Abstract
Introduction:
Poly (ADP-ribose) polymerase inhibitors (PARPis) are an exciting class of agents that have shown efficacy, particularly for BRCA-mutant triple-negative breast cancer (TNBC) and high-grade serous ovarian cancer (HGSOC). However, most patients who receive PARPi as their standard of care therapy inevitably develop resistance and this underscores the need to identify additional targets that can circumvent such resistance. Combination treatment strategies have been developed in preclinical and clinical studies to address the challenges of efficacy and resistance.
Areas covered:
This review examines completed or ongoing clinical trials of PARPi mono- and combination therapies. PARPi monotherapy in HER2 negative breast (HR+ and TNBC subtypes) and ovarian cancer is a focal point. The authors propose potential strategies that might overcome resistance to PARPi and discuss key questions and future directions.
Expert Opinion:
While the advent of PARPis has significantly improved the treatment of tumors with defects in DNA damage and repair pathways, careful patient selection will be essential to enhance these treatments. The identification of molecular biomarkers to predict disease response and progression is an endeavor.
Keywords: clinical trial, combination therapy, DNA damage repair (DDR), drug resistance, germline BRCA-mutation, high-grade serous ovarian cancer (HGSOC), homologous recombination deficiency (HRD), niraparib, olaparib, poly (ADP-ribose) polymerase (PARP) inhibitor, preclinical study, somatic BRCA-mutation, triple-negative breast cancer (TNBC)
1. Introduction
Triple-negative breast cancer (TNBC), a subtype of breast cancer without expression of hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2), accounts for approximately 15% of invasive breast cancers [1]. Patients with the TNBC subtype cannot obtain clinical benefits from endocrine therapy or anti-HER2 therapy. Unfortunately, this subtype of breast cancer is more aggressive with decreased overall survival and poorer prognosis than the other subtypes. Lehmann et al. analyzed gene expression profiles of 21 breast cancer databases and 587 TNBC cases. They identified 6 TNBC subtypes: 2 basal-like (BL-1 and BL-2), an immunomodulatory (IM), a mesenchymal (M), a mesenchymal stem-like (MSL), and a luminal androgen receptor (LAR) subtype [2]. Another study analyzed RNA and DNA profiles of 198 TNBC tumors identified 4 TNBC subtypes: a luminal androgen receptor (AR; LAR), a mesenchymal (MES), a basal-like immunosuppressed (BLIS), and a basal-like immune-activated (BLIA) subtype [3]. However, despite the increasing knowledge of TNBC biology, no evidence supports their use in clinical practice for treatment selection. Conventional chemotherapy, including taxane-, alkylating, and anthracycline-based regimens, is the mainstay of TNBC treatment, but the clinical response to chemotherapy is limited and concomitant with substantial toxicities. Very recently, FDA approved pembrolizumab (Keytruda), a humanized antibody targeting the PD-1 receptor, for treating early-stage TNBC in combination with chemotherapy in the neoadjuvant setting, followed by its use as a single agent in the adjuvant setting [4]. In addition, sacituzumab govitecan (Trodelvy), a conjugate of the humanized anti-Trop-2 monoclonal antibody linked with a topoisomerase inhibitor, has also been recently approved by FDA for the treatment of metastatic TNBC [5].
TNBC is a highly heterogeneous tumor with constitutive activation of oncogenes and increased levels of DNA damage, resulting in its high dependence on the existing DNA damage repair (DDR) pathways compared to normal cells. The DDR pathway, responsible for DNA damage repair and maintenance of genomic stability, consists of an elegantly orchestrated network of pathways for repairing DNA damage lesions and for activation of checkpoints that arrest the cell at key points in the cell cycle [6]. DDR pathways are considered the ‘Achilles heel’ of TNBC, making the TNBC more susceptible to DDR drugs. The most investigated DDR pathways in TNBC are the homologous recombination repair (HRR) and the ATR-CHK1-WEE1 pathways.
2. HRR deficiency, BRCA1/2 mutations, and BRCAness in TNBC
BRCA1 and BRCA2 are tumor suppressor genes that play essential roles in maintaining genomic stability by participating in an error-free, homology-directed DNA repair (HDR) when DNA double-strand breaks (DSBs) occur. In cells with germline BRCA1 or BRCA2 mutation, DNA repair of DSB through homologous recombination (HR) is defective [7]. Furthermore, patients with germline mutations in BRCA1/2 genes have a 70% risk of developing breast cancer by age 80 [8]. The increased risk of developing ovarian cancer is 47% in women with the germline BRCA1 mutation and 14% with germline BRCA2 mutations, respectively [8]. Moreover, 60% to 80% of all breast cancer patients with germline BRCA1 mutations develop breast cancers that are molecularly classified as the TNBC subtype [9, 10].
The prevalence of BRCA mutations varies by ethnic and racial background. In the general population, the presence of these germline mutations is rare, while the highest rate occurs among Ashkenazi Jewish women (8.3%), followed by Hispanic women (3.5%) [11]. Founder mutations of BRCA genes (185delAG, 538insC in BRCA1; 6174delT in BRCA2) are identified among Ashkenazi Jewish women [12]. Interestingly, some sporadic breast cancers without germline mutations in BRCA display similar alterations as those tumors harboring germline BRCA mutations with consequent defective HR. Breast cancer cases where sporadic BRCA mutations occur in the absence of germline mutations are referred to as cases with BRCA like properties or “BRCAness”. The overall frequency of BRCAness phenotypes in sporadic breast cancer is unknown. However, data on BRCA1 methylation, FANCF methylation, and EMSY amplification indicate that approximately up to 25% of sporadic breast cancer could show BRCAness phenotypes [13]. Other biomarkers for assessing BRCAness include mutational genes associated with HR repair, mutational or transcriptomic signatures related to HRD, and functional biomarkers of HRD [7].
Genomic instability and DNA scarring are the consequences of BRCA mutations, BRCAness, and HRD. Previous studies suggest that approximately 50% of TNBCs can be classified as HRD regardless of the status of BRCA mutations [14, 15]. Breast cancers with HRD are likely to be more sensitive to drugs that induce DNA damage. Clinical trials using the family of poly (ADP-ribose) polymerase (PARP) inhibitors revealed that these agents are effective in cancers with HRD, especially those breast and ovarian cancers with BRCA1/2 mutations.
3. PARP inhibitors and mechanisms of action (Figure 1)
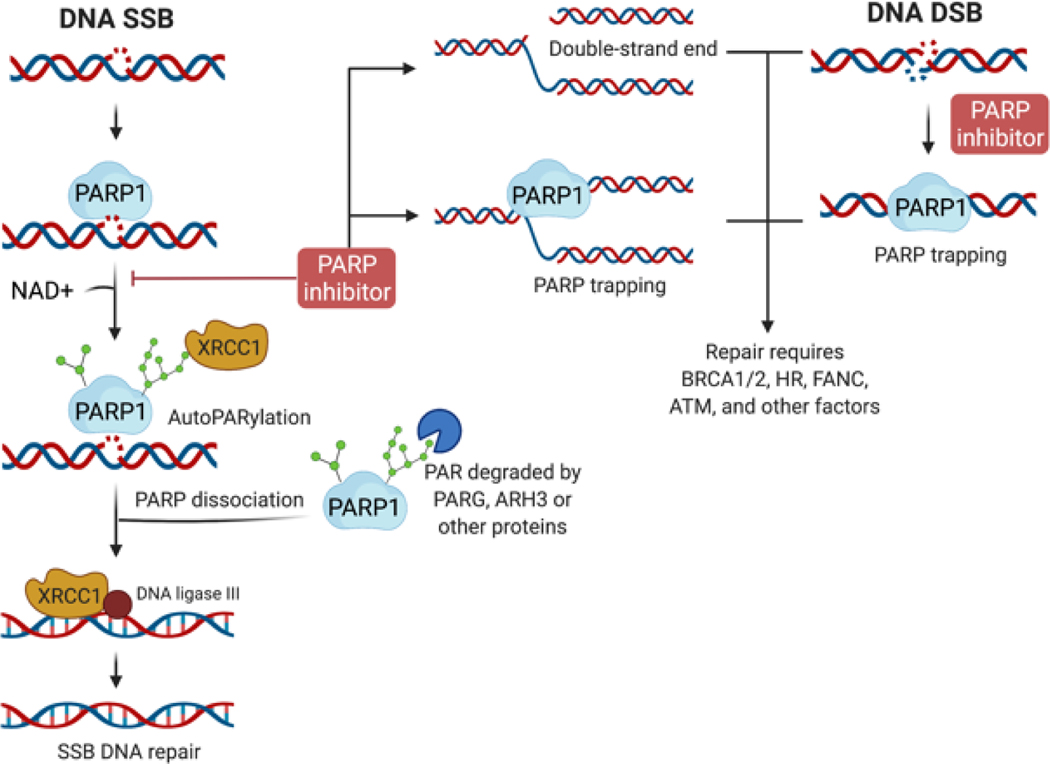
Figure 1. DNA repair and the effects of PARP inhibitor.
When the DNA single-strand break (SSB) occurs, PARP1 binds to the DNA lesion and uses nicotinamide adenine dinucleotide (NAD+) to generate PAR chains on itself (autoPARylation) and recruits additional DNA repair effectors, such as XRCC1 for SSB repair. Meanwhile, the autoPARylated PARP is released from DNA, and the PAR chains can be degraded by PARG, ARH3, or other proteins, which allows PARP protein reactivation. The recruited DNA repair proteins further complete the SSB DNA repair process. When the PARP inhibitor is exposed, the autoPARylation or PARylation are inhibited, resulting in the double-strand end or PARP trapping, with SSB DNA repair incomplete. PARP1 is also involved in the DNA double-strand break (DSB), and PARP inhibitors trap PARP at double-strand ends (DSEs) or DSBs. Repairing DSB and PAPR trapping requires BRCA1, BRCA2, HRR proteins, ATM, Fanconi, and other factors for cell survival.
PARP is a family of proteins that comprise 17 members with diverse functions (Table 1) [16]. Only four PARPs generate poly-ADP-ribose (PAR) chains: PARP1, PARP2, and the two tankyrases, PARP5a and PARP5b, while the other PARPs add only a mono-ADP-ribose [17]. The most studied PARP proteins are PARP1, 2, and 3, involved in DNA damage repair. PARP1 is a DNA damage sensor protein and an enzyme that functions in an error-free, base excision repair pathway. Once single-strand breaks (SSB) occur, PARP1 binds to DNA through zinc finger domains, undergoes a conformational change, and synthesizes a polymetric adenosine diphosphate ribose (poly-ADP-ribose), also called PAR chains on itself (autoPARylation) or on DNA damage response proteins (PARylation) for repair single-strand breaks [18] (Figure 1). Meantime, the nicotinamide adenine dinucleotide (NAD+), a cofactor of PARP1, binds to the active site of the enzyme PARP1. These PAR chains lead to the recruitment of additional DNA repair effectors, such as a scaffolding protein XRCC1, to complete the DNA repair process [19]. After repairing, the PAR chains are degraded via Poly(ADP-ribose) glycohydrolase (PARG) and other proteins [20] (Figure 1). This PARylation of proteins around the DNA breaks also mediates DNA repair by modifying chromatin structure, such as histone-PARylation [21]. Pharmacological PARP inhibitors (PARPis) structurally mimic nicotinamide and have two general effects: (1) catalytic inhibition of PARP1/2 via preventing PARylation and (2) ‘trapping’ PARP1/2 on damaged DNA (Figure 1). Of note, the differential trapping potency does not correlate with the catalytic inhibitory properties for each drug [22]. The potency in trapping PARPs differs among inhibitors, with the highest potency reported for talazoparib, followed by niraparib, olaparib, and then veliparib [22–24] (Table 2). Even though the mechanisms of PARP trapping to stall the progression of replication forks are still unclear, the current hypothesis includes enhanced PARP1 and DNA binding avidity [22, 23] and inhibited PARP1 release due to autoPARylation [25].
Table 1.
Catalytic activity and cellular functions of PARP family proteins.
| PARP | Activity | Localization | Function | References |
|---|---|---|---|---|
| 1 | Poly- | Nucleus | DNA repair enzyme for single- and double-strand breaks of DNA; replication fork damage | [126] |
| 2 | Poly- | Nucleus, cytoplasm | DNA repair enzyme for single- strand breaks of DNA | [127] |
| 3 | Mono- | Nucleus, cytoplasm | DNA damage and repair, energy metabolism | [128] |
| 4 | Mono- | Nucleus, cytoplasm | Limited results | |
| 5a | Poly- | Cytoplasm | Tankyrase enzyme involved in the WNT pathway, telomere length, and cellular proliferation and differentiation | [129] |
| 5b | Poly- | Cytoplasm | Tankyrase enzyme involved in NHEJ and tumorigenesis | [130] |
| 6 | Mono- | Cytoplasm | Tumor suppressor, negative regulation of cell proliferation | [131, 132] |
| 7 | Mono- | Nucleus, cytoplasm | Negative regulation of type I interferon | [133] |
| 8 | Mono- | Cytoplasm | Limited results | |
| 9 | Inactive | Nucleus, cytoplasm | A noncanonical RNA virus sensor to produce type I interferon in dendritic cells | [134] |
| 10 | Mono- | Cytoplasm | Promote tumorigenesis by alleviating replication stress; suppresses tumor metastasis through negatively regulating Aurora A activity | [135, 136] |
| 11 | Mono- | Nucleus, cytoplasm | Modulating the type I interferon antiviral response by mono-ADP-ribosylating the ubiquitin E3 ligase β-TrCP | [137] |
| 12 | Mono- | Cytoplasm | An interferon-induced gene with a role in cellular defense against viral infections | [138] |
| 13 | Inactive | Cytoplasm | An RNA-binding protein with a role in cellular response to stress; protective against malignant transformation by repressing oncogenic viruses | [139] |
| 14 | Mono- | Nucleus, cytoplasm | Play roles in cellular responses and signaling pathways in the immune system | [140] |
| 15 | Mono- | Not clear | Limited results | |
| 16 | Mono- | Cytoplasm | An endoplasmic reticulum protein required for the PERK- and IRE1α-mediated unfolded protein response during ER stress | [141] |
Mono-, Poly-: Mono- or Poly-(ADP)ribose polymerases
Table 2.
Different PARP Inhibitors Comparison and Summarized Clinical Benefits
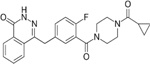
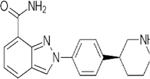
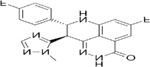
| PARP Inhibitors | Olaparib | Rucaparib | Niraparib | Talazoparib | Veliparib | Pamiparib | Fluzoparib |
|---|---|---|---|---|---|---|---|
|

|
|
|
|
|

|
|
| IC50 (nM) | 1 | 1 | 4 | 0.6 | 2 | 0.83 | 1.5 |
| Targets | PARP1/2/3/4 | PARP1/2/3/4, TNKS1 | PARP1/2 | PARP1/2 | PARP1/2/3 | PARP1/2 | PARP1/2 |
| Trapping capacity [1–5] | ++ | ++ | ++ | +++ | - | ++ | N.A. |
| Single-agent dose | 300 mg PO BID | 600 mg PO BID | 300 mg PO BID | 1 mg PO QD | 400 mg PO BID | 60 mg PO BID | N.A. |
| CNS penetration | + | + | ++ | - | + | ++ | N.A. |
| T1/2 (hours) | 11.9 | 25.9 | 48–51 | 90 ± 58 | 4.1–8.2 | N.A. | N.A. |
| P-glycoprotein substrate | Yes | Yes | Weak | Weak | No | No | N.A. |
| Off-target interactions | CYP3A4 | CYP enzymes | Dopamine, norepinephrine and serotonin transporters | None | N.A. | N.A. | N.A. |
| Toxicities (most frequent) | Nausea, fatigue, vomiting, diarrhea, dysgeusia, headache | Nausea, fatigue, vomiting, diarrhea, dysgeusia, LFT elevation | Nausea, fatigue, LFT elevation, vomiting, headache, insomnia, HTN | Nausea, fatigue, headache, vomiting, alopecia, diarrhea | Nausea, fatigue, lymphopenia | Nausea, fatigue | Limited early-phase trial data |
| Grade higher than 3 hematologic toxicities | Anemia, neutropenia | Anemia, neutropenia | Thrombocytopenia, anemia, neutropenia | Anemia, neutropenia, thrombocytopenia | NTD | Anemia, neutropenia | NTD |
| Clinical benefits | SOLO1 (ovarian maintenance), HR 0.30, PFS benefit; SOLO2 (relapsed ovarian maintenance), HR 0.30, PFS benefit; OympiAD (HER2 BC), HR 0.58, PFS benefit | ARIEL2 (relapsed ovarian), HR 0.27, PFS benefit; ARIEL3 (relapsed ovarian maintenance), HR 0.23, PFS benefit | NOVA (relapsed ovarian maintenance), HR 0.27, PFS benefit | EMBRACA (HER2 BC), HR 0.54, PFS benefit | BROCADE 3 (advanced BC), HR, 0.71, PFS benefit | Ongoing, data not mature (NCT03427814) | NTD |
| Approvals | Ovarian, Breast | Ovarian | Ovarian | Breast (FDA) | NTD | NTD | NTD |
CNS, central nervous system; CYP, cytochrome P450; IC50, half maximal inhibitory concentration; N.A., not available; PARP, poly(ADP-ribose) polymerase; TNKS1, tankyrase 1; -, poor; +, modest; ++, good; HR, hazard ratio; PFS, progression-free survival
In normal proliferating cells, PARPi-induced replication fork stalling would be repaired by the homologous recombination (HR) mechanism. However, in tumor cells lacking the critical HR proteins, such as BRCA1, BRCA2, PALB2, or RAD51, alternative DNA repair pathways are activated, primarily through the error-prone, non-homologous end joining (NHEJ) or microhomology-mediated end joining (MMEJ). Instead of restoring the damaged DNA sequence to its native form, the use of error-prone DNA repair pathways leads to fragmentation of the genome that ultimately leads the cell death [26].
4. Clinically approved PARP inhibitors
To date, four PARPis have been approved as maintenance treatment in several types of cancers, and in total seven PARPis are currently under clinical investigation, which includes olaparib, rucaparib, niraparib, talazoparib, veliparib, pamiparib, and fluzoparib (Table 2). Many of these PARPis are being used for various cancers, with or without germline BRCA mutations (Table 3). For example, olaparib is the first PARPi approved by the Food and Drug Agency (FDA) and European Medicines Agency (EMA) as monotherapy to treat germline BRCA-mutant metastatic ovarian cancer (NCT0107662, SOLO-1) [27], or advanced ovarian, fallopian and primary peritoneal tumors independent of BRCA mutational status (NCT01874353, SOLO-2, and NCT00753545, Study 19). Olaparib has also been approved for the treatment of metastatic TNBC and HER2 negative breast cancer with germline BRCA mutations (NCT02000622, OlympiAD) [28] and first-line treatment of BRCA-mutant advanced pancreatic cancer (NCT02184195, POLO) [29]. Most recently, its use has been expanded in combination with bevacizumab for first-line maintenance treatment of HRD advanced ovarian, fallopian tube (FTC) and primary peritoneal carcinomas (PPC) (NCT02477644, PAOLA-1) [30], and HRD positive metastatic castration-resistant prostate cancer (NCT02987543, PROfound) [31] (Table 3).
Table 3.
Summary of Phase II/III Clinical Trials of PARP Inhibitors
| Clinical trial identifier | Treatment Arms | Phase | Status | Locations | Time | Study population and numbers | Treatment | Median Follow-up Duration | Outcomes | Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | ||||||||||
| NCT02000622 (OlympiAD) | Olaparib vs. physician’s choice of Capecitabine, Vinorelbine or Eribulin | III | Completed | 19 countries | 2014–2016 | BRCA-mutant, HER2 negative metastatic breast cancer ( N = 302) | Patients were administered olaparib orally twice daily (bid) at 300 mg. | Median PFS was significantly longer in the olaparib group than in the standard therapy group (7.0 vs. 4.2 mo). The response rate was 59.9% in the olaparib group vs. 28.8% in the standard therapy group. | The grade 3 or higher AE rate was 36.6% in olaparib group vs. 50.5% in the standard-therapy group. The rate of treatment discontinuation was 4.9% and 7.7%, respectively. | |
| NCT01945775 (EMBRACA) | Talazoparib vs. physician’s choice of Capecitabine, Eribulin, Gemcitabine or Vinorelbine | III | Completed | United States | 2013–2017 | Breast neoplasms, HER2 negative, BRCA mutations (N = 431) | Patient were randomized 2:1 to receive talazoparib oral capsules (1.0 mg) once daily for 21 continuous days. | Until 2018 | Median PFS: 8.6 mo vs. 5.6 mo, HzR 0.54, 0.41 to 0.71, P < 0.001; The ORR: 62.2% vs. 27.2%, OR 5.0, 2.9–8.8, P < 0.01; Overall improvement was seen for talazoparib compared to PCT group (3.0, 1.2 to 4.8 vs. (−5.4), (−8.8 to −2.0), P < 0.0001. Greater delay was observed in definitive clinically meaning full deterioration (TTD) favoring talazoparib vs. PCT (HzR, 0.38, 2.26 to 0.55); median 24.3 mo vs. 6.3 mo, P < 0.0001 | Hematologic grade 3–4 AE: 55% of the patients received talazoparib vs. 38% of the patients received standard therapy; nonhematologic grade 3 AE occurred: 32% vs. 38%, respectively. |
| NCT03575065 | Pamiparib/BGB-290 | II | Completed | China | 2018–2021 | Metastatic HER2 negative, BRCA-mutant breast cancer; TNBC cohort (N = 62); HR+/HER2- cohort (N = 26) | Patents received pamiparib 60 mg orally twice daily in 28-day cycles | In the TNBC cohort: confirmed ORR = 38.2% (95% CI: 25.4–52.3); median DOR = 6.97 months; median PFS = 5.49 months (95% CI: 3.65–7.33); median OS = 17.08 months (95% CI:13.70–NE). In the HR+ cohort: confirmed ORR = 61.9% (95% CI: 38.4–81.9); mDOR = 7.49 months (95% CI: 5.55–14.75); mPFS = 9.20 months (95% CI: 7.39–11.93); mOS was not reached | Grade 3 TEAEs occurred in 54 pts (61.4%); anemia was the most common TEAE, occurring in 77 pts (87.5%). Dose reduction due to TEAEs occurred for 57 pts (64.8%); discontinuation due to TEAEs occurred for 2 pts (2.3%) | |
| NCT02032823 (OlympiA) | Olaparib vs. Placebo | III | Ongoing | 23 countries | 2014-current | gBRCA1/2 mutations and high risk HER2 negative primary breast cancer (N = 1836) | Olaparib tablets 300mg b.i.d. p.o. | Adjuvant olaparib after completion of local treatment and neoadjuvant/adjuvant chemotherapy significantly increased survival free of invasive or distant disease than was placebo. | Most common reasons for discontinuation of olaparib: nausea (2.0%), anemia (1.8%), fatigue (1.3%), and decreased neutrophil count (1.0%). | |
| NCT01905592 (BRAVO) | Niraparib vs. physician’s choice of single-agent chemotherapy | III | Ongoing | United States | 2014-current | Neoplasms, breast and BRCA mutant, HER2 negative (N = 306) | 300 mg (3×100 mg capsules) once daily until progression or unacceptable toxicity develops | The hypothesis of median PFS improvement is 3 versus 6 months for physician’s choice and niraparib, respectively, with a hazard ratio of 0.5 and power of 99.6% for the primary PFS analysis. | ||
| Ovarian Cancer | ||||||||||
| NCT01844986 (SOLO1) | Olaparib vs. Placebo | III | Ongoing | 15 countries | 2013-current | Patients with BRCA mutated, newly diagnosed advanced high-grade serous or endometrioid ovarian cancer, primary peritoneal cancer, or fallopian tube cancer following first-line platinum-based chemotherapy (N = 457) | Olaparib tablets p.o. 300mg twice daily | 41 mo | The risk of disease progression or death was 70% lower with olaparib than with placebo. Mean quality-adjusted PFS: olaparib 29.75 vs placebo 17.58 months, P < 0.0001; mean duration of time without significant symptoms of toxicity (olaparib 33.15 vs placebo 20.24 months, P < 0.0001). | Consistent with the known toxic effects of olaparib |
| NCT01874353 (SOLO2/ENGOT-Ov21) | Olaparib vs. Placebo | III | Ongoing | 16 countries | 2013-current | Platinum sensitive, BRCA mutated relapsed ovarian cancer (N = 327) | Olaparib 300mg in two 150 mg tablets twice daily | Median OS: 51.7 mo with olaparib vs. 38.8 mo with placebo | Anemia (21%) was the most common adverse effect. 26% of patients reported severe treatment-emergent adverse effects with olaparib versus 8% with placebo. Fatal outcomes occurred in 4% of patients with olaparib attributed to myelodysplastic syndrome (n = 3) and acute myeloid leukemia (n = 3). | |
| NCT02477644 (PAOLA-1) | (1) Bevacizumab + Olaparib (p.o. 300 mg bid) vs. Bevacizumab + Placebo; (2) Olaparib vs. Placebo | III | Ongoing | 11 countries | 2015-current | Advanced FIGO Stage IIIB-IV high grade serious or endometrioid ovarian, fallopian tube, or peritoneal cancer treated standard first-line treatment | Tablets, per os, 300 mg twice daily | 22.9 mo | Median PFS: (1) 22.1 mo vx. 16.6 mo; HzR, 0.59: (0.49 to 0.72); P<0.001; (2) 37.2 mo vs. 17.7 mo; HzR, 0.33; (0.25 to 0.45) in patients with HRD including BRCA-mutation; 28.1 mo vs. 16.6 mo; HzR, 0.43; (0.28 to 0.66) in patients with HRD without BRCA mutations | |
| NCT02282020. (SOLO3) | Olaparib vs. physician’s choice of single agent chemotherapy | III | Ongoing | United States | 2015-current | gBRCA-mutant platinum-sensitive relapsed ovarian cancer (N = 266) | Olaparib 300mg oral tablets; twice daily or single-agent nonplatinum chemotherapy (paclitaxel, gemcitabine, pegylated liposomal doxorubicin, or topotecan). | ORR was significantly higher with olaparib than with chemotherapy (72.2% vs. 51.4%). BICR-assessed PFS also significantly favored olaparib vs. chemotherapy (13.4 mo vs. 9.2 mo). | Consistent with the established safety profiles of olaparib and chemotherapy. | |
| NCT03402841 (OPINION) | Olaparib maintenance monotherapy | IIIb | Ongoing | 17 countries | 2018-current | Non-germline BRCA mutated ovarian cancer (N = 279) | 300 mg twice daily - oral until disease progression or unacceptable toxicity. | Until Oct 2020 (75.3% data maturity) | Median PFS was 9.2 months. PF at 6, 12 and 18 months were 65.3%, 38.5% and 24.3%, respectively. A grade higher than 3 treatment-emergent AEs occurred in 29% of patients and serious TEAEs in 19.7% of patients. | Consistent with the known toxic effects of olaparib |
| NCT02476968 (ORZARA) | Olaparib maintenance monotherapy | IV | Ongoing | 8 countries | 2015–2021 | Platinum sensitive relapsed somatic or germline BRCA mutated ovarian cancer patients with the complete or partial response following platinum-based chemotherapy (N = 181) | Olaparib capsules orally 400 mg twice daily within 8 weeks after their last dose of platinum-containing chemotherapy until disease progression or discontinuation. | Until April 2020 (60% data maturity) | In the BRCA-mutant cohort, the median PFS was 18.9 months, and the PFS rate was 67% at 1 year and 30% at 2 years. In patients with non-BRCA HRR mutations, the median PFS was 16.4 months and the PFS rate at 1 and 2 years were 68% and 26%, respectively. Treatment-emergent AEs of any grade, a grade higher than 3, and serious events were 93%, 38.2%, and 23.6, respectively, among all BRCA-mutant patients. | The most common AEs of any grade included nausea (53.7%), fatigue (53.7%), anemia (42.4%), and vomiting (27.7%) |
| NCT01891344 (ARIEL2) | Rucaparib | II | Completed | 7 countries | 2013–2019 | Part I (N = 204) and Part II (N = 287): patients with recurrent, platinum-sensitive, HGSOC were classified into one of three predefined HRD subgroups: BRCA mutant, BRCA WT and LOH high (LOH high group), or BRCA WT and LOH low (LOH low group). | 600 mg twice per day for continuous 28-day cycles until disease progression or any other reason for discontinuation | Between Oct 2013 and Aug 2016, 491 patients were enrolled. | Part I: median PFS: 12.8 mo, (9.0–14.7) in BRCA mutant group vs. 5.7 (5.3–7.6) in LOH high group vs/ 5.2 (3.6–5.5) in LOH low group. PFS is significantly longer in BRCA mutant (HzR 0.27, 0.16–0.44, P < 0.0001) and LOH high (HzR 0.62, 0.42–0.9, P = 0.011) compared to LOH low group. Part II: confirmed ORR: 31% (21.3–42), 6.8% (2.3–15.3) and 5.6% (2.1–11.8), respectively. Median PFS: 7.8 mo (7.3–9.2) vs 4.3 mo (3.5–5.7) vs 4.0 mo (3.5–5.3), P < 0.001) | The most common grade 3 or worse AE were anemia or decreased hemoglobin (22% patients), followed by elevated ALT or AST (12%). Common serious AE included small intestine obstruction (5%), malignant neoplasm progression (5%). No treatment-related deaths occurred. |
| NCT01968213 (ARIEL3) | Rucaparib vs. Placebo | III | Completed | 11 countries | 2014–2016 | Platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma (N = 564) | Randomly allocated patients 2:1 to receive oral rucaparib 600 mg twice daily or placebo in 28-day cycles using a computer-generated sequence | Between April 7, 2014, and July 19, 2016 | Median PFS in BRCA-mutant carcinoma was 16.6 months in the rucaparib group vs. 5.4 months in the placebo group. In patients with a HR deficient carcinoma, it was 13.6 vs. 5.4 months respectively. | TEAE of grade 3 or higher were reported in 56% of patients in the rucaparib group and 15% in the placebo group. The most common AEs included anemia or decreased hemoglobin concentration (19% vs 1%) and increased alanine or aspartate aminotransferase concentration (10% vs. none). |
| NCT01847274 (ENGOT-OV16/NOVA Study) | Niraparib | III | Ongoing | GSK, US | 2013-current | Patients with platinum sensitive ovarian cancer (N = 578) | Niraparib vs placebo at 2:1 ratio administered once daily continuously during a 28-day cycle. | |||
| NCT02354586 (QUARA) | Niraparib | II | Completed | US, Canada | Ovarian Cancer Who Have Received Three or Four Previous Chemotherapy Regimens | Between April 1, 2015 to Nov 1, 2017 | Median OS = 12.2 months | Small intestinal obstruction, thrombocytopenia, vomiting | ||
| NCT02655016 (PRIMA) | Niraparib | III | Ongoing | 2016-current | Maintenance Treatment in Participants with Advanced Ovarian Cancer Following Response on Front-Line Platinum-Based Chemotherapy | Median PFS in patients treated with niraparib vs. placebo = 21.9 vs 10.4 months (HR = 0.43, 0.31–0.59) and OS = 13.8 vs. 8.2 months, respectively. | Anemia, thrombocytopenia and neutropenia | |||
| NCT03519230 | Pamiparib/BGB-290 | III | Ongoing | China | 2018-current | Platinum-sensitive recurrent ovarian cancer (N = 216) | ||||
| NCT01540565 | Veliparib/ABT-888 | II | Completed | United States | 2012–2018 | Persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer (N = 52) | Veliparib orally twice daily on days 1–28. Courses repeat every 28 days in the absence of disease progression or unacceptable toxicity. | |||
| NCT02470585 (VELIA) | Veliparib plus Carboplatin and Paclitaxel | III/IV | Ongoing | United States | 2015-current | Patients with newly diagnosed stage III or IV, high-grade serous, epithelial ovarian, fallopian tube, or primary peritoneal cancer (N = 1140) | Placebo + Carboplatin + Paclitaxel (C/P) -> Placebo; Veliparib + C/P -> Placebo; Veliparib + C/P -> Veliparib. Veliparib: 150 mg orally. Paclitaxel: intravenous infusion, either 80 mg/m2 of body-surface area (BSA) on Days 1, 8, and 15 of each 21-day cycle (weekly dosing), or 175 mg/m2 of BSA on Day 1 of each 21-day cycle (3-week dosing). Carboplatin: intravenous infusion at an area under the curve (AUC) of 6 mg/mL/min every 3 weeks. | In the BRCA-mutation cohort, the median PFS = 34.7 (veliparib) vs. 22 (control), HR = 0.44, 0.28–0.68. In the HRD cohort, the median PFS = 31.9 vs. 20.5 respectively, HR = 0.57, 95% CI: 0.43–0.76 | Anemia, thrombocytopenia, nausea, and fatigue | |
| Prostate Cancer | ||||||||||
| NCT02987543 (PROfound Study) | Olaparib vs. physician’s choice of enzalutamide or abiraterone plus prednisone | III | Ongoing | 21 countries | 2017-current | Metastatic castration-resistant prostate cancer who have failed prior treatment with a new hormonal agent and have HR repair gene mutations | 300 mg (2× 150 mg tablets) twice daily; enzalutamide (160 mg once daily) or abiraterone (1000 mg once daily) plus prednisone (5 mg twice daily) | Analysis data cutoff date of June 4, 2019 | Median PFS: Cohort A (at least one alteration in BRCA1, BRCA2, or ATM): 19.1 mo vs. 14.7 mo; HzR, 0.69; (0.50 to 0.97); P<0.02; Cohort B (at least one alteration in any of the other 12 prespecified genes): 14.1 mo vs. 11.5 mo | |
| NCT02952534 (TRITON2) | Rucaparib | II | Completed | 12 countries | 2016–2021 | Metastatic castration-resistant prostate cancer a BRCA alteration (N = 115) | Rucaparib 600 mg twice daily | Confirmed ORRs per independent radiology review and investigator assessment were 43.5% (95% CI, 31.0% to 56.7%; 27 of 62 patients) and 50.8% (95% CI, 38.1% to 63.4%; 33 of 65 patients), respectively. ORRs were similar for patients with a germline or somatic BRCA alteration and patients with a BRCA1 or BRCA2 alteration. | The most frequent grade ≥ 3 treatment-emergent adverse event was anemia (25.2%; 29 of 115 patients). | |
| Pancreatic Cancer | ||||||||||
| NCT02184195 (POLO) | Olaparib vs. Placebo | III | Completed | United States | 2014–2019 | Patients with gBRCA mutated metastatic pancreatic cancer without progression on first-line platinum-based chemotherapy (N = 154) | Olaparib tablets po. 300 mg twice daily | Median PFS was significantly longer in the olaparib group than in the placebo group (7.4 vs. 3.8 mo). | The incidence of grade 3 or higher AE was 40% in the olaparib group and 23% in the placebo group. | |
| Gastric Cancer | ||||||||||
| NCT03427814 | Pamiparib/BGB-290 | III | Ongoing | United States | 2018-current | Metastatic gastric cancer (N = 136) | ||||
HRD, homologous recombination deficiency; HGSOC, high-grade serous ovarian carcinoma; LOH, loss of heterozygosity; HRR, homologous recombination repair; PFS, progression-free survival; ORR, objective response rate; DOR, duration of response; OS, overall survival; PCT, physician’s choice of chemotherapy; AE, adverse event; TEAE, treatment-emergent adverse event; HzR/HR, hazard ratio; CI, confidence interval; BID/b.i.d., twice a day; p.o., per os/oral; mo, month
In the OlympiAD trial, 302 metastatic HER2 negative breast cancer patients were randomized in a 2:1 ratio to receive monotherapy of olaparib or treatment physician’s choice (TPC) among capecitabine, eribulin, or vinorelbine. The primary analysis showed that PFS was significantly increased in the olaparib arm (7.0 months) than in the TPC group (4.2 months, HR: 0.58; 95% CI: 0.43–0.80; P < 0.001). The overall response rate (ORR) was also higher in the olaparib group than in the standard therapy group (59.8% vs. 29.8%). In contrast, the overall survival (OS) was not different between the two arms [28]. The recent updated OS data also showed no improvement in the olaparib treatment compared to the standard chemotherapy (19.3 vs. 17.1 months; HR: 0.90, 95% CI: 0.66–1.23; p = 0.513). However, when the patients were stratified according to pre-defined subgroups (with or without prior chemotherapy for mBC, receptor status (HR+ and TNBC), and with or without prior platinum), an OS benefit was observed in patients who had not received prior chemotherapy for metastatic breast cancer (22.6 versus 14.7 months; HR: 0.51; 95% CI: 0.29–0.90) [32, 33]. In addition, olaparib was generally well-tolerated, without evidence of cumulative toxicity during extended exposure [32, 33].
Talazoparib is another FDA and EMA-approved PARPi to treat metastatic BRCA1/2-mutant HER2 negative breast cancers (NCT01945775, EMBRACA study) [34]. In the EMBRACA trial, a total of 431 patients were randomized in a 2:1 ratio to receive talazoparib and standard chemotherapy of the physician’s choice (capecitabine, eribulin, gemcitabine, or vinorelbine). 40% of the enrolled patients were TNBC subtype. This phase III clinical study demonstrated that talazoparib as monotherapy had significantly greater median progression-free survival (PFS) in patients with metastatic HER2-negative breast cancer (8.6 months) than standard chemotherapy treatment (5.6 months, HR 0.54, 95% CI [0.41, 0.71], P < 0.001) [34]. The PFS HRs were consistent among prespecified subgroups of HR+ (0.47, 95% CI: 0.32 to 0.71) and TNBC (0.60, 95% CI: 0.41 to 0.87) [35]. The subgroup analyses showed that across all patient subgroups with gBRCA-mutant advanced breast cancer, talazoparib demonstrated clinical benefit in outcomes compared with standard chemotherapy. The response rate by the investigators was also higher in the talazoparib arm than the chemotherapy arm (62.6% vs. 27.2%). The most recent OS analysis showed that talazoparib did not significantly improve OS over chemotherapy, and subsequent treatments may impact analysis. HR for OS with talazoparib versus chemotherapy was 0.848 (95% CI 0.670–1.073; P = 0.17) with median (95% CI) 19.3 months (16.6–22.5 months) versus 19.5 months (17.4–22.4 months) [36]. Safety was consistent with previous observations. Taken together, in patients with metastatic HER2-negative, gBRCA-mutant breast cancer, PARPis offer an improvement in PFS and tumor response rate [37].
Rucaparib is FDA- and/or EMA-approved in use of treating BRCA-mutant metastatic castration-resistant prostate cancer (NCT02952534, TRITON2) [38] and first-line maintenance treatment of advanced ovarian cancer dependent (NCT01891344, ARIEL2, and NCT01482715, Study 10) [39, 40], or those patients independent of BRCA mutations (NCT01968213, ARIEL3) [41] Similarly, niraparib is another FDA or EMA-approved PARPi to treat reoccurring ovarian, TFC, and PPC with complete or partial chemotherapy response (NCT01847274, ENGOT-OV16/NOVA Study). Its use has also been expanded to the same cancer types with HRD positive but regardless of chemotherapy response (NCT02354586, QUADRA study). Most recently, niraparib has been approved to be used in advanced ovarian, TFC, and PPC independent of biomarkers status (NCT02655016, PRIMA Study) [42]. Pamiparib has been used in phase II trials to treat advanced ovarian cancer and metastatic castration-resistant prostate cancer [43], and a randomized phase III trial for maintenance therapy in advanced gastric cancer that responds to platinum-based chemotherapy [44]. Of note, the results from a phase II completed study of pamiparib in the treatment of patients with locally advanced or metastatic HER2 negative breast cancer with gBRCA mutations showed that in the TNBC cohort, confirmed objective response rate (ORR) was 38.2% (95% CI: 25.4–52.3) versus 61.9% (95% CI: 38.4–81.9) in the HR+ cohort [45]. The median duration of response (DOR) was 6.97 and 7.49 months in the TNBC and HR+ cohort, respectively. Additionally, mPFS was 5.49 (95% CI: 3.65–7.33) and 9.20 months (95% CI: 7.39–11.93) in these two cohorts [45]. These data suggest pamiparib had a favorable response in patients with locally advanced/metastatic HER2 negative breast cancer with gBRCA mutations. Although veliparib has yet to be FDA-approved, promising preclinical data lead to multiple early clinical settings [46].
Different PARPis in clinical development have shown different potencies as PARP1 catalytic inhibitors and PARP trappers (Table 2). For example, olaparib reversibly inhibits PARP1, 2, and 3 isoforms and demonstrates an intense PARP trapping [22]. On the other hand, in preclinical studies, veliparib has been reported to be a weak PARP1 trapper, with a decreased synthetic lethality than more effective trappers, such as rucaparib, olaparib, talazoparib, and niraparib [13]. At present, no clinical trial has compared head-to-head different PARPis or assessed their PARP1 trapping potency in the clinical setting. Of note, iniparib (BiPar Sciences/Sanofi) was a compound developed as PARPi but based on the alternative premise of altering PARP1 zinc fingers (disturbing its activation by DNA breaks). After an unsuccessful phase III study in TNBCs, preclinical work demonstrated that iniparib was not a bona fide PARPi [14]. In addition, PARP1 also binds directly to DNA to act as a transcription factor and regulate chromatin structure remodeling. Recently, the role of PARP1 as a transcriptional regulator for nuclear factors has raised some interest due to the role of estrogen/progesterone and androgen receptors (all nuclear transcriptional factors) in breast and prostate cancer, respectively [15].
5. PARP inhibitor resistance and combination therapy
Even though several PARPis have been approved for advanced ovarian, breast, and pancreatic cancer indications, more than 40% of BRCA-mutant patients fail to respond to PARPi initially or develop resistance following treatment [47, 48]. The PARPi resistance is nearly inevitable. Several major mechanisms of PARPi resistance have been identified, such as restoration of HR and BRCA1/2 functions by reversion mutations and epigenetic modification, restoration of PARPylation and fork stability, upregulation of drug efflux pumps, and pharmacological alterations, which suggest potential strategies to overcome PARPi resistance through modulation of synthetic lethality mediated by PARPis [49, 50].
Based on the discovery of PARPi induced-synthetic lethality in BRCA-mutant and HRD cancer cells, the genetic concept of synthetic lethality can be further broadened into many therapeutic strategies. Synthetic lethality occurs when simultaneous perturbations of two genes or molecular processes significantly lose cell viability. Numerous studies have shown that strategy provoking a “BRCAness” phenotype can expand the applicability of PARPi in HR proficient tumors. The inhibitors of CDK12/13, PI3K, mTOR, ATM, CHK, WEE1, DNA methyltransferase, and histone deacetylase have been shown to suppress HR and lead to synergetic effects against TNBCs by decreasing HR-associated proteins or preventing HR proteins recruitment to DSB sites [51–55]. In addition, combining immunotherapy with PARPi also provides a new perspective for TNBC treatment. Below, the current status of the above-mentioned inhibitors in combination with PARPi in clinical trials are summarized (Table 4).
Table 4.
Combinations therapy of PARPi with Inhibitors of DDR, CDKs, epigenetics drugs and others in Clinical Trials
| Drugs | Clinical Trial ID | Phase | Treatment | Status | Study Population | Outcomes |
|---|---|---|---|---|---|---|
| NCT02723864 | I | Veliparib + VX-970 | Active, not recruiting | Refractory solid tumors | ||
| NCT04267939 | I | Niraparib + BAY1895344 | Recruiting | Advanced solid tumors and ovarian cancer | ||
| NCT02576444 (OLAPCO) | II | Olaparib + AZD6738 | Active, not recruiting | Tumors harboring HDR gene mutations, including ATM, CHK2, APOBEC, MRE11 complex | ||
| NCT04065269 (ATARI) | II | Olaparib + AZD6738 | Recruiting | Gynecological cancers with ARID1A loss or no loss | ||
| NCT03787680 (TRAP) | II | Olaparib + AZD6738 | Active, not recruiting | Metastatic Castration-Resistant Prostate Cancer | ||
| NCT03878095 | II | Olaparib + AZD6738 | Recruiting | IDH1 and IDH2 mutant tumors | ||
| NCT03462342 (CAPRI) | II | Olaparib + AZD6738 | Recruiting | HGSC | ||
| PARPi-ATRi | NCT03428607 (SUKSES-N2) | II | Olaparib + AZD6738 | Completed | SCLC | |
| NCT03682289 | II | Olaparib + AZD6738 | Recruiting | Clear cell renal cell cancer; metastatic renal cell cancer; metastatic urothelial cancer; metastatic pancreatic cancer; locally advanced pancreatic cancer | ||
| NCT04417062 | II | Olaparib + Ceralasetib | Recruiting | Recurrent or refractory sarcoma | ||
| NCT04090567 | II | Olaparib + Ceralasetib/AZD6738 | Recruiting | BRCA mutant advanced breast cancer | ||
| NCT04655183 | II | Niraparib + M4344/VX-803 | Withdrawal | Advanced solid tumors | ||
| NCT04239014 (DUETTE) | II | Olaparib + Ceralasetib | Withdrawal | Platinum sensitive relapsed epithelial ovarian cancer | ||
| NCT03330847 (VIOLETTE) | II | Olaparib + AZD6738 | Active, not recruiting | Metastatic triple negative breast cancer stratified by alterations of HRR-related genes | ||
| PARPi-CHK1i | NCT03057145 | I | Olaparib + Prexasertib | Completed | Advanced solid tumors | |
| PARPI-ATMi | No clinical trials | |||||
| NCT03579316 (EFFORT) | II | Olaparib + AZD1775/Adavosertib | Recruiting | Recurrent fallopian tube, ovarian and primary peritoneal cancers | Adavosertib: ORR = 23%; median PFS = 5.5 mo; median duration of response = 5.5 mo; Adavosertib + Olaparib: ORR = 29%; median PFS = 6.8 mo; median duration of response = 6.4 mo | |
| NCT04197713 (STAR study) | I | Olaparib + AZD1775 | Recruiting | Advanced solid tumors with selected mutations and PARP Resistance | ||
| PARPi-WEE1i | NCT02576444 (OLAPCO) | II | Olaparib + AZD1775 | Active, not recruiting | Tumors harboring either TP53 or KRAS mutations or mutations in KRAS and TP53 | |
| NCT02511795 | Ib | Olaparib + AZD1775 | Completed | Refractory solid tumors | ORR for the total population, cohort 4.2 (A: 175 mg BID + O: 200 mg BID) and cohort 7.4 (A: 200 mg QD + O: 200 mg BID) was 11.1%, 30.8% and 0%, respectively, and the DCR was 55.7%, 76.9% and 53.8%. | |
| NCT03330847 (VIOLETTE) | II | Olaparib + AZD1775 | Active, not recruiting | Metastatic triple negative breast cancer stratified by alterations of HRR-related genes | ||
| PARPi-MYT1i | No clinical trials | |||||
| PARPi-POLqi | NCT04991480 | I/II | Talazoparib + ART4215 | Recruiting | Metastatic breast cancer | |
| PARPi- HSP90i | NCT02627430 | I | Talazoparib + AT13387 | Withdrawal | Metastatic solid tumor or recurrent ovarian, fallopian tube, primary peritoneal, or triple negative breast cancer | |
| NCT02898207 | I | Olaparib + AT13387/Onalespib | Active, not recruiting | Metastatic solid tumor or recurrent ovarian, fallopian tube, primary peritoneal, or triple negative breast cancer | ||
| PARPi-AKTi | NCT02576444 (OLAPCO) | II | Olaparib + AZD5363/capivasertib | Active, not recruiting | Tumors harboring PTEN, PIK3CA, AKT, or ARID1A mutations or other molecular aberrations leading to dysregulation of the PI3K/AKT pathway | |
| NCT02208375 | I/II | Olaparib + AZD5363/capivasertib | Active, not recruiting | BRCA mutant recurrent endometrial and ovarian cancer | ||
| PARPi-CDKi | NCT01434316 | I | Veliparib/ABT888 + Dinaciclib | Recruiting | Advanced solid tumors | |
| NCT04355858 (MULAN) | II | SHR3162 + SHR6390 (CDK4/6i) | Recruiting | HR+/HER2- endocrine-resistant advanced breast cancer | ||
| PARPi-mTOR1/2i | NCT02208375 | Ib/II | Olaparib + AZD2014/vistusertib | Active, not recruiting | BRCA mutant recurrent endometrial and ovarian cancer | Mean response rate = 19%; median duration = 14 mos.; clinical benefit rate = 34% |
| PARPi-MEK/ERKi | NCT04005690 | I | Olaparib + Cobimetinib | Recruiting | Pancreatic cancer | |
| PARPi-MEKi-PD1 | NCT03182673 | I | SHR3162 + SHR7390 + SHR1210 | N.A. | Advanced solid tumors | |
| NCT04586335 | I | Olaparib + CYH33 | Recruiting | Ovarian, breast, prostate and endometrial cancers | ||
| PARPi-PI3Ki | NCT01623349 | I | Olaparib + BKM120 or BYL719 | Completed | Recurrent TNBC or HGSOC | 36% patients achieved a partial response and 50% patients had stable disease. |
| NCT03586661 | I | Niraparib + Copanlisib | Recruiting | Recurrent endometrial, ovarian, primary peritoneal, or fallopian tube cancer | ||
| PARPi-VEGFi | NCT01116648 | II | Olaparib + cediranib/AZD2171 | Completed | Non-BRCA mutant recurrent platinum resistant ovarian cancer (N = 90) | Median PFS was 17.7 months for women with cediranib plus olaparib combination treatment compared with 9.0 months for those with olaparib monotherapy. |
| NCT02889900 (CONCERTO) | Iib | Olaparib + cediranib/AZD2171 | Completed | Non-BRCA mutant recurrent platinum resistant ovarian cancer (N = 62) | Objective response rate = 15.3%; median PFS = 5.1 months; median duration of response = 8.3 months; median OS = 13.2 months. | |
| NCT03742245 | I/Ib | Olaparib + Vorinostat/SAHA | Recruiting | Relapsed/refractory and/or metastatic breast cancer | ||
| NCT04703920 | I | Talazoparib + Belinostat | Recruiting | Metastatic breast cancer, metastatic castration resistant prostate cancer, and metastatic ovarian cancer | ||
| PARPi- epigenetic drugs | NCT03259503 | I | Olaparib + multiple chemo including Vorinostat | Recruiting | Relapsed or refractory lymphomas undergoing stem cell transplant | |
| NCT02878785 | I/II | Talazoparib + Decitabine/Dacogen (DAC) | Active, not recruiting | Untreated AML and Relapsed/refractory AML | ||
| NCT04355858 (MULAN) | II | SHR3162 + SHR2554 (EZH2i) | Recruiting | HR+/HER2- endocrine-resistant advanced breast cancer |
HGSC: high-grade serous carcinoma; SCLC, small cell lung cancer; TNBC, triple-negative breast cancer; AML, acute myeloid leukemia; HRR, homologous recombination repair; DCR, disease control rate; ORR, objective response rate; PFS, progression-free survival; BID/b.i.d., twice a day; QD/q.d., every day; mo, month
a. PARPi and cell-cycle checkpoints inhibitors
Previous studies suggest that abrogation of cell cycle checkpoint signaling may alleviate resistance to PARPis. ATM and ATR are the two major kinases regulating cell-cycle checkpoint activations and arrest cells in response to DNA damage. ATR is activated by stalled replication forks and plays a key role in the DNA replication stress response through facilitating the recovery from stalled replication forks and preventing premature mitosis [56]. Several mechanisms of PARPi’s resistance, including BRCA1-independent HR and fork protection, are dependent on ATR activity by promoting RAD51 loading onto DSBs and stalled forks [57]. Like ATR, ATM kinase activity is also required for the early stage of HR, and the inhibition of ATR in cancer cells with certain DDR defects (e.g., ATM loss) induces rapid cell death with synthetic lethality. Germline ATM mutations predispose an estimated 20–60% increased risk for breast cancer and are associated with HRD in BRCA-wild type breast cancer patients [58]. Thus, ATR inhibitors (ATRis) in ATM-deficient tumor cells or combination treatment comprising PARPi and ATRi are currently being investigated in several preclinical studies, as a synthetical lethal combination [57, 59–61]. In addition to the above-mentioned agents, PARPi can also be combined with CHK1 inhibitors[59]. CHK1 and 2 kinases are downstream of the checkpoint kinases, particularly ATM and ATR. In response to PARPi-induced replication stress, CHK1 is activated to relay the signal to S and G2/M cell cycle checkpoint kinases, and inhibition of CHK1 increases PARPi-induced DNA damage and inhibits RAD51 focus formation and HRR [62]. Since PARPi increases the reliance on ATR/CHK1 for maintaining genome stability, the combination of PARPi with ATR/CHK1 inhibition suppressed ovarian cancer cell growth regardless of the status of HRR [63]. There are currently several early-phase clinical trials combining ATRis and PARPis to treat advanced cancers of the breast, ovarian, prostate, sarcoma, pancreatic, renal cell with or without HDR gene mutations. These ongoing early-phase clinical trials are summarized in Table 4 (NCT02723864, NCT04267939, NCT02576444 (OLAPCO), NCT04065269 (ATARI), NCT03787680 (TRAP), NCT03878095, NCT03462342 (CAPRI), NCT03428607 (SUKSES-N2), NCT03682289, NCT04417062, NCT04090567, NCT04655183, NCT04239014 (DUETTE), NCT03330847 (VIOLETTE)). Final results are still pending from these studies, and there is no available clinical trial combining ATMi and PARPis to treat cancer patients.
Another inhibitor that modulates DNA damage/repair pathway is the WEE1 kinase inhibitor. WEE1 kinase regulates G2/M progression by inhibiting CDK1 and CDK2, resulting in cell-cycle arrest facilitating DNA repair [64]. Recent studies demonstrate that the G2/M checkpoint WEE1 inhibitor, adavosertib or AZD1775, sensitizes TNBC cells to olaparib by modulating DNA damage response [65]. Another study shows synergistic effects between WEE1 and PARPi (rucaparib) in BRCA2-mutant cells [66]. In addition, combining WEE1 inhibitor (AZD-1775) and PARPi (MK-4837) demonstrates synergistic effects in BRCA1-mutant TNBC with high levels of Cyclin E protein expression [67]. Currently, five clinical trials are using WEE1 inhibitor AZD-1775 combined with olaparib to treat metastatic TNBC, SCLC, recurrent fallopian tube, ovarian, and primary peritoneal cancers with or without HRR-related genes alteration [NCT03579316 (EFFORT), NCT04197713 (STAR ), NCT02576444 (OLAPCO), NCT02511795, NCT03330847 (VIOLETTE)] (Table 4).
In the EFFORT (NCT03579316) study, a randomized two-arm phase II clinical trial investigated the efficacy of adavosertib in PARP-resistant ovarian cancer. The efficacy results show that the objective response rate (ORR) is 23% on the adavosertib arm versus 29% on the adavosertib plus olaparib arm. The duration response to the treatment also increased from 5.5 months on the adavosertib arm to 6.4 months on the combination treatment arm. Additionally, median PFS was increased from 5.3 months to 6.8 months, indicating that the patients benefit from the treatment of adavosertib combined with olaparib [68]. Another phase Ib study (NCT02511795) of adavosertib in combination with olaparib in patients with refractory solid tumors showed the antitumor activity with combination treatment. The maximum tolerated dose was determined to be adavosertib (A) 175 mg for 2/3 weeks plus olaparib (O) 200mg BID. The ORR for the total population, cohort 4.2 (A: 175 mg BID + O: 200 mg BID) and cohort 7.4 (A: 200 mg QD + O: 200 mg BID) was 11.1%, 30.8% and 0%, respectively, and the disease control rate (DCR) was 55.7%, 76.9% and 53.8%. Common adverse effects were anemia, neutropenia, and thrombocytopenia [69].
Another kinase in the same class as WEE1 kinase is the MYT1 kinase, which also inhibits CDK1 by phosphorylation, contributing to checkpoint recovery in a WEE1-independent manner [70]. Specifically, cyclin B-CDK1 complexes are inactivated before mitosis through phosphorylation of Thr14 and Tyr15 by WEE1 and MYT1. WEE1 specifically phosphorylates Tyr15 on both CDK1 and CDK2 [64], while MYT1 preferentially phosphorylates Thr14 on CDK1 [71, 72]. A previous study suggested that upregulation of MYT1 promotes acquired resistance of cancer cells to WEE1 inhibition while downregulating MYT1 enhances ectopic CDK1 activity and restores sensitivity to the WEE1 inhibitor (adavosertib) [73]. These data suggest MYT1 could be a potential predictive biomarker of acquired resistance to adavosertib. Currently, there is only one recent phase-I clinical trial of MYT1 inhibitor (RP-6306) as monotherapy in treating advanced solid tumors, which was initiated in late 2021, and no results are yet available (NCT04855656).
Very recently, one novel DDR target, the DNA polymerase theta (POLθor POLQ) inhibitor, ART4215, entered a phase I/II clinical trial as monotherapy or combination therapy with talazoparib for the treatment of metastatic breast cancers (NCT04991480). It has been shown that Polθ is highly expressed in HR-deficit breast and ovarian cancers [74], and plays a role in DNA DSB repair, and can compensate for the HR loss in tumor cells [75]. Both in vitro and in vivo studies demonstrate that POLθ inhibitor shows a synthetic lethal effect in HR-deficit tumor cells or cells with acquired PARPi resistance and elicits PARPi synergy [76, 77]. These studies suggest that Polθ inhibitors not only have clinical potential in targeting HR defective cancers but could also be used to target PARPi resistance.
PARPi is also suggested to be combined with other drugs such as heat shock protein 90 (HSP90) inhibitors. Previous studies show that HSP90 promotes the stabilization of HR proteins, including BRCA1, BRCA2, and RAD51 [78, 79]. Furthermore, the HSP90 inhibitor, 17-AAG, suppresses HR DNA repair and enhances sensitivity to carboplatin and olaparib in ovarian and breast cancer cells [79, 80]. Another in vivo study shows the synergistic effect of olaparib and HSP90 inhibitor (AT13387) using HGSOC patients-derived xenograft mouse models [81]. Currently, only one early-phase clinical trial is still accruing patients to investigate the combination treatment of HSP90 inhibitor (AT13387) with PARPi olaparib (NCT02898207), while another study using HSP90 inhibitor combined with talazoparib to treat recurrent epithelial ovarian, fallopian tube, peritoneal cancer to advanced TNBC has been withdrawn due to low participants (NCT02627430).
b. PARPi-CDKs inhibitors
Another class of proteins that regulate DNA repair pathways are the CDKs [82]. Previous studies reported that when DSB occurs, 53BP1 and RIF1 proteins inhibit BRCA1 recruitment to the DSB lesions, leading to the inhibition of DNA end resection and promoting the activation of the NHEJ repair pathway [83]. DNA end resection, which generates a long 3′ single-stranded DNA (ssDNA) tail that can invade the homologous DNA strand [84], is a vital step in HR repair and is dependent on the kinase activities of at least two CDKs (CDK5 and CDK12). Both these CDKs participate in the PARPi resistance [85]. For example, when CDK5 is knocked out in HeLa cells, these cells become more sensitive to PARPi [85]. Furthermore, the analysis of genome-wide profiling of synthetic genetic lethality in HGSOC also identified CDK12 as a novel marker of PARP1/2 sensitivity [86]. Consistently, knockout of CDK12 disrupts HR repair and sensitizes ovarian cancer cells to cisplatin and PARPi [87]. CDK12 inhibition can also reverse intrinsic or required PARPi resistance in TNBC cells, and patient-derived xenograft (PDX) models derived from TNBC patients [88]. Other studies have reported that other CDKs with regulatory activities it the G1 to S transition (CDK2, 4, and 6) may also be involved in modulating PARPi. For example, a recent study suggests that CDK4/6 inhibitor (palbociclib) combined with PARPi was synergistic in mediating a therapeutic response as compared to single agents in treating BRCA-mutant ER (+) breast cancer cells [89]. Additionally, CDK2 combined with PARPi display synergistic effects in BRCA1-mutant breast cancer due to the identification of convergence between BRCA1 loss and high cyclin E1 expression using a basal-like breast cancer cohort [90]. Intriguingly, when the CDKs inhibitor dinaciclib is combined with PARPi (niraparib) it can resensitize niraparib-resistant TNBC cells through downregulating MYC expression and inhibiting HR, EMT, and cancer stem-like phenotype [91]. This synthetic lethal strategy can further expand in effectively treating ovarian, prostate, colon, and lung cancer cells independent of BRCA status [91]. The only early-phase clinical trial currently investigates the combination treatment of veliparib and the CDK inhibitor dinaciclib in advanced solid tumors (NCT01434316).
In addition to the above mentioned, PARPi is likely to synergize with anti-angiogenic agents (bevacizumab or cediranib) [92], mTORC1/2 kinase inhibitors of mTORC1/2 (AZD2014 and everolimus), AKT (AZD5363) [93], MEK (cobimetinib), and PI3K (BKM120, BYL719, and copanlisib). These combination trials are conducted in the neoadjuvant setting and advanced diseases, all early Phase I trials (Table 4). The efficacy results from one phase I clinical trial (NCT02208375) of olaparib and visusertib (mTOR1/2i) in recurrent endometrial, ovarian, and TNBC show that the combination therapy was well tolerated with durable anti-tumor activity, and a favorable response was observed in endometrial cancers. In the continuous BID cohort, the mean response rate was 19% with a median duration of 14 months, and the clinical benefit rate was 34%. The data of BID 2 days on and 5 days off group were similar. In addition, the most common adverse effects were nausea, followed by anemia, hyperglycemia, fatigue, leukopenia, increased creatinine, and headaches [94]. The results from another early phase Ia clinical trial (NCT03586661) of olaparib and alpelisib (α-specific PI3Ki) in patients with epithelial ovarian cancer demonstrated that 36% of patients achieved a partial response and 50% of patients had stable disease, suggesting a promising therapeutic strategy particularly in epithelial ovarian cancer. In addition, combining alpelisib and olaparib is feasible with no unexpected toxic effects, and the common adverse effects are hyperglycemia and nausea [95].
c. PARPi-epigenetic and post-translational modification
Epigenetic modifications may be an alternative mechanism leading to PARPi resistance. For example, it was recently reported that loss of BRCA1 promoter methylation can restore the BRCA1 expression resulting in resistance to PARPi (rucaparib) in ovarian cancer [96]. Another study shows that hyperacetylation of 53BP1, similar to depletion of 53BP1, inhibits NHEJ and restores the HR DNA repair pathway leading to PARPi resistance in BRCA1-mutant cells [97]. In addition, phosphorylation of PARP1 at Tyr907, mediated by c-Met, increases PARP1 enzymatic activity and inhibits its binding to PARPi, thereby rendering cells resistant to PARPis [98]. In fact, the combination of c-Met inhibitor (crizotinib and foretinib) and PARPi (ABT-888 and AG014699) synergistically inhibit TNBC cells in vitro and TNBC xenograft models in vivo [98]. Furthermore, PAR glycohydrolase (PARG) has been identified as an alternative major resistance mechanism of PARPi using genetic screens and multi-omics analysis in BRCA2-mutant mouse mammary tumors. PARG depletion restores PAR formation and rescues the PARP1 pathway [99].
Intriguingly, numerous studies have shown that the combination of histone deacetylation inhibitor (HDACi) with PARPi exhibits synergistic effects and resensitizes different types of cancer cells to PARPi [100–103]. For example, studies in TNBC tumors show that pan-histone deacetylase inhibitors (Vorinostat/VS and Panobinostat/PS) induce hyperacetylation of the nuclear HSP90 and cause deletion DDR and HR proteins, such as ATR and CHK1, leading to BRCAness in the BRCA1-mutant TNBC cells [101]. Another study also shows that when olaparib is combined with the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamic acid, SAHA), it results in synergistic growth inhibition of TNBC cells [103]. Moreover, low doses of DNA methyltransferase inhibitor (DNMTi), vidaza (5-AZA), or dacogen (DAC) down-regulate HR gene expression and induce BRCAness phenotype, which results in the sensitization of NSCLC to PARPi [51]. Epigenetic-PARPi combination therapy also enhances the therapeutic effects in breast and ovarian cancers regardless of BRCA mutational status [104]. A recent report suggests the next generation of DNMTi (GSK3685032) more specifically targets DNMT1 with a longer half-life, a higher durable hypomethylating activity, and less off-target effects, such as cytotoxicity and DNA damage, compared to nucleoside analogs AZA or DAC [105]. Currently, four early-phase clinical trials are accruing patients to investigate the combination treatment of PARPi with the HDACi (vorinostat) or DNMTi (dacogen) in patients with metastatic breast cancer, castration-resistant prostate cancer, ovarian cancer, refractory AML, and lymphomas (NCT03742245, NCT04703920, NCT03259503, NCT02878785) (Table 4). Results are pending for these trials until accrual are completed.
d. PARPi with DNA-damage agents
Although platinum or other alkylating chemotherapies represent the backbone of anti-cancer therapy for several malignancies, their efficacy is overshadowed by a vast of side effects. Theoretically, PARP may become critical for repairing SSBs induced by alkylating agents, and inhibition of catalytic PARP and PARP trapping display synergistic effects with DNA-damage agents. The BROCADE3 trial (NCT02163694) evaluated the veliparib with carboplatin/paclitaxel (C/P) versus placebo plus C/P in patients with HER2 negative, gBRCA-mutant metastatic breast cancer. In patients who had no prior cytotoxic chemotherapy in the metastatic setting, median PFS was increased in patients with veliparib plus C/P compared to patients with placebo plus C/P (16.6 vs. 13.1 months) [106]. Addition of veliparib to C/P treatment significantly prolonged PFS in patients with HER2-negative, gBRCA-mutant metastatic breast cancer compared to the placebo plus C/P group (HR = 0.71; 95% CI, 0.57–0.88; P = 0.002). The increased PFS was also observed in the subgroup of HR+/HER2- (HR = 0.69; 95% CI, 0.52–0.93; P = 0.013) and TNBC patients (HR = 0.72; 95% CI, 0.52–1.00; P = 0.051) [107].
However, the combination of PARPis and platinum agents have development challenges due to overlapping toxicities, including myelosuppression [108]. In the BROCADE3 trial, adverse events led to study drug discontinuation in 8.0% vs. 3.3% of HR+/HER2- patients and 10.5% vs. 7.5% of TNBC patients in the veliparib plus C/P and placebo plus C/P arms, respectively [107]. A major issue with the strategy of enhancing the activity of chemotherapy using PARPi is a narrow therapeutic window due to the off-target effects of both drugs on normal cells, thereby enhancing toxicity from chemotherapy.
6. PARP inhibitors and immunotherapy
a. PARPi with Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs), featured by PD1/PD-L1 (programmed cell death (ligand) 1) and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) blocking antibodies have demonstrated clinical efficacy and generated a durable response in a variety of cancers [109]. To date, FDA has approved the clinical use of up to eight ICIs, including one anti-CTLA-4 antibody (ipilimumab), four anti-PD1 antibodies (pembrolizumab, nivolumab, cemiplimab and dostarlimab) and three anti-PD-L1 antibodies (atezolizumab, avelumab and durvalumab) for treating different tumors include TNBC in both monotherapy and combination therapy regimens [110]. It has been shown that TNBC tumors bear defects in the DNA damage repair pathway, resulting in genomic instability [111], and in conjunction with the PARPis, induce synthetic lethality in patients with the BRCA1/2 mutations. Recent studies also demonstrate that combination therapy with ICIs and PARPi is a promising therapeutic strategy for the treatment of TNBC [112]. Both in vitro and in vivo studies demonstrate that PARPi upregulates PD-L1 expression in breast cancer cells and activates tumor-infiltrating T cells [113]. Currently, there is one ongoing clinical trial to evaluate the changes of PD-L1 expression levels in patients with early-stage TNBC after being treated with rucaparib in a window of opportunity study (NCT03911453). The successful completion of this trial will provide rationale and guidance if the sequential treatment of PARPi followed by PD-L1 is more effective than a concomitant treatment strategy.
In KEYNOTE-162 (TOPACIO/NCT02657889), a Phase 1/2 study evaluating the safety and efficacy of combination treatment with PARPi (niraparib) and anti-PD1 antibody (pembrolizumab) in patients with advanced or metastatic TNBC or recurrent ovarian cancer, an ORR of 21% has been reported. Of note, in the patients with BRCA mutations, a higher ORR of 47% was observed [114]. In addition, studies have been designed to explore the combination of PARPi with anti-PD-L1 antibody, which include two ongoing Phase 2 studies (DORA/NCT03167619 and NCT03801369) assessing the efficacy of the combination of olaparib and durvalumab in the treatment of patients with metastatic TNBC. Moreover, the benefit of combining ICI, PARPi, and VEGFRi is being explored in a Phase I/II study (NCT02484404) of durvalumab in combination with olaparib and/or cediranib for treatment of advanced solid tumors including TNBC. Current clinical trials for the ICI and PARPi combination therapy for TNBC treatment are summarized in Table 5. Interim results from the study (NCT02484404) have been reported, which showed limited efficacy of durvalumab in combination olaparib in relapsed SCLC patients [115]. Therefore, pretreatment and during-treatment biopsy specimens reflecting the tumor immune phenotypes may provide predictive values.
Table 5.
Different PARP Inhibitors Comparison and Summarized Clinical Benefits
| Drugs | Clinical Trial ID | Phase | Treatment | Status | Study Population |
|---|---|---|---|---|---|
| NCT02734004 | I/II | Olaparib + MED14736 | Active, not recruiting | Ovarian, breast, SCLCand gastric cancers | |
| NCT03824704 (ARIES) | II | Rucaparib + Nivolumab | Terminated | Epithelia ovarian cancer, fallopian tube cancer, primary peritoneal cancer, HGSCand endometrioid adenocarcinoma | |
| NCT02849496 | II | Olaparib + Atezolizumab | Recruiting | Locally advanced unresectable; metastatic non-HER2-positive breast cancer | |
| NCT04782089 | I | Fluzoparib + Camrelizumab | Not yet recruiting | Extensive stage small cell lung cancer | |
| NCT04790955 | N.A. | PARPi + Temozolomide | Not yet recruiting | SCLC after initial treatment failure | |
| NCT04483544 | II | Olaparib + Pembrolizumab | Recruiting | Advanced cervical cancer | |
| NCT03639935 | II | Rucaparib + Nivolumab | Recruiting | Advanced or metastatic biliary tract cancer following platinum therapy | |
| NCT03651206 | II/III | Niraparib + Dostarlimab/TSR-042 | Recruiting | Recurrent ovarian carcinosarcoma | |
| NCT02660034 | I | Pamiparib/BGB-290 + tislelizumab/BGB-A317 | Completed | Advanced solid tumors | |
| NCT04701307 | II | Niraparib + Dostarlimab/TSR-042 | Recruiting | SCLC and other high-grade neuroendocrine carcinomas | |
| NCT03308942 | II | Niraparib + Dostarlimab or Pembrolizumab | Completed | NSCLC | |
| PARPi-PD1 | NCT03574779 (OPAL) | II | Niraparib + Dostarlimab + Bevacizumab | Active, not recruiting | Recurrent ovarian cancer |
| NCT05065021 | II | Niraparib + Dostarlimab + Bevacizumab | Not yet recruiting | Ovarian, fallopian tube, primary peritoneal, high grade serous and endometrioid cancers | |
| NCT05093231 | II | Olaparib + Pembrolizumab | Not yet recruiting | Pancreatic cancer | |
| NCT03955471 (MOONSTONE) | II | Niraparib + Dostarlimab/TSR-042 | Active, not recruiting | Platinum resistant ovarian cancer | |
| NCT04779151 (NIRADO) | II | Niraparib + Dostarlimab/TSR-042 | Not yet recruiting | Patients with DNA repair-deficient or platinum-sensitive solid tumors | |
| NCT04508803 (CHANGEABLE) | II | Niraparib + HX008 ± Trastuzumab | Not yet recruiting | Germline-mutated metastatic breast cancer | |
| NCT04380636 (KEYLYNK-012) | III | pembrolizumab+chemoradiation→pembrolizumab±olaparib | Recruiting | NSCLC | |
| NCT04978012 | II | Fluzoparib + Camrelizumab | Not yet recruiting | Nasopharyngeal carcinoma | |
| NCT02657889 (TOPACIO/KEYNOTE-162) | I/II | Niraparib + Pembrolizumab | Completed | TNBC or ovarian cancer | |
| NCT03654833 (MiST) | II | Niraparib + Dostarlimab | Recruiting | Malignant mesothelioma | |
| NCT03951415 (DOMEC) | II | Olaparib + Durvalumab | Active, not recruiting | Metastatic or recurrent endometrial cancer | |
| NCT04276376 (ARIANES) | II | Rucaparib + Atezolizumab | Recruiting | Patients with DNA repair-deficient or platinum-sensitive solid tumors | |
| NCT03308942 | II | Niraparib + Pembrolizumab or Dostarlimab | Completed | HSCLC | |
| NCT04739800 | II | Olaparib + Durvalumab + Cediranib | Recruiting | Recurrent or refractory solid tumors | |
| NCT04837209 (NADiR) | II | Niraparib + Dostarlimab | Recruiting | TNBC | |
| NCT03851614 (DAPPER) | II | Olaparib + Durvalumab or Cediranib | Active, not recruiting | Mismatch repair proficient colorectal cancer, pancreatic adenocarcinoma, leiomyosarcoma | |
| NCT02734004 (MEDIOLA) | I/II | Olaparib + MEDI4736 + Bevacizumab | Completed | Ovarian, breast, SCLC, gastric cancers | |
| NCT02849496 | II | Olaparib + Atezolizumab | Recruiting | BRCA mutant HER2-negative breast cancer | |
| PARPi-PD-L1 | NCT02484404 | I/II | Olaparib + Durvalumab ± Cediranib | Recruiting | Advanced solid tumors and advanced or recurrent ovarian, TNBC, lung, prostate and colorectal cancers |
| NCT03330405 (Javelin Parp Medley) | II | Talazoparib + Avelumab | Active, not recruiting | Locally advanced or metastatic solid tumors | |
| NCT03167619 (DORA) | II | Olaparib + Durvalumab | Active, not recruiting | Platinum treated advanced TNBC | |
| NCT03801369 | II | Olaparib + Durvalumab | Recruiting | Advanced TNBC | |
| NCT03025035 | II | Olaparib + Pembrolizumab | Recruiting | Advanced BRCA-mutated or HDR-defect breast cancer | |
| NCT05033756 (COMPRENDO) | II | Olaparib + Pembrolizumab | Not yet recruiting | Unresectable or metastatic HER2 negative breast cancer and a deleterious germline mutation or an HRD | |
| NCT04683679 | II | Olaparib + Pembrolizumab + Radiation | Recruiting | TNBC | |
| NCT03594396 | II | Olaparib + Durvalumab | Active, not recruiting | Before standard neoadjuvant chemotherapy for stage II/III TNBC or Low ER+ breast cancer | |
| NCT04191135 (KEYLYNK-009) | II | Olaparib + Pembrolizumab | Active, not recruiting | TNBC | |
| NCT02571725 | I/II | Olaparib + Tremelimumab | Active, not recruiting | BRCA-deficient ovarian cancer | |
| PARPi-CTLA4 | NCT04034927 | II | Olaparib + Tremelimumab | Active, not recruiting | Recurrent ovarian, fallopian tube or peritoneal cancer |
| NCT02953457 | II | Olaparib + Tremelimumab + Duralumab | Active, not recruiting | Recurrent or refractory ovarian, fallopian tube or primary peritoneal cancer with BRCA1 or BRCA2 mutation | |
| PARPi-ADCs | NCT04039230 | I/II | Talazoparib + Sacituzumab Govitecan/SG | Recruiting | Metastatic breast cancer |
| NCT04585958 | I | Olaparib + Trastuzumab Deruxtecan/T-DXd | Recruiting | HER2 expressing tumors, endometrial cancer | |
| PARPi-NKs | No clinical trials |
SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; TNBC, triple-negative breast cancer; HRD, homologous recombination deficiency
b. PARPi with Antibody Drug Conjugate
Compared to small molecule inhibitors, antibody therapy usually demonstrates high target specificity, long serum half-life, and multiple mechanisms of action such as blocking receptor-ligand interaction, antibody-dependent cellular cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC) [116, 117]. In the last decade, antibody therapy has evolved into a new design called antibody-drug conjugate (ADC), which combines features of the high specificity of antibodies and toxicity of small molecule drugs [118]. The antibody and small molecule toxin in ADC are conjugated through a well-designed chemical linker [119]. Upon binding to its target on tumor cells, ADCs are internalized into cells, and the toxin is released from the antibody moiety through cleavage of the linker in intracellular compartments such as endosomes and lysosomes. The released toxin then destroys tumor cells from inside by inhibiting cellular processes, including DNA synthesis and microtubule assembly [120].
As of the writing of this review, twelve different ADCs have been approved by the FDA, including the recent approval in 2020 of sacituzumab govitecan (Trodelvy), which targets the Trop-2 tumor antigen for the treatment of metastatic TNBC patients who have received at least two prior therapies. In a preclinical study, a combination of sacituzumab govitecan with PARPis (olaparib or talazoparib) results in significantly-improved antitumor efficacy and delay in tumor progression time compared to monotherapy in a mouse model bearing BRCA1/2-mutated HCC1806 TNBC tumors [121]. Of importance, similarly increased survival and inhibition of tumor progression have also been observed in mice bearing BRCA1/2-wild-type MDA-MB-468 or MDA-MB-231 tumors with sacituzumab govitecan plus olaparib treatment [121]. Another ongoing phase I/II clinical trial investigates the effect of combination treatment of sacituzumab govitecan with talazoparib in metastatic breast cancer patients (NCT04039230). These encouraging results indicate a combination of PARPi with topoisomerase I inhibition by sacituzumab govitecan leads to synthetic lethality in TNBC tumors and suggest that such therapy is a new promising combination regimen for treating TNBC tumors regardless of the BRCA1/2 status.
c. PARPi with Engineered Tumor-Infiltrating Lymphocytes
Although classified as a unique group in breast cancers based on genetic features, TNBC is a highly heterogeneous tumor. In two independent studies, over 22–28% of TNBC tumors showed ≥ 50–60% (lymphocyte-predominant breast cancer, LPBC) phenotype, and the presence of tumor-infiltrating lymphocytes (TILs) was associated with better prognosis, including improved disease-free survival as well as overall survival [122–124]. Especially, breast cancer patients with BRCA1/2 mutations are usually associated with increased genomic instability and a high number of stromal TILs [125]. Thus, pairing the PARPi with TILs isolated from the tumor and expanded ex vivo can be a promising combination therapy for treating TNBC. However, it has been shown that PARPi can upregulate PD-L1 expression and enhance cancer-associated immunosuppression [113]. To overcome such immunosuppressive tumor microenvironment, before infusion back to patients, TILs expanded ex vivo can be engineered with PD-L1 targeting molecules, either in a membrane-bound form as a chimeric antigen receptor (CAR) or in a soluble format such as antibody or antibody fragment. Such armed TILs and PARPi present a novel approach and are worthy of investigation in preclinical and clinical studies for TNBC patients with BRAC1/2 mutations in the future.
7. Conclusion
There is now mounting evidence, from both preclinical and clinical data suggesting that PARPis have the potential to be used as monotherapy and combination strategies across a broad spectrum of molecular backgrounds and tumor types. While HRD tumors are most favored in PARPi treatment, there is clinical evidence to support their use in other molecular subsets of cancers, such as SCLC tumors with high levels of replication stress. Beyond PARPi, multiple DDR agents also demonstrate synthetic lethal effects in HRD tumors, broadening a massive opportunity based on the success of PARPi. Different combination strategies further strengthen the treatment efficacy and address the drug resistance.
8. Expert opinion
The development of PARPis drugs that block the activity of enzymes involved in DNA repair has been a game-changer for the treatment of cancers, such as ovarian cancer, with germline BRCA1/2 mutations. Since their introduction, numerous clinical trials with different agents in this class of inhibitors have shown significant improvement in progression-free survival compared to placebo controls in these patients. Due to their tremendous clinical success in BRCA1/2 mutation carriers, the use of these drugs has also been approved for the treatment of patients who do not harbor germline mutations in BRCA1/2. To this end, several combination therapies have been clinically examined in different types of cancer, with or without BRCA1/2 mutations, but with alterations in other DNA damage repair pathways or cell signaling pathways. These studies have substantially increased our knowledge of the mechanism of action and possible resistance to PARPis. Studies have shown that while many patients initially respond to PARPi, their use over time results in acquired resistance. The acquired resistance can manifest as early as 3 months (early-stage therapy) up to 2 years and beyond continuous treatment (late-stage therapy). Additionally, some patients may already have propagated tumor subclones that are intrinsically resistant to this class of agents. Therefore, identifying which patients fall into these three categories (intrinsically resistant, early-stage progressors, late-stage progressors) will be critical to determine how best to treat them. Also, determining whether patients can benefit from PARPi treatment early during treatment and the early detection of subclones that are likely to confer resistance to PARPis is crucial to the success of a treatment regimen. To this end, we need to identify reliable biomarkers to (i) predict the response to PARPi as monotherapy and in combination therapy and with specific agents, (ii) to distinguish the early- versus late-progressors and (iii) to identify those patients who are intrinsically resistant to PARPi. To identify such biomarkers, a non-bias multi-pronged multi-omics approach is required to closely monitor treatment response and invasive samplings, such as tumor biopsies of pre-treatment, ongoing treatment, and progression. In addition, non-invasive methods of sample collection that would identify secreted chemokines and cytokines, circulating tumor cells, exosomes, or cell-free DNA using blood or plasma samples are needed. Integration of the data from each of these approaches is also required to validate and identify novel pathways and specific genes/proteins that could identify different modes of resistance. Once these pathways or individual genes have been identified in the patients, their functionalities need to be addressed in preclinical models to narrow down the final responsive or resistance signatures to a few genes signature that can be used in future clinical trials to identify the responders from non-responders. Lastly, to understand how the intratumor and immune microenvironment is altered in response to PARPis in the monotherapy or combination therapy, the use of single-cell omics technologies in pre/post-progression samples will be critical.
In addition to identifying the biomarkers in patients, it is also important to select patients based on their treatment history. A patient who has undergone multiple rounds of therapies and sequentially progressed on each of these treatments, will have generated multiple downstream drivers that could alter their tumor biomarker portfolio. To ensure that the biomarker studies can be applied to specific treatment strategies with PARPi as single agents or in combination therapy, patient selection should take into account if similar prior lines of treatments have been administered to these patients.
While the advent of PARPis has significantly improved the field of cancer therapy of tumors with defects in DNA damage and repair pathways, patient selection will be essential to enhance further to individualize these treatments. We believe that with the multitude of clinical trials with different PARPis and the increased sensitivity of the multi-omics approaches, these key questions will likely be addressed successfully in the very near future.
Article Highlights:
Several PARP inhibitors have been approved to treat advanced, relapsed patients with BRCA-mutant triple-negative breast and high-grade serous ovarian cancers.
The use of PARP inhibitors is being expanded beyond patients with ovarian or breast cancers harboring germline or somatic BRCA1/2 mutations to those with homologous recombination deficient (HRD) tumors and other types of cancers.
Approaches to enhance PARP inhibitor efficacy and overcome resistance are focused on combining a PARP inhibitor with other inhibitors of DNA damage repair (DDR), cell cycle checkpoints, immune checkpoints, and therapies targeting specific cell signaling pathways.
Several clinical trials have demonstrated the benefits of PARP inhibitors as maintenance treatment in patients with BRCA mutations or HRD; however, there is a lack of clinical trials comparing PARP inhibitor treatment in the first line setting to the other first or second line of treatment options for breast cancer patients.
Future directions include exploring the benefits of PARP inhibitors in neoadjuvant settings and broadening the significant benefits of PARP inhibitors in combination with other targets.
Identifying molecular biomarkers to predict the response to PARP inhibitor monotherapy or combination strategies is an area of unmet need.
Funding
This paper was not funded.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer has received honoraria from AstraZeneca, Pfizer, GSK, Clovis and Gilead.
Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Anders C. and Carey LA, Understanding and treating triple-negative breast cancer. Oncology (Williston Park, NY), 2008. 22(11): p. 1233. [PMC free article] [PubMed] [Google Scholar]
- 2.Lehmann BD, et al. , Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of clinical investigation, 2011. 121(7): p. 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein MD, et al. , Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clinical Cancer Research, 2015. 21(7): p. 1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid P, et al. KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab D chemotherapy vs. placebo D chemotherapy, followed by adjuvant pembrolizumab vs. placebo for earlystage TNBC. ELSEVIER RADARWEG 29, 1043 NX AMSTERDAM, NETHERLANDS. [Google Scholar]
- 5.Bardia A, et al. , Biomarker Analyses in the Phase 3 ASCENT Study of Sacituzumab Govitecan Versus Chemotherapy in Patients with Metastatic Triple-Negative Breast Cancer. Annals of Oncology, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Curtin NJ, DNA repair dysregulation from cancer driver to therapeutic target. Nature Reviews Cancer, 2012. 12(12): p. 801–817. [DOI] [PubMed] [Google Scholar]
- 7.Lord CJ and Ashworth A, BRCAness revisited. Nature Reviews Cancer, 2016. 16(2): p. 110–120. [DOI] [PubMed] [Google Scholar]
- 8.G Anglian Breast Cancer Study., Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. British Journal of Cancer, 2000. 83(10): p. 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, et al. , Association between BRCA status and triple-negative breast cancer: a meta-analysis. Frontiers in pharmacology, 2018. 9: p. 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atchley DP, et al. , Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. Journal of Clinical Oncology, 2008. 26(26): p. 4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly MB, et al. , Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 2021. 19(1): p. 77–102. [DOI] [PubMed] [Google Scholar]
- 12.Levy-Lahad E, et al. , Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: frequency and differential penetrance in ovarian cancer and in breast-ovarian cancer families. American journal of human genetics, 1997. 60(5): p. 1059. [PMC free article] [PubMed] [Google Scholar]
- 13.Turner N, Tutt A, and Ashworth A, Hallmarks of’BRCAness’ in sporadic cancers. Nature reviews cancer, 2004. 4(10): p. 814–819. [DOI] [PubMed] [Google Scholar]
- 14.Telli ML, et al. , Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clinical cancer research, 2016. 22(15): p. 3764–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaklamani VG, et al. , Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579). Breast cancer research and treatment, 2015. 151(3): p. 629–638. [DOI] [PubMed] [Google Scholar]
- 16.Manasaryan G, et al. , Bioinformatic Analysis of the Nicotinamide Binding Site in Poly (ADP-Ribose) Polymerase Family Proteins . Cancers, 2021. 13(6): p. 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas S, et al. , Family-wide analysis of poly (ADP-ribose) polymerase activity. Nat Commun 5: 4426. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai P, Biology of poly (ADP-ribose) polymerases: the factotums of cell maintenance. Molecular cell, 2015. 58(6): p. 947–958. [DOI] [PubMed] [Google Scholar]
- 19.Wei L, et al. , Damage response of XRCC1 at sites of DNA single strand breaks is regulated by phosphorylation and ubiquitylation after degradation of poly(ADP-ribose). J Cell Sci, 2013. 126(Pt 19): p. 4414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isabelle M, et al. , Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome science, 2010. 8: p. 22-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri AR and Nussenzweig A, The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nature reviews Molecular cell biology, 2017. 18(10): p. 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murai J, et al. , Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer research, 2012. 72(21): p. 5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murai J, et al. , Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Molecular cancer therapeutics, 2014. 13(2): p. 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pommier Y, O’Connor MJ, and De Bono J, Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Science translational medicine, 2016. 8(362): p. 362ps17-362ps17. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins TA, et al. , Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Molecular Cancer Research, 2015. 13(11): p. 1465–1477. [DOI] [PubMed] [Google Scholar]
- 26.Radhakrishnan SK, Jette N, and Lees-Miller SP, Non-homologous end joining: emerging themes and unanswered questions. DNA repair, 2014. 17: p. 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim G, et al. , FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clinical cancer research, 2015. 21(19): p. 4257–4261. [DOI] [PubMed] [Google Scholar]
- 28. Robson M, et al. , Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. New England Journal of Medicine, 2017. 377(6): p. 523–533. • The critical clinical study led to the FDA-approved olaparib treatment in germline BRCA-mutant advanced breast cancers.
- 29.Golan T, et al. , Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. New England Journal of Medicine, 2019. 381(4): p. 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray-Coquard I, et al. , Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. New England Journal of Medicine, 2019. 381(25): p. 2416–2428. [DOI] [PubMed] [Google Scholar]
- 31.de Bono J, et al. , Olaparib for metastatic castration-resistant prostate cancer. New England Journal of Medicine, 2020. 382(22): p. 2091–2102. [DOI] [PubMed] [Google Scholar]
- 32. Robson ME, et al. , OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol, 2019. 30(4): p. 558–566. •• The recent critical clinical study, OlympiAD, stratified the patients who received monotherapy of olaparib or chemotherapy into subgroups based on prior chemotherapy and receptor status.
- 33.Robson M, et al. , Abstract PD4–03: OlympiAD extended follow-up for overall survival and safety: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Cancer Research, 2020. 80(4 Supplement): p. PD4–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Litton JK, et al. , Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. New England Journal of Medicine, 2018. 379(8): p. 753–763. • The critical clinical study led to the approval of talazoparib in germline BRCA-mutant advanced breast cancers.
- 35. Rugo HS, et al. , Outcomes in Clinically Relevant Patient Subgroups From the EMBRACA Study: Talazoparib vs Physician’s Choice Standard-of-Care Chemotherapy. JNCI Cancer Spectr, 2020. 4(1): p. pkz085. •• The recent analysis of phase III clinical study, EMBRACA, stratified the patients who received monotherapy of talazoparib or chemotherapy into subgroups of HR+/HER2- and TNBC.
- 36. Litton JK, et al. , Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol, 2020. 31(11): p. 1526–1535. •• Critical final overall survival analysis of talazoparib from the EMBRACA study.
- 37.Taylor AM, et al. , PARP (Poly ADP-Ribose Polymerase) inhibitors for locally advanced or metastatic breast cancer. Cochrane Database Syst Rev, 2021. 4(4): p. Cd011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abida W, et al. , Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clinical Cancer Research, 2020. 26(11): p. 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swisher EM, et al. , Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol, 2017. 18(1): p. 75–87. [DOI] [PubMed] [Google Scholar]
- 40.Swisher EM, et al. , Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat Commun, 2021. 12(1): p. 2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oza AM, et al. , Patient-Centered Outcomes in ARIEL3, a Phase III, Randomized, Placebo-Controlled Trial of Rucaparib Maintenance Treatment in Patients With Recurrent Ovarian Carcinoma. J Clin Oncol, 2020. 38(30): p. 3494–3505. •• The recent analysis of phase III clinical study, ARIEL3, with different cohorts of BRCA-mutant, HRD, and BRCA-WT recurrent ovarian carcinoma patients who received monotherapy of rucaparib or placebo.
- 42. Gonzalez-Martin A, et al. , Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med, 2019. 381(25): p. 2391–2402. • The critical phase III clinical study led to the approval of niraparib in advanced ovarian cancer.
- 43.Chowdhury S, et al. , Pamiparib, an investigational PARP inhibitor, in patients with metastatic castration-resistant prostate cancer (mCRPC) and a circulating tumor cell (CTC) homologous recombination deficiency (HRD) phenotype or BRCA defects: A trial in progress. 2019, American Society of Clinical Oncology. [Google Scholar]
- 44.Ciardiello F, et al. , A phase 3, double-blind, randomized study of pamiparib versus placebo as maintenance therapy in patients with inoperable, locally advanced, or metastatic gastric cancer that responded to platinum based first-line chemotherapy. Ann Oncol, 2018. 29: p. viii205–viii270. [Google Scholar]
- 45. Sun T, et al. , A phase 2 study of pamiparib in the treatment of patients with locally advanced or metastatic HER2-negative breast cancer with germline BRCA mutation. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 1087-1087. •• The recent analysis of phase II clinical study stratified the locally advanced/metastatic HER2- breast cancer patients who received monotherapy of Pamiparib into subgroups of HR+/HER2- and TNBC.
- 46.Boussios S, et al. , Veliparib in ovarian cancer: a new synthetically lethal therapeutic approach. Investigational new drugs, 2020. 38(1): p. 181–193. [DOI] [PubMed] [Google Scholar]
- 47.Audeh MW, et al. , Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. The lancet, 2010. 376(9737): p. 245–251. [DOI] [PubMed] [Google Scholar]
- 48.Fong PC, et al. , Poly (ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. Journal of clinical oncology, 2010. 28(15): p. 2512–2519. [DOI] [PubMed] [Google Scholar]
- 49.Zhou P, et al. , Recent advancements in PARP inhibitors-based targeted cancer therapy. Precision Clinical Medicine, 2020. 3(3): p. 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, et al. , PARP inhibitor resistance: the underlying mechanisms and clinical implications. Molecular cancer, 2020. 19(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbotts R, et al. , DNA methyltransferase inhibitors induce a BRCAness phenotype that sensitizes NSCLC to PARP inhibitor and ionizing radiation. Proceedings of the National Academy of Sciences, 2019. 116(45): p. 22609–22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hopkins JL and Zou L, Induction of BRCAness in triple-negative breast cancer by a CDK12/13 inhibitor improves chemotherapy. Cancer cell, 2019. 36(5): p. 461–463. [DOI] [PubMed] [Google Scholar]
- 53.Wiegmans AP, et al. , Differences in expression of key DNA damage repair genes after epigenetic-induced BRCAness dictate synthetic lethality with PARP1 inhibition. Molecular cancer therapeutics, 2015. 14(10): p. 2321–2331. [DOI] [PubMed] [Google Scholar]
- 54.Ibrahim YH, et al. , PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer discovery, 2012. 2(11): p. 1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mo W, et al. , mTOR inhibitors suppress homologous recombination repair and synergize with PARP inhibitors via regulating SUV39H1 in BRCA-proficient triple-negative breast cancer. Clinical Cancer Research, 2016. 22(7): p. 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith J, et al. , The ATM–Chk2 and ATR–Chk1 pathways in DNA damage signaling and cancer. Advances in cancer research, 2010. 108: p. 73–112. [DOI] [PubMed] [Google Scholar]
- 57.Yazinski SA, et al. , ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes & development, 2017. 31(3): p. 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renwick A, et al. , ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nature genetics, 2006. 38(8): p. 873–875. [DOI] [PubMed] [Google Scholar]
- 59.Kim H, et al. , Targeting the ATR/CHK1 axis with PARP inhibition results in tumor regression in BRCA-mutant ovarian cancer models. Clinical Cancer Research, 2017. 23(12): p. 3097–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim H, et al. , Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nature communications, 2020. 11(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd RL, et al. , Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene, 2020. 39(25): p. 4869–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith HL, Prendergast L, and Curtin NJ, Exploring the synergy between PARP and CHK1 inhibition in matched BRCA2 mutant and corrected cells. Cancers, 2020. 12(4): p. 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gralewska P, et al. , PARP Inhibition Increases the Reliance on ATR/CHK1 Checkpoint Signaling Leading to Synthetic Lethality—An Alternative Treatment Strategy for Epithelial Ovarian Cancer Cells Independent from HR Effectiveness. International journal of molecular sciences, 2020. 21(24): p. 9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt M, et al. , Regulation of G2/M transition by inhibition of WEE1 and PKMYT1 kinases. Molecules, 2017. 22(12): p. 2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ha D-H, et al. , Antitumor effect of a WEE1 inhibitor and potentiation of olaparib sensitivity by DNA damage response modulation in triple-negative breast cancer. Scientific reports, 2020. 10(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith H, Prendergast L, and Curtin N, Investigating synergy between WEE1 and PARP inhibitors in BRCA2 mutant and corrected cells. European Journal of Cancer, 2020. 138: p. S36–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, et al. , Targeting Replicative Stress and DNA Repair by Combining PARP and Wee1 Kinase Inhibitors Is Synergistic in Triple Negative Breast Cancers with Cyclin E or BRCA1 Alteration. Cancers, 2021. 13(7): p. 1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westin SN, et al. , EFFORT: EFFicacy Of adavosertib in parp ResisTance: A randomized two-arm non-comparative phase II study of adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 5505-5505. [Google Scholar]
- 69.Hamilton E, et al. , Phase Ib study of adavosertib in combination with olaparib in patients with refractory solid tumors: dose escalation. Cancer Res, 2019. 79(13 Suppl). [Google Scholar]
- 70.Chow JPH and Poon RYC, The CDK1 inhibitory kinase MYT1 in DNA damage checkpoint recovery. Oncogene, 2013. 32(40): p. 4778–4788. [DOI] [PubMed] [Google Scholar]
- 71.Booher RN, Holman PS, and Fattaey A, Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. Journal of Biological Chemistry, 1997. 272(35): p. 22300–22306. [DOI] [PubMed] [Google Scholar]
- 72.Parker LL and Piwnica-Worms H, Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science, 1992. 257(5078): p. 1955–1957. [DOI] [PubMed] [Google Scholar]
- 73.Lewis CW, et al. , Upregulation of Myt1 promotes acquired resistance of cancer cells to Wee1 inhibition. Cancer research, 2019. 79(23): p. 5971–5985. [DOI] [PubMed] [Google Scholar]
- 74.Ceccaldi R, et al. , Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature, 2015. 518(7538): p. 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ceccaldi R, Rondinelli B, and D’Andrea AD, Repair pathway choices and consequences at the double-strand break. Trends in cell biology, 2016. 26(1): p. 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou J, et al. , A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors. Nature Cancer, 2021: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zatreanu D, et al. , Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nature Communications, 2021. 12(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stecklein SR, et al. , BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proceedings of the National Academy of Sciences, 2012. 109(34): p. 13650–13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang J, et al. , Ganetespib overcomes resistance to PARP inhibitors in breast cancer by targeting core proteins in the DNA repair machinery. Investigational new drugs, 2017. 35(3): p. 251. [DOI] [PubMed] [Google Scholar]
- 80.Choi YE, et al. , Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget, 2014. 5(9): p. 2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Konstantinopoulos P, et al. , In vivo synergism between PARP-inhibitor olaparib and HSP90-inhibitor AT13387 in high grade serous ovarian cancer patient derived xenografts. 2016, American Society of Clinical Oncology. [Google Scholar]
- 82.Hustedt N. and Durocher D, The control of DNA repair by the cell cycle. Nature cell biology, 2017. 19(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 83.Symington LS and Gautier J, Double-strand break end resection and repair pathway choice. Annual review of genetics, 2011. 45: p. 247–271. [DOI] [PubMed] [Google Scholar]
- 84.Liu T. and Huang J, DNA End Resection: Facts and Mechanisms. Genomics Proteomics Bioinformatics, 2016. 14(3): p. 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tomimatsu N, et al. , Phosphorylation of EXO1 by CDKs 1 and 2 regulates DNA end resection and repair pathway choice. Nature communications, 2014. 5(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bajrami I, et al. , Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer research, 2014. 74(1): p. 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joshi PM, et al. , Ovarian cancer-associated mutations disable catalytic activity of CDK12, a kinase that promotes homologous recombination repair and resistance to cisplatin and poly (ADP-ribose) polymerase inhibitors. Journal of Biological Chemistry, 2014. 289(13): p. 9247–9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson SF, et al. , CDK12 inhibition reverses de novo and acquired PARP inhibitor resistance in BRCA wild-type and mutated models of triple-negative breast cancer. Cell reports, 2016. 17(9): p. 2367–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Militello AM, et al. , Mechanism of action and clinical efficacy of CDK4/6 inhibitors in BRCA-mutated, estrogen receptor-positive breast cancers: case report and literature review. Frontiers in oncology, 2019. 9: p. 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aziz D, et al. , Cyclin E1 protein is stabilized in BRCA1 mutated breast cancers leading to synergy between CDK2 and PARP inhibitors. bioRxiv, 2020. [Google Scholar]
- 91.Carey JPW, et al. , Synthetic lethality of PARP inhibitors in combination with MYC blockade is independent of BRCA status in triple-negative breast cancer. Cancer research, 2018. 78(3): p. 742–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ivy SP, et al. , Cediranib, a pan-VEGFR inhibitor, and olaparib, a PARP inhibitor, in combination therapy for high grade serous ovarian cancer. Expert opinion on investigational drugs, 2016. 25(5): p. 597–611. [DOI] [PubMed] [Google Scholar]
- 93.Westin SN, et al. , Phase Ib Dose Expansion and Translational Analyses of Olaparib in Combination with Capivasertib in Recurrent Endometrial, Triple-Negative Breast, and Ovarian Cancer. Clinical Cancer Research, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westin SN, et al. , Phase I trial of olaparib (PARP inhibitor) and vistusertib (mTORC1/2 inhibitor) in recurrent endometrial, ovarian and triple negative breast cancer. 2018, American Society of Clinical Oncology. [Google Scholar]
- 95.Konstantinopoulos PA, et al. , Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. The Lancet Oncology, 2019. 20(4): p. 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kondrashova O, et al. , Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nature communications, 2018. 9(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo X, et al. , Acetylation of 53BP1 dictates the DNA double strand break repair pathway. Nucleic acids research, 2018. 46(2): p. 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du Y, et al. , Blocking c-Met–mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitors. Nature medicine, 2016. 22(2): p. 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gogola E, et al. , Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer cell, 2018. 33(6): p. 1078–1093. [DOI] [PubMed] [Google Scholar]
- 100.Jasek E, et al. , Combinatorial effects of PARP inhibitor PJ34 and histone deacetylase inhibitor vorinostat on leukemia cell lines. Anticancer Research, 2014. 34(4): p. 1849–1856. [PubMed] [Google Scholar]
- 101.Ha K, et al. , Histone deacetylase inhibitor treatment induces ‘BRCAness’ and synergistic lethality with PARP inhibitor and cisplatin against human triple negative breast cancer cells. Oncotarget, 2014. 5(14): p. 5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valdez BC, et al. , Combination of a hypomethylating agent and inhibitors of PARP and HDAC traps PARP1 and DNMT1 to chromatin, acetylates DNA repair proteins, down-regulates NuRD and induces apoptosis in human leukemia and lymphoma cells. Oncotarget, 2018. 9(3): p. 3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Min A, et al. , Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Research, 2015. 17(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pulliam N, et al. , An effective epigenetic-PARP inhibitor combination therapy for breast and ovarian cancers independent of BRCA mutations. Clinical Cancer Research, 2018. 24(13): p. 3163–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mehdipour P, Chen R, and De Carvalho DD, The next generation of DNMT inhibitors. Nature Cancer, 2021. 2(10): p. 1000–1001. [DOI] [PubMed] [Google Scholar]
- 106.Arun BK, et al. , Abstract PD4–01: First-line veliparib plus carboplatin/paclitaxel in patients with HER2-negative advanced/metastatic g<em>BRCA</em>-associated breast cancer: Planned subgroup analysis from the phase 3 BROCADE3 trial. Cancer Research, 2020. 80(4 Supplement): p. PD4–01. [Google Scholar]
- 107.Ayoub JP, et al. , 140O veliparib plus carboplatin-paclitaxel in patients with HER2-negative advanced/metastatic gBRCA-associated breast cancer: results in hormone receptor-positive and triple-negative breast cancer subgroups from the phase III BROCADE3 trial. Annals of Oncology, 2020. 31: p. S65. [Google Scholar]
- 108.Matulonis UA and Monk BJ, PARP inhibitor and chemotherapy combination trials for the treatment of advanced malignancies: does a development pathway forward exist? Annals of Oncology, 2017. 28(3): p. 443–447. [DOI] [PubMed] [Google Scholar]
- 109.Thallinger C, et al. , Review of cancer treatment with immune checkpoint inhibitors. Wiener Klinische Wochenschrift, 2018. 130(3): p. 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thomas R, Al-Khadairi G, and Decock J, Immune Checkpoint Inhibitors in Triple Negative Breast Cancer Treatment: Promising Future Prospects. Front Oncol, 2020. 10: p. 600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Couch FJ, et al. , Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. Journal of clinical oncology, 2015. 33(4): p. 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCann KE and Hurvitz SA, Advances in the use of PARP inhibitor therapy for breast cancer. Drugs in context, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiao S, et al. , PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clinical Cancer Research, 2017. 23(14): p. 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vinayak S, et al. , Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA oncology, 2019. 5(8): p. 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas A, et al. , Durvalumab in combination with olaparib in patients with relapsed SCLC: results from a phase II study. Journal of Thoracic Oncology, 2019. 14(8): p. 1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu R-M, et al. , Development of therapeutic antibodies for the treatment of diseases. Journal of biomedical science, 2020. 27(1): p. 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh S, et al. , Monoclonal antibodies: a review. Current clinical pharmacology, 2018. 13(2): p. 85–99. [DOI] [PubMed] [Google Scholar]
- 118.Beck A, et al. , Strategies and challenges for the next generation of antibody–drug conjugates. Nature reviews Drug discovery, 2017. 16(5): p. 315–337. [DOI] [PubMed] [Google Scholar]
- 119.Polakis P, Antibody drug conjugates for cancer therapy. Pharmacological reviews, 2016. 68(1): p. 3–19. [DOI] [PubMed] [Google Scholar]
- 120.Khongorzul P, et al. , Antibody–drug conjugates: a comprehensive review. Molecular Cancer Research, 2020. 18(1): p. 3–19. [DOI] [PubMed] [Google Scholar]
- 121.Cardillo TM, et al. , Synthetic Lethality Exploitation by an Anti–Trop-2-SN-38 Antibody–Drug Conjugate, IMMU-132, Plus PARP Inhibitors in BRCA1/2–wild-type Triple-Negative Breast Cancer. Clinical Cancer Research, 2017. 23(13): p. 3405–3415. [DOI] [PubMed] [Google Scholar]
- 122.Denkert C, et al. , Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin oncol, 2015. 33(9): p. 983–991. [DOI] [PubMed] [Google Scholar]
- 123.Pruneri G, et al. , Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Annals of oncology, 2016. 27(2): p. 249–256. [DOI] [PubMed] [Google Scholar]
- 124.Vihervuori H, et al. , Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. Journal of cancer research and clinical oncology, 2019. 145(12): p. 3105–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lakhani SR, et al. , Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. Journal of the National Cancer Institute, 1998. 90(15): p. 1138–1145. [DOI] [PubMed] [Google Scholar]
- 126.Ummarino S, Hausman C, and Di Ruscio A, The PARP Way to Epigenetic Changes. Genes, 2021. 12(3): p. 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Obaji E, et al. , Activation of PARP2/ARTD2 by DNA damage induces conformational changes relieving enzyme autoinhibition. Nature Communications, 2021. 12(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boehler C, et al. , Poly (ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proceedings of the National Academy of Sciences, 2011. 108(7): p. 2783–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haikarainen T, Krauss S, and Lehtio L, Tankyrases: structure, function and therapeutic implications in cancer. Current pharmaceutical design, 2014. 20(41): p. 6472–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu J. and Crowe DL, PARP5B is required for nonhomologous end joining during tumorigenesis in vivo. Molecular Carcinogenesis, 2021. 61(1): p. 85–98. [DOI] [PubMed] [Google Scholar]
- 131.Tuncel H, et al. , PARP6, a mono (ADP-ribosyl) transferase and a negative regulator of cell proliferation, is involved in colorectal cancer development. International journal of oncology, 2012. 41(6): p. 2079–2086. [DOI] [PubMed] [Google Scholar]
- 132.Qi G, et al. , PARP6 acts as a tumor suppressor via downregulating Survivin expression in colorectal cancer. Oncotarget, 2016. 7(14): p. 18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gozgit JM, et al. , PARP7 negatively regulates the type I interferon response in cancer cells and its inhibition triggers antitumor immunity. Cancer Cell, 2021. 39(9): p. 1214–1226. [DOI] [PubMed] [Google Scholar]
- 134.Xing J, et al. , Identification of poly (ADP-ribose) polymerase 9 (PARP9) as a noncanonical sensor for RNA virus in dendritic cells. Nature communications, 2021. 12(1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schleicher EM, et al. , PARP10 promotes cellular proliferation and tumorigenesis by alleviating replication stress. Nucleic acids research, 2018. 46(17): p. 8908–8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao Y, et al. , PARP10 suppresses tumor metastasis through regulation of Aurora A activity. Oncogene, 2018. 37(22): p. 2921–2935. [DOI] [PubMed] [Google Scholar]
- 137.Guo T, et al. , ADP-ribosyltransferase PARP11 modulates the interferon antiviral response by mono-ADP-ribosylating the ubiquitin E3 ligase β-TrCP. Nature microbiology, 2019. 4(11): p. 1872–1884. [DOI] [PubMed] [Google Scholar]
- 138.Welsby I, et al. , PARP12, an interferon-stimulated gene involved in the control of protein translation and inflammation. Journal of Biological Chemistry, 2014. 289(38): p. 26642–26657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Todorova T, Bock FJ, and Chang P, Poly (ADP-ribose) polymerase-13 and RNA regulation in immunity and cancer. Trends in molecular medicine, 2015. 21(6): p. 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qin W, et al. , Research progress on PARP14 as a drug target. Frontiers in pharmacology, 2019. 10: p. 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jwa M. and Chang P, PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK-and IRE1α-mediated unfolded protein response. Nature cell biology, 2012. 14(11): p. 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steffen JD, et al. , Structural implications for selective targeting of PARPs. Frontiers in oncology, 2013. 3: p. 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pilié PG, et al. , PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clinical Cancer Research, 2019. 25(13): p. 3759–3771. [DOI] [PubMed] [Google Scholar]
- 144.Murai J. and Pommier Y, PARP trapping beyond homologous recombination and platinum sensitivity in cancers. Annual Review of Cancer Biology, 2019. 3: p. 131–150. [Google Scholar]