Abstract
Alzheimer’s disease (AD) is the most common cause of dementia in late life. It is difficult to precisely diagnose AD at early stages, making biomarker search essential for further developments. The objective of this study was to identify protein biomarkers associated with aluminum ions toxicity (AD-like toxicity) in a human neuroblastoma cell model, SH-SY5Y and assess potential prevention by NAP (NAPVSIPQ). Complete proteomic techniques were implemented. Four proteins were identified as up-regulated with aluminum ion treatment, CBP80/20-dependent translation initiation factor (CTIF), Early endosome antigen 1 (EEA1), Leucine-rich repeat neuronal protein 4 (LRRN4) and Phosphatidylinositol 3-kinase regulatory subunit beta (PI3KR2). Of these four proteins, EEA1 and PI3KR2 were down-regulated after NAP-induced neuroprotective activity in neuroblastoma cells. Thus, aluminum ions may increase the risk for neurotoxicity in AD, and the use of NAP is suggested as a treatment to provide additional protection against the effects of aluminum ions, via EEA1 and PI3KR2, associated with sorting and processing of the AD amyloid precursor protein (APP) through the endosomal system.
Keywords: Aluminum ion, Proteomics, Alzheimer’s disease (AD), Activity-dependent neuroprotective protein (ADNP), NAP
1. Introduction
Alzheimer’s disease (AD), first described by a German neurologist Alois Alzheimer in 1906, dramatically affects the brain. The symptoms of AD include language problems, short-term memory loss, slow loss of memory, and unpredictable behavior. Dr. Alzheimer found postmortem brain tissue changes in a woman who had died of an unusual mental illness. The original findings included multiple abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary tangles). Plaques, tangles and loss of connections between nerve cells (neurons) in the brain are the main features of AD. AD is the most common cause of dementia late in life and it is a devastating neurological disorder accounting for about 50% of all dementias, which involve multiple pathologies in the complex biological system of the brain [1]. AD is a neurodegenerative disease, which means progressive brain damage coupled with gradually increasing symptomatic severity. The processes resulting in the catastrophic brain damages associated with AD is still an enigma, and it is difficult and time-consuming to diagnose AD at its very early stage [2,3].
Aluminum is the third ranking element (after oxygen and silicon) in the crust of earth, and is the most abundant metallic element. Previously, studies have indicated that physiological concentrations (30 and 300 μmol/L) of aluminum ions can promote the mitosis of epithelial cells. Less than physiological concentrations of aluminum ions may interact with DNA and protein synthesis in cells, similar to other toxic trace elements. It is also indicated that excessive intake of aluminum ions may be one of the risk factors for AD and damage to the nervous system [4].
Previous studies have also indicated some candidate screening biomarkers for AD may be tau and phosphorylated tau in saliva [5], and α-2-macroglobulin, amyloid, and apolipoproteins in serum [6–8]. The most reproducible biomarker findings were decreased expression of β-amyloid1–42 and increased expression of tau and phospho-tau (ptau-181) in cerebrospinal fluid (CSF) samples [5,9–12]. However, obtaining CSF for clinical diagnosis in the early stage of AD is challenging. A diagnosis is made primarily on clinical grounds and, to date, there are no well-defined biochemical biomarkers available for early stage diagnosis.
Previously, complete proteomics of AD serum identified activity-dependent neuroprotective protein (ADNP, 124 kDa) as the only protein decreasing in AD vs. matched controls [13]. Furthermore, serum ADNP levels paralleled intelligence test performance in elderly individuals [14]. Originally, ADNP was discovered as a glial cell mediator of a vasoactive intestinal peptide associated with neuroprotection and essential for brain development [15–17]. In the hippocampus, cerebral cortex, and cerebellum, the highly conserved ADNP gene is abundantly expressed [18,19]. It is implicated inmaintenance of cell survival through chromatin remodeling, cytoskeletal protection modulation of p53 expression [20–25]. In a previous study, the mRNA expression of ADNP in hippocampus and cerebellum in early stage of AD was detected using mouse model [19]. NAP also protects cerebral cortical neurons against the major AD toxic peptide β-amyloid1–42 and inhibits β-amyloid1–40 aggregation [26,27]. Several studies have shown that ADNP was involved in neuroprotection, central nervous system (CNS) development and immunoregulation for the brain damage [17,18,24,25,28]. The expression of ADNP may be related to neurogenesis in the hippocampus as part of endogenous compensatory mechanisms triggered by AD onset and β-amyloid toxicity [19,21–23].
Structure activity studies identified a short octapeptide sequence in ADNP, NAP, as a multi-functional protective peptide, (Asn-Ala-Pro-Val-Ser-Ile-Pro-Gln; single-letter code, NAPVSIPQ, ADNP amino acid sequence from 354 to 361) [15]. NAP plays an important role in neuronal differentiation and the survival of neurons in different pathological conditions [29]. The structure of NAP allows membrane penetration, which interacts with tubulin binding and enhances microtubule assembly toward cellular protection in astrocytes to increase neurite outgrowth and to protect neurons and glial cells against toxicities [30,31]. NAP directly interacts with microtubule end-binding proteins (EBs) and has an impact on the microtubular processes mediated by EBs, such as taumicrotubule association, microtubule dynamics and assembly, and neurite outgrowth [32–34]. It also presents anti-oxidative and anti-apoptotic properties and exerts protective actions against different types of cholinergic neurotoxicity. NAP also prevents neuronal death associated with chronic neurodegenerative disorders [31]. Thus, the mechanisms of NAP may include anti-inflammatory effects, antioxidant activity, inhibition of protein aggregation and interaction with microtubules [35]. In previous studies, it has been indicated that NAP could prevent ethanol-induced developmental toxicity, embryotoxicity, and has a potential importance of decreasing ethanol effects on the pathophysiology of fetal alcohol syndrome (FAS) [36,37]. In AD research, the apolipoprotein E (APOE) E4 allele has been implicated as a risk factor [38]. NAP also provides neuroprotection in Apo E deficiency [15] and is able to break amyloid aggregation and protect against β-amyloid toxicity [27,38,39]. Neurofibrillary tangles, composed of hyperphosphorylated tau aggregation, are one of the major pathological hallmarks of AD [40]. NAP treatment may reduce the tau pathology and the extent of phosphorylation at the early stages of AD [41]. NAP mimics the neuroprotective activity protein and can cross the blood-brain barrier (BBB). Thus, the use of NAP is suggested for treatment as an additional protection in injured brain patients [28,42].
In this study, we will set out to elucidate the neuroprotective activity of NAP in vitro. To understand the physiological significance of NAP, the SH-SY5Y cell model was developed by using aluminum ions as the toxic element. We aimed to investigate the effect of NAP on neuroprotection and neurogeneration in a prodromal stage of AD, and set out to elucidate the pathways involving NAP activities during aluminum exposure in a neuronal model system, utilizing proteomic approaches.
2. Methods and materials
2.1. SH-SY5Y cell culture
The human neuroblastoma cell line, SH-SY5Y was obtained from Bioresource Collection and Research Center of Taiwan. Cells were grown in a 1:1 mixture of ATCC-formulated Eagle’s Minimum Essential Medium and F12 Medium, which was complemented by: 100 U/ml penicillin and fetal bovine serum to a final concentration of 10%. SH-SY5Y cells were incubated at 37 °C in 5% CO2 for 12 h for investigation of the adhesion of the cells. After 24 h incubation, the aluminum chloride (AlCl3) and NAP were added into the medium for cell culture for 24 h. Previous studies have shown Zinc and NAP effect on microtubule dynamics in shorter periods of time (e.g. 2–4 h) [32–34]. However, effects on cell shape were seen after 24 h [32]. We therefore chose the 24 h time period with plans to also investigate shorter and longer time periods of incubation in follow up studies. It should be noted though that aluminum ion concentrations higher than 300 or 100 μM were toxic to cells for the exposure of 48 and 72 h (see results).
2.2. BrdU and LDH assay
Cell proliferation was determined by BrdU cell proliferation assay kit (Millipore). The assay was performed according to the manufacturer’s instructions. Briefly, 1 × 103 SH-SY5Y cells were seeded in a sterile 96-well tissue culture plate and incubated for 48–72 h. Then, SH-SY5Y cells were incubated in the culture medium containing BrdU reagent for 2 h. Fixing solution had been added before the absorbance was measured at 450 nm by ELISA reader.
The cell cytotoxicity study was performed with the lactate dehydrogenase (LDH) leakage assay leakage into the culture medium. The LDH assay was based on the conversion of lactate to pyruvate in the presence of LDH with a parallel reduction of NAD. The formation of NADH from the above reaction resulted in a change of absorbance at 340 nm.
2.3. Determination of malondialdehyde concentration
Lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA) using a TBARS Assay Kit (Thiobarbituric Acid Reactive Substances Assay Kit, Cayman Chemical). To prepare the color reagent, 530 mg of thiobarbituric acid (TBA) was weighed and added into a beaker containing 50 mL of diluted TBA acetic acid solution. Subsequently, 50 mL of diluted TBA sodium hydroxide was added and mixed until the TBA was completely dissolved. Each cell lysate sample (100 μL) was added into a 15 mL centrifuge tube and thoroughly mixed with 100 μL of TBA SDS solution. Then, 4 mL of the color Reagent was added into the 15 mL centrifuge tube, and the mixture was incubated in boiling water for a 1 h reaction time. The 15 mL centrifuge tube was then removed and placed in an ice bath for 10 min to stop the reaction. The resulting solution was centrifuged at 1600 g at 4 °C for 10 min, and then set at room temperature for 30 min. The supernatant (100 μL) was loaded into the wells of a microplate, and the absorbance was measured at 520 nm using an ELISA reader.
2.4. Free radical scavenging activity using DPPH
The antioxidant activity of the aluminum ion with NAP treatment was determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. DPPH is a stable free radical with purple coloring and has a maximum absorption at 520 nm. The free radical-scavenging assay was based on the decoloration of the compound when reduced by a free radical scavenger. Briefly, 0.5 μM DPPH ethanolic solution was freshly prepared in reserve. Each cell lysate sample (50 μL) was added to 750 μL of DPPH ethanolic solution and diluted with an extra 200 μL of ethanol. Then, 750 μL of DPPH ethanolic solution and 250 μL of ethanol were mixed as a control. The resulting solution (150 μL) was loaded into the wells of a microplate, and the absorbance was then measured at 520 nm using an ELISA reader. Quintuplicates of the control and the cell lysate samples were performed.
The DPPH radical scavenging activity (%) was calculated using the following formula:
where A0 and Ai are the absorbance of control and test samples, respectively.
2.5. ELISA analysis
Each cell lysate sample was analyzed for the concentrations of expectant and candidate proteins in duplicate, using commercially available enzyme-linked immunosorbent assay (ELISA) kits. The protein concentrations were tested by standard protocols, as suggested by the manufacturer. The ELISA reader model was Multiskan EX (Thermo scientific, Vantaa, Finland). Statistical significance was determined by using the two-tailed Fisher exact test at p < 0.05 and ROC analysis.
2.6. Protein identification by proteomic approaches
After incubation with aluminum ion and NAP for 24 h, SH-SY5Y cells are washed using phosphate-buffered saline and lysed using radioimmunoprecipitation assay (RIPA) cell lysis buffer. Cell lysates were centrifuged at 1500 g for 10 min at 4 °C and filtered through a 0.8 μmfilter. Cell lysate samples (100 μL) were reduced, alkylated, and then digested with trypsin according to standard protocols [43]. Formic acid (2 μL) was added to each sample prior to mass spectrometric analysis for protein identification. Complex peptide mixtures were separated using RP-nano-HPLC-ESI-MS/MS (nanoACQUITY UPLC, Waters, Milford, MA, USA coupled to an ion trap mass spectrometer LTQ Orbitrap Discovery Hybrid FTMS, Thermo, San Jose, CA, USA). Each cycle of one full scan mass spectrum (m/z 400–2000) was followed by four data-dependent tandem mass spectra, with collision energy set at 35%. For protein identification, Mascot software (Version 2.2.1, Matrix Science, London, UK) was used to search the Swiss-Prot human protein sequence database. Positive protein identifications were defined when the Mowse scores were considered significant (p < 0.05). All Mascot results were manually confirmed by visual assessment of the MS/MS spectra for overall quality. It required a readily observable series of at least 4 y ions. Only a small fraction of searches produced significant matches according to the inclusion criteria. Proteins were annotated by similar searches using UniProtKB/Swiss-Prot databases (SIB Swiss Institute of Bioinformatics, Lausanne, Switzerland). The protein–protein interaction pathways were performed by String 9.1 Web software (SIB Swiss Institute of Bioinformatics, Lausanne, Switzerland).
2.7. Western blot
Protein extracts were prepared in RIPA cell lysis buffer and each protein sample (1 μg/μL, 10 μL) was electrophoresed through a precast gel (NuPAGE®Novex® 4–12% Bis-Tris Gel, 1.5 mm, 10 wells, Invitrogen™, Carlsbad, CA). Proteins were transferred from the gel to a polyvinyldifluoride (PVDF) membrane by means of the semidry technique using the Criterion Blotter (Bio-Rad) at 100 V for 60 min, and blocked with 5% milk in PBS (adjusted to pH 7.4) containing 0.05% Tween-20. Membranes were then separately incubated overnight with primary antibodies (CTIF, LRRN4, 1 μg/μL, 1:500). After washing, membranes were incubated with HRP-conjugated secondary antibodies for one hour (1:10000). Proteins were detected with an enhanced chemiluminscent (ECL) system, and quantitative analysis of Western blotting was carried out using the ImageQuant-TL-7.0 software.
2.8. Cell morphology observed by immunochemical straining
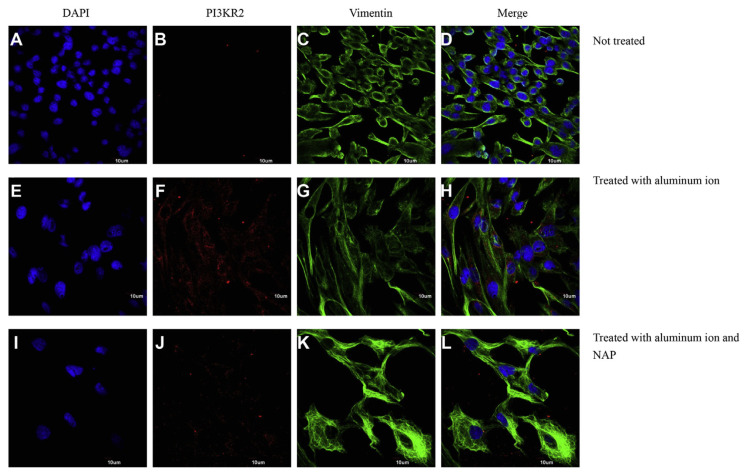
The nuclei and cytoskeleton of the cells were stained with 4′-6-diamidino-2-phenylindole (DAPI, Sigma–Aldrich, USA) and vimentin (vimentin DyLight®488 antibody, Epitomics, USA). For immunochemical staining of the morphology of SH-SY5Y cells via a confocal microscope, two kinds of antibodies (anti-EEA1, PA1-063A, Thermo Fisher Scientific and anti-PI3KR2, ab28356, abcam) were incubated separately and followed by staining with Alexa Fluor®568 goat anti-rabbit IgG (A-11011, Invitrogen). Cells were visualized and their images were taken using a laser confocal microscope (IX81, Olympus, UK).
2.9. Statistical analysis
All calculations used the SigmaStat statistical software (Jandel Science Corp., San Rafael, CA, USA). All statistical significances were evaluated at 95% of confidence level or better. Data are presented as mean ± standard error.
3. Results
3.1. Cell proliferation and viability of SH-SY5Y cells treated with aluminum ion
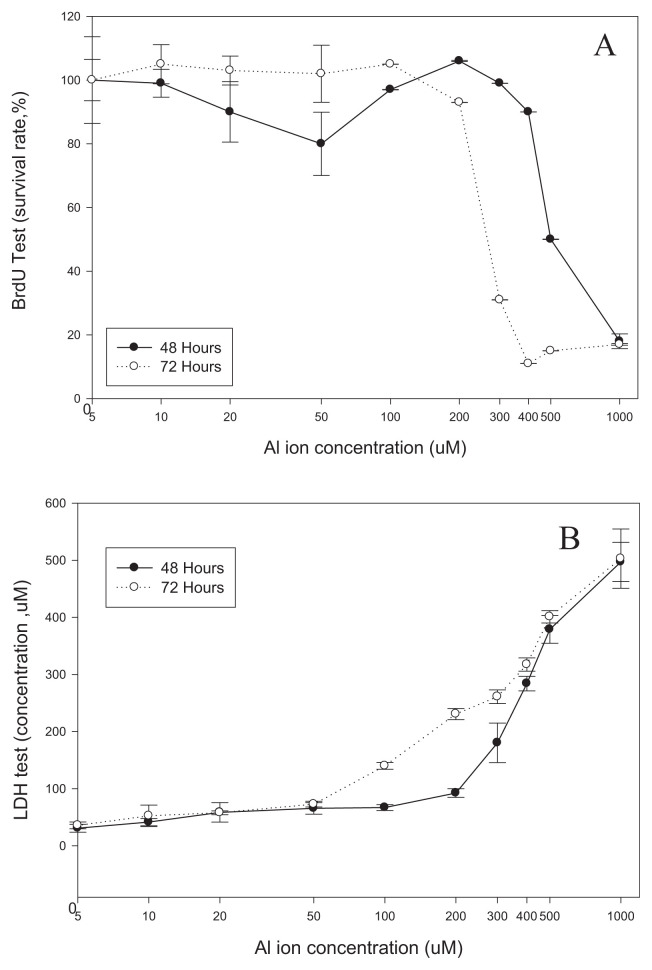
To study the effect of aluminum ion on SH-SY5Y cells, BrdU and LDH assays were used to test the viability of cells cultured in different concentrations of aluminum ion. Fig. 1A shows the proliferation rate of SH-SY5Y cells in the presence of 0–1000 μM of aluminum ions for 48 and 72 h with BrdU assay. No significant inhibitions to cell proliferation were observed when cells were treated with up to 400 μM of aluminum ions for 48 h or 200 μM of aluminum ion for 72 h. Nevertheless, dramatic inhibition of cell proliferation was noted in cells treated with aluminum ions at concentrations higher than 500 μMand 300 μM, particularly in the cells exposed for 48 and 72 h. Viability of SH-SY5Y cells in the presence of aluminum ions tested by the LDH assay, showed that aluminum ion concentrations higher than 300 or 100 μM were toxic to cells for the exposure of 48 and 72 h, respectively (Fig. 1B).
Fig. 1.
The cell proliferation and viability of SH-SY5Y cells treated with aluminum were measured by (A) BrdU and (B) LDH assays. (mean ± standard error, 6 repeats, *p < 0.05, t-test).
3.2. Aluminum ion exposure induces stress response
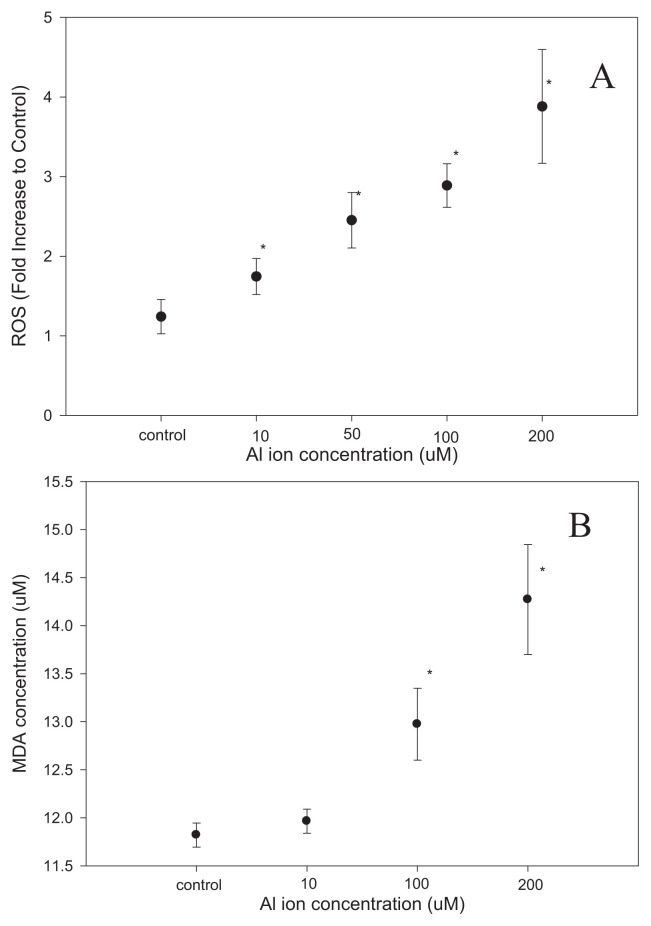
Potential stress responses driven by the exposure to aluminum ions were studied in SH-SY5Y cells. The ROS in the cells were quantified with amethod based on DPPH, showing a dose-dependent increment of ROS induced by aluminum ion (Fig. 2A). Measurement of MDA levels also indicated that cells exposed to aluminum ions exhibited higher level of MDA (Fig. 2B), the indicator of lipid peroxidation during oxidative stress. In the following experiments, treatment of 100 μM of aluminum ions for 48 h was taken as the standard procedure to study the impact of aluminum ions in SH-SY5Y cells.
Fig. 2.
The concentration of ROS and MDA of the SH-SY5Y cells with aluminum ion treatment were measured by ELISA methods. It was significantly higher with a dose-dependent increment of ROS and MDA induced by aluminum ion. (mean ± standard error, 6 repeats, *p < 0.05, t-test).
3.3. The level of β-amyloid in SH-SY5Y cells treated with aluminum ion
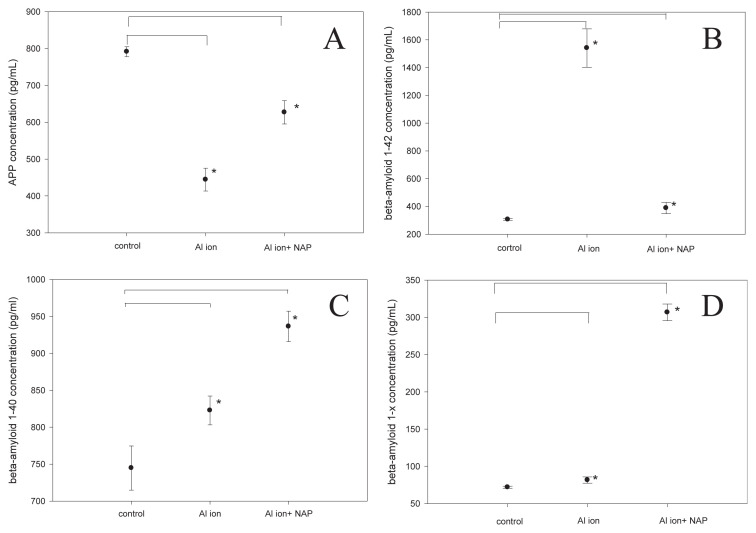
Further studies explored how the exposure to aluminum ions affected the cellular level of amyloid precursor protein (APP) and β-amyloid in SH-SY5Y cells. The generation of the neurotoxic β-amyloid peptide from sequential APP proteolysis is the crucial step in the development of AD. The concentration of APP in SH-SY5Y cells was found to be lowered when the cells were cultured in the presence of aluminum ions, and such aluminum-mediated APP reduction was partially diminished by the concurrent treatment of NAP (Fig. 3A). The APP down-regulation with aluminum ion may be related to the change in APP metabolism to β-amyloid sequences. Thus, the concentration of β-amyloid1–42 was found higher in the cells treated with aluminumions, and the supplement of NAP diminished such increment of β-amyloid1–42 level (Fig. 3B). The level of β-amyloid1–40 was also higher when the cells were treated with aluminumions, and the supplement NAP, (unlike what has been found for β-amyloid1–42) further increased β-amyloid1–40 level 1.3 folds higher (Fig. 3C). The level of β-amyloid1–x showed a similar trend as β-amyloid1–40, in that the presence of aluminum ion slightly increased the β-amyloid1–x level in SH-SY5Y cells while the supplement of NAP further boosted β-amyloid1–x level about 3 folds higher (Fig. 3D). In summary, treatments of aluminum ion increased the level of all the 3 types of β-amyloid in SH-SY5Y cells, yet supplements of NAP exclusively diminished the raise of β-amyloid1–42 but further increased the level of β-amyloid1–40 and β-amyloid1–x. It may due to the fact that the NAP enhanced the decomposition of the β-amyloid1–42, which is caused by the aluminum ion and thus caused increases in the fragments of β-amyloid1–40 and β-amyloid1–x.
Fig. 3.
Confirmations of APP and β-amyloid (1–42, 1–40 and 1–X) expression in SH-SY5Y cells with aluminum ion and NAP treatment were measured by ELISA methods. (mean ± standard error, 6 repeats, *p < 0.05, t-test).
3.4. The level of Tau protein and activity-dependent neuroprotective protein (ADNP) in SH-SY5Y cells
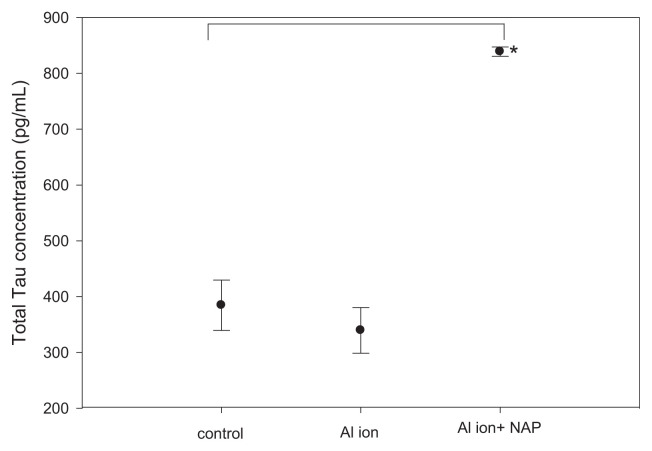
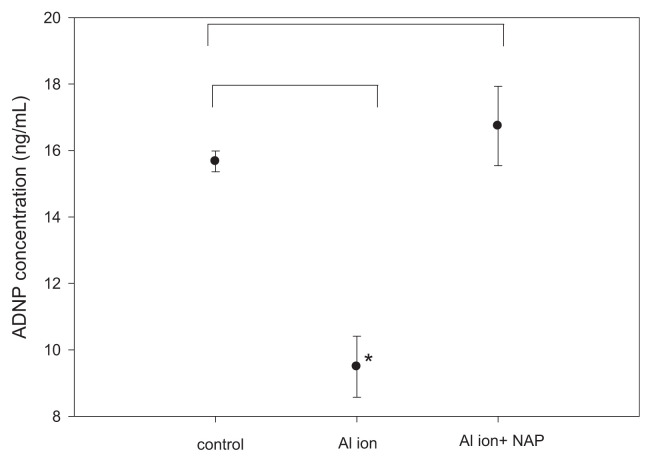
We also quantified the level of Tau protein, one of the key pathological factors of Alzheimer’s disease, and ADNP, a negative regulator of β-amyloid precipitation, in SH-SY5Y cells treated with aluminum ions by ELISA methods. The exposure to aluminum ion did not alter Tau protein levels in SH-SY5Y cells, but the addition of NAP increased the level of Tau by 2.5 fold (Fig. 4). Measurements of the level of ADNP indicated that the treatment with aluminum ion decreased the ADNP level, while the supplement of NAP could completely diminish such decrement of ADNP induced by aluminum ion (Fig. 5).
Fig. 4.
Confirmations of Tau expression in SH-SY5Y cells with aluminum ion and NAP treatment were measured by ELISA methods. (mean ± standard error, 6 repeats, *p < 0.05, t-test).
Fig. 5.
Confirmations of ADNP expression in SH-SY5Y cells with aluminum ion and NAP treatment were measured by ELISA methods. (mean ± standard error, 6 repeats, *p < 0.05, t-test).
3.5. Identification of differentially expressed proteins induced by aluminum ion
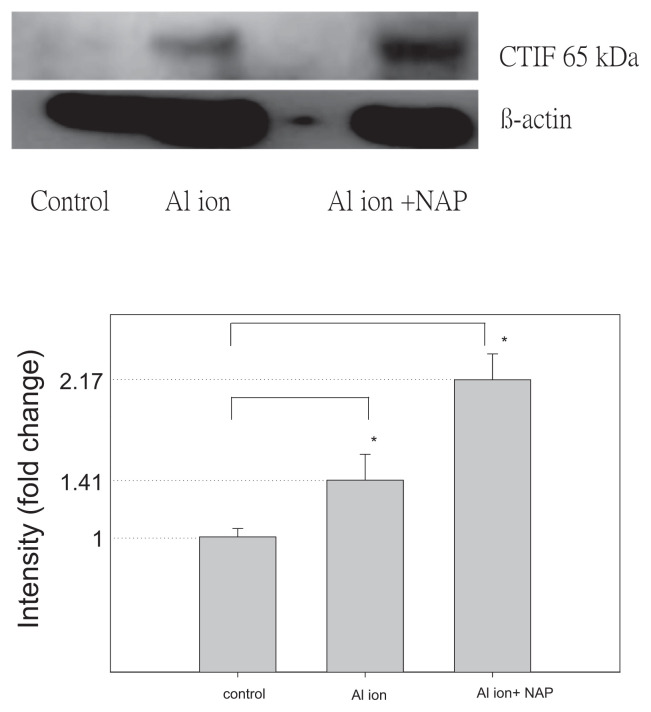
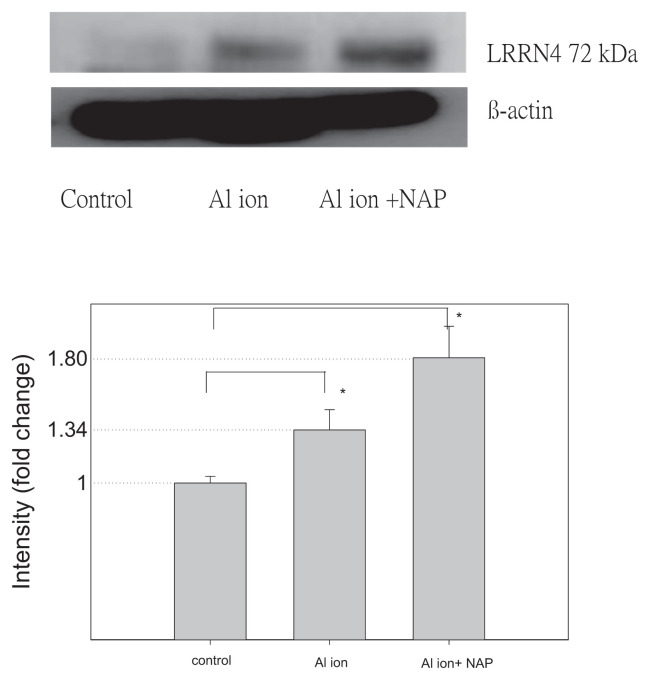
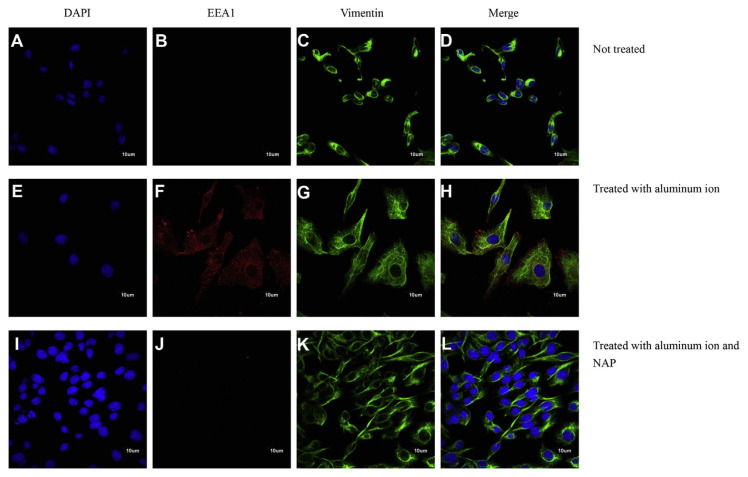
To identify the candidate proteins that may be related to aluminum ions, we identified differential protein expression by nano-HPLC-ESI-MS/MS. The experimental results demonstrated that a total of 2953 unique proteins, were identified from SH-SY5Y cell. When a protein was identified by three or more unique peptides possessing Mascot scores that passed the above criteria, the protein was considered present in the sample. Most of the 2953 proteins were identified at minimal confidence level, that is, only one unique peptide sequence was matched. Among these 2953 proteins, 256 proteins were identified with higher confidence levels (at least three unique peptide sequences matched). Of these 256 proteins, CTIF, EEA1, LRRN4, PI3KR2 were up-regulated and found in the samples of SH-SY5Y cell with aluminum ion treatment (Table 1). Expression of EEA1 and PI3KR2 proteins (but not of CTIF and LRRN4) was reduced by NAP (Figs. 6 and 7). Thus, the EEA1 and PI3KR2 were further studied for their expression and cellular localization. Fig. 8 demonstrated the expression and the localization of EEA1 proteins, showing cytosolic presence of EEA1 in aluminum ion treated cells but not non-treated cells (Fig. 8A–H). Interestingly, the EEA1 expression induced by aluminum ion was found diminished when cells were co-treated with NAP (Fig. 8I–L). Similar results were noted for the expression of PI3KR2, in that aluminum ion treatment induced high expression of the protein (Fig. 9A–H) yet concurrent treatment with NAP largely diminished such induction (Fig. 9I–L).
Table 1.
Four differentially expressed proteins identified in aluminum ion treated SH-SY5Y cells.
| Accession No.a | Protein name | MW (Da) | Mascot score | Match queries | pI | Sequence coverage | Peptide | Protein function |
|---|---|---|---|---|---|---|---|---|
| O43310 | CBP80/20-dependent translation initiation factor | 67,544 | 29 | 6 | 6.10 | 16% | R.NNSSDVDTK.L + 2 Deamidated (NQ) R.SSTGEPFR.V + 2 Phospho (ST) R.DKMLCPSESMLTR.S + Oxidation (M); Phospho (ST) - MENSSAASASSEAGSSR.S + Deamidated (NQ); Oxidation (M); 2 Phospho (ST) M.ENSSAASASSEAGSSR.S + Deamidated (NQ); 6 Phospho (ST) - MENSSAASASSEAGSSR.S + Deamidated (NQ); 5 Phospho (ST) |
Specifically required for the pioneer round of mRNA translation mediated by the cap-binding complex (CBC), that takes place during or right after mRNA export via the nuclear pore complex (NPC). Acts via its interaction with the NCBP1/CBP80 component of the CBC complex and recruits the 40S small subunit of the ribosome via eIF3. In contrast, it is not involved in steady state translation, that takes place when the CBC complex is replaced by cytoplasmic cap-binding protein eIF4E. Also required for nonsense-mediated mRNA decay (NMD), the pioneer round of mRNA translation mediated by the cap-binding complex playing a central role in nonsense-mediated mRNA decay (NMD). |
| Q15075 | Early endosome antigen 1 | 162,367 | 21 | 4 | 5.5 | 4% | M.LRRILQR.T + Deamidated (NQ) R.ENQSLQIKHTQALNRK.W + Deamidated (NQ) R.ENQSLQIKHTQALNRK.W + 4 Deamidated (NQ) K.MEKEALMTELSTVKDK.L + Oxidation (M); 2 Phospho (ST) |
Binds phospholipid vesicles containing phosphatidylinositol 3-phosphate and participates in endosomal trafficking. |
| Q8WUT4 | Leucine-rich repeat neuronal protein 4 | 78,794 | 34 | 3 | 6.82 | 6% | R.AFACFPALQLLNLSCTALGR.G + 2 Carbamidomethyl (C); 2 Deamidated (NQ) R.STYAQGTTVAPSAAPATRPAGDQQSVSK.A + 6 Phospho (ST) R.QTLPLLLLTVLRPSWADPPQEK.V |
May play an important role in hippocampus-dependent long-lasting memory |
| O00459 | Phosphatidylinositol 3-kinase regulatory subunit beta | 81,574 | 35 | 6 | 6.2 | 16% | R.LKSRIAEIHESR.T + 2 Phospho (ST) R.APALGPAVRALGATFGPLLLR.A + Phospho (ST) R.TWYVGKINRTQAEEMLSGK.R R.TWYVGKINRTQAEEMLSGK.R + Deamidated (NQ) R.TWYVGKINRTQAEEMLSGK.R + 2 Deamidated (NQ) K.IRDQYLVWLTQKGAR.Q + 2 Deamidated (NQ); Phospho (Y) |
Binds to activated (phosphorylated) protein-tyrosine kinases, through its SH2 domain, and acts as an adapter, mediating the association of the p110 catalytic unit to the plasma membrane. |
Swiss-Prot/TrEMBL accession number was given from https://world-2dpage.expasy.org/swiss-2dpage/.
Fig. 6.
The detection of CTIF protein expression on SH-SY5Y cells. Western blotting of CTIF and β-actin from SH-SY5Y cells cultured with aluminum ion and NAP. The signals were quantified and the data are presented as the mean ± standard error; p < 0.05 indicates statistical significance, as determined by the unpaired Student’s t-test.
Fig. 7.
The detection of LRRN4 protein expression on SH-SY5Y cells. Western blotting of LRRN4 and β-actin from SH-SY5Y cells cultured with aluminum ion and NAP. The signals were quantified and the data are presented as the mean ± standard error; p < 0.05 indicates statistical significance, as determined by the unpaired Student’s t-test.
Fig. 8.
Analyses of representative samples of SH-SY5Y cells expression of DAPI, Vimentin and EEA1 were shown. Immunochemical stains for DAPI (blue), vimentin (green) and EEA1 (red) for SH-SY5Y cells cultured with aluminum ion and NAP for 24 h. (Scale bars, 10 μm, Confocal microscope, 400X).
Fig. 9.
Analyses of representative samples of SH-SY5Y cells expression of DAPI, Vimentin and PI3KR2 were shown. Immunochemical stains for DAPI (blue), vimentin (green) and PI3KR2 (red) for SH-SY5Y cells cultured with aluminum ion and NAP for 24 h. (Scale bars, 10 μm, Confocal microscope, 400X).
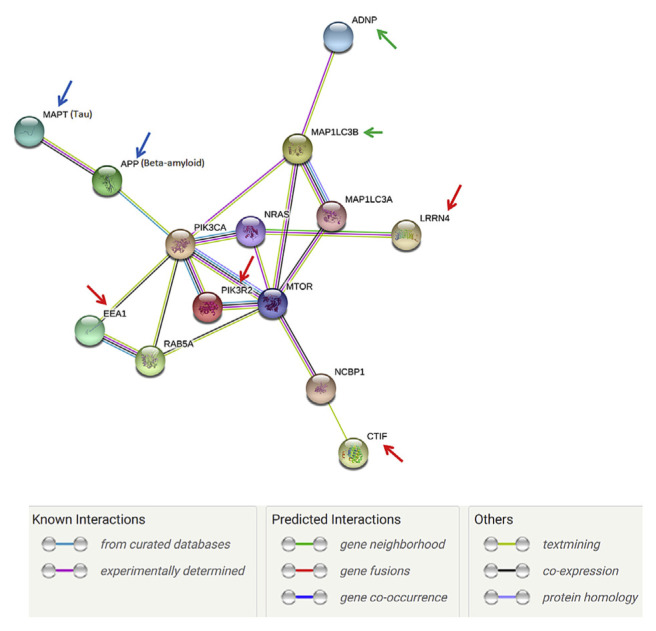
The protein–protein interaction pathways were performed by String 9.1 Web software, and four proteins identified in this study were marked by red arrows (Fig. 10). Using the protein–protein interaction pathway analysis, the main finding of aluminum ion treated cells is that influence of the mTOR pathway in SH-SY5Y cells which may result in cell proliferation which is required for survival of the majority of cell. We have also shown that ADNP interacts with MAP1LC3B [44], and NAP enhances this interaction (green arrows) and an indirect interaction between EEA1 and MAP1LC3A, suggest an interaction with the autophagy process at the level of protein expression and at the level of protein binding [45,46]. Furthermore, NAP was shown to enhance protective autophagy [44]. Tau and β-amyloid are marked by blue arrows (Fig. 10).
Fig. 10.
The protein–protein interaction pathways were illustrated. Proteins identified in this study are marked by arrows. For those 4 proteins, they may effect the mTOR pathway, which is responsible for the proliferation and is required for survival of the majority of cell.
4. Discussion
4.1. Toxicity of aluminum ion to SH-SY5Y cells
Aluminum ions have been reported to influence numerous cellular reactions that may cause various adverse effects on the mammalian central nervous system [47]. The only oxidation state of aluminum, aluminum ion, has strong affinity for negatively charged and/or oxygen-donor ligands, including inorganic and organic phosphates, carboxylate, and deprotonated hydroxyl groups, that facilitate its binding to many biological (macro-)molecules in cells, e.g. DNA, RNA, Proteins, ATP, amino acids. As a result, exposure to aluminum ion lead to altered gene expression, energy metabolism, enzymatic catabolism, that causes apoptotic death of neurons and glial cells [48]. As we have shown above, the exposure of SH-SY5Y cells to aluminum ions higher than 100 μM causes significant decrease in cell proliferation and viability (Fig. 1). We also observed an increased level of ROS when SH-SY5Y cells were treated with higher concentration of aluminum ion (Fig. 2A). Moreover, aluminum ion concentrations correlated with oxidative stress in SH-SY5Y cells (Fig. 2B), in accordance to previously reported in vivo results [49,50]. We suggest that aluminum ions increase oxidative stress which in turn compromises viability of neuronal cells, and chronic and/or high-dose administration of aluminum ion may impair brain functions, such as cognition and memory [51–55].
4.2. Aluminum ion alters the level of β-amyloid in SH-SY5Y cells
The accumulation of β-amyloid and hyper-phosphorylated Tau proteins in senile plaques were generally recognized as a hallmark of AD, and recently quite a few bio-chemical, toxicological, and genetic studies have suggested that the AD-like pathological symptoms caused by aluminumion were related to amyloid accumulation [56,57]. We have found that in SH-SY5Y cells the level of APP dropped when aluminumion was administered (Fig. 3A), though some studies in mice and rats have shown that oral/injective administration of aluminum ion increases the APP levels [58,59] and other studies of PC12 and NBP2 cells have found that the APP level was not altered by aluminum ion exposure [60,61]. The drop of APP level may be a consequence of the substantial increases in β-amyloid level (Fig. 3); the level of β-amyloid1–42 was about 5 fold higher when cells were treated with aluminum ion (Fig. 3B), though the aluminum ions induced only about a 20% increment of β-amyloid1–40 and β-amyloid1–x (Fig. 3C, D). Interestingly, Castorina et al., have reported that in SH-SY5Y cells the beta-site APP-cleaving enzyme 1, BACE1 and BACE2, two of the main enzymes participating in the processing of β-amyloid, were slightly down-regulated by the exposure of aluminum ion [62]. Thus, we suggest that aluminum ions promote APP to be processed to β-amyloid without up-regulations of BACE1 and BACE 2, resulting particularly the accumulation of β-amyloid1–42.
4.3. The effects of NAP in aluminum ion-mediated alteration of amyloid and Tau proteins
NAP has been reported for its neuroprotective effects against β-amyloid25–35/1–40 toxicity/aggregation [41], Tau hyper-phosphorylation associated with ADNP deficiency, and excitoxicity [27,63,64]. NAP has also been shown to promote neuronal survival in cerebral cortical cultures derived from newborn rats [65], and reduced the level of β-amyloid1–40 and β-amyloid1–42 in mice [66]. Here we showed that NAP partially diminished the drop of APP level induced by aluminum ion (Fig. 3A), and nearly completely relieved the boost of β-amyloid1–42 induced by aluminum ion (Fig. 3B). However, the increments of β-amyloid1–40 and β-amyloid1–x induced by aluminum ion have not been diminished by the concurrently administrated NAP (Fig. 3C, D). On the contrary, NAP treatments further increased the levels of β-amyloid1–40 and β-amyloid1–x about 1.3–3 fold. We concluded that NAP is able to diminish aluminum ion-induced β-amyloid1–42 and changes the composition of β-amyloid proteins, and such alterations in β-amyloid may be the key of NAP’s neuroprotective activity.
It has been reported that NAP is able to reduce hyper-phosphorylation of Tau proteins and prevent the deposition of neurofibrillary tangles without significant alteration of Tau level [18,67–69]. In SH-SY5Y cells, we have found that NAP induced high accumulation of Tau protein in the background of aluminum ion exposure (Fig. 4), though the impact of such elevated Tau in vivo needed to be further investigated. It is possible that NAP inhibition of PI3KR2, which resulted in the activation of CDC42 and GSK3B, thus the concentration of total Tau was increased. These results suggested the potential role played by NAP in the biogenesis of Tau protein, perhaps by increasing Tau-microtubule interaction and reducing the free available Tau [34,42].
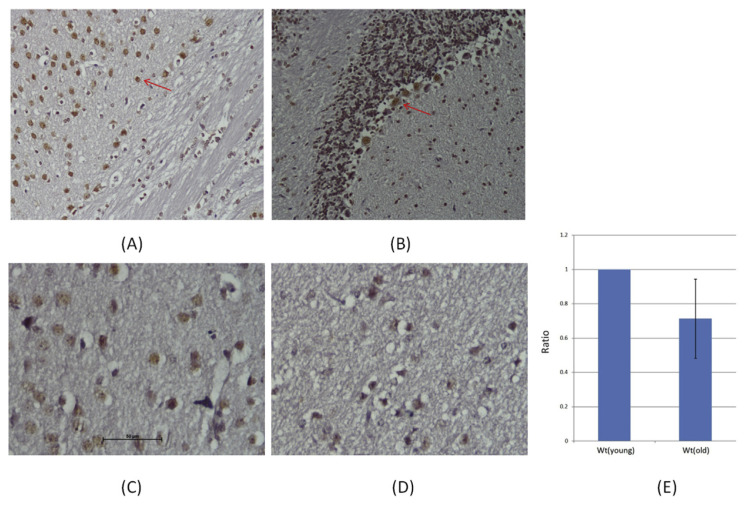
We also found that NAP diminished aluminum ion-mediated down regulation of ADNP (Fig. 5). Interestingly, the NAP-mediated ADNP regulation was found counteractive to the level of β-amyloid1–42 (Fig. 3B), in agreement with our suggestion that NAP might be capable to rescue aluminum ion-induced neurodegeneration. In our previous animal study, we also found the ADNP was down-regulated in the brain of old mice [70]. IHC stain showed the major locations of ADNP in neuron and Purkinje cell of brain (Fig. 11A, B). In Fig. 11C, D, the ADNP showed lower expression and neuronal cells also appeared to shrink in the old mouse brains. The same reductions were observed by Western blotting (Fig. 11E). Our results place ADNP at a pivotal regulatory point suggesting that changes in ADNP expression may affect aging sensitivity. More studies are needed to clarify the mechanism of how NAP could possibly protect neuronal cells during aluminum ion exposure.
Fig. 11.
The ADNP expression in the brains of mice. Histopathological examination of mice brain. (A) neuron and (B) Purkinje cell (Red arrow). Analyses of representative samples of the mice brains expression of ADNP were shown. Immunochemical stains for ADNP (red) for 3 months old mice (C) was showed the significal increased than 1 year old mice (D) (200X, Scale bars, 50 μm). The results of Western blotting which were showed the ADNP concentration in mice brain (E).
4.4. Aluminum ion-induced protein expression
CBP80/20-dependent translation initiation factor (CTIF) is the starting factor of translation. A previous report indicated that CTIF may affect the methylation of DNA, causing a mutation of APP protein and onset of AD [71]. Another study also reported that the expression of CTIF was higher in the AD-associated gene mutant H4-SW cell line and increased the secretion of β-amyloid [72]. In our experiment, the expression of CTIF in the treatment group of aluminum ion was higher than that of the control group. This result showed that aluminum ions may promote the expression of CTIF to induce DNA methylation, which causes APP protein to produce large amounts of beta-amyloid deposition in the brain, but NAP did not inhibit the performance of CTIF.
Leucine-rich repeat neuronal protein 4, referred to as LRRN4, was found on human chromosome 20 and composed of 740 amino acids containing fibronetin type III protein and 10 repeats of leucine-rich type I transfer membrane protein in the central nervous system. The function of LRRN4 is essential in the hippocampus [73]. The NLRR4 gene is associated with learning ability and long-term memory [74,75]. Animal experiments indicated that LRRN4 gene is related to long-and short-term memory [74]; diseases associated with LRRN4 are within the nervous system but the pathogenesis of AD is still unclear.
Four up-regulated proteins were identified with the proteomic analysis in aluminum ion-treated SH-SY5Y cells in this study, and two of these proteins, EEA1 and PI3KR2, were observed for their expression and localization under confocal microscopy with immunostaining (Figs. 8 and 9). EEA1 is one of the crucial protein group members that bind to phospholipid vesicles and participate in endosomal trafficking [76,77]. It has been suggested that AD is related to defects of endosomal sorting [78,79]. Recently, it has been reported that phosphatidylinositol-3-phosphate (PI3P) regulates sorting and processing of APP through the endosomal system, which is the binding ligand of EEA1 [80], implying an involvement of EEA1 in amyloidogenesis. In our study, aluminum ions were found to induce EEA1 expression that was diminished by concurrent administration of NAP (Fig. 8). Interestingly, the aluminum ion-induced expression of EEA1 positively correlated to the level of β-amyloid1–42 in the background of aluminum ion exposure (Fig. 3B). Thus, we suggested that EEA1 is a novel NAP-sensitive biomarker correlated to cellular β-amyloid1–42 level, though the functional impact of upregulated EEA1 expression to neurodegeneration requires more experimental evidences in vivo.
The expression of PI3P-kinase regulatory subunit (PI3KR2) was also found to be positively correlated to β-amyloid1–42 (Fig. 9), which was reported to be an activator of many signaling cascades involved in cell growth, survival, proliferation, motility and morphology, including PI3K/Akt/mTOR pathway that controls cell proliferation and apoptosis [81,82]. In neuronal cells, several studies have suggested that β-amyloid may trigger PI3K/Akt/mTOR signaling to promote cell proliferation to counteract β-amyloid’s apoptotic neurotoxicity [83–85]. This could be one of the reasons that PI3KR2 was found positively correlated to aluminum ion-induced β-amyloid1–42 accumulation (Fig. 9). Moreover, we highlight the potential interplay between EEA1 and PI3KR2, i.e. PI3KR2 regulates the production of PI3P which is the endosomal binding ligand of EEA1. Future, further studies may focus on the relevance of such interplay to aluminum ion-induced β-amyloid1–42 accumulation, NAP-mediated neuronal protection, and phosphorylation of Tau protein. We look forward to these new insights that may shed light on the development of AD.
We assume that aluminum is a key risk factor for AD [45–62], however many other risk factors exist and it is an open question if those share similar mechanisms, Furthermore, our study is limited in the time/dose/model choice aspects. Regardless, the discoveries made here open new horizons for AD study toward better understanding of the disease.
5. Conclusions
Many investigations have focused on the development of biomarkers as a noninvasive diagnostic tool. The use of proteomic techniques to identify disease-specific protein biomarkers is a powerful tool for defining the prognosis of disease and gaining deep insight into disease mechanisms in which proteins play major roles. Our present study further demonstrated a relationship between aluminumion, NAP and neuroblastoma cells. In our preliminary proteomic data, the aluminum ion-induced accumulation of β-amyloid1–42 may enhance the risk for AD. According to our previous proteomic data, ADNP levels may serve as a new and direct therapeutic indicator for NAP treatment, as also demonstrated in the recent study [86]. In this study, the protein expression of EEA1, PI3KR2, CTIF and LRRN4 was up-regulated in the aluminum ion treated cells, but only that of EEA1, PI3KR2 was diminished after NAP addition. These results pave the path to further tests, mandated for demonstrating the reproducibility of the potential biomarkers in disease models and in clinical trials. In conclusion, these proteins are valuable for the identification of differentially expressed proteins involved in the proteomics database and for screening biomarkers for further study of AD.
Acknowledgments
This work was supported by Research Grants: MOST 107-2320-B-037-003 from the Ministry of Science and Technology; KMU-TP105E12, 105KMUOR05 and KMU-O104003 (Aim for the Top 500 Universities Grant) from Kaohsiung Medical University; 105-CCH-KMU-005 from CCH-KMU Research Project; NSY-SUKMU106-P011 from NSYSU-KMU Research Project; AS-KPQ-105-TPP Taiwan Protein Project and the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout
Project by the Ministry of Education (MOE) in Taiwan. Professor Illana Gozes, the incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors and heads the Elton Laboratory for Neuroendocrinology, is also affiliated with Sagol School of Neuroscience and the Adams Super Center for Brain Studies at Tel Aviv University. Additional support is from AMN Foundation (Israel).
Funding Statement
This work was supported by Research Grants: MOST 107-2320-B-037-003 from the Ministry of Science and Technology; KMU-TP105E12, 105KMUOR05 and KMU-O104003 (Aim for the Top 500 Universities Grant) from Kaohsiung Medical University; 105-CCH-KMU-005 from CCH-KMU Research Project; NSY-SUKMU106-P011 from NSYSU-KMU Research Project; AS-KPQ-105-TPP Taiwan Protein Project and the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout
Footnotes
Conflicts of interest
NAP (also called CP201) is under several patent protection and is licensed to Coronis Neurosciences (http://www.coronisns.com/)., Professor Gozes, Chief Scientific Officer.
REFERENCES
- 1. Irizarry MC, Hyman BT. Alzheimer disease therapeutics. J Neuropathol Exp Neurol. 2001;60:923–8. doi: 10.1093/jnen/60.10.923. [DOI] [PubMed] [Google Scholar]
- 2. Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 3. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and National Institute on Aging Working Group. Consensus report of the Working Group on: molecular and biochemical markers of Alzheimer’s disease. Neurobiol Aging. 1998;19:109–16. [PubMed] [Google Scholar]
- 4. Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi M, Sui YT, Peskind ER, Li G, Hwang HJ, Devic I, et al. Salivary tau species are potential biomarkers of Alzheimer disease. J Alzheimers Dis. 2011;27:299–305. doi: 10.3233/JAD-2011-110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zabel M, Schrag M, Mueller C, Zhou W, Crofton A, Petersen F, et al. Assessing candidate serum biomarkers for Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2012;29:1–11. doi: 10.3233/JAD-2012-112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mueller C, Zhou W, VanMeter A, Heiby M, Magaki S, Ross MM, et al. The heme degradation pathway is a promising serum biomarker source for the early detection of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1081–91. doi: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Bryant SE, Xiao G, Barber R, Reisch J, Hall J, Cullum CM, et al. A blood-based algorithm for the detection of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;32:55–62. doi: 10.1159/000330750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010;1357:184–94. doi: 10.1016/j.brainres.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain. 2006;129:3042–50. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- 11. Zilka N, Korenova M, Kovacech B, Novak M. CSF phospho-tau correlates with behavioural decline and brain insoluble phospho-tau levels in a rat model of tauopathy. Acta Neuropathol. 2011;119:679–87. doi: 10.1007/s00401-010-0680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ewers M, Mielke MM, Hampel H. Blood-based biomarkers of microvascular pathology in Alzheimer’s disease. Exp Gerontol. 2010;45:75–9. doi: 10.1016/j.exger.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang MH, Yang YH, Lu CY, Jong SB, Chen LJ, Lin YF, et al. Activity-dependent neuroprotector homeobox protein: a candidate protein identified in serum as diagnostic biomarker for Alzheimer’s disease. J Proteomics. 2012;75:3617–29. doi: 10.1016/j.jprot.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 14. Malishkevich A, Marshall GA, Schultz AP, Sperling RA, Aharon-Peretz J, Gozes I. Blood-borne activity-dependent neuroprotective protein (ADNP) is correlated with premorbid intelligence, clinical stage, and alzheimer’s disease biomarkers. J Alzheimers Dis. 2016;50:249–60. doi: 10.3233/JAD-150799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–93. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- 16. Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, et al. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–14. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- 17. Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 18. Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323:438–49. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- 19. Fernandez-Montesinos R, Torres M, Baglietto-Vargas D, Gutierrez A, Gozes I, Vitorica J, et al. Activity-dependent neuroprotective protein (ADNP) expression in the amyloid precursor protein/presenilin 1 mouse model of Alzheimer’s disease. J Mol Neurosci. 2010;41:114–20. doi: 10.1007/s12031-009-9300-x. [DOI] [PubMed] [Google Scholar]
- 20. Patocka J, Slaninova J, Kunesova G. Neuroprotective peptides as drug candidates against Alzheimer’s disease. J Appl Biomed. 2005;3:67–73. [Google Scholar]
- 21. Mandel S, Rechavi G, Gozes I. Activity-dependent neuroprotective protein (ADNP) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol. 2007;303:814–24. doi: 10.1016/j.ydbio.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 22. Mandel S, Gozes I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J Biol Chem. 2007;282:34448–56. doi: 10.1074/jbc.M704756200. [DOI] [PubMed] [Google Scholar]
- 23. Ostapcuk V, Mohn F, Carl SH, Basters A, Hess D, Iesmantavicius V, et al. Activity-dependent neuroprotective protein recruits HP1 and CHD4 to control lineage-specifying genes. Nature. 2018;557:739–43. doi: 10.1038/s41586-018-0153-8. [DOI] [PubMed] [Google Scholar]
- 24. Amram N, Hacohen-Kleiman G, Sragovich S, Malishkevich A, Katz J, Touloumi O, et al. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol Psychiatry. 2016;21:1467–76. doi: 10.1038/mp.2015.208. [DOI] [PubMed] [Google Scholar]
- 25. Steingart RA, Gozes I. Recombinant activity-dependent neuroprotective protein protects cells against oxidative stress. Mol Cell Endocrinol. 2006;252:148–53. doi: 10.1016/j.mce.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 26. Gozes I, Divinski I, Piltzer I. NAP and D-SAL: neuroprotection against the β amyloid peptide (1–42) BMC Neurosci. 2008;9:1–5. doi: 10.1186/1471-2202-9-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ashur-Fabian O, Segal-Ruder Y, Skutelsky E, Brenneman DE, Steingart RA, Giladi E, et al. The neuroprotective peptide NAP inhibits the aggregation of the beta-amyloid peptide. Peptides. 2003;24:1413–23. doi: 10.1016/j.peptides.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 28. Gozes I, Zaltzman R, Hauser J, Brenneman DE, Shohami E, Hill JM. The expression of activity-dependent neuroprotective protein (ADNP) is regulated by brain damage and treatment of mice with the ADNP derived peptide, NAP, reduces the severity of traumatic head injury. Curr Alzheimer Res. 2005;2:149–53. doi: 10.2174/1567205053585873. [DOI] [PubMed] [Google Scholar]
- 29. Jehle T, Dimitriu C, Auer S, Knoth R, Vidal-Sanz M, Gozes I, et al. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefe’s Arch Clin Exp Ophthalmol. 2008;246:1255–63. doi: 10.1007/s00417-007-0746-7. [DOI] [PubMed] [Google Scholar]
- 30. Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem. 2004;279:28531–8. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- 31. Divinski I, Holtser-Cochav M, Vulih-Schultzman I, Steingart RA, Gozes I. Peptide neuroprotection through specific interaction with brain tubulin. J Neurochem. 2006;98:973–84. doi: 10.1111/j.1471-4159.2006.03936.x. [DOI] [PubMed] [Google Scholar]
- 32. Oz S, Ivashko-Pachima Y, Gozes I. The ADNP derived peptide, NAP modulates the tubulin pool: implication for neurotrophic and neuroprotective activities. PLoS One. 2012;7:e51458. doi: 10.1371/journal.pone.0051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oz S, Kapitansky O, Ivashco-Pachima Y, Malishkevich A, Giladi E, Skalka N, et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol Psychiatry. 2014;19:1115–24. doi: 10.1038/mp.2014.97. [DOI] [PubMed] [Google Scholar]
- 34. Ivashko-Pachima Y, Sayas CL, Malishkevich A, Gozes I. ADNP/NAP dramatically increase microtubule end-binding protein-Tau interaction: a novel avenue for protection against tauopathy. Mol Psychiatry. 2017;22:1335–44. doi: 10.1038/mp.2016.255. [DOI] [PubMed] [Google Scholar]
- 35. Magen I, Gozes I. Davunetide: peptide therapeutic in neurological disorders. Curr Med Chem. 2014;21:2591–8. doi: 10.2174/0929867321666140217124945. [DOI] [PubMed] [Google Scholar]
- 36. Wilkemeyer MF, Chen SY, Menkari CE, Brenneman DE, Sulik KK, Charness ME. Differential effects of ethanol antagonism and neuroprotection in peptide fragment NAPVSIPQ prevention of ethanol-induced developmental toxicity. Proc Natl Acad Sci USA. 2003;100:8543–8. doi: 10.1073/pnas.1331636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yenjerla M, LaPointe NE, Lopus M, Cox C, Jordan MA, Feinstein SC, et al. The neuroprotective peptide NAP does not directly affect polymerization or dynamics of reconstituted neural microtubules. J Alzheimers Dis. 2010;19:1377–86. doi: 10.3233/JAD-2010-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidativeinsults and beta-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 39. Zemlyak I, Furman S, Brenneman DE, Gozes I. A Novel peptide prevents death in enriched neuronal cultures. Regul Pept. 2000;96:39–43. doi: 10.1016/s0167-0115(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 40. Nisbet RM, Polanco JC, Ittner LM, Götz J. Tau aggregation and its interplay with amyloid-β. Acta Neuropathol. 2015;129:207–20. doi: 10.1007/s00401-014-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of alzheimer’s disease. J Pharmacol Exp Ther. 2008;325:146–53. doi: 10.1124/jpet.107.130526. [DOI] [PubMed] [Google Scholar]
- 42. Gozes I, Ivashko-Pachima Y, Sayas CL. ADNP, a microtubule interacting protein, provides neuroprotection through end binding proteins and tau: an amplifier effect. Front Mol Neurosci. 2018;11:151. doi: 10.3389/fnmol.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. León IR, Schwämmle V, Jensen ON, Sprenger RR. Quantitative assessment of in-solution digestion efficiency identifies optimal protocols for unbiased protein analysis. Mol Cell Proteomics. 2013;12:2992–3005. doi: 10.1074/mcp.M112.025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Merenlender-Wagner A, Malishkevich A, Shemer Z, Udawela M, Gibbons A, Scarr E, et al. Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry. 2015;20:126–32. doi: 10.1038/mp.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Merenlender-Wagner A, Shemer Z, Touloumi O, Lagoudaki R, Giladi E, Andrieux A, et al. New horizons in schizophrenia treatment: autophagy protection is coupled with behavioral improvements in a mouse model of schizophrenia. Autophagy. 2014;10:2324–32. doi: 10.4161/15548627.2014.984274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Esteves AR, Gozes I, Cardoso SM. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson’s disease. Biochim Biophys Acta. 2014;1842:7–21. doi: 10.1016/j.bbadis.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 47. Kawahara M, Kato M, Kuroda Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of beta-amyloid protein. Brain Res Bull. 2001;55:211–7. doi: 10.1016/s0361-9230(01)00475-0. [DOI] [PubMed] [Google Scholar]
- 48. Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10(Suppl 1):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia T, Esparza JL, Giralt M, Romeu M, Domingo JL, Gomez M. Protective role of melatonin on oxidative stress status and RNA expression in cerebral cortex and cerebellum of AbetaPP transgenic mice after chronic exposure to aluminum. Biol Trace Elem Res. 2010;135:220–32. doi: 10.1007/s12011-009-8490-y. [DOI] [PubMed] [Google Scholar]
- 50. Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J – Offic Publ Fed Am Soc Exp Biol. 2002;16:1138–40. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- 51. Kaneko N, Takada J, Yasui H, Sakurai H. Memory deficit in mice administered aluminum-maltolate complex. Biometals –Int J Role Metal Ions Biol Biochem Med. 2006;19:83–9. doi: 10.1007/s10534-005-6965-7. [DOI] [PubMed] [Google Scholar]
- 52. Walton JR. Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology. 2009;30:182–93. doi: 10.1016/j.neuro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 53. Walton JR. A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neurosci Lett. 2007;412:29–33. doi: 10.1016/j.neulet.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 54. Sethi P, Jyoti A, Singh R, Hussain E, Sharma D. Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology. 2008;29:1069–79. doi: 10.1016/j.neuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 55. Rodella LF, Ricci F, Borsani E, Stacchiotti A, Foglio E, Favero G, et al. Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histol Histopathol. 2008;23:433–9. doi: 10.14670/HH-23.433. [DOI] [PubMed] [Google Scholar]
- 56. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 57. Wirths O, Multhaup G, Bayer TA. A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide–the first step of a fatal cascade. J Neurochem. 2004;91:513–20. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- 58. Li XB, Zhang ZY, Yin LH, Schluesener HJ. The profile of beta-amyloid precursor protein expression of rats induced by aluminum. Environ Toxicol Pharmacol. 2012;33:135–40. doi: 10.1016/j.etap.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 59. Ribes D, Colomina MT, Vicens P, Domingo JL. Effects of oral aluminum exposure on behavior and neurogenesis in a transgenic mouse model of Alzheimer’s disease. Exp Neurol. 2008;214:293–300. doi: 10.1016/j.expneurol.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 60. Lin R, Chen X, Li W, Han Y, Liu P, Pi R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin. Neurosci Lett. 2008;440:344–7. doi: 10.1016/j.neulet.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 61. Campbell A, Kumar A, La Rosa FG, Prasad KN, Bondy SC. Aluminum increases levels of beta-amyloid and ubiquitin in neuroblastoma but not in glioma cells. Proc Soc Exp Biol Med Soc Exp Biol Med. 2000;223:397–402. doi: 10.1046/j.1525-1373.2000.22356.x. [DOI] [PubMed] [Google Scholar]
- 62. Castorina A, Tiralongo A, Giunta S, Carnazza ML, Scapagnini G, D’Agata V. Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of beta-amyloid toxicity. Cell Biol Toxicol. 2010;26:367–77. doi: 10.1007/s10565-009-9149-3. [DOI] [PubMed] [Google Scholar]
- 63. Sokolowska P, Passemard S, Mok A, Schwendimann L, Gozes I, Gressens P. Neuroprotective effects of NAP against excitotoxic brain damage in the newborn mice: implications for cerebral palsy. Neuroscience. 2011;173:156–68. doi: 10.1016/j.neuroscience.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 64. Shiryaev N, Pikman R, Giladi E, Gozes I. Protection against tauopathy by the drug candidates NAP (davunetide) and D-SAL: biochemical, cellular and behavioral aspects. Curr Pharmaceut Des. 2011;17:2603–12. doi: 10.2174/138161211797416093. [DOI] [PubMed] [Google Scholar]
- 65. Gozes I, Divinski I, Piltzer I. NAP and D-SAL: neuroprotection against the beta amyloid peptide (1–42) BMC Neurosci. 2008;9:S3. doi: 10.1186/1471-2202-9-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP, et al. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer’s disease at early pathological stage. J Mol Neurosci. 2007;31:165–70. doi: 10.1385/jmn/31:02:165. [DOI] [PubMed] [Google Scholar]
- 67. Gozes I. NAP (davunetide) provides functional and structural neuroprotection. Curr Pharmaceut Des. 2011;17:1040–4. doi: 10.2174/138161211795589373. [DOI] [PubMed] [Google Scholar]
- 68. Quraishe S, Cowan CM, Mudher A. NAP (davunetide) rescues neuronal dysfunction in a Drosophila model of tauopathy. Mol Psychiatr. 2013;18:834–42. doi: 10.1038/mp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Idan-Feldman A, Ostritsky R, Gozes I. Tau and caspase 3 as targets for neuroprotection. Int J Alzheimer’s Dis. 2012;2012:493670. doi: 10.1155/2012/493670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schirer Y, Malishkevich A, Ophir Y, Lewis J, Giladi E, Gozes I. Novel marker for the onset of frontotemporal dementia: early increase in activity-dependent neuroprotective protein (ADNP) in the face of Tau mutation. PLoS One. 2014;9:e87383. doi: 10.1371/journal.pone.0087383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kennedy BP, Bottiglieri T, Arning E, Ziegler MG, Hansen LA, Masliah E. Elevated S-adenosylhomocysteine in Alzheimer brain: influence on methyltransferases and cognitive function. J Neural Transm. 2004;111:547–67. doi: 10.1007/s00702-003-0096-5. [DOI] [PubMed] [Google Scholar]
- 72. Sung HY, Choi EN, Ahn Jo S, Oh S, Ahn JH. Amyloid protein-mediated differential DNA methylation status regulates gene expression in Alzheimer’s disease model cell line. Biochem Biophys Res Commun. 2011;414:700–5. doi: 10.1016/j.bbrc.2011.09.136. [DOI] [PubMed] [Google Scholar]
- 73. Li R, Fang J, Huo B, Su YS, Wang J, Liu LG, et al. Leucine-rich repeat neuronal protein 4 (LRRN4) potentially functions in dilated cardiomyopathy. Int J Clin Exp Pathol. 2017;10:9925–33. [PMC free article] [PubMed] [Google Scholar]
- 74. Bando T, Sekine K, Kobayashi S, Watabe AM, Rump A, Tanaka M, et al. Neuronal leucine-rich repeat protein 4 functions in hippocampus-dependent long-lasting memory. Mol Cell Biol. 2005;25:4166–75. doi: 10.1128/MCB.25.10.4166-4175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kanamori-Katayama M, Kaiho A, Ishizu Y, Okamura-Oho Y, Hino O, Abe M, et al. LRRN4 and UPK3B are markers of primary mesothelial cells. PLoS One. 2011 doi: 10.1371/journal.pone.0025391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mishra A, Eathiraj S, Corvera S, Lambright DG. Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of Early Endosomal Autoantigen 1 (EEA1) Proc Natl Acad Sci USA. 2010;107:10866–71. doi: 10.1073/pnas.1000843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barysch SV, Aggarwal S, Jahn R, Rizzoli SO. Sorting in early endosomes reveals connections to docking-and fusion-associated factors. Proc Natl Acad Sci USA. 2009;106:9697–702. doi: 10.1073/pnas.0901444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cataldo AM, Paskevich PA, Kominami E, Nixon RA. Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10998–1002. doi: 10.1073/pnas.88.24.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26:373–82. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 80. Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat Commun. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–27. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 82. Chen S, Fisher RC, Signs S, Molina LA, Shenoy AK, Lopez MC, et al. Inhibition of PI3K/Akt/mTOR signaling in PI3KR2-overexpressing colon cancer stem cells reduces tumor growth due to apoptosis. Oncotarget. 2016;8:50476–88. doi: 10.18632/oncotarget.9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ito S, Sawada M, Haneda M, Fujii S, Oh-Hashi K, Kiuchi K, et al. Amyloid-beta peptides induce cell proliferation and macrophage colony-stimulating factor expression via the PI3-kinase/Akt pathway in cultured Ra2 microglial cells. FEBS Lett. 2005;579:1995–2000. doi: 10.1016/j.febslet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 84. Liu T, Jin H, Sun QR, Xu JH, Hu HT. Neuroprotective effects of emodin in rat cortical neurons against beta-amyloid-induced neurotoxicity. Brain Res. 2010;1347:149–60. doi: 10.1016/j.brainres.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 85. Bartl J, Meyer A, Brendler S, Riederer P, Grunblatt E. Different effects of soluble and aggregated amyloid beta42 on gene/protein expression and enzyme activity involved in insulin and APP pathways. J Neural Transm. 2013;120:113–20. doi: 10.1007/s00702-012-0852-5. [DOI] [PubMed] [Google Scholar]
- 86. Hacohen-Kleiman G, Sragovich S, Karmon G, Gao AYL, Grigg I, Pasmanik-Chor M, et al. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J Clin Invest. 2018;128:4956–69. doi: 10.1172/JCI98199. [DOI] [PMC free article] [PubMed] [Google Scholar]