Abstract
Diminishing homeostatic proliferation of memory T cells is essential for improving the efficacy of lymphoablation in transplant recipients. Our previous studies in a mouse heart transplantation model established that B lymphocytes secreting proinflammatory cytokines are critical for T cell recovery after lymphoablation. The goal of the current study was to identify mediators of B cell activation following lymphoablation in allograft recipients. Transcriptome analysis revealed that macrophage‐inducible C‐type lectin (Mincle, Clec4e) expression is up‐regulated in B cells from heart allograft recipients treated with murine anti‐thymocyte globulin (mATG). Recipient Mincle deficiency diminishes B cell production of pro‐inflammatory cytokines and impairs T lymphocyte reconstitution. Mixed bone marrow chimeras lacking Mincle only in B lymphocytes have similar defects in T cell recovery. Conversely, treatment with a synthetic Mincle ligand enhances T cell reconstitution after lymphoablation in non‐transplanted mice. Treatment with agonistic CD40 mAb facilitates T cell reconstitution in CD4 T cell‐depleted, but not in Mincle‐deficient, recipients indicating that CD40 signaling induces T cell proliferation via a Mincle‐dependent pathway. These findings are the first to identify an important function of B cell Mincle as a sensor of damage‐associated molecular patterns released by the graft and demonstrate its role in clinically relevant settings of organ transplantation.
Keywords: B cell biology, basic (laboratory) research/science, immunobiology, immunosuppressive regimens ‐ induction, lymphocyte biology, solid organ transplantation, T cell biology
Short abstract
In mouse heart allograft recipients, macrophage inducible C‐type lectin is an important pattern recognition receptor that drives proinflammatory cytokine production in B cells following lymphoablative treatment.
Abbreviations
- Bcl10
B cell lymphoma/leukemia 10
- CARD9
caspase recruitment domain family member 9
- DAMPs
damage‐associate molecular patterns
- FcRγ
Fc receptor gamma chain
- IL
interleukin
- ITAM
immunoreceptor tyrosine‐based activation motif
- LPS
lipopolysaccharide
- Malt1
Mucosa‐associated lymphoid tissue lymphoma translocation protein 1
- mATG
murine anti‐thymocyte globulin
- Mincle
macrophage‐inducible C‐type lectin
- NFκB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- PAMPs
pathogen‐associated molecular patterns
- pMHC
peptide plus major histocompatibility complex molecule
- PRR
pattern recognition receptor
- SAP‐130
spliceosome‐associated protein 130
- Syk
spleen tyrosine kinase
- TCR
T cell receptor
- TLR
Toll‐like receptor
- TNF
tumor necrosis factor
- β‐GlcCer
β‐glucosylceramide
1. INTRODUCTION
Antibody‐mediated lymphoablation is a commonly used therapy in solid organ and stem cell transplantation and autoimmunity. 1 Its efficacy is determined by the balance between the removal of pathogenic lymphocytes and preserving the individual's ability to respond to opportunistic infections. In transplant recipients, T cell reconstitution following lymphoablation is the sum of potentially detrimental homeostatic proliferation of depletion‐resistant memory T cells and beneficial thymopoiesis that replenishes non‐alloreactive T cells necessary for host protection. Diminishing the extent of homeostatic T cell proliferation is, therefore, essential for improving the efficacy of clinical lymphoablation.
Previously, several factors were implicated in driving T cell homeostatic expansion, including costimulatory signals, IL‐7, and antigens from commensal microorganisms. However, these studies typically used whole‐body irradiation, antibody‐mediated depletion, or adoptive T cell transfer into lymphopenic hosts in the absence of additional systemic inflammation induced by autoimmunity or transplantation. Our recent studies in mouse heart transplantation models using a murine analog of anti‐thymocyte globulin (mATG) revealed distinct mechanisms underlying homeostatic T cell expansion in the recipients. We found that the rapid T cell reconstitution in transplant recipients does not depend on specificity for donor alloantigens suggesting an important role for posttransplant inflammation. 2 , 3 Depletion‐resistant memory CD4+ T cells provide helper signals essential for CD8+ T cell homeostatic reconstitution. Furthermore, B cells activated via CD40 signaling are central mediators of this help. 2 While cognate TCR‐pMHC interactions between B cells and T cells are dispensable, B cell expression of proinflammatory cytokines IL‐1β, IL‐6, and IL‐27 is critical for T cell reconstitution following lymphoablation. In particular, B cell‐derived gp‐130 signaling cytokines IL‐6 and IL‐27 directly target T cell proliferation, while IL‐1β most likely acts by promoting B cell cytokine production. 4 , 5 The goal of the current study was to identify mediators linking post‐transplant inflammation with the activation of depletion‐resistant B lymphocytes that ultimately drive T cell homeostatic proliferation following mATG induction therapy.
During the search of additional mediators of B cell activation in mATG‐treated allograft recipients, we identified and validated the importance of the C‐type lectin receptor Clec‐4e (macrophage‐inducible C‐type lectin, or Mincle). 6 , 7 , 8 , 9 , 10 In addition to being a sensor of microbial pathogen‐associated molecular patterns (PAMPs), both human and mouse Mincle has been implicated in non‐infectious inflammatory conditions including rheumatoid arthritis, 11 , 12 , 13 multiple sclerosis, 14 and post‐ischemic inflammation. 15 , 16 , 17 , 18 , 19 The best‐studied endogenous Mincle ligand is nuclear spliceosome‐associated protein 130 (SAP‐130) released by dying cells, 20 although an additional glycolipid ligand, β‐glucosylceramide (β‐GlcCer), was recently identified. 21 Mincle signals via association with ITAM‐containing FcRγ leading to recruitment of Syk kinase and NFκB activation through the CARD9/Bcl10/Malt1 signalosome. 6 , 9 , 10 To date, Mincle signaling and functions have been characterized exclusively in myeloid cells, with only one report of Mincle expression in human B cells. 22 Here, we report that following heart allograft transplant and mATG lymphoablation, Mincle expression is markedly upregulated on B cells in a CD40‐dependent manner. Mincle deficiency diminishes B cell production of pro‐inflammatory cytokines and impairs T lymphocyte reconstitution in transplant recipients. Mixed bone marrow chimeras lacking Mincle only in B lymphocytes have similar defects in T cell recovery after mATG treatment. Taken together, these findings are the first to identify an important function of Mincle in B lymphocytes, and demonstrate its role in clinically relevant settings of lymphoablation and organ transplantation.
2. METHODS
2.1. Animals
Male and female C57BIL/6J (H‐2b) [B6.WT], BALB/cJ (H‐2d) [BALB/c], SJL/J‐Pde6brd1 (H2s) [SJL], B6.129S2‐Ighmtm1Cgn/j (H‐2b) [B6.µMT], and B6N.129S2‐Casp1tm1Flv /J [caspase‐1 KO] mice aged 6–8 weeks, were purchased from The Jackson Laboratories (Bar Harbor, ME). C57BL/6‐Clec4etm1.1Cfg/Mmucd (B6.Mincle−/−) mice were generously provided by Dr. Holly Rosenzweig at the Oregon Health and Science University via MMRRC Consortium for Functional Glycomics. All animals were maintained and bred in the pathogen‐free facility at the Cleveland Clinic. All animal procedures were approved by the Institutional Animal Care and Use Committee.
2.2. Heart transplantation
Vascularized heterotopic cardiac transplants were performed as described previously. 23 Rejection was defined as a loss of palpable heartbeat and confirmed by laparotomy.
2.3. mATG preparation and animal treatment
Rabbit anti‐mouse thymocyte serum was generated by the Hybridoma Core at the Cleveland Clinic Lerner Research Institute as previously described. 4 Heart allograft recipients were treated with mATG (0.5 mg i.p.) on days 0 and 4 posttransplant, and non‐transplanted mice were injected with 0.5 mg mATG i.p. on days 0 and 4 of the experiment. For CD4 T cell depletion, anti‐mouse CD4 mAb (clones GK1.5 and YTS191, BioXCell) were injected i.p. on days −3, −2, −1 relative to mATG treatment, 200 μg each per animal per day. When indicated, the recipients were treated i.v. with 1 mg anti‐CD154 mAb MR1 1 day prior to the surgery or with 0.1 mg agonistic anti‐CD40 mAb FGK4.5 on days 0 and 1 posttransplant (both from BioXCell). For the administration of synthetic Mincle agonist, non‐transplanted B6 mice treated with 0.5 mg mATG on days 0 and 4 were additionally injected with trehalose‐6,6‐dibehenate (TDB, InvivoGen) at 4 mg/kg i.p. twice weekly for the experiment duration starting on day 2.
2.4. Bone marrow chimeras
Bone marrow was isolated from femurs of wild type B6, B6.Mincle−/− and B6.μMT mice. Female B6.μMT mice were irradiated (1100 rads) and injected i.v. with bone marrow cells so that each recipient received 5 × 106 μMT−/− cells plus 5 × 106 either WT or Mincle−/− cells as we previously described. 2 4–5 weeks later, the resulting chimeras were transplanted with BALB/c heart allografts and treated with mATG.
2.5. In vitro B cell stimulation
B cells were isolated from naïve B6.WT, or B6.Mincle−/− mice by EasySep immunomagnetic kit and incubated for 20 h in RPMI 1640 supplemented with 10% FBS and 2mM L‐glutamine with or without the addition of 1 μg/ml LPS (Lipopolysaccharide from Escherichia coli O55:B5) (MilliporeSigma) or agonistic anti‐CD40 mAb (10 μg/ml, clone FGK4.5; BioXcell). For intracellular cytokine staining, after 16 h of incubation cells were treated PMA (10 ng/ml, MilliporeSigma) and ionomycin (1 μM, MilliporeSigma) for 4 h plus 2 μM monensin (Biolegend) during the last 2 h of culture to inhibit cytokine release from the Golgi/endoplasmic reticulum complex. After incubation, cells were analyzed by flow cytometry for the expression of Mincle, IL‐1β, IL‐6, and TNFα.
2.6. NanoString gene expression analysis
Gene expression analysis was performed using NanoString Mouse Immunology Panel (561 genes) as previously published 4 (Supplementary Methods).
2.7. Real‐time PCR
RNA was isolated from spleen B220+ cells and subjected to reverse transcription with the High‐Capacity cDNA Reverse Transcription Kit. Real‐time PCR was performed on a 7500 Fast Real‐Time PCR System instrument with the following probes and primers: Clec4e (Mm01183703_m1) and Card9 (Mm01327594_m1). All reagents were obtained from Applied Biosystems. Data were normalized to Mrpl 32 (Mm00777741‐sH) RNA amplification and the expression of the target gene in naïve spleen B cells.
2.8. Flow cytometry
Fluorochrome‐conjugated antibodies used in this study are listed in Table S1. Single‐cell suspensions were prepared from peripheral blood and spleen. Following red blood cell lysis, the cells were aliquoted at 1 × 106/sample and stained as previously described. 3 , 24 Cell viability was determined by staining with either SytoxTM‐Orange Nucleic Acid Stain or LIVE/DEADTM Fixable Aqua Dead Cell Stain (Invitrogen) as described in Supplementary Methods. At least 50,000 live events per sample were collected on an LSR Fortessa X‐20 (BD Bioscience). The data were analyzed with FlowJo 10.8.1 (Tree Star Inc.).
2.9. Statistics
GraphPad Prism 9.1 was used for all statistical analyses, and the results are presented as the mean ± SD. The comparison between groups was performed by two‐tailed Student's t‐test (two groups), one‐way analysis of variance (ANOVA) (three or more groups), and multiple t‐tests (kinetics experiments). The graft survival was analyzed using the log‐rank Mantel‐Cox test.
3. RESULTS
3.1. Mincle expression is up‐regulated in B cells following heart allograft transplantation and lymphoablation
We recently reported that IL‐1β and IL‐6 are produced by activated B cells and are critical for rapid homeostatic T cell reconstitution in allograft recipients treated with anti‐mouse thymocyte globulin (mATG). 5 To identify potential mediators of B cell activation and proinflammatory functions following lymphoablation, we purified B cells from the spleens of mATG treated B6 recipients of BALB/c heart allografts on day 12 posttransplant, isolated mRNA, and performed NanoString Gene Expression analysis (Mouse Immunology Panel, 561 genes). The control samples included B cells from untreated B6 recipients of BALB/c heart allografts and from allograft recipients treated with a combination of mATG and anti‐CD4 depleting mAbs to eliminate helper signals to B cells. mATG lymphoablation upregulated B cell expression of over 30 genes >10‐fold. 4 In addition to proinflammatory cytokines, C‐type lectin receptor Mincle and its signaling mediator CARD9 were among the top gene candidates expressed in B cells following mATG treatment in a CD4‐dependent manner (Figure 1A). The increases in the Mincle gene and protein expression in B cells were further confirmed by qRT‐PCR and by flow cytometry (Figure 1B,C; Figure S1B). It is worth noting that the numbers of spleen B cells are only transiently reduced by mATG treatment and are restored to pre‐depletion levels by day 8 after transplantation 2 (not shown). By day 8 posttransplant, mATG treatment increased the proportion of spleen B220+Mincle+ cells in allograft recipients compared to heart allograft transplantation alone (Figure 1D). Thus, the total numbers of B220+Mincle+ cells were also increased in mATG‐treated vs untreated heart allograft recipients (Figure 1E). A similar increase in B220+Mincle+ cells was observed after mATG treatment of heart isograft recipients (Figure 1E), with <1.5 × 106 per spleen B220+Mincle+ cells detected in untreated heart isograft recipients (not shown).
FIGURE 1.
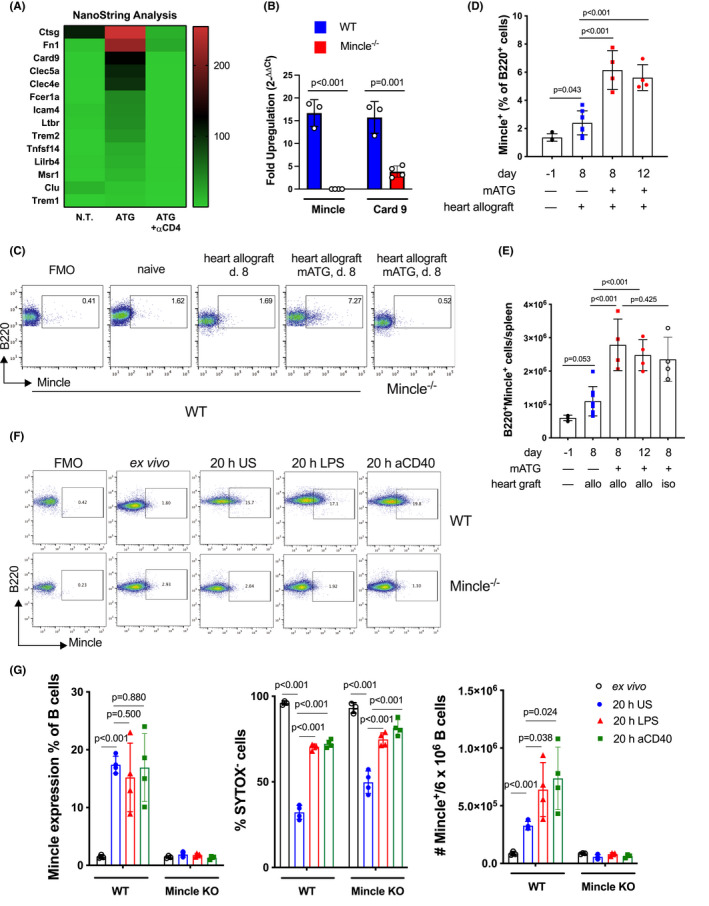
Mincle expression is upregulated in activated B cells. (A, B) C57BL/6 mice were transplanted with BALBc hearts and either left untreated or treated with mATG (days 0 and 4) alone or in combination with CD4 T cell depleting antibody. B cells were isolated on day 12 posttransplant, and gene expression was analyzed by NanoString (A) or by real‐time PCR (B). The fold upregulation was calculated relative to the gene expression in B220+ spleen cells from naïve C57BL/6 mice. n = 4–8 animals/group. (C–E) Mincle expression was measured by flow cytometry on spleen B cells isolated from B6.WT or B6.Mincle−/− heart allograft recipients or from B6.WT heart isograft recipients. (C) Representative dot plots are shown after gating on B220+ cells. The detailed gating strategy is presented in Figure S1. (D–E) Flow cytometry data quantification is shown as a percentage of Mincle+ among B220+ spleen cells (D) or numbers of B220+Mincle+ cells per spleen (E). (F–G) B cells isolated from naive B6.WT and B6.Mincle−/− mice were analyzed for Mincle expression ex vivo or after 20 h culture with or without LPS or agonistic anti‐CD40 mAb stimulation. (F) Representative dot plots. (G) Flow cytometry data quantification is shown as the proportion of Mincle+ cells among live B220+ lymphocytes (left), cell viability (middle), and the numbers of Mincle+ live B cells (right). Data shown from one of two independent experiments, n = 4 animals/group, error bars represent SD
The analysis of CD11+CD11c−, CD11b+CD11c+, and CD11b+CD11c+ myeloid cells revealed that Mincle expression in all these subsets is markedly up‐regulated after heart allotransplantation alone and is further enhanced by concurrent mATG treatment (Figure S2). However, as mATG treatment significantly reduced the numbers of myeloid cells (not shown), more than half of Mincle+ cells in the spleen were B220+ (Figure 1E; Figure S2B).
While Mincle was initially identified as a pattern recognition receptor induced in macrophages by inflammatory stimuli including LPS, TNFα, IL‐6, and IFNγ, 10 , 25 the pattern of Mincle expression in mouse B lymphocytes has not been previously reported. Analogous to resting macrophages, B cells isolated from spleens of naïve non‐transplanted mice do not express Mincle. However, Mincle expression on B lymphocytes is induced after 20 h in vitro culture even in the absence of stimulation. While additional TLR4 or CD40 stimulation did not increase the proportion of Mincle‐expressing cells or the level of Mincle expression on individual cells (as evidenced by MFI), it significantly enhanced B cell viability resulting in higher numbers of Mincle+ B cells at the end of the culture (Figure 1F–G). Taken together, these results demonstrated the ability of murine B cells to express Mincle both in vitro and in vivo and suggested that the Mincle pathway may contribute to B cell activation in mATG treated transplant recipients.
3.2. Mincle is required for B cell proinflammatory cytokine production and rapid T cell reconstitution following lymphoablation
Analysis of cytokine production at day 8 after heart allotransplantation in mATG treated mice revealed that a significant proportion of Mincle+ B cells expressed IL‐1β and TNFα (with a minority of IL‐6 producers), suggesting that these cells contribute to the proinflammatory environment following lymphoablation (Figure 2A,B). To test the functional relevance of Mincle during T cell reconstitution, B6.WT or B6.Mincle−/− mice were transplanted with BALB/c heart allografts and treated with mATG. Recipient Mincle deficiency significantly decreased frequencies of B cells producing IL‐1β, IL‐6, and TNFα on days 8 and 12 posttransplant (Figure 2C,D). The reduced B cell activation in B6.Mincle−/− recipients was associated with delayed CD4+ and CD8+ T cell reconstitution in peripheral blood and the spleen (Figure 3A,B). Consistent with slower T cell recovery, the heart allograft survival following mATG treatment was significantly prolonged in B6.Mincle−/− compared to B6.WT recipients (MST of 25 vs. 11 days, respectively, Figure 3C). To specifically test the role of Mincle in B cells, we generated two types of mixed bone marrow chimeras: WT + μMT BM → μMT (BWT, B cells express Mincle) and Mincle−/− + μMT BM →μMT (BΔMincle, Mincle is absent only in B cells) as we previously published. 4 B cell‐specific Mincle deletion resulted in significantly delayed T cell recovery after BALB/c heart transplantation and mATG treatment compared to control BWT chimeras (Figure 3D,E).
FIGURE 2.
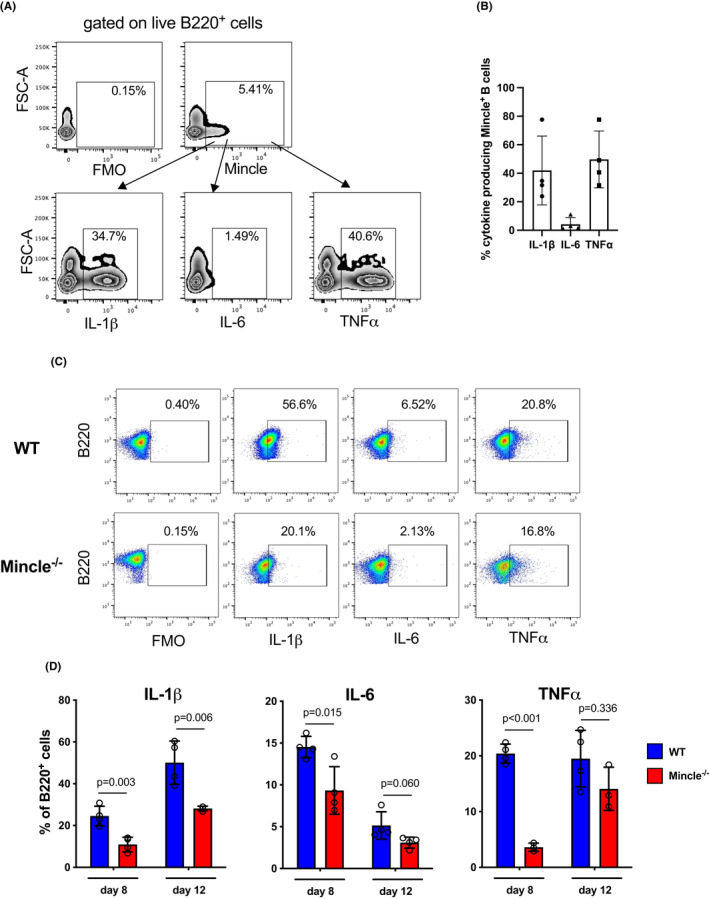
Mincle deficiency reduces B cells cytokine production in mATG treated heart allograft recipients. (A, B) B6.WT recipients of BALB/c heart allografts were treated with mATG. Spleen cells were analyzed for Mincle and IL‐1β, IL‐6, and TNFα expression at day 8 posttransplant. (A) Representative zebra plots after gating on B220+ cells. (B) Proportion of cytokine‐producing B220+Mincle+ cells. Symbols represent individual recipients from one of two independent experiments. (C, D) B6.WT or B6.Mincle−/− mice were transplanted with BALB/c heart allografts and treated with mATG. The cytokine production by B cells was determined by intracellular flow cytometry on days 8 and 12 posttransplant. (C) Representative dot plots (day 12) after gating on B220+ cells. (D) Quantification of the flow cytometry analysis at indicated time points. n = 4 animals/group; error bars represent SD
FIGURE 3.
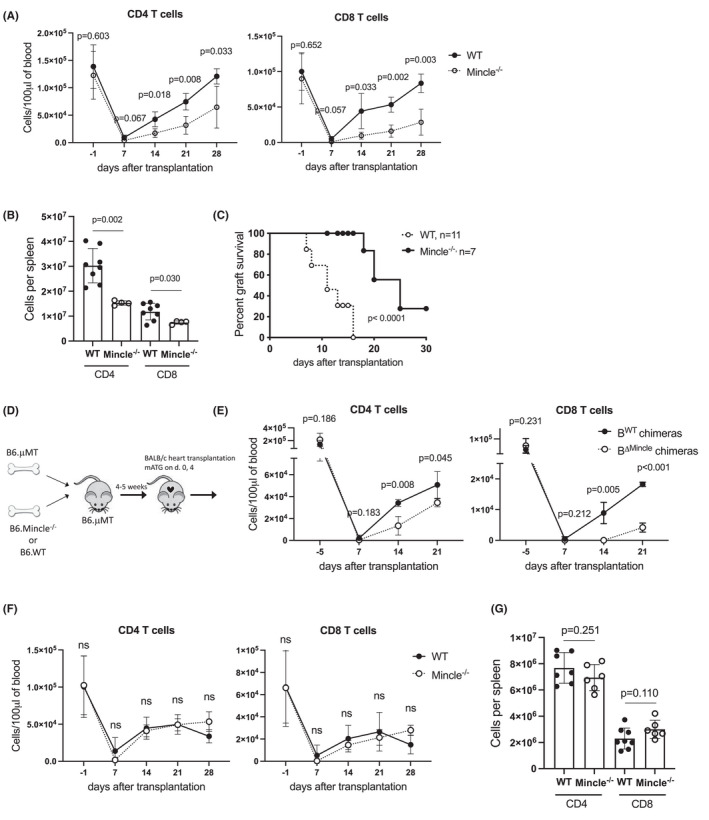
B cell Mincle deficiency impairs T cell reconstitution after mATG lymphoablation in heart allograft recipients. (A–C) B6.WT or B6.Mincle−/− mice were transplanted with BALB/c heart allografts and treated with mATG (day 0 and 4). (A) Numbers of CD4+ and CD8+ T cells per 100 μl of peripheral blood. (B) Numbers of CD4+ and CD8+ T cells per recipient's spleen at day 28 after transplantation. Symbols represent individual recipients. (C) Heart allograft survival. n = 7–11 animals/group. (D–E) Lethally irradiated B6.μMT mice were co‐injected with bone marrow cells from either B6.μMT and B6.WT (BWT chimeras) or B6.μMT and B6.Mincle−/− mice (BΔMincle chimeras), followed by BALB/c heart transplantation and mATG treatment 4–5 weeks later. (D) Experimental design. (E) Numbers of CD4+ and CD8+ T cells per 100 μl of peripheral blood. The results of the representative experiment with n = 3–4 animals/group are shown. The experiment was performed one more time with similar results. (F, G) Non‐transplanted B6.WT or B6.Mincle−/− mice were treated with mATG on days 0 and 4. The numbers of CD4+ and CD8+ T cells in peripheral blood (F) and spleen (G, day 28). n = 6–7 animals/group; error bars represent SD
In contrast to the heart allograft model, recipient Mincle deficiency had a less prominent effect on the tempo of T cell reconstitution after transplantation of allogeneic skin grafts. B6.Mincle−/− recipients had lower spleen CD4+ T cell numbers after skin transplantation, while the numbers of CD8+ T cells were similar in both groups throughout the experiment (Figure S3). While skin graft tissue is mostly eliminated upon rejection, transplanted heart allografts remain in the recipient for the duration of the experiment. The observed differences in Mincle requirement between vascularized heart and non‐vascularized skin allograft recipients suggest that Mincle signaling is activated via continuous release of DAMPs from the allograft into the recipient circulation. In line with this scenario, non‐transplanted B6.WT and B6.Mincle−/− mice treated with mATG had similar kinetics of T cell reconstitution (Figure 3F,G). Taken together, these data indicate that Mincle serves as a sensor of persistent allograft‐induced inflammation.
We next tested whether the delivery of exogenous Mincle ligand at the time of lymphoablation can substitute for heart allotransplantation in accelerating T cell reconstitution. Non‐transplanted B6.WT mice were treated with two doses of mATG and additionally injected twice weekly with a synthetic Mincle agonist trehalose‐6,6‐dibehenate (TDB). While TDB treatment did not alter the efficacy of T cell depletion by mATG, it significantly increased CD4+ and CD8+ T cell recovery in the peripheral blood and spleen (Figure 4). These results indicate that Mincle‐induced proinflammatory signaling is sufficient to drive T cell reconstitution even in the absence of ongoing alloresponse.
FIGURE 4.
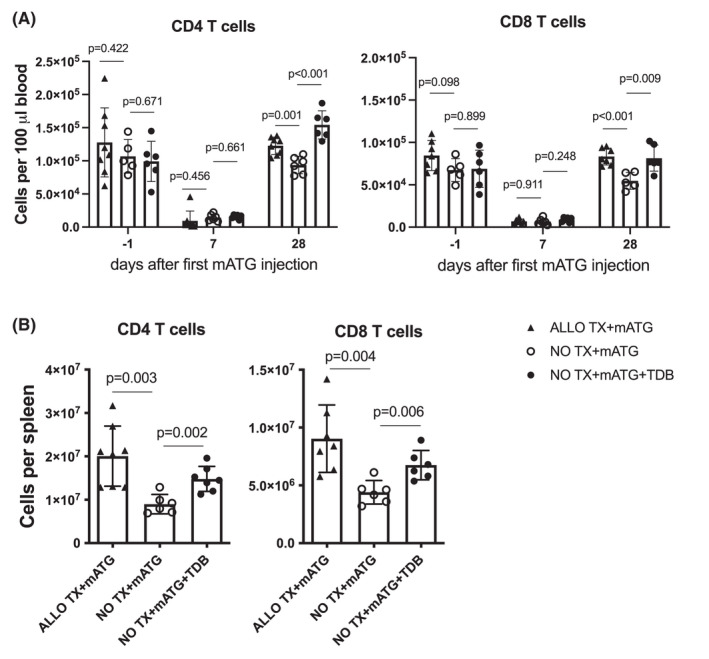
Treatment with Mincle agonist accelerates T cell reconstitution in the absence of heart transplantation. Non‐transplanted B6.WT was injected with mATG on day 0 and 4 with or without treatment with synthetic Mincle agonist trehalose‐6,6‐dibehenate (TDB, twice weekly for 4 weeks). B6 recipients of BALB/c heart allografts treated with mATG are shown for comparison. The numbers of CD4+ and CD8+ T cells in peripheral blood (A) and spleen (B, day 28). The symbols represent individual animals; error bars represent SD
3.3. CD40 signaling facilitates T cell recovery via a Mincle‐dependent pathway
We have previously reported that homeostatic reconstitution of recipient T cell repertoire following mATG lymphoablation is critically dependent on interactions between CD154 expressed by residual memory helper T cells and CD40 on B cells. 2 The initial gene expression analysis showed that CD4 T cell help was required for Mincle up‐regulation in B cells (Figure 1A). We further tested the relationship between CD40 and Mincle signaling pathways following lymphoablation. Administration of blocking anti‐CD154 mAb at the time of mATG treatment and heart transplantation significantly reduced the proportion of Mincle+ B cells and B cells producing proinflammatory cytokines to the levels observed in recipients in the absence of lymphoablation (Figure 5A,B).
FIGURE 5.
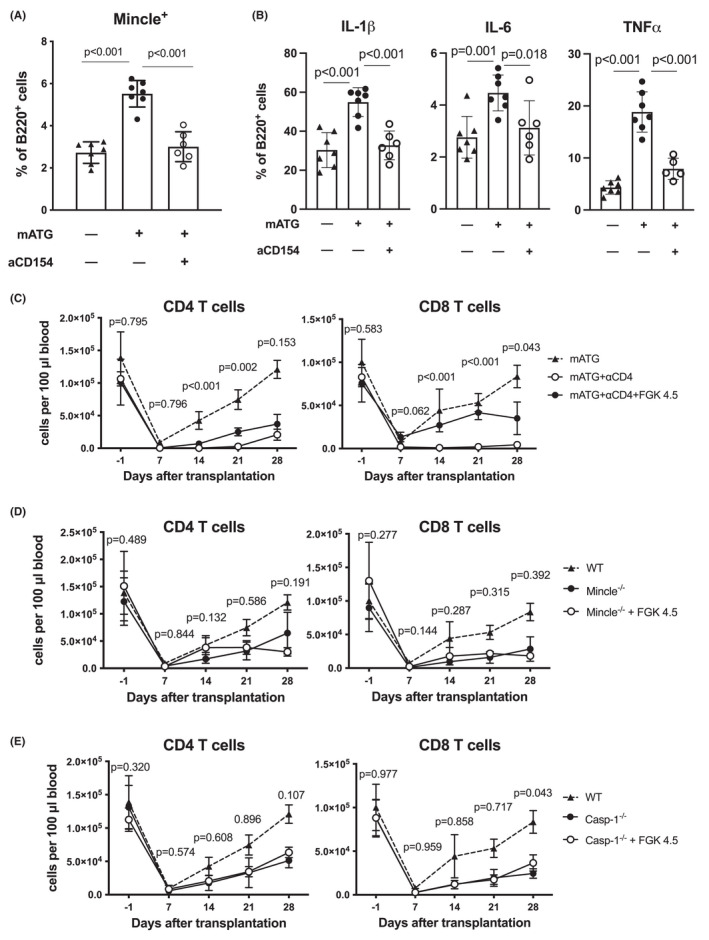
CD40 signaling facilitates T cell recovery via a Mincle‐dependent pathway. (A, B) B6 recipients transplanted with BALB/c heart allografts were treated with mATG (days 0 and 4) with or without blocking anti‐CD154 mAb MR1 (day −1) or left untreated. The proportion of Mincle+, IL‐1β+, IL‐6+, or TNFα+ spleen B cells was determined by flow cytometry at day 12 after transplantation. The symbols represent individual animals; error bars represent SD. (C–E) Effect of agonistic anti‐CD40 mAb FGK4.5 treatment on T cell reconstitution in peripheral blood of allograft recipients treated with mATG. (C) B6.WT recipients of BALB/c heart allografts were treated with mATG alone (dotted line), mATG + depleting anti‐CD4 mAbs, or mATG + anti‐CD4 mAbs + FGK4.5. The p‐values are shown for the comparison between mATG + anti‐CD4 vs mATG + anti‐CD4 + FGK4.5 groups. (D, E) B6.Mincle−/− (D) or B6.Casp1−/− (E) recipients of BALB/c heart allografts were treated with mATG alone or in combination with FGK4.5. T cell reconstitution in B6.WT heart allograft recipients treated with mATG alone is shown for comparison (dotted line). n = 4–6 recipients/group; error bars represent SD. The p‐values are shown for comparisons between knockout recipients treated with mATG alone vs mATG + FGK4.5
To test whether the critical role of CD40 signaling after lymphoablation is dependent on Mincle and proinflammatory cytokines, we treated heart allograft recipients with agonistic anti‐CD40 mAb at the time of lymphoablation. As we previously reported, 2 recipient CD4 T cell depletion markedly diminished CD8 T cell homeostatic expansion, but anti‐CD40 mAb FGK4.5 injection compensated for the lack of help in CD4‐depleted recipients and partially restored CD8 T cell recovery (Figure 5C). In contrast, administration of agonistic anti‐CD40 mAb had no effect on T cell reconstitution in Mincle−/− recipients (Figure 5D). Furthermore, anti‐CD40 mAb treatment failed to promote T cell recovery in Casp‐1−/− recipients unable to produce mature IL‐1β (Figure 5E). In conjunction with our previous findings, these results suggest that CD40/CD154 pathway in this model acts primarily via enhancing Mincle signaling and proinflammatory cytokine production by B cells.
4. DISCUSSION
Despite the well‐documented benefits of peritransplant lymphoablation for early graft function and prevention of acute rejection, the impact on long‐term outcomes remains controversial. 26 , 27 , 28 , 29 , 30 Our previous work established inflammation induced by transplant surgery and recipient B cells producing proinflammatory cytokines as important drivers of T cell reconstitution contributing to allograft rejection. In the current study, we identify C‐type lectin Mincle as a receptor linking posttransplant inflammation and B cell activation in mATG treated allograft recipients.
Since the cloning of the Mincle gene in 1999, its expression, signaling, and functions in myeloid cells have been extensively characterized. To date, a single study demonstrated Mincle up‐regulation in human B cells treated with a TLR9 ligand. 22 Kawata et al. reported that the addition of a synthetic Mincle ligand trehalose‐6,6’‐dibehenate (TDB) decreased in vitro IgA and IgG release induced by TLR9 activation. 22 Analogous to previous findings in myeloid cells and human B cells, we found that mouse B lymphocytes up‐regulate Mincle expression after 20 h of in vitro culture even in the absence of stimulation (presumably via sensing DAMPs released by dying cells) and that the numbers of Mincle‐expressing B cells are further increased by TLR4 or CD40 ligation. Importantly, our study is the first to detect up‐regulation of Mincle on B lymphocytes in vivo and to show its functional relevance during an immune response. The studies are currently ongoing in the laboratory to address the functions of Mincle+ vs Mincle− B cells in lymphopenic recipients with high resolution.
The current study is the first to investigate Mincle expression and contribution during the alloimmune response to transplanted organs. Presumably, the impact of Mincle and other pattern recognition receptors (PRRs) is more prominent in the settings of increased inflammation such as ischemia/reperfusion injury or massive cell death due to lymphoablation. Following mATG treatment, recipient Mincle deficiency results in decreased B cytokine production and delayed T cell reconstitution ultimately leading to prolonged heart allograft survival (Figures 2 and 3). Conversely, providing exogenous Mincle ligand to mATG treated, non‐transplanted mice enhanced T cell reconstitution indicating that Mincle ligation is both necessary and sufficient for this process (Figure 4).
While measuring T cell reconstitution in lymphopenic heart allograft recipients, it is important to consider the contribution of ongoing alloresponse as well as posttransplant inflammation. We have previously established that the rate of T cell expansion in heart allograft recipients treated with mATG does not depend on reactivity to donor alloantigens. 3 In the current study, Mincle expression is equally upregulated in B cells from either allo‐or isograft recipients (Figure 1E). This was unexpected given that the T cell reconstitution in heart isograft recipients treated with mATG is delayed compared to that in allograft recipients. 5 One explanation for such results is that the early posttransplant injury and DAMPs release (including Mincle ligands) by an allograft exceeds that of an isograft and can thus have a stronger influence on T cell proliferation. This possibility is consistent with the similar T cell recovery in WT and Mincle−/− recipients of skin allografts that have a limited time of DAMPs release compared to heart transplants.
Our findings raise several questions including the precise mechanisms leading to Mincle up‐regulation in B lymphocytes after transplantation with or without lymphoablation, the identity of Mincle ligand(s) in heart allograft recipients, and the potential contribution of other Mincle‐expressing cells besides B lymphocytes. Our data rule out the role of BCR signaling in Mincle up‐regulation. Spleen B cells from isograft recipients increase Mincle expression after mATG treatment (Figure 1E), whereas B cells up‐regulate Mincle expression in culture in the absence of BCR ligation (Figure 1G). Mincle expression in myeloid cells can be induced by a variety of factors, including TLR ligands, IL‐6, TNFα, and IFNγ (reviewed in Refs. 7, 8, 9, 10), all of which are generated after allotransplantation. In addition, our data indicate that the B cell Mincle expression and cytokine production is critically dependent on CD40 signaling (Figure 5). Notably, heart transplantation without lymphoablation is not sufficient to significantly up‐regulate Mincle expression (Figure 1) most likely because CD40 signaling in lymphoreplete animals is limited to rare antigen‐specific B cells. Mincle is one of the targets of the C/EBPβ transcription factor that can be activated by CD40 signaling in B cells. 9 , 25 , 31 It remains to be determined whether CD40 signals in our model induce B cell Mincle expression directly or indirectly, for example via enhancing TLR signaling. Regardless of the exact mechanism, the ability of CD40 signaling to facilitate T cell reconstitution is critically dependent on the presence of Mincle (Figure 5).
While Mincle was originally described as a sensor of glycolipids from microbial pathogens, several ligands released by dying mammal cells have been eventually identified. To date, the established endogenous ligands include SAP‐130, 20 β‐GlcCer, 21 and cholesterol crystals, 32 and it is likely that more Mincle ligands will be described in the future. Transplantation surgery, early alloresponses, and lymphoablation‐induced cell death all result in the release of various DAMPs. 33 , 34 , 35 , 36 Based on our previously published findings and the data presented in this manuscript, we propose that after lymphoablation, B cells receive CD40 signals from residual helper T cells leading to Mincle up‐regulation and sensing of graft‐derived circulating DAMPs through TLRs and Mincle. This results in the secretion of proinflammatory cytokines that either directly enhance T cell proliferation or amplify inflammation by further enhancing B cell cytokine production (Figure 6). Consistent with this model, T cell reconstitution is markedly accelerated in heart allograft recipients compared to non‐transplanted mice implicating the graft as a major source of DAMPs. 2 , 5 It is, therefore, not surprising that Mincle facilitates T cell recovery after heart allotransplantation, but not in non‐transplanted mice or recipients of non‐vascularized skin allografts (Figure 3). Ongoing studies in the lab are investigating the biochemical nature of potential Mincle ligand(s) released by heart allografts with the ultimate goal of targeting these mediators to diminish posttransplant inflammation. As Mincle was shown to regulate antigen‐presenting functions of myeloid cells, 8 limiting this signaling pathway may lead to an additional benefit of decreased alloreactive T cell priming further promoting allograft survival.
FIGURE 6.
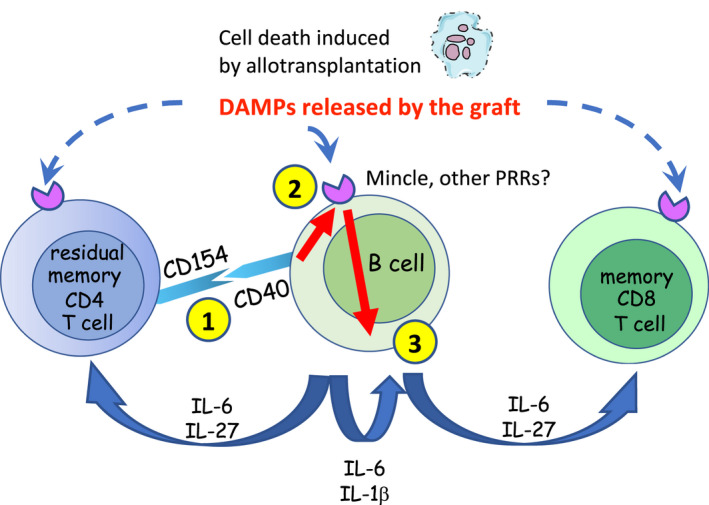
Proposed model. 1: interaction of CD40 on B cells with CD154 on depletion‐resistant memory helper T cells. 2: up‐regulation of Mincle and other pattern recognition receptors. 3: production of proinflammatory cytokines by B cells
We have previously established the central role of B lymphocytes following mATG lymphoablation, as B cell depletion or genetic deficiency significantly impairs T cell reconstitution. 2 , 5 The absence of Mincle only in recipient B cells led to markedly delayed T cell recovery indicating the importance of this PRR in B lymphocyte activation following lymphoablation (Figure 3). Nevertheless, these data do not rule out the potential contribution of Mincle signaling in myeloid cells. In our previous studies, the depletion of CD11c+ cells using CD11cDTR mice treated with diphtheria toxin (DT) did not influence T cell reconstitution 2 (not shown). It is, therefore, suggestive that the numbers of Mincle‐expressing CD11b+CD11c− but not CD11b−CD11c+ or CD11b+CD11c+ cells are increased in mATG‐treated heart allograft recipients compared to heart transplantation alone (Figure S2). The role of Mincle‐expressing CD11b+ cells in the process of T cell recovery will be addressed in our future studies. It should be noted that the levels of Mincle expression in T lymphocytes were minimal after heart allotransplantation, and not affected by mATG treatment (not shown) making unlikely the direct influence of Mincle ligands on T lymphocytes.
In summary, our data indicate that following lymphoablation in the settings of transplantation, Mincle is required for recipient B cells to initiate production of proinflammatory cytokines and thus facilitate homeostatic T cell reconstitution. To our knowledge, this is the first report of the role of Mincle function in B lymphocytes in vivo. These findings identify Mincle and its endogenous ligands as potential therapeutic targets for minimizing the effects of posttransplant inflammation and improving the efficacy of lymphoablation in transplant recipients.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Ms. Ashley Beavers for managing mouse colonies and generating animals for this study. The study was supported by NIAID R01 AI113142 (AV) and CTOT NanoString Core U01 AI063594 (RLF).
Hasgur S, Yamamoto Y, Fan R, et al. Macrophage‐inducible C‐type lectin activates B cells to promote T cell reconstitution in heart allograft recipients. Am J Transplant. 2022;22:1779–1790. doi: 10.1111/ajt.17033
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mohty M, Bacigalupo A, Saliba F, Zuckermann A, Morelon E, Lebranchu Y. New directions for rabbit antithymocyte globulin (Thymoglobulin((R))) in solid organ transplants, stem cell transplants and autoimmunity. Drugs. 2014;74(14):1605‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayasoufi K, Fan R, Fairchild RL, Valujskikh A. CD4 T cell help via B cells is required for lymphopenia‐induced CD8 T cell proliferation. J Immunol. 2016;196(7):3180‐3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor‐reactive memory T cells in mice. Am J Transplant. 2013;13(3):589‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayasoufi K, Zwick DB, Fan R, et al. Interleukin‐27 promotes CD8+ T cell reconstitution following antibody‐mediated lymphoablation. JCI Insight. 2019;4(7):e125489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasgur S, Fan R, Zwick DB, Fairchild RL, Valujskikh A. B cell‐derived IL‐1beta and IL‐6 drive T cell reconstitution following lymphoablation. Am J Transplant. 2020;20:2740‐2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Fresno C, Iborra S, Saz‐Leal P, Martinez‐Lopez M, Sancho D. Flexible signaling of myeloid C‐type lectin receptors in immunity and inflammation. Front Immunol. 2018;9:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kingeter LM, Lin X. C‐type lectin receptor‐induced NF‐kappaB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9(2):105‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu X, Nagata M, Yamasaki S. Mincle: 20 years of a versatile sensor of insults. Int Immunol. 2018;30(6):233‐239. [DOI] [PubMed] [Google Scholar]
- 9. Ostrop J, Lang R. Contact, collaboration, and conflict: signal integration of Syk‐coupled C‐type lectin receptors. J Immunol. 2017;198(4):1403‐1414. [DOI] [PubMed] [Google Scholar]
- 10. Richardson MB, Williams SJ. MCL and Mincle: C‐type lectin receptors that sense damaged self and pathogen‐associated molecular patterns. Front Immunol. 2014;5:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ribbhammar U, Flornes L, Backdahl L, Luthman H, Fossum S, Lorentzen JC. High resolution mapping of an arthritis susceptibility locus on rat chromosome 4, and characterization of regulated phenotypes. Hum Mol Genet. 2003;12(17):2087‐2096. [DOI] [PubMed] [Google Scholar]
- 12. Wu XY, Guo JP, Yin FR, et al. Macrophage‐inducible C‐type lectin is associated with anti‐cyclic citrullinated peptide antibodies‐positive rheumatoid arthritis in men. Chin Med J (Engl). 2012;125(17):3115‐3119. [PubMed] [Google Scholar]
- 13. Nakamura N, Shimaoka Y, Tougan T, et al. Isolation and expression profiling of genes upregulated in bone marrow‐derived mononuclear cells of rheumatoid arthritis patients. DNA Res. 2006;13(4):169‐183. [DOI] [PubMed] [Google Scholar]
- 14. N’diaye M, Brauner S, Flytzani S, et al. C‐type lectin receptors Mcl and Mincle control development of multiple sclerosis‐like neuroinflammation. J Clin Invest. 2020;130(2):838‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arumugam TV, Manzanero S, Furtado M, et al. An atypical role for the myeloid receptor Mincle in central nervous system injury. J Cereb Blood Flow Metab. 2017;37(6):2098‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki Y, Nakano Y, Mishiro K, et al. Involvement of Mincle and Syk in the changes to innate immunity after ischemic stroke. Sci Rep. 2013;3:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greco SH, Torres‐Hernandez A, Kalabin A, et al. Mincle signaling promotes Con A hepatitis. J Immunol. 2016;197(7):2816‐2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Te Velde AA. The C‐type lectin Mincle: Clues for a role in Crohn's disease adjuvant reaction. Front Immunol. 2017;8:1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou H, Yu M, Zhao J, et al. IRAKM‐Mincle axis links cell death to inflammation: Pathophysiological implications for chronic alcoholic liver disease. Hepatology. 2016;64(6):1978‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM‐coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9(10):1179‐1188. [DOI] [PubMed] [Google Scholar]
- 21. Nagata M, Izumi Y, Ishikawa E, et al. Intracellular metabolite beta‐glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc Natl Acad Sci USA 2017;114(16):E3285‐E3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawata K, Illarionov P, Yang G‐X, et al. Mincle and human B cell function. J Autoimmun. 2012;39(4):315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor‐specific transfusion and anti‐CD40 ligand therapy. J Immunol. 2004;172(9):5456‐5466. [DOI] [PubMed] [Google Scholar]
- 24. Rabant M, Gorbacheva V, Fan R, Yu H, Valujskikh A. CD40‐independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long‐lasting humoral immunity. Am J Transplant. 2013;13(11):2831‐2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumoto M, Tanaka T, Kaisho T, et al. A novel LPS‐inducible C‐type lectin is a transcriptional target of NF‐IL6 in macrophages. J Immunol. 1999;163(9):5039‐5048. [PubMed] [Google Scholar]
- 26. Bunnapradist S, Takemoto SK. Multivariate analysis of antibody induction therapy and their associated outcomes in deceased donor transplants. Transplant Proc. 2005;37(2):889‐891. [DOI] [PubMed] [Google Scholar]
- 27. Hardinger KL, Schnitzler MA, Koch MJ, et al. Thymoglobulin induction is safe and effective in live‐donor renal transplantation: a single center experience. Transplantation. 2006;81(9):1285‐1289. [DOI] [PubMed] [Google Scholar]
- 28. Libório AB, Mendoza TR, Esmeraldo RM, et al. Induction antibody therapy in renal transplantation using early steroid withdrawal: long‐term results comparing anti‐IL2 receptor and anti‐thymocyte globulin. Int Immunopharmacol. 2011;11(11):1832‐1836. [DOI] [PubMed] [Google Scholar]
- 29. Martins L, Fonseca I, Almeida M, et al. Immunosuppression with antithymocyte globulin in renal transplantation: better long‐term graft survival. Transplant Proc. 2005;37(6):2755‐2758. [DOI] [PubMed] [Google Scholar]
- 30. Opelz G, Naujokat C, Daniel V, Terness P, Dohler B. Disassociation between risk of graft loss and risk of non‐Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation. 2006;81(9):1227‐1233. [DOI] [PubMed] [Google Scholar]
- 31. Baccam M, Woo SY, Vinson C, Bishop GA. CD40‐mediated transcriptional regulation of the IL‐6 gene in B lymphocytes: involvement of NF‐kappa B, AP‐1, and C/EBP. J Immunol. 2003;170(6):3099‐3108. [DOI] [PubMed] [Google Scholar]
- 32. Kiyotake R, Oh‐Hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S. Human mincle binds to cholesterol crystals and triggers innate immune responses. J Biol Chem. 2015;290(42):25322‐25332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Braza F, Brouard S, Chadban S, Goldstein DR. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol. 2016;12(5):281‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dwyer GK, Turnquist HR. Untangling local pro‐inflammatory, reparative, and regulatory damage‐associated molecular‐patterns (DAMPs) pathways to improve transplant outcomes. Front Immunol. 2021;12:611910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and damage‐associated molecular patterns (DAMPs). Am J Transplant. 2016;16(12):3338‐3361. [DOI] [PubMed] [Google Scholar]
- 36. Matta BM, Reichenbach DK, Blazar BR, Turnquist HR. Alarmins and their receptors as modulators and indicators of alloimmune responses. Am J Transplant. 2017;17(2):320‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


