Abstract
High-density lipoprotein (HDL) carbamylation has been known in uremia patients. Paraoxonase-1 (PON-1) is an important HDL protein responsible for HDL anti-oxidant, arylesterase and lactonase activities. PON-1 carbamylation in uremic HDL has never been explored. We isolated HDL from uremia patients and control healthy subjects for study. Sandwich ELISA was used to estimate carbamylated PON-1 protein expression in HDL, and nanoflow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) was applied to identify the amino acid in PON-1 carbamylated. PON-1 enzyme activities were estimated by substrates conversion method. HDL anti-oxidant activity was gauged by fluorescence changes of indicator dye in the presence of H2O2. Our study results proved that the degree of PON-1 carbamylation was higher in uremic HDL than in control HDL. Sandwich ELISA study showed that carbamylated PON-1 concentration in uremic HDL was 1.49 ± 0.08 fold higher than that in HDL from controls (p < 0.05). The nanoLC-MS/MS showed that the carbamylation of lysine 290 (K290) of PON-1, a residue adjacent to PON-1 activity determining site, was detected in uremic HDL but not detected in control HDL. K290 carbamylation leads to local conformation changes that reduce accessible solvent accessibility. The HDL paraoxonase, arylesterase, and lactonase activities were all significantly lower in uremia patients than in control subjects. Additionally, HDL anti-antioxidant ability was also lower in uremia patients. Carbamylation of PON-1 in uremia patients could be one of the factors in impairing PON-1 enzyme activities and HDL anti-oxidation function.
Keywords: HDL, Paraoxonase-1, Mass spectrometry, Uremia, Carbamylation
1. Introduction
Uremia is a risk factor of cardiovascular disease. The incidence of atherosclerotic coronary artery disease (CAD) of uremia patients is much higher than that of general population after adjustment of conventional risk factors [1]. High-density lipoprotein (HDL) is a complex made up of half protein and half lipid, with apoliporotein A1 (ApoA1), apoliporotein A2 and other apolipoproteins being the major protein component. HDL also contains some HDL-associated enzymes such as paraoxonase-1 (PON-1), which is important for HDL function [2]. Studies have shown that HDL is the main plasma anti-atherosclerosis lipoprotein, which possesses anti-oxidative, anti-inflammation, anti-platelet, and endothelial protection effects [3,4]. The anti-atherosclerosis effect of HDL comes largely from its reverse cholesterol transport (RCT) effect, a process mobilizing cholesterol from macrophage or peripheral tissue to liver for biliary excretion [5]. HDL dysfunction has been postulated as a possible pathogenic cause of CAD in hemodialysis patients [6]. HDL composition and function are altered in uremic milieu and RCT is compromised in HDL of uremia patients [7].
PON-1 belongs to the paraoxonase enzyme family, which also includes PON-2 and PON-3. Previous studies have shown that PON-1 is a calcium-dependent enzyme with a wild range of substrates such as paraoxon and phenylacetate [8], and PON-1 also has arylesterase or lactonase activities [9]. Human PON-1 is 355 amino acids in length with amolecular weight of 43 kDa [10]. The architecture of PON-1 is a six-bladed β-propeller with 3 helices and is folded into a velcro closure. There is an active site lid for HDL binding and a central tunnel with 2 calcium ions. One calcium involves in catalytic function and the other is important for structural stability [11].
PON-1 is also essential for the anti-oxidation function of HDL [12]. PON-1 knockout mice showed increased lipoprotein peroxidation [13]. HDL isolated from PON-1 transgenic mice was more protective than HDL of control wild type littermate from oxidation by oxidized LDL [14,15]. In human study, PON-1 gene polymorphism can affect PON-1 blood level and its enzyme catalytic activities [15]. Through the inhibition of HDL oxidation, PON-1 can help preserve RCT activity and decrease the risk of atherosclerosis [16]. On the contrary, decreased PON-1 activity is associated with atherosclerosis [17,18].
Serum PON-1 activity has been known to decrease in uremia patients [19,20]. The mechanism of decreased PON-1 activity in uremia patients is still not clear. Taking the fact that PON-1 activity of uremia patients can increase after hemodialysis treatment [19], blood soluble substance removed by hemodialysis may affect PON-1 activity.
Protein carbamylation is a post-translational modification commonly seen in uremia patients [15]. Urea in the uremic blood degrades into cynate and isocynate. The electrophilic pair of cynate and isocynate will react with nucleophilic amino acid such as lysine (k) to induce protein carbamylation, which impairs protein function [15].
HDL of uremia patients is carbamylated and associated with cardiovascular mortality [7]. Most of the previous studies found that ApoA1 can be carbamylated in human HDL or in recombinant ApoA1 [21,22]. Chemically-induced carbamylated HDL also presented a reduced PON-1 activity when compared with native HDL isolated from healthy subjects [21]. However, to the best of our knowledge, there is no study reporting the existence of carbamylated PON-1 protein in uremia patients.
As the blood urea level in uremia patients is higher than in healthy subjects, we suppose PON-1 can be carbamylated in uremia patients. In the present study, we used western blot, ELISA and nanoLC-MS/MS methods to show high degree of carbamylation of PON-1 in uremia patients and identify its carbamylated site on sequence. The impaired biofunctions based on carbamylated PON-1 were also investigated.
2. Material and methods
2.1. Patients
Sixteen uremia patients on maintenance hemodialysis over 6 months and 16 age- and sex-matched healthy control subjects were enrolled in this study. All these uremia patients received a 4-h session trice weekly hemodialysis therapy. The dialysis doses of all these patients obtained were above the recommended urea reduction ratio of 65% per hemodialysis treatment session [23]. We excluded patients with serum high sensitive C-reactive protein (HS-CRP) above reference level (0.8 mg/dl) and we also excluded smokers and diabetes patients. Venous blood was collected from all study subjects after fasting and, for uremia patients, before hemodialysis treatment. All patients signed written informed consent before blood sampling. The study protocols were reviewed and approved by China Medical University & Hospital Research Ethics Committee (Reference number: CMUH102-REC3-142), and the study began only after getting study approval.
2.2. Western blotting
HDL was isolated by ultracentrifugation as we previously described [2]. The isolated HDL was electrophoresis by 10% SDS gels for 1.5 h. The gels were then transferred to a polyvinylidene difluoride (PVDF) membrane and blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20. Immunoblotting was conducted by incubating the membranes with antibodies (1:1000) PON-1 (GeneTex, Irvine, CA, USA) overnight followed by incubating with an anti-rabbit secondary antibody for 1 h. The blots were developed using electrochemiluminescence regents (Millipore) and analyzed using densitometry.
2.3. Immunoprecipitation
HDL protein (1 mg) and 5 μl anti-carbamyl antibody (anti-CBL) (Academy Bio-medical Co., Houston, USA) were incubated in buffer A solution (0.02 M Tris-HCl, 0.5 mM EDTA, pH = 8) at 4 °C overnight. Dynabeads (Sigma, Invitrogen) (30 μl) were added in the solution and incubated for another 4 h at 4 °C. The beads were then precipitated after centrifuge at 3000 rpm for 5 min and washed with 1 ml buffer A solution for 3 times. Sample buffer (50 μl) were added in the precipitate and incubated 5 min at 4 °C for denature. Samples were subjected to SDS gel electrophoresis and western blotting study.
2.4. Sandwich ELISA
Anti-carbamyl antibody was placed in 100 μl coating buffer B (Invitrogen) finally diluted to 1:250 in a 96-well plate at 4 °C overnight. The plate was then washed with 200 μl wash buffer 2 times. HDL was mixed in assay buffer for 2 h and then washed 5 times followed by incubation with 100 μl HRP-conjugated anti-human PON-1 (1:1000) in assay buffer for one hour. After 5 wash cycles, 100 μl TMB (3,3′,5,5′-Tetrame-thylbenzidine) was added for 30 min and 100 μl stop solution was added thereafter. The signals were monitored using a microplate reader at OD 450 nm.
2.5. In gel digestion
PON-1 bands were excised from SDS gel, cut into small pieces and washed twice with 25 mM ammonium bicarbonate (ABC) solution containing 50% acetonitrile for 15 min. Gel pieces were then dehydrated, reduced, alkylated and digested with trypsin (1:50 trypsin to protein ratio in weight) at 4 °C. Digested peptides were extracted from the gel using 0.1% trifluoroacetic acid (TFA) in 50% acetonitrile [4]. The digested peptides were then purified by Zip-Tip (Milipore, Billerica, Massachusetts, USA).
2.6. NanoLC-MS/MS
The NanoLC-MS/MS was performed with a nanoflow UPLC system (Ultimate 3000, RSLCnano system, Dionex, Amsterdam, Netherland) coupled with coupled with a captive spray ion source and a Q-TOF mass spectrometer (maXis impact II, Bruker). The samples were injected into a tunnel frit trap column (C18, 5 μC, 100 Å, packed length of 2 cm, 375 μ od × 180 μ × id) [2] with a flow rate of 10 μl/min and then separated by an analytical column (Acclaim PepMap C18, 2 μm, 100 Å, 75 μm × 250 mm, Thermo Scientific, USA) with a flow rate of 300 nl/min. A gradient elution of 5% ACN (0.1% FA) to 35% ACN (0.1%) within 80 min was used for peptide separation. Eight precursors of charge +2, +3 and +4 from each TOF-MS scan (400–2000m/z) were dynamically selected and isolated for MS/MS fragment ion scan (50–2000m/z). Selected precursors were then actively excluded for 20 s. MS and MS/MS accumulation were set at 1 and 2 Hz.
2.7. Protein database search
The nanoLC–MS/MS spectra were processed and converted into xml files using DataAnalysis (version 4.1, Bruker). The xml files were searched against the Swissprot (release 51.0) database using MASCOT (version 2.2.07). The search parameters for MASCOT for precursor ion and fragment ion tolerance were 50 ppm and 0.05 Da, respectively. Search parameters were selected as Taxonomy-Human; missed cleavages-2; enzyme-trypsin; fixed modifications-carbamidomethyl (C); variable modifications-oxidation (M), deamidation and carbamyl (K). To give a better sensitivity for the peptide identification in MASCOT database search, individual peptide matches with scores above 25 were considered statistically significant.
2.8. Structural remodeling
To know the structural remodeling after PON-1 carbamylation, we used the crystal structure of Serum Paraoxonase (PDB ID:1v04, Resolution 2.2 Å) as a template for modeling the amino acid substitution. Mutagenesis was performed by using Pymol to choose the most common rotamer that did not cause clashes at position 290 for lysine (Lys 290 or K290) and carbamylated lysine (LysC) [24]. Simultaneously, these structures were analyzed for the polar contacts, hydrogen bonds and surface area changes. To calculate the differences parameters, we applied DSSP (or Stride) to calculate the accessible solvent accessibility (ASA) for each residue [25]. Therefore, using the silico virtual screening approaches can help to identify the local conformational structure upon mutations.
2.9. PON-1 enzyme activity assays
PON-1 paraoxonase activity was measured by the conversion of paraoxon to p-nitrophenol, which can be monitored by the change in absorbance at 405 nm. HDL (100 μg) was added into 200 μl assay solution containing 1 mM paraoxon, 2 mM CaCl2, and 100 mM Tris at pH8.0. The assay was done on a plate reader with readings at 405 nm to generate a kinetic plot and the slope was determined. An extinction coefficient of 17,000 M/cm was used to calculate paraoxonase activity. PON-1 arylesterase activity was gauged by monitoring the rate of hydrolysis of phenyl acetate into phenol at 270 nm using microplate reader. HDL 50 μg was added in 200 μl solution containing 10 mM phenyl acetate, 2 mM CaCl2, and 50 mM Tris-HCl at pH 8.0. Arylesterase enzyme activity was calculated with an extinction coefficient of 1310 M/cm. Lactonase activity was measured in 20 μg HDL by using 1 mM 2-coumaranone as substrates and computed with the formation of 2-hydroxyphenyl acetic acid (2-HPAA) with an extinction coefficient of 876 M/cm.
2.10. HDL anti-oxidative stress effect
DCFH-DA (Invitrogen) dye, highly fluorescent in the presence of oxidants [26], was used to determine the anti-oxidative stress of PON-1. RAW264.7 cells were grown in 96-well culture plates overnight, and 50 μg/mL of HDL and 100 μM DCFH-DA were added to the medium and incubated at 37 °C for 60 min. H2O2 (10 mM) was then added, and the fluorescence of each well was measured at 480 nm/530 nm every minute for 60 min by using a microplate reader. All the studies were run in triplicate.
2.11. Statistical analysis
IBM®SPSS® Statistics version 21 for Macintosh was used for statistical analysis. Data was presented as mean ± SEM (standard error mean). A t-test was used to compare the difference of mean values between uremia patients and control subjects. A p-value of <0.05 was deemed significant.
3. Results
3.1. Demographic results
Both groups were sex-and age-matched. BMI (body mass index), serum cholesterol and low-density lipoprotein-c (LDL-c) were not different between groups. BUN and serum creatinine were higher in uremia group. Mean serum HS-CRP levels, although higher in uremia patients, were within normal reference range in both groups. Serum triglyceride was significantly higher and HDL-c was statistically lower in uremia patients than in control subjects. HDL enzyme activities such as Paraoxonase, Arylesterase, and Lactonase activities from equal amount of HDL were all lower in uremia patients than in control healthy subjects (Table 1).
Table 1.
Demographic data of uremia patients and control healthy subjects.
| Control (16) | Uremia (16) | p-value | |
|---|---|---|---|
| Male: Female (Number) | 10:6 | 12:4 | 0.704 |
| Age (years) | 53.8 ± 3.1 | 55.8 ± 1.9 | 0.527 |
| BMI (Kg/m2) | 22.3 ± 0.3 | 22.2 ± 0.5 | 0.917 |
| BUN (mg/dl) | 17 ± 0.6 | 66.9 ± 5.4 | <0.001 |
| Creatinine (mg/dl) | 0.7 ± 0.1 | 10.5 ± 0.6 | <0.001 |
| HS-CRP (mg/dl)a | 0.10 ± 0.01 | 0.45 ± 0.05 | <0.001 |
| Total Cholesterol (mg/dl) | 163.7 ± 7.8 | 183.4 ± 5.8 | 0.052 |
| Triglyceride (mg/dl) | 79.6 ± 9.9 | 161.5 ± 15.1 | <0.001 |
| HDL-c (mg/dl) | 56.6 ± 2.2 | 38.6 ± 2.1 | <0.001 |
| LDL-c (mg/dl) | 90.6 ± 5.1 | 98.2 ± 5.5 | 0321 |
| Paraoxonase activity (nmol/min/100 μg) | 694 ± 23 | 517 ± 18 | <0.001 |
| Arylesterase activity (μmol/min/50 μg) | 194 ± 6 | 118 ± 5 | <0.001 |
| Lactonase activity (μmol/min/20 μg) | 107 ± 4 | 81 ± 3 | <0.001 |
BMI, body mass index; BUN, blood urea nitrogen; HS-CRP, high sensitive C-reactive protein; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol.
Reference level of HS-CRP, (<0.8 mg/dl).
3.2. Uremia cohort has a lower PON-1 protein level but a higher carbamylated PON-1 level in HDL
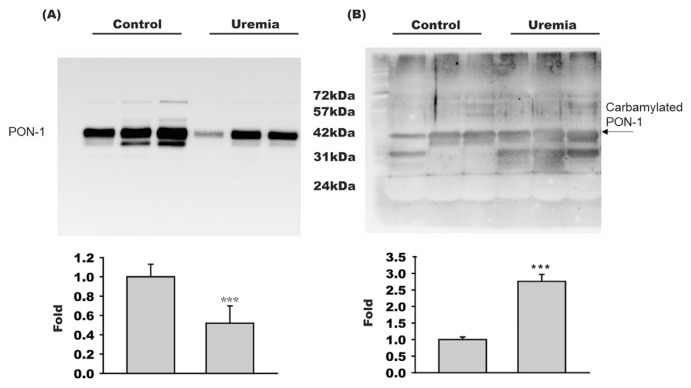
Equal amount of HDL proteins from control subjects or uremia patients were subjected to SDS/PAGE and then blotted with anti-PON-1 antibody. Resulting blots showed that the PON-1 protein level in HDL of uremia patients was only 0.48 ± 0.21 fold of that in HDL of healthy subjects (n = 6, p < 0.001) (Fig. 1A). However, when HDL protein was subjected to SDS-PAGE and blotted with anti-CBL, the level of carbamylated PON-1 protein in HDL of uremia patients was 2.69 ± 0.27 fold higher than that in HDL of control subjects (n = 6, p < 0.001) (Fig. 1B). Because uremia cohort has lower PON-1 protein level but higher carbamylated PON-1 level in HDL, the carbamylated degree of PON-1 is significantly higher in uremia patients than in control subjects.
Fig. 1.
PON-1 concentration decreased but carbamylated PON-1 concentration increased in HDL of uremia patients. Western blotting studies of HDL showed that (A) PON-1 concentration was lower, but (B) carbamylated PON-1 concentration was higher in uremia patients (n = 6) than in control subjects (n = 6). ***, p < 0.001.
3.3. Co-immune-precipitation and ELISA studies showed highly carbamylated PON-1 in HDL of uremia group
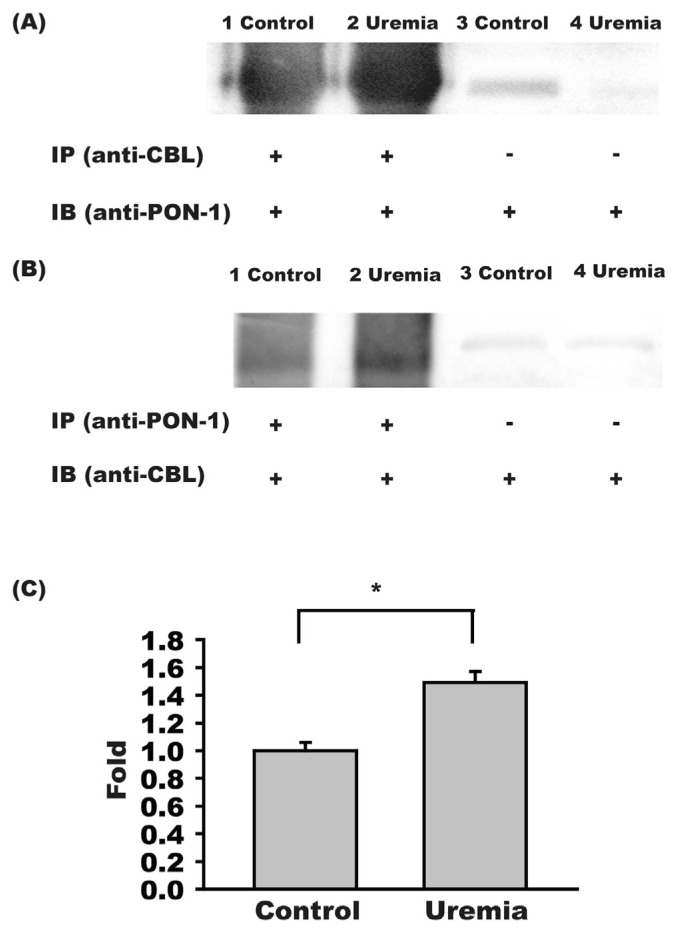
We first immune-precipitated carbamylated proteins in HDL and then blotted precipitated proteins with PON-1 antibody. The carbamylated PON-1 expression was higher in HDL of uremia patients than in control group (Fig. 2A). In a reverse approach, PON-1 was first immune-precipitated and the precipitated proteins were later blotted with anti-carbamyl antibody. Similarly, the carbamylated PON-1 protein expression amount was higher in uremia patients then in control subjects (Fig. 2B). We further confirmed the immuneprecipitation study results with sandwich ELISA method, which showed that the concentration of carbamylated PON-1 was 1.49 ± 0.08 fold higher in uremia patients than in control subjects (n = 16, p < 0.05) (Fig. 2C).
Fig. 2.
PON-1 carbamylation increased in uremia patients. (A) Immune precipitated carbamylated HDL proteins then blotted the precipitated proteins with anti-PON-1 antibody revealed a higher density of carbamylated PON-1 in uremia patients than in control subjects. (B) Immune precipitated PON-1 from HDL then blotted the precipitated PON-1 with anti-carbamyl antibody (anti-CBL) showed a higher concentration of carbamylated PON-1 in uremia patients than in control subjects. (C) Sandwich study of carbamylated PON-1 showed the concentration of carbamylated PON-1 in HDL was higher in uremia patients (n = 16) than in control subjects (n = 16). *,p < 0.05.
3.4. Identification of carbamylated sites of PON-1 using nanoLC-MS/MS
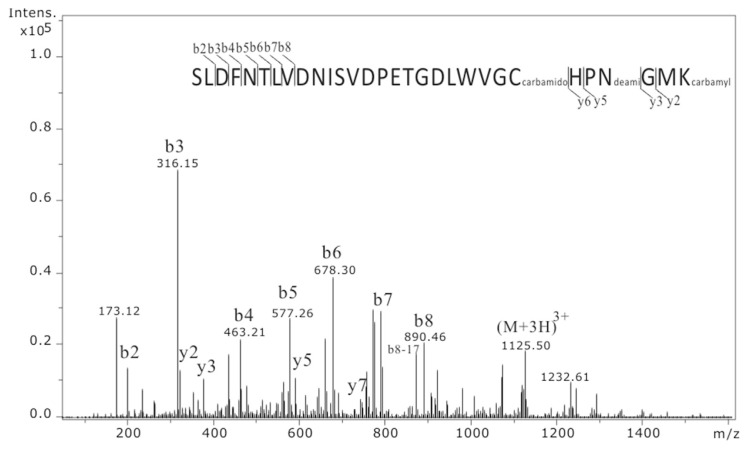
In order to identify the carbamylated sites of PON-1, we separated HDL proteins by SDS-PAGE. PON-1 band was excised and trypsin digested, followed by peptide extraction and nanoLC-MS/MS analysis. The sequence coverage of PON-1 from uremic PON-1 and normal PON-1 was 76.1% (Supplementary Table 1) and 57.5% (Supplementary Table 2), respectively. There is only one carbamylated modification on lysine (K290) of uremic PON-1, and there is no detectable carbamylated modification on normal PON-1. A MS/MS ion spectrum showed that a carbamylated peptide of (SLDFNTLVDNISVDPETGDLWVGC(carbamidomethyl) HPN(deamidated)GMK(carbamyl)290, [M+3H]3+ = 1125.5 Da) from PON-1 was identified (Fig. 3).
Fig. 3.
Identification of the carbamylated peptide on PON-1 by nanoLC-MS/MS analysis. The peptide peak of 1125.5 m/z ([M+3H]3+) was identified as peptide sequence of SLDFNTLVDNISVDPETGDLWVGCHPNGMK with modifications at C284 (carbamidomethylation), N287 (deamidation) and K290 (carbamylation) with Mascot ion score of 52.4. K represents lysine.
3.5. Local conformational changes
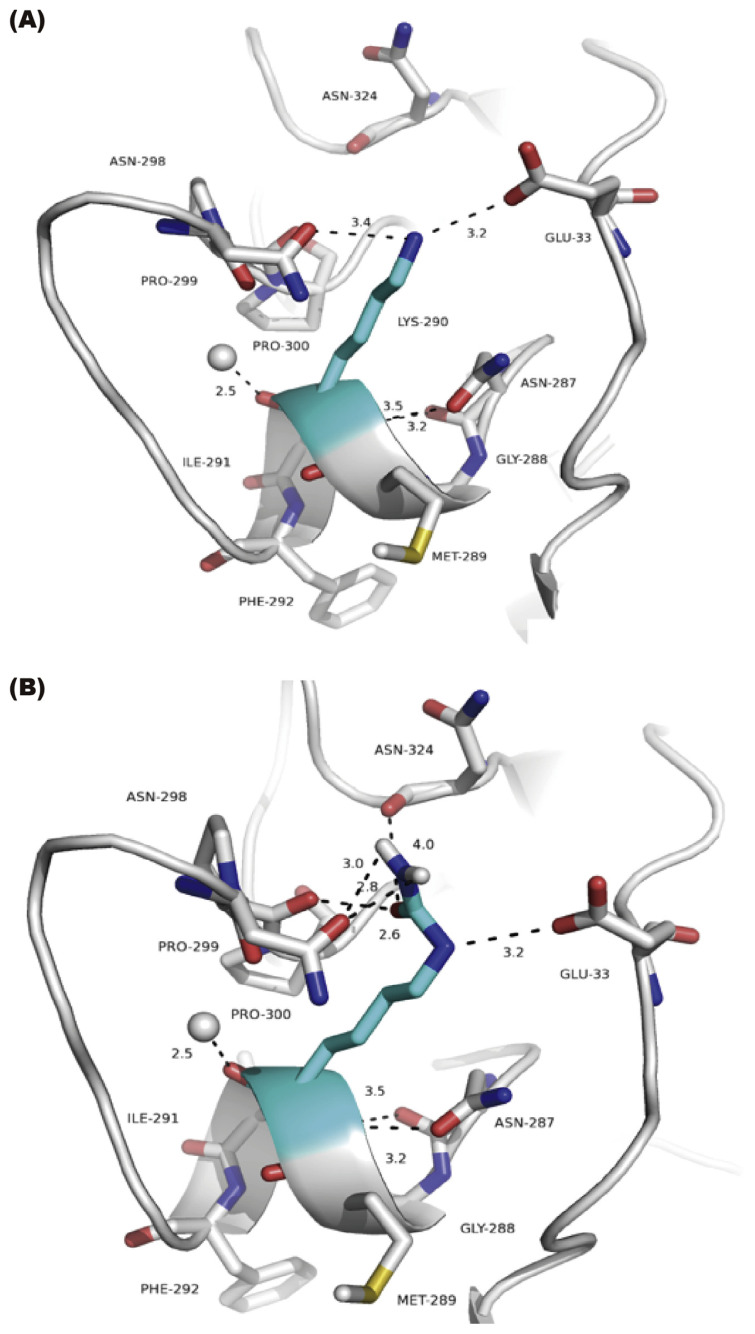
Within the range of 4 Å as a distance cutoff, all of the residues were selected and analyzed including the polar contacts and hydrogen bonds formations. The fundamental differences between the neighboring residues in the Lys290 (lysine 290 or K290) and LysC290 (Carbamylated lysine 290) protein were shown as in Fig. 4. Firstly, the potency of LysC290 (Fig. 4B) than Lys290 (Fig. 4A) is likely due to additional polar contact to Asn298 (asparagine 298), Pro299 (proline 299) and Asn324 (asparagine 324). Then, the elongation of side chain groups in LysC290 leads to an increase of the accessible solvent accessibility (ASA) about 11 Å2. At the same time, the surrounding residues, G33/V34/N298/P299/G301/N324 decrease the total ASA about 45 Å2, mainly because of the contributions from Asn298 and Asn324 residues that decrease the ASA about 29 Å2 (Supplementary Table 3). Therefore, this carbamylated Lysine may lead to the remarkable local conformational changes that reduce surface area, affect tertiary structure and result in the formation of interchain bonding.
Fig. 4.
Local conformation changes of PON-1 after Lysine 290 m (Lys 290) carbamylation. Side chain of residue Lys290 (A) and carbamylated Lys290 (B) of PON-1 by mutagenesis are shown as sticks (cyan) by Pymol. The dashed line was labeled the distance and shown to contact within 4 Å to neighboring residues (gray) and H2O (gray sphere).
3.6. The HDL anti-oxidant ability was lower in uremia patients
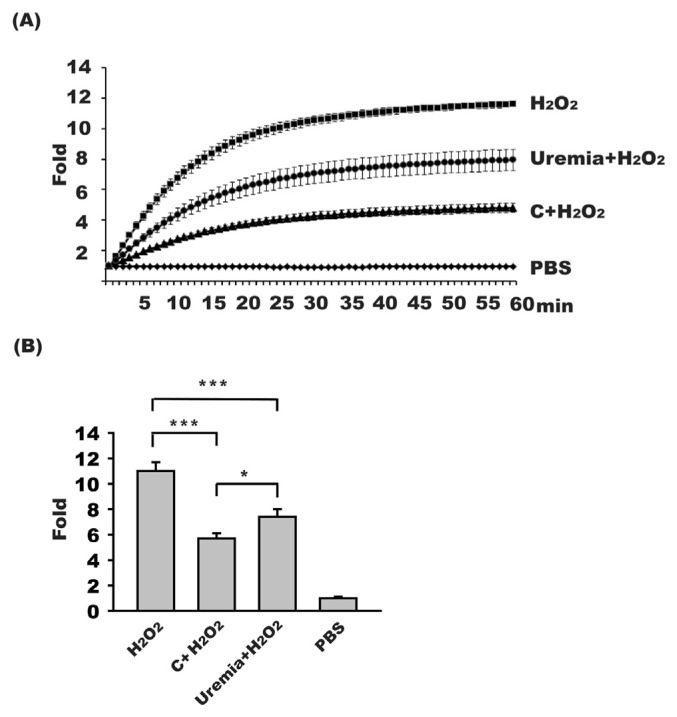
PON-1 is the major enzyme responsible for anti-oxidant activity of HDL. To determine the anti-oxidant ability of HDL, an assay by measuring the fluorescent yield of DCFH-DA was used. When RAW264.7 was treated with 10 mM H2O2, which served as positive control, the fluorescence increased to 11.0 ± 0.7 fold, compared to the fluorescence of negative control of PBS-treatment group (Fig. 5). When RAW264.6 cells treated with 10 mM H2O2 were added with HDL isolated from uremia or control subjects, the fluorescence decreased to 7.4 ± 0.6 fold and 5.7 ± 0.4 fold, respectively (uremia vs control, p < 0.01). These results may indicate that the anti-oxidant ability of PON-1 was lower in HDL of uremia patients than in HDL of control subjects.
Fig. 5.
HDL anti-oxidant activity decreased in uremia patients. DCFH-DA was used as fluorescence indicator of oxidant. (A) A representative triplicated study results of the time-course fluorescence changes from RAW264.7 cells treated with H2O2 (10 mM) (n = 16), HDL of uremia patients with H2O2 (10 mM) (n = 16), HDL of control healthy subjects with H2O2 (10 mM) (n = 16), or PBS, respectively. (B) The fluorescence at 60 min of RAW264.7 cells treated with H2O2 (10 mM) can increase the fluorescence 11.0 ± 0.7 fold compared to that of cells treated with PBS. RAW 264.7 treated with HDL from uremia patients plus H2O2 (10 mM) can increase the fluorescence higher than the fluorescence of cells treated with HDL isolated from control subjects plus H2O2 (10 mM) (p < 0.01). The fluorescence of cells treated with PBS (1.0 ± 0.1 fold) served as negative control. C, HDL of control subjects; *, p < 0.05; ***, p < 0.001.
4. Discussion
In this study, based on western blotting, immuneprecipitation, and ELISA results, PON-1 quantity is found to be lower-expressed but highly carbamylated in uremia patients than in control subjects. K290 site of PON-1 sequence is carbamylated in uremia subjects based on nanoLC-MS/MS analysis. It has been reported that carbamylation at lysine is at higher frequency when lysine is adjacent to G (glycine), A (alanine) and I (isoleucine), than adjacent to other amino acids [27,28]. In PON-1, K290 (Lys 290) is adjacent to I 291; also, K290 is in an exposed feature rather than in a centrally buried position and can access to isocyanate [11]. The detection of K290 carbamylation only in uremia subjects but not in control subjects by nano-LC/MS/MS may further indicate that the degree of PON-1 carbamylation is significantly higher in uremia patients than in control subjects.
The association between PON-1 carbamylation and PON-1 activity changes is still not clear. However, K290 is adjacent to PON-1 activity determine site of IFF(291–293). Point mutation of the individual site at I291 or F293 has been proved to result in changes of PON-1 phosphesterase, lactonase or esterase activities [11]. K290 (Lys290) carbamylation results in local conformation changes, which lead to a reduced surface area and a decrease of solvent accessibility or enzyme substrate accessibility. Our results also showed decreased activities of paraoxonase, arylesterase and lactonase in uremia patients. It is possible that carbamylation of K290 may disrupt the stereo structure that can influence the activity or substrate accessibility of PON-1, similar to negative effect brings from IFF (291–293) mutation.
Paraxonase is the enzyme responsible for the hydrolysis of many organophosphate compounds such as paraxon. Arylesterase is responsible for the hydrolysis carboxylic esters such as phenyl acetate. Lactonase is responsible for hydrolysis of many esters such as thiolactone. The impairment of these enzyme activities may lead to defect in the metabolism of these PON-1 substrates [8,29]. For example, decreased PON-1 lactonase activity can diminish homocysteine-thiolactone degradation and results in its accumulation, which promotes incorporation of homocysteine into proteins and subsequently alters protein structure and functions [30].
As PON-1 is also a strong anti-oxidant enzyme, PON-1 dysfunction may contribute to the lower anti-oxidant ability of HDL in uremia patients. Furthermore, a lower HDL PON-1 quantity and an impaired HDL PON-1 bio-functions based on carbamylation on HDL may increase the high cardiovascular risk seen in uremia patients.
To avoid factors that may affect carbamylation formation, patients with known co-morbid risk factors of protein carbamylation such as smoking and diabetes were excluded from the study [31,32]. To minimize the possible effect of systemic inflammation on carbamylation, we also included only uremia patients with serum HS-CRP within the recommended normal reference ranges, although the mean HS-CRP level was higher in uremia than in control subjects. Soluble substance in uremic blood, especially blood urea nitrogen (BUN), plays an important role in the process of protein carbamylation [32]. BUN in the presence of oxidative stress and uremia-associated high myeloperoxidase activity will non-enzymatically catalyze carbamylation formation [7]. Although all the uremia subjects we enrolled for study received hemodialysis therapy, their PON-1 activities were still lower than those of healthy controls. More adequate of dialysis therapy for BUN removal such as prolonging the dialysis treatment time, hemodialfiltration therapy (combining both hemodialysis and hemofiltration with enhanced BUN clearance) [33], or adequate diet modulation (such as vegetarian diets that lower BUN input) may be suggested for PON-1 activities rescue.
5. Conclusion
The current study demonstrated that PON-1 concentration in HDL of uremia patients decreased but the degree of PON-1 carbamylation increased as compared to HDL of control healthy subjects. Our results showed that the PON-1 enzyme activities and its anti-oxidant ability were both impaired in HDL of uremia patients. Increased PON-1 carbamylation may contribute to the PON-1 and HDL dysfunction observed in uremia patients.
Acknowledgments
This work was supported by grants from the China Medical University Hospital (DMR-107-121), the Ministry of Science and Technology (MOST 106-2113-M-039-001), and Academia Sinica’s Stroke Biosignature Project (BM10701010021).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfda.2018.09.007.
Funding Statement
This work was supported by grants from the China Medical University Hospital (DMR-107-121), the Ministry of Science and Technology (MOST 106-2113-M-039-001), and Academia Sinica’s Stroke Biosignature Project (BM10701010021).
REFERENCES
- 1. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–45. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 2. Hsieh JY, Chang CT, Huang MT, Chang CM, Chen CY, Shen MY, et al. Biochemical and functional characterization of charge-defined subfractions of high-density lipoprotein from normal adults. Anal Chem. 2013;85:11440–8. doi: 10.1021/ac402516u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ Res. 2014;114:171–82. doi: 10.1161/CIRCRESAHA.114.300935. [DOI] [PubMed] [Google Scholar]
- 4. Chang CT, Liao HY, Chang CM, Chen CY, Chen CH, Yang CY, et al. Oxidized ApoC1 on MALDI-TOF and glycated-ApoA1 band on gradient gel as potential diagnostic tools for atherosclerotic vascular disease. Clin Chim Acta. 2013;420:69–75. doi: 10.1016/j.cca.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 5. Bailey D, Ruel I, Hafiane A, Cochrane H, Iatan I, Jauhiainen M, et al. Analysis of lipid transfer activity between model nascent HDL particles and plasma lipoproteins: implications for current concepts of nascent HDL maturation and genesis. J Lipid Res. 2010;51:785–97. doi: 10.1194/jlr.M001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–9. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, et al. Uremia alters HDL composition and function. J Am Soc Nephrol. 2012;22:1631–41. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sztanek F, Seres I, Harangi M, Locsey L, Padra J, Paragh GJ, et al. Decreased paraoxonase 1 (PON1) lactonase activity in hemodialyzed and renal transplanted patients. A novel cardiovascular biomarker in end-stage renal disease. Nephrol Dial Transplant. 2012;27:2866–72. doi: 10.1093/ndt/gfr753. [DOI] [PubMed] [Google Scholar]
- 9. Gugliucci A, Kinugasa E, Ogata H, Caccavello R, Kimura S. Activation of paraoxonase 1 after hemodialysis is associated with HDL remodeling and its increase in the HDL2 fraction and VLDL. Clin Chim Acta. 2014;430:9–14. doi: 10.1016/j.cca.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 10. Hassett C, Richter RJ, Humbert R, Chapline C, Crabb JW, Omiecinski CJ, et al. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991;30:10141–9. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- 11. Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol. 2004;11:412–9. doi: 10.1038/nsmb767. [DOI] [PubMed] [Google Scholar]
- 12. Cuellar LA, Prieto ED, Cabaleiro LV, Garda HA. Apolipoprotein A-I configuration and cell cholesterol efflux activity of discoidal lipoproteins depend on the reconstitution process. Biochim Biophys Acta. 2014;1841:180–9. doi: 10.1016/j.bbalip.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 13. Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275:17527–35. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 14. Bielicki JK, Oda MN. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 2002;41:2089–96. doi: 10.1021/bi011716p. [DOI] [PubMed] [Google Scholar]
- 15. Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, Tang WH, Hazen SL. Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol. 2013;24:853–61. doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed Z, Ravandi A, Maguire GF, Emili A, Draganov D, La Du BN, et al. Apolipoprotein A-I promotes the formation of phosphatidylcholine core aldehydes that are hydrolyzed by paraoxonase (PON-1) during high density lipoprotein oxidation with a peroxynitrite donor. J Biol Chem. 2001;276:24473–81. doi: 10.1074/jbc.M010459200. [DOI] [PubMed] [Google Scholar]
- 17. Yunoki K, Naruko T, Inaba M, Inoue T, Nakagawa M, Sugioka K, et al. Gender-specific correlation between plasma myeloperoxidase levels and serum high-density lipoprotein-associated paraoxonase-1 levels in patients with stable and unstable coronary artery disease. Atherosclerosis. 2013;231:308–14. doi: 10.1016/j.atherosclerosis.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 18. Ikeda Y, Suehiro T, Itahara T, Inui Y, Chikazawa H, Inoue M, et al. Human serum paraoxonase concentration predicts cardiovascular mortality in hemodialysis patients. Clin Nephrol. 2007;67:358–65. doi: 10.5414/cnp67358. [DOI] [PubMed] [Google Scholar]
- 19. Gugliucci A, Mehlhaff K, Kinugasa E, Ogata H, Hermo R, Schulze J, et al. Paraoxonase-1 concentrations in end-stage renal disease patients increase after hemodialysis: correlation with low molecular AGE adduct clearance. Clin Chim Acta. 2007;377:213–20. doi: 10.1016/j.cca.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 20. Gugliucci A, Kinugasa E, Kotani K, Caccavello R, Kimura S. Serum paraoxonase 1 (PON1) lactonase activity is lower in end-stage renal disease patients than in healthy control subjects and increases after hemodialysis. Clin Chem Lab Med. 2011;49:61–7. doi: 10.1515/CCLM.2011.004. [DOI] [PubMed] [Google Scholar]
- 21. El-Gamal D, Holzer M, Gauster M, Schicho R, Binder V, Konya V, et al. Cyanate is a novel inducer of endothelial icam-1 expression. Antioxid Redox Signal. 2012;16:129–37. doi: 10.1089/ars.2011.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hadfield KA, Pattison DI, Brown BE, Hou L, Rye KA, Davies MJ, et al. Myeloperoxidase-derived oxidants modify apolipoprotein A-I and generate dysfunctional high-density lipoproteins: comparison of hypothiocyanous acid (HOSCN) with hypochlorous acid (HOCl) Biochem J. 2013;449:531–42. doi: 10.1042/BJ20121210. [DOI] [PubMed] [Google Scholar]
- 23. Collins AJ, Ma JZ, Umen A, Keshaviah P. Urea index and other predictors of hemodialysis patient survival. Am J Kidney Dis. 1994;23:272–82. doi: 10.1016/s0272-6386(12)80984-x. [DOI] [PubMed] [Google Scholar]
- 24.Hibbert F, Emsley J. Hydrogen bonding and chemical reactivity. In: Bethell D, editor. Advances in physical organic chemistry. Vol. 26. Academic Press; 1990. pp. 255–379. [Google Scholar]
- 25. Frishman D, Argos P. Knowledge-based protein secondary structure assignment. Proteins. 1995;23:566–79. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- 26. Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV, et al. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro. 2013;27:954–63. doi: 10.1016/j.tiv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 27. Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–90. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pang CN, Hayen A, Wilkins MR. Surface accessibility of protein post-translational modifications. J Proteome Res. 2007;6:1833–45. doi: 10.1021/pr060674u. [DOI] [PubMed] [Google Scholar]
- 29. Kennedy DJ, Tang WH, Fan Y, Wu Y, Mann S, Pepoy M, et al. Diminished antioxidant activity of high-density lipoprotein-associated proteins in chronic kidney disease. J Am Heart Assoc. 2013;2:e00014. doi: 10.1161/JAHA.112.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perla-Kajan J, Jakubowski H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J. 2010;24:931–6. doi: 10.1096/fj.09-144410. [DOI] [PubMed] [Google Scholar]
- 31. Shiu SW, Xiao SM, Wong Y, Chow WS, Lam KS, Tan KC, et al. Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clin Sci (Lond) 2014;126:175–81. doi: 10.1042/CS20130369. [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–84. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 33. Maduell F, Arias M, Duran CE, Vera M, Fontsere N, Azqueta M, et al. Nocturnal, every-other-day, online haemodiafiltration: an effective therapeutic alternative. Nephrol Dial Transplant. 2012;27:1619–31. doi: 10.1093/ndt/gfr491. [DOI] [PubMed] [Google Scholar]