Abstract
Blood sampling by the dried blood spot (DBS) technique has become commonly applied in newborn screening. It is often used for analysis of small molecules, such as metabolites. Recently, DBS sampling has been applied for quantification of post-translational protein modifications. Glyoxal and methylglyoxal are two simple oxoaldehydes released from glycated proteins in the Maillard reaction. They are widely distributed in the environment (e.g. cigarette smoke) and found in foods and beverages. Glyoxal and methylglyoxal are shown to react with biomolecules including DNA and proteins. In this laboratory, we previously identified the sites of modification by these two oxoaldehydes in human hemoglobin and found that the extents of modification at certain sites of lysine and arginine residues are significantly higher in type 2 diabetes mellitus patients than in nondiabetic individuals. In this study, we examine the stability of these modifications of hemoglobin stored on DBS cards at room temperature or 4 °C in the ambient air. After hemoglobin was extracted from the DBS cards, it was digested by trypsin and analyzed by nanoflow liquid chromatography coupled with nanospray ionization tandem mass spectrometry. The results show that the extents of all these PTMs are stable within 14 and 21 days when stored on DBS at room temperature and at 4 °C, respectively. Extraction of globin from DBS cards is mostly advantageous for hemolytic blood samples. This assay is sensitive as only a quarter of a DBS card containing ca. 12 μL of blood is required. Thus, it is practically useful to measure the extents of glyoxal- and methylglyoxal-induced hemoglobin modifications from DBS cards.
Keywords: Blood, Dried blood spot, Glyoxal, Hemoglobin, Mass spectrometry, Methylglyoxal, Post-translational modifications
1. Introduction
Electrophiles from endogenous and exogenous sources can react with biomolecules, including DNA and proteins, leading to adverse effects. DNA adducts have attracted much attention because of the direct association with the mutagenic activity. These covalently linked adducts are useful in assessing the biological consequences and the exposure of the toxic chemicals. However, it is hardly plausible to measure DNA and protein adducts in target tissues. In addition, protein adducts are not repaired and proteins are much abundant than DNA. Consequently, measuring protein adducts from the abundant blood proteins, hemoglobin (Hb) and serum albumin, is a conceivable alternative [1,2]. Due to the fact that hemoglobin has a longer life time (126 days in human) than serum albumin (half-life of 20 days) [1], we aim to study hemoglobin adducts as exposure biomarkers.
The requirement to acquire venous blood samples limited the use of hemoglobin or albumin adducts in studies involving large populations. Dried blood spots (DBS) can be obtained by a simple skin prick as sources of blood. Currently, the advancement in the specificity and sensitivity of mass spectrometry-based assays has contributed greatly to identification and quantification of targets in DBS cards, not limited to newborn screening [3]. Other advantages of DBS include simple sample collection, decrease biohazards, and cost effective storage and shipment. In addition, DBS sampling is minimally invasive and is thus an ideal self-sampling method for clinical screening in epidemiological studies [4,5].
Traditionally, analysis of from DBS has focused on small molecules, such as drugs and metabolites. A decade ago, Funk et al. developed a procedure to specifically isolate hemoglobin from DBS and demonstrated that levels of hemoglobin adducts of benzene oxide are comparable in human globin whether it is extracted from DBS or isolated from conventional venous blood [6]. In this laboratory, we examined the stability of oxidative associated hemoglobin modifications on a total of 11 sites and types of hemoglobin modifications, including nitration and nitrosylation of tyrosine and oxidation of cysteine and methionine residues at various temperatures, and compared the levels with those from direct extraction from fresh blood. We found that the extents of these hemoglobin adducts were stable for 14 days when stored on DBS at room temperature and at 4 °C [7].
The simple oxoaldehydes glyoxal (gx) and methylglyoxal (Mgx) are found in the environment as well as in beverages, foods, and cigarette smoke [8–10]. They can be produced endogenously from conjugation of glucose with proteins (Maillard reaction) [11–14]. These aldehydes react with biological targets forming irreversible advanced glycation end-products (AGEs), which are implicated in complications originated from hyperglycemia [12,13,15]. Glyoxal reacts with DNA forming monoadduct as well as crosslinked adducts [16–19]. Damage of protein and DNA structures are associated with various types of diseases, including diabetes mellitus [20,21]. In this current study, we evaluate the stability of glyoxal- and methylglyoxal-derived hemoglobin adducts stored on DBS cards using the highly sensitive nanoflow liquid chromatography coupled with nanospray ionization tandem mass spectrometry (nanoLC–NSI/MS/MS) assay previously developed [22].
2. Methods
2.1. Materials
Trypsin was obtained from Promega Corporation (Madison, WI). Sodium dodecyl sulfate (SDS) and 2-vinylpyridine were purchased from Sigma Chemical Co. (St. Louis, MO). Perki-nElmer 226 spot saver cards were from PerkinElmer Life Sciences (Boston, MA). All reagents used in this study were of reagent grade or above.
2.2. DBS blotting and storage
Human blood was obtained with approval by the Institutional Review Boards (IRB) of the National Chung Cheng University (IRB No. 100112902) and the IRB of. Buddhist Dalin Tzu Chi General Hospital (IRB No. B10203014-1).
Fresh blood (50 μL) was blotted onto a PerkinElmer 226 spot saver card and air-dried at room temperature for 2 h. The DBS cards were kept in a plastic bag containing of anhydrous calcium sulfate (ca. 35 g, with moisture indicator) and stored at room temperature or 4 °C for 1, 7, 14, 21, and 28 days.
2.3. Isolation of globin from DBS cards
Globin was isolated following the previously reported procedures with minor modification [6,23]. Typically, deionized water (1 mL) was added to a quarter of the DBS card contained in a vial, which was shaken at 160 rpm for 90 min. The filter paper was removed with tweezers, and the solution was filtered by a 0.22 μm Nylon syringe filter to remove cell debris, followed by concentration with a centrifugal vacuum concentrator to 0.2 mL. The solution was added 1 mL of 43% ethanol (v/v) with vigorous shaking, left at −20 °C for 1 h, transferred to a 1.5 mL Eppendorf tube, and centrifuged at 23 000 g at 4 °C for 30 min. The precipitate was dissolved in 0.1 mL of deionized water with the addition of 0.1% HCl (1 mL) in cold acetone and the mixture was allowed to stand at −20 °C for 4 h, followed by centrifugation at 3680g at 10 °C for 10 min. The precipitate was washed twice with cold acetone (0.5 mL) and once with ethyl acetate (0.5 mL), followed by centrifugation at 3680g for 10 min at 10 °C for each washing step. The precipitate was dissolved in deionized water (0.5 mL) and the concentration of globin was determined by fluorescence excited at 280 nm and emitted at 353 nm [24].
2.4. Trypsin digestion of globin
Typically, globin (50 μg) solution in ammonium bicarbonate (100 mM, pH 8.0) with 1% SDS (w/v) (total volume 100 μL) was incubated at 95 °C for 10 min. Cold acetone (900 μL) was added to the solution and let it stand at −20 °C for 15 min to precipitate the globin, followed by centrifugation at 23 000 g for 20 min. The precipitate was dissolved in ammonium bicarbonate (100mM, pH8.0), added trypsin (5 μg), and incubated at 37 °C for 18 h. Trifluoroacetic acid (0.1%, 50 μL) was added to stop the digestion and the solution was filtered through a Nylon syringe filter (0.22 μm) before nanoLC–NSI/MS/MS analysis.
2.5. NanoLC–NSI/MS/MS analysis
A 4 μL of the trypsin digest equivalent to 2 μg of globin was injected into an LC system with an UltiMate 3000 RSLCnano system (Dionex, Amsterdam, Netherlands) with a reversed phase C18 precolumn (100 μm × 20 mm) packed in-house (Magic C18, 5 μm, 100 Å, Michrom BioResource, Auburn, CA) connected to a C18 tip column (75 μm × 110 mm) packed in-house with Magic C18AQ (5 μm, 200 Å, Michrom Bio-Resource). The mobile phases A and B composed of 5% and 80% acetonitrile (v/v) in 0.1% formic acid (v/v, pH 2.6), respectively. The column was eluted with 4% B for 4.5 min, followed by a linear gradient from 4% B to 40% B in the next 38 min, then from 40% B to 90% B in the next 20 min, and maintained at 90% B for 10 min at a flow of 300 nL/min. The starting conditions were equilibrated with 4% B (v/v) for 20 min before the next injection. The column was connected to an LTQ linear ion trap mass spectrometer (Thermo Electron Corp., San Jose, CA) fitted with a nanospray ionization (NSI) source. The mass spectrometry conditions were reported previously [22].
2.6. Extents of modifications
Relative quantification of the extents of modification was performed using the selected reaction monitoring (SRM) experiments selecting the precursor ion and acquire the product ion scan spectra. The formation of a specific fragment ion from each precursor ion was used to construct the chromatogram. The specific SRM transitions for the modified lysine- and arginine-containing peptides and their reference peptides are reported [22]. The extent of modification on a peptide was expressed as the peak area ratio of the modified peptide versus the sum of the peak areas of the modified peptide and the corresponding reference peptide in the SRM chromatograms.
3. Results and discussion
Glyoxal was shown to react with the lysine and arginine residues of proteins forming Nɛ-carboxymethyllysine and Nɛ-carboxymethylarginine, respectively [25]. In addition to Nɛ-carboxyethylation on lysine and arginine residues, the modification of proteins by methylglyoxal gives a cyclic dehydration product on arginine, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)ornithine (H-Mgx) [22,26–28]. With high resolution nanoLC–NSI/MS/MS under the data-dependent scan mode, we have identified sites of carboxymethylation (+58 Da) on 6 lysine and 2 arginine residues in the tryptic digest of human hemoglobin incubated with glyoxal; no cyclic dehydration product on arginine was identified. In the in vitro reaction mixture of hemoglobin with methyglyoxal, carboxyethylation (+72 Da) was observed on 2 lysine and 2 arginine residues; the hydroimidazolone formation (+54 Da) on the same sites of arginine was also identified. However, only 5 lysine and 1 arginine sites modified with glyoxal and 1 lysine and 1 arginine modified with methylglyoxal were detected in human hemoglobin isolated from study subjects (diabetics and non-diabetics) [22]. Among them, both carboxyethylation and hydroimidazolone formation were detected on α-Arg-92. Thus, only these 9 sites and types of modification, namely, carboxymethylated α-Lys-11, α-Lys-16, α-Lys-56, α-Arg-92, β-Lys-17, β-Lys-66, carboxyethylated α-Arg-92, β-Lys-66, and hydroimidazolone of α-Arg-92, were analyzed under the SRM mode in this study.
To assess the stability of these glyoxal- and methylglyoxal-induced modifications on the DBS, a fixed amount of blood (50 μL) was blotted on a DBS card and air-dried. The card was sealed in a plastic bag containing drying agent and stored at room temperature (RT) and at 4 °C (in the refrigerator) in the dark. Our previous studies suggested that hemoglobin isolated from a quarter of DBS should be enough for the assay [22,23]. The amount of globin obtained from a quarter of a DBS spot was enough for the assay, which started from digestion of 50 μg of globin with trypsin, and only an equivalent of 2 μg of the hydrolysate was injected into the nanoLC-NSI/MS/MS system. Thus, a quarter of a DBS card was removed after 24 h (day 1), 1, 2, 3, and 4 weeks and hemoglobin was isolated from it. During the precipitation-washing process, HCl solution was added and the heme moiety fell off, giving globin as the apoprotein. The isolated globin was digested with trypsin and the modified and the reference peptides were analyzed by nanoLC–NSI/MS/MS. Relative quantification of the extent of modification was achieved as the peak area ratio of the modified peptide versus the sum of the peak areas of the modified peptide and its corresponding reference (unmodified) peptide in the SRM chromatograms [7]. Using the native unmodified peptide present in the trypsin digest of a protein as the reference peptide for the modified peptide is referred as the native reference peptide (NRP) method, which permits correction for variations in protein amounts and in peptide recovery during the digestion procedures [29]. To achieve absolute quantification of the extent of modification, it requires the use stable isotope-labeled reference peptide and the modified peptide as internal standards, which are very costly, especially when multiple sites of modification are analyzed simultaneously.
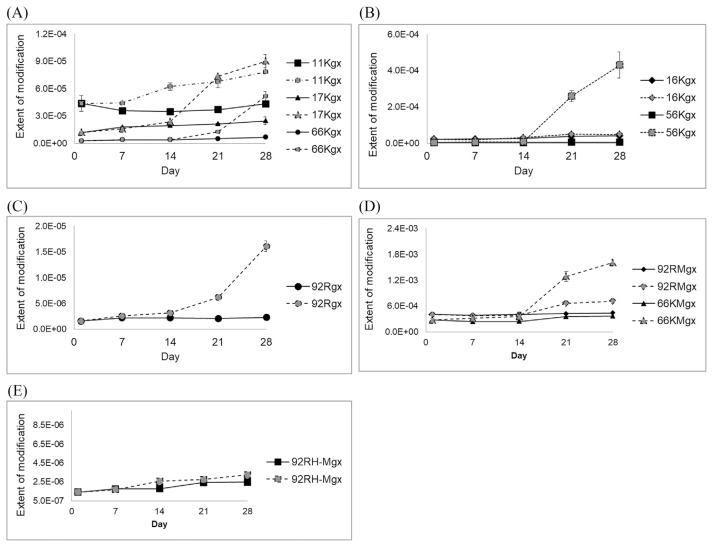
Samples from four subjects were analyzed with triplicates. The analysis is precise, based on the relative standard deviation (RSD) of the triplicated experiments are all <20% (ranging from 0.1% to 19%), with an average of 7.8%. The results showed that most of the glyoxal- and methylglyoxal-induced modifications were stable for 28 days when the DBS cards were stored in a 4 °C refrigerator. The exception was the increase in the extents of α-Lys-56-gx and β-Lys-66-Mgx in one sample at day 28. When the DBS cards were stored at room temperature, most of the glyoxal- and methylglyoxal-induced modifications remained constant except α-Lys-56-gx, α-Arg-92-Mgx, and β-Lys-66-Mgx, which increased after 14 days. Thus, we concluded that all the modifications are stable within 21 days when stored at 4 °C and they are stable for 14 days when stored at room temperature. Fig. 1 showed the relative extent of modification on hemoglobin extracted from a representative sample on DBS stored at 4 °C and at room temperature. The possible reason for the increase of the extent of modifications might be due to degradation of hemoglobin during storage on the DBS cards in the ambient air at room temperature. Degradation of the unmodified hemoglobin can lead to the increase in the extent of modifications because the unmodified peptides are present in much larger amounts than the modified peptides.
Fig. 1.
Relative extent of modification on hemoglobin extracted from a dried blood spot stored at 4 °C and at room temperature. (A) α-Lys-11-gx, β-Lys-17-gx, β-Lys-66-gx, (B) α-Lys-16-gx, α-Lys-56-gx, (C) α-Arg-92-gx, (D) α-Arg-92-Mgx, β-Lys-66-Mgx, (E) α-Arg-92-HMgx. The dark solid symbols are for 4 °C; the grey dotted symbols are for room temperature.
During blood collection and handling, hemolysis can take place and it can be due to certain diseases [30]. Extraction of globin from the DBS cards is particularly beneficial for hemolytic blood samples, as it is difficult to obtain intact red blood cells to isolate globin by the conventional method.
The extent of glyoxal-derived modifications at α-Lys-16, α-Arg-92, β-Lys-17, β-Lys-66) and methyl glyoxal-modified hydroimidazolone at α-Arg-92 are significantly higher in diabetic patients than in healthy control subjects [22]. Thus, the stability of glyoxal- and methylglyoxal-induced hemoglobin modifications on DBS should be helpful in assessing the exposure of these two aldehydes in studies involving large populations.
Acknowledgments
This work was supported by Ministry of Science and Technology, Taiwan (Grants MOST 103-2113-M-194-003-MY3 and NSC-100-2113-M-194-002-MY3) and by National Chung Cheng University (to H.-J.C.C.).
Funding Statement
This work was supported by Ministry of Science and Technology, Taiwan (Grants MOST 103-2113-M-194-003-MY3 and NSC-100-2113-M-194-002-MY3) and by National Chung Cheng University (to H.-J.C.C.).
REFERENCES
- 1. Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 2. Skipper PL, Tannenbaum SR. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990;11:507–18. doi: 10.1093/carcin/11.4.507. [DOI] [PubMed] [Google Scholar]
- 3. Wagner M, Tonoli D, Varesio E, Hopfgartner G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. 2016;35:361–438. doi: 10.1002/mas.21441. [DOI] [PubMed] [Google Scholar]
- 4. McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 5. Wilhelm AJ, den Burger JC, Swart EL. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet. 2014;53:961–73. doi: 10.1007/s40262-014-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Funk WE, Waidyanatha S, Chaing SH, Rappaport SM. Hemoglobin adducts of benzene oxide in neonatal and adult dried blood spots. Cancer Epidemiol Biomark Prev. 2008;17:1896–901. doi: 10.1158/1055-9965.EPI-08-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen HJ, Chen YC. Reactive nitrogen oxide species-induced post-translational modifications in human hemoglobin and the association with cigarette smoking. Anal Chem. 2012;84:7881–90. doi: 10.1021/ac301597r. [DOI] [PubMed] [Google Scholar]
- 8. Nagao M, Fujita Y, Wakabayashi K, Nukaya H, Kosuge T, Sugimura T. Mutagens in coffee and other beverages. Environ Health Perspect. 1986;67:89–91. doi: 10.1289/ehp.866789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- 10. Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bugel S, Nielsen J, et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 11. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 12. Odani H, Iijima K, Nakata M, Miyata S, Kusunoki H, Yasuda Y, et al. Identification of N(omega)-carboxymethylarginine, a new advanced glycation endproduct in serum proteins of diabetic patients: possibility of a new marker of aging and diabetes. Biochem Biophys Res Commun. 2001;285:1232–6. doi: 10.1006/bbrc.2001.5322. [DOI] [PubMed] [Google Scholar]
- 13. Thorpe SR, Baynes JW. Role of the Maillard reaction in diabetes mellitus and diseases of aging. Drugs Aging. 1996;9:69–77. doi: 10.2165/00002512-199609020-00001. [DOI] [PubMed] [Google Scholar]
- 14. Thornalley PJ. Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci. 2005;1043:111–7. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 15. Han Y, Randell E, Vasdev S, Gill V, Gadag V, Newhook LA, et al. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol Cell Biochem. 2007;305:123–31. doi: 10.1007/s11010-007-9535-1. [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Cao H, Wang Y. Quantification of N2-carboxymethyl-2′-deoxyguanosine in calf thymus DNA and cultured human kidney epithelial cells by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope dilution method. Chem Res Toxicol. 2010;23:74–81. doi: 10.1021/tx900286c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brock AK, Kozekov ID, Rizzo CJ, Harris TM. Coupling products of nucleosides with the glyoxal adduct of deoxyguanosine. Chem Res Toxicol. 2004;17:1047–56. doi: 10.1021/tx049906z. [DOI] [PubMed] [Google Scholar]
- 18. Chen HJ, Chen YC. Analysis of glyoxal-induced DNA cross-links by capillary liquid chromatography nanospray ionization tandem mass spectrometry. Chem Res Toxicol. 2009;22:1334–41. doi: 10.1021/tx900129e. [DOI] [PubMed] [Google Scholar]
- 19. Chen HC, Chang YL, Teng YC, Hsiao CF, Lin TS. A stable isotope dilution nanoflow liquid chromatography tandem mass spectrometry assay for the simultaneous detection and quantification of glyoxal-induced DNA cross-linked adducts in leukocytes from diabetic patients. Anal Chem. 2017;89:13082–8. doi: 10.1021/acs.analchem.6b04296. [DOI] [PubMed] [Google Scholar]
- 20. Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metabol Drug Interact. 2008;23:125–50. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niwa T. Mass spectrometry for the study of protein glycation in disease. Mass Spectrom Rev. 2006;25:713–23. doi: 10.1002/mas.20089. [DOI] [PubMed] [Google Scholar]
- 22. Chen HJ, Chen YC, Hsiao CF, Chen PF. Mass spectrometric analysis of glyoxal and methylglyoxal-induced modifications in human hemoglobin from poorly controlled type 2 diabetes mellitus patients. Chem Res Toxicol. 2015;28:2377–89. doi: 10.1021/acs.chemrestox.5b00380. [DOI] [PubMed] [Google Scholar]
- 23. Chen HC, Fan CH, Yang YF. Stability and application of reactive nitrogen and oxygen species-induced hemoglobin modifications in dry blood spots as analyzed by liquid chromatography tandem mass spectrometry. Chem Res Toxicol. 2016;29:2157–63. doi: 10.1021/acs.chemrestox.6b00334. [DOI] [PubMed] [Google Scholar]
- 24. Boys BL, Konermann L. Folding and assembly of hemoglobin monitored by electrospray mass spectrometry using an on-line dialysis system. J Am Soc Mass Spectrom. 2007;18:8–16. doi: 10.1016/j.jasms.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 25. Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyllysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, et al. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287–92. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed N, Thornalley PJ. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann N Y Acad Sci. 2005;1043:260–6. doi: 10.1196/annals.1333.031. [DOI] [PubMed] [Google Scholar]
- 28. Gao Y, Wang Y. Site-selective modifications of arginine residues in human hemoglobin induced by methylglyoxal. Biochemistry. 2006;45:15654–60. doi: 10.1021/bi061410o. [DOI] [PubMed] [Google Scholar]
- 29. Ruse CI, Willard B, Jin JP, Haas T, Kinter M, Bond M. Quantitative dynamics of site-specific protein phosphorylation determined using liquid chromatography electrospray ionization mass spectrometry. Anal Chem. 2002;74:1658–64. doi: 10.1021/ac0157122. [DOI] [PubMed] [Google Scholar]
- 30. Gayler M. Haemolysis of blood samples: what it is and how to avoid it. Nurs Times. 1999;95:54–5. [PubMed] [Google Scholar]