Abstract
Objective:
To investigate the co-infections with human rhinovirus (HRV) among patients with asthma exacerbation and COVID-19 in Jordan. Also, to determine the frequency of acute asthma exacerbation before and during the COVID-19 pandemic on a matched basis.
Methods:
The data of this prospective cohort research consisted of clinical variables. During the first visit, and after 14-days, nasopharyngeal swabs were taken and the quantitative polymerase chain reaction was performed for HRV and SARS-CoV-2 detection.
Results:
Forty-seven out of 175 (26.9%) COVID-19 adult cases have been diagnosed with asthma. The number of asthma exacerbations among the study participants was higher during 2021 than in 2020 (p=0.035). Most of the included asthmatic participants (61.7%) were only positive for SARS-CoV-2 and 38.3% were co-infected with HRV. The SARS-CoV-2 cycle threshold value was lower in samples infected with both viruses compared to samples infected with SARS-CoV-2 alone, p<0.005.
Conclusion:
Our findings indicate that HRV and SARS-CoV-2 were significantly more prevalent in asthma exacerbations than stable asthma. Thus, HRV and/or SARS-CoV-2 infections were potentially cofactors or contributors to the asthma exacerbation in this cohort. This is the first study, in Jordan, to investigate the HRV co-infection in COVID-19 asthmatic patients and HRV could be related with a higher severity of COVID-19.
Keywords: Asthma exacerbation, COVID-19, Rhinovirus, Co-infection
INTRODUCTION
Viral respiratory infections are an important cause of increased pulmonary symptoms in children and adults with pre-existing asthma.1,2 The prevalence and severity of viral exacerbations vary by patient age and season. Viral infection is responsible for around three-quarters of asthma exacerbations in children3 and more than 50% in adults.4,5
Human rhinovirus (HRV) is known as one of the most important and prevalent respiratory pathogens.6 HRV primarily affects the upper respiratory tract, causing common colds. The majority of persons with HRVs infection exhibit no or mild symptoms, as HRV typically causes a short self-limiting illness in immunocompetent hosts.7 However, HRV infections can have serious consequences in asthmatic immunocompromised and elderly patients8 and have also been linked to asthma as well as chronic obstructive pulmonary disease (COPD) exacerbations, wheezing, and pneumonia.9-11 As a result, strategies enabling the management and control of viral-induced events are a priority for preventing disease exacerbations.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that leads to coronavirus disease 2019 (COVID-19) is spreading widely among humans all over the globe.12 The symptoms of COVID-19 can vary from mild symptoms to severe pneumonia requiring mechanical ventilation, and the clinical characteristics of HRV infection might be similar to those of COVID-19.13 COVID-19 may be more severe in patients with chronic lung diseases, such as asthma.14 Biological factors may influence an asthmatic patient’s susceptibility to SARS-COV-2 or the severity of COVID-19.
In many countries, including the United States and Canada, asthma hospitalizations during viral infections, particularly with HRV, are the main cause of increased asthma hospitalization rates that are consistently found in late summer and early fall.15-17 These findings underscore the crucial role of viral infection in respiratory disease morbidity and mortality. Patients with asthma were at a higher risk of hospitalization and respiratory morbidity.18,19 During the 2009 H1N1 influenza pandemic, children with asthma were at an elevated risk of ICU stays and pneumonia.
A recent study in Australia20 and South-eastern Wisconsin21 have demonstrated that HRV is the highest frequent SARS-CoV-2 co-infection, representing 63.2% and 41.51%, respectively. In Turkey, HRV was reported in 14.2% and 10.0% of the COVID-19 cases in men and women, respectively.22 However, a study performed in Brazil reported a relatively normal frequency of HRV detection from March to December 2020.23
The susceptibility to asthma exacerbations were linked with asthma severity and co-morbidities in a recent study based in Jordan.24 However, no regional studies were performed to examine the role of HRV and COVID-19 in asthma exacerbations. Understanding these principles is critical for public health practice in terms of reducing seasonal respiratory virus outbreaks and informing response to potential respiratory virus pandemics.
We can learn more about the pathophysiology and clinical manifestations of COVID-19 and polymicrobial infection associated with COVID-19 by examining the link between COVID-19 and asthma and by looking at HRV co-infections in SARS-CoV-2 patients. The present research is therefore aimed, first, to determine the prevalence of HRV during and 14-days following asthma exacerbation in COVID-19 patients to specify if any may be implicated in asthma exacerbations. Second, to investigate the prevalence of acute asthma exacerbation before and during the COVID-19 pandemic on a matched basis and examine whether hand washing, face masks, and social/physical distancing during COVID-19 decreased the prevalence of asthma exacerbations in Jordanian patients.
METHODS
Study design and participants
This cohort study, a branch of a prospective observational project, took place over an 8-month period from March 10, 2021, to November 15, 2021. Based in Jordan, the participants in this study were with suspected COVID-19 aged 18 years and above with a history of breathing problems categorized as asthma (excluding severe persistent asthma) at the time of informed consent. Having more than 12% reversibility in FEV1 (forced expiratory volume in one second) and more than 200 ml increase in FEV1 following the administration of salbutamol (200 μg) as documented in the medical report was the used criteria to confirm the presence of asthma. Exclusion criteria included those under the age of 18 and those who had any other pulmonary disease, like COPD or cystic fibrosis. The study participants were gathered from a respiratory consultant clinic or were recommended by primary care workers.
The approval of the study was sought and gained from the Research Ethics Board of Applied Science Private University (ASU), Amman in Jordan (2021-PHA-35), in accordance with the Research Ethics and Governance Policies and Procedures at the Al-Rayhan Medical Center (2021-IRB-9-1). Informed consent was obtained from every participant.
Data collection
The case report form consisted of demographic and clinical variables such as gender, age, number of years since diagnosis of asthma, co-morbidity, previously inhaled corticosteroid (ICS) uses and other asthma medications, and asthma severity. The frequency of exacerbation, unscheduled physician visits, emergency room (ER)/hospitalization visits, treatment for exacerbation, hospital and intensive care unit (ICU) admission during three years (2019-2021) were reported. The clinical variables also include presenting complaints (for example, rhinorrhea, wheezing, cough and fever). Additionally, the extent of the application of precaution measures during the COVID-19 pandemic such as face masks, hand washing, and social or physical distancing were reported. The study included the proportion of patients who received the COVID-19 vaccine. Data security was maintained by encrypting data before transmission with the only date and subject ID as identifiers.
Acute asthma exacerbation is an episode of deteriorating asthma symptoms and lung function in patients with a documented asthma diagnosis, requiring a change in treatment. This exacerbation occurs as a result of a viral respiratory infection, noncompliance with controller medication, irritant or allergen exposure, or other triggers.25-27 Asthma severity was assessed using the Global Initiative for Asthma (GINA) standards based on the frequency of symptoms and controller treatments employed categorized as intermittent, mild persistent, moderate persistent, or severe persistent asthma.27
Clinical samples and detection of SARS-CoV-2 and HRV
During the first visit, the health care provider requested SARS-CoV-2 testing for those with suspected COVID-19. After 14 days, another nasopharyngeal swab was taken from those with confirmed COVID-19. The samples were processed by a research nurse. The samples were immediately transferred to the Regional Laboratory, in Amman, Jordan on dry ice and tested for SARS-CoV-2 according to a previous study.28 The fully automated total nucleic acid extraction was performed by BIOBASE Kit from 200-μl samples using the magnetic beads method (Biobase Biodustry (Shandong) co. ltd). Following extraction, the 100-μl eluted nucleic acid samples were used for SARS-CoV-2 detection. Then, the remaining volume of samples were transferred to 1.5-ml conical tubes and deeply frozen at -80°C for HRV retrospective detection at the end of the study.
SARS-CoV-2 RT-qPCR assay was performed in the TIANLONG: Real-Time PCR System with 48-well block equipment using LiliF™ COVID-19 Multi Real-time RT-PCR Kit depending on the CDC protocol. A SARS-CoV2 assay result was registered positive if the ribonuclease P (RNP) and either the N1 or N2 gene were identified, and negative if only the RNP gene was identified. Samples were tested for RNP, a gene that maintains essential cellular function, to identify PCR inhibition and evaluate the quality of the samples. HRV detection was performed according to a recent publication.29
The absolute quantification / second derivative maximum strategy was used to analyze the samples. A positive result was registered for Ct values less than or equal to 40 cycles, while a negative result was registered in the absence of a Ct value and for Ct values were greater than 40 cycles. A further criterion was a positive RNP value with Ct ≤ 37 cycles. In the case of RNP, Ct values greater than 37 cycles, the sample had been frozen, thawed, re-extracted, and tested again. Samples having RNP Ct values greater than 37 cycles for the second test had eliminated from the analysis because they were either poor quality or include a component that inhibits the PCR.
Statistical analysis
Data were analyzed using SPSS Statistics v.25 (IBM Corporation, New York, NY, USA). All data are presented as numbers (%) unless otherwise noted. The Shapiro-Wilk test for normality was used. The statistically significant p value was considered if it is less than 0.05.
Comparison of proportions of positive samples for the SARS-CoV-2 and HRV between the exacerbation (recruitment) and 14-days follow-up states was conducted with the McNemar’s test for related samples (the chi-squared distribution)30 with continuity correction.31
A Friedman test was applied to uncover the differences in the number of asthma exacerbations during 2019, 2020, and 2021 years. Pairwise comparisons were conducted with a Bonferroni correction for multiple comparisons.
Viral Ct values were assessed by ANCOVA and post-hoc analysis with a Bonferroni adjustment to determine any significant difference by viral type.
RESULTS
Study population and their clinical characteristics
Of the 223 individuals with suspected COVID-19, 212 (95.1%) continued participation in the study and provided nasopharyngeal swabs for SARS-CoV-2 detection. Overall, 175 (82.5%) positive cases of SARS-CoV-2 were reported, among them, 47 (26.9%) have been already diagnosed with asthma. The majority of the included asthmatic patients (n = 29, 61.7%) showed positivity only to SARS-CoV-2. Interestingly, 38.3% (n = 18) patients were infected with both SARS-CoV-2 and HRV. Two weeks following the initial real-time qPCR, only 3 samples (from 3 participants) remained positive to SARS-CoV-2 alone, one sample was positive to both SARS-CoV-2 and HRV, and three samples with HRV detection alone (1/3 was HRV-positive at recruitment and 2/3 were HRV-negative becoming positive after two weeks) (Figure 1).
Figure 1.
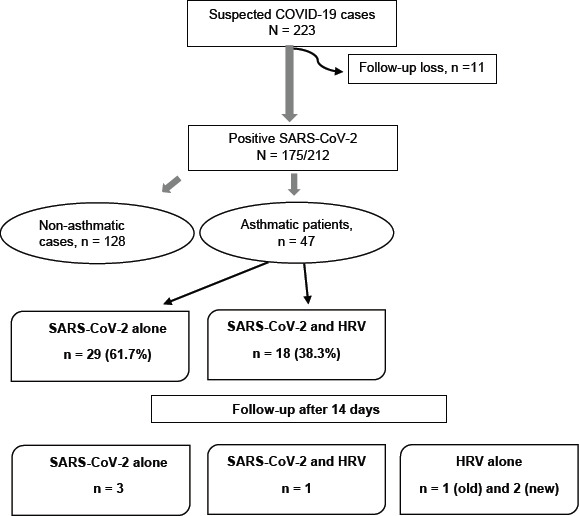
Flow chart of the study participants.
Table 1 represents the demographics and clinical data for the study participants. Among those 47 asthmatic patients, 17 (36.2%) were male and 30 (63.8%) were females. The mean age (SD) was 42.53 (±16.02) years. Overall, 8 (17.0%) had comorbidity; 4 (8.5%) diabetes mellitus and 4 (8.5%) cardiovascular disease. Around three quarters were non-smokers (76.6%). More than one-half of the included asthmatic patients were second-hand smokers (66.0%). The majority (72.3%) of participants did not take two doses of the COVID-19 vaccine at the time of recruitment. Family history of lung diseases (asthma, COPD), allergic rhinitis and eczema, was 15 (31.9%), 14 (29.8%), and 10 (21.3%), respectively. The ICS/LABA was the most frequent used medications (n = 43, 91.5%) followed by LTRA (n = 32, 68.1%). During the study period for every patient (day 0 until day 14), supplemental oxygen was used for 18 patients (38.3%), 10/18 (55.6%) were HRV-positive (p = 0.055) (Table 1). None of the study participants required hospitalization during the 14 days follow-up.
Table 1.
Demographics and clinical data for the study participants (N=47)
| Variables | N (%) or M (±SD) |
|---|---|
| Age | 42.53 (±16.02) |
| Years since diagnosis of asthma | 19.52 (±12.19) |
| Gender | |
| Male | 17 (36.2%) |
| Female | 30 (63.8%) |
| Smoking | |
| Current smoker | 8 (17.0%) |
| Previous smoking history | 3 (6.4%) |
| Non-smoker | 36 (76.6%) |
| Second-hand smoking | 31 (66.0%) |
| COVID-19 vaccine | |
| None | 34 (72.3%) |
| One dose | 0 (0%) |
| Two doses | 13 (27.7%) |
| Family history of lung diseases (asthma, COPD) | 15 (31.9%) |
| Family history of allergic rhinitis | 14 (29.8%) |
| Family history of eczema | 10 (21.3%) |
| Comorbidities | |
| Diabetes mellitus | 4 (8.5%) |
| Allergic rhinitis | 0 (0%) |
| Eczema | 0 (0%) |
| Immunocompromised | 0 (0%) |
| Cardiovascular disease | 4 (8.5%) |
| Maintenance medication | |
| ICS | 23 (48.9%) |
| ICS/LABA | 43 (91.5%) |
| SABA | 21 (44.7%) |
| LTRA | 32 (68.1%) |
| LAMA | 1 (2.1%) |
| OCS | 6 (12.8%) |
| Theophylline | 0 (0%) |
| Supplemental oxygen (0-14 days) | 18 (38.3%) 10/18 (0.56) HRV-positive (p = 0.055) |
| Magnesium sulfate | 0 (0%) |
Abbreviations: Inhaled corticosteroids (ICS), Inhaled corticosteroids /Long-acting beta-agonist (ICS) /(LABA), short-acting beta-agonist (SABA), Leukotriene receptor antagonists (LTRA), Long-acting muscarinic antagonists (LAMA), Oral corticosteroids (OCS)
The most frequently reported symptoms at the initial visit were shortness of breath and cough, 40 (85.1%) and 38 (80.9%), respectively. Although sputum production does not always accompany asthma exacerbation, the frequency of sputum production was high (n = 31, 66.0%).
Figure 2 shows the difference in the number of asthma exacerbations, ER visits, hospital and ICU admissions among the three years 2019, 2020, and 2021. To investigate if there were differences in the number of asthma exacerbations during 2019, 2020, 2021 years, the Friedman test was used. Pairwise comparisons were applied with a Bonferroni correction for multiple comparisons. The number of asthma exacerbations during the three years was significantly different, χ2(2) = 7.987, p = 0.018. Post hoc analysis uncovered a significant difference from 2020 (Median = 1.00) to 2021 (Median = 2.00) (p =0.035) (Figure 3). The mean number of asthma exacerbations was decreased from 2.19 in 2019 to 1.74 in 2020, however, this difference was statistically insignificant. Insignificant differences were observed in the ER visit, hospital, and ICU admissions among the three years.
Figure 2.
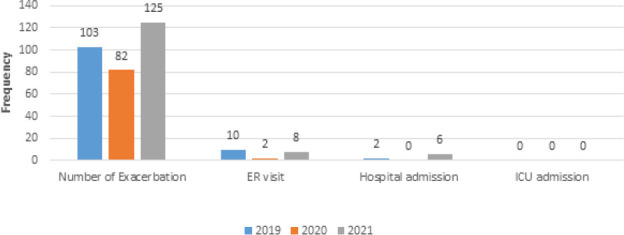
History of asthma exacerbation in the last three years among the study participants.
Figure 3.
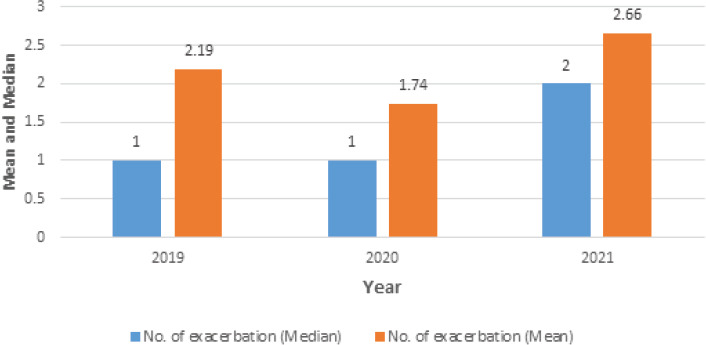
Mean and median of the number of asthma exacerbations during the last three years.
It is apparent that the face mask, hand washing, and social and physical distancing were not always implemented. The number of patients that wears face mask was 21 (44.7%). As well as, implementation of hand washing and social and physical distancing were reported in 17 (36.2%) and 22 (46.8%), respectively of the included asthmatic patients (Figure 4).
Figure 4.
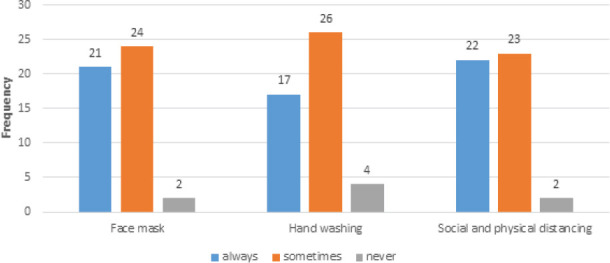
MPrecautions during COVID-19 pandemic.
SARS-CoV-2 and HRV detection
Forty-seven patients were investigated, on a matched basis, to compare the proportion of SARS-CoV-2 and HRV detection between the initial presentation (exacerbation) states and after 14 days.
Out of the 47 patients during the exacerbations, a total of 29 patients were positive only to SARS-CoV-2 (CoV-2-group) (61.7%) and three samples (6.4%) remained positive for SARS-CoV-2 at the follow-up (Table 3). The related samples McNemar’s test was performed to conclude whether a difference exists in the proportion of SARS-CoV-2 positivity at the initial presentation (exacerbation) states and after 14 days on a matched basis. The proportion of only SARS-CoV-2-positive (CoV-2-group) reduced at the follow-up to 6.4% of patients from the initial state value of 61.7%, a statistically significant difference, χ2(1) = 15.059, p < 0.005 (Table 3).
Table 3.
Proportions of positive samples for the SARS-CoV-2 and HRV between the exacerbation (recruitment) and 14-days follow-up on a matched basis (N = 47 patients)
| Groups | Exacerbation, n (%) | 14 days following the exacerbation, n (%) | P value (using McNemar’s test) |
|---|---|---|---|
| CoV-2-grou p | 29 (61.7%) | 3 (6.4%) | 0.001 |
| HRV- group | 0 (0%) | 3* (6.4%) | < 0.005 |
| CoV-2-HRV-group | 18 (38.3%) | 1 (2.1%) | < 0.005 |
| None | 0 (0%) | 40 (85.1%) | - |
| Total | 47 (100%) | 47 (100%) | - |
1/3 was HRV-positive at recruitment and 2/3 were HRV-negative becoming positive after two weeks.
CoV-2-HRV-group refers to those samples infected with both SARS-CoV-2 and HRV, CoV-2-group refers to samples infected only with SARS-CoV-2, HRV- group refers to sample infected only with HRV.
Table 2.
Presented symptoms at the time of initial visit
| Symptoms | N (%) |
|---|---|
| Shortness of breath | 40 (85.1%) |
| Cough | 38 (80.9%) |
| Wheezing | 22 (46.8%) |
| Chest tightness | 22 (46.8%) |
| Sputum production | 31 (66.0%) |
| Runny nose | 20 (42.6%) |
| Fever | 37 (78.7%) |
| Headache | 30(63.8%) |
| Myalgia | 20 (42.6%) |
| Diarrhea | 28 (59.6%) |
| Loss of smell and taste | 36 (76.6%) |
On recruitment, 38.3% (n = 18) of the patients were HRV-positive. Two weeks following the exacerbation, the number of HRV detection had decreased to 4/47 (8.5%) patients. This change was a consequence of 16/18 HRV-positive patients at the recruitment state becoming HRV-negative in 14 days following the initial sampling, and two HRV-negative became positive at the follow-up time. The related samples McNemar’s test30 with continuity correction31 was used to conclude whether a difference exists in the proportion of HRV-positive at the initial presentation (exacerbation) state and after 14 days. The proportion of HRV-positive decreased from the exacerbation state value of 38.3% to 8.5% at the follow-up, a statistically significant difference, χ2(1)= 5.263, p= 0.019 (Table 3).
To better understand the role of the respiratory virus in asthmatic patients, we investigated the difference in the SARS-CoV-2 Ct values, which refer to the viral load, of the SARS-CoV-2-positive samples. To determine if a significant difference exists between the Ct values of SARS-CoV-2 positive specimens “CoV-2-group” with the SARS-CoV-2 Ct values of those specimens positive to both SARS-CoV-2 and HRV “CoV-2-HRV-group”, analysis of covariance (ANCOVA) and post hoc analysis with a Bonferroni adjustment was applied.
The independent variable had two groups; CoV-2-group versus CoV-2-HRV-group. In the ANCOVA analysis, positive viral tests’ Ct values are the dependent variable, while the RNP assay Ct values, a function of the sample quality, is the covariate. Preliminary assumptions of ANCOVA were tested and no violations were uncovered. The mean Ct values and 95% CI for each group are shown in Table 4 (CoV-2-group versus CoV-2-HRV-group).
Table 4.
Descriptive statistics summary of the Ct values of CoV-2-group and CoV-2-HRV-group
| CoV-2-HRV-group (n=18 samples) | CoV-2-group (n=29 samples) | |
|---|---|---|
| Mean (SD) Viral Ct value | 21.48 (4.959) | 25.86 (4.200) |
| 95% CI LB | 19.08 | 24.91 |
| 95 % CI UB | 23.88 | 26.8 |
| Minimum value | 14.12 | 16.28 |
| Maximum value | 29.73 | 33.39 |
| Mean (SD) RNP Ct value | 23.91 (2.608) | 23.55 (3.201) |
| 95% CI LB | 22.59 | 22.83 |
| 95% CI UB | 25.23 | 24.27 |
| Minimum value | 20.85 | 20.23 |
| Maximum value | 28.04 | 32.49 |
| ANCOVA adjusted mean Viral Ct value | 21.23 | 25.91 |
| 95% CI LB | 19.65 | 25.16 |
| 95% CI UB | 22.81 | 26.67 |
CI: confidence interval; LB: lower bound; UB: upper bound
Following adjustment for the RNP Ct values, significant difference was observed between the CoV-2- and CoV-2-HRV-groups, with the CoV-2-HRV-group producing (4.69) lower Ct values than the CoV-2-group, F (1, 34) = 28.22, p < 0.005, partial η2= 0.233.
By performing the standard curve of the four concentrations of the standard nucleic acid with five replicates, the mean amplification efficiency (E) of the absolute quantification was 96.37%. The mean (± SD) of the correlation coefficient (r2) was −0.996 (± 0.001).
DISCUSSION
This is the first study, based in Jordan, to investigate the HRV co-infection in COVID-19 asthmatic patients. The main purpose of this study was to investigate the co-infections with HRV among COVID-19 asthmatic patients to determine if any may be implicated in asthma exacerbation and to determine the frequency of asthma exacerbation before and during the COVID-19 pandemic. To better understand the polymicrobial infection associated with COVID-19, we examined HRV co-infection in SARS-CoV-2 individuals. Our study has thoroughly examined the HRV and SARS-CoV-2 roles at asthma exacerbation compared with the stable (14-days following exacerbation) states using nasopharyngeal swab specimens with a particular reference to the Ct values of the SARS-CoV-2.
Evidence from this research displays that HRV and SARS-CoV-2 infections were significantly higher in asthma exacerbation compared with the stable (14-days following exacerbation) states (p < 0.05). These findings suggest that HRV and/or SARS-CoV-2 infections, when presented in this Jordanian patients’ cohort, were potentially cofactors or contributors to the asthma exacerbation. At present, not much is known about how the respiratory virus, particularly HRV and SARS-CoV-2, infection develops and progresses during asthma exacerbation episodes. Despite this knowledge gap, it is clear that asthma progress is a consequence of exacerbations, and HRV and SARS-CoV-2 might be associated with these episodes. The mechanisms that cause some asthma patients to be especially vulnerable to exacerbation episodes are not clear. Collecting more information about the role of the viruses, including HRV and SARS-CoV-2, in asthma exacerbation on a wider scale using molecular methods may aid the development of targeted therapies, potentially reducing the exacerbation severity. This study may suggest the need for effective prevention or therapy for those respiratory viruses. Effective prophylaxis and treatment for the respiratory virus could decrease the length of their infections. Economically, this could decrease the cost of long hospital stays and other healthcare expenses.
The results of the ANCOVA test illustrate that the SARS-CoV-2 Ct values of those samples positive to both SARS-CoV-2 and HRV were smaller than that recorded for the samples positive only to SARS-CoV-2 which shows that HRV might be associated with the greater levels of SARS-CoV-2 shedding than noticed in those samples without HRV. Although the reason for this is unclear and needs further investigation, the results indicate that HRV possibly makes SARS-CoV-2 more able to overcome the immunity in these asthmatic patients. However, this finding has to be confirmed using a large-scale study. Even though it is yet not clear if viral co-infection is associated with more severe COVID-19, this study indicates that detection of HRV with SARS-CoV-2 was associated with a higher tendency to use supplemental oxygen, although this association was not, but close to, statistically significant (p = 0.055).
Since the pandemic outset, there have been conflicting views on patients’ infectiousness, as a positive RT-PCR test cannot determine whether or not a patient is infectious or when the infection began. In the majority of cases, virus load rapidly decreases following symptom onset, and most patients will no longer have SARS-CoV-2 RNA detected in nasopharyngeal swabs after a median of 14-25 days. However, nasopharyngeal swabs and lower respiratory tract specimens from elderly, critically ill or immunocompromised patients can still be positive for SARSCoV-2 RNA detection several weeks after symptom onset.32 Overall, this study specimens have low Ct values, indicating a high virus load. The question was raised as to whether this is infectious virus material or only viral genomic material. Thereby, simply detecting the presence of a viral genome is insufficient. Therefore, some scientists believe that Ct values are an imprecise and low reproducible, laboratory-dependent, and ineffective measure. Several studies, that attempted to quantify the relationship between the Ct and the likelihood of culturing live viruses, indicating that the probability of recovering a live virus from specimens with a Ct higher than 34 is low. While RT-qPCR was critical in the response to COVID-19, a study based on three clinical laboratories in UK, Belgium and the Republic of Korea, recommend that the Ct values not be used to establish clinical cut-offs or diagnostic performance targets due to their low reproducibility across laboratories; calibrated copy-based units offer more reproducible alternatives.33
Defining a general Ct cut-off is a significant challenge. Additionally, infectivity and RT-PCR Ct cut-off values are dependent on the time interval between sampling and analysis. Obviously, prior to analysis, samples are transported and stored at 4 °C for several hours. WHO recommends storing samples at 4 °C for up to 5 days, and the use of storage buffers is believed to preserve the samples quality.34 Defining RT-PCR cut-off levels is furthermore necessary for evaluating the performance of other SARS−CoV2 tests compared to RT-PCR.35
Although this study was closed around 45 days before the end of the 2021 year, the number of asthma exacerbations of the study participants is significantly higher than in 2020. These findings probably reflect the loosening of physical distancing measures of the study participants during the 2021 year or these measures failed to prevent the spread of HRV. Despite our initial hypothesis that hand washing, face masks, and social/physical distancing during COVID-19 supposed to reduce the incidence of other respiratory infections related to asthma exacerbations, the decrease in the mean number of exacerbations between 2019 and 2020 was statistically insignificant. Changes in the prevalence of asthma exacerbations and circulating respiratory viruses may be a valuable indicator of the effectiveness of continuing efforts, such as physical distancing restrictions. Another possible justification for this finding could be the role of other respiratory viruses not studied in our study.
In spring 2020, anti-SARS-CoV-2 measures in Finland temporarily halted the spread of HRV.36 HRV incidence quickly returned to normal levels following the lifting of the COVID-19 restrictions. These loosened social restrictions prevented respiratory syncytial virus (RSV) and influenza seasons but did not prevent HRV propagation.36 In Germany, an almost similar pattern of HRV prevalence was observed in its relation with anti-SARS-CoV-2 measures.37 South Korea’s widespread use of social distance throughout the COVID-19 pandemic slowed the spread of common respiratory virus infections.38 In 2020, HRV levels in Korea increased significantly compared to 2019 levels and were negatively related with the confirmed COVID-19 cases in 2020.39 This could be because the virus is resistant to environmental conditions due to its non-enveloped nature, as well as the protracted period of viral shedding from patients.
In this study, based in Jordan, 38.3% of patients were positive for both SARS-CoV-2 and HRV. In accordance with the present finding, a recent study in Australia20 and South-eastern Wisconsin21 have demonstrated that HRV is the highest frequent SARS-CoV-2 co-infection, representing 63.2% and 41.51%, respectively. The most common co-infection was HRV also in Korea.40 HRV infection was the most frequently encountered additional pathogen in 116 SARS-COV-2-positive specimens, according to Kim and co-workers’ study based in the United States.41 In Spain, HRV was the most common detected virus in adult (n=18/20, 90.0%) and co-infection with COVID-19 (n=15/20, 83.3%). Also, HRV was predominant in children (n=120/157, 76.4%) but co-infection with SARS-CoV-2 was less frequent (n = 26, 21.7%).42 In Italy, HRV was reported in 130/583 (22.3%).43 In Turkey, HRV was reported in 14.2% and 10.0% of the COVID-19 cases in men and women, respectively.22 However, a study performed in Brazil reported a relatively normal frequency of HRV detection from March to December 2020.23 After the influenza virus, HRV was the most detected virus in Canada.44
The reasons underlying these findings are unknown and could be due to a combination of variables. It is likely that stricter controls on the spread of SARS-CoV-2 resulted in significant reductions in HRV transmission as well. However, the role of SARS-CoV-2-mediated viral displacement and interference is unknown. Furthermore, the hypothesis of a rebound in HRV levels following the relaxation of lockdown measures merits additional examination. It is unknown whether the absence of exposure to non-SARS-CoV-2 respiratory viruses throughout the COVID-19 pandemic resulted in bigger epidemics of other respiratory viral infections in Jordan following the relaxation of existing public health restrictions. Understanding these principles is critical for public health practice in terms of reducing seasonal respiratory virus outbreaks and informing response to potential respiratory virus pandemics.
While HRV infection is typically associated with minor and limited consequences in immunocompetent hosts, it has been linked to bronchiolitis in paediatrics, pneumonia in those with immunosuppression states, and exacerbation of pre-existing asthma or COPD in immunocompromised individuals.10,11
Infectious respiratory viruses attack the airway epithelium and interact with host factors to cause an inflammatory response that can lead to wheezing.3,45-47 The virus-induced immune response may modulate host responses to various microorganisms, infections, allergens, stress, and pollution, leading to wheeze and asthma exacerbations in vulnerable individuals. Patients with asthma had a longer duration of symptoms and a more severe viral-induced respiratory disease than healthy controls.46
In many countries, including the United States and Canada, asthma hospitalizations during viral infections, particularly with HRV, are the main cause of increased asthma hospitalization rates that are consistently found in late summer and early fall.15-17 Tan and co-worker reported a viral detection rate of 59% in the ICU patients admitted for severe asthma.1 The most frequently encountered virus in near-fatal attacks was HRV, and co-infection with adenovirus was detected. These findings underscore the crucial role of viral infection in respiratory disease morbidity and mortality. Children and adults with asthma were at a higher risk of hospitalization and respiratory morbidity.18,19 During the 2009 H1N1 influenza pandemic, children with asthma were at an elevated risk of ICU stays and pneumonia.
It is challenging to distinguish symptoms between asthma exacerbations and COVID-19. Clinicians often administer antibiotics to people who have asthma exacerbations ‘simply to be safe,’ and this practice should be avoided.48,49 While fever may aid in the differentiation of these two illnesses in young and elderly populations, caution should be maintained because fever may be present in other virus-induced asthma exacerbations. Additionally, headache, myalgia, pharyngitis, runny nose, loss of smell and taste, nausea, vomiting, and diarrhoea may help differentiate COVID-19 from asthma. Additionally, travel history, intimate contact with someone infected with COVID-19, and a child’s absence of prior atopic history can aid in the differentiation of asthma exacerbation from COVID-19. COVID-19 testing is indicated for any asthmatic patient experiencing increasing cough or shortness of breath.50
Taken together, the findings of this study, as well as recent research,51 demonstrate significant alterations in the circulation of respiratory pathogens other than SARS-CoV-2, including HRV. The long-term effects of these alterations are unknown, but efforts aimed at mitigating the spread of COVID 19 are predicted to continue to affect many respiratory infections. Understanding current viral circulation enables clinicians to identify the most likely infections that may affect patients, and ongoing surveillance is critical for guiding mitigation methods.
HRV is a collection of genetically heterogeneous non-enveloped, positive-stranded RNA viruses divided into three serotypes: HRV-A, HRV -B and HRV -C.52 It is well established that HRV is highly diverse, with HRV-B infection typically resulting in mild or asymptomatic symptoms, whereas HRV-A or HRV-C infection is typically associated with more severe illness or exacerbation of asthma and hospitalizations.53,54 Therefore, future work will aim to investigate the prevalence of different HRV species and their role in respiratory diseases in Jordan.
With such a large population of asthma patients, it is critical to define the risk of SARS-CoV-2 infection and severity in asthma, which is a major concern during this potentially extended COVID-19 pandemic. A greater knowledge of the link between COVID-19 and asthma may help shed light on the aetiology and clinical characteristics of COVID-19.
CONCLUSION
Our findings indicate that HRV and SARS-CoV-2 were significantly more prevalent in asthma exacerbations than stable asthma. These findings indicate that HRV and/or SARS-CoV-2 infections were potentially cofactors or contributors to the asthma exacerbation in this Jordanian cohort. This is the first study, based in Jordan, to investigate the HRV co-infection in COVID-19 asthmatic patients. HRV could be associated with increasing COVID-19 severity. However, a multi-center research is required.
CONFLICTS OF INTEREST
All Authors declare no conflicts of interest related to this article.
ABBREVIATIONS
COPD Chronic obstructive pulmonary disease
COVID-19 Coronavirus disease 2019
FEV1 Forced expiratory volume in one second
HRV Human Rhinovirus
SARS-CoV-2 Severe acute respiratory syndrome
coronavirus 2
ACKNOWLEDGMENTS
A very special thank you goes out to the respiratory consultant clinic members and Alrayhan Medical Center and the regional molecular laboratory in Amman, Jordan, and to the respiratory consultant Dr. Mamoun Zihlif.
Contributor Information
Abdullah Al-Dulaimi, MSc. Department of Clinical Pharmacy and Therapeutics, Faculty of Pharmacy, Applied Science Private University, Amman 11931-166, Jordan. abedhatem97@gmail.com.
Ahmad R. Alsayed, PhD, MSc, PharmD. Department of Clinical Pharmacy and Therapeutics, Faculty of Pharmacy, Applied Science Private University, Amman 11931-166, Jordan. a_alsayed@asu.edu.jo, a.alsayed.phd@gmail.com
Mohammed Al Maqbali, PhD. Department of Nursing Midwifery and Health, Northumbria University, Newcastle-Upon-Tyne, UK. mohammed.maqbali@northumbria.ac.uk.
Malek Zihlif, PhD. Department of Pharmacology, School of Medicine, The University of Jordan, Amman, Jordan. m.zihlif@ju.edu.jo.
FUNDING
This work was supported by the Applied Science Private University [grant number DRGS-2020-2021-7].
HIGHLIGHTS
The number of asthma exacerbations was higher during 2021 than in 2020 in Jordan.
SARS-CoV-2 and HRV were more in asthma exacerbations than in stable states.
HRV SARS-CoV-2 and/or HRV infections are cofactors in asthma exacerbation.
This is the first study, in Jordan, to examine the HRV and COVID-19 in asthma.
HRV could be associated with the more severe form of COVID-19.
References
- 1.Tan WC, Xiang X, Qiu D, et al. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med. 2003;115(4):272–277. doi: 10.1016/s0002-9343(03)00353-x. [DOI] [PubMed] [Google Scholar]
- 2.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010;10(2):133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Bmj. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Bmj. 1993;307(6910):982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy JL, Pham S, Borish L. Rhinovirus and Asthma Exacerbations. Immunol Allergy Clin North Am. 2019;39(3):335–344. doi: 10.1016/j.iac.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Self WH, Williams DJ, Zhu Y, et al. Respiratory Viral Detection in Children and Adults:Comparing Asymptomatic Controls and Patients with Community-Acquired Pneumonia. J Infect Dis. 2016;213(4):584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles CH, Yelmene M, Luo GX. Recent advances in rhinovirus therapeutics. Curr Drug Targets Infect Disord. 2004;4(4):331–337. doi: 10.2174/1568005043340551. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos N, Johnston S. The rhinovirus--not such an innocent? Qjm. 2001;94(1):1–3. doi: 10.1093/qjmed/94.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Brzezińska-Pawłowska OE, Rydzewska AD, Łuczyńska M, et al. Environmental factors affecting seasonality of ambulance emergency service visits for exacerbations of asthma and COPD. The Journal of asthma:official journal of the Association for the Care of Asthma. 2016;53(2):139–145. doi: 10.3109/02770903.2015.1075547. [DOI] [PubMed] [Google Scholar]
- 11.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140(4):895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie JS, Smith DW. COVID-19:a novel zoonotic disease caused by a coronavirus from China:what we know and what we don't. Microbiol Aust. 2020:Ma20013. doi: 10.1071/MA20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe Y, Kamioka Y, Seki M. Rhinovirus-Infected Patients in the COVID-19 Pandemic Period. Infect Drug Resist. 2021;14:609–611. doi: 10.2147/IDR.S300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates DV, Baker-Anderson M, Sizto R. Asthma attack periodicity:a study of hospital emergency visits in Vancouver. Environ Res. 1990;51(1):51–70. doi: 10.1016/s0013-9351(05)80182-3. [DOI] [PubMed] [Google Scholar]
- 16.Weiss KB. Seasonal trends in US asthma hospitalizations and mortality. Jama. 1990;263(17):2323–2328. [PubMed] [Google Scholar]
- 17.Kimes D, Levine E, Timmins S, et al. Temporal dynamics of emergency department and hospital admissions of pediatric asthmatics. Environ Res. 2004;94(1):7–17. doi: 10.1016/s0013-9351(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 18.Dawood FS, Kamimoto L, D'Mello TA, et al. Children with asthma hospitalized with seasonal or pandemic influenza, 2003-2009. Pediatrics. 2011;128(1):e27–32. doi: 10.1542/peds.2010-3343. [DOI] [PubMed] [Google Scholar]
- 19.Merckx J, Ducharme FM, Martineau C, et al. Respiratory Viruses and Treatment Failure in Children with Asthma Exacerbation. Pediatrics. 2018;142(1) doi: 10.1542/peds.2017-4105. [DOI] [PubMed] [Google Scholar]
- 20.Marriott D, Beresford R, Mirdad F, et al. Concomitant Marked Decline in Prevalence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Other Respiratory Viruses Among Symptomatic Patients Following Public Health Interventions in Australia:Data from St Vincent's Hospital and Associated Screening Clinics, Sydney, NSW. Clin Infect Dis. 2021;72(10):e649–e651. doi: 10.1093/cid/ciaa1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott SJ, Pfotenhauer B, Weiner JJ, et al. Respiratory Pathogen Coinfections in SARS-CoV-2-Positive Patients in Southeastern Wisconsin:A Retrospective Analysis. Microbiol Spectr. 2021;9(2):e0083121. doi: 10.1128/Spectrum.00831-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Çelebi Ö, Çelebi D. Viral Respiratory Tract Pathogens During the COVID-a19 Pandemic. Eurasian J Med. 2021;53(2):123–126. doi: 10.5152/eurasianjmed.2021.20459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisen AKA, Gularte JS, Demoliner M, et al. Low circulation of Influenza A and coinfection with SARS-CoV-2 among other respiratory viruses during the COVID-19 pandemic in a region of southern Brazil. J Med Virol. 2021;93(7):4392–4398. doi: 10.1002/jmv.26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altawalbeh SM, Manoon NA, Ababneh MA, et al. Respiratory tract infection-induced asthma exacerbations in adults with asthma:assessing predictors and outcomes. The Journal of asthma:official journal of the Association for the Care of Asthma. 2020;57(3):231–240. doi: 10.1080/02770903.2019.1568454. [DOI] [PubMed] [Google Scholar]
- 25.BTS/SIGN. British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192. [PubMed] [Google Scholar]
- 26.NAEPP. 2020 Focused Updates to the Asthma Management Guidelines:A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. 2020 doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.27. GINA. Global Initiative for Asthma. 2020; GINA. Global Initiative for Asthma. 2020 [Google Scholar]
- 28.Alsayed A, Talib W, Al-Dulaimi A, et al. The first detection of Pneumocystis jirovecii in asthmatic patients post COVID-19 in Jordan. Bosn J of Basic Med Sci. 2022 May 17; doi: 10.17305/bjbms.2022.7335. [cited 2022 May 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alsayed A, Al-Doori A, Al-Dulaimi A, et al. Influences of bovine colostrum on nasal swab microbiome and viral upper respiratory tract infections–A case report. Respiratory medicine case reports. 2020;31:101189. doi: 10.1016/j.rmcr.2020.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 31.Edwards AL. Note on the “correction for continuity”in testing the significance of the difference between correlated proportions. Psychometrika. 1948;13:185–187. doi: 10.1007/BF02289261. [DOI] [PubMed] [Google Scholar]
- 32.Omar S, Bartz C, Becker S, et al. Duration of SARS-CoV-2 RNA detection in COVID-19 patients in home isolation, Rhineland-Palatinate, Germany, 2020 - an interval-censored survival analysis. Euro Surveill. 2020;25(30) doi: 10.2807/1560-7917.ES.2020.25.30.2001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans D, Cowen S, Kammel M, et al. The dangers of using Cq to quantify nucleic acid in biological samples;a lesson from COVID-19. Clin Chem. 2021;68(1):153–162. doi: 10.1093/clinchem/hvab219. [DOI] [PubMed] [Google Scholar]
- 34.Engelmann I, Alidjinou EK, Ogiez J, et al. Preanalytical issues and cycle threshold values in SARS-CoV-2 real-time RT-PCR testing:should test results include these? 2021;6(10):6528–6536. doi: 10.1021/acsomega.1c00166. https://doi.org/10.1021/acsomega.1c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelmann I, Alidjinou EK, Lazrek M, et al. Comparison of two commercial quantitative PCR assays and correlation with the first WHO International Standard for human CMV. Diagn Microbiol Infect Dis. 2018;91(1):27–33. doi: 10.1016/j.diagmicrobio.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Kuitunen I, Artama M, Haapanen M, Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-A nationwide register study in Finland. J Med Virol. 2021;93(10):6063–6067. doi: 10.1002/jmv.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitanovski S, Horemheb-Rubio G, Adams O, et al. Rhinovirus prevalence as indicator for efficacy of measures against SARS-CoV-2. BMC Public Health. 2021;21(1):1178. doi: 10.1186/s12889-021-11178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MC, Kweon OJ, Lim YK, et al. Impact of social distancing on the spread of common respiratory viruses during the coronavirus disease outbreak. PLoS One. 2021;16(6):e0252963. doi: 10.1371/journal.pone.0252963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HM, Lee EJ, Lee NJ, et al. Impact of coronavirus disease 2019 on respiratory surveillance and explanation of high detection rate of human rhinovirus during the pandemic in the Republic of Korea. Influenza Other Respir Viruses. 2021;15(6):721–731. doi: 10.1111/irv.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roh KH, Kim YK, Kim SW, et al. Coinfections with Respiratory Pathogens among COVID-19 Patients in Korea. Can J Infect Dis Med Microbiol. 2021;2021:6651045. doi: 10.1155/2021/6651045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Quinn J, Pinsky B, et al. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. Jama. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brotons P, Jordan I, Bassat Q, et al. The Positive Rhinovirus/Enterovirus Detection and SARS-CoV-2 Persistence beyond the Acute Infection Phase:An Intra-Household Surveillance Study. Viruses. 2021;13(8):1598. doi: 10.3390/v13081598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderaro A, De Conto F, Buttrini M, et al. Human respiratory viruses, including SARS-CoV-2, circulating in the winter season 2019-2020 in Parma, Northern Italy. Int J Infect Dis. 2021;102:79–84. doi: 10.1016/j.ijid.2020.09.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groves HE, Piché-Renaud PP, Peci A, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada:A population-based study. Lancet Reg Health Am. 2021;1:100015. doi: 10.1016/j.lana.2021.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis:new rationales for asthma prevention and treatment. Nat Med. 2012;18(5):726–735. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 46.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 47.Gern JE. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2008;27:S97–103. doi: 10.1097/01.inf.0000108196.46134.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bousquet J, Jutel M, Akdis CA, et al. ARIA-EAACI statement on asthma and COVID-19 (June 2, 2020) Allergy. 2021;76(3):689–697. doi: 10.1111/all.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alsayed A, El Hajji FD, Al-Najjar MA, et al. Patterns of antibiotic use, knowledge, and perceptions among different population categories:A comprehensive study based in Arabic countries. Saudi Pharmaceutical Journal. 2022;30(3):317–328. doi: 10.1016/j.jsps.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrams EM, Szefler SJ. Managing Asthma during Coronavirus Disease-2019:An Example for Other Chronic Conditions in Children and Adolescents. J Pediatr. 2020;222:221–226. doi: 10.1016/j.jpeds.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodgers L, Sheppard M, Smith A, et al. Changes in Seasonal Respiratory Illnesses in the United States During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin Infect Dis. 2021;73:S110–s117. doi: 10.1093/cid/ciab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bochkov YA, Watters K, Ashraf S, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci USA. 2015;112(17):5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee WM, Lemanske RF, Evans MD, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox DW, Bizzintino J, Ferrari G, et al. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188(11):1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


