Abstract
The Kromdraai site in South Africa has yielded numerous early hominin fossils since 1938. As a part of recent excavations within Unit P, a largely complete early hominin calcaneus (KW 6302) was discovered. Due to its role in locomotion, the calcaneus has the potential to reveal important form/function relationships. Here, we describe KW 6302 and analyze its preserved morphology relative to human and nonhuman ape calcanei, as well as calcanei attributed to Australopithecus afarensis, Australopithecus africanus, Australopithecus sediba, Homo naledi, and the Omo calcaneus (either Paranthropus or early Homo). KW 6302 calcaneal morphology is assessed using numerous quantitative metrics including linear measures, calcaneal robusticity index, relative lateral plantar process position, Achilles tendon length reconstruction, and a three‐dimensional geometric morphometric sliding semilandmark analysis. KW 6302 exhibits an overall calcaneal morphology that is intermediate between humans and nonhuman apes, although closer to modern humans. KW 6302 possesses many traits that indicate it was likely well‐adapted for terrestrial bipedal locomotion, including a relatively flat posterior talar facet and a large lateral plantar process that is similarly positioned to modern humans. It also retains traits that indicate that climbing may have remained a part of its locomotor repertoire, such as a relatively gracile tuber and a large peroneal trochlea. Specimens from Kromdraai have been attributed to either Paranthropus robustus or early Homo; however, there are no definitively attributed calcanei for either genus, making it difficult to taxonomically assign this specimen. KW 6302 and the Omo calcaneus, however, fall outside the range of expected variation for an extant genus, indicating that if the Omo calcaneus was Paranthropus, then KW 6302 would likely be attributed to early Homo (or vice versa).
Keywords: bipedalism, foot, Homo, Paranthropus, tarsals
A new early hominin calcaneus (KW 6302) that exhibits a mix of modern human and nonhuman ape morphologies was found within Unit P at the Kromdraai site (South Africa). Its suite of morphological characteristics indicates that it was well‐adapted for terrestrial bipedalism, but that climbing remained a part of its locomotor repertoire. Previous Kromdraai specimens have been attributed to either Paranthropus robustus or early Homo and while no early hominin calcanei have been definitively attributed to either genus, the Omo calcaneus (either Paranthropus or early Homo) falls outside of the range of expected variation for an extant genus with KW 6302, indicating that if one specimen is Paranthropus than the other may be early Homo.

1. INTRODUCTION
1.1. Kromdraai site
The Kromdraai fossiliferous site (26°00′41”S, 27°44′60″E) is one of the palaeokarsts truncated by surficial erosion, situated along the southern flank of the Blaauwbank River (Gauteng Province, South Africa). The Plio‐Pleistocene site has borne many early hominin fossils and is listed among the UNESCO World Heritage Sites often referred to as the “Cradle of Humankind” (e.g., Braga et al., 2013, 2017, 2021; Braga & Thackeray, 2003; Broom, 1938a; Broom, 1938b; Thackeray et al., 2001; Vrba, 1981). Some fossiliferous deposits in the Kromdraai area were discovered in 1895 by David Draper, who collected fossiliferous breccias and sent them to the British Museum of Natural History (de Graaff, 1961; Malan, 1959). The Kromdraai site became well known in 1938, when it yielded a partial skull and dentition (TM 1517) designated as the type specimen of a new genus and species, Paranthropus robustus (Broom, 1938a), and subsequently associated with a few postcranial elements to represent the partial skeleton of a single individual (Braga et al., 2017; Broom, 1938b, 1942, 1943; Cazenave et al., 2020). With the exception of the ongoing excavations (since 2014), the work that Broom initiated in 1938 at the “Kromdraai B" (KB) locality (or “hominid site”) was the first in a series of four phases of field research activities (not detailed here; see Braga et al., 2017; Braga & Thackeray, 2016).
The latest phase of systematic excavation at Kromdraai was initiated in 2014 by the Kromdraai Research Project (KRP) (Braga & Thackeray, 2016; Braga et al., 2017), and discovered that the fossiliferous deposits extended for a considerable distance to the north, past what was previously recognized by Brain (1958, 1975) or Vrba (1981). The northern “Extension Site” revealed about 600 m2 of new breccia deposits, resulting in a revised stratigraphic framework for Kromdraai (Bruxelles et al., 2016). The term “Unit” was subsequently used to replace the term “Member” to designate stratigraphic units at Kromdraai (Ngoloyi et al., 2020). This change follows the conventional lithostratigraphic terminology used by the International Commission on Stratigraphy (stratigraphy.org), in which a “Member” does not designate “the smallest formal unit in the hierarchy of sedimentary lithostratigraphic units.” The stratigraphic sequence at Kromdraai starts with Unit A, which contains no fossils and corresponds to the “Stony breccia” as described in Brain (1958), or “Member 1” as described in Vrba (1981), Partridge (1982), and Bruxelles et al. (2016). Unit P represents one of the two newly recognized hominin‐bearing sedimentary deposits that have been identified during fieldwork at Kromdraai since 2014. Unit P was previously identified as “Member 2” (Partridge, 1982) when it was considered as “sterile” (Vrba, 1981). It was described in detail and still named “Member 2” in the most recent description of the stratigraphy of Kromdraai (Bruxelles et al., 2016), as well as when the discovery of new fossil hominin specimens was subsequently announced (Braga et al., 2017). Unit P is stratigraphically older than the immediately overlying sedimentary deposits of “Member 3” (Bruxelles et al., 2016) (now divided into Units Q‐R).
Paleomagnetic data suggest that Unit P is older than 2 million years (Ma). Three samples from one flowstone revealed an interval of reversed polarity interpreted as older than the normal Olduvai Event (at 1.95–1.78 Ma; Thackeray et al., 2002). Units Q‐R underlie this flowstone (Bruxelles et al., 2016), such that an unknown interval of time separates the flowstone from the underlying older Unit P. Based on Thackeray et al.’s (2002) paleomagnetic data, this evidence suggests that Units Q‐R are older than the Olduvai SubChron at 1.95 Ma, and that the stratigraphically older hominin‐bearing assemblage from Unit P would correspond to an early Pleistocene period dated in excess of 2 Ma. Consistent with this evidence, biochronological data (e.g., the occurrence of the small mustelid Prepoecilogale bolti) also suggest that Unit P predates 2 Ma (Braga et al., 2017).
The hominin assemblage from Kromdraai that was available prior to the commencement of excavations in 2014 by the KRP consists of 17 individually numbered craniodental specimens, and six postcranial bones, five of which may derive from a single individual, TM 1517 (Braga et al., 2013, 2017). Isolated teeth comprise the bulk of this assemblage which, with the sole exception of the KB 5223 mandibular dentition, have been attributed to P. robustus. While Grine (1982) argued that KB 5223 represents a juvenile of P. robustus, Braga and Thackeray (2003) suggested that it could be accommodated in Homo. Only a few of these hominin specimens were recovered in situ (Braga et al., 2016, 2017). Before 2014, no diagnostic postcranial remains recovered in situ were available in the hominin assemblage from Kromdraai. For instance, the type specimen of P. robustus (TM 1517) is unprovenanced, even though it could be tied to “significantly younger layers” than “Member 3” (Braga et al., 2013). Among the fossil hominin specimens published between 1938 and 2013, only two small samples can be precisely tied to the revised stratigraphy from the site (Bruxelles et al., 2016). These were discovered in two distinct deposits that are separated from one another by another two deposits. The first sample comprises three specimens, KB 5223 and KB 5226 (Vrba, 1981) and KB 6067 (Braga et al., 2013), which were recovered from the calcified deposits identified as “Member 3”, or Unit Q‐R (Partridge, 1982; Vrba, 1981). The second sample comprises two specimens, KB 5522 (Thackeray et al., 2005) and KB 5524 (Braga et al., 2013), that were recovered in situ in 2002 from younger deposits identified as “Members 5 and 6,” respectively, by Bruxelles et al. (2016). These are referred to here as Units X and Y and may represent the deposits from which the holotype of P. robustus (TM 1517) (Broom, 1938a) was recovered. Thus, the previously recovered KB fossils cannot be regarded as representing a temporally homogeneous sample.
The renewed excavations at Kromdraai since 2014 resulted in the in situ recovery of faunal (Fourvel et al., 2016, 2018; Pavia, 2020) and fossil hominin assemblages (Braga et al., 2017, 2021) from Unit P. The fossil remains from Unit P are fragmented but with a very good degree of preservation. Although cranial and postcranial fossil hominin specimens have been recovered, there is no definitive association between them. The spatial patterning of the fossil hominin and non‐hominin assemblage recovered in situ from Unit P since 2014 describes four main clusters interpreted as either areas of higher density of fossils, or as accumulations resulting from particular processes (e.g., distinct entrances) (Ngoloyi et al., 2020). As a part of this assemblage, a newly isolated fossil hominin calcaneus (KW 6302), that is stratigraphically older than TM 1517, was recovered in situ from Unit P (Figure 1).
FIGURE 1.

The KW 6302 calcaneus from superior (a), inferior (b), anterior (c), lateral (d), medial (e), and posterior (f) views
1.2. Importance of the calcaneus
Skeletal elements of the foot and ankle have played a critical role in reconstructing the locomotor repertoires of early hominins (e.g., Day & Napier, 1964; Latimer et al., 1987; Kidd, 1999; Harcourt‐Smith & Aiello, 2004; Gebo & Schwartz, 2006; Prang, 2016a, 2016b; McNutt et al., 2018; DeSilva et al., 2019; Sorrentino et al., 2020a). Within the foot, the calcaneus is a particularly relevant element to study because of its role in weight‐bearing and proximate contact with the substrate during locomotion (e.g., Boyle et al., 2018; Gebo, 1992; Harper et al., 2021a, 2021b; Holowka & Lieberman, 2018; Latimer & Lovejoy, 1989; Prang, 2015; Schmitt & Larson, 1995; Webber & Raichlen, 2016; Zeininger et al., 2020). Heel strike, for example, represents the beginning of the modern human bipedal gait cycle and is utilized by nonhuman great apes during both arboreal and terrestrial locomotor bouts (Carrier & Cunningham, 2017; Gebo, 1992; Schmitt & Larson, 1995; Vereecke et al., 2003; Zeininger et al., 2020). Heel strike is often simultaneous with lateral midfoot ground contact in chimpanzees and bonobos, while orangutans primarily walk with an inverted foot in which the heel contacts the ground prior to the rest of the foot in most terrestrial steps (Gebo, 1992; Schmitt & Larson, 1995; Vereecke et al., 2003). Recent pressure data has shown that gorillas utilize a heel‐only heel strike, similar to what is seen in modern humans (Wunderlich et al., 2019).
The subtalar and calcaneocuboid joints also play critical roles in facilitating foot inversion/eversion, which has been shown to be important in both arboreal and terrestrial contexts and facilitating midfoot mobility in chimpanzees (Close et al., 1967; Holowka et al., 2017; Inman, 1976; Lewis, 1980; Thompson et al., 2014). Chimpanzees utilize an inverted foot position during vertical climbing, which serves to increase the contact area between the plantar surface of the foot and the substrate (DeSilva, 2009; Holowka et al., 2017). Modern humans are thought to exhibit a close‐packed position of the calcaneocuboid joint during inversion, which has been suggested to aid in the transformation of the human foot (along with the windlass mechanism) into a rigid lever during toe‐off (Bojsen‐Møller, 1979). It is important to note, however, that recent experimental work by Holowka and Lieberman (2018) has suggested that both stiffness and mobility of the midfoot characterize the human foot during stance.
Calcanei from the Plio‐Pleistocene are relatively rare in the fossil record and the KW 6302 calcaneus represents the tenth adult calcaneus published from this geologic time period from Africa (Table 1; Figure 2). Analyses of external calcaneal morphology have played an essential role in reconstructing the locomotor repertoires of early hominins (e.g., Stern Jr. & Susman, 1983; Latimer & Lovejoy, 1989; Gebo, 1992; Zipfel et al., 2011; Prang, 2015, 2016a.b; DeSilva et al., 2019; Harper et al., 2021a). Many aspects of calcaneal morphology, such as tuber robusticity, posterior talar facet curvature, cuboid facet depth, and lateral plantar process position, have been related to locomotor behavior (e.g., Boyle et al., 2018; DeSilva et al., 2019; Gebo, 1992; Harper et al., 2021a, 2021b; Latimer & Lovejoy, 1989; Prang, 2015, 2016a, 2016b). A more robust calcaneal tuber and the presence of a prominent lateral plantar process has been related to bipedality in humans, while a deeper cuboid facet pivot region and a posterior talar facet with a high degree of curvature have been associated with increased midfoot mobility and inversion/eversion abilities, respectively, for arboreality in nonhuman primates (e.g., Boyle et al., 2018; DeSilva et al., 2019; Gebo, 1992; Harper et al., 2021a, 2021b; Holowka & Lieberman, 2018; Latimer & Lovejoy, 1989; Prang, 2015, 2016a, 2016b). Here, we describe and analyze the preserved external morphology of the KW 6302 calcaneus relative to modern humans, nonhuman primates, and other early hominins to discuss its potential functional and taxonomic affinities.
TABLE 1.
Plio‐Pleistocene adult hominin calcanei
| Specimen number | Species attributed | Date | Locality |
|---|---|---|---|
| A.L. 333–8 a , b , c | Australopithecus afarensis | 3.2 MA | Afar Locality 333, Hadar Formation, Ethiopia |
| A.L. 333–37 a , b , c | Australopithecus afarensis | 3.2 MA | Afar Locality 333, Hadar Formation, Ethiopia |
| A.L. 333–55 a , b , c | Australopithecus afarensis | 3.2 MA | Afar Locality 333, Hadar Formation, Ethiopia |
| Omo 33‐74‐896 d , e , f | Homo habilis/Paranthropus aethopithicus | 2.36 MA | Omo Shungura Formation, Ethiopia |
| StW 352 g | Australopithecus africanus | 2.0–2.6 MA | Sterkfontein, South Africa |
| KW 6302 | Early Homo/Paranthropus | >2 MA | Kromdraai, Gauteng Province, South Africa |
| U.W. 88–99 h | Australopithecus sediba | 1.98 MA | Malapa, South Africa |
| OH 8i.j | Homo habilis/Paranthropus boisei | 1.85 MA | Olduvai Gorge, Tanzania |
| U.W. 101–724 k , l | Homo naledi | 0.24–0.34 MA | Dinaledi Chamber, Rising Star Cave, Gauteng, South Africa |
| U.W. 101–1322 k , l | Homo naledi | 0.24–0.34 MA | Dinaledi Chamber, Rising Star Cave, Gauteng, South Africa |
FIGURE 2.

Known early hominin calcanei from the Plio‐Pleistocene from both a superior and lateral view. Surface models were flipped to represent right calcanei for ease of visual comparison. Adapted from Harper et al., 2021a
2. MATERIALS AND METHODS
2.1. Anatomical description and linear measurements
An anatomical description of the KW 6302 calcaneus is presented. Linear measures were collected on the original specimen using plastic digital calipers following DeSilva et al. (2018; Table 2) and are reported in millimeters. Linear measures are compared to those previously collected on all known Plio‐Pleistocene hominin calcaneus fossils (DeSilva et al., 2018; Latimer et al., 1982). Total calcaneal length was estimated based on the relationship between calcaneal tuber length and total calcaneal length in modern humans, chimpanzees/bonobos, and gorillas using least‐squares regression analyses (extant relationship in Figure S1; linear measures from Harper et al., 2022).
Although the anatomical description and linear measures are primarily based on the original specimen, the KW 6302 calcaneus was additionally micro‐computed tomography (micro‐CT) scanned for use in other analyses (described below). The specimen was scanned at a voxel size of 27.9 μm using an X‐Tek (Metris) XT H225L industrial CT system housed at the South African Nuclear Energy Corporation in Pelindaba, South Africa. A surface model was generated from micro‐CT data in Avizo Lite 9.0.1 (FEI Visualization Sciences Group, 2015).
2.2. Tuber robusticity
The KW 6302 calcaneal tuber robusticity was investigated using two quantitative methods (Latimer & Lovejoy, 1989; Prang, 2015). As such, tuber robusticity was analyzed relative to different comparative samples depending on which of the metrics was used (described below in more detail). Tuber robusticity calculated following Latimer and Lovejoy (1989) was analyzed relative to the sample from the original study, which included modern humans (n = 20), Pan troglodytes (n = 20), and Gorilla gorilla (n = 20), as well as A.L. 333–8 and A.L. 333–55. For the analysis investigating the calcaneal robusticity index following Prang (2015), tuber robusticity was assessed relative to modern humans (n = 34), P. troglodytes (n = 25), G. gorilla (n = 25), and P. pygmaeus (n = 11) (see Prang, 2015 for more details). In addition, the KW 6302 calcaneal robusticity index analysis included values from A.L. 333–8, A.L., 333–55, U.W. 88–99, StW 352, and Omo 33–74‐896 (Prang, 2015).
Robusticity was first assessed as minimal calcaneal tuber volume relative to estimated body mass (Latimer & Lovejoy, 1989). Minimal calcaneal tuber volume was calculated as the product of the minimal cross‐sectional area of the calcaneal tuber and calcaneal tuber length (Latimer & Lovejoy, 1989). Minimal cross‐sectional area of the tuber was obtained by taking a virtual slice through the minimum dorsoplantar region of the tuber in Geomagic Control (3D Systems Inc., 2015). Unfortunately, body mass could not be directly estimated for the specimen, as no body mass estimation equations exist for partial calcanei. Body mass was instead estimated using the KNM‐ER 1476 talus following the intra Homo equation outlined in McHenry (1992), as no associated tali were found with the KW 6302 calcaneus. The KNM‐ER 1476 talus was chosen because it is comparable in size and articulates well with KW 6302 (Figure 3). Calcaneal robusticity index was calculated as the minimal cross‐sectional area of the calcaneal tuber divided by calcaneal tuber length squared following Prang (2015).
FIGURE 3.

The KW 6302 calcaneus cast articulated with the KNM‐ER 1476 talus cast from a posterior view. The KNM‐ER 1476 talus was used for analyses that necessitated a talus because it is of a similar size and articulates well with the KW 6302 calcaneus
2.3. Achilles tendon reconstruction
The extant nonhuman primate sample for Achilles tendon length reconstruction includes wild‐collected Ateles spp. (n = 8), Cebus capucinus (n = 11), Chlorocebus aethiops (n = 4), G. g. gorilla (n = 24), Hylobates lar (n = 20), Lagorthrix lagotricha (n = 2), Macaca mulatta (n = 4), P. paniscus (n = 4), P. troglodytes (n = 20), Papio hamadryas (n = 5), and P. pygmaeus (n = 10) curated by the American Museum of Natural History (AMNH) and the Museum of Comparative Zoology (MCZ) (McNutt & DeSilva, 2020). The human sample consists of 31 adult calcanei from biological anthropology laboratories at Boston University and Dartmouth College, as well as Florisbad Quaternary Research Station, National Museum, Bloemfontein, South Africa (McNutt & DeSilva, 2020). In addition, KW 6302 reconstructed Achilles tendon length was investigated relative to the previously reconstructed lengths of A. afarensis and A. sediba (McNutt & DeSilva, 2020).
To reconstruct the length of the Achilles tendon, the area of the superior facet (a proxy for the retrocalcaneal bursa) and the total area of the superior plus middle facets were first calculated in ImageJ (McNutt & DeSilva, 2020; Schneider et al., 2012). The ratio of these two facet areas was then used to estimate the length of the Achilles tendon (specifically percent total muscle tendon unit) based on previously established relationships between these two metrics in the extant sample described above (McNutt & DeSilva, 2020). In addition, the Kromdraai calcaneus was compared to modern human and great ape calcanei using the single observation with the mean test (Sokal & Rohlf, 1998).
2.4. Lateral plantar process positioning
The relative height of the lateral plantar process (LPP) in the KW 6302 calcaneus was assessed relative to measures of LPP height collected by Boyle et al. (2018). Their comparative nonhuman primate sample was represented by G. beringei beringei (n = 7), G. g. gorilla (n = 27), P. paniscus (n = 4), P. troglodytes (n = 24), and P. pygmaeus (n = 5) housed at the MCZ, AMNH, and the National Museum of Natural History (NMNH) (Boyle et al., 2018). The human sample consisted of 154 calcanei curated by the Peabody Museum of Archaeology and Ethnology (PMAE), the University of the Witwatersrand, the Florisbad Quaternary Research Station, National Museum and the University Museum, University of Tokyo (Boyle et al., 2018). KW 6302 relative lateral plantar process height was also assessed relative to that of A.L. 333–8, A.L. 333–55, and U.W. 88–99 (Boyle et al., 2018).
Lateral plantar process position was calculated following Boyle et al. (2018). This method requires the calcaneus to be articulated with an associated talus in order to standardize calcaneal orientation (Boyle et al., 2018). As no associated talus was found with the KW 6302 calcaneus, a cast of the KNM‐ER 1476 talus was used because of the size and shape of the posterior calcaneal articular surface of KNM‐ER 1476 complemented the posterior talar articular surface of KW 6302 (described above; Figure 3). High‐quality casts of the KNM‐ER 1476 talus and the KW 6302 calcaneus were articulated and photographed from a posterior view. The height of the LPP was measured in ImageJ as the distance from the midpoint of the LPP (identified as the most laterally protruding point on the lateral aspect of the tuber) to the base of the calcaneus (Boyle et al., 2018; Schneider et al., 2012).
2.5. Three‐dimensional geometric morphometric sliding semilandmark analysis
Calcaneal external shape was analyzed relative to an extant nonhuman primate comparative sample represented by calcanei of G. b. beringei (n = 11), G. b. graueri (n = 20), G. g. gorilla (n = 51), P. paniscus (n = 23), P. t. troglodytes (n = 50), P. t. schweinfurthii (n = 29), P. t. verus (n = 10), P. abelii (n = 12), P. pygmaeus (n = 19), Hylobates spp. (n = 27), Symphalangus syndactylus (n = 6), and Papio spp. (n = 28). The extant nonhuman primate sample is curated by the AMNH, the MCZ, the NMNH, the Cleveland Museum of Natural History (CMNH), and the Royal Museum for Central Africa (RMCA). All specimens are wild‐collected, skeletally adult, and free from obvious pathology. The human comparative sample is made up of 130 calcanei curated by the NMNH and the Iziko Museums of South Africa (IMSA). The sample consists of a sedentary population (n = 60; Terry collection; Hunt & Albanese, 2005), two Native American agriculturalist populations (Puye [n = 47] and Illinois [n = 10]; Perino, 1968; Chirchir et al., 2017), and a Late Stone Age hunter‐gatherer population (n = 13; Pfeiffer, 2003). Human populations were selected to sample a wide variety of activity levels and subsistence strategies because both aspects of behavior affect lower limb cortical bone, as well as the internal and external structure of tarsal elements (DeMars et al., 2021; Saers et al., 2019; Sorrentino et al., 2020b; Sorrentino et al., 2021; Stock & Pfeiffer, 2001). The preserved morphology of the KW 6302 calcaneus was also analyzed relative to overlapping morphology of the U.W. 88–99, A.L. 333–8, A.L. 333–55, StW 352, U.W. 101–1322, and Omo 33–74‐896 calcanei.
For this analysis, the calcanei were either surface scanned with a NextEngine laser scanner (0.1 mm resolution; NextEngine Inc.) or were micro‐CT scanned (see Harper et al., 2021a for voxel size information). As calcanei were originally scanned for a different study (Harper et al., 2021a), right calcanei were preferentially selected among the extant specimens. As the majority of the sample was represented by right calcanei, the KW 6302 calcaneus was mirrored for the purpose of this analysis; however, no shape information was lost in this process. Surface models from micro‐CT scans were produced in Avizo Lite 9.0.1 (FEI Visualization Sciences Group, 2015), and scans from all modalities were cleaned (e.g., small holes filled) in Geomagic Wrap (3D Systems Inc., 2015).
The external shape of the KW 6302 calcaneus was quantified through a series of three‐dimensional geometric morphometric (3D GM) sliding semilandmark analyses. The complete KW 6302 calcaneus was represented by 621 semilandmarks (Figure 4). Twenty‐five curve semilandmarks were used to represent the border of the posterior talar facet. Previous analyses of complete calcaneal shape carried out on the extant sample utilized 1007 sliding semilandmarks to represent an entire calcaneus (Harper et al., 2021a, 2021b). To determine the number of semilandmarks necessary to quantify the shape of the KW 6302 calcaneus, a comparative specimen was scaled and aligned to the fossil specimen following Harper et al. (2021a). The comparative specimen was then trimmed to match the damage of the incomplete fossil and any semilandmarks associated with the trimmed areas were removed. The curve semilandmarks representing the border of the posterior talar facet were placed manually on the KW 6302 surface model and the remaining surface semilandmarks were warped to the fossil specimen using the thin plate spline (TPS) interpolation function (Yang, 2012). The TPS interpolation function was established using the curve semilandmarks and 17 unanalyzed orientation landmarks. Landmark sets for the extant sample were reduced (from Harper et al., 2021a) to match the KW 6302 calcaneus landmark set. To analyze the shape of KW 6302 calcaneus relative to other fossil specimens, additional analyses were run using 492 semilandmarks (A.L. 333–8, A.L. 333–55, Omo‐33‐74‐896) and 415 semilandmarks (StW 352, U.W. 101–1322), respectively, based on overlapping preserved morphology.
FIGURE 4.

The fully landmarked KW 6302 calcaneus, represented by 621 semilandmarks
To create final landmark configurations, curve, and surface semilandmarks were allowed to slide along tangent vectors and planes, respectively, to minimize the bending energy of the TPS interpolation function relative to a reference specimen (Gunz et al., 2005). As semilandmarks may slide off of the bone during this process, semilandmarks were then projected back onto the bone surface (Gunz et al., 2005). A Generalized Procrustes Analysis (GPA) was used to remove the effects of size, location, and orientation (Gower, 1975; Rohlf & Slice, 1990; Zelditch et al., 2012). The average specimen post‐GPA was calculated and used as the reference specimen for the next round of sliding. This process of sliding, projecting, and GPA was carried out until stable landmark configurations were achieved.
Principal component analysis (PCA) was used to summarize shape variation captured by Procrustes coordinates. The shape of the posterior talar facet was also individually summarized with a separate PCA because it is the only preserved articular facet and has been previously shown to be related to locomotion behavior (e.g., DeSilva et al., 2019; Harper et al., 2021a; Prang, 2016a, 2016b). To determine which extant taxon/human population the KW 6302 calcaneus was most similar to in shape, Procrustes distances between the KW 6302 fossil and the average specimen of each taxon for the nonhuman primates and each population for the human sample were calculated. A previous GM analysis of these specimens found Blomberg's K statistic to be <1, indicating variation among the extant taxa is less than would be expected under a Brownian Motion model of evolution, allowing for functional interpretations to be made (Blomberg et al., 2003; Harper et al., 2021a).
To determine if the amount of variation between the KW 6302 calcaneus and each other early hominin calcaneus are within the range of variation for an extant genus/(sub)species (i.e., the two fossils can be accommodated by a single (sub)species or genus), Mahalanobis distances were calculated using the first six principal components (PC) scores. The first six PCs were chosen based on ANOVAs run testing for significant differences among humans, chimpanzees/bonobos, and gorillas on each PC (p‐value PC 7 = 0.5601) and the inflection point of the scree plot (Figure S2). The lack of a significant p‐value on PC 7 indicates that PCs seven and above represent shared morphological variation among the sample. To determine the covariance matrix for each genus/(sub)species, pairwise Mahalanobis distances between each specimen of the genus or (sub)species of interest were calculated. These covariance matrices were then used to calculate the Mahalanobis distance between the KW 6302 calcaneus and the fossil of interest. The Mahalanobis distance between the KW 6302 and the fossil calcaneus being tested was then compared to the distribution of pairwise Mahalanobis distances for a (sub)species/genus to estimate a p‐value to determine if the two fossils were outside the range of variation for the taxon of interest. Humans, chimpanzees/bonobos, and gorillas were included in this analysis on the genus level and P. t. troglodytes, P. t. schweinfurthii, and G. g. gorilla were included on the (sub)species level, based on sample sizes.
3. RESULTS
3.1. Anatomical description and linear measurements
KW 6302 is a left calcaneus that preserves 46.8 mm from the most proximal aspect of the calcaneal tuberosity to a break just beyond the distal margin of the posterior talar facet (Figure 1). Linear measurements of the KW 6302 and other Plio‐Pleistocene hominin calcanei are presented in Table 2. The overall (gross) size of the specimen is marginally smaller than the U.W. 88–99 calcaneus from Malapa. Based on the relationships established among extant taxa (Table S1; Figure S1), the total length of the KW 6302 calcaneus is estimated to be between 57.8 and 59.6 mm.
TABLE 2.
Comparative calcaneal linear measurements
| KW 6302 | U.W. 88–99 | A.L. 333–8 | A.L. 333–37 | A.L. 333–55 | StW 352 | Omo 33–74‐896 | OH 8 | U.W. 101–1322 | |
|---|---|---|---|---|---|---|---|---|---|
| Preserved length of fragment | 46.8 | 56.9 | 61.9 | 41.2 | 52 | 50.1 | 69.4 | 32.5 | 57 |
| Posterior calcaneal length (Martin, 1928) | 42.3 | 43.7 | 46.8 | — | 46.4 | — | 51.9 | — | 42.7 |
| Minimum Dorsoplantar Height of Distal Body (at base of posterior talar facet) | 15.6 | 19.4 | 17.3 | — | 17.6 | 16 | 19.5 | 13.8 | 15.6 |
| Maximum dorsoplantar height of calcaneal tuberosity | 31 | 33 | 38.1 | 33.5 a | 38.8 | — | — | — | — |
| Maximum mediolateral breadth of calcaneal tuberosity | 20.1 | 21.8 | 22.7 a | 24.6 | 23.6 | — | — | — | — |
| Maximum mediolateral breadth of the posterior talar facet | 16.7 a | 17.3 | 24.4 a | — | 24.5 a | 14.5 | 19.4 | — | 14.4 |
| Maximum proximodistal length of the posterior talar facet | 22.5 | 19.8 | 29.8 a | — | — | 22 | 28.4 | — | 20.8 |
| Subtended angle of the posterior talar facet | 94° | 118° | 82° | — | — | 120° | 87° | — | 89° |
| Volume of the posterior calcaneus (cm3) | 11.4 | 13.4 | 28.7 | — | 29.8 | — | — | — | 15.4 |
Unless otherwise indicated, all measurements are in millimeters. Measurements of Hadar fossils from Latimer et al. (1982). Measurements of all other comparative early hominin specimens from DeSilva et al. (2018). (Adapted from DeSilva et al., 2018).
Indicates estimates that approximate the actual value.
The entire distal portion of the calcaneus is missing, including the anterior and middle talar articular surfaces, and the cuboid articular surface. Medially, the entire sustentaculum tali is missing and there is some damage along the central medial margin of the posterior talar facet. The plantolateral portion of the calcaneal tuberosity is eroded; however, most of the medial plantar process is present.
The posterior talar facet is convex proximodistally and measures 22.5 mm, slightly concave to flat mediolaterally and is estimated to measure 16.7 mm. The posterior talar facet faces slightly distolaterally, almost in line with the long axis of the calcaneus, as in humans.
The calcaneal tuberosity is gracile and is a minimum of 23.5 mm superoinferiorly and 17.6 mm mediolaterally. In proximal view, the tuberosity is ovoid in shape and a maximum of 31 mm superoinferiorly and 20.1 mm mediolaterally. The superior portion of the calcaneal tuberosity is large, flat, and angled dorsodistally to plantoproximally. The upper third of the posterior surface, corresponding to the retrocalcaneal bursa, is clearly distinguishable from the middle and lower thirds. The tuberosity flexes at its lower third, below which it curves plantarly to become convex and weakly beaked. The superior facet for the retrocalcaneal bursa and the middle facet are divided by a groove progressing plantomedially to dorsolaterally, similar to U.W. 88–99. The middle facet terminates inferiorly in a rugose insertion for the Achilles tendon (Kachlik et al., 2008). This rugosity is angled dorsolaterally (parallel to the superior groove), separating the middle facet from the inferior facet. Weak Sharpey's fibers can be felt within this rugosity and are oriented perpendicular to the long axis of the calcaneal body. The superior facet for the retrocalcaneal bursa is 8.8 mm superoinferiorly (SI) at the midpoint of the calcaneus (28% of total tuberosity height), the middle facet is 9.8 mm SI (31.2% of the total tuberosity height), and the inferior portion of the bone 10.8 mm SI. These proportions are human‐like (Zipfel et al., 2011).
In the dorsal view, the tuberosity is long and narrow. Relative to the long axis of the bone, the insertion for the triceps surae muscle complex is medially deviated 14 degrees. Unlike in apes and A. afarensis, the calcaneus does not deviate medially at the peroneal trochlea, but remains straight, as it does in most humans, A. sediba, A. africanus (based on the fragmentary StW 352 calcaneus), and H. naledi. The calcaneal tuber is concave proximodistally and generally smooth. Calcaneal tuber length (measured from the middle of the posterior talar facet to the most distal point on the calcaneal tuberosity) is 25.6 mm in length. Plantarly, the medial plantar process extends 17.8 mm distally from the calcaneal tuberosity. There is a rugosity 29.6 mm from the proximal end of the calcaneal tuberosity for the origin of the long plantar ligament, an important ligament stabilizing the midfoot (e.g., Gomberg, 1985). This rugosity is also present on calcanei from Malapa, Hadar, and Omo (Gebo & Schwartz, 2006; Zipfel et al., 2011).
The LPP is well‐developed and plantarly positioned. There is an obliquely‐oriented retrotrochlear eminence connecting the LPP and the peroneal trochlea similar to what is found in humans and the Hadar calcanei (A.L. 333–8 and A.L. 333–55), but in contrast with U.W. 88–99, which has a nearly horizontal retrotrochlear eminence (similar to chimpanzees) (Zipfel et al., 2011). The peroneal trochlea is well‐developed, projecting approximately 4.7 mm laterally from the main body of the calcaneus and is prominent according to the Hyer et al. (2005) classification in humans. Superior to the peroneal trochlea there is a sulcus for the passage of the peroneus brevis muscle. Inferior to the peroneal trochlea there is a smoothed sulcus for the peroneal longus muscle tendon. Plantarly to the LPP, there is a small concavity separating the LPP from the medial plantar process.
3.2. Tuber robusticity
Body mass for KW 6302 was estimated to range from 33.8 to 35.9 kg based on the size of the KNM‐ER 1476 talus. The minimal cross‐sectional area of the KW 6302 calcaneal tuber was 335.9mm2 and the tuber length was 25.6 mm. The relationship of these two variables relative to those of humans, extant great apes, and other early hominins can be seen in Figure 5 (comparative data from Prang, 2015). Following Latimer and Lovejoy's (1989) method of calculating tuber robusticity, the ratio is 0.24–0.25, which is ape‐like (Table 3). Following Prang's (2015) method, the robusticity index is 51.3, which falls between African apes and modern humans (Table 3). Relative to other fossil hominin calcanei, tuber robusticity is similar to that of A. africanus, higher than A. sediba and the Omo calcaneus, and lower than in A. afarensis (Table 3).
FIGURE 5.

Calcaneal tuber cross‐sectional area relative to calcaneal tuber length. Comparative extant and fossil hominin data from Prang (2015)
TABLE 3.
Tuber robusticity measures
| Extant species and early hominin calcanei | Tuber volume/estimated body mass (Latimer & Lovejoy, 1989) | Calcaneal robusticity index (Prang, 2015) |
|---|---|---|
| KW 6302 | 0.24–0.25 | 51.3 |
| Homo sapiens | 0.742 | 73.1 |
| Pan troglodytes | 0.198 | 44.3 |
| Gorilla gorilla | 0.216 | 23.2 |
| A.L. 333–8 | 0.468–0.702 | 79.2 |
| A.L. 333–55 | 0.497–0.745 | 57.1 |
| U.W. 88–99 | 0.38–0.52 | 43 |
| StW 352 | — | 50.6 |
| Omo 33–74‐896 | — | 40.2 |
3.3. Achilles tendon length reconstruction
The relative Achilles tendon length of the KW 6302 calcaneus was predicted to be 44.2% (37%–51%). This estimated relative length falls between that of A. afarensis (63% [54%–70%]) and A. sediba (40% [33–47%]) (McNutt & DeSilva, 2020). Based on a single observation with the mean test, the length of the KW 6302 Achilles tendon is not significantly different from either nonhuman apes (p = 0.14) or humans (p = 0.78).
3.4. Lateral plantar process positioning
The KW 6302 calcaneus has an LPP position of 14.9 (calculated as the distance from the midpoint of the LPP to the base of the calcaneus; Boyle et al., 2018). This is within the human range of variation, indicating a human‐like LPP superoinferior position, and, of the early hominin calcanei, is most similar to that of the Hadar calcanei (Boyle et al., 2018; Table 4).
TABLE 4.
Lateral plantar process position
| Extant species and early hominin calcanei | Mean LPP position |
|---|---|
| KW 6302 | 14.9 |
| G. b. beringei | 50.6 |
| G. g. gorilla | 44.5 |
| P. paniscus | 32.7 |
| P. troglodytes | 43.9 |
| P. pygmaeus | 48.5 |
| H. sapiens (Jomon, Prehistoric Japanese) | 13.2 |
| H. sapiens (modern Japanese) | 15.7 |
| H. sapiens (Merida) | 19.1 |
| H. sapiens (prepastoral South African) | 14.4 |
| H. sapiens (Zulu) | 16.4 |
| A. afarensis (Hadar) | 14.7 |
| A. sediba (Malapa) | 30.2 |
Lateral plantar process positions for all comparative specimens from Boyle et al. (2018).
3.5. Three‐dimensional geometric morphometric sliding semilandmark analysis
The KW 6302 calcaneus falls in between humans and nonhuman primates along PC 1 (Figure 6). PC 1 largely represents the separation between humans and nonhuman primates (except for hylobatids) and is driven by the anteroposterior length of the calcaneal tuber relative to that of the posterior talar facet. Relative to the extant taxa, the KW 6302 calcaneus is most similar to modern humans (specifically sedentary humans from the Terry collection), and of the nonhuman primates, is closest to P. paniscus based on Procrustes distances (Table 5). The KW 6302 posterior talar facet lies on the edge of the nonhuman primate range of variation, indicating its overall shape is nonhuman primate‐like (Figure 7). The posterior talar facet of KW 6302 is anteroposteriorly and mediolaterally flatter than the gorilla condition and is more similar to modern humans (Figure 8a,b). Compared to modern humans, the KW 6302 calcaneus also exhibits a relatively large peroneal trochlea (more similar to gorillas in degree of lateral projection), a relatively narrower calcaneal tuber, and a smaller and more posteriorly‐positioned lateral plantar process (Figure 8a,b; superior view for LPP size). Relative to P. paniscus, the KW 6302 calcaneus has a flatter posterior talar facet, a wider calcaneal tuber (similar in breadth to G. b. beringei relative to the size of the calcaneus), and a smaller peroneal trochlea that is similarly positioned on the bone (Figure 8a,c).
FIGURE 6.

PC plots of complete KW 6302 calcaneal morphology for PCs 1–3. For the extant comparative sample, females are represented by open symbols and males by closed symbols. Specimens of unknown sex are represented by xs
TABLE 5.
Procrustes distances between KW 6302 and the average of each human population and nonhuman primate taxon
| Comparative sample | Procrustes distance |
|---|---|
| H. sapiens (Illinois) | 0.0194 |
| H. sapiens (Puye) | 0.0207 |
| H. sapiens (Khoesan) | 0.0197 |
| H. sapiens (Terry) | 0.0131 |
| P. t. schweinfurthii | 0.0221 |
| P. t. troglodytes | 0.0205 |
| P. t. verus | 0.0166 |
| P. paniscus | 0.0161 |
| G. b. beringei | 0.0197 |
| G. b. graueri | 0.0281 |
| G. g. gorilla | 0.0220 |
| P. abelii | 0.0223 |
| P. pygmaeus | 0.0211 |
| Papio | 0.0324 |
| Hylobates spp. | 0.0510 |
| Siamangs | 0.0370 |
FIGURE 7.
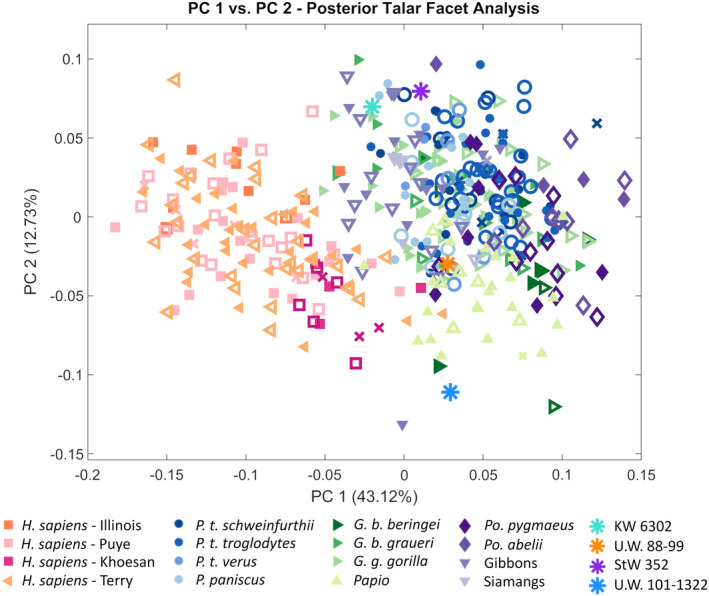
PC plot of PC 1 versus PC 2 for posterior talar facet variation (analyzed individually). For the extant comparative sample, females are represented by open symbols and males by closed symbols. Specimens of unknown sex are represented by xs
FIGURE 8.
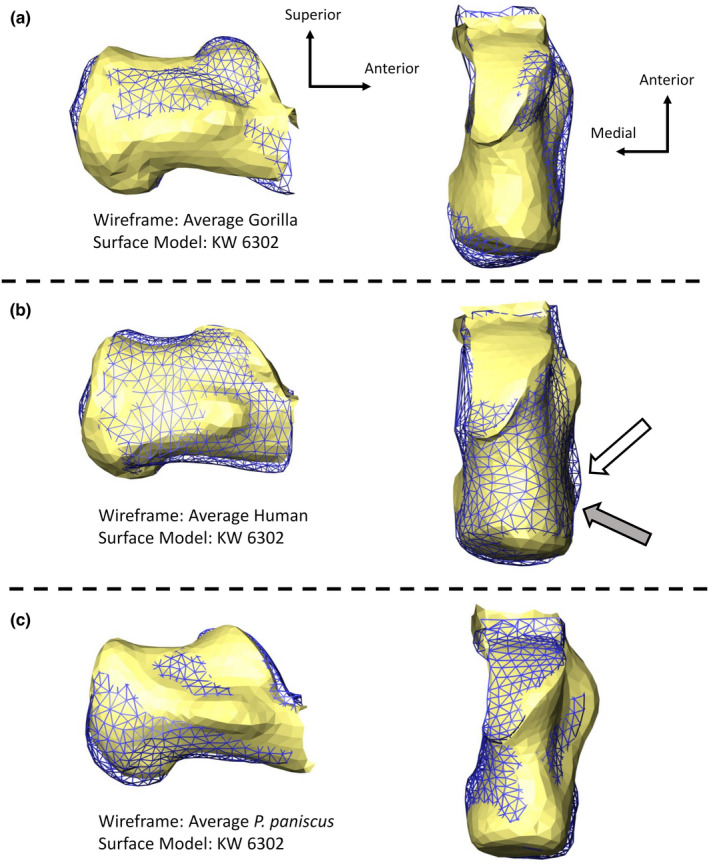
KW 6302 calcaneus relative to the average gorilla (a), the average sedentary modern human (b), and the average pan paniscus (c). The white arrow points to the location of the modern human LPP and the gray arrow points to the KW 6302 LPP
The KW 6302 calcaneus exhibits some unique morphologies relative to all other fossil calcanei included in the sample (Figures 9 and 10). Based on Mahalanobis distances, however, KW 6302 falls within the range of expected variation for a genus (based on Homo, Gorilla, and Pan) and (sub)species (based on G. g. gorilla, P. t. troglodytes, and P. t. schweinfurthii) with the U.W. 88–99, A.L. 333–55, and the StW 352 calcanei (Table 6). Relative to the U.W. 88–99 calcaneus, the KW 6302 calcaneus has a flatter posterior talar facet, a smaller peroneal trochlea, and a calcaneal tuber that is similar in mediolateral width (Figure 10). The KW 6302 calcaneus is mediolaterally narrower than the A.L. 333–55 calcaneus (Figure 10). For centroid size, the KW 6302 calcaneus has similar overall dimensions to StW 352 but exhibits a smaller peroneal trochlea and a relatively flatter posterior talar facet (Figure 10). The KW 6302 and A.L. 333–8 calcanei are within the range of variation for all three genera and G. g. gorilla, but outside of the range of variation seen in P. t. troglodytes and P. t. schweinfurthii (Table 6). The KW 6302 calcaneus differs from the A.L. 333–8 calcaneus in demonstrating a mediolaterally narrower posterior calcaneus for centroid size and a relatively smaller peroneal trochlea (Figure 10). The Omo 33–74‐896 and KW 6302 calcanei are within the range of humans and gorillas at the genus level (but not chimpanzees/bonobos) and outside of the range of morphological variation for all three (sub)species (Table 6). KW 6302 exhibits a relatively smaller and more anteriorly‐positioned peroneal trochlea than Omo 33–74‐896 and a posterior calcaneus that is similar in size (Figure 10). The KW 6302 calcaneus is outside the range of variation of the U.W. 101–1322 calcaneus at both the genus and (sub)species‐level (Table 6). The KW 6302 has a relatively wider posterior calcaneus than the U.W. 101–1322, with a more prominent peroneal trochlea (Figure 10). Compared to all other fossil calcanei in the sample, the KW 6302 posterior talar facet is more superiorly positioned relative to the rest of the bone (Figure 10).
FIGURE 9.

PC plots of the first two PCs for overlapping calcaneal morphology of KW 6302, Omo 33–74‐896, A.L. 333–8, and A.L. 333–55 (a), as well as the overlapping calcaneal morphology of KW 6302, StW 352, and U.W. 101–1322 (b)
FIGURE 10.

KW 6302 calcaneal morphology relative to overlapping morphology of fossil calcanei included in the extinct comparative sample from a superior view. For all comparisons, the KW 6302 calcaneus is represented by a surface model and the comparative fossil calcaneus is represented by a wireframe
TABLE 6.
Mahalanobis distance p‐values for significant differences between the KW 6302 calcaneus and other fossils calculated based on covariance matrices of different genera/(sub)species
| Humans | Pan | Gorilla | P. t. troglodytes | P. t. schweinfurthii | G. g. gorilla | |
|---|---|---|---|---|---|---|
| A.L. 333–8 | 0.0785 | 0.0609 | 0.1923 | 0.0016 | 0.0393 | 0.2453 |
| A.L. 333–55 | 0.3803 | 0.2213 | 0.3805 | 0.0698 | 0.4226 | 0.4781 |
| StW 352 | 0.3497 | 0.5425 | 0.6269 | 0.2623 | 0.5799 | 0.5964 |
| Omo 33–74‐896 | 0.2204 | 0.0454 | 0.0536 | 0.0294 | <0.001 | 0.0125 |
| U.W. 88–99 | 0.3456 | 0.4541 | 0.2473 | 0.3613 | 0.3538 | 0.3135 |
| U.W. 101–1322 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Bold values indicate a significant difference at p < 0.05.
4. DISCUSSION
4.1. Functional considerations
The KW 6302 calcaneus exhibits an intermediate morphology between modern humans and nonhuman great apes, however, is overall closer to modern humans. The functional implications of this suite of morphological traits will be discussed below.
The calcaneal tuber is relatively narrower than in modern humans and is more similar to G. b. beringei, indicating that there may be a difference in the magnitude of mechanical loading through the posterior calcaneus compared to modern humans. Gorillas have been recently shown to use a heel‐only heel strike with an impact spike, however, suggesting that KW 6302 may have utilized a modern human‐like heel strike (Zeininger et al., 2019; Wunderlich et al., 2019). The relatively narrow tuber may also be the product of KW 6302′s small body size (reconstructed to be 33.3–35.9 kg) as calcaneal tuber breadth scales to estimated body mass with positive allometry in both modern humans and nonhuman primates (Harper et al., 2022). The KW 6302 calcaneal robusticity index is close to what is preserved in A. africanus, another small‐bodied hominin (Prang, 2015; Ruff et al., 2018), indicating that the two specimens may have experienced similar loading regimes. Despite findings of intermediate tuber breadth in both the 3D GM and calcaneal robusticity index analyses, based on Latimer and Lovejoy (1989) tuber robusticity metric, KW 6302 has a nonhuman ape‐like tuber. The calcaneal tuber is relatively anteroposteriorly short, however, which may be influencing this result. The combination of these findings in the tuber indicates that KW 6302 likely experienced less frequent loading or a lower magnitude of loading relative to modern humans.
The KW 6302 calcaneus exhibits an LPP superoinferior position that falls within the range of human variation (Boyle et al., 2018). The LPP is slightly smaller (although more pronounced than the ape condition) and more posteriorly positioned on the calcaneus than in modern humans. We do not yet understand the functional relevance of LPP size or anteroposterior position; however, anteroposterior position has been suggested to be variable in modern humans (Laidlaw, 1905; Edwards, 1928; Elftman & Manter, 1935; Gill et al., 2014). The LPP has been suggested to increase the heel contact area with the ground during bipedal walking, lowering peak compressive forces at any given location during heel strike (Boyle et al., 2018; Latimer & Lovejoy, 1989). The presence of a large, relatively human‐like LPP indicates that, at least while traveling on the ground, this specimen was likely engaging in bipedal locomotion.
The length of the KW 6302 Achilles tendon has been reconstructed to be intermediate between A. afarensis and A. sediba (McNutt & DeSilva, 2020). Tendon length likely limits the length of muscle fibers, which have been previously shown to be proportional to joint excursion (Butler & Dominy, 2016; Venkataraman et al., 2013). This indicates that variation in Achilles tendon length may be related to the range of dorsiflexion possible. Given this assumption, KW 6302 exhibits an intermediate degree of dorsiflexion possible between A. afarensis and A. sediba, suggesting that KW 6302 may have engaged in less climbing than A. sediba.
The posterior talar facet exhibits a similar degree of anteroposterior and mediolateral curvature to modern humans, indicating a limited range of motion at the subtalar joint, which is consistent with the formation of a stiff foot for push‐off (e.g. Prang, 2016a, 2016b) and thus a potentially bipedally adapted calcaneus. Yet, the overall shape of the posterior talar facet is more great‐ape‐like, indicating a potentially different set of the subtalar joint than is seen in modern humans. Without a preserved sustentaculum tali, or talus, however, it is difficult to assess this fully. A different set to the subtalar joint could serve to facilitate climbing in a more terrestrially adapted foot.
The peroneal trochlea is larger than is seen in the average modern human calcaneus and is more gorilla‐like in overall size. Although previous researchers have suggested that a large peroneal trochlea is indicative of increased lateral leg muscle mass for increased eversion during arboreal behaviors (Gebo & Schwartz, 2006), a recent analysis found no consistent relationship between the relative size of the peroneal trochlea and degree of arboreality among humans and nonhuman great apes (Harper et al., 2021a). It is rather suggested that the size of the peroneal trochlea may be influenced by foot positioning or the size of the peroneal musculature (Harper et al., 2021a). In this specimen, it is possible that the relatively large peroneal trochlea may be a consequence of climbing with a relatively bipedally adapted calcaneus. This would likely increase the stress on the lateral ligamentous structures, such as the calcaneofibular ligament, which could explain the larger peroneal trochlea (see also, Harper et al., 2021a, 2021b).
Based on this suite of morphological characteristics, the KW 6302 calcaneus appears to be adapted for terrestrial bipedal locomotion but retains some features that indicate that climbing remained a part of its locomotor repertoire. KW 6302 falls within the range of variation expected for an extant species with U.W. 88–99 and StW 352, which have both been interpreted by various authors to use both bipedal locomotion and climbing as parts of their locomotor repertoires (e.g., DeSilva et al., 2018; DeSilva et al., 2019; Harcourt‐Smith & Aiello, 2004; Prang, 2016b). Relative to A. afarensis, KW 6302 falls within the range of variation of an extant species with the A.L. 333–55 calcaneus, and extant genus with the A.L. 333–8 calcaneus. Although A. afarensis is largely regarded to have used modern human‐like bipedalism (e.g., DeSilva et al., 2019; Harper et al., 2021a; Latimer & Lovejoy, 1989; Prang, 2015), the upper limb indicates some degree of maintained climbing (e.g., Green & Alemseged, 2012; Ruff et al., 2016; Susman & Stern, 1984). It is possible that the KW 6302 specimen utilized a similar locomotor repertoire; however, without more skeletal elements this is difficult to establish.
4.2. Taxonomic attribution
Fossils from the Kromdraai site have been primarily attributed to P. robustus (Braga et al., 2013, 2021; Braga & Thackeray, 2016; Broom, 1938a, 1938b, 1943; Grine, 1982); however, some specimens have been suggested to belong to early Homo (potentially H. habilis) (e.g., Braga & Thackeray, 2003). Unfortunately, there are no published calcanei that are definitively attributed to either species, making it difficult to provide the KW 6302 calcaneus with a taxonomic affiliation. KW 6302 does have a similar morphology to the South African australopiths, however, no australopithecine dentition has been found at Kromdraai, further limiting our taxonomic choices. The Omo 33–74‐896 calcaneus (attributed to either H. habilis or P. aethiopicus) and the OH 8 calcaneus (attributed to either H. habilis or P. boisei) are thus the closest specimens for making taxonomic comparisons because they have been attributed to genera found at the Kromdraai site (Deloison, 1986; Feibel et al., 1989; Braga & Thackeray, 2003; Deloison, 2004; Gebo & Schwartz, 2006). KW 6302 and the Omo calcaneus are outside of the range of variation of a species and one of the genera tested, indicating that if one of the calcanei belongs to Paranthropus, the other could potentially be attributed to early Homo.
Unfortunately, the KW 6302 and OH 8 calcanei were not able to be analyzed in this manner because the two specimens preserve little overlapping morphology. A previous analysis directly compared the OH 8 and Omo 33–74‐896 calcanei and found that they were within the range of variation for an extant species and genus (Harper et al., 2021a). Although the phylogenetic affinity of OH 8 is still debated (e.g., DeSilva et al., 2019; Leakey et al., 1964), a recent review article surveying the evolution of the foot throughout the Plio‐Pleistocene suggested that it likely represents a P. boisei specimen based primarily on the preserved morphology of the associated talus and first metatarsal (DeSilva et al., 2019). If this supposition is correct, it would suggest that KW 6302 represents an early Homo calcaneus. Until this calcaneus can be compared to a known early Homo calcaneus, however, it is not possible to definitively attribute this specimen. An early Homo calcaneus from Ileret, Kenya has been preliminarily announced (Jungers et al., 2015) and eventual comparison of the two calcanei may help to shed light on the taxonomy of KW 6302.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
AUTHOR CONTRIBUTIONS
JB, JMD, BZ, and CMH designed the study. CMH, BZ, JMD, EJM, FT, and JB acquired the data. CMH, JMD, EJM, and BZ conducted data analysis and interpretation. CMH, BZ, and JB drafted the manuscript. CMH, BZ, JMD, EJM, FT, and JB revised and approved the manuscript.
Supporting information
Table S1
Figure S1
Figure S2
ACKNOWLEDGMENTS
We thank the Cradle Management Authority, the South African Heritage Resources Agency (SAHRA) and M. Riaan Lotz for their continuous support. We warmly acknowledge M.M Moleko Monyama, Levy Modise, Sipho Makhele, Shadrack Molefe, Clopus Seshoene, Andrew Phaswana, Benjamin Lans, and Raphaël Hautefort for their valuable assistance during the field work. We also thank the Evolutionary Studies Institute, University of the Witwatersrand, for curating fossils from Kromdraai. The Kromdraai Research Project is supported by the French Ministry of Foreign Affairs, the Institut des Déserts et des Steppes (Paris), the “Patrick Mathieu Recherche et Conseil” company, the Institut Picot de Lapeyrouse (Toulouse) and the “AESOP plus” programme of Erasmus Mundus (European Union). We thank Sifelani Jirah and the Fossil Access Advisory Committee at the University of the Witwatersrand for access to the Australopithecus sediba, A. africanus, and Homo naledi calcanei; Bill Kimbel, Zeray Almseged, and Fred Spoor for access to the A. afarensis calcanei. We would like to thank the following people for access to the nonhuman primate specimens: Darrin Lunde at the National Museum of Natural History (Washington, D.C.), Lyman Jellema at the Cleveland Museum of Natural History (Cleveland, OH), Emmanuel Gilisseen at the Royal Museum for Central Africa (Tervuren, Belgium), Mark Omura at the Museum of Comparative Zoology (Cambridge, MA), and Eleanor Hoeger at the American Museum of Natural History (New York, NY). We also thank the Rwandan government for permission to access specimens curated by the Mountain Gorilla Skeletal Project and Shannon McFarlin and Angel Zeininger for providing us with said scan data. We thank Dave Hunt at the National Museum of Natural History (Washington, D.C.) and Wendy Black and Wihelmina Seconna at the Iziko Museums of South Africa for access to human specimens. For assistance with micro‐CT scanning, we thank Greg Lin and Jim Reynolds at the Harvard's Center for Nanoscale Technology, Anton du Plessis, Stephan le Roux, and Muofhe Tshibalangand at Stellenbosch University CT Facility, and Kudawashe Jakata at the University of Witwatersrand. For assistance in 3D printing, we would like to thank Darren Boehning. We additionally thank Kaya G. Zelazny for assistance in the development of the full bone‐sliding code, as well as Evan Wang and Vincent Hou for data processing assistance.
This project was supported by NSF grant BCS‐1824630.
Harper, C.M. , Zipfel, B. , DeSilva, J.M. , McNutt, E.J. , Thackeray, F. & Braga, J. (2022) A new early hominin calcaneus from Kromdraai (South Africa). Journal of Anatomy, 241, 500–517. Available from: 10.1111/joa.13660
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 3D Systems Inc . (2015) Geomagic warp. Rock Hill: 3D Systems Inc. [Google Scholar]
- Berger, L.R. , Ruiter, D.J.d. , Churchill, S.E. et al. (2010) Australopithecus sediba: a new species of homo‐like Australopith from South Africa. Science, 328, 195–204. 10.1126/science.1184944 [DOI] [PubMed] [Google Scholar]
- Berger, L.R. , Hawks, J. , de Ruiter, D.J. et al. (2015) Homo Naledi, a new species of the genus homo from the Dinaledi chamber, South Africa. eLife, 4, e09560. 10.7554/eLife.09560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg, S.P. , Garland, T. , Ives, A.R. & Crespi, B. (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57, 717–745. [DOI] [PubMed] [Google Scholar]
- Bojsen‐Møller, F. (1979) Calcaneocuboid joint and stability of the longitudinal arch of the foot at high and low gear push off. Journal of Anatomy, 129, 165–176. [PMC free article] [PubMed] [Google Scholar]
- Boyle, E.K. , McNutt, E.J. , Sasaki, T. , Suwa, G. , Zipfel, B. & DeSilva, J.M. (2018) A quantification of calcaneal lateral plantar process position with implications for bipedal locomotion in Australopithecus. Journal of Human Evolution, 123, 24–34. 10.1016/j.jhevol.2018.05.008 [DOI] [PubMed] [Google Scholar]
- Braga, J. & Thackeray, J.F. (2016) Kromdraai, a birth place of Paranthropus in the cradle of humankind (sun media metro). Johannesburg: Sun Metro Media. [Google Scholar]
- Braga, J. & Thackeray, J.F. (2003) Early homo at Kromdraai B: probabilistic and morphological analysis of the lower dentition. Comptes Rendus Palevol, 2, 269–279. [Google Scholar]
- Braga, J. , Fourvel, J.B. , Lans, B. , Bruxelles, L. & Thackeray, J.F. (2016) Evolutionary, chrono‐cultural and palaeoenvironmental backgrounds to the Kromdraai site: a regional perspective. In: Braga, J. & Thackeray, J.F. (Eds.) Kromdraai, a birth place of Paranthropus in the cradle of humankind. Sun Media Metro: Johannesburg, South Africa, pp. 1–16. [Google Scholar]
- Braga, J. , Thackeray, J.F. , Dumoncel, J. et al. (2013) A new partial temporal bone of a juvenile hominin from the site of Kromdraai B (South Africa). Journal of Human Evolution, 65, 447–456. 10.1016/j.jhevol.2013.07.013 [DOI] [PubMed] [Google Scholar]
- Braga, J. , Thackeray, J.F. , Bruxelles, L. , Dumoncel, J. & Fourvel, J.‐B. (2017) Stretching the time span of hominin evolution at Kromdraai (Gauteng, South Africa): recent discoveries. Comptes Rendus Palevol, 16, 58–70. 10.1016/j.crpv.2016.03.003 [DOI] [Google Scholar]
- Braga, J. , Samir, C. , Fradi, A. et al. (2021) Cochlear shape distinguishes southern African early hominin taxa with unique auditory ecologies. Scientific Reports, 11, 17018. 10.1038/s41598-021-96543-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain, C.K. (1958). The Transvaal ape‐man‐bearing cave deposits: Makapan limeworks (Vol. 11). Pretoria: Transvaal Museum, pp. 101–118. [Google Scholar]
- Brain, C.K. (1975) An interpretation of the bone assemblage from the Kromdraai australopithecine site, South Africa. In: Tuttle, R.H. (Ed.) Palaeontology, morphology and Palaeoecology. The Haugue: Mouton, pp. 225–243. [Google Scholar]
- Broom, R. (1938a) The Pleistocene anthropoid apes of South Africa. Nature, 142, 377–379. [Google Scholar]
- Broom, R. (1938b) Further evidence on the structure of the south African Pleistocene anthropoids. Nature, 142, 897–899. [Google Scholar]
- Broom, R. (1942) The hand of the ape‐man, Paranthropus robustus . Nature, 149, 513–514. 10.1038/149513a0 [DOI] [Google Scholar]
- Broom, R. (1943) An ankle‐bone of the ape‐man, Paranthropus robustus . Nature, 152, 689–690. [Google Scholar]
- Bruxelles, L. , Maire, R. , Couzens, R. , Thackeray, F. & Braga, J. (2016) A revised stratigraphy of Kromdraai. In: Braga, J. & Thackery, J.F. (Eds.) Kromdraai, a birthplace of Paranthropus in the cradle of humankind. Sun Metro Media: Johannesburg, South Africa, pp. 31–47. [Google Scholar]
- Butler, E.E. & Dominy, N.J. (2016) Architecture and functional ecology of the human gastrocnemius muscle‐tendon unit. Journal of Anatomy, 228, 561–568. 10.1111/joa.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier, D.R. & Cunningham, C. (2017) The effect of foot posture on capacity to apply free moments to the ground: implications for fighting performance in great apes. Biology Open, 6, 269–277. 10.1242/bio.022640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave, M. , Dean, C. , Zanolli, C. et al. (2020) Reassessment of the TM 1517 odonto‐postcranial assemblage from Kromdraai B, South Africa, and the maturational pattern of Paranthropus robustus . American Journal of Physical Anthropology, 172, 714–722. 10.1002/ajpa.24082 [DOI] [PubMed] [Google Scholar]
- Chirchir, H. , Ruff, C.B. , Junno, J.‐A. & Potts, R. (2017) Low trabecular bone density in recent sedentary modern humans. American Journal of Physical Anthropology, 162, 550–560. 10.1002/ajpa.23138 [DOI] [PubMed] [Google Scholar]
- Close, J.R. , Inman, V.T. , Poor, P.M. & Todd, F.N. (1967) The function of the subtalar joint. Clinical Orthopedics and Related Research, 50, 159–179. [PubMed] [Google Scholar]
- Day, M.H. & Napier, J.R. (1964) Hominid fossils from bed 1, Olduvai Gorge, Tanzania: fossil foot bones. Nature, 211, 929–930. [DOI] [PubMed] [Google Scholar]
- Day, M.H. & Wood, B.A. (1968) Functional affinities of the Olduvai hominid 8 talus. Man, 3, 440–455. [Google Scholar]
- de Graaff, G. (1961) On the fossil mammalian microfauna collected at Kromdraai by Draper in 1895. South African Journal of Science, 57, 259–260. [Google Scholar]
- Deloison, Y. (1986) Description d'un calcanéum fossile de Primate et sa comparison avec des calcanéums de Pongidés, d'Australopithèques et d'Homo . Comptes Rendus des Séances de l'Académie des Sciences Paris, Série III, 302, 257–262. [Google Scholar]
- Deloison, Y. (2004) A new hypothesis on the origin of hominoid locomotion. In: Meldrum, D.J. & Hilton, C.E. (Eds.) From biped to strider. Boston, MA: Springer, pp. 35–47. [Google Scholar]
- DeMars, L.J.D. , Stephens, N.B. , Saers, J.P.P. , Gordon, A. , Stock, J.T. & Ryan, T.M. (2021) Using point clouds to investigate the relationship between trabecular bone phenotype and behavior: an example utilizing the human calcaneus. American Journal of Human Biology, 33, e23468. 10.1002/ajhb.23468 [DOI] [PubMed] [Google Scholar]
- DeSilva, J.M. (2009) Functional morphology of the ankle and the likelihood of climbing in early hominins. Proceedings of the National Academy of Sciences, 106, 6567–6572. 10.1073/pnas.0900270106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva, J.M. , Carlson, K.J. , Claxton, A.G. et al. (2018) The anatomy of the lower limb skeleton of Australopithecus sediba . PaleoAnthropology, 2018, 357–405. [Google Scholar]
- DeSilva, J. , McNutt, E. , Benoit, J. & Zipfel, B. (2019) One small step: a review of Plio‐Pleistocene hominin foot evolution. American Journal of Physical Anthropology, 168, 63–140. 10.1002/ajpa.23750 [DOI] [PubMed] [Google Scholar]
- Dirks, P.H. , Roberts, E.M. , Hilbert‐Wolf, H. et al. (2017) The age of homo Naledi and associated sediments in the rising star cave, South Africa. eLife, 6, e24231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, M.E. (1928) The relations of the peroneal tendons to the fibula, calcaneus, and cuboideum. American Journal of Anatomy, 42, 213–253. [Google Scholar]
- Elftman, H. & Manter, J. (1935) The evolution of the human foot, with especial reference to the joints. Journal of Anatomy, 70, 56. [PMC free article] [PubMed] [Google Scholar]
- FEI Visualization Sciences Group . (2015) Avizo lite 9.0.1. Hillsboro: FEI Company. [Google Scholar]
- Feibel, C.S. , Brown, F.H. & McDougall, I. (1989) Strategic context of fossil hominids from the Omo group deposits: northern Turkana Basin, Kenya, and Ethiopia. American Journal of Physical Anthropology, 78, 595–622. 10.1002/ajpa.1330780412 [DOI] [PubMed] [Google Scholar]
- Fourvel, J.‐B. , Brink, J. , O'Regan, H. , Beaudet, A. & Pavia, M. (2016) Some preliminary interpretations of the oldest faunal assemblage from Kromdraai. In: Braga, J. & Thackeray, J.F. (Eds.) Kromdraai, a birthplace of Paranthropus in the cradle of humankind. Sun Media Metro: Johannesburg, South Africa, pp. 71–104. [Google Scholar]
- Fourvel, J.‐B. , Thackeray, J.F. , Brink, J.S. , O'Regan, H. & Braga, J. (2018) Taphonomic interpretations of a new Plio‐Pleistocene hominin‐bearing assemblage at Kromdraai (Gauteng, South Africa). Quaternary Science Reviews, 190, 81–97. 10.1016/j.quascirev.2018.04.018 [DOI] [Google Scholar]
- Gebo, D.L. (1992) Plantigrady and foot adaptation in African apes: implications for hominid origins. American Journal of Physical Anthropology, 89, 29–58. 10.1002/ajpa.1330890105 [DOI] [PubMed] [Google Scholar]
- Gebo, D.L. & Schwartz, G.T. (2006) Foot bones from Omo: implications for hominid evolution. American Journal of Physical Anthropology, 129, 499–511. 10.1002/ajpa.20320 [DOI] [PubMed] [Google Scholar]
- Gill, C.M. , Taneja, A.K. , Bredella, M.A. , Torriani, M. & DeSilva, J.M. (2014) Osteogenic relationship between the lateral plantar process and the peroneal tubercle in the human calcaneus. Journal of Anatomy, 224, 173–179. 10.1111/joa.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomberg, D.N. (1985) Functional differences of three ligaments of the transverse tarsal joint in hominoids. Journal of Human Evolution, 14, 553–562. 10.1016/S0047-2484(85)80080-4 [DOI] [Google Scholar]
- Gower, J.C. (1975) Generalized procrustes analysis. Psychometrika, 40, 33–51. 10.1007/BF02291478 [DOI] [Google Scholar]
- Green, D.J. & Alemseged, Z. (2012) Australopithecus afarensis scapular ontogeny, function, and the role of climbing in human evolution. Science, 338, 514–517. [DOI] [PubMed] [Google Scholar]
- Grine, F.E. (1982) A new juvenile hominid from member 3, Kromdraai formation, Transvaal, South Africa. Annals of the Transvaal Museum, 33, 165–239. [Google Scholar]
- Gunz, P. , Mitteroecker, P. & Bookstein, F.L. (2005) Semilandmarks in three dimensions. In: Slice, D.E. (Ed.) Modern morphometrics in physical anthropology. New York, NY: Springer, pp. 73–98. [Google Scholar]
- Harcourt‐Smith, W.E.H. & Aiello, L.C. (2004) Fossils, feet and the evolution of human bipedal locomotion. Journal of Anatomy, 204, 403–416. 10.1111/j.0021-8782.2004.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, C.M. , Ruff, C.B. & Sylvester, A.D. (2021a) Calcaneal shape variation in humans, nonhuman primates, and early hominins. Journal of Human Evolution, 159, 103050. 10.1016/j.jhevol.2021.103050 [DOI] [PubMed] [Google Scholar]
- Harper, C.M. , Ruff, C.B. & Sylvester, A.D. (2021b) Gorilla calcaneal morphological variation and ecological divergence. American Journal of Physical Anthropology, 174, 49–65. 10.1002/ajpa.24135 [DOI] [PubMed] [Google Scholar]
- Harper, C.M. , Ruff, C.B. & Sylvester, A.D. (2022) Scaling and relative size of the human, nonhuman ape, and baboon calcaneus. The Anatomical Record, 305, 100–122. 10.1002/ar.24642 [DOI] [PubMed] [Google Scholar]
- Holowka, N.B. & Lieberman, D.E. (2018) Rethinking the evolution of the human foot: insights from experimental research. Journal of Human Evolution, 221, jeb174425. [DOI] [PubMed] [Google Scholar]
- Holowka, N.B. , O'Neill, M.C. , Thompson, N.E. & Demes, B. (2017) Chimpanzee ankle and foot joint kinematics: arboreal versus terrestrial locomotion. American Journal of Physical Anthropology, 104, 23–31. [DOI] [PubMed] [Google Scholar]
- Hunt, D.R. & Albanese, J. (2005) History and demographic composition of the Robert J. Terry anatomical collection. American Journal of Physical Anthropology, 127, 406–417. 10.1002/ajpa.20135 [DOI] [PubMed] [Google Scholar]
- Hyer, C.F. , Dawson, J.M. , Philbin, T.M. , Berlet, G.C. & Lee, T.H. (2005) The peroneal tubercle: description, classification, and relevance to peroneus longus tendon pathology. Foot & Ankle International, 26, 947–950. 10.1177/107110070502601109 [DOI] [PubMed] [Google Scholar]
- Inman, V.T. (1976) The joints of the ankle. Baltimore: Williams & Wilkins. [Google Scholar]
- Johanson, D.C. , Lovejoy, C.O. , Kimbel, W.H. et al. (1982) Morphology of the Pliocene partial hominid skeleton (a.L. 288‐1) from the Hadar formation, Ethiopia. American Journal of Physical Anthropology, 57, 403–451. 10.1002/ajpa.1330570403 [DOI] [Google Scholar]
- Jungers, W.L. , Grine, F.E. , Leakey, M.G. et al. (2015) New hominin fossils from Ileret (Kolom Odiet), Kenya. American Journal of Physical Anthopology, 156, 181. [Google Scholar]
- Kachlik, D. , Baca, V. , Capelik, M. , Hajek, P. , Mandys, V. & Musil, V. (2008) Clinical anatomy of the calcaneal tuberosity. Annals of Anatomy—Anatomischer Anzeiger, 190, 284–291. [DOI] [PubMed] [Google Scholar]
- Kidd, R. (1999) Evolution of the rearfoot. A model of adaptation with evidence from the fossil record. Journal of the American Podiatric Medical Association, 89, 2–17. 10.7547/87507315-89-1-2 [DOI] [PubMed] [Google Scholar]
- Laidlaw, P.P. (1905) The os calcis: part II. Journal of Anatomy and Physiology, 39, 161. [PMC free article] [PubMed] [Google Scholar]
- Latimer, B. & Lovejoy, C.O. (1989) The calcaneus of Australopithecus afarensis and its implications for the evolution of bipedality. American Journal of Physical Anthropology, 78, 369–386. 10.1002/ajpa.1330780306 [DOI] [PubMed] [Google Scholar]
- Latimer, B. , Lovejoy, C.O. , Johanson, D.C. & Coppens, Y. (1982) Hominid tarsal, metatarsal, and phalangeal bones recovered from the Hadar formation: 1974‐1977 collections. American Journal of Physical Anthropology, 57, 701–719. [Google Scholar]
- Latimer, B. , Ohman, J.C. & Lovejoy, C.O. (1987) Talocrural joint in African hominoids: implications for Australopithecus afarensis. American Journal of Physical Anthropology, 74, 155–175. 10.1002/ajpa.1330740204 [DOI] [PubMed] [Google Scholar]
- Leakey, L.S.B. , Tobias, P.V. & Napier, J.R. (1964) A new species of genus homo from Olduvai Gorge. Nature, 202, 7–9. [DOI] [PubMed] [Google Scholar]
- Lewis, O.J. (1980) The joints of the evolving foot. Part I. the ankle joint. Journal of Anatomy, 130(Pt 3), 527–543. [PMC free article] [PubMed] [Google Scholar]
- Malan, B.D. (1959) Early references to the Sterkfontein caves. South African Journal of Science, 55, 321–324. [Google Scholar]
- McHenry, H.M. (1992) Body size and proportions in early hominids. American Journal of Physical Anthropology, 87, 407–431. 10.1002/ajpa.1330870404 [DOI] [PubMed] [Google Scholar]
- McNutt, E.J. & DeSilva, J.M. (2020) Evidence for an elongated Achilles tendon in Australopithecus. The Anatomical Record, 303, 2382–2391. 10.1002/ar.24387 [DOI] [PubMed] [Google Scholar]
- McNutt, E.J. , Zipfel, B. & DeSilva, J.M. (2018) Evolution of the human foot. Evolutionary Anthropology, 27, 197–217. [DOI] [PubMed] [Google Scholar]
- Ngoloyi, N.M. , Dumoncel, J. , Thackeray, J.F. & Braga, J. (2020) A new method to evaluate 3D spatial patterns within early hominin‐bearing sites. An example from Kromdraai (Gauteng Province South Africa). Journal of Archaeological Science: Reports, 32, 102376. 10.1016/j.jasrep.2020.102376 [DOI] [Google Scholar]
- Partridge, T.C. (1982) Some preliminary observations on the stratigraphy and sedimentology of the Kromdraai B hominid site. Palaeoecology of Africa and the Surrounding Islands, 15, 3–12. [Google Scholar]
- Pavia, M. (2020) Palaeoenvironmental reconstruction of the cradle of humankind during the Plio‐Pleistocene transition, inferred from the analysis of fossil birds from member 2 of the hominin‐bearing site of Kromdraai (Gauteng, South Africa). Quaternary Science Reviews, 248, 106532. 10.1016/j.quascirev.2020.106532 [DOI] [Google Scholar]
- Perino, G. (1968) The Pete Klunk mound group, Calhoun County, Illinois: The Archaic and Hopewell occupations. In: Hopewell and Woodland site archaeology in Illinois, Vol. 6. Illinois Archaeological Survey Bulletin, pp. 9–124. [Google Scholar]
- Prang, T.C. (2015) Calcaneal robusticity in Plio‐Pleistocene hominins: implications for locomotor diversity and phylogeny. Journal of Human Evolution, 80, 135–146. 10.1016/j.jhevol.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Prang, T.C. (2016a) Conarticular congruence of the hominoid subtalar joint complex with implications for joint function in Plio‐Pleistocene hominins. American Journal of Physical Anthropology, 160, 446–457. 10.1002/ajpa.22982 [DOI] [PubMed] [Google Scholar]
- Prang, T.C. (2016b) The subtalar joint complex of Australopithecus sediba. Journal of Human Evolution, 90, 105–119. 10.1016/j.jhevol.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Rohlf, F.J. & Slice, D. (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59. 10.2307/2992207 [DOI] [Google Scholar]
- Ruff, C.B. , Burgess, M.L. , Ketcham, R.A. & Kappelman, J. (2016) Limb bone structural proportions and locomotor behavior in a.L. 288‐1 (“Lucy”). PLoS One, 11, e0166095. 10.1371/journal.pone.0166095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, C.B. , Burgess, M.L. , Squyres, N. , Junno, J.‐A. & Trinkaus, E. (2018) Lower limb articular scaling and body mass estimation in Pliocene and Pleistocene hominins. Journal of Human Evolution, 115, 85–111. 10.1016/j.jhevol.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Saers, J.P.P. , Ryan, T.M. & Stock, J.T. (2019) Trabecular bone function adaptation and sexual dimorphism in the human foot. American Journal of Physical Anthropology, 168, 154–169. 10/1002/ajpa.23732 [DOI] [PubMed] [Google Scholar]
- Schmitt, D. & Larson, S.G. (1995) Heel contact as a function of substrate type and speed in primates. American Journal of Physical Anthropology, 96, 39–50. 10.1002/ajpa.1330960105 [DOI] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. & Eliceiri, K.W. (2012) NIH image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal, R.R. & Rohlf, F.J. (1998) Biometry: the principles and practice of statistics in biological research, 3rd edition. New York: W.H. Freeman and Company. [Google Scholar]
- Sorrentino, R. , Carlson, K.J. , Bortolini, E. et al. (2020a) Morphometric analysis of the hominin talus: evolutionary and functional implications. Journal of Human Evolution, 142, 102747. 10.1016/j.jhevol.2020.102747 [DOI] [PubMed] [Google Scholar]
- Sorrentino, R. , Stephens, N.B. , Carlson, K.J. et al. (2020b) The influence of mobility strategy on the modern human talus. American Journal of Physical Anthropology, 171, 456–469. 10.1002/ajpa.23976 [DOI] [PubMed] [Google Scholar]
- Sorrentino, R. , Stephens, N.B. , March, D. et al. (2021) Unique foot posture in Neanderthals reflects their body mass and high mechanical stress. Journal of Human Evolution, 161, 103093. 10.1016/j.jhevol.2021.103093 [DOI] [PubMed] [Google Scholar]
- Stern, J.T., Jr. & Susman, R.L. (1983) The locomotor anatomy of Australopithecus afarensis . American Journal of Physical Anthropology, 60, 279–317. 10.1002/ajpa.1330600302 [DOI] [PubMed] [Google Scholar]
- Stock, J. & Pfeiffer, S. (2001) Linking structural variability in long bone diaphysis to habitual behaviors: foragers from the southern African later stone age and the Adaman Islands. American Journal of Physical Anthropology, 115, 337–348. [DOI] [PubMed] [Google Scholar]
- Susman, R.L. & Stern, J.T. (1984) Arboreality and bipedality in the Hadar hominins. Folia Primatologica, 43, 113–156. [DOI] [PubMed] [Google Scholar]
- Thackeray, J.F. , de Ruiter, D.J. , Berger, L.R. & van der Merwe, N.J. (2001) Hominid fossils from Kromdraai: a revised list of specimens discovered since 1938. Annals of the Transvaal Museum, 38, 43–56. [Google Scholar]
- Thackeray, J.F. , Kirschvink, J.L. & Raub, T.D. (2002) Palaeomagnetic analysis of calcified deposits from the Plio‐Pleistocene hominid site of Kromdraai, South Africa. South African Journal of Science, 98, 537–540. [Google Scholar]
- Thackeray, J.F. , Braga, J. , Sénégas, F. , Gommery, D. , Potze, S. & Senut, B. (2005) Discovery of a humerus shaft from Kromdraai B: part of the skeleton of the type specimen of Paranthropus robustus (Broom 1938)? Annals of the Transvaal Museum, 42, 92–93. [Google Scholar]
- Thompson, N.E. , Holowka, N.B. , O'Neill, M.C. & Larson, S.G. (2014) Brief communication: cineradiographic analysis of the chimpanzee (pan troglodytes) talonavicular and calcaneocuboid joints. American Journal of Physical Anthropology, 154, 604–608. 10.1002/ajpa.22529 [DOI] [PubMed] [Google Scholar]
- Venkataraman, V.V. , Kraft, T.S. & Dominy, N.J. (2013) Tree climbing and human evolution. Proceedings of the National Academy of Sciences, 110, 1237–1242. 10.1073/pnas.1208717110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke, E. , D'Août, K. , De Clercq, D. , Van Elsacker, L. & Aerts, P. (2003) Dynamic plantar pressure distribution during terrestrial locomotion of bonobos (pan paniscus). American Journal of Physical Anthropology, 120, 373–383. 10.1002/ajpa.10163 [DOI] [PubMed] [Google Scholar]
- Vrba, E. (1981) The Kromdraai australopithecine site revisited in 1980: recent investigations and results. Annals of the Transvaal Museum, 33, 17–60. [Google Scholar]
- Webber, J.T. & Raichlen, D.A. (2016) The tolr of plantirgady and heel‐strike in the mechanics and energetics of human walking with implications for the evolution of the human foot. Journal of Experimental Biology, 219, 3729–3737. [DOI] [PubMed] [Google Scholar]
- Wunderlich, R.E. , Zeininger, A. & Schmitt, D. (2019) Plantar pressure distribution in gorilla . American Journal of Physical Anthropology, 168, 275. [Google Scholar]
- Yang, Y. (2012). 3D thin plate spline warping function. MATLAB central file Exchange.
- Zeininger, A. , Schmitt, D. & Wunderlich, R.E. (2019) Impact forces and hindlimb vertical impulses in Gorilla . American Journal of Physical Anthropology, 168, 280. [Google Scholar]
- Zeininger, A. , Schmitt, D. & Wunderlich, R.E. (2020) Mechanics of heel‐strike plantigrady in African apes. Journal of Human Evolution, 145, 102840. [DOI] [PubMed] [Google Scholar]
- Zelditch, A. , Swiderski, D.L. & Sheets, H.D. (2012) Geometric morphometrics for biologists: a primer, 2nd edition. London, UK: Elsevier Science. [Google Scholar]
- Zipfel, B. , DeSilva, J.M. , Kidd, R.S. , Carlson, K.J. , Churchill, S.E. & Berger, L.R. (2011) The foot and ankle of Australopithecus sediba. Science, 333, 1417–1420. 10.1126/science.1202703 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1
Figure S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


