |
| [0.0070, 0.074] | [0.011, 0.075] | |
| Observations | 1,672 | 1,667 |
| Panel E: Pregnant again since index birth | ||
| Treatment | –0.038** | –0.037** |
| [–0.0618,–0.0137] | [–0.0621,–0.0111] | |
| Observations | 1,581 | 1,475 |
Each observation is a woman. The results presented are from intent-to-treat linear probability models, and 95% CIs, which are calculated with heteroskedasticity-robust SEs, are presented in brackets. The adjusted regressions, reported in column 2, include the following covariates at baseline: the woman’s total number of children who are alive, her educational attainment (primary or less versus secondary and higher), her age (in three age groups), age of sexual debut, ever use of FP her religion, work status, and tribal group. The adjusted regressions also include neighborhood fixed effects and control for baseline levels of the outcome. For Panel E, the time since the index birth (in months) is included as an additional control variable.
**P < 0.05.
In our adjusted analysis, we find a 5.9-percentage-point (p.p.) increase [95% CI: 2.4, 9.4] in contraceptive use among women in the intervention group after 2 y of exposure to our intervention. Panels B–D report the effect of the intervention on long-acting contraceptive methods, injectables, and implants, respectively. As can be seen from these results, the increase in contraceptive use is almost entirely due to a rise in the use of long-acting contraceptive methods, in particular, implants. On the other hand, our adjusted analyses (presented in SI Appendix, Table S3) find strong and significant correlations between baseline contraceptive use, particularly long-acting method use, and endline use. Taken together, we find that the intervention may have impacted the contraceptive method mix, but only marginally, given women’s strong underlying method preferences and high use at baseline. In Panel E of Table 2, we report estimates of the effect of the intervention on the probability of becoming pregnant again since the index birth after 2 y of intervention exposure. The sample size for this analysis is smaller than for contraceptive use since we exclude 74 women who were pregnant at baseline, but did not subsequently have an index birth. We estimate a 3.7-p.p. reduction [95% CI: –6.2, –1.1] in the probability of becoming pregnant within 2 y after the index birth. SI Appendix, Table S3 presents the full set of estimates of the key exposure and control variables for each of the adjusted regressions that are presented in Table 2.
Survival Analysis Results.
A total of 1,772 women were included in the survival analysis; Fig. 1 presents the flowchart to identify the analytic sample for this analysis. The sample is larger than for the second-year follow-up since we have partial data on time to pregnancy from wave one for some additional women. Fig. 2 presents the Kaplan–Meier survival plots of the probability of not having a pregnancy within 24 mo following the index birth and also presents the number of women who were at risk at each time point. Table 3 presents estimates from unadjusted and adjusted Cox proportional hazard models of the intervention impact on the risk of subsequent pregnancy within 24 mo from the index birth. The adjusted estimates show that, on average, the intervention reduced the risk of subsequent pregnancy by 43.5%, based on a hazard rate of 0.565 [95% CI: 0.387, 0.824]. The coefficient estimates for the full set of covariates in the adjusted model are presented in SI Appendix, Table S4.
Fig. 2.
Kaplan–Meier survival plot of the probability of not having a short pregnancy interval (pregnancy within 24 mo after birth).
Table 3.
Hazard rate estimates of pregnancy within 24 mo after index birth
| Model | Unadjusted | Adjusted |
|---|---|---|
| Treatment | 0.565** | 0.575** |
| [0.387,0.824] | [0.393,0.843] | |
| Observations | 1,772 | 1,767 |
Each observation is a woman. Columns 1 and 2, respectively, report unadjusted and adjusted hazard rates from a Cox proportional hazards model, and 95% CIs, which are calculated with heteroskedasticity-robust SEs, are presented in brackets. The adjusted regression, reported in column 2, includes the following covariates at baseline: the woman’s total number of children who are alive, her educational attainment (primary or less versus secondary and higher), her age (in three age groups), age of sexual debut, ever use of FP, religion, work status, and tribal group. The adjusted regression also includes neighborhood fixed effects.
**P < 0.05.
Discussion
Our experimental findings show that improving access to postpartum FP has a significant effect on contraceptive use, particularly on the adoption of implants (a long-acting reversible method), and on pregnancy spacing in the 2 y following the index birth. These results are consistent with prior studies that show the positive impact of postpartum FP interventions on postpartum contraceptive use and method mix (11, 12, 26, 28). The increase in implant use relative to other methods may also reflect the intervention’s potential to improve contraceptive concordance for women in the intervention group. When asked which method features or attributes were most important in choosing a method, women reported that they preferred methods that were effective, easy to use, and did not require resupply or revisits to the clinic; SI Appendix, Table S9 presents the distribution of women’s preferred method attributes. While injectables, oral contraceptives, and implants are all effective modern contraceptive methods, the longer duration of the implant relative to the injectable and pill, combined with its limited need for resupply following insertion, may make implants a more preferred choice, even relative to injectables, which remain the most widely used method in Malawi. This preference for more effective and easier-to-use methods may be more easily realized for women in the intervention group, who all received free access to a full range of methods, including clinical insertion as well as removal of long-acting methods, at our partner clinic. In addition to the increase in contraceptive use, we find that the intervention led to a significant decrease in the likelihood of becoming pregnant again within 2 y of a birth; our survival-analysis results indicate that the intervention nearly halved the probability of a pregnancy in the 24 mo after the index birth. This finding highlights the positive and sustained impacts of FP access on both a woman’s contraceptive use and her control over birth spacing and postpartum fertility.
Our trial has several limitations. Given that the treatment assignment was conducted at the individual level to women within the same neighborhoods, we cannot exclude the possibility of spillover effects between intervention and control women, which might attenuate our overall estimated treatment effects (40). The loss to follow-up, while smaller than expected in our analysis plan, introduces a potential bias if it was related to the outcomes of interest; an analysis of women who were both reinterviewed as well as lost to follow-up suggests that attrition is not differential by treatment status (refer to SI Appendix, Table S5). While we had independent teams separately carry out the intervention components (counseling, transport, and reimbursement activities) and data-collection activities (baseline and follow-up interviews), and although our teams were not directly informed of treatment assignment, we cannot rule out potential assessment biases by both interviewers and respondents since the trial was not fully blinded (41). Another limitation of our intervention design is that it does not allow us to disentangle the effects of the different components that, taken together, comprise the intervention package. Such an approach would require a much larger, multifactorial trial, through which both the independent and joint effects of each component on outcomes could be estimated.
From a policy standpoint, our intervention estimates may not be generalizable to other settings. The limits to external validity may be significant, given the observed heterogeneity in the impacts of FP interventions in different settings (11, 12, 26, 28). When comparing our findings with other FP interventions, particularly the Matlab and Navrongo programs, we find that baseline contraceptive use in Lilongwe was significantly higher in 2016 (58%) than what was found in Matlab (1%) and Navrongo (10%) prior to the introduction of FP programs in those settings. This suggests that we may observe a larger intervention effect (and possibly even larger than what was observed in Matlab and Navrongo) if our intervention were rolled out in a setting where baseline contraceptive prevalence is not as high. As such, our observed treatment effects provide an estimate for a lower bound of the underlying effect of our intervention. On the other hand, our intervention was more intensive relative to other FP programs (though not as intensive or costly as the Matlab and Navrongo programs) and was intended to overcome multiple barriers to access simultaneously. A less intensive program that might be more feasible to scale up might therefore have a smaller impact on outcomes. Finally, we note that our eligibility criteria, as well as our follow-up study design, were established specifically to conduct an FP intervention in the postpartum period and to measure the effect on birth spacing, as well as contraceptive use. Restricting our study eligibility to pregnant and immediate postpartum women limits our ability to generalize our findings to other populations, as well as to compare our intervention impacts with those from larger programs, such as Matlab and Navrongo, which targeted all women of reproductive age over a longer period.
The relevance and significance of FP and FP programs on pregnancy and fertility-related outcomes has been historically questioned and debated (42) since women may use other methods of fertility control. Our results demonstrate that there are strong and significant effects from expanding access to FP, contributing to an existing, but limited, evidence base on the effects of FP interventions on both short-term outcomes, such as contraceptive use, and longer-term outcomes related to pregnancy, fertility, and birth spacing. Our study also provides an opportunity to investigate the direct benefits of a comprehensive, multicomponent FP intervention on longer-term measures of health and well-being, for which high-quality empirical and policy evidence is limited and mixed (13, 14, 43). Further investigation in these domains is warranted to uncover the overall welfare and programmatic impacts of the intervention. More broadly, evidence from this study will contribute both to the design of FP programs and policies that work to increase contraceptive access and to the debate about how such programs can affect contraceptive use, health, and longer-term development.
Materials and Methods
Ethical Considerations and Trial Registration.
Ethical approval to conduct the study was received from the Harvard University Institutional Review Board (Protocol No. IRB16-0421) and from the Malawi National Health Sciences Research Committee (Protocol No. 16/7/1628). Written informed consent to participate in the study was obtained, and only women who consented were recruited into the study. This trial was registered at the American Economics Association Registry for randomized controlled trials on May 7, 2015 (AEARCTR-0000697) and at the Registry for International Development Impact Evaluations (RIDIE) on May 28, 2015 (RIDIE-STUDY-ID-556784ed86956). The initial trial registration specified the study to be conducted in Burundi; however, this proved to be infeasible due to political unrest in the country. The study registration was therefore updated to reflect a change of location to Malawi on April 27, 2016, in both registries.
No harms were reported from the study. However, in two cases, baseline interviews with women who had consented were interrupted by their husbands, and the interviews were not able to be completed. These cases were reported to both Institutional Review Boards, and they approved removal of these women from the study and further follow-up.
Power Calculations.
Our target recruitment sample size was 2,000 women, which was based on an expected attrition rate of 27% over 2 y, given the high migration rates in poor urban settings in Malawi. This sample size would give 90% statistical power to detect a 6.5-p.p. increase in modern contraceptive prevalence and 99% power to detect an odds ratio of 0.88, a 12% reduction in the odds of pregnancy within 2 y of the previous birth. We refer to our preanalysis plan and published study protocol for additional details on our power calculations for our main outcomes (36).
Supplementary Material
Acknowledgments
We are grateful to Günther Fink, Jessica Cohen, Maggie McConnell, Jocelyn Finlay, Iqbal Shah, Helena Choi, Ruth Levine, Abiba Longwe-Ngwira, Fannie Kachale, Modesta Kasawala, Mihira Karra, Marlene Lee, James Gribble, John Townsend, Beth Brogaard-Allen, Laetitia Lemoine, Elina Pradhan, Akshar Saxena, Livia Montana, and participants at the Department of Global Health and Population at the Harvard T. H. Chan School of Public Health, the Harvard Center for Population and Development Studies, the Center for African Studies, the International Health Economics Association Congress, the Population Association of America Annual Meetings, the International Conference on Family Planning, the McGill University Center for Population Dynamics Seminar, the International Food Policy Research Institute Malawi Research Seminar, and the Ninth Annual Population and Poverty Research Network Conference for their helpful comments, support, and feedback over the course of the project. This research makes use of original data collected by D.C. and M.K. with support from Innovations for Poverty Action in Malawi. Field implementation would not have been possible without the support of the Malawi Ministry of Health and Reproductive Health Directorate, Population Services International Malawi, Faison Mussa and the Good Health Kauma Clinic, and Carly Farver, Patrick Baxter, Reginald Chunda, and the entire Malawi Family Planning Study team, which comprised 22 enumerators and 7 FP counselors over a 3-y study period. We also thank Fatima Aqeel and Xiao Chen for their research assistance. Finally, we would like to especially acknowledge the dedication and support of Viola Nyirongo, Violet Chitsulo, and Macdonald Salamu. This study was funded through William and Flora Hewlett Foundation Grants 2014-9952 and 2017-5795. Supplemental funding support for piloting, travel, and fieldwork was provided by the Global Development Policy Center at Boston University, the Harvard Center for Population and Development Studies, the Harvard Center for African Studies, and the Harvard Institute for Quantitative Social Science. Finally, M.K. received travel support from the Harvard T. H. Chan School of Public Health’s Uwe Brinkmann Travel Fellowship and research support from a Max Planck Society Sabbatical Award. The study funders had no role in study design, data collection, analysis, interpretation, or writing of the results.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2200279119/-/DCSupplemental.
Data Availability
A replication package, which includes: 1) two deidentified datasets that replicate the findings for the main paper; 2) four Stata do-files, which reproduce the results presented in the main paper; and 3) a Read-Me file for users, have been deposited to the Harvard Dataverse database, https://doi.org/10.7910/DVN/KNNYZN (44).
References
- 1.Conde-Agudelo A., Rosas-Bermudez A., Castaño F., Norton M. H., Effects of birth spacing on maternal, perinatal, infant, and child health: A systematic review of causal mechanisms. Stud. Fam. Plann. 43, 93–114 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Ball S. J., Pereira G., Jacoby P., de Klerk N., Stanley F. J., Re-evaluation of link between interpregnancy interval and adverse birth outcomes: Retrospective cohort study matching two intervals per mother. BMJ 349, g4333 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molitoris J., Barclay K., Kolk M., When and where birth spacing matters for child survival: An international comparison using the DHS. Demography 56, 1349–1370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaya S., Uthman O. A., Ekholuenetale M., Bishwajit G., Adjiwanou V., Effects of birth spacing on adverse childhood health outcomes: Evidence from 34 countries in sub-Saharan Africa. J. Matern. Fetal Neonatal Med. 33, 3501–3508 (2020). [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, “Report of a WHO technical consultation on birth spacing” (Tech. Rep., World Health Organization, Geneva, 2007).
- 6.Moore Z., et al., Missed opportunities for family planning: An analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception 92, 31–39 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Morroni C., Glasier A., Increasing the use of effective postpartum contraception: Urgent and possible. Lancet Glob. Health 8, e316–e317 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Brown W., Ahmed S., Roche N., Sonneveldt E., Darmstadt G. L., Impact of family planning programs in reducing high-risk births due to younger and older maternal age, short birth intervals, and high parity. Semin. Perinatol. 39, 338–344 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Casterline J. B., Odden C., Trends in inter-birth intervals in developing countries 1965–2014. Popul. Dev. Rev. 42, 173–194 (2016). [Google Scholar]
- 10.Gahungu J., Vahdaninia M., Regmi P. R., The unmet needs for modern family planning methods among postpartum women in Sub-Saharan Africa: A systematic review of the literature. Reprod. Health 18, 35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleland J., Shah I. H., Daniele M., Interventions to improve postpartum family planning in low- and middle-income countries: Program implications and research priorities. Stud. Fam. Plann. 46, 423–441 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Blazer C., Prata N., Postpartum family planning: Current evidence on successful interventions. Open Access J. Contracept. 7, 53–67 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller G., Singer Babiarz K., Family planning program effects: Evidence from microdata. Popul. Dev. Rev. 42, 7–26 (2016). [Google Scholar]
- 14.Mwaikambo L., Speizer I. S., Schurmann A., Morgan G., Fikree F., What works in family planning interventions: A systematic review. Stud. Fam. Plann. 42, 67–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaaban O. M., et al., Counseling and in-advance provision of levonorgestrel emergency contraceptive pills decrease the rate of unplanned pregnancy during breastfeeding: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 32, 1250–1255 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Bayoumi Y. A., et al., Post-placental intrauterine device insertion vs puerperal insertion in women undergoing caesarean delivery in Egypt: A 1 year randomised controlled trial. Eur. J. Contracept. Reprod. Health Care 25, 439–444 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Debpuur C., et al., The impact of the Navrongo Project on contraceptive knowledge and use, reproductive preferences, and fertility. Stud. Fam. Plann. 33, 141–164 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Phillips J. F., Bawah A. A., Binka F. N., Accelerating reproductive and child health programme impact with community-based services: The Navrongo experiment in Ghana. Bull. World Health Organ. 84, 949–955 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips J. F., et al., The long-term fertility impact of the Navrongo Project in Northern Ghana. Stud. Family Planning 43, 175–190 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Desai J., Tarozzi A., Microcredit, family planning programs, and contraceptive behavior: Evidence from a field experiment in Ethiopia. Demography 48, 749–782 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Kosgei R. J., et al., Impact of integrated family planning and HIV care services on contraceptive use and pregnancy outcomes: A retrospective cohort study. J. Acquir. Immune Defic. Syndr. 58, e121–e126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha N., Fertility, child work, and schooling consequences of family planning programs: Evidence from an experiment in Rural Bangladesh. Econ. Dev. Cult. Chang. 54, 97–128 (2005). [Google Scholar]
- 23.Joshi S., Schultz T. P., Family planning and women’s and children’s health: Long-term consequences of an outreach program in Matlab, Bangladesh. Demography 50, 149–180 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Joshi S., Schultz T. P., Family planning as an investment in development: Evaluation of a program’s consequences in Matlab, Bangladesh. SSRN [Preprint] ( 2007). https://papers.ssrn.com/sol3/papers.cfm?abstract_id=962938 (Accessed 10 May 2022).
- 25.Miller G., Contraception as development? New evidence from family planning in Colombia. Econ. J. 120, 709–736 (2010). [Google Scholar]
- 26.Tran N. T., et al., Post-partum family planning in Burkina Faso (Yam Daabo): A two group, multi-intervention, single-blinded, cluster-randomised controlled trial. Lancet Glob. Health 7, e1109–e1117 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Coulibaly A., et al., Yam Daabo interventions’ effects on postpartum family planning use in Burkina Faso at 24 months after childbirth. BMC Public Health 21, 946 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran N. T., et al., Effectiveness of post-partum family planning interventions on contraceptive use and method mix at 1 year after childbirth in Kinshasa, DR Congo (Yam Daabo): A single-blind, cluster-randomised controlled trial. Lancet Glob. Health 8, e399–e410 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber-Krum S., et al., The effect of antenatal counseling and intrauterine device insertion services on postpartum contraceptive use in Nepal: Results from a stepped-wedge randomized controlled trial. Contraception 101, 384–392 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Baqui A. H., et al.; Projahnmo Study Group in Bangladesh, Impact of integrating a postpartum family planning program into a community-based maternal and newborn health program on birth spacing and preterm birth in rural Bangladesh. J. Glob. Health 8, 020406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NSO Malawi, ICF, “Malawi Demographic and Health Survey 2015–16” (Tech. Rep., NSO and ICF, Zomba, Malawi, 2017).
- 32.Sedgh G., Hussain R., Reasons for contraceptive nonuse among women having unmet need for contraception in developing countries. Stud. Fam. Plann. 45, 151–169 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Chilinda I. T., Cooke A., Lavender D. T., Experiences of women, men and healthcare workers accessing family planning services in Malawi: A grounded theory. S. Afr. Fam. Pract. 62, e1–e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angotti N., Dionne K. Y., Gaydosh L., An offer you can’t refuse? Provider-initiated HIV testing in antenatal clinics in rural Malawi. Health Policy Plann. 26, 307–315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz K. F., Altman D. G., Moher D.; CONSORT Group, CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. Trials 11, 32 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karra M., Canning D., The effect of improved access to family planning on postpartum women: Protocol for a randomized controlled trial. JMIR Res. Protoc. 9, e16697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broglio K., Randomization in clinical trials: Permuted blocks and stratification. JAMA 319, 2223–2224 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Assaf S., Davis L. M., Women’s modern contraceptive use in Sub-Saharan Africa: Does men’s involvement matter? J. Glob. Health Rep. 3, e2019013 (2019). [Google Scholar]
- 39.Freedman D. A., Survival analysis: A primer. Am. Stat. 62, 110–119 (2008). [Google Scholar]
- 40.Benjamin-Chung J., et al., Spillover effects in epidemiology: Parameters, study designs and methodological considerations. Int. J. Epidemiol. 47, 332–347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins J. P., Savović J., Page M. J., Elbers R. G., Sterne J. A., “Assessing risk of Bias in a randomized trial” in Cochrane Handbook for Systematic Reviews of Interventions, Higgins J. P. T. et al., Eds. (John Wiley and Sons, Chichester, UK, 2nd ed., 2019). pp. 205–228. [Google Scholar]
- 42.Pritchett L., Summers L. H., Desired fertility and the impact of population policies. Popul. Dev. Rev. 20, 1–55 (1994). [Google Scholar]
- 43.Canning D., Schultz T. P., The economic consequences of reproductive health and family planning. Lancet 380, 165–171 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Karra M., Canning D., Replication Data for: The causal effect of a family planning intervention on women’s contraceptive use and birth spacing. Harvard Dataverse. 10.7910/DVN/KNNYZN. Deposited 10 May 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A replication package, which includes: 1) two deidentified datasets that replicate the findings for the main paper; 2) four Stata do-files, which reproduce the results presented in the main paper; and 3) a Read-Me file for users, have been deposited to the Harvard Dataverse database, https://doi.org/10.7910/DVN/KNNYZN (44).