Abstract
Benzo [a]pyrene (BaP) is a model compound for the study of polycyclic aromatic hydrocarbon (PAH) carcinogenesis. Upon metabolism, BaP is metabolized to the ultimate metabolite, BaP trans-7,8-diol-anti-9,10-epoxide (BPDE), that reacts with cellular DNA to form BPDE-dG adducts responsible for BaP-induced mutagenicity, carcinogenicity, and teratogenicity. In this study, we employed our developed LC-MS/MS method to detect and quantity BPDE-dG adducts present in 42 normal human umbilical cord blood samples and 42 birth defect cases. We determined that there is no significant difference in the level of BPDE-dG formation between the normal and birth defect groups. This represents the first time to use an LC-MS/MS method to quantify BPDE-dG in human umbilical blood samples. The results indicated that under experimental conditions, BPDE-dG adducts were detected in all the human umbilical cord blood samples from the normal and birth defect groups.
Keywords: BPDE-dG, Human biomonitoring, LC-MS; MS, Molecular epidemiology
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous carcinogenic environmental pollutants present in the environment, e.g. air, water, soil and sediment [1–5]. PAHs are present in tobacco smoke in high concentrations, in foods, and occupational exposure [6,7]. PAHs induce malignant tumors in rodents, including mammary gland, lung, bladder, and skin [8–10]. International Agency for Research of Cancer (IARC) classified a group of PAHs as Group 1 human carcinogens, Group 2A probably carcinogens, or Group 2B possible human carcinogens [2,3]. It has also been reported that consumption of PAHs in foods increases risks of colorectal adenoma and pancreatic cancer [2,3,11–14].
Benzo [a]pyrene (BaP) is a model compound for the study of PAH carcinogenesis. Carcinogenic PAHs, including BaP, require metabolic activation to exert mutagenicity, teratogenicity, and carcinogenicity. BaP is metabolized to form the ultimate metabolite, trans-7,8-dihydroxy-anti-9, 10-epoxy-7, 8, 9, 10-tetrahydrobenzo [a]pyrene (BPDE). BPDE can bind with cellular DNA to form BPDE-DNA adducts, with 10- (deoxyguanosin-N2-yl)-7, 8, 9-trihydroxy-7,8,9,10-tetrahydrobenzo [a]pyrene (BPDE-dG) as a major DNA adduct [5]. Therefore, development of sensitive and reliable analytical methods to identify and quantitate BPDE-dG adducts in human samples is required for molecular epidemiological studies [15–18].
Molecular epidemiology studies have evidenced that the fetus is more susceptible than the adults to the toxic effects of PAHs and other toxicants [19–22]. The previous studies have suggested that the developing fetus may be 10 times more susceptible than the mother to PAH-induced DNA damage [20]. Perera et al. measured PAH-DNA and other bulky aromatic adducts in umbilical cord white blood cells using the 32P-postlabeling assay [20]. They determined the association between this molecular dosimeter and behavioral attention problems in childhood and the results suggested that PAH exposure, measured by DNA adducts, may adversely affect child behavior, potentially affecting school performance [20].
In this study, we measured the BPDE-dG adducts in the DNA of umbilical cord white blood cell samples from a subset of birth cohort. A total of 84 umbilical cord white blood cell samples, with 42 birth defect cases and 42 normal cases, were studied.
2. Materials and methods
2.1. Chemicals
[15N5]-2′-Deoxyguanosine was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Calf thymus DNA, deoxyribonuclease I from bovine pancreas (DNase I), phosphodiesterase I from Crotalusadamanteus venom (phosphodiesterase I), phosphatase, alkaline from Escherichia coli (alkaline phosphatase) were obtained from Sigma–Aldrich Corp. (St. Louis, MO, USA). BPDE, synthesized by reaction of BaP trans-7,8-dihydrodiol with DDQ [23,24], was kindly provided by Dr. Peter P. Fu of the National Center for Toxicological Research (NCTR). All solvents were HPLC grade.
2.2. Umbilical cord white blood samples
Umbilical cord white blood samples were collected between November 2013 and June 2014 from pregnant women enrolled from the local hospital of the Shenqiu county, Henan province (Huaihe River Basin). A total of 84 human umbilical cord blood samples, including 42 birth defect cases and 42 normal cases, were randomly selected from a subset of birth cohort. Before umbilical cord blood was obtained, the pregnant women were required to sign a consent form after receiving a detailed explanation of the study. Approximately 25–30 mL of umbilical cord blood was collected in SST coagulation tubes at the time of delivery, and the centrifuged plasma samples were stored at −80 °C before use. This study was reviewed and approved by the Ethics Committee of the National Institute for Occupational Health and Poison Control, Chinese Center for Disease Control and Prevention.
2.3. DNA isolation from umbilical cord blood
Umbilical cord white blood cell DNA preparation were collected in vacuum blood collection tube with EDTA-K2 (Becton, Dickinson and Company, USA) (0.05 M sodium citrate, pH 7.0), cooled to 4–8 °C and followed by centrifugation (2900 rpm, 20 min). Buffy coat (PBMC, mainly leukocytes) and plasma fractions were separated and stored at −80 °C for further analyses. The isolation of genomic DNA from buffy coats was achieved using the DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol except for the modification of the supplied pH to 7.4 and adjusting the NaCl concentration in the elution buffer to 1.4 M.
2.4. Synthesis of BPDE-dG and [15N5]BPDE-dG standards
BPDE-dG was synthesized followed the protocol reported earlier by Beland et al. with minor modifications [25]. Briefly, BPDE (3.0 mg) was dissolved in 5.0 mL THF and added 18 mg of 2′-deoxyguanosine (dG) dissolved in 1.0 mM Tris–HCl buffer (pH 6.8). The mixture was incubated 37 °C for 4 h and extracted sequentially with 10 mL of water saturated n-butanol and washed with 10 mL of n-butanol saturated water three times respectively. The n-butanol extracts were evaporated and the residue was dissolved in methanol and purified by reverse phase HPLC using linear gradient program with acetonitrile (A) and 0.1% formic acid (B) as follows: 0–5 min, 5% A isocratic; 5–30 min, 5%–35% A in B; 30–35 min, 35%–90% A in B; 35–40 min, 90% A isocratic; 40–41 min, 90%–5% A in B; 41–50 min, 5% A isocratic. The flow rate was 0.3 mL/min. The purified BPDE-dG was quantitated by UV spectrometry using the absorption coefficients as an external standard [26]. [15N5] BPDE-dG was synthesized by the reaction of BPDE with [15N5] 2′-deoxyguanosine ([15N5]dG) followed by same procedures described above as an internal standard (I.S.). The purified [15N5]BPDE-dG was quantitated by UV spectrometry using the absorption coefficients [26].
2.5. LC-MS/MS analysis for BPDE-dG adduct in umbilical cord white blood cell samples
LC-MS/MS analysis of BPDE-dG adduct was conducted following the protocol reported earlier with minor modifications [25]. Briefly, umbilical cord white blood cell DNA (20 μg) was added 10 μL DNase I (0.5 unit/μL) and 10 pg of [15N5]BPDE-dG as I.S. for correcting recovery of BPDE-dG adduct. The mixture was incubated at 37 °C for 3 h. After incubation, the mixture was added 10 μL phosphodiesterase I (0.0002 unit/μL) and 10 μL alkaline phosphatase (0.004 unit/μL). After incubation at 37 °C for 4 h, the mixture was extracted by adding 500 μL of water-saturated n-butanol three times. The nbutanol extracts were evaporated and dissolved in 50 μL of methanol and injected into LC-MS/MS for BPDE-dG adduct analysis. The liquid chromatography handling system consisted of a Thermo TSQ Quantum Access Max module (Thermo Fisher, Milford, MA, USA) connecting with a Hypersil Gold 1.9 μm C18 column (100 × 2.10 mm, Thermo Fisher, Milford, MA, USA). Each sample was eluted at 0.35 mL/min using a linear program with acetonitrile (A) and 0.1% formic acid (B) gradient as follows: 0–1 min, 5% A isocratic; 1–1.5 min, 5–20% A in B; 1.5–20 min, 20–28% A in B; 20–21 min, 28–90% A in B; 21–22 min, 90% A isocratic; 22–22.5 min, 90–5% A in B; 22.5–24 min, 5% A isocratic. A Thermo TSQ Access Max mass spectrometer (Thermo Fisher, Milford, MA, USA) equipped with an HESI interface, was used with a spray voltage of 3500 v, a vaporizer temperature of 300 °C and a capillary temperature of 270 °C. Nitrogen was the sheath gas (45 arb), aux gas (10 arb), and collision gas (0.12 mL/min). Positive ions were acquired in the multiple reaction monitoring (MRM) mode (collision energy, 10 eV).
2.6. Standard calibration curve
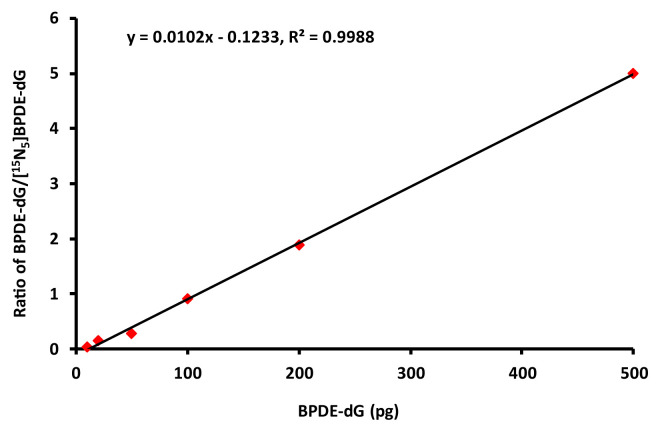
Standard curve was generated by plotting the amounts of standard compounds against peak area. Each sample was tested at 10 μL injection volume containing 10 pg [15N5]BPDE-dG internal standard. The calibration curve was linear over the concentration range of 1–500 pg BPDE-dG. The best linear fit and least variability for the calibration curve was achieved with a weighting factor of 1/X, with the correlation coefficients (r2) above 0.99 (Fig. 1).
Fig. 1.
Standard calibration curve of BPDE-dG.
3. Results
3.1. Analysis of BPDE-dG and [15N5]BPDE-dG adducts
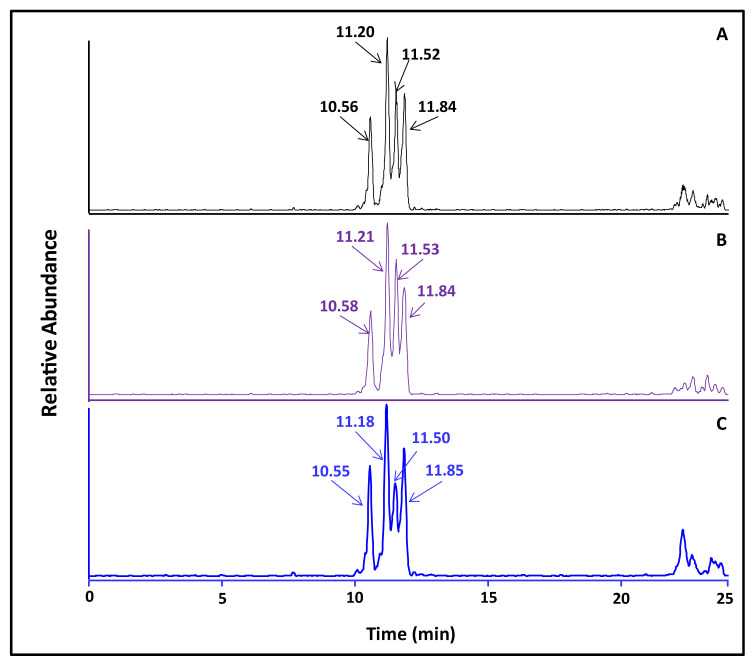
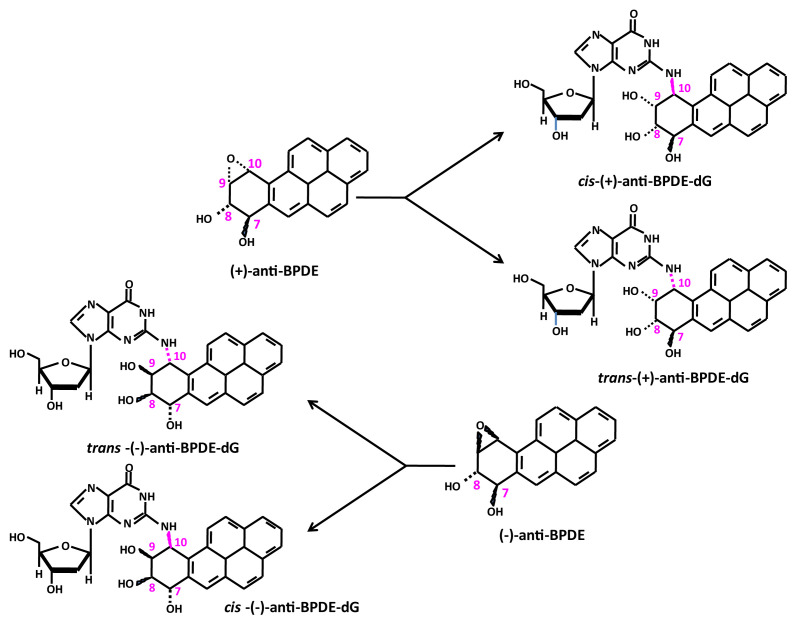
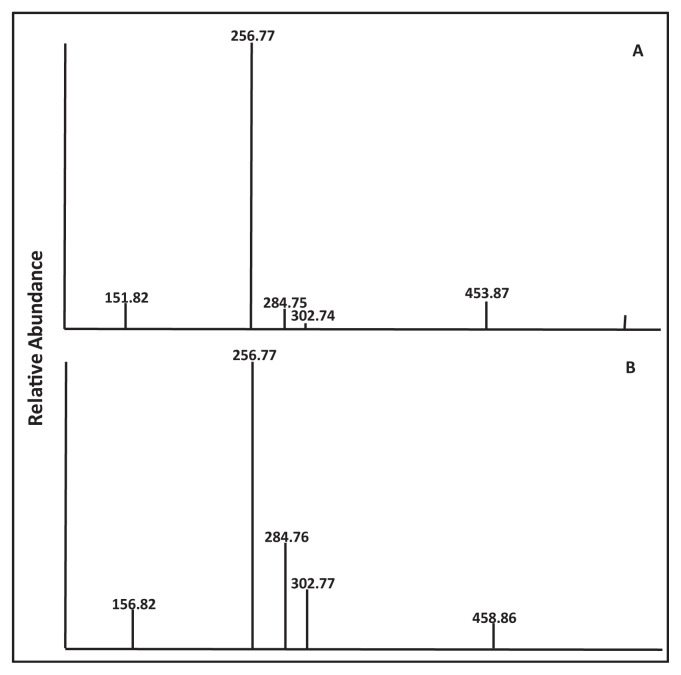
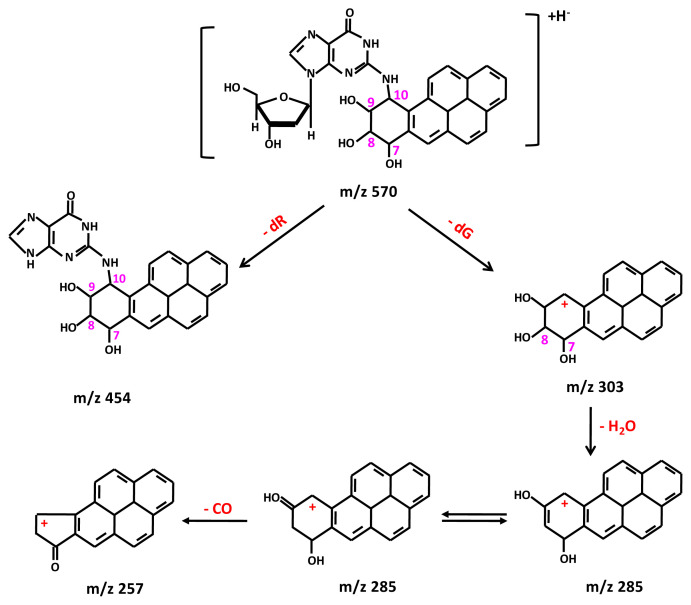
Following the method of Beland et al. with modification [25], reaction of BPDE in THF with 2′-deoxyguanosine dissolved in Tris–HCl buffer (pH 6.8) at 37 °C for 4 h resulted in the formation of four enantiomeric BPDE-dG adducts, with the retention times at 10.56, 11.20, 11.52, and 11.84 min, respectively (Fig. 2). The structures of these enantiomeric BPDEdG adducts are cis-(+)-anti-BPDE-dG; trans-(−)-anti-BPDE-dG; cis- (−)-anti-BPDE-dG, and trans-(+)-anti-BPDE-dG, respectively (Fig. 3). These BPDE-dG adducts had a protonated molecule [(M + H)+] at m/z 570 (data not shown). The full scan LC-MS/MS spectrometric analysis indicated that these adducts all had identical fragment ions pattern, with fragment ions atm/z 454, m/z 303, m/z 285, m/z 257 and m/z 152 (Fig. 4A). The mass spectrum of [15N5]BPDE-dG shown in Fig. 2B also exhibited four chromatographic peaks, with the retention times at 10.58, 11.21, 11.53, and 11.84 min, respectively.
Fig. 2.
LC-MS/MS chromatographic profiles of BPDE-dG adducts: (A) BPDE-dG standard; (B) [15N5]BPDE-dG internal standard; and (C) BPDE-dG detected in cord blood cell DNA. 10 pg of [15N5]BPDE-dG was added to each sample as an internal standard for correcting recovery of each sample.
Fig. 3.
Structures of four enantiomeric of BPDE-dG adducts formed from organic reactions.
Fig. 4.
Mass spectra of synthesized standards: (A) BPDEdG; and (B) [15N5]–BPDE-dG.
Product ion analysis of BPDE-dG gave fragments consistent with sequential loss of deoxyguanosine (dG) (m/z 303), loss of H2O (m/z 285), and loss of CO (m/z 257) as well as the fragment indicative of guanine (m/z 152) (Fig. 4A). The structures of the fragment ions from BPDE-dG are shown in Fig. 5. Similarly, in the full scan mass spectrum of [15N5]BPDE-dG, four chromatographic peaks were also observed (Fig. 4B). The fragment ion at m/z 459 is the loss of a ribose [(M-ribose)+].
Fig. 5.
Structures of protonated molecular ions of BPDE-dG and its fragment ions formed from the LC-MS/MS full scan of BPDE-dG.
3.2. Response curve of LC-MS/MS analysis
The substrate concentration-dependent response curve of LC-MS/MS analysis was obtained by adding 2.5, 5, 10, 15, 20 and 25 pg of BPDE-dG standards in calf thymus DNA samples and each added 10 pg of [15N5]BPDE-dG as internal standards. After incubation and enrichment, these single calf thymus DNA samples were then subjected into LC-MS/MS (data not shown). There is a linear correlation between the quantity of BPDE-dG and area ratios of BPDE-dG/[15N5]BPDE-dG), within the range studied. It was determined that the limit of detection (s/n = 3) of BPDE-dG is 2.7 BPDE-dG/109 dG. BPDE-dG in an aliquot equivalent about 10 μg of DNA could be detected.
3.3. Reproducibility of LC-MS/MS method
The LC-MS/MS method was validated with respect to intra-assay and inter-assay by adding 5 pg and 10 pg of [15N5] BPDE-dG as internal standards in calf thymus DNA. The calf thymus DNA samples were injected into LC-MS/MS at the same time for the intra-assay. The relative standard deviation (RSD) was 13.05%. For the inter-assay, the calf thymus DNA samples were injected into LC-MS/MS with a 24 h interval, RSD was 23.1%.
3.4. Recovery of LC-MS/MS method
The recovery of LC-MS/MS method was achieved by adding 5 pg and 10 pg of [15N5]BPDE-dG in calf thymus DNA, respectively. These single DNA samples were injected into LC-MS/MS for the calculation of the recovery. The recovery of [15N5]BPDE-dG with adding 5 pg ranged from 74.6% to 89.6% and the mean recovery was 83.7% ± 6.1, RSD was 9.54%. The recovery of [15N5]BPDE-dG with adding 10 pg ranged from 80.6% to 96.4%, mean recovery was 88.3% ± 5.4, RSD was 8.96%.
3.5. Analysis of BPDE-dG adduct in umbilical cord white blood cell DNA samples
Analysis of umbilical cord white blood cell DNA samples was conducted by LC-MS/MS method, with 84 human umbilical cord blood samples, including 42 birth defect cases and 42 normal cases, randomly selected from a subset of birth cohort. The results are summarized in Table 1. A representative autoradiogram of BPDE-dG detected in human umbilical cord blood samples is shown in Fig. 2C. The results indicate that there is no significant difference in the level of BPDE-dG adducts formed from the normal and the birth defect groups.
Table 1.
Detection of BPDE-DNA adduct in human umbilical cord blood by LC/MS analysis.
| Case ID (healthy) | BPDE-dG/108 nucleotides | Case ID (birth defect) | Type of defect | BPDE-dG/108 nucleotides |
|---|---|---|---|---|
| 0737002 | 0.27 | 0409019 | pigmented nevus | 0.27 |
| 1118001 | 0.27 | 0712004 | cleft lip | 0.27 |
| 1701001 | 0.27 | 1020001 | melanoma | 0.27 |
| 2004001 | 0.27 | 1908004 | umbilical hernia | 0.27 |
| 2023003 | 0.27 | 0523005 | hemangioma | 0.84 |
| 0408006 | 1.04 | 0524005 | fissura auris congenita | 1.04 |
| 0409002 | 0.98 | 0529007 | locus coeruleus buttock | 0.96 |
| 0409003 | 1.01 | 0536002 | pigmented nevus | 0.84 |
| 0523001 | 0.84 | 0702003 | pigmented nevus | 0.96 |
| 0524002 | 1.04 | 0718011 | fissura auris congenita | 0.96 |
| 0529001 | 0.82 | 0724004 | polydactyly | 0.93 |
| 0529002 | 0.87 | 0913005 | strephexopodia | 0.96 |
| 0536003 | 0.93 | 1009001 | fissura auris congenita | 1.01 |
| 0706002 | 0.96 | 1013001 | pigmented nevus | 0.98 |
| 0706004 | 0.96 | 1122001 | accessory auricles, hydrocele of tunica vaginalis | 0.96 |
| 0712002 | 0.82 | 1210001 | fissura auris congenita | 0.98 |
| 0718003 | 0.82 | 1318002 | pigmented nevus | 0.87 |
| 0724002 | 0.96 | 1605004 | fissura auris congenita | 0.96 |
| 0913001 | 0.82 | 1610001 | pigmented nevus | 1.01 |
| 0913002 | 0.82 | 1702001 | pigmented nevus | 0.96 |
| 1014001 | 0.82 | 1702003 | hypospadias | 0.98 |
| 1030005 | 1.01 | 1712003 | fissura auris congenita | 0.96 |
| 2406002 | 0.98 | 2003004 | locus coeruleus buttock | 1.01 |
| 1111001 | 1.01 | 2018001 | locus coeruleus buttock | 1.04 |
| 1210002 | 0.98 | 2222006 | aural deformity, accessory auricles | 1.01 |
| 1605001 | 0.79 | 2406003 | accessory auricles | 1.01 |
| 1605002 | 0.93 | 2229003 | fissura auris congenita | 0.96 |
| 1610002 | 0.93 | 2304001 | ectrodactyly (left) | 0.98 |
| 1703001 | 1.01 | 2304003 | chin deformity (right) | 0.98 |
| 1908001 | 0.87 | 2318001 | fissura auris congenita | 0.98 |
| 2017001 | 0.93 | 2321002 | hemangioma | 1.01 |
| 2022001 | 0.98 | 2401002 | hydrocele of tunica vaginalis | 0.98 |
| 2027001 | 0.96 | 2228002 | hydrocele of tunica vaginalis | 1.10 |
| 2027002 | 0.84 | 0724001 | down’s syndrome | 1.12 |
| 2303002 | 1.01 | 0706005 | pigmented nevus | 1.21 |
| 2315003 | 0.87 | 1041005 | fissura auris congenita | 1.29 |
| 2321001 | 0.82 | 1107002 | pigmented nevus | 1.35 |
| 2406001 | 0.82 | 0902005 | pigmented nevus | 1.41 |
| 1041001 | 1.18 | 2222005 | retained testicle | 1.43 |
| 2402001 | 1.15 | 0408004 | pigmented nevus (black) | 2.53 |
| 1713001 | 1.27 | 1032001 | pigmented nevus (black) | 6.38 |
| 1023001 | 1.55 | 0736002 | cleft lip | 11.84 |
4. Discussion
PAHs are mutagenic, teratogenic, and carcinogenic widespread environmental pollutants [1–7]. BPDE, the ultimate metabolite of BaP, covalently bound with DNA to form four enantiomeric BPDE-dG adducts in vivo (Fig. 2). The biological activities of trans-BPDE-dG enantiomers are extensively studied than the cis enantiomers [27,28]. Several analytical methods, including 32P-postlabeling, immunoassay, and LCMS/MS-based methods, have been used for the analysis of BPDE-DNA adducts [29–32]. Among which, the best analytical method is the LC-MS/MS. The recent improvements in LC-MS/MS method have been utilized in high-throughput applications for BPDE-DNA adducts analysis [33–35]. To analyze BPDE-dG adducts in small amounts in the umbilical cord white blood cell DNA, we developed an ultrasensitive LC-MS/MS method that is highly sensitive. The improved method is based on the effective purification and enrichment of BPDE-dG adducts in human umbilical cord blood samples followed by solvent extraction to efficiently enrich BPDE-dG adducts in cord blood samples. The sensitivity of this developed method can be applied to use human cord blood cell DNA in a quantity as low as ~10 μg of DNA. For improving the reliability of the method, [15N5]BPDE-dG was added to each human umbilical cord blood sample as internal standards allowing the good correction of the recovery.
Our study represents the first time to use the LC-MS/MS method to quantify BPDE-dG adducts in human umbilical cord blood samples. As the data shown in Table 1, the levels of BPDE-DNA adducts present in 42 normal human umbilical cord blood samples and the 42 birth defect cases are generally close, with the adduct level in only three birth defect cases a little higher than all the others. Since only one measurement was conducted for each sample, it is reasonably to conclude that there is no significant difference in the BPDE-dG formation between the normal and the birth defect groups.
It is important to point out that all the 82 human umbilical cord blood samples, from both normal and birth defect groups, contained BPDE-dG adducts. Thus, it is important to determine whether this is a general phenomenon, or a specific case. This warrants further investigation.
Acknowledgement
The research work was supported by National Science and Technology Infrastructure Program, China (2013BAI12B03).
Funding Statement
The research work was supported by National Science and Technology Infrastructure Program, China (2013BAI12B03).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
REFERENCES
- 1. Phillips DH. Fifty years of benzo (a) pyrene. Nature. 1983;303(5917):468–72. doi: 10.1038/303468a0. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer. Polynuclear aromatic compounds, part 1, chemical, environmental, and experimental data. IARC (Int Agency Res Cancer) Monogr Eval Carcinog Risk Chem Hum. 1983;32:1–453. [PubMed] [Google Scholar]
- 3. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 4. Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–88. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. 1999 Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91(14):1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 6. Larsson BK, Sahlberg GP, Eriksson AT, Busk LA. Polycyclic aromatic hydrocarbons in grilled food. J Agric Food Chem. 1983;31(4):867–73. doi: 10.1021/jf00118a049. [DOI] [PubMed] [Google Scholar]
- 7. Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2001;45(1):3–13. [PubMed] [Google Scholar]
- 8. Cavalieri EL, Rogan EG, Higginbotham S, Cremonesi P, Salmasi S. Tumor-initiating activity in mouse skin and carcinogenicity in rat mammary gland of dibenzo [a] pyrenes: the very potent environmental carcinogen dibenzo [a,l] pyrene. J Cancer Res Clin Oncol. 1989;115(1):67–72. doi: 10.1007/BF00391602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo [a] pyrene in a 2-year bioassay. Carcinogenesis. 1998;19(1):117–24. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein LS, Weyand EH, Safe S, Steinberg M, Culp SJ, Gaylor DW, et al. Tumors and DNA adducts in mice exposed to benzo [a] pyrene and coal tars: implications for risk assessment. Environ Health Perspect. 1998;106(Suppl 6):1325–30. doi: 10.1289/ehp.98106s61325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kriek E, Rojas M, Alexandrov K, Bartsch H. Polycyclic aromatic hydrocarbon-DNA adducts in humans: relevance as biomarkers for exposure and cancer risk. Mutat Res. 1998;400(1–2):215–31. doi: 10.1016/s0027-5107(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 12. Li D, Firozi PF, Wang LE, Bosken CH, Spitz MR, Hong WK, et al. Sensitivity to DNA damage induced by benzo(a)pyrene diol epoxide and risk of lung cancer: a case-control analysis. Cancer Res. 2001;61(4):1445–50. [PubMed] [Google Scholar]
- 13. Gyorffy E, Anna L, Gyori Z, Segesdi J, Minárovits J, Soltész I, et al. DNA adducts in tumour, normal peripheral lung and bronchus, and peripheral blood lymphocytes from smoking and non-smoking lung cancer patients: correlations between tissues and detection by 32P-postlabelling and immunoassay. Carcinogenesis. 2004;25(7):1201–9. doi: 10.1093/carcin/bgh131. [DOI] [PubMed] [Google Scholar]
- 14. Bosetti C, Boffetta P, La Vecchia C. Occupational exposures to polycyclic aromatic hydrocarbons, and respiratory and urinary tract cancers: a quantitative review to 2005. Ann Oncol. 2007;18(3):431–46. doi: 10.1093/annonc/mdl172. [DOI] [PubMed] [Google Scholar]
- 15. Yang SK, McCourt DW, Leutz JC, Gelboin HV. Benzo [a] pyrene diol epoxides: mechanism of enzymatic formation and optically active intermediates. Science. 1977;196(4295):1199–201. doi: 10.1126/science.870975. [DOI] [PubMed] [Google Scholar]
- 16. Shimada T, Gillam EM, Oda Y, Tsumura F, Sutter TR, Guengerich FP, et al. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7, 8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem Res Toxicol. 1999;12(7):623–9. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- 17. Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos. 2001;29(9):1176–82. [PubMed] [Google Scholar]
- 18. Kramata P, Zajc B, Sayer JM, Jerina DM, Wei CS. A single site-specific trans-opened 7, 8, 9, 10-tetrahydrobenzo [a] pyrene 7, 8-diol 9, 10-epoxide N2-deoxyguanosine adduct induces mutations at multiple sites in DNA. J Biol Chem. 2003;278(17):14940–8. doi: 10.1074/jbc.M211557200. [DOI] [PubMed] [Google Scholar]
- 19. Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children’s health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect. 2000;108(suppl 3):573–94. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 21. Perera FP, Tang D, Jedrychowski W, Hemminki K, Santella RM, Cruz LA, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004;112:1133–6. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, et al. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect. 2011;119(8):1176–81. doi: 10.1289/ehp.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu PP, Harvey RG. Synthesis of the diols and diolepoxides of carcinogenic hydrocarbons. Tetrahedron Lett. 1977:2059–62. [Google Scholar]
- 24. Fu PP, Harvey RG. Dehydrogenation of polycyclic hydroaromatic compounds. Chem Rev. 1978;78:317–61. [Google Scholar]
- 25. Beland FA, Churchwell MI, Von Tungeln LS, Chen S, Fu PP, Culp SJ, et al. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo [a] pyrene–DNA adducts. Chem Res Toxicol. 2005;18(8):1306–15. doi: 10.1021/tx050068y. [DOI] [PubMed] [Google Scholar]
- 26. Pulkrabek P, Leffler S, Weinstein IB, Grunberger D. Conformation of DNA modified with a dihydrodiol epoxide derivative of benzo[a]pyrene. Biochemistry. 1977;16:3127–32. doi: 10.1021/bi00633a014. [DOI] [PubMed] [Google Scholar]
- 27. Buening MK, Wislocki PG, Levin W, Yagi H, Thakker DR, Akagi H, et al. Tumorigenicity of the optical enantiomers of the diastereomeric benzo [a] pyrene 7, 8-diol-9, 10-epoxides in newborn mice: exceptional activity of (+)-7β, 8α-dihydroxy-9α, 10α-epoxy-7, 8, 9, 10-tetrahydrobenzo [a] pyrene. Proc Natl Acad Sci U S A. 1978;75(11):5358–61. doi: 10.1073/pnas.75.11.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geacintov NE, Cosman M, Hingerty BE, Amin S, Broyde S, Patel DJ. NMR solution structures of stereoisomeric covalent polycyclic aromatic carcinogen–DNA adducts: principles, patterns, and diversity. Chem Res Toxicol. 1997;10(2):111–46. doi: 10.1021/tx9601418. [DOI] [PubMed] [Google Scholar]
- 29. Garner RC, Tierney B, Phillips DH. A comparison of 32P-postlabelling and immunological methods to examine human lung DNA for benzo [a] pyrene adducts. IARC Sci Publ. 1988;(89):196–200. [PubMed] [Google Scholar]
- 30. Baan RA, Steenwinkel MJ, van Asten S, Roggeband R, van Delft JH. The use of benzo[a]pyrene diolepoxide-modified DNA standards for adduct quantification in 32P-postlabelling to assess exposure to polycyclic aromatic hydrocarbons: application in a biomonitoring study. Mutat Res. 1997;378(1–2):41–50. doi: 10.1016/s0027-5107(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 31. van Schooten FJ, Hillebrand MJ, Scherer E, den Engelse L, Kriek E. Immunocytochemical visualization of DNA adducts in mouse tissues and human white blood cells following treatment with benzo[a]pyrene or its diol epoxide. A quantitative approach. Carcinogenesis. 1991;12(3):427–33. doi: 10.1093/carcin/12.3.427. [DOI] [PubMed] [Google Scholar]
- 32. Beach AC, Gupta RC. Human biomonitoring and the 32P-postlabeling assay. Carcinogenesis. 1992;13(7):1053–74. doi: 10.1093/carcin/13.7.1053. [DOI] [PubMed] [Google Scholar]
- 33. Andrews CL, Vouros P, Harsch A. Analysis of DNA adducts using high-performance separation techniques coupled to electrospray ionization mass spectrometry. J Chromatogr A. 1999;856(1–2):515–26. doi: 10.1016/s0021-9673(99)00779-7. [DOI] [PubMed] [Google Scholar]
- 34. Koc H, Swenberg JA. Applications of mass spectrometry for quantitation of DNA adducts. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778(1–2):323–43. doi: 10.1016/s1570-0232(02)00135-6. [DOI] [PubMed] [Google Scholar]
- 35. Singh R, Farmer PB. Liquid chromatography-electrospray ionization-mass spectrometry: the future of DNA adduct detection. Carcinogenesis. 2006;27(2):178–96. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]