Abstract
Bacterial biomass production is often estimated from incorporation of radioactively labeled leucine into protein, in both oxic and anoxic waters and sediments. However, the validity of the method in anoxic environments has so far not been tested. We compared the leucine incorporation of bacterial assemblages growing in oxic and anoxic waters from three lakes differing in nutrient and humic contents. The method was modified to avoid O2 contamination by performing the incubation in syringes. Isotope saturation levels in oxic and anoxic waters were determined, and leucine incorporation rates were compared to microscopically observed bacterial growth. Finally, we evaluated the effects of O2 contamination during incubation with leucine, as well as the potential effects of a headspace in the incubation vessel. Isotope saturation occurred at a leucine concentration of above about 50 nM in both oxic and anoxic waters from all three lakes. Leucine incorporation rates were linearly correlated to observed growth, and there was no significant difference between oxic and anoxic conditions. O2 contamination of anoxic water during 1-h incubations with leucine had no detectable impact on the incorporation rate, while a headspace in the incubation vessel caused leucine incorporation to increase in both anoxic and O2-contaminated samples. The results indicate that the leucine incorporation method relates equally to bacterial growth rates under oxic and anoxic conditions and that incubation should be performed without a headspace.
Bacterial production in aquatic systems is commonly estimated from the rate of incorporation of radioactively labeled leucine into bacterial protein (10, 13). This method has been extensively tested in oxic waters (12, 14, 30, 31). It is fast and technically simple and has gradually become very popular compared to methods such as those based on the frequency of dividing cells (9) or incorporation of radioactively labeled thymidine into bacterial DNA (7, 20).
Bacterial production methods cannot be applied to anoxic environments without caution. It has been shown that several important metabolic groups of bacteria in anoxic environments do not incorporate thymidine (8, 11, 24–26, 34). In field studies, the thymidine method frequently yields lower estimates of bacterial production than the leucine method in anoxic environments (18, 33). A proposed reason, apart from differential uptake, is that thymidine to a large extent is incorporated into proteins instead of DNA under anoxic conditions (18, 33). The leucine method has been used in anoxic water columns (2, 18) and has recently also been applied to sediment bacteria (5, 6, 17, 33), but no systematic comparison of how it performs under different oxygen conditions has been presented.
In addition to potential metabolic constraints on leucine incorporation into anaerobic bacteria, there is also the issue of sensitivity to O2 contamination. It seems to be very difficult to create anoxic conditions during the leucine incubation when following the protocols developed for oxic conditions (15, 31), and any headspace in the incubation vessels containing O2 may corrupt the samples.
The purpose of this study was to assess the applicability of the leucine method to both anoxic and oxic conditions in freshwater. We developed a modified protocol to avoid O2 contamination, assessed isotope saturation levels of bacterial assemblages developing in oxic and anoxic waters, and simultaneously measured bacterial growth by microscopy and by the [3H]leucine incorporation method in similar lake water with or without the presence of O2. In addition, we evaluated the effect of short-term O2 contamination during [3H]leucine incubations of anoxic samples.
MATERIALS AND METHODS
Bacterial regrowth cultures.
Bacterial production was measured in cultures containing natural bacterial assemblages growing in filtered water, which was retrieved from three different lakes during summer stratification in 1999. The lakes were chosen to be different in terms of nutrient and dissolved organic carbon concentrations. In situ average water column total phosphorous concentrations were 15, 52, and 38 mg liter−1, and dissolved organic carbon concentrations were 19.8, 17.9, and 9.4 mg liter−1, in Lillsjön, Mårn, and Illersjön, respectively. All lakes had anoxic hypolimnetic water. Water column composite samples were collected in 10-liter polyethylene carboys using a submersible pump (Amazon 10; Awimex International). The water was then transported to the laboratory and stored at 2°C until used in the experiments. In the isotope dilution experiments the water was transferred directly into infusion bottles (see below) without nutrient addition and filtering, and isotope dilution experiments were performed after 1 week at 15°C. The water for other experiments was spiked with phosphate (Na2HPO4) and ammonium (NH4Cl) to make the organic carbon the limiting resource (additions gave minimal final concentrations of 350 μg of P liter−1 and 1.5 mg of N liter−1, respectively) and filtered through a glass fiber filter (Gelman A/E) to reduce bacterial numbers and remove bacterivores. As a first step to remove O2, the water was then purged with N2 (N52; Air Liquide) (<1 ppm of O2) for 1 h. Thereafter, the water was transferred to 330-ml infusion bottles (Laboratory Service Provider B.V.; 250 ml of water per bottle) with a dispenser pump while continuously flushing the bottles with N2. Directly after transfer the bottles were capped with 17-mm-thick butyl rubber stoppers, which were tested to be gas tight after being pierced more than 100 times with syringe needles (Microlance, 0.6 by 25 mm; Becton-Dickinson). The stoppers were secured with aluminum caps.
In a second oxygen removal step, the capped bottles for the anoxic treatments were attached via syringe needles to a gas exchange device with which the bottles were evacuated until vigorous bubble formation occurred upon tapping of the bottles with a plastic rod. Evacuation was followed by N2 addition to about 2 atm of pressure. The cycle of evacuation and N2 addition was repeated at least nine times. The purging and the gas exchange cycles removed oxygen to below the levels of detection by both Winkler titration and an oxygen electrode (Orion model 835; detection limit of 0.005 mg liter−1) with little disturbance of the organic matter and bacteria compared to alternative oxygen removal methods such as boiling at 100°C or addition of reducing agents such as sulfide. After the second removal step, excess N2 was released from the anoxic bottles through a syringe needle until ambient air pressure was reached. To ensure that bottles for oxic treatments were oxygenated, they were subjected to gas exchange cycles with air instead of N2.
During the experiments, samples for measuring leucine incorporation, bacterial biomass, and biovolume or for measuring oxygen content were withdrawn from the bottles with plastic syringes (see below for details). To maintain atmospheric pressure within the bottles, air or N2 corresponding to the sample volume was added upon sampling in the oxic and anoxic bottles, respectively. Syringes used for sampling anoxic bottles were flushed at least three times with N2 prior to sampling.
Leucine incorporation method.
The leucine incorporation protocols of Smith and Azam (31) and Kirchman (15) were used with some minor modifications. Instead of using Eppendorf tubes, we incubated the samples in 2-ml plastic syringes (Plastipak syringes having a rubber seal of the piston and Discardit II syringes made only of plastic, both from Becton-Dickinson). Syringes were kept in plastic bags with N2 for at least 24 h prior to use to reduce the amount of O2 dissolved in the plastic. Samples of 1.7 ml were drawn directly from the experiment bottles into the 2-ml syringes, and 50 μl of leucine solution (purged with N2 to remove O2) was injected into each syringe through its tip using a Hamilton syringe. For control samples, 90 μl of 100% trichloroacetic acid (TCA) was injected prior to the leucine injection. Immediately after injections, syringe needles (Microlance, 0.5 by 16 mm; Becton-Dickinson) were connected to the incubation syringes and the air in the needles was replaced with sample water by gently pushing the syringe piston until the first droplet of the sample could be seen at the end of the needle. The syringe was then capped by insertion halfway into a 10-mm-thick rubber stopper. Syringes were thoroughly shaken and rotated to mix the sample and then incubated in the dark at 15°C. Incubations were stopped after about 60 min by transferring the samples from the syringes into 2-ml Eppendorf vials holding 90 μl of 100% TCA. Control samples were transferred to similar vials without TCA. The subsequent processing of the samples, including washing in 5% TCA and 80% ethanol, was performed as described by Smith and Azam (31). Radioactivity was measured after addition of scintillation cocktail in a Beckman LS 1801 scintillation counter, correcting quench by the H number method.
Bacterial production was estimated as described by Kirchman (15), according to the equation BP = LI · 131.2 · (% Leu)−1 · (C/protein) · ID, where BP is bacterial production, LI is the leucine incorporation rate (moles liter−1 h−1), 131.2 is the formula weight of leucine, % Leu is the fraction of leucine in protein (0.073) (15), C/protein is the ratio of cellular carbon to protein (0.86) (15), and ID is the isotope dilution (a value of 1 is used here when comparing bacterial estimates from leucine incorporation and microscopy). [3H]leucine (37 MBq/mol; Amersham Pharmacia Biotech product no. TRK 510) was diluted to various extents with cold leucine prior to experiments due to economic reasons. The hot leucine comprised 10 to 100% of the total leucine, depending on the final leucine concentration chosen. The final leucine concentration varied in the isotope saturation experiment (see below) but was 100 nM in the other experiments.
Isotope saturation.
Unlabeled leucine, naturally occurring in the water or from intracellular leucine synthesis, causes dilution of the added radioactively labeled leucine. Such isotope dilution significantly decreases the uptake of labeled leucine unless leucine is added at a concentration high enough to make the isotope dilution negligible. To find such saturating concentrations, we compared leucine incorporation at final added concentrations ranging from 5 to 257 nM in both oxic and anoxic bacterial regrowth cultures originating from the three different lakes studied. For each bottle and leucine addition, leucine incorporation was measured in triplicate samples and one killed control using Plastipak syringes as described above.
Leucine incorporation versus observed change in biomass.
To compare how leucine incorporation relates to bacterial growth in oxic and anoxic waters, leucine incorporation and the change in bacterial biomass were measured simultaneously in five oxic and five anoxic bacterial regrowth cultures from each lake. Twice daily, 5-ml samples for determination of bacterial abundance and biovolume were withdrawn from all cultures and preserved by adding borax-buffered formaldehyde to a 5% final concentration. Bacterial abundance was determined daily by counts of DAPI (4′,6′-diamidino-2-phenylindole)-stained cells using an epifluorescence microscope (27) in two replicates per lake and oxygen regimen to monitor bacterial growth. When microscopic counting indicated that the cultures had reached the exponential growth phase, leucine incorporation was measured in triplicate samples and one killed control per bottle using Plastipak syringes as described above.
The experiment was terminated after about 10 days. Bacterial abundances in all samples were determined using flow cytometry (Becton-Dickinson FACSCalibur and CellQuest 3.1 software) after staining with Syto 13 (Molecular Probes) as described by del Giorgio et al. (3). Volumes (V) of 100 to 400 bacterial cells per replicate bottle during the exponential growth phase were determined using image-analyzed fluorescence microscopy as described by Bertilsson et al. (1) and converted to bacterial dry weight (mb) using the equation mb = 435 × V0.86 (16). To estimate bacterial carbon biomass, we assumed that carbon comprised 50% of bacterial dry weight.
For each culture the natural logarithm of bacterial abundance was plotted against time. The slope of this curve, representing the intrinsic growth rate (k) (19), was calculated using at least three of the data points closest in time to the leucine incorporation measurements. The change in bacterial biomass during the leucine incorporation experiments was calculated according to the equation ΔBB = BA0 · k · t · Ccell, where ΔBB is the change in bacterial carbon biomass during the leucine incorporation experiment, BA0 is the bacterial abundance at the start of the leucine incorporation experiment, k is the intrinsic growth rate, t is the time of the leucine incorporation experiment, and Ccell is the average carbon content in each bacterial cell.
Effects of O2 contamination, headspace, and different incubation vessels.
The effect of short-term exposure to O2 during incubation was investigated using an anoxic regrowth culture (water from Lillsjön). Samples were incubated in syringes (six replicates and one killed control) that were deliberately contaminated with different amounts of O2. Contamination with O2 corresponding to 5 to 76% atm saturation was achieved by introducing a 0.3-ml headspace of the appropriate mixture of N2 and air, right after the leucine addition but before mixing of the sample. Each level of O2 contamination was introduced into three additional syringes (not amended with radioactive leucine) for control of the attained O2 concentration. We also tested different incubation vessels (Plastipak syringes or Discardit II syringes from Becton-Dickinson or screw cap Eppendorf vials from Sarstedt).
The oxygen concentration in the syringes was measured spectrophotometrically at 430 nm (28). Appropriate amounts of Winkler reagents were introduced through syringe tips using 50-μl Hamilton syringes. Spectrophotometric O2 measurements were calibrated against oxygen electrode measurements (Orion model 835; detection limit, 0.005 mg liter−1) in N2-purged anoxic water and against Winkler titration in water with an O2 content of 25 to 100% atm saturation. The resulting calibration curve was linear over the whole interval (0 to 100% atm saturation; O2 concentration [% atm saturation] = 80.83 · A430 − 1.14; r2 = 0.995).
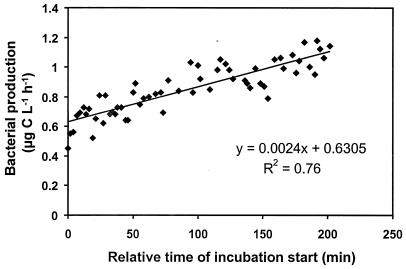
It took almost 4 h to start all of the leucine incubations for the experiment on effects of O2 contamination, and the bacterial production rate in the sampled culture increased slowly during this period. Hence, replicates of each O2 contamination level were started individually in a semirandomized design to make sure that there was no bias among the different contamination levels regarding initial bacterial production. To account for the drift in the bacterial production during the experiment, a linear regression analysis of all bacterial production measurements versus incubation start times was done. Individual correction factors for each starting time were derived from the regression slope (i.e., the residuals) to normalize bacterial production to the average production based on all measurements.
RESULTS AND DISCUSSION
Experimental setup and O2 concentrations.
The procedure for deoxygenating and sealing the infusion bottles allows water to be kept anoxic for very long times, and in previous tests of diffusion through the stoppers, no transport of O2 into bottles could be detected during the test period (2 months). At various stages during the experiments, the O2 level was checked using three methods: (i) with an O2 electrode during preparation of cultures, (ii) by Winkler titration of water in infusion bottles prior to and after experiments, and (iii) by spectrophotometric measurements of water in syringes used for measuring leucine incorporation. O2 concentrations in the anoxic bottles or syringes were always below the detection limit regardless of the method used. In addition, a calculation based on the biomass yield in the anoxic batch cultures, oxic respiration of glucose, and a bacterial growth efficiency of 40% (a conservative value since the median for freshwaters is reported to 26% [4]) shows that any O2 contamination sufficient to support significant bacterial growth would have been detected during O2 measurements. This confirms that anoxic conditions were reached and maintained during the experiments.
Isotope saturation.
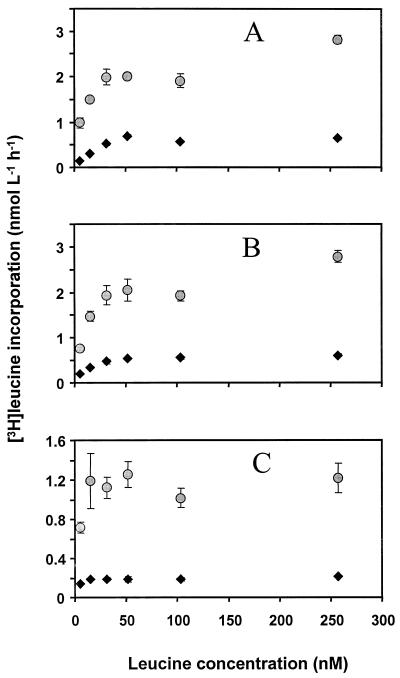
The changes in leucine incorporation rate with increasing leucine concentration were similar in waters from all lakes (Fig. 1). The leucine incorporation reached a saturation level at a leucine concentration of about 50 nM under both oxygen conditions. Hence, a leucine concentration of 100 nM was chosen for both oxic and anoxic treatments to ensure saturation. Jørgensen (12) also found isotope saturation at above 50 nM in waters from two eutrophic lakes, and the saturating concentration of leucine in humic water is typically high (30 nM or more) (32) compared to saturating leucine concentrations commonly found in marine waters (15, 30).
FIG. 1.
Leucine uptake at different concentrations by bacteria in waters from three lakes, Illersjön (A), Mårn (B), and Lillsjön (C), under both oxic and anoxic conditions (circles and diamonds, respectively). Bars show ±1 standard deviation (n = 3).
In waters from two of the lakes, the oxic leucine incorporation appeared to be higher at a leucine concentration of about 250 nM than at 50 to 100 nM (Fig. 1A and B), indicating that there may be a secondary increase in the leucine incorporation rate at high leucine concentrations. Bi- or multiphasic modes of leucine incorporation in both pelagic and sediment environments have been reported (reference 6 and references therein). This may be due to diffusion gradients of enzymes and substrates or to physiological heterogeneity of the microbial community, including the presence of eucaryotes taking up leucine at higher concentrations. A secondary increase in leucine incorporation was not seen in the anoxic cultures, indicating a difference in leucine biochemistry between oxic and anoxic microbial communities.
Leucine incorporation versus observed change in biomass.
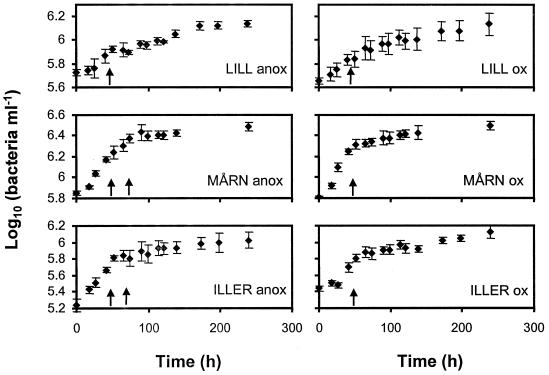
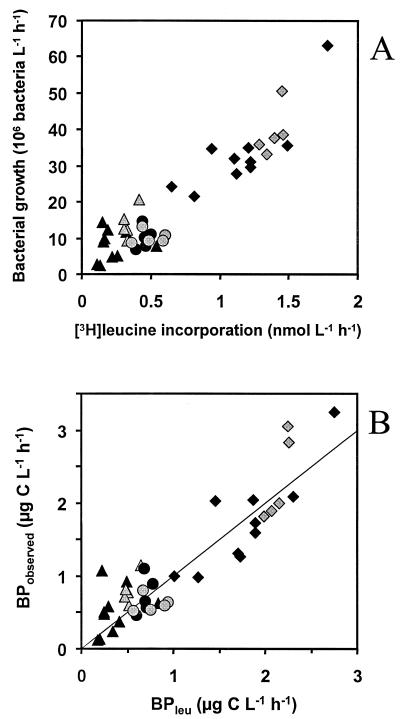
Exponential growth started within 48 h in all cultures. The leucine incorporation was measured at least once per bottle during the exponential growth phase (Fig. 2). Leucine incorporation rates were proportional to rates of increase in bacterial abundance during the period of [3H]leucine incorporation (Fig. 3A). When analyzed separately, the rates of increase in cell numbers and leucine incorporation were as closely related in oxic as in anoxic cultures (by linear regression, r2=0.86 under both oxygen conditions). In addition, the slopes of the regressions were not significantly different (by two-way analysis of variance [ANOVA] with the method [leucine incorporation or bacterial counting] and the O2 regimen as independent factors, P = 0.58), suggesting similar ratios of leucine incorporation to increase in cell number under both O2 conditions. Similar results are obtained when comparing bacterial carbon production derived from leucine measurements and from the increase in bacterial carbon biomass calculated from cell abundance and size, but the use of bacterial volume estimates introduced some minor additional variation (oxic, r2 = 0.84; anoxic, r2 = 0.82) (Fig. 3B). These results imply that leucine incorporation corresponded well to bacterial biomass production under both oxic and anoxic conditions in waters from three very different lakes.
FIG. 2.
Bacterial growth curves during the batch culture experiments. The arrows indicate the time of leucine incorporation measurements. Bars show ±1 standard deviation (n = 5). Curve labels refer to lake names (see the text) and to anoxic (anox) and oxic (ox) treatments.
FIG. 3.
(A) Leucine incorporation versus change in bacterial abundance (y = 27.79 · x + 0.77 and r2 = 0.86 for the anoxic samples; y = 26.96 · x + 1.17 and r2 = 0.86 for the oxic samples [linear regression]). (B) Bacterial production from the leucine incorporation (BPleu) versus observed biomass change (BPobserved) (y = 0.90 · x + 0.15 and r2 = 0.82 for the anoxic samples; y = 1.05 · x + 0.05 and r2 = 0.84 for the oxic samples [linear regression]). Grey and black symbols denote oxic and anoxic conditions, respectively. Triangles, circles, and squares represent experiments with water from Illersjön, Lillsjön, and Mårn, respectively (see text for information about lakes). The line corresponds to the 1:1 relationship.
Effects of O2 contamination, headspace, and different incubation vessels.
Throughout the 3.5-h experimental period the bacterial production increased by about 70% (Fig. 4). When this drift was corrected for (see Materials and Methods), the results showed that deliberate O2 contamination did not affect leucine incorporation (by ANOVA, P = 0.57) (Table 1).
FIG. 4.
Drift of bacterial production (determined by leucine incorporation) with time in the experiment testing impacts of O2 contamination, type of incubation vessel, and headspace in the incubation vessels.
TABLE 1.
Drift-corrected bacterial production in the experiment testing impacts of O2 contamination, type of incubation vessel, and presence of a headspace during incubation
| Incubation vessela | O2 concn (% saturation) | Headspace | Bacterial production (μg of C liter−1 h−1)b |
|---|---|---|---|
| Discardit II | 0 | Yes | 0.90 ± 0.088 |
| 6 | Yes | 0.87 ± 0.049 | |
| 12 | Yes | 0.85 ± 0.031 | |
| 20 | Yes | 0.92 ± 0.053 | |
| 43 | Yes | 0.89 ± 0.054 | |
| 76 | Yes | 0.89 ± 0.085 | |
| 0 | No | 0.81 ± 0.059 | |
| Plastipak | 0 | No | 0.76 ± 0.086 |
| 76 | Yes | 0.75 ± 0.042 | |
| Eppendorf | 100 | Yes | 0.84 ± 0.098 |
See text for a description of the incubation vessels.
Results are means ± standard deviations; (n = 6).
Theoretically, O2 contamination could affect bacterial production in several ways. The sudden introduction of O2 into the samples may inhibit certain anaerobic bacteria that are sensitive to changes in the composition of dissolved gases. Alternatively, O2 may increase substrate availability by allowing the use of oxygenases, a group of powerful extracellular enzymes used in the primary attack of substrates (29). The sudden presence of O2 possibly also induces a metabolic shift in the microbial community favoring oxygen respiration at the expense of anoxic respiration (nitrate, iron, manganese, and sulfate respiration), methanogenesis, and fermentation processes, which all yield less energy per unit of substrate used than oxygen respiration (35). Thus, an O2 intrusion causing such a metabolic shift should result in altered bacterial production. However, we found that the O2 saturation level did not significantly affect the leucine incorporation by bacteria from anoxic water during the 60 min of incubation with leucine. One possible explanation of these results is that effects of O2 contamination develop too slowly to be registered during short incubation times. Alternatively, it is possible that most of the bacteria active in the anoxic water were facultative anaerobes. Interestingly, microbial processes regarded to be obligately anaerobic, such as methanogenesis and denitrification, seem to be quite tolerant to temporary O2 intrusion, although process rates are often reduced under oxic conditions (21–23).
The presence of a headspace during incubation yielded a small increase (9%) in leucine incorporation (Table 1) compared to incubation with no headspace (by ANOVA, P = 0.031). Possibly this was due to more vigorous mixing of the samples when bubbles were present, causing increased substrate availability. This headspace effect can easily be avoided by the use of syringes instead of Eppendorf tubes when measuring leucine incorporation under both oxic and anoxic conditions.
Leucine incorporation was on average 11% lower in Plastipak syringes than in Discardit II syringes and Eppendorf vials (by ANOVA, P = 0.002) (Table 1). The rubber sealing the piston in the Plastipak syringes may have caused a decrease in bacterial growth rates. Inhibition of bacterial production by rubber stoppers has been previously reported (24). We thus recommend the use of Discardit II or other syringes without rubber sealing.
Anoxic leucine incorporation rates were consistently lower than oxic rates in the saturation level experiments (Fig. 1), while oxic and anoxic rates were equal in all other experiments. This difference is probably due to the different pretreatments of the water. In the isotope dilution experiment, original lake water was transferred directly into the experimental bottles, and possibly bacterial growth was limited to different extents in oxic and anoxic waters by remineralization processes. Presumably, bacterivores were more active in the oxic cultures, yielding a greater remineralization and therefore higher bacterial growth rates in the presence of O2. The other experiments included addition of inorganic nutrients and filtration, resulting in low initial numbers of bacteria, and a period of exponential growth without limitation by nutrients or organic substrate in any of the oxygen regimens.
Comparative studies of bacterial production in oxic and anoxic environments, e.g., at different depths of stratified water bodies, rely on methods that deliver comparable results for all samples. Despite the widespread use of the leucine method for anoxic waters, the validity of the method in these environments has not been evaluated previously. We describe a convenient procedure for headspace-free incubation in plastic syringes, and we conclude that the leucine incorporation method used in this way works equally well in both oxic and anoxic waters.
ACKNOWLEDGMENTS
We thank Stefan Bertilsson, Sofia Kallner, and Ramunas Stepanauskas for assistance during various parts of the work. Håkan Olsson generously provided information which helped us select lakes to sample.
This study was funded by the Swedish Natural Science Research Council.
REFERENCES
- 1.Bertilsson S, Stepanauskas R, Cuadros-Hansson R, Granéli W, Wikner J, Tranvik L. Photochemically induced changes in bioavailable carbon and nitrogen pools in a boreal watershed. Aquat Microb Ecol. 1999;19:47–56. [Google Scholar]
- 2.Cole J J, Pace M L. Bacterial secondary production in oxic and anoxic freshwaters. Limnol Oceanogr. 1995;40:1019–1027. [Google Scholar]
- 3.del Giorgio P A, Bird D B, Prairie Y T, Planas D. Flow cytometric determination of bacterial abundance in lake plankton with the green nucleic acid stain Syto 13. Limnol Oceanogr. 1996;41:783–789. [Google Scholar]
- 4.del Giorgio P A, Cole J J. Bacterial growth efficiency in natural aquatic systems. Annu Rev Ecol Syst. 1998;29:503–541. [Google Scholar]
- 5.dos Santos Furtado A L, Casper P. Different methods for extracting bacteria from freshwater sediment and a simple method to measure bacterial production in sediment samples. J Microbiol Methods. 2000;41:249–257. doi: 10.1016/s0167-7012(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 6.Fischer H, Pusch M. Use of the [14C]leucine incorporation technique to measure bacterial production in river sediments and the epiphyton. Appl Environ Microbiol. 1999;65:4411–4418. doi: 10.1128/aem.65.10.4411-4418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhrman J A, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface freshwaters: evaluation and field results. Mar Biol. 1982;66:109–120. [Google Scholar]
- 8.Gilmour C G, Leavitt M E, Shiaris M P. Evidence against incorporation of exogenous thymidine by sulfate-reducing bacteria. Limnol Oceanogr. 1990;35:1401–1409. [Google Scholar]
- 9.Hagström Å, Larsson U, Hörstedt P, Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979;33:1225–1228. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst C J, editor. Manual of environmental microbiology. Washington, D.C.: ASM Press; 1997. [Google Scholar]
- 11.Johnstone B H, Jones R D. A study on the lack of [methyl-3H]thymidine uptake and incorporation by chemolitotrophic bacteria. Microb Ecol. 1989;18:73–77. doi: 10.1007/BF02011697. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen N O G. Incorporation of [3H]leucine and [3H]valine into protein of freshwater bacteria: uptake kinetics and intracellular isotope dilution. Appl Environ Microbiol. 1992;58:3638–3646. doi: 10.1128/aem.58.11.3638-3646.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. [Google Scholar]
- 14.Kirchman D, K'nees E, Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol. 1985;49:599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirchman D L. Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 509–512. [Google Scholar]
- 16.Loferer-Kröβbacher M, Klima J, Psenner R. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl Environ Microbiol. 1998;64:668–694. doi: 10.1128/aem.64.2.688-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marxen J. Measurement of bacterial production in stream-bed sediments via leucine incorporation. FEMS Microbiol Ecol. 1996;21:313–325. [Google Scholar]
- 18.McDonough R J, Sanders R W, Porter K G, Kirchman D L. Depth distribution of bacterial production in a stratified lake with an anoxic hypolimnion. Appl Environ Microbiol. 1986;52:992–1000. doi: 10.1128/aem.52.5.992-1000.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McManus G B. Growth rates of natural populations of heterotrophic nanoplankton. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 557–562. [Google Scholar]
- 20.Moriarty D J W. Measurement of bacterial growth rates in aquatic systems from rates of nucleic acid synthesis. Adv Microb Ecol. 1986;9:245–292. [Google Scholar]
- 21.Oh J, Silversein J. Oxygen inhibition of activated sludge denitrification. Water Res. 1999;33:1925–1937. [Google Scholar]
- 22.O'Keefe D M, Brigmon R L, Chynoweth D P. Influence of methane enrichment by aeration of recirculated supernatant on microbial activities during anaerobic digestion. Bioresource Technol. 2000;71:217–224. [Google Scholar]
- 23.Öquist M, Sundh I. Effects of a transient oxic period on mineralization of organic matter to CH4 and CO2 in anoxic peat incubations. Geomicrobiology. 1998;15:325–333. [Google Scholar]
- 24.Pedrós-Alió C, García-Cantizano J, Calderón J I. Bacterial production in anaerobic water columns. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 519–530. [Google Scholar]
- 25.Piker L, Reichardt W. Do sulfate-reducing bacteria respond to thymidine incorporation assays in marine sediments? Kieler Meeresforsch Sonderh. 1991;8:102–106. [Google Scholar]
- 26.Pollard P C, Moriarty D J W. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurements of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984;48:1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 28.Roland F, Caraco N F, Cole J J. Rapid and precise determination of dissolved oxygen by spectrophotometry: evaluation of interference from colour and turbidity. Limnol Oceanogr. 1999;44:1148–1154. [Google Scholar]
- 29.Schink B. Principles and limits of anaerobic degradation: environmental and technological aspects. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons; 1988. pp. 771–846. [Google Scholar]
- 30.Simon M, Azam F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser. 1989;51:201–213. [Google Scholar]
- 31.Smith D C, Azam F. A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine. Marine Microb Food Webs. 1992;6:107–114. [Google Scholar]
- 32.Tulonen T. Bacterial production in a mesohumic lake estimated from [14C]leucine incorporation rate. Microb Ecol. 1993;26:201–217. doi: 10.1007/BF00176953. [DOI] [PubMed] [Google Scholar]
- 33.Tuominen L. Comparison of leucine uptake methods and a thymidine incorporation method for measuring bacterial activity in sediment. J Microbiol Methods. 1995;24:125–134. [Google Scholar]
- 34.Winding A. [3H]thymidine incorporation to estimate growth rates of anaerobic bacterial strains. Appl Environ Microbiol. 1992;58:2660–2662. doi: 10.1128/aem.58.8.2660-2662.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zehnder A J B, Stumm W. Geochemistry and biogeochemistry of anaerobic habitats. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: John Wiley & Sons; 1988. pp. 1–38. [Google Scholar]