Abstract
Background:
Therapy management in patients suffering from mental health disorders is complex and the risks derived from changes or interruptions of treatment should not be ignored. Medication reconciliation in psychiatry may reduce medication errors and promote patient safety during transitions of care.
Objective:
To identify the influence of complementary information sources in the construction of the best possible medication history, and to ascertain the potential clinical impact of discrepancies identified in a medication reconciliation service.
Methods:
An observational study was conducted in an acute mental hospital unit, with a further validation in an internal medicine unit. Adult patients taking at least one medicine admitted in the unit were included. Patients/caregivers were interviewed upon admission and the information gathered was compared with hospital medical and shared electronic medical records. Once the best possible medication history was gathered, therapeutic information was reconciled against the prescription on admission to identify discrepancies. Potential clinical impact of medication errors was classified using the International Safety Classification.
Results:
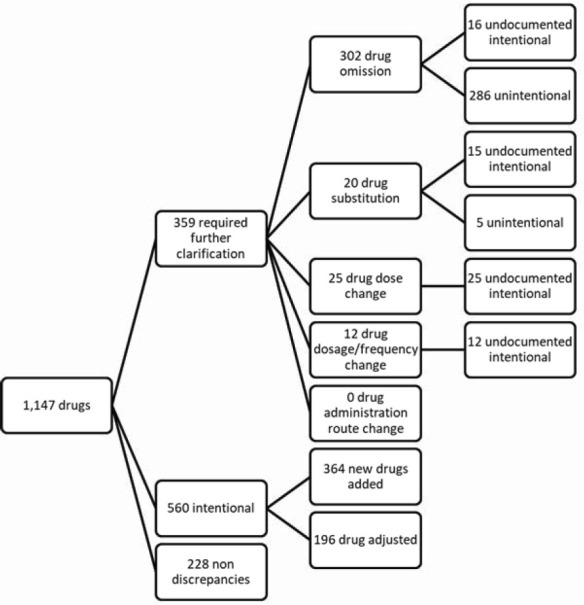
During the study period, 148 patients were admitted, 50.7% females, mean age 54.6 years (SD=16.3). Collaboration of a caregiver was a needed in 74% of the interviews. In total, 1,147 drugs were considered to obtain patients’ best possible medication history. After reconciliation, 560 clinically sound intentional discrepancies were identified and 359 discrepancies required further clarification from prescribers: 84.12% “drug omission”, 5.57% “drug substitution”, 6.96% “dose change”, and 3.34% “dosage frequency change”. Potential clinical impact of these medication discrepancies was classified as: 95 mild, 100 moderate, and 29 severe medication errors.
Conclusion:
About 1 in three intentional discrepancies observed in a pharmacists-led medication reconciliation service required further clarification from prescribers, being 80% of them unintentional discrepancies. Results highlight the importance of the caregiver as source of information for the psychiatric patient, the relevance of analyzing shared electronic health records until 6 months before, and the need to use hospital medical records efficiently. Additionally, 29 discrepancies were classified as errors with potentially severe clinical impact. A medication reconciliation service is concluded to be feasible and necessary in a mental health unit.
Keywords: Medication Errors, Medication Reconciliation, Patient Safety, Transitional Care
INTRODUCTION
Medication reconciliation (MR) is an intervention aiming to promote the risk reduction during transitions of care, revealing as a promising strategy to reduce medication errors and to promote of patient safety.1 MR is a systematic process to create an accurate list of the patient’s medicines, known as the best possible medication history, and to compare it with the medication instituted at each time of transition of care.2,3
In many countries, healthcare institutions should have established medication reconciliation policies at patient admission.4,5 The Prescribing Observatory for Mental Health in the UK, which aims to improve the quality of mental health prescribing practice, reported that medication reconciliation improves the quality of these inpatient health programs in a psychiatric unit.6
Unfortunately, many psychiatric services do not perform a medication reconciliation yet, and medication errors are still frequent. Many mental health units fail to recognize medication reconciliation as an essential process in medication management and patient safety, because MR is considered complex and time-consuming.7,8 However, the psychiatric patients, due to their frequent transitions between settings, are privileged targets of MR.4
Literature recommend that MR should be carried in the first 24 hours after admission.4 However, admission at psychiatric units presents some specificities that may affect the MR process. In mentally ill patients, detecting medication errors may be a challenge, because the clinical interview commonly used to create the best possible medication history, is often difficult or even impossible to occur, and the caregiver is needed to complete the information about medicines used by the patient. Additionally, other factors such as the complexity of the therapy due to the psychopathologies associated with the diagnosis of other morbidities increase the required duration of the interview. 9
To eliminate the stigma of the complexity to perform a medication reconciliation in a mental illness service, and to highlight the relevance of it, we developed a project to implement medication reconciliation upon admission to an acute psychiatric unit. As part of a project aiming to implement MR in an acute psychiatric unit, the objective of this study was to assess the influence of health information technologies to obtain the best possible medication history, and evaluate the potentially clinical impact of discrepancies identified.
METHODS
A two-stage observational study was conducted comprising a main study in an acute psychiatric unit, and a further validation in an internal medicine department at the Coimbra University Hospital.
Main study
The first stage, the main study, was conducted in an acute care unit of the Center for Integrated Responsibility of Psychiatry and Mental Health, at the Coimbra University Hospital (CHUC) (January 2015 – February 2016). The study was approved by the Ethics Committees of Hospital University Center of Coimbra (CHUC-008-15) and the University of Coimbra, Faculty of Medicine (CE 109/2014). All participants signed informed consent before their inclusion in the study.
Patients older than 18 years of age and taking at least one medicine at the moment of admission in the unit were invited to participate in the study. Exclusion criteria comprised pregnancy, or patients unable to communicate by themselves or through a caregiver (due to cognitive impairment or language barriers).
A standardized face-to-face interview with the patient was conducted by a trained pharmacist, using a data collection form, within 72 hours after admission. When it was not possible to interview the patient due to serious mental impairment or other situations compromising the reliability of the information collected, the caregiver was invited. The data collected in the interview were: medications currently taken, including prescription and non-prescription medicines, patient’s medical conditions, allergies, and information about previous adverse drug reactions. Hospital medical records were used to collect information on the clinical information at admission to the mental unit, including admission diagnoses, and medicines prescribed at admission, as well as medicines identified during the admission process as in use by the patient. Patient’s shared electronic health record was online accessed to obtain the patient’s medication history from the preceding 6 months, including duration and dose and frequency. The ALERT® - LifeSscience Computing software used at the hospital emergency department was also accessed. The agreement between the sources of information was evaluated and the best possible medication history was obtained using the methods of comparison, combination, and confirmation between the different sources of information.
Then, the best possible medication history created was compared to the medicines prescribed to the patient at admission and discrepancies were recorded. Discrepancies were classified into clinically justified intentional discrepancies and discrepancies that needed clarification by the prescriber. After clarification by the prescriber, these discrepancies were classified according to their intentionality into unintentional discrepancies or undocumented intentional discrepancies.4 These discrepancies were also classified according to their typology: drug omission, drug substitution, drug dose change, drug administration route change, and drug dosage frequency change.4
Potential degrees of harm, or potential severity, were considered according to the Conceptual Framework of the International Safety Classification of Sick: none, mild, moderate, severe and death.10
Validation study
To compare the specific findings obtained in the psychiatric unit, a small validation study in the Internal Medicine department at the Coimbra University Hospital (CHUC) was conducted between October 2019 – December 2019. The part of the study was approved by the Ethics Committees of Hospital University Center of Coimbra (CHUC-133-19). All participants signed informed consent before their inclusion in the study.
The first 100 patients older than 18 years of age, admitted to the internal medicine department were considered for the validation study. Patients with communication difficulties not accompanied by an acquaintance caregiver, were excluded. All the methods and procedures used in the validation study were identical to those used in the main study.
RESULTS
Main study
In the main study at the psychiatric unit, 148 patients were admitted to the acute mental health unit, coming from different stages of transitions: 135 (91%) came from the psychiatric emergency service, 10 (7%) from the outpatient consultation, 2 (1.4%) from in-hospital transition, and 1 (0.7%) from the appointment, when there was no availability of beds. With a mean age of 54.6 years (SD=16.3), 75 (50.7%) were females. The most prevalent conditions among these patients were affective mood disorders (59 patients - 39.9%), followed by organic psychopathologies, including symptomatic mental disorders (42 patients - 28.4%). The presence of a caregiver was required to perform the interview in 109 (74%) of the interviews.
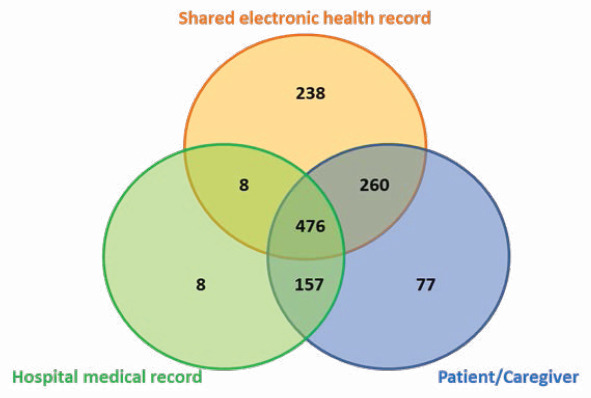
In total, 1,147 drugs were considered to obtain the best possible medication histories of included patients, but 77 drugs from interviews with patients/caregivers were excluded due to inconsistencies with other sources of information (Figure 1). Cardiovascular system drugs (13.9%) stood out, namely cardiac therapy, antihypertensives, diuretics, vasoprotectors, beta-blockers, calcium channel blockers, drugs that act on the renin-angiotensin system, and drugs with lipid-lowering properties. Followed by drugs of the alimentary tract and metabolism (10.6%), such as antacids and drugs for peptic ulcer and flatulence, as well as drugs used in diabetes; drugs acting on blood and hematopoietic organs (4.2%), such as antithrombotic and antiemetic preparations. Respiratory system drugs (1.75%) were also reported, namely used in airway obstruction; drugs for use in the sense organs (1.4%), such as ophthalmic products and general anti-infective for systemic use (1.0%), such as antibacterials and antivirals for systemic use.
Figure 1. Venn diagram with the contribution of different data sources for the construction of the best possible medication history.
When reconciling the best possible medication history with prescription list at admission, 560 clinically justified intentional discrepancies were identified and 359 discrepancies were sent to the prescriber for justification of the intentionality. Regarding the typology of the discrepancies: 302 (84.12%) “drug omission”, 20 (5.57%) “drug substitution”, 25 (6.96%) “drug dose change”, 12 (3.34%) “drug dosage frequency change”. There were no “drug administration route change” discrepancies. The distribution of these discrepancies between unintentional discrepancies and undocumented intentional discrepancies is presented in Figure 2.
Figure 2. Distribution of the discrepancies found in the study.
The severity analysis of the discrepancies revealed that, from the 302 drug omissions, six could not be evaluated due to incomplete information, 75 were mild, 80 moderate, 18 severe while 123 were classified as with no consequences. From the 20 drug substitution discrepancies 6 were mild, 11 moderate, and 3 severe. Among the 25 drug dose change discrepancies, 12 were mild, 7 moderate and 6 severe. Finally, from the 12 drug dosage frequency change discrepancies 2 were mild, 2 moderate, 2 severe and 6 considered as no relevant. Table 1 presents the potentially severe medication errors identified.
Table 1. Potentially severe medication errors according to the Anatomical Therapeutic Chemical classification (ATC 1 and 2).
| ATC 1 code | ATC 2 code | (n) | Potentially severe medication errors |
|---|---|---|---|
| A | A10 | 5 | Drug omission in diabetes mellitus |
| 2 | Drug substitution in diabetes mellitus | ||
| 4 | Drug dose change in diabetes mellitus | ||
| B | B01 | 2 | Drug omission - antithrombotic drug |
| 1 | Drug dosage frequency change – antithrombotic drug | ||
| C | C01 | 1 | Drug omission in cardiac therapy |
| 2 | Drug dose change in cardiac therapy | ||
| C03 | 1 | Drug omission – diuretic drug | |
| C07 | 1 | Drug dosage frequency change – beta blocker | |
| C09 | 2 | Drug omission – renin-angiotensin system | |
| 1 | Drug substitution – renin-angiotensin system | ||
| J | J01 | 7 | Drug omission – antibacterial drug for systemic use |
| Total | 29 | ||
Validation study
The 100 patients included in the validation study (33 women) have 77.0 years of age (SD 13.7) with 80 patients over 65 years. These patients had a mean of 7.7 (SD 3.0) medicines prescribed in ambulatory care and suffered 7.8 (SD 2.5) medical conditions.
After the reconciliation process, 791 discrepancies were identified, with 757 (95.7%) classified as intentional discrepancies. Among the 34 (4.3% of the total) unintentional discrepancies identified, 26 (76.5%) were “drug omissions”.
When applying the Conceptual Framework of the International Safety Classification of Sick, 4 (11.7%) were considered as potentially serious.
DISCUSSION
This study focused on the process of MR at admission of a psychiatric unit. The contribution of different sources of information to obtain the best possible medication history was analyzed, demonstrating the importance of the caregiver as a source of information to the psychiatric patient, the relevance of analyzing shared electronic health records in the 6 months preceding hospital admission, and the need to use the electronic medical record efficiently. The classification of discrepancies between the patient’s best possible medication history and the prescription at admission revealed that about one in three discrepancies required clarification by the prescriber and, of these, about one in five were intentional but undocumented. The rest were unintentional discrepancies, mainly drug omissions. The analysis of potential clinical impact of these discrepancies found 29 potentially severe discrepancies.
Conducting interviews requires the use of skills such as intensive listening, careful planning, and preparation for data collection.11 Lertxundi et al. described that clinical experience suggests that patients with mental conditions difficult medication error identification, because clinical interviews are difficult or even impossible in these patients.9 This is coincident with the fact that 74% patients in our study needed the caregiver as a source of information.
Our study combined health information technologies with the clinical interview, increasing efficiency in obtaining the best possible medication history. When analyzing the general contribution of the various sources of information, the hospital medical record presented the smallest contribution, with only 56% drugs (649/1147). According to Joon et al.,12 several drawbacks were found that may justify the fact that the electronic medical record presents a lower number of drugs as a source of information: inaccurate and incomplete histories, which delayed the assessment and treatment of patients. From these 649 drugs, only 199 (31%) corresponded to drugs used in acute and chronic situations prescribed by other specialists. Several authors suggested different timeframes for collecting medication information from sources supported by health information technology. Our previous work revealed that electronic health records represent the most comprehensive source of information to create the patient’s best possible medication history, with the most efficient data retrieval process considering a 6-month retrospective analysis. 3
The results we obtained about intentionality (19% undocumented intentional discrepancies) differ from other studies, such as 41% undocumented intentional discrepancies found by Cornish et al.,13 49% found by Poornima et al.,14 or 38% undocumented intentional discrepancies described by Graabæk et al.15 These differences may be associated to the characteristics of the clinical service chosen, the types of drugs used by the patients included in the sample, and the characteristics of the study.16 In any case, the existence of a significant number of undocumented intentional discrepancies indicates that the pharmacist, when performing reconciliation during admission, should never allow an undocumented intentional discrepancy identified during hospital admission to go unclarified, to prevent future medication errors.4
Our study found an important amount of unintentional discrepancies classified as drug omissions. According to Mekonnen et al. systematic review, unintentional drug omission discrepancies are the most common medication discrepancies found.17 Another systematic review on predicting factors for unintentional discrepancies found that unintentional drug omission discrepancies appear in several studies as the most frequent medication discrepancies.18 Finally, 29 severe omissions, substitutions, dose changes, and dosage frequency changes of drugs used in the treatment of other comorbidities were found. Our study also found an important number of severe omissions. This may reflect a major focus of psychiatrists on mental conditions, paying less attention to other patient comorbidities. 9,19 The results of the validation study reinforced the specific characteristics of the acute psychiatric unit, where the probability to identify unintentional discrepancies is much higher, with a substantially higher presence of drug omissions.
Limitations
Our study included patients admitted to an acute mental hospital unit. We cannot ensure that the results are similar to those that could be obtained with patients with other medical conditions or recruited in different hospital units in Portugal.
CONCLUSIONS
This study revealed that performing medication reconciliation in an acute psychiatric unit is feasible and can be helpful to identify medication errors and to promote patient safety during the transition of care.
Footnotes
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
FUNDING
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES. Brasília - Brasil. (Reg. num. BEX:11915/13-7).
Service Information Études Supérieures du Ministère de l’Enseignement Supérieur et de la Recherche. Luxembourg.
Contributor Information
Joelizy Oliveira, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra, Portugal. joelizy@hotmail.com.
Thaís Costa E Silva, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra, Portugal. thaaiscolombo@hotmail.com.
Ana C. Cabral, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Coimbra, Portugal. anacgcabral@gmail.com
Marta Lavrador, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Coimbra, Portugal. martalavrador@hotmail.com.
Filipe F. Almeida, Serviço de Psiquiatria. Centro Hospitalar e Universitário de Coimbra. Coimbra. Portugal. f.felix.almeida@gmail.com
António Macedo, Serviço de Psiquiatria. Centro Hospitalar e Universitário de Coimbra. Coimbra. Portugal. amacedo@ci.uc.pt.
Carlos Saraiva, Faculty of Medicine, University of Coimbra, Coimbra, Portugal. cbsaraiva@ci.uc.pt.
Fernando Fernandez-Llimos, Laboratory of Pharmacology, Faculty of Pharmacy, University of Porto. Porto (Portugal). fllimos@ff.up.pt.
M. Margarida Caramona, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Coimbra, Portugal. caramona@ci.uc.pt.
Isabel V. Figueiredo, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Coimbra, Portugal. isabel@ff.uc.pt
M. Margarida Castel-Branco, Pharmacology and Pharmaceutical Care Laboratory, Faculty of Pharmacy, University of Coimbra, Coimbra Institute for Clinical and Biomedical Research (iCBR), Coimbra, Portugal. mmcb@ci.uc.pt.
References
- 1.World Health Organization. Medication Safety in Transitions of Care. [accessed Feb 7, 2022]. Available at: https://www.who.int/patientsafety/medication-safety/TransitionOfCare.pdf?ua=1 .
- 2.Penm J, Vaillancourt R, Pouliot A. Defining and identifying concepts of medication reconciliation:An international pharmacy perspective. Res Social Adm Pharm. 2019;15(6):632–640. doi: 10.1016/j.sapharm.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira J, Cabral AC, Lavrador M, et al. Contribution of different patient information sources to create the best possible medication history. Acta Med Port. 2020;33(6):384–389. doi: 10.20344/amp.12082. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO);Action on Patient Safety (WHO) The High 5 Project:Standard Operating Protocol Assuring Medication Accuracy at Transitions in Care. [Accessed Feb 7, 2022]. Available at: https://www.who.int/patientsafety/implementation/solutions/high5s/h5s-sop.pdf?ua=1 .
- 5.NICE guideline. Medicines optimisation:the safe and effective use of medicines to enable the best possible outcomes. [accessed Feb 7, 2022]. Available at: www.nice.org.uk/guidance/ng5 .
- 6.Paton C, McIntyre S, Bhatti SF, et al. Medicines Reconciliation on Admission to Inpatient Psychiatric Care:Findings from a UK Quality Improvement Programme. Ther Adv Psychopharmacol. 2011;1(4):101–110. doi: 10.1177/2045125311417299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothschild JM, Mann K, Keohane CA, et al. Medication safety in a psychiatric hospital. Gen Hosp Psychiatry. 2007;29(2):156–162. doi: 10.1016/j.genhosppsych.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.MARQUIS investigators - Marquis implementation manual. A guide for medication reconciliation quality improvement. Society of Hospital Medicine. [accessed Feb 8, 2022]. Available at: https://www.hospitalmedicine.org/globalassets/clinical-topics/clinical-pdf/shm_medication_reconciliation_guide.pdf .
- 9.Lertxundi U, Corcostegui B, Prieto M, et al. Medication reconciliation in psychiatric hospitals:some reflections. J Pharm Pract Res. 2017;47(1):47–50. doi: 10.1002/jppr.1224. [DOI] [Google Scholar]
- 10.World Health Organization:Patient Safety (WHO) Conceptual Framework for the International Classification for Patient Safety Version 1.1. [accessed Feb 8, 2022]. Available at: https://www.who.int/patientsafety/taxonomy/icps_full_report.pdf .
- 11.Qu SQ, Dumay J. The qualitative research interview. Qualitative R)esearch in Account Manag. 2011;8(3):238–264. doi: 10.1108/11766091111162070. [DOI] [Google Scholar]
- 12.Hong CJ, Kaur MN, Farrokhyar F, et al. Accuracy and completeness of electronic medical records obtained from referring physicians in a Hamilton, Ontario, plastic surgery practice:A prospective feasibility study. Plast Surg (Oakv) 2015;23(1):48–50. doi: 10.4172/plastic-surgery.1000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–429. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 14.Poornima P, Reshma P, Ramakrishnan TV, et al. Medication reconciliation and medication error prevention in an emergency department of a tertiary care hospital. J Young Pharm. 2015;7(3):242–249. [Google Scholar]
- 15.Graabæk T, Terkildsen BG, Lauritsen KE, et al. Frequency of undocumented medication discrepancies in discharge letters after hospitalization of older patients:a clinical record review study. Ther Adv Drug Saf. 2019;10:204209↫ↄ49. doi: 10.1177/204209↫ↄ49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellström LM, Bondesson Å, Höglund P, et al. Errors in medication history at hospital admission:prevalence and predicting factors. BMC Clin Pharmacol. 2012;12:9. doi: 10.1186/1472-6904-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions:a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003. doi: 10.1136/bmjopen-2015-010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hias J, Van der Linden L, Spriet I, et al. Predictors for unintentional medication reconciliation discrepancies in preadmission medication:a systematic review. Eur J Clin Pharmacol. 2017;73(11):1355–1377. doi: 10.1007/s00228-017-2308-1. [DOI] [PubMed] [Google Scholar]
- 19.Kukreja S, Kalra G, Shah N, et al. Polypharmacy in psychiatry:A review. Mens Sana Monogr. 2013;11(1):82–99. doi: 10.4103/0973-1229.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]


