Abstract
Background
About 40% of patients who have had COVID-19 still have symptoms three months later whereas a 10% may experience physical and/or psychological consequences two years later. Therefore, it is necessary to perform preventive interventions when patients are discharged from the hospital to decrease the aforementioned sequelae. The purpose of this pilot-controlled trial will be to determine the efficacy of a rehabilitation program on functional status and psychosocial factors for post-COVID-19 patients when it is delivered through a tele-care platform versus a booklet-based rehabilitation.
Methods
The estimated sample size will be of 50 participants who have been discharged after COVID-19 and have a level of fatigue equal or greater than 4 on the Fatigue Severity Scale. The primary outcome will be the severity of fatigue. Participants will be randomly allocated to an “asynchronous telerehabilitation group” or to a “booklet-based rehabilitation group”. Treatment in both groups will be the same and will consist of a combination of therapeutic exercise and an educative program. Treatment outcomes will be evaluated the last day of the intervention and at three- and six-months follow-up.
Discussion
The telerehabilitation intervention appears to be a viable and efficacy option in decreasing severe fatigue and other fitness variables such as strength and aerobic capacity, similar to other traditional rehabilitation formats such as through an explanatory booklet.
Clinical trial registration
This trial has been prospectively registered at clinialtrials.gov identifier: NCT04794036.
Introduction
About 40%-70% of patients still have symptoms three months later whereas a 10% may experience physical and/or psychological consequences two years later [1–3]. In addition, it is known that patients who have been hospitalized or home-bound have greater physical consequences [4]. Therefore, these symptoms can be perpetuated over time and may lead to long-term consequences called “post-COVID condition” in approximately 10% of post-COVID-19 patients [2, 5].
Fever, cough, generalized pain, gastrointestinal disturbances, dyspnea, and fatigue are amongst the most frequent symptoms in the acute phase [6]. In the post-COVID-19 phase, when the infection has disappeared, the most common symptom was fatigue, which was present from 46 to 53% of the cases [1–3].
Furthermore, psychosocial factors such as the experience of illness and isolation, have entailed to higher levels of depression, stress and anxiety in post-COVID-19 patients affecting their health and recovery [7–10]. Both, physical and psychological sequelae affect quality of life, increasing the risk of comorbidities, and slowing down the recovery of these patients [11]. This might be very detrimental, especially in the elderly population [12, 13].
So, it is necessary to perform early interventions based on therapeutic exercise to prevent and improve all these sequelae after post-acute phase [4, 14–16]. This may lead to improvements in the functional status, which ultimately may reduce dyspnea, fatigue and other associated symptoms [17], even for patients with respiratory disease [18]. However, the health system overload due to the high number of people affected, the avoidance of face-to-face attendance, and the difficulty to provide health services due to the dispersion of the population, may limit the access of people to treatment after hospital discharge.
The use of telerehabilitation, defined as the delivery of rehabilitation services via technologies, appears to be a viable option [4, 13]. Furthermore, it could reduce the possible consequences of disease and prevent disability without the need to attend in person [12, 14, 19]. In addition, asynchronous telerehabilitation allows to incorporate home-based rehabilitation for the treatment of patients who have been discharged and do not require face-to-face treatment. This benefits especially to different population groups such as those who live in rural areas and has less access to health services, who experience any mobility limitations or have to be confined at their homes [20, 21].
According to the current scientific evidence, the efficacy of telerehabilitation has been demonstrated in many other pathologies such as the cardiac, neurological, respiratory, and musculoskeletal [19, 22–26]. There is already evidence of the efficacy of telerehabilitation interventions in the acute phase of COVID-19, at hospital setting patients and at home setting patients without hospitalization [27–30]. On the other hand, several studies have shown that telerehabilitation interventions can lead to an increased patient adherence and satisfaction [24, 31, 32]. To the authors’ knowledge, no previous studies have assessed if an asynchronous telerehabilitation program consisting of a combined therapeutic exercise and education program is effective to improve the functional status and the patient´s adherence and satisfaction of post-COVID-19 patients.
Therefore, the aim of this pilot study will be to analyze the efficacy on fatigue, functional status and psychosocial factors of a 12-weeks therapeutic exercise and education program, when this is delivered through an asynchronous telerehabilitation format versus a booklet-based rehabilitation format in post-COVID-19 patients.
Methods
Study design
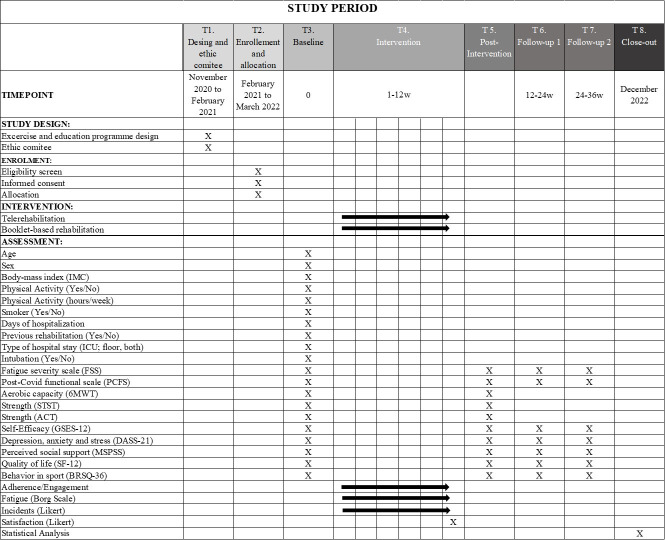
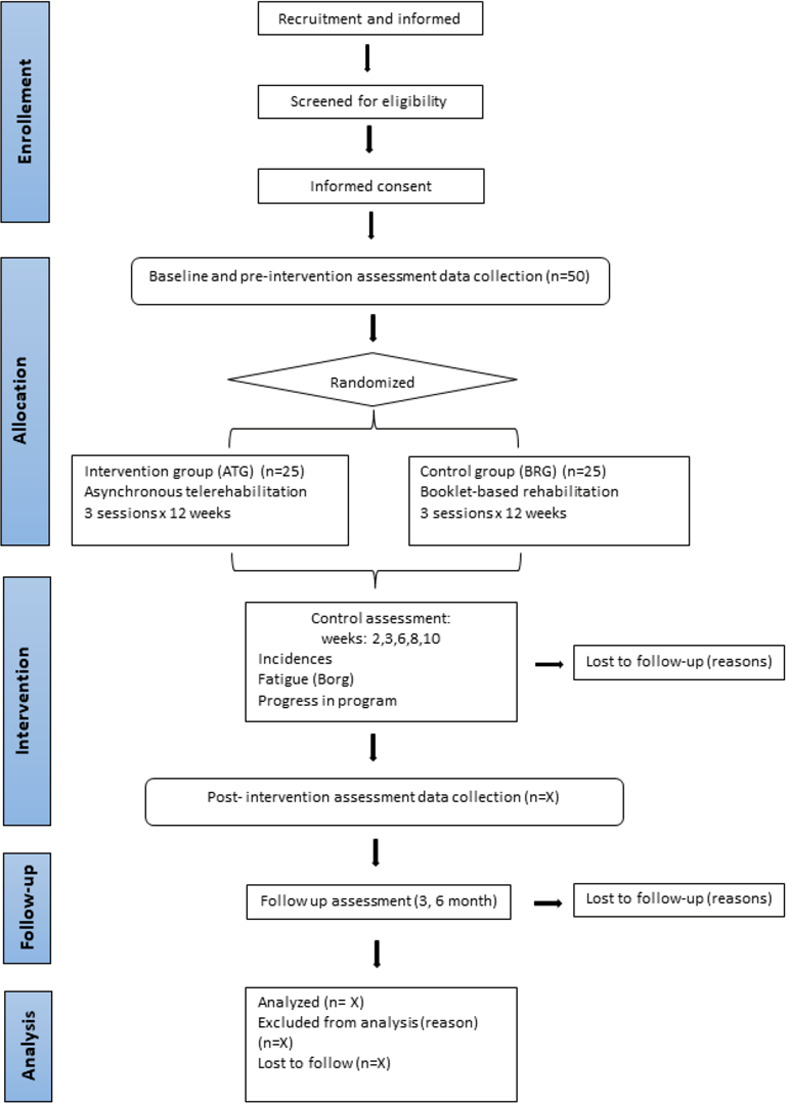
This protocol will be a pilot and feasibility study with a single-blind randomized clinical trial design. The trial has been designed according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) and will compare two parallel interventions: an intervention group (asynchronous telerehabilitation group, (ATG)) and a control group (booklet-based rehabilitation group, (BRG)). The duration of the study will be 12 months, with 12 weeks of intervention and two follow-ups at three- and six-months post-intervention. Figs 1 and 2 illustrate the pilot trial design. The study protocol has been approved by the Ethics Committee of Aragón (reference number: PI21/019, current protocol version date April 04, 2021). The protocol has been registered at clinialtrials.gov (NCT04794036).
Fig 1. SPIRIT statement.
Description of the study steps: 6MWT: 6 Meter Walk Test; STST: Sit-To-Stand Test; ACT: Arm Curl Test; GSES-12: General Self Efficacy Scale; DASS-21: Depression, Anxiety and Stress Scale; MSPSS: Multidimensional Scale of Perceived Social Support; SF-12: Self-reported Quality of Life Questionnaire-version 2; BRSQ-36: Behavioral Regulation in Sport Questionnaire.
Fig 2. Flow chart.
Setting and population
This study will be conducted at the Hospital Real y Provincial Nuestra Señora de Gracia (HPNSG) and the Hospital Royo Villanova (HRV), both in Zaragoza (Spain). Recruitment will be carried out at post-COVID-19 rehabilitation unit. Participants will be patients who had been infected by the COVID-19 and have been discharged from the hospital.
Eligibility criteria
All potential participants will be informed before and will have to give their consent to participate in the study. The inclusion criteria will be: 1) post-COVID-19 patients discharged after more than 5 days hospitalized; 2) aged 18 to 75 years; 3) independent standing with or without technical aids; and 4) present a degree of fatigue ≥4 points in the FSS. The exclusion criteria will be: 1) having any other central and/or peripheral neurological disorders; 2) a previous history of rheumatic pathology or acute musculoskeletal injury; 3) patients with severe hypoxemia, defined as having a SaO2 less than 90% or a respiratory rate ≥30; 4) having any cardiac comorbidities or signs of cardiovascular instability as uncontrolled arrythmia, blood pressure and/or effort angina; 5) having any other contraindicated pathology for moderate-intensity aerobic or strength exercise; 6) a score ≤24 evaluated with the validated Spanish version of Mini-mental State Examination (MMSE-MEC) [33]; 7) no access to internet; and 8) to be unable to follow oral and written instructions in the Spanish language.
Allocation and blinding
Participants will be assigned to the intervention or the control group through the software www.randomizer.org by an independent researcher, not involved in the treatment or evaluation, conducted at 1:1 ratio, giving each participant an identification code (IC) to guarantee their anonymity. The envelopes will be securely stored and will be opened in sequence to reveal group allocation.
Both, participants, and main researchers, will be masked to the randomization sequence. The evaluator will be unaware of the allocation and the intervention researcher will not participate in the evaluations.
Procedure
At the time of hospital discharge all potential participants will receive the study information with a telephone number to contact. Those who agree to participate will call to the information number provided in the information sheet. One of the researchers of the study will ensure that the patient meets the inclusion and exclusion criteria and will schedule the patient for the baseline and pre-intervention assessment. In the first meeting with the researcher, the patient will be asked to sign the informed consent form.
All assessments will be always performed at the same time and place in the hospital to maximally preserve the participant conditions. Each assessment will last about 60 min. This evaluation shall be carried out according to the same procedure previously trained.
Once the baseline and pre-intervention evaluation is completed, a second researcher will assign each patient to the ATG or BRG according to the 1:1 randomization previously done with a web page (www.randomizer.org). This same researcher will help the ATG patients to install the telerehabilitation platform in the mobile (through an app) or will explain the home booklet-based program to the BRG patients. The intervention will be prescribed considering the starting level of the program which will depend on the pre-intervention FSS score.
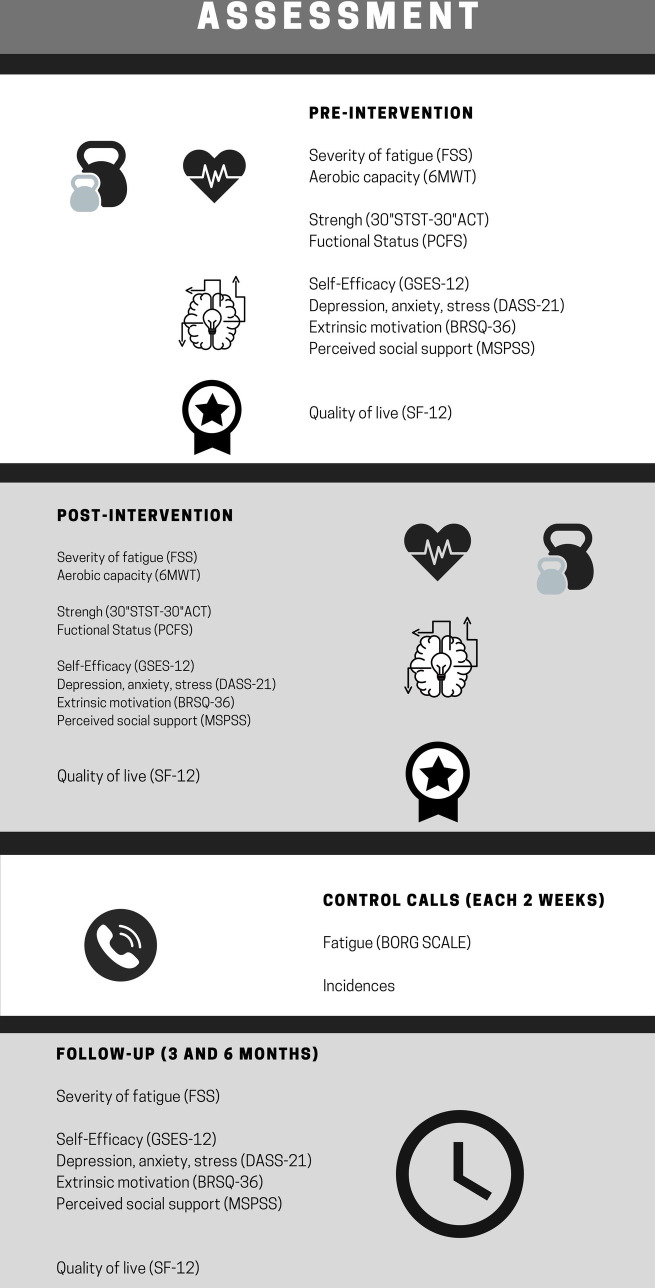
Every two weeks during the intervention, all patients will receive a control call from the second researcher to ask them about possible incidents and decide if the patient must progress to the next exercise phase based on the intensity of fatigue perceived by each patient. Three- and six- months after the intervention, patients will receive a follow-up call to analyze the level of fatigue (FSS), the functional status (PCFS), the quality of life (SF-12) and the psychosocial factors (GSES-12, DASS-21, BRSQ-36, MSPSS) (Figs 1 and 2).
Intervention
The ATG and BRG will receive the same therapeutic exercise and education program. It has been designed following the recommendations of the Spanish physiotherapy associations, the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and the American Thoracic Society (ATS) [34–36]. The program will be performed for 12 weeks (three sessions/week). The therapeutic exercise program will include aerobic, strength and lung capacity exercises [37]. It will be composed of three levels of difficulty with different exercises in each one, except for the pulmonary exercises (S1 Appendix). The first level will include patients with a score equal to or greater than 6 on the FSS, the second level will include those with a score between 5–5.99 and the third one will include those patients with a score between 4–4.99. Every patient, regardless of their initial level, will start with 10 minutes of the proposed aerobic exercise and 3 sets of strength exercises doing as many repetitions as the patient can. The lung capacity exercises will be the same throughout the entire intervention and will be performed in 2 sets of 10 repetitions with a fatigue level below 3 on the Borg Scale.
The intensity will be measured with the modified Borg Scale. All participants will start for the first two weeks with a fatigue intensity between 3 and 4 and, after 2 weeks, the intensity will be increasing to levels between 5–7 level. Patients will progress every two weeks according to the information provided during the control calls. The appearance of symptoms such as chest pain, lot of coughing, high fatigue, fever, dizziness, or any others deemed relevant, will be taken into account not to progress with the exercises.
The therapeutic education program will be available to all participants during the 12-week intervention. It will consist of 3 blocks of health advice. The first block will focus on how to prevent reinfection by COVID-19 though hygienic recommendations. The second block will focus on how to plan daily life when the patient is at home for a longer period and the third block will focus on psychological advice to reduce levels of anxiety and stress during the recovery period.
Intervention group
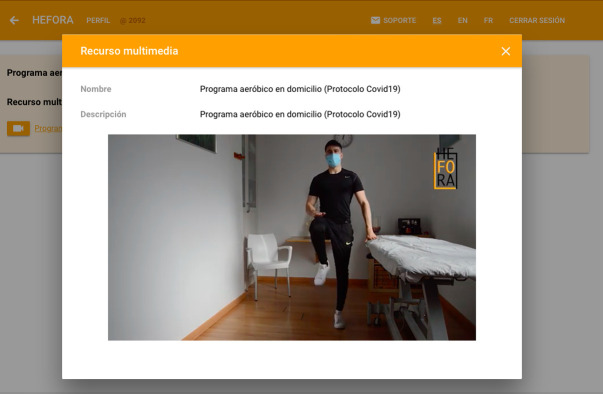
The ATG will perform the program through a telerehabilitation platform which is accessible via www.hefora.net or with an app mobile that will be installed in the patients´ mobile (HEFORA, Fisio Consultores, Zaragoza, Spain). Therapeutic exercises will be presented in the form of an explanatory video with a specific description (Fig 3). The platform allows the physical therapist to customize the number of sets, repetitions, speed, and observations for each patient. The therapeutic education recommendations will be presented through animated educational videos, explaining to the patients the health and emotional tips to improve their quality of life after COVID-19.
Fig 3. HEFORA interface.
Control group
The BRG will perform the same therapeutic exercise and education program than the ATG but through a booklet-based rehabilitation, which will include key pictures and descriptions for every exercise of each level. In addition, patients in the BRG will receive the same therapeutic education recommendations that in the ATG but in text format.
Outcome measures
Primary outcome: Fatigue
The primary outcome measure will be the fatigue, measured with the Fatigue Severity Scale (FSS). The FSS is a self-reported scale that allows assessing the severity of fatigue as a sense of physical tiredness, muscle weakness and lack of energy [38–40]. It is composed of 9 items with scores ranging from 1 = strongly disagree to 7 = strongly agree. The higher the number, the greater the severity of fatigue. The most common cut-off point is a mean score of 4 point, considering equal or more than 4 as severe fatigue [38, 41–45]. Three levels of severity will be considered: 4 = borderline fatigue; 5 = high fatigue; 6–7 = very high fatigue [44]. It has been shown to be reliable [38].
Secondary outcomes: Functional status, psychosocial factors, quality of live and feasibility
Functional status. It will be measured using the main recommended test for post-COVID-19 patients [46].
- Aerobic capacity
- 6MWT is a sub-maximal exercise test used and recommended to assess the maximum distance possible for six minutes, in a 30-meter corridor, allowing the patient to rest as needed. It has been shown to be reliable [47–49]. The distance covered by each participant will be compared with the estimated distance for their gender, weight, and age according to the Troosters equation [50–52].
- Strength
- 30” STST is part of the Senior Fitness Test (SFT) designed by Rikli and Jones and it will be used as a stand-alone test, especially to assess weakness in respiratory patients who have passed COVID-19 [53–55]. It has been shown to be reliable in adults with asthma or pulmonary hypertension [53, 56–58]. This test will be performed with a chair, with the patient´s feet resting on the floor and the arms crossed on the chest. Once in this position, the patients will sit down and stand up to the starting position as many times as possible within 30 seconds [59].
- 30” ACT is part of the SFT and is also used as a stand-alone test to assess strength. It has been shown to be reliable [53, 60, 61] in deconditioned patients and in the elderly population. From a sitting position, the patient is asked to do as many elbow flexion-extension movements as possible with his/her dominant limb during 30 seconds with a two kg weight. The higher number of repetitions the better strength [53, 59].
- Functional Status post-COVID
- PCFS is a scale specially designed and that has been shown to be reliable to assess the functional status of patients who have gone through COVID-19 at discharge [50, 62–64]. It consists of four levels of post-COVID-19 functional limitations been: D = death; 0 = no limitation; 1 = no significant functional limitation; 2 = mild functional limitation; 3 = moderate functional limitation; 4 = severe functional limitation. This scale can be administered by telephone or self-administered [12].
Psychosocial factors.
Self-efficacy will be measured with the General Self-Efficacy Scale (GSES-12), which is an abbreviated scale developed by Bosscher and Smit [65]. It is composed of 10 items scored on a Likert scale from 1 = never happens to me, to 5 = always happens to me. It has been shown to be reliable [66]. The higher the score, the greater the perceived self-efficacy [65, 67–70].
The level of depression, anxiety and stress will be measured with the Depression, Anxiety, and Stress Scale (DASS-21), which is a set of 3 self-reported questionnaires designed to measure the negative emotional states of depression, anxiety, and stress. Each of these DASS subscales contains 7 Likert-type items with a score from 0 = did not apply to me, to 3 = applied to me very much or most of the time. The total score for the global scale score ranges from 0 to 21 points. The higher scores indicate higher levels of stress, depression, and anxiety [71–73]. The DASS-21 has been shown to be reliable [74, 75].
The Behavioral Regulation in Sport Questionnaire (BRSQ-36) will be used to measure the motivation to practice physical activity. It has been shown to be reliable [76, 77]. The BRSQ-36 is composed of 36 items, and it is divided in three main domains: extrinsic motivation: integrated regulation (items 5,14,23,32), identified regulation (items 6,15,24,33), introjected regulation (items 7,16,25,34) and external regulation (items 8,17,26,35); intrinsic motivation: knowledge (items 2,11,20,29), performance (items 4,13,22,31), stimulation (items 3,12,21,30) and general (items 1,10,19,28) and 3); and amotivation (items 9,18,27,36). Its items are valued using a Likert scale that ranges from 1 = not at all true, to 7 = very true. A higher total score on each of the factors indicates a higher dominance of that motivational factor [78].
The perceived social support will be measured with the Multidimensional Scale of Perceived Social Support (MSPSS) [79]. It is considered a priority scale to be applied in people who are in the process of recovery. It has been translated and validated in different languages [80]. It measures social support in 3 domains, family, friends, and significant others. It has a three factors structure with each subscale comprising four items addressing practical help. Emotional support, availability to discuss problems and help in decision making. The version used will consist of 12 items that are answered using a Likert scale that ranges from 1 = totally disagree, to 7 = totally agree. Total scores from 12–14 indicate low social support, scores from 49 to 68 indicate moderate social support, and scores from 69 to 84 indicate high social support [81, 82].
Quality of life. It will be measured with the Self-Reported Quality of Life questionnaire, version 2 (SF-12). The SF-12 is a shortened version of the SF-36 conducted in 2002. It consists of 12 questions covering 8 dimensions which are grouped into two components, physical and mental. The scores for each item are different and range from 1–2 to 1–6. The overall score ranges from 0 to 100 considering both components. Its interpretation is based on the reference values for the Spanish population, indicating higher scores a higher self-perceived quality of life [83–85]. The version 2 of SF-12 has been shown to be reliable in a student population during COVID-19 [86, 87].
Feasibility. It will be measured by the recruitment and adherence rate, the report of satisfaction and the number of incidences. It will be reported with a diary in the telerehabilitation platform or in a diary in the booklet [88, 89].
Adherence is the degree to which a person’s behavior, with respect to taking medication or lifestyle change recommendations, is in accordance with the prescriptions of the healthcare professional. It will be recorded in the activity logs of the brochure or through the platform questionnaire and will be coded by means of a Likert scale, with 0 = if do not has performed the exercises, 1 = if has have carried out some exercises, 2 = if has performed all recommended exercise, and 3 = if they have carried out more additional activity. Additional physical activity will be understood as voluntary physical activity that exceeds the recommended daily activity. A high adherence will be considered when the participant performs at least the 80% of the sessions, and they have done all recommended exercised or more. Non-adhesion will be considered to perform less than 20% of the sessions [90, 91].
Recruitment rate, will be calculated based on the percentage of the sample size recruited.
Satisfaction with the pilot process will be recorded with a predetermined questionnaire on a Likert scale, from 0 = very dissatisfied, to 4 = very satisfied [92–94]. Participants will evaluate the overall aspects, the brochure, the platform, the attention received, and the exercises given.
Incidents will be assessed during the control calls: 0 = no incidences; 1 = some incidence but it has been solved; 2 = incidences that makes it impossible to follow up that period.
All the clinical outcomes (FSS, PCFS, GSES-12, DASS-21, BRSQ-36, MSPSS, SF-12) will be measured at pre-intervention, post-intervention and at three- and six-months follow-up. The functional status outcomes (6MWT, 30” ACT and 30” STST) only will be measured pre- and post-intervention. Regarding the feasibility outcomes, incidences and adherence will be collected during the control calls every two weeks. Satisfaction will be measured in the post-intervention evaluation. The timeline is shown in Fig 4.
Fig 4. Timeline of outcome measurement.
FSS: Fatigue Severity Scale; 6MWT: 6 Meters Walk Test; STST; Sit-To-Stand Test; ACT: Arm Curl Test; PCFS: Post-Covid Functional Scale; GSES: General Self Efficacy Scale; DASS: Depression, Anxiety and Stress Scale; BRSQ: Behavioral Regulation in Sport Questionnaire; MSPSS: Multidimensional Scale of Perceived Social Support.
Sample size calculation
A sample size of 50 subjects will be required, 20 in each group, plus 10 in anticipation of future losses according to the recommendations for RCT pilot studies [95, 96].
Data management
A detailed database will be set up to track each participant’s progress, including all evaluations. All the generated data will be recorded and stored on password-protected computers, that will be only accessible to the researchers involved in this study. An independent researcher will monitor data collection progress and safety. Data will be analyzed when all recruitment and data collection has been performed.
Statistical analysis
Statistical analysis will be performed with Statistical Package for the Social Sciences version 25.0 (SPSS Inc, Chicago, IL). The significance level will be 0.05 for all statistical analyses. Descriptive statistics, including frequency counts for categorical variables and measurements of central tendency and dispersion for continuous variables (standard deviation, 95% confidence interval) will be calculated to summarize the data.
The Shapiro-Wilk test will be used to determine the normality of the data.
General lineal models will be performed to compare the intervention effects [time (pre-intervention vs post-intervention vs follow-up 1 and 2 assessments) x group interaction] on the primary and secondary outcomes: severity of fatigue, functional status, self-efficacy, depression, anxiety and stress, quality of live, behavioral regulation in sport and perceived social support. It will be examined with analysis of variance (ANOVA) mixed model for repeated measures when normal distribution will be detected. A post hoc test will be used to locate the differences between the groups, if necessary. If data are not normally distributed, statistical analysis will be performed using non-parametric correspondent tests like Friedman test to compare the three moments involved and Wilcoxon test if statistics significance will be reached in previous Friedman test. In Wilcoxon test, type I error will be divided by the number of tests done.
For the aerobic capacity, strength, functional status post-COVID and feasibility outcomes, comparisons between-groups outcomes will be using t-tests for independent samples and Levene test for parametric data, or Mann-Whitney test U tests for non-parametric data. Chi-square and Fisher tests will be used of independence for categorical data. Finally, in the event of possible dropouts, analyses will be conducted as intention to treat.
Between- and within-group effect sizes using Cohen’s d or r for main outcomes will also be computed.
Chi square test for qualitative data and t-test or Mann-Whitney for normal or not normal data respectively will be used to check the comparability between the two groups at baseline.
Ethical aspects and dissemination
The study will be conducted following the ethical guidelines of the Declaration of Helsinki. This protocol has been approved by the Ethics Committee of Aragón (reference number: PI21/019, current protocol version date April 04, 2021). This research was registered at ClinicalTrials.gov Protocol Registration System with the number NCT04794036.
Only the researcher that will be performed the analysis will have access to the data set. To ensure confidentiality, data will be codified to blinded of any identifying participant information.
Discussion
This protocol describes a pilot and feasibility study to analyze the efficacy of an exercise and education therapeutic program using asynchronous telerehabilitation for post-COVID-19 patients. The aim of the pilot study will be to analyze if there are improvements in the participants´ level of fatigue and if these changes are maintained in the short (3 months) and medium term (6 months) and compare it with traditional home rehabilitation using a booklet. The secondary aim will be to analyze the feasibility of this pilot study.
Telerehabilitation is well accepted in terms of overall user experience, adherence, and satisfaction [94]. So, it represents a great opportunity for the treatment and monitoring of long-term patients. Currently, the large increase of COVID-19 cases during 2020 and 2021 has made telerehabilitation-based consultation a viable and reliable option in several pathologies. Scientific evidence shows how telerehabilitation is already effective in post-COVID-19 patients, as reported in the systematic review of da Silva et al. [97] where it was found that a physical programme using telerehabilitation can improve functional capacity, dyspnea and quality of life. Similarly, Dalbosco-Salas et al. [98], found that a group of post-COVID-19 patients undergo remote rehabilitation using synchronous telerehabilitation, achieved improvements in physical condition and quality of life. Although their model is on-line and there is no control group, the study shows positive results for post-COVID-19 patients in lower limb strength and quality of life. Furthermore, in most of the studies carried out in the acute and post-COVID-19 patients, the control group does not receive treatment.
Moreover, telerehabilitation has also shown to be effective in many other different conditions such as musculoskeletal pain, chronic obstructive pulmonary disease (COPD) or cardiovascular disease [99]. The study of Lewis et al. [100] showed that telerehabilitation is effective in COPD patients on multiple variables such as lower limb strength, fatigue, dyspnea, and anxiety, including improvements also in patient engagement and exercise progression. Similar results were obtained by Batalik et al. [101], who performed a study with asynchronous telerehabilitation in cardiac patients and showed also to be effective to improve the aerobic capacity assessed with the 200 meter fast-walk test.
When telerehabilitation is compared to other formats, most of the studies about telerehabilitation reported clinical improvements in favor of telerehabilitation, but no statistically significant changes compared to other ways, which leads us to expect similar results in our study. Suso-Martí et al. [102] in their systematic review with meta-analysis, showed how telerehabilitation generated clinical improvements in physical condition variables in cardiorespiratory, musculoskeletal, and neurological patients, even comparable to conventional face-to-face rehabilitation. Other studies such as Dias et al. or Hansen et al. [103, 104] reported how telerehabilitation was not different to other interventions for physical function. The systematic review of Dias et al. [103] concluded that exercise via telerehabilitation can be an alternative to improve physical function and quality of live in persons with disabilities. Moreover, Hansen et al. [104] also found how telerehabilitation was similar to face-to-face rehabilitation in severe COPD patients to improve the aerobic capacity and the lower limb strength.
Regarding the psychosocial factors, several trials, such as ours, have implemented in their protocols a therapeutic education program together with the exercise program, with the aim of improving the emotional components by telerehabilitation [105]. These interventions seem to show better results than those based on isolated therapeutic exercise [103, 106]. However, although it seems that emotional factors can be improved with therapeutic education, it has no effect on decreasing the perception of fatigue [107]. In the cross-sectional study by Milani et al. [108] in adult population with a physical disability, it was observed that those patients who were able to undergo synchronous telerehabilitation via Skype had higher self-efficacy with respect to the control group. In addition, Hansen et al. [104] demonstrated improvements in the depression scale, compared to the group without telerehabilitation, measured with the HADS-D scale.
Finally, we also expect to find clinical changes in the quality of life, especially in the physical component, as was found in the study by Milani et al. [108] where a slight improvement was observed in the telerehabilitation group. Likewise, the systematic review by Dias et al. [103] showed that telerehabilitation is similar in efficacy in terms of improving quality of life, assessed with the SF-36 scale, to other systems in both the short and long term.
Strengths and weaknesses of the study
The main limitation is that the two modalities of the program make it impossible to blind the patients. To avoid this intervention bias, all participants will be told that both programs are the same and with the same personalized control-calls, stressing that the only difference is the format. Other limitation will be that in the home booklet-based program will be not possible to control the execution of the exercises of each patient. Although there is a diary to record the activity on the platform and in the booklet, in order to reduce patient memory bias, adherence will be monitored during the control calls to control the dates when patients have not recorded their activity.
Supporting information
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
We would like to thank all patients and families who will be collaborating in this project. We would also like to give special thanks to the service of rehabilitation of the Hospital Real Provincial Nuestra Señora de Gracia and the Hospital Royo Villanova.
Data Availability
Deidentified research data will be made publicly available when the study is completed and published.
Funding Statement
This study has been supported by a research funding from Universidad San Jorge (ID 2122036). The funders had and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kim SY, Kumble S, Patel B, Pruski AD, Azola A, Tatini AL, et al. Managing the Rehabilitation Wave: Rehabilitation Services for COVID-19 Survivors. Arch Phys Med Rehabil. 2020. Dec;101(12):2243–9. doi: 10.1016/j.apmr.2020.09.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int J Environ Res Public Health. 2021. Mar 5;18(5). doi: 10.3390/ijerph18052621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Long covid could be four different syndromes, review suggests. Bmj. 2020. Oct 14;371:m3981. doi: 10.1136/bmj.m3981 [DOI] [PubMed] [Google Scholar]
- 4.CF DL. Adoption of telerehabilitation in a developing country before and during the COVID-19 pandemic. Ann Phys Rehabil Med. 2020. Nov;63(6):563–4. doi: 10.1016/j.rehab.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PÚBLICA SDGDS. Información clínica COVID-19. 2021 [cited 2022 31 may 2022]; Available from: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/20211028_CLINICA.pdf.
- 6.Alimohamadi Y, Sepandi M, Taghdir M, Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. Journal of preventive medicine and hygiene. 2020. Sep;61(3):E304–e12. doi: 10.15167/2421-4248/jpmh2020.61.3.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nehme M, Braillard O, Alcoba G, Aebischer Perone S, Courvoisier D, Chappuis F, et al. COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings. Annals of internal medicine. 2021. May;174(5):723–5. doi: 10.7326/M20-5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan L, Zhu G. Psychological interventions for people affected by the COVID-19 epidemic. The lancet Psychiatry. 2020. Apr;7(4):300–2. doi: 10.1016/S2215-0366(20)30073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Gerez JJ, Bernal-Utrera C, Anarte-Lazo E, Garcia-Vidal JA, Botella-Rico JM, Rodriguez-Blanco C. Therapeutic pulmonary telerehabilitation protocol for patients affected by COVID-19, confined to their homes: study protocol for a randomized controlled trial. Trials. 2020. Jun 29;21(1):588. doi: 10.1186/s13063-020-04494-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SJ, Barradell AC, Greening NJ, Bolton C, Jenkins G, Preston L, et al. British Thoracic Society survey of rehabilitation to support recovery of the post-COVID-19 population. BMJ Open. 2020. Dec 2;10(12):e040213. doi: 10.1136/bmjopen-2020-040213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Sire A, Andrenelli E, Negrini F, Negrini S, Ceravolo MG. Systematic rapid living review on rehabilitation needs due to COVID-19: update as of April 30th, 2020. Eur J Phys Rehabil Med. 2020. Jun;56(3):354–60. doi: 10.23736/S1973-9087.20.06378-9 [DOI] [PubMed] [Google Scholar]
- 12.Curci C, Pisano F, Bonacci E, Camozzi DM, Ceravolo C, Bergonzi R, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehabil Med. 2020. Oct;56(5):633–41. doi: 10.23736/S1973-9087.20.06339-X [DOI] [PubMed] [Google Scholar]
- 13.Richmond T, Peterson C, Cason J, Billings M, Terrell EA, Lee ACW, et al. American Telemedicine Association’s Principles for Delivering Telerehabilitation Services. International journal of telerehabilitation. 2017. Fall;9(2):63–8. doi: 10.5195/ijt.2017.6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowden Davies KA, Pickles S, Sprung VS, Kemp GJ, Alam U, Moore DR, et al. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Therapeutic advances in endocrinology and metabolism. 2019;10:2042018819888824. doi: 10.1177/2042018819888824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney S, Webster A, Paul L. Systematic Review of Changes and Recovery in Physical Function and Fitness After Severe Acute Respiratory Syndrome-Related Coronavirus Infection: Implications for COVID-19 Rehabilitation. Phys Ther. 2020. Sep 28;100(10):1717–29. doi: 10.1093/ptj/pzaa129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceravolo MG, de Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid "living" review on rehabilitation needs due to COVID-19: update to March 31st, 2020. Eur J Phys Rehabil Med. 2020. Jun;56(3):347–53. doi: 10.23736/S1973-9087.20.06329-7 [DOI] [PubMed] [Google Scholar]
- 17.Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F, Sánchez-Alcaraz Martínez BJ, et al. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int J Environ Res Public Health. 2021. May 17;18(10). doi: 10.3390/ijerph18105329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American journal of respiratory and critical care medicine. 2013. Oct 15;188(8):e13–64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 19.Cottrell MA, Russell TG. Telehealth for musculoskeletal physiotherapy. Musculoskeletal science & practice. 2020. Aug;48:102193. doi: 10.1016/j.msksp.2020.102193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhaskar S, Bradley S, Chattu VK, Adisesh A, Nurtazina A, Kyrykbayeva S, et al. Telemedicine as the New Outpatient Clinic Gone Digital: Position Paper From the Pandemic Health System REsilience PROGRAM (REPROGRAM) International Consortium (Part 2). Frontiers in public health. 2020;8:410. doi: 10.3389/fpubh.2020.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim MA, Pranata R. Teleorthopedic: A Promising Option During and After the Coronavirus Disease 2019 (COVID-19) Pandemic. Frontiers in surgery. 2020;7:62. doi: 10.3389/fsurg.2020.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbosa MT, Sousa CS, Morais-Almeida M, Simões MJ, Mendes P. Telemedicine in COPD: An Overview by Topics. Copd. 2020. Oct;17(5):601–17. doi: 10.1080/15412555.2020.1815182 [DOI] [PubMed] [Google Scholar]
- 23.Piotrowicz E, Stepnowska M, Leszczyńska-Iwanicka K, Piotrowska D, Kowalska M, Tylka J, et al. Quality of life in heart failure patients undergoing home-based telerehabilitation versus outpatient rehabilitation—a randomized controlled study. European journal of cardiovascular nursing. 2015. Jun;14(3):256–63. doi: 10.1177/1474515114537023 [DOI] [PubMed] [Google Scholar]
- 24.Piotrowicz E, Piotrowicz R, Opolski G, Pencina M, Banach M, Zaręba W. Hybrid comprehensive telerehabilitation in heart failure patients (TELEREH-HF): A randomized, multicenter, prospective, open-label, parallel group controlled trial-Study design and description of the intervention. American heart journal. 2019. Nov;217:148–58. doi: 10.1016/j.ahj.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 25.Thompson DA, Leimig R, Gower G, Winsett RP. Assessment of depressive symptoms during post-transplant follow-up care performed via telehealth. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2009. Sep;15(7):700–6. doi: 10.1089/tmj.2009.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agostini M, Moja L, Banzi R, Pistotti V, Tonin P, Venneri A, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. Journal of telemedicine and telecare. 2015. Jun;21(4):202–13. doi: 10.1177/1357633X15572201 [DOI] [PubMed] [Google Scholar]
- 27.Mukaino M, Tatemoto T, Kumazawa N, Tanabe S, Katoh M, Saitoh E, et al. Staying Active in Isolation: Telerehabilitation for Individuals With the Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Am J Phys Med Rehabil. 2020. Jun;99(6):478–9. doi: 10.1097/PHM.0000000000001441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Blanco C, Bernal-Utrera C, Anarte-Lazo E, Saavedra-Hernandez M, De-La-Barrera-Aranda E, Serrera-Figallo MA, et al. Breathing exercises versus strength exercises through telerehabilitation in coronavirus disease 2019 patients in the acute phase: A randomized controlled trial. Clin Rehabil. 2022. Apr;36(4):486–97. doi: 10.1177/02692155211061221 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Blanco C, Gonzalez-Gerez JJ, Bernal-Utrera C, Anarte-Lazo E, Perez-Ale M, Saavedra-Hernandez M. Short-Term Effects of a Conditioning Telerehabilitation Program in Confined Patients Affected by COVID-19 in the Acute Phase. A Pilot Randomized Controlled Trial. Medicina (Kaunas, Lithuania). 2021. Jul 3;57(7). doi: 10.3390/medicina57070684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin I, Braem F, Baudet L, Poncin W, Fizaine S, Aboubakar F, et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respiratory medicine. 2021. Jul;183:106438. doi: 10.1016/j.rmed.2021.106438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal-Utrera C, Anarte-Lazo E, De-La-Barrera-Aranda E, Fernandez-Bueno L, Saavedra-Hernandez M, Gonzalez-Gerez JJ, et al. Perspectives and Attitudes of Patients with COVID-19 toward a Telerehabilitation Programme: A Qualitative Study. Int J Environ Res Public Health. 2021. Jul 24;18(15). doi: 10.3390/ijerph18157845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseiniravandi M, Kahlaee AH, Karim H, Ghamkhar L, Safdari R. Home-based telerehabilitation software systems for remote supervising: a systematic review. International journal of technology assessment in health care. 2020. Apr;36(2):113–25. doi: 10.1017/S0266462320000021 [DOI] [PubMed] [Google Scholar]
- 33.Lobo A, Saz P, Marcos G, Día JL, de la Cámara C, Ventura T, et al. [Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental Status Examination) in the general geriatric population]. Med Clin (Barc). 1999. Jun 5;112(20):767–74. [PubMed] [Google Scholar]
- 34.Madrid ICPdFd. Recomendaciones de Fisioterapia Respiratoria y Ejercicio Terapéutico para personas confinadas en fase de recuperación por COVID-19. 2020.
- 35.Aragón ICOdFd. Guía de intervención del Fisioterpeuta en la Atención hospitalaria del paciente infectado por el nuevo coronavirus (SARS-COOV-2). 2020.
- 36.SEPAR. Fisioterapia respiratoria en el manejo del paciente con COVID-19: recomendaciones generales: SEPAR. 2020 [cited 2021 14 december 2021]; Available from: http://svmefr.com/wp-content/uploads/2020/03/COVID19-SEPAR-26_03_20.pdf.
- 37.Blokland IJ, Ilbrink S, Houdijk H, Dijkstra JW, van Bennekom CAM, Fickert R, et al. [Exercise capacity after mechanical ventilation because of COVID-19: Cardiopulmonary exercise tests in clinical rehabilitation]. Nederlands tijdschrift voor geneeskunde. 2020. Oct 29;164. [PubMed] [Google Scholar]
- 38.Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013. Aug 15;331(1–2):102–7. doi: 10.1016/j.jns.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 39.Bernal-Vargas L, Riveros-Munévar F, Vinaccia-Alpi S, Quiceno-Sierra J-M. Estructura factorial y consistencia interna de la Escala de severidad de fatiga en población colombiana con enfermedades crónicas %J Enfermería Global. 2017;16:37–49. [Google Scholar]
- 40.Ortelli P, Ferrazzoli D, Sebastianelli L, Engl M, Romanello R, Nardone R, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci. 2021. Jan 15;420:117271. doi: 10.1016/j.jns.2020.117271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis care & research. 2011. Nov;63 Suppl 11:S263–86. doi: 10.1002/acr.20579 [DOI] [PubMed] [Google Scholar]
- 42.Stone P, Richards M, A’Hern R, Hardy J. Fatigue in patients with cancers of the breast or prostate undergoing radical radiotherapy. Journal of pain and symptom management. 2001. Dec;22(6):1007–15. doi: 10.1016/s0885-3924(01)00361-x [DOI] [PubMed] [Google Scholar]
- 43.Schneeberger EE, Marengo MF, Dal Pra F, Maldonado Cocco JA, Citera G. Fatigue assessment and its impact in the quality of life of patients with ankylosing spondylitis. Clin Rheumatol. 2015. Mar;34(3):497–501. doi: 10.1007/s10067-014-2682-3 [DOI] [PubMed] [Google Scholar]
- 44.Lerdal A, Wahl A, Rustøen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scandinavian journal of public health. 2005;33(2):123–30. doi: 10.1080/14034940410028406 [DOI] [PubMed] [Google Scholar]
- 45.Andreasen AK, Stenager E, Dalgas U. The effect of exercise therapy on fatigue in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2011. Sep;17(9):1041–54. doi: 10.1177/1352458511401120 [DOI] [PubMed] [Google Scholar]
- 46.Vasconcello-Castillo L, Torres-Castro R, Solís-Navarro L, Rivera G, Puppo H. Evaluación Funcional y Respiratoria en Pacientes post COVID-19: ¿Cuáles son las mejores pruebas? 2020. 12/02:109–15. [Google Scholar]
- 47.Uszko-Lencer N, Mesquita R, Janssen E, Werter C, Brunner-La Rocca HP, Pitta F, et al. Reliability, construct validity and determinants of 6-minute walk test performance in patients with chronic heart failure. International journal of cardiology. 2017. Aug 1;240:285–90. doi: 10.1016/j.ijcard.2017.02.109 [DOI] [PubMed] [Google Scholar]
- 48.Klein SR, Gulart AA, Venâncio RS, Munari AB, Gavenda SG, Martins ACB, et al. Performance difference on the six-minute walk test on tracks of 20 and 30 meters for patients with chronic obstructive pulmonary disease: validity and reliability. Brazilian journal of physical therapy. 2021. Jan-Feb;25(1):40–7. doi: 10.1016/j.bjpt.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arcuri JF, Borghi-Silva A, Labadessa IG, Sentanin AC, Candolo C, Pires Di Lorenzo VA. Validity and Reliability of the 6-Minute Step Test in Healthy Individuals: A Cross-sectional Study. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2016. Jan;26(1):69–75. doi: 10.1097/JSM.0000000000000190 [DOI] [PubMed] [Google Scholar]
- 50.Holland AE, Malaguti C, Hoffman M, Lahham A, Burge AT, Dowman L, et al. Home-based or remote exercise testing in chronic respiratory disease, during the COVID-19 pandemic and beyond: A rapid review. Chronic respiratory disease. 2020. Jan-Dec;17:1479973120952418. doi: 10.1177/1479973120952418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. The European respiratory journal. 1999. Aug;14(2):270–4. doi: 10.1034/j.1399-3003.1999.14b06.x [DOI] [PubMed] [Google Scholar]
- 52.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002. Jul 1;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 53.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. The Gerontologist. 2013. Apr;53(2):255–67. doi: 10.1093/geront/gns071 [DOI] [PubMed] [Google Scholar]
- 54.Warden SJ, Liu Z, Moe SM. Sex- and Age-Specific Centile Curves and Downloadable Calculator for Clinical Muscle Strength Tests to Identify Probable Sarcopenia. Phys Ther. 2022. Mar 1;102(3). doi: 10.1093/ptj/pzab299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cobo-Mejía A, Ochoa González ME, Ruiz Castillo LI, Vargas Niño DM, Sáenz Pacheco AM, Sandoval-Cuellar C. Confiabilidad del "Senior Fitness Test" versión en español, para población adulta mayor en Tunja-Colombia. Archivos de medicina del deporte: revista de la Federación Española de Medicina del Deporte y de la Confederación Iberoamericana de Medicina del Deporte. 2016;33(176):382–6. [Google Scholar]
- 56.Oliveira JM, Spositon T, Cerci Neto A, Soares FMC, Pitta F, Furlanetto KC. Functional tests for adults with asthma: validity, reliability, minimal detectable change, and feasibility. The Journal of asthma: official journal of the Association for the Care of Asthma. 2022. Jan;59(1):169–77. doi: 10.1080/02770903.2020.1838540 [DOI] [PubMed] [Google Scholar]
- 57.McAllister LS, Palombaro KM. Modified 30-Second Sit-to-Stand Test: Reliability and Validity in Older Adults Unable to Complete Traditional Sit-to-Stand Testing. Journal of geriatric physical therapy (2001). 2020 Jul/Sep;43(3):153–8. doi: 10.1519/jpt.0000000000000227 [DOI] [PubMed] [Google Scholar]
- 58.Ozcan Kahraman B, Ozsoy I, Akdeniz B, Ozpelit E, Sevinc C, Acar S, et al. Test-retest reliability and validity of the timed up and go test and 30-second sit to stand test in patients with pulmonary hypertension. International journal of cardiology. 2020. Apr 1;304:159–63. doi: 10.1016/j.ijcard.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 59.Langhammer B, Stanghelle JK. The Senior Fitness Test. Journal of physiotherapy. 2015. Jul;61(3):163. doi: 10.1016/j.jphys.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 60.Boshnjaku A, Bahtiri A, Feka K, Krasniqi E, Tschan H, Wessner B. Test-retest reliability data of functional performance, strength, peak torque and body composition assessments in two different age groups of Kosovan adults. Data in brief. 2021. Jun;36:106988. doi: 10.1016/j.dib.2021.106988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carbonell-Baeza A, Álvarez-Gallardo IC, Segura-Jiménez V, Castro-Piñero J, Ruiz JR, Delgado-Fernández M, et al. Reliability and feasibility of physical fitness tests in female fibromyalgia patients. International journal of sports medicine. 2015. Feb;36(2):157–62. doi: 10.1055/s-0034-1390497 [DOI] [PubMed] [Google Scholar]
- 62.Çalik Kütükcü E, Çakmak A, Kinaci E, Uyaroğlu OA, Vardar Yağli N, Sain Güven G, et al. Reliability and validity of the Turkish version of Post-COVID-19 Functional Status Scale. Turkish journal of medical sciences. 2021. Oct 21;51(5):2304–10. doi: 10.3906/sag-2105-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran VT, Riveros C, Clepier B, Desvarieux M, Collet C, Yordanov Y, et al. Development and Validation of the Long Coronavirus Disease (COVID) Symptom and Impact Tools: A Set of Patient-Reported Instruments Constructed From Patients’ Lived Experience. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2022. Jan 29;74(2):278–87. doi: 10.1093/cid/ciab352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorca LA, Leão Ribeiro I, Torres-Castro R, Sacomori C, Rivera C. [Psychometric properties of the Post-COVID 19 Functional Status scale for adult COVID 19 survivors]. Rehabilitacion. 2021. Jul 31. doi: 10.1016/j.rh.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bosscher RJ, Smit JH. Confirmatory factor analysis of the General Self-Efficacy Scale. Behaviour research and therapy. 1998. Mar;36(3):339–43. doi: 10.1016/s0005-7967(98)00025-4 [DOI] [PubMed] [Google Scholar]
- 66.Clavijo M, Yévenes F, Gallardo I, Contreras AM, Santos C. [The general self-efficacy scale (GSES): Reevaluation of its reliability and validity evidence in Chile]. Revista medica de Chile. 2020. Oct;148(10):1452–60. doi: 10.4067/S0034-98872020001001452 [DOI] [PubMed] [Google Scholar]
- 67.Sherer M, Maddux JE, Mercandante B, Prentice-Dunn S, Jacobs B, Rogers RW. The Self-Efficacy Scale: Construction and Validation. 1982;51(2):663–71. doi: 10.2466/pr0.1982.51.2.663 [DOI] [Google Scholar]
- 68.López-Torrecillas F, García J, Cañadas GA, Uclés IR, De La Fuente EI. Validity of self-efficacy scale scores for a Spanish sample. Psychological reports. 2006. Apr;98(2):437–50. doi: 10.2466/pr0.98.2.437-450 [DOI] [PubMed] [Google Scholar]
- 69.Herrero R, Espinoza M, Molinari G, Etchemendy E, Garcia-Palacios A, Botella C, et al. Psychometric properties of the General Self Efficacy-12 Scale in Spanish: general and clinical population samples. Comprehensive psychiatry. 2014. Oct;55(7):1738–43. doi: 10.1016/j.comppsych.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 70.Van der Maas LC, Köke A, Pont M, Bosscher RJ, Twisk JW, Janssen TW, et al. Improving the Multidisciplinary Treatment of Chronic Pain by Stimulating Body Awareness: A Cluster-randomized Trial. The Clinical journal of pain. 2015. Jul;31(7):660–9. doi: 10.1097/AJP.0000000000000138 [DOI] [PubMed] [Google Scholar]
- 71.Daza P, Novy DM, Stanley MA, Averill P. The Depression Anxiety Stress Scale-21: Spanish Translation and Validation with a Hispanic Sample. Journal of Psychopathology and Behavioral Assessment. 2002. 2002/09/01;24(3):195–205. doi: 10.1023/A:1016014818163 [DOI] [Google Scholar]
- 72.Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological Assessment. 1998;10(2):176–81. doi: 10.1037/1040-3590.10.2.176 [DOI] [Google Scholar]
- 73.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour research and therapy. 1995. Mar;33(3):335–43. doi: 10.1016/0005-7967(94)00075-u [DOI] [PubMed] [Google Scholar]
- 74.Osman A, Wong JL, Bagge CL, Freedenthal S, Gutierrez PM, Lozano G. The Depression Anxiety Stress Scales-21 (DASS-21): further examination of dimensions, scale reliability, and correlates. Journal of clinical psychology. 2012. Dec;68(12):1322–38. doi: 10.1002/jclp.21908 [DOI] [PubMed] [Google Scholar]
- 75.Zanon C, Brenner RE, Baptista MN, Vogel DL, Rubin M, Al-Darmaki FR, et al. Examining the Dimensionality, Reliability, and Invariance of the Depression, Anxiety, and Stress Scale-21 (DASS-21) Across Eight Countries. Assessment. 2021. Sep;28(6):1531–44. doi: 10.1177/1073191119887449 [DOI] [PubMed] [Google Scholar]
- 76.Moreno-Murcia JA, Marzo JC, Martínez-Galindo C, Marín LC. Validación de la Escala de "Satisfacción de las Necesidades Psicológicas Básicas" y del Cuestionario de la "Regulación Conductual en el Deporte" al contexto español. [Validation of Psychological Need Satisfaction in Exercise Scale and the Behavioural Regulation in Sport Questionnaire to the Spanish context.]. RICYDE Revista internacional de Ciencias del Deporte / The International Journal of Sport Science. 2012;7(26):355–69. doi: 10.5232/ricyde2011.02602 [DOI] [Google Scholar]
- 77.Viladrich C, Torregrosa M, Cruz J. [Psychometric quality supporting the Spanish adaptation of the Behavioral Regulation in Sport Questionnaire]. Psicothema. 2011. Nov;23(4):786–94. [PubMed] [Google Scholar]
- 78.da Cruz MMA, Ricci-Vitor AL, Borges GLB, da Silva PF, Turri-Silva N, Takahashi C, et al. A Randomized, Controlled, Crossover Trial of Virtual Reality in Maintenance Cardiovascular Rehabilitation in a Low-Resource Setting: Impact on Adherence, Motivation, and Engagement. Phys Ther. 2021. May 4;101(5). doi: 10.1093/ptj/pzab071 [DOI] [PubMed] [Google Scholar]
- 79.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. Journal of personality assessment. 1990. Winter;55(3–4):610–7. doi: 10.1080/00223891.1990.9674095 [DOI] [PubMed] [Google Scholar]
- 80.Dambi JM, Corten L, Chiwaridzo M, Jack H, Mlambo T, Jelsma J. A systematic review of the psychometric properties of the cross-cultural translations and adaptations of the Multidimensional Perceived Social Support Scale (MSPSS). Health Qual Life Outcomes. 2018. May 2;16(1):80. doi: 10.1186/s12955-018-0912-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grey I, Arora T, Thomas J, Saneh A, Tohme P, Abi-Habib R. The role of perceived social support on depression and sleep during the COVID-19 pandemic. Psychiatry research. 2020. Nov;293:113452. doi: 10.1016/j.psychres.2020.113452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landeta O, Calvete E. Adaptación y Validación de la Escala Multidimensional de Apoyo Social Percibido. Ansiedad y Estrés. 2002. 01/01;8:173–82. [Google Scholar]
- 83.Vilagut G, Valderas JM, Ferrer M, Garin O, López-García E, Alonso J. [Interpretation of SF-36 and SF-12 questionnaires in Spain: physical and mental components]. Medicina clinica. 2008. May 24;130(19):726–35. doi: 10.1157/13121076 [DOI] [PubMed] [Google Scholar]
- 84.Vilagut G, Ferrer M, Rajmil L, Rebollo P, Permanyer-Miralda G, Quintana JM, et al. [The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments]. Gaceta sanitaria. 2005. Mar-Apr;19(2):135–50. doi: 10.1157/13074369 [DOI] [PubMed] [Google Scholar]
- 85.Guerra-Tapia A, Buendía-Eisman A, Ferrando Barbera J. Final Phase in the Validation of the Cross-Cultural Adaptation of the Hair-Specific Skindex-29 Questionnaire Into Spanish: Sensitivity to Change and Correlation With the 12-Item Short-Form Health Survey. Actas dermo-sifiliograficas. 2019. Dec;110(10):819–29. doi: 10.1016/j.ad.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 86.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996. Mar;34(3):220–33. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 87.Ruotolo I, Berardi A, Sellitto G, Panuccio F, Polimeni A, Valente D, et al. Criterion Validity and Reliability of SF-12 Health Survey Version 2 (SF-12v2) in a Student Population during COVID-19 Pandemic: A Cross-Sectional Study. Depression research and treatment. 2021;2021:6624378. doi: 10.1155/2021/6624378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Resnick B. The definition, purpose and value of pilot research. Geriatr Nurs. 2015. Mar-Apr;36(2 Suppl):S1–2. doi: 10.1016/j.gerinurse.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 89.Morris NS, Rosenbloom DA. CE: Defining and Understanding Pilot and Other Feasibility Studies. The American journal of nursing. 2017. Mar;117(3):38–45. doi: 10.1097/01.NAJ.0000513261.75366.37 [DOI] [PubMed] [Google Scholar]
- 90.Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, et al. Effects of a 9-Week Hybrid Comprehensive Telerehabilitation Program on Long-term Outcomes in Patients With Heart Failure: The Telerehabilitation in Heart Failure Patients (TELEREH-HF) Randomized Clinical Trial. JAMA cardiology. 2020. Mar 1;5(3):300–8. doi: 10.1001/jamacardio.2019.5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of Statin Adherence With Mortality in Patients With Atherosclerotic Cardiovascular Disease. JAMA cardiology. 2019. Mar 1;4(3):206–13. doi: 10.1001/jamacardio.2018.4936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polinski JM, Barker T, Gagliano N, Sussman A, Brennan TA, Shrank WH. Patients’ Satisfaction with and Preference for Telehealth Visits. Journal of general internal medicine. 2016. Mar;31(3):269–75. doi: 10.1007/s11606-015-3489-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li HL, Chan YC, Huang JX, Cheng SW. Pilot Study Using Telemedicine Video Consultation for Vascular Patients’ Care During the COVID-19 Period. Annals of vascular surgery. 2020. Oct;68:76–82. doi: 10.1016/j.avsg.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mouli TC, Davuluri A, Vijaya S, Priyanka ADY, Mishra SK. Effectiveness of simulation based teaching of ventilatory management among non-anaesthesiology residents to manage COVID 19 pandemic—A Quasi experimental cross sectional pilot study. Indian journal of anaesthesia. 2020. May;64(Suppl 2):S136–s40. doi: 10.4103/ija.IJA_452_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Statistical Methods in Medical Research. 2015. 2016/06/01;25(3):1057–73. doi: 10.1177/0962280215588241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kieser M, Wassmer GJBJ. On the Use of the Upper Confidence Limit for the Variance from a Pilot Sample for Sample Size Determination. 1996;38:941–9. [Google Scholar]
- 97.Vieira A, Pinto A, Garcia B, Eid RAC, Mól CG, Nawa RK. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: a systematic review. Journal of physiotherapy. 2022. Apr;68(2):90–8. doi: 10.1016/j.jphys.2022.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalbosco-Salas M, Torres-Castro R, Rojas Leyton A, Morales Zapata F, Henríquez Salazar E, Espinoza Bastías G, et al. Effectiveness of a Primary Care Telerehabilitation Program for Post-COVID-19 Patients: A Feasibility Study. Journal of clinical medicine. 2021. Sep 27;10(19). doi: 10.3390/jcm10194428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turolla A, Rossettini G, Viceconti A, Palese A, Geri T. Musculoskeletal Physical Therapy During the COVID-19 Pandemic: Is Telerehabilitation the Answer? Phys Ther. 2020. Aug 12;100(8):1260–4. doi: 10.1093/ptj/pzaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis A, Knight E, Bland M, Middleton J, Mitchell E, McCrum K, et al. Feasibility of an online platform delivery of pulmonary rehabilitation for individuals with chronic respiratory disease. BMJ open respiratory research. 2021. Mar;8(1). doi: 10.1136/bmjresp-2021-000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Batalik L, Konecny V, Dosbaba F, Vlazna D, Brat K. Cardiac Rehabilitation Based on the Walking Test and Telerehabilitation Improved Cardiorespiratory Fitness in People Diagnosed with Coronary Heart Disease during the COVID-19 Pandemic. Int J Environ Res Public Health. 2021. Feb 24;18(5). doi: 10.3390/ijerph18052241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suso-Martí L, La Touche R, Herranz-Gómez A, Angulo-Díaz-Parreño S, Paris-Alemany A, Cuenca-Martínez F. Effectiveness of Telerehabilitation in Physical Therapist Practice: An Umbrella and Mapping Review With Meta-Meta-Analysis. Phys Ther. 2021. May 4;101(5). doi: 10.1093/ptj/pzab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dias JF, Oliveira VC, Borges PRT, Dutra F, Mancini MC, Kirkwood RN, et al. Effectiveness of exercises by telerehabilitation on pain, physical function and quality of life in people with physical disabilities: a systematic review of randomised controlled trials with GRADE recommendations. Br J Sports Med. 2021. Feb;55(3):155–62. doi: 10.1136/bjsports-2019-101375 [DOI] [PubMed] [Google Scholar]
- 104.Hansen H, Bieler T, Beyer N, Kallemose T, Wilcke JT, Østergaard LM, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax. 2020. May;75(5):413–21. doi: 10.1136/thoraxjnl-2019-214246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsutsui M, Gerayeli F, Sin DD. Pulmonary Rehabilitation in a Post-COVID-19 World: Telerehabilitation as a New Standard in Patients with COPD. International journal of chronic obstructive pulmonary disease. 2021;16:379–91. doi: 10.2147/COPD.S263031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2013. Oct;19(10):800–5. doi: 10.1089/tmj.2012.0288 [DOI] [PubMed] [Google Scholar]
- 107.Vink M, Vink-Niese A. Could Cognitive Behavioural Therapy Be an Effective Treatment for Long COVID and Post COVID-19 Fatigue Syndrome? Lessons from the Qure Study for Q-Fever Fatigue Syndrome. Healthcare (Basel, Switzerland). 2020. Dec 11;8(4). doi: 10.3390/healthcare8040552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milani G, Demattè G, Ferioli M, Dallagà G, Lavezzi S, Basaglia N, et al. Telerehabilitation in Italy During the COVID-19 Lockdown: A Feasibility and Acceptability Study. International journal of telerehabilitation. 2021;13(1):e6334. doi: 10.5195/ijt.2021.6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
Deidentified research data will be made publicly available when the study is completed and published.