Abstract
Cholesterol is an important lipid molecule in cell membranes and lipoproteins. Cholesterol is also a precursors of steroid hormones, bile acids, and vitamin D. Abnormal levels of cholesterol or its precursors have been observed in various human diseases, such as heart diseases, stroke, type II diabetes, brain diseases and many others. Therefore, accurate quantification of cholesterol is important for individuals who are at increased risk for these diseases. Multiple analytical methods have been developed for analysis of cholesterol, including classical chemical methods, enzymatic assays, gas chromatography (GC), liquid chromatography (LC), and mass spectrometry (MS). Strategy known as ambient ionization mass spectrometry (AIMS), operating at atmospheric pressure, with only minimal sample pretreatments for real time, in situ, and rapid interrogation of the sample has also been employed for quantification of cholesterol. In this review, we summarize the most prevalent methods for cholesterol quantification in biological samples and foods. Nevertheless, we highlight several new technologies, such as AIMS, used as alternative methods to measure cholesterol that are potentially next-generation platforms. Representative examples of molecular imaging of cholesterol in tissue sections are also included in this review article.
Keywords: Cholesterol, Quantification, Mass spectrometry, AIMS, DESI, DART
1. Introduction
Cholesterol is a sterol lipid produced by animal cells. It is a relatively small biological molecule that plays important roles in human body. Most of cholesterol is produced in liver, adrenal glands, intestines, and in gonads, whereas 20–25% of cholesterol comes from the diet of animal origin [1]. Cholesterol is an essential component of all cell membranes - it helps to maintain structural integrity and fluidity of cell membranes, allowing animal cells to change shapes easily without cell walls [2]. One of the major functions of cholesterol is to participate in the biosynthesis of bile acids in the liver [3]. Bile can break down dietary fats into smaller droplets and helps the subsequent digestion. Cholesterol also participates in the absorption and the production of vitamin D [4]. Moreover, cholesterol is a precursor of steroids, such as testosterone and estrogen [5]. Besides, cholesterol is also a key component in lipoproteins, which are used to transport hydrophobic molecules (such as fats) in hydrophilic media, like blood. Lipoproteins, classified as very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL), and high-density lipoprotein (HDL), consist of esterified and free cholesterol, proteins, phospholipids, and triglycerides. Cholesteryl ester transfer protein (CETP) facilitates transfer of cholesterol esters and triglycerides among different classes of lipoproteins [1].
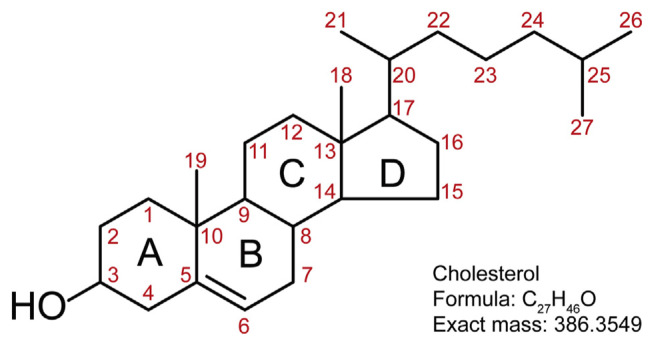
A cholesterol molecule contains three major parts: 1) tetracyclic carbon ring (A–D) as the core of steroids, 2) polar hydroxyl group attached to ring A, and 3) short non-polar carbon chain attached to ring D (Fig. 1) [6,7]. All four rings of the sterol group are in a trans conformation, making cholesterol a planner molecule. The double bond between C5 and C6 helps to keep the rigidity of cholesterol.
Fig. 1.
Structure of cholesterol with the numbering of the carbon atoms.
Cholesterol is required for viability and cell proliferation. More than 90% of cellular cholesterol is located at the plasma membrane [8]. The cholesterol molecule is inserted into lipid bilayers, where it aligns itself parallel to the glycerophospholipids and sphingophospholipids. The hydroxyl group is very close to the polar lipid head but is insufficient to shield the hydrophobic ring from water, thus, cholesterol tails are very closely associated with saturated phospholipid and sphingolipids where the enzymes, substrates, and products involved tends to be extremely hydrophobic [7]. Thus, cholesterol has vital structural roles in membranes and in lipid metabolism with the extraordinary diversity of biological roles [9–11].
In this article, we discuss the importance of cholesterol measurement in clinical and food sciences, introducing the most prevalent methods used for cholesterol quantification in the related fields. In the end, we highlight several new analytical platforms that are potentially the next-generation techniques in clinical laboratories.
2. Why measuring cholesterol?
2.1. Cholesterol in clinical sciences
Cholesterol is an essential building block for cell membranes and also important precursor for production of hormones [1]. Up to certain levels, cholesterol is beneficial for human body. Meanwhile, higher levels of cholesterol are associated with many diseases. According to the National Heart Lung and Blood Institute (NHLBI, USA), the higher the level of LDL cholesterol in our system, the greater our chances of getting heart diseases. On the other hand, people with higher level of HDL cholesterol have lower chances of getting heart diseases [12,13]. LDL carries cholesterol around the body to the parts where it is demanded. However, if there is too much of cholesterol in the bloodstream, it tends to cling to the walls of the arteries and may eventually lead to the clogging. Excessive cholesterol can deposit in the arteries and promote formation of plaques, leading to atherosclerosis [14]. Therefore, LDL is referred to as “bad cholesterol”. In the contrary, HDL is called as “good cholesterol” due to its capability to gobble up cholesterol and to take it back to the liver [15]. To determine lipoprotein level and obtain complete lipoprotein profile, blood sample is withdrawn after 12 h fasting, while to determine total level of cholesterol - fasting is not required [13]. The concentration of total cholesterol, occurring in the free form and as cholesterol esters (esterified to long-chain fatty acids), in human serum is highly variable among individuals. The American Heart Association (AHA, USA) recommends all adults at age between 40 and 75, without cardiovascular diseases and with LDL-C between 70 mg/dL (1.81 mmol/L) and 189 mg/dL (4.90 mmol/L), to take tests for cholesterol levels every 4–6 years [15,16].
The association between elevated total cholesterol and the risk of cardiovascular disease – one of the leading causes of death worldwide – is well documented [17,18]. In addition, abnormal levels of cholesterol or its precursors also have been observed in various human diseases, such as Smith-Lemli-Opitz syndrome (SLOT) [19], non-insulin-dependent diabetes mellitus [20], brain diseases [21], and cancers [22]. Therefore, regular monitoring of cholesterol level is important for individuals who are at increased risk of these diseases. As cholesterol is an early-warning indicator of various diseases, screening tests for cholesterol are mandatory in adult healthcare in clinical laboratories, and its levels are determined in routine blood tests. According to the National Cholesterol Education Program (NCEP, USA), optimal LDL and total cholesterol concentrations are < 100 mg/dL and <200 mg/dL, respectively. HDL concentration <40 mg/dL is considered as low, while ≥60 mg/dL is considered as high [13].
2.2. Cholesterol in food sciences
Public interests in dietary cholesterol had increased considerably due to the association of plasma cholesterol levels with the risk of heart diseases. As around 20–25% of cholesterol in our body comes from the food of animal origin, such as egg, meat, milk products, etc., it is important to know the cholesterol concentration in our dietary intake [23]. Food nutritional information need to be provided by laboratories to food producers for precise food labeling, as a nutrition food label has to inform customers about healthy eating and appropriate food choices to reduce nutrient-related diseases [24]. Thus, evaluation of the exact cholesterol content in foods is crucial and drives the technological development for cholesterol quantification [25,26].
3. Methods for cholesterol measurements
Multiple methods have been developed for cholesterol levels determination. Those methods can be divided into three major categories: 1) classical chemical methods based on the Abell-Kendall protocol, 2) fluorometric and colorimetric enzymatic assays that are commonly employed for assay kits and automated plate readers, and 3) analytical instrumental approaches such as gas and liquid chromatography or mass spectrometry. Each of those methods has its distinctive advantages. Classical chemical methods are relatively simple and inexpensive to perform but multi-step procedures are required. Enzymatic assays involve the use of costly enzymes, although limits of detection (LOD) are usually low. Chromatographic and mass spectrometric methods are the most accurate and sensitive ones, however they require costly equipment and extensive sample pretreatment. National Institute of Standards and Technology (NIST, USA) has approved an isotope-dilution mass spectrometry (ID-MS) method [27] and modified Abell-Kendall protocol [28] as two reference measurement procedures (RMPs) for blood cholesterol quantification. Based on those methods, various analytical protocols have thus been developed. Recently, ambient ionization mass spectrometry techniques (AIMS) have been proposed as alternative approaches for high-throughput screening as they do not require tedious sample pretreatments. We summarize several noteworthy methods for cholesterol analysis in Table 1 and discuss them in more details in the following paragraphs. In this review article, we focus more on description of analytical instrumental approaches and we highlight several new technologies, such as AIMS, used as alternative methods to measure cholesterol that are potentially next-generation platforms. Representative examples of molecular imaging of cholesterol in tissue sections are also included here.
Table 1.
Summary of the methods described in the article.
| Method | Characteristics | Ref. |
|---|---|---|
| Modified Abell-Kendall method | This is a standard reference method for total blood cholesterol measurement. It is a multi-step classical chemical method, which involves saponification of cholesterol esters by hydroxide, extraction with hexane, and color development with acetic anhydride–sulfuric acid. | [28] |
| Fluorometric-enzymatic assay | This assay is based on an enzyme-coupled reaction for determining free cholesterol and cholesterol esters. Esterified cholesterol is converted to cholesterol by cholesterol esterase. The resulting cholesterol is then acted upon by cholesterol oxidase to produce cholest-4-en-3-one and hydrogen peroxide and the corresponding ketone product. Hydrogen peroxide is then detected using sensitive and stable fluorescence probe. | [29] [30] [31] |
| GC/GC-MS: gas chromatography – mass spectrometry | This method is used for routine cholesterol determination. It is used to separate cholesterol from other interfering species. It can be used for determination of free, esterified and total cholesterol levels (based on sample pretreatment). More accurate than classical chemical and enzymatic methods. Extensive sample preparation (including derivatization) prior analysis is required. Labor and resource intensive. | [32] [33] [34] |
| GC-ID-MS | This is a standard reference method for total blood cholesterol measurement. Isotopically labeled cholesterol is added to a sample in an amount approximately equal to the actual amount of analyte present in a sample. Additionally, two calibration mixtures of a known cholesterol and labeled cholesterol concentrations are prepared. Quantification is based on calculating peak area ratio corresponding to unlabeled and labeled cholesterol for unknown sample and comparing it with the ratios calculated for calibration mixtures. | [27] |
| LC/LC-MS: liquid chromatography – mass spectrometry | This method is used for routine cholesterol determination. Similarly to GC, it is used to separate cholesterol from other interfering species. It can be used for determination of free, esterified and total cholesterol level (based on sample pretreatment). More accurate than classical chemical and enzymatic methods. Extensive sample preparation prior analysis is required. Labor and resource intensive. | [35] [36] |
| ESI-MS/MS: electrospray ionization tandem mass spectrometry | This method requires derivatization to increase ionization efficiency. Free cholesterol may be converted to cholesteryl acetate using acetyl chloride. Sample is directly injected to MS. Fragment at m/z 369 is used for quantification (SRM mode and precursor ion scan). | [37] |
| MALDI-MS: matrix-assisted laser desorption ionization-time of flight | This method requires use of matrix. Signal at m/z 369 corresponding to dehydrated molecule [M-H2O+H]+ can be observed. Lipoprotein lipid signals in 369–910 m/z range can be detected in human serum. | [38] |
| MALDI-IM-MS: Matrix-assisted laser desorption/ionization-ion-mobility mass spectrometry | This method takes advantage of ion-mobility to separate chemical noise from the analytes of interest. Coating samples with metal increases the conductivity of insulating substrates, such as tissues or cells. Using sputtered silver nanoparticles instead of matrix provides lower limit of detection based on signals of silver coordinated molecules. Profiling of cholesterol and 7-dehydrocholesterol in SLOS human fibroblast can be done. | [39] |
| DESI-MS: Desorption electrospray ionization mass spectrometry | This method often requires derivatization to increase ionization efficiency. Derivatising reagent can be incorporated into DESI spray. Betaine aldehyde reacts with cholesterol forming charge-labeled cholesterol. | [40] |
| DART: Direct analysis in real time mass spectrometry | This method is suitable for the analysis of non-polar compounds. Signal at m/z 369 corresponding to dehydrated molecule [M-H2O+H]+ can be observed. This method can be used for food and biological samples analysis. Endogenous cholesterol from serum spotted onto the chromatography paper can be desorbed and rapidly ionized. Fast quantitative screening can be achieved. | [26] [41] |
| MALDI-MSI: Matrix-assisted laser desorption/ionization mass spectrometry imaging | This method requires covering tissue section with a thin layer of matrix. It can be used to determine the distribution of endogenous cholesterol sulfate as well as exogenous compounds in human skin tissue sections. Spatial resolution is around 30 μm. | [42] |
| DESI-MSI: Desorption electrospray ionization mass spectrometry imaging | This method allows interrogation of the sample in its native environment. No matrix is required. Reactive DESI (with a betaine aldehyde as a reagent) can be used to image cholesterol in rat brain tissue. Spatial resolution is around 200 μm. DESI can be used to image cholesterol sulfate that is a differentiating compound between healthy and prostate cancer tissue. | [22] [40] |
In addition to the selection of the appropriate analytical technique, a proper sample preparation is also crucial in cholesterol analysis. Cholesterol esters constitute about two-thirds of the cholesterol in the blood and can be hydrolyzed chemically by a hydroxide (saponification) or enzymatically by cholesterol esterase to free cholesterol for total cholesterol quantification. For serum cholesterol quantification, at least 0.5 mL of blood specimen is drawn and around 15 μL of serum is prepared for analysis. For plant- and animal-derived foods cholesterol determination, a sterol fraction is isolated from lipid extract by thin-layer or column chromatography, following hydrolysis [43,44]. All these methods commonly share the same procedure for sample preparation that includes saponification extraction step of total lipids and a multistage solvent extraction followed by purification and concentration.
Quantitation of cholesterol in foods is often achieved by gas chromatography with flame ionization (GC-FID) or mass spectrometry (GC-MS) detection, spectrophotometry, and high performance liquid chromatography (HPLC). For milk and related products, cholesterol is initially isolated from the crude materials by solid-phase extraction (SPE). Such protocols have been established by The Association of Official Analytical Chemists (AOAC, USA) and International Dairy Federation (IDF) [45–47]. Recently, a rapid and reliable method for cholesterol determination in egg-containing foods by direct analysis in real time mass spectrometry (DART-MS) was reported [26].
High throughput liquid chromatography tandem mass spectrometry (LC-MS/MS) has become a gold standard method for measurement of steroid substances in biological fluids and foods. Gas chromatography mass spectrometry (GC-MS) is also widely used to quantify cholesterol. Details of these methods are elaborated in the following paragraphs.
3.1. Modified Abell-Kendall method
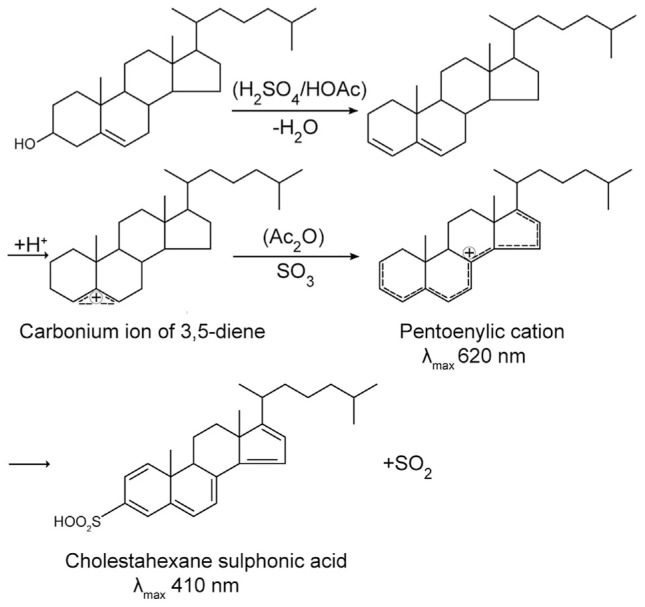
The modified Abell-Kendall method is a standard reference method for determination of total cholesterol for clinical use approved by NIST. It is a multi-step classical chemical method involving Liebermann-Burchard (L-B) reaction for color development [28]. It includes saponification of cholesterol ester with alcoholic potassium hydroxide, extraction of hydrolyzed cholesterol with hexane (instead of petroleum ether as in original Abell-Kendall method) followed by evaporation of the solvent, and finally color development with acetic anhydride and concentrated sulfuric acid. The scheme of L-B reaction is shown in Fig. 2. Appearance of the color is due to the reaction of hydroxyl group of cholesterol. Ultra-violet (UV) spectrophotometry (λ = 410 nm) is commonly used for measurement in order to quantify cholesterol. Although, modified Abell-Kendall reaction is considered as a standard reference method, its use is not accepted for routine tests nowadays since highly corrosive reagents are used for L-B reaction.
Fig. 2.
The scheme of the Liebermann-Burchard reaction for color development in modified Abell-Kendall method [28].
3.2. Enzymatic assays
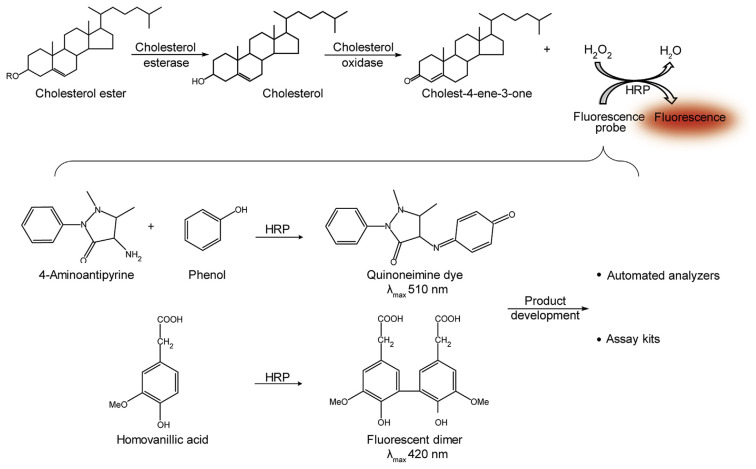
Those types of assays are based on the enzyme-coupled reaction to determine total cholesterol levels. The first enzymatic assay to measure cholesterol in serum was introduced in 1974 [29]. Since then, enzymatic assays started being commonly used and they are widely employed for assay kits and automated analyzers nowadays [48]. At first, esterified cholesterol is hydrolyzed to free cholesterol by cholesterol esterase (see Fig. 3) [29–31]. The free cholesterol is subsequently oxidized to cholest-4-en-3-one by cholesterol oxidase. Hydrogen peroxide is produced in this reaction as a side product and can be easily detected using high sensitive colorimetric or fluorometric probes. Multiple fluorometric probes have been developed and therefore enzymatic assays have become a common strategy. For example, 4-aminoantipyrine (with addition of phenol known as Trinder reagent) [29], homovanillic acid [49], and 10-acetyl-3,7-dihydroxyphenoxazine (known as Amplex Red) [30] can be catalyzed by horse radish peroxidase (HRP) to form the fluorescent product in a presence of hydrogen peroxide (see Fig. 3). For routine analysis, many laboratories use commercial cholesterol quantitation assay kits, handheld point-of-care testing (POCT) devices and automated analyzers based on enzymatic assays [31,48]. It should be mentioned, that enzymatic assays may not be strictly selective for cholesterol determination. In fact, cholesterol oxidase may also react with other sterols [50]. Moreover, some chemicals present in a sample (such as ascorbic acid and bilirubin) may consume hydrogen peroxide and some bias during indirect cholesterol quantification may occur.
Fig. 3.
The scheme of the enzyme-coupled reaction of fluorometric enzymatic assay.
3.3. Gas and liquid chromatography
Chromatographic techniques such as gas (GC, where gas is a mobile phase) and liquid (LC, where liquid is a mobile phase) chromatography are commonly used for cholesterol quantification [32,51]. Those methods are widely accepted to be more reliable, sensitive and accurate than the other methods. Additional selectivity is achieved due to the fact that cholesterol is separated from interfering species (such as sterols) on chromatographic columns. Moreover, low volumes of samples (tens of μL) are required for analysis. Meanwhile, as mentioned before, the performance of chromatographic techniques are more dependent on sample preparation, such as saponification and analyte extraction. Analysis of cholesterol and cholesterol esters is the most often accomplished by GC. In 1964, GC coupled with hydrogen flame ionization detector was employed to analyze cholesterol in serum [32]. In 1979, reverse phase high-performance liquid chromatography (HPLC) with an UV detection at λ = 210 nm was used for determination of free, esterified and total cholesterol in serum [35]. Linear range from 0.09 to 0.31 g/L for quantification of cholesterol was achieved.
Cholesterol is readily ionized by electron ionization (EI), but other MS ionization techniques including electrospray ionization (ESI) are less effective due to low proton affinity and low acidity of cholesterol [40]. Methods based on mass spectrometry usually use isotopically labeled cholesterol (2D labeled: cholesterol-D6, cholesterol-D7 or 13C labeled) as internal standards to compensate for signal variability. Using internal standard, more accurate quantification by MS is achieved. GC coupled with MS detection was used for analysis of cholesterol, in which total free cholesterol was extracted from serum with chloroform and derivatized with bis(trimethylsilyl) trifluoroacetamide (BSTFA) [34]. Commonly used step of saponification was omitted. Linear range from 0.1 to 15 mmol/L for cholesterol quantification was achieved. The limit of detection (LOD) was 0.04 mmol/L and intra-day and inter-day precision and recovery were 92.5%–98.5% (n = 6). It was fund that total cholesterol levels in rats serum positively correlated with high cholesterol diet.
Isotope-dilution mass spectrometry (ID-MS) is a reference measurement procedure for cholesterol quantification approved by NIST. In 1980, the first GC-ID-MS method was presented [27]. In this report, cholesterol-D7 was added to the serum sample in a specific amount, so that unlabeled to labeled cholesterol peak area ratio was approximately equal to 1. Then, cholesterol esters were hydrolyzed and resulting free cholesterol, along with cholesterol-D7, were extracted and derivatized for GC-MS analysis. Additionally, two calibration mixtures of a known cholesterol and cholesterol-D7 concentrations, with unlabeled to labeled cholesterol peak area ratio from 0.8 to 1.2, were prepared following the same protocol. Quantification was based on calculating peak area ratio corresponding to unlabeled and labeled cholesterol for unknown sample and comparing it with the ratios calculated for calibration mixtures (so called bracketing protocol). Later on, various modifications of this method were developed and presented. For example, liquid chromatography–isotope dilution mass spectrometry (LC-ID-MS) employing cholesterol-13C3 was reported [36]. Initially, alkaline hydrolysis and cholesterol extraction were performed. No derivatization was used this time, making it a convenient and popular method. High accuracy and precision were achieved and good stability of the method was reported.
3.4. Electrospray ionization tandem mass spectrometry
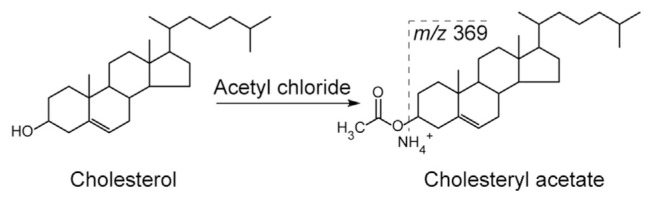
Electrospray ionization (ESI) is not an effective ionization technique for neutral free sterol molecules such as cholesterol. Molecular ions [M+H]+ of sterol molecules fragment easily in the ion source, therefore it is difficult to detect them. In contrary, cholesterol ester species tend to form more stable ammonium adduct ions [M+NH4]+ that can be successfully detected. Derivatization of free cholesterol is required to improve ionization efficiency in ESI-MS. For example, cholesterol can be converted to cholesteryl acetate in a reaction with acetyl chloride (see Fig. 4). Such a procedure was used to simultaneously analyze free cholesterol and cholesterol esters in plasma samples and cell homogenates by ESI-MS/MS [37]. In this report, free cholesterol was converted to cholesteryl acetate. Upon collision induced dissociation, derivatized cholesterol as well as cholesterol esters ([M+NH4]+ ions), formed the same fragment ion at m/z 369 and thus it was used for further quantification. Cholesterol-D7 served as internal standard. Selected reaction monitoring (SRM) in a combination with precursor ion scan mode was used.
Fig. 4.
Scheme of cholesterol derivatization with acetyl chloride to form cholesteryl acetate. Fragment at m/z 369 is used for quantitative purposes in ESI-MS/MS analysis [37].
Alternatively, silver cationization approach can be implemented for direct cholesterol ionization due to high affinity of Ag+ ions to π orbitals. Electrolytically generated metal cations (such as Ag+) can be injected from the primary electrospray towards an open-tube secondary capillary containing the sample. Such a method defined as relay ESI (rESI) was successfully applied to form stable [M+Ag]+ cholesterol ionsat m/z 493 and to perform MS/MS analysis for 100 ppb of the analyte [52].
3.5. Matrix-assisted laser desorption/ionization mass spectrometry
Matrix-assisted laser desorption/ionization (MALDI) in not an effective ionization technique for neutral free sterol molecules. Nevertheless, there are increasing evidences showing that MALDI-MS can be used in cholesterol measurements. In the study by Hidaka et al., MALDI-TOF (time-of-flight) MS was employed for the analysis of cholesterol in human serum lipoproteins [38]. Lipids were isolated and extracted from serum and further mixed with the organic matrix (2,5-dihydroxybenzoic acid; 2,5-DHB) dissolved in chloroform-methanol (Folch’s solution). Lipoprotein lipid signals were detected in a range of 369–910 m/z, while signals from 2,5-DHB matrix were observed in a range of 154–551 m/z. Signal from dehydrated cholesterol was detected at m/z 369 [MH2O+H]+. Quantitative measurement was achieved by using 4-cholesten-3-one as an internal standard to compensate the instrument variation.
Silver cationization was implemented to enhance ionization of cholesterol by infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI). Trace amounts of silver nitrate (AgNO3) were doped into the ESI solvent. Authors observed that adduct ion intensity was proportional to the concentration of the dopant in the solvent up to specific value (100 μmol/L). Selectivity of this approach was reasonable and cholesterol detection was possible in complex sample such as human serum [53].
In addition, sputtered silver matrix-assisted laser desorption/ionization-ion mobility-mass spectrometry (MALDI-IM-MS) strategy for profiling cholesterol and 7-dehydrocholesterol (7-DHC) in human fibroblast cells was reported [39]. In the study by Xu et al., sputtered silver nanoparticles provided a LOD as low as 0.5 pmol/mm2. Ion-mobility was implemented to separate chemical noise from the analytes of interest to improve LOD. The 7-DHC/cholesterol ratios were compared between Smith-Lemli-Opitz syndrome (SLOS) and control human fibroblasts, revealing a higher cholesterol level in SLOS cells. This result indicates the possibility to investigate cholesterol biosynthesis disorders using mass spectrometry-based methods [39].
4. Ambient ionization mass spectrometry
In the past decade, a strategy known as ambient ionization mass spectrometry (AIMS) was developed for chemical analysis on biological systems [54]. Ambient ionization techniques operate at atmospheric pressure and they require only minimal sample pretreatment for real time, in situ, and rapid mass spectrometric analysis. Desorption electrospray ionization (DESI) [55] and direct analysis in real time (DART) [56] are two rapid AIMS-based technologies that can be directly used for quantitative cholesterol measurements and are more effective than direct ESI approaches for cholesterol ionization.
4.1. Desorption electrospray ionization mass spectrometry
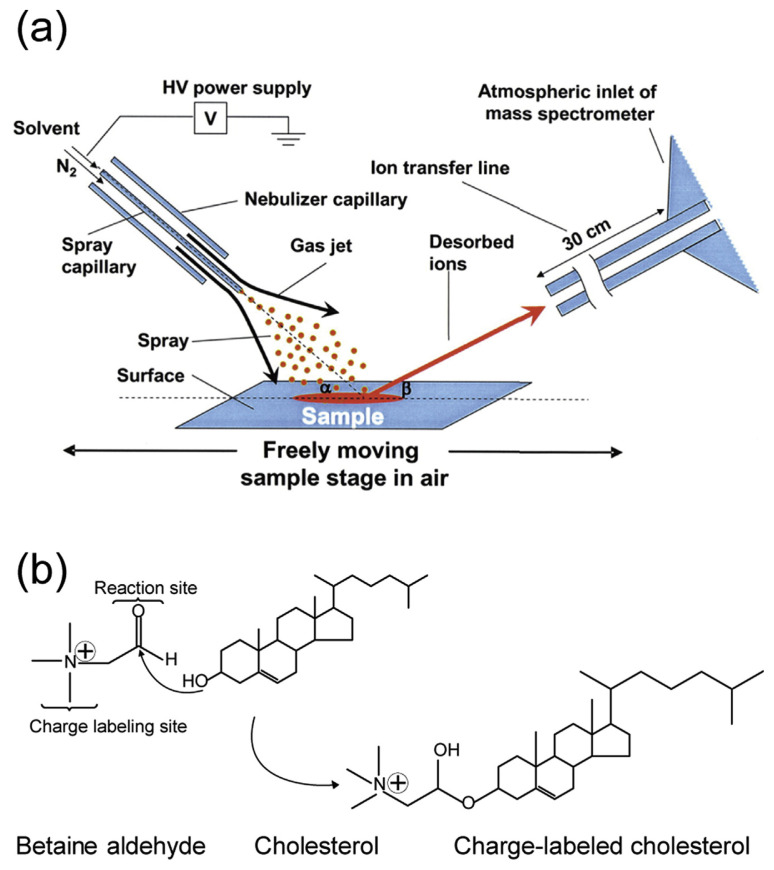
DESI is an electrospray-based ion source developed in 2004 by Takáts et al. [55]. Streams of electrically charged droplets are directed toward the sample surfaces (see Fig. 5a). Charged micro-droplets desorb molecules from the surface, where gas-phase ions are generated and subsequently transferred toward the mass spectrometer. Although DESI MS can be used for analysis of non-polar compounds, yet ionization efficiency is not satisfactory. Therefore, derivatization is usually required for cholesterol measurement.
Fig. 5.
(a) Desorption electrospray ionization mass spectrometry instrumental setup. Reprinted with permission from Ref. [55]. (b) Charge labeling reaction using betaine aldehyde for reactive DESI [40].
In this regard, application of reactive DESI is utilized to improve the sensitivity and selectivity. For example, betaine aldehyde was incorporated into the DESI spray solvent to react with the hydroxyl group of cholesterol through nucleophilic addition and form a hemiacetal salt (see Fig. 5b) [40]. Using this approach – called charge labeling, free cholesterol was quantified in serum samples with LOD reduced from over 200 ng for conventional DESI to 1 ng for reactive DESI.
4.2. Direct analysis in real time mass spectrometry
DART is a plasma-based ion source developed in 2005 by Cody et al. [56]. Metastable species that are generated by an excited helium or argon gas stream react with analytes transforming them into gaseous ions. DART is mainly used for ionization of relatively non-polar compounds with molecular weight lower than 1500 Da. Due to the fact that DART ion source is available commercially, it has become the most commonly adopted AIMS technique in the world. For cholesterol, signal at m/z 369 corresponding to dehydrated molecule ions [M-H2O+H]+ can be observed.
A rapid method, based on DART MS, was developed for quantitation of cholesterol in egg-containing food products such as dried eggs or egg pasta [26]. Following simple solvent extraction, samples were introduced to the ionization region using “Dip-it” tips. The sampling tips were immersed into vials containing sample solutions and transferred to the optimized position on the holder. The mounted tips were sequentially exposed to helium gas stream for ionization of analytes. Cholesterol-D6 was used as internal standard for quantification. Although the LOD for cholesterol in real samples (0.03 mg/g) was not as low as for conventional GC-FID analysis (0.002 mg/g), this new methodology based on DART is much simpler and faster. Capabilities for quantitative analysis of the method are suitable for preliminary screening.
4.3. Direct analysis in real time mass spectrometry of serum cholesterol
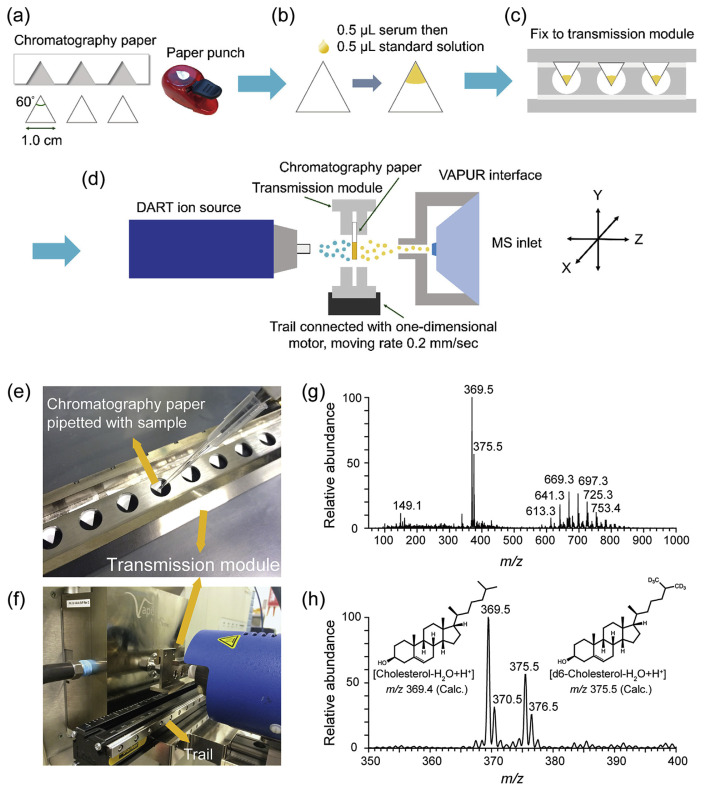
A method for rapid and cost-effective screening of endogenous free cholesterol in human serum samples was presented using DART ion source (see Fig. 6) by Hsieh et al. [41]. Small volume of the serum (0.5 μL) was loaded onto a tiny piece of chromatography paper followed by loading the same volume of internal standard (Fig. 6a,b). Subsequently, the paper substrates were mounted on the transmission module that was moved on the trail by a motor towards DART ionization region for mass spectrometric interrogation (Fig. 6c–f). Such methodology was abbreviated as pDART-MS. Cholesterol from serum was efficiently desorbed from the paper substrate and rapidly reacted with the plasma-induced metastable species. Signal at m/z 369.5, corresponding to dehydrated cholesterol, was the dominant signal in the mass range of 50–1000 m/z (Fig. 6g–h). The optimized method enabled the detection of cholesterol at the level of around 20 μg/mL in serum. The concentration of cholesterol in pooled serum samples was estimated to be 250 μg/mL and it was in very close agreement with the results obtained by LC-MS and fluorometric-enzymatic assays. Using this technology, concentration of cholesterol in each serum samples could be assessed within only 1 min. Therefore, large number of samples can be analyzed in short period of time. Presented methodology was applied to test blood samples collected from 21 recreational runners participating in the ultramarathon race. As a result, pDART-MS demonstrates a potential complementary tool for cholesterol tests in clinical laboratories and could possibly be translated to the use for POCT.
Fig. 6.
Workflow of pDART-MS for serum cholesterol quantification: (a) cutting chromatography papers, (b) loading sample and internal standard onto paper, (c) fixing paper substrate with loaded sample on the transmission module, (d) scanning across the samples. Experimental setup of pDART-MS: (e) and (f). Typical mass spectra of serum samples: (g) positive ion mode spectrum of a mixture of serum sample and cholesterol-D6, (h) magnification of the mass spectrum. Adapted with permission from Ref. [41].
5. Mass spectrometry imaging of cholesterol in biological tissues
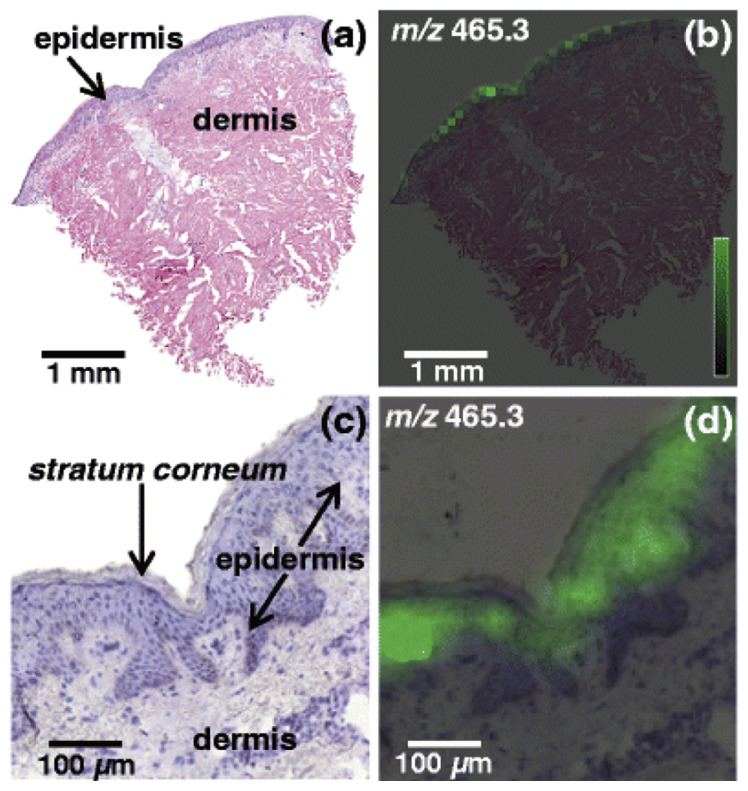
Visualizing the distribution of molecules in biological tissues enables the understanding of physiological processes and disease pathology [57,58]. The first generation of molecular imaging of tissue sections mainly targets for proteins and is largely relied on the use of immunohistochemistry [59]. However, using this traditional method it was difficult to assess spatial distribution of other compounds such as lipids. However, the second generation of molecular imaging, mass spectrometry imaging (MSI) in specific, aims for determining in situ spatial localization of a wide variety of molecules including metabolites, lipids, and polypeptides. MALDI-MSI was formally introduced in 1997 by Caprioli group and has soon become an effective and reliable technique [60]. MALDI-MSI uses organic matrix to cover tissue sections so as to facilitate ablation and ionization of biomolecules. However, matrix causes interferences in low mass range and therefore imaging of low molecular weight species is troublesome and therefore MALDI-MSI was mostly employed to localize peptides and proteins [60]. MALDI-MSI was also used to determine localization of endogenous cholesterol sulfate, which is formed from cholesterol by steroid sulfotransferases, in human skin tissue sections [42]. For this purpose, corona discharge treatment was used to modify the indium–tin oxide-coated glass slide surface to improve adhesion of the tissue. Localization of cholesterol sulfate in skin tissue was demonstrated with spatial resolution of 30 μm (see Fig. 7). Cholesterol sulfate was found to be present predominantly in the epidermis since it plays an important role in regulation of the barrier formation.
Fig. 7.
Localization of cholesterol sulfate in human skin tissue section. (a) H&E stained section, (b) MALDI-MSI imaging result for m/z 465.3, (c) magnification of H&E stained section, (d) magnification of MALDI-MSI result. Reprinted with permission from Ref. [42].
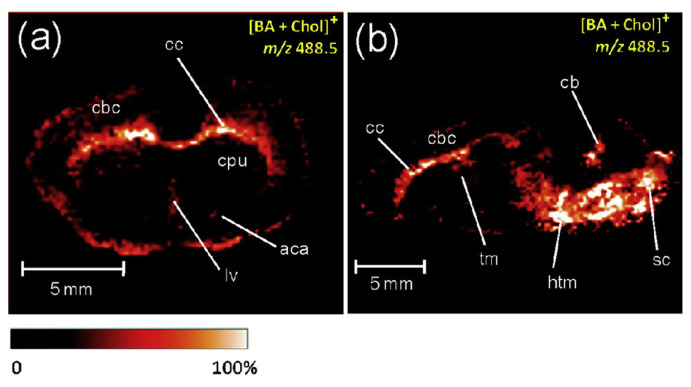
Compared to MALDI-MSI, DESI-MSI works under ambient conditions and therefore it allows interrogation of the sample in its native environment. It provides in situ, real time, and rapid mass spectrometric analysis. DESI-MSI does not require use of the matrix, allowing detection of low molecular weight species. Reactive DESI, incorporating betaine aldehyde (BA) into the DESI spray solvent to form easily ionized product (see Fig. 5b), was used for cholesterol imaging in rat brain with a spatial resolution of 200 μm [40]. Signal at m/z 488.5, corresponding to [BA+Chol]+ ion, had much higher intensity in the white matter as compared to the gray matter of rat brain (see Fig. 8).
Fig. 8.
Localization of cholesterol in rat brain section observed by reactive DESI. Distribution of the signal at m/z 488.5 in (a) coronal section and (b) sagittal section of rat brain. Reprinted with permission from Ref. [40].
In another study, DESI imaging was used to localize cholesterol sulfate in prostate cancer and normal tissue sections [22]. Cholesterol sulfate was identified as a differentiating compound between cancer and normal tissues. This compound was found almost exclusively in cancerous tissues, thus it is a promising biomarker candidate to serve as a tool for prostate cancer diagnosis.
6. Conclusions
Up to date, multiple analytical methods have been developed for analysis of cholesterol, including classical chemical methods, enzymatic assays, gas chromatography (GC), liquid chromatography (LC), and mass spectrometry (MS). Several golden standard methods have been established for clinical and industrial use. Nevertheless, new analytical platforms, such as ambient ionization mass spectrometry, are being developed and utilized. DART-MS was presented as a promising tool for rapid and cost-effective quantitation of cholesterol that could be possibly used in POCT. On the other hand, techniques such as MALDI-MSI and DESI-MSI were used to visualize spatial distribution of cholesterol and its derivatives in tissue sections. Mass spectrometry-based molecular imaging will increase the understanding of physiological processes and disease pathology towards to a more precise absolute quantitation of molecules in situ.
Acknowledgments
C.-C. H. would like to acknowledge funding supports from Ministry of Science and Technology, R.O.C. and Center for Emerging Materials and Advanced Devices, NTU. E. P. D. would like to acknowledge Dr. Yu-Liang Yang from the Agricultural Biotechnology Research Center, Academia Sinica, for the financial support to her postdoctoral research.
Funding Statement
C.-C. H. would like to acknowledge funding supports from Ministry of Science and Technology, R.O.C. and Center for Emerging Materials and Advanced Devices, NTU. E. P. D. would like to acknowledge Dr. Yu-Liang Yang from the Agricultural Biotechnology Research Center, Academia Sinica, for the financial support to her postdoctoral research.
Footnotes
Conflicts of interest
None.
Notes/Thanks/Other declarations
None.
REFERENCES
- 1. Norum KR, Berg T, Helgerud P, Drevon CA. Transport of cholesterol. Physiol Rev. 1983;63:1343–419. doi: 10.1152/physrev.1983.63.4.1343. [DOI] [PubMed] [Google Scholar]
- 2. Maekawa M, Fairn GD. Complementary probes reveal that phosphatidylserine is required for the proper transbilayer distribution of cholesterol. J Cell Sci. 2015;128:1422–33. doi: 10.1242/jcs.164715. [DOI] [PubMed] [Google Scholar]
- 3. Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035–78. doi: 10.1002/cphy.c120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faridi KF, Lupton JR, Martin SS, Banach M, Quispe R, Kulkarni K, et al. Vitamin D deficiency and non-lipid biomarkers of cardiovascular risk. Arch Med Sci. 2017;13:732–7. doi: 10.5114/aoms.2017.68237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauvais-Jarvis F, editor. Sex and gender factors affecting metabolic homeostasis, diabetes and obesity. Vol. 1043. Cham: Springer International Publishing; 2017. [DOI] [Google Scholar]
- 6. Zhou Y, Briand V, Sharma N, Ahn S, Kasi R. Polymers comprising cholesterol: synthesis, self-assembly, and applications. Materials (Basel) 2009;2:636–60. doi: 10.3390/ma2020636. [DOI] [Google Scholar]
- 7. Song Y, Kenworthy AK, Sanders CR. Cholesterol as a co-solvent and a ligand for membrane proteins. Protein Sci. 2014;23:1–22. doi: 10.1002/pro.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goluszko P, Nowicki B. Membrane cholesterol: a crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect Immun. 2005;73:7791–6. doi: 10.1128/IAI.73.12.7791-7796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alphonse PAS, Jones PJH. Revisiting human cholesterol synthesis and absorption: the reciprocity paradigm and its key regulators. Lipids. 2016;51:519–36. doi: 10.1007/s11745-015-4096-7. [DOI] [PubMed] [Google Scholar]
- 10. Cartocci V, Servadio M, Trezza V, Pallottini V. Can cholesterol metabolism modulation affect brain function and behavior? J Cell Physiol. 2017;232:281–6. doi: 10.1002/jcp.25488. [DOI] [PubMed] [Google Scholar]
- 11. Cortes VA, Busso D, Maiz A, Arteaga A, Nervi F, Rigotti A. Physiological and pathological implications of cholesterol. Front Biosci. 2014;19:416–28. doi: 10.2741/4216. [DOI] [PubMed] [Google Scholar]
- 12. Xu S, Liu Z, Liu P. HDL cholesterol in cardiovascular diseases: the good, the bad, and the ugly? Int J Cardiol. 2013;168:3157–9. doi: 10.1016/j.ijcard.2013.07.210. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Cleeman JI, Bairey Merz CN, Brewer HB, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the national cholesterol education Program adult treatment panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 14. Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–72. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu H, Han Q, Zhang D, Wang Y, Gao J, Geng W, et al. A diagnostic model for minimal change disease based on biological parameters. Peer J. 2018;6:e4237. doi: 10.7717/peerj.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics - 2017 update: a report from the american heart association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256:2823–8. doi: 10.1001/jama.256.20.2823. [DOI] [PubMed] [Google Scholar]
- 18. Ballantyne CM. Primary prevention of coronary heart disease. J Clin Endocrinol Metab. 2000;85:2089–92. doi: 10.1210/jcem.85.6.6642-1. [DOI] [PubMed] [Google Scholar]
- 19. Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cassader M, Ruiu G, Gambino R, Alemanno N, Veglia F, Pagano G. Hypercholesterolemia in non-insulin-dependent diabetes mellitus: different effect of simvastatin on VLDL and LDL cholesterol levels. Atherosclerosis. 1993;99:47–53. doi: 10.1016/0021-9150(93)90049-Z. [DOI] [PubMed] [Google Scholar]
- 21. Martin MG, Pfrieger F, Dotti CG. Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. 2014;15:1036–52. doi: 10.15252/embr.201439225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eberlin LS, Dill AL, Costa AB, Ifa DR, Cheng L, Masterson T, et al. Cholesterol sulfate imaging in human prostate cancer tissue by desorption electrospray ionization mass spectrometry. Anal Chem. 2010;82:3430–4. doi: 10.1021/ac9029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clayton ZS, Fusco E, Kern M. Egg consumption and heart health: a review. Nutrition. 2017;37:79–85. doi: 10.1016/j.nut.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 24. Rimpeekool W, Yiengprugsawan V, Kirk M, Banwell C, Seubsman S, Sleigh A. Nutrition label experience, obesity, high blood pressure, and high blood lipids in a cohort of 42,750 Thai adults. PloS One. 2017;12:e0189574. doi: 10.1371/journal.pone.0189574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park J-M, Jeong I-S, Kwak B-M, Ahn J-H, Leem D, Jeong J, et al. Application of rapid sample preparation method and monitoring for cholesterol content in chicken egg and egg powder. Korean J Food Sci Anim Resour. 2013;33:672–7. doi: 10.5851/kosfa.2013.33.5.672. [DOI] [Google Scholar]
- 26. Al-Balaa D, Rajchl A, Grégrová A, Ševčík R, Čížková H. DART mass spectrometry for rapid screening and quantitative determination of cholesterol in egg pasta. J Mass Spectrom. 2014;49:911–7. doi: 10.1002/jms.3465. [DOI] [PubMed] [Google Scholar]
- 27. Cohen A, Hertz HS, Mandel J, Paule RC, Welch MJ, White E, et al. Total serum cholesterol by isotope dilution/mass spectrometry: a candidate definitive method. Clin Chem. 1980;26:854–60. [PubMed] [Google Scholar]
- 28. Abell LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357–66. [PubMed] [Google Scholar]
- 29. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 30. Amundson DM, Zhou M. Fluorometric method for the enzymatic determination of cholesterol. J Biochem Biophys Meth. 1999;38:43–52. doi: 10.1016/S0165-022X(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 31. dos Santos Ferreira CE, França CN, Correr CJ, Zucker ML, Andriolo A, Scartezini M. Clinical correlation between a point-of-care testing system and laboratory automation for lipid profile. Clin Chim Acta. 2015;446:263–6. doi: 10.1016/j.cca.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 32. Kanai M. Gas chromatographic separation of sterols and its clinical application. J Biochem. 1964;56:266–72. doi: 10.1093/oxfordjournals.jbchem.a127988. [DOI] [PubMed] [Google Scholar]
- 33. Beggio M, Cruz-Hernandez C, Golay P-A, Lee LY, Giuffrida F. Quantification of total cholesterol in human milk by gas chromatography. J Sep Sci. 2018;41:1805–11. doi: 10.1002/jssc.201700833. [DOI] [PubMed] [Google Scholar]
- 34. Lian K, Zhang P, Wang W, Dai T, Li E. Determination of total cholesterol in serum by gas chromatography-mass spectrometry. Asian J Chem. 2014;26:2646–8. doi: 10.14233/ajchem.2014.15780. [DOI] [Google Scholar]
- 35. Duncan IW, Culbreth PH, Burtis CA. Determination of free, total, and esterified cholesterol by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1979;162:281–92. doi: 10.1016/S0378-4347(00)81515-7. [DOI] [PubMed] [Google Scholar]
- 36. Kock R, Delvoux B, Greiling H. Determination of total cholesterol in serum by liquid chromatography-isotope dilution mass spectrometry. Clin Chem. 1997;43:1896–903. [PubMed] [Google Scholar]
- 37. Liebisch G, Binder M, Schifferer R, Langmann T, Schulz B, Schmitz G. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS) Biochim Biophys Acta Mol Cell Biol Lipids. 2006;1761:121–8. doi: 10.1016/j.bbalip.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38. Hidaka H, Hanyu N, Sugano M, Kawasaki K, Yamauchi K, Katsuyama T. Analysis of human serum lipoprotein lipid composition using MALDI-TOF mass spectrometry. Ann Clin Lab Sci. 2007;37:213–21. [PubMed] [Google Scholar]
- 39. Xu L, Kliman M, Forsythe JG, Korade Z, Hmelo AB, Porter NA, et al. Profiling and imaging ion mobility-mass spectrometry analysis of cholesterol and 7-dehydrocholesterol in cells via sputtered silver MALDI. J Am Soc Mass Spectrom. 2015;26:924–33. doi: 10.1007/s13361-015-1131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu C, Ifa DR, Manicke NE, Cooks RG. Rapid, direct analysis of cholesterol by charge labeling in reactive desorption electrospray ionization. Anal Chem. 2009;81:7618–24. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh H-Y, Li L-H, Hsu R-Y, Kao W-F, Huang Y-C, Hsu C-C. Quantification of endogenous cholesterol in human serum on paper using direct analysis in real time mass spectrometry. Anal Chem. 2017;89:6146–52. doi: 10.1021/acs.analchem.7b00943. [DOI] [PubMed] [Google Scholar]
- 42. Enthaler B, Pruns JK, Wessel S, Rapp C, Fischer M, Wittern KP. Improved sample preparation for MALDI–MSI of endogenous compounds in skin tissue sections and mapping of exogenous active compounds subsequent to ex-vivo skin penetration. Anal Bioanal Chem. 2012;402:1159–67. doi: 10.1007/s00216-011-5562-6. [DOI] [PubMed] [Google Scholar]
- 43. Abidi SL. Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A. 2001;935:173–201. doi: 10.1016/S0021-9673(01)00946-3. [DOI] [PubMed] [Google Scholar]
- 44. Dinh TTN, Thompson LD, Galyean ML, Brooks JC, Patterson KY, Boylan LM. Cholesterol content and methods for cholesterol determination in meat and poultry. Compr Rev Food Sci Food Saf. 2011;10:269–89. doi: 10.1111/j.1541-4337.2011.00158.x. [DOI] [Google Scholar]
- 45.Horwitz W. AOAC official method 976 26, cholesterol in multicomponent foods. In: William Horwitz, George Latimer JE., editors. Off Methods Anal AOAC Int – 18th ed Revis 3. 18th ed. AOAC International; 2010. [Google Scholar]
- 46.Horwitz W. AOAC official method 994 10, cholesterol in foods. In: William Horwitz, George Latimer JE., editors. Off Methods Anal AOAC Int – 18th ed Revis 3. 18th ed. AOAC International; 2010. [Google Scholar]
- 47.ISO 18252:2006(en) Anhydrous milk fat - determination of sterol composition by gas liquid chromatography (routine method) 2006. [Google Scholar]
- 48. Owen WE, Thatcher ML, Crabtree KJ, Greer RW, Strathmann FG, Straseski JA, et al. Body fluid matrix evaluation on a Roche cobas 8000 system. Clin Biochem. 2015;48:911–4. doi: 10.1016/j.clinbiochem.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 49. Paital B. A modified fluorimetric method for determination of hydrogen peroxide using homovanillic acid oxidation principle. Biomed Res Int. 2014;2014:342958. doi: 10.1155/2014/342958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. MacLachlan J, Wotherspoon AT, Ansell RO, Brooks CJ. Cholesterol oxidase: sources, physical properties and analytical applications. J Steroid Biochem Mol Biol. 2000;72:169–95. doi: 10.1016/S0960-0760(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 51. Takatsu A, Nishi S. Total cholesterol in serum determined by isotope dilution/mass spectrometry, with liquid-chromatographic separation. Clin Chem. 1987;33:1113–7. [PubMed] [Google Scholar]
- 52. Li A, Hollerbach A, Luo Q, Cooks RG. On-demand ambient ionization of picoliter samples using charge pulses. Angew Chem Int Ed. 2015;54:6893–5. doi: 10.1002/anie.201501895. [DOI] [PubMed] [Google Scholar]
- 53. Meier F, Garrard KP, Muddiman DC. Silver dopants for targeted and untargeted direct analysis of unsaturated lipids via infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) Rapid Commun Mass Spectrom. 2014;28:2461–70. doi: 10.1002/rcm.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu CC, Dorrestein PC. Visualizing life with ambient mass spectrometry. Curr Opin Biotechnol. 2015;31:24–34. doi: 10.1016/j.copbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–3. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 56. Cody RB, Laramée JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 57. Miyamoto S, Hsu CC, Hamm G, Darshi M, Diamond-Stanic M, Declèves AE, et al. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 2016;7:121–34. doi: 10.1016/j.ebiom.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hsu C-C, Chou P-T, Zare RN. Imaging of proteins in tissue samples using nanospray desorption electrospray ionization mass spectrometry. Anal Chem. 2015;87:11171–5. doi: 10.1021/acs.analchem.5b03389. [DOI] [PubMed] [Google Scholar]
- 59. Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70:46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 60. Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–60. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]