Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Inhaled, Treatment, Combinational, Device
Abbreviations: ACE-2, Angiotensin Converting Enzyme-2; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease; COVID-19, Coronavirus Disease-19; CVD, Cardiovascular Disease; DOPC, 1, 2-Dioleoyl-sn-glycero-3-phosphocholine; DPI, Dry Powder Inhaler; DPPC, Dipalmitoylphosphatidylcholine; DSPC, Distearoylphosphatidylcholine; DSPE-PEG2000, 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly (ethylene glycol2000); ED, Emitted Dose; EUA, Emergency Use Authorization; FPD, Fine Particle Dose; FPF, Fine Particle Fraction; HIV, Human Immunodeficiency Virus; hsACE2, Human Soluble Angiotensin Converting Enzyme 2; ICTV, International Committee on Taxonomy of Viruses; IND, Investigational New Drug application; MERS-CoV, Middle East Respiratory Syndrome; MMAD, Mass Median Aerodynamic Diameter; PEG, Polyethylene Glycol; PLGA, Poly Lactic co Glycolic Acid; pMDl, Pressurized Metered-Dose Inhaler; PVP, Polyvinylpyrrolidone; RdRp, RNA-Dependent RNA-Polymerase; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SBEβ-CD, Sulfobutylether Beta Cyclodextrin; SFD, Spray Freeze Drying; SMI, Soft Mist Inhaler; TFF, Thin-Film Freezing; TMPRSS2, Transmembrane Protease, Serine 2; U.S. FDA, United States Food and Drug Administration
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent responsible for the COVID-19 pandemic, has outspread at full tilt across the world. Although several effective vaccines continue to be deployed, reliable antiviral treatments have yet to be developed against this disease. Currently, available therapeutics for COVID-19 include repurposed, and a few novel drugs. Many drugs have been promising in preclinical studies, but a majority of these drugs have shown little or no efficacy in clinical studies. One of the major reasons is the insufficient drug concentration in the lung, the primary target site of infection for SARS-CoV-2, from the administration of drugs through oral or intravenous routes. Higher effective doses administered through these routes could also lead to adverse side effects. For this reason, inhaled treatments are being tested as an efficient approach for COVID-19, allowing lower doses of drugs ensuring higher concentrations of the drug(s) in the lung. The inhaled treatment combining two or more antiviral drugs will increase potency and reduce the possibility of selecting for SARS-CoV-2 variants with reduced drug susceptibility. Finally, the appropriate drug combination needs to be delivered using a suitable system. Here, we review the current treatment for COVID-19 and their limitations, discussing the advantages of mono and combinational inhaled therapy with a brief outline of the recently reformulated anti-SARS-CoV-2 agents as inhaled formulations. The selection of appropriate delivery devices for inhalation and associated key considerations including the formulation challenges are also discussed.
1. Introduction
The emergence of coronavirus disease-19 (COVID-19) was first detected in December 2019 in China’s Wuhan state, and this ongoing pandemic has affected the whole world with millions of deaths (World Health Organization (WHO), 2022a; Ji et al., 2020). The International Committee on Taxonomy of Viruses (ICTV) termed the causative agent of COVID-19 as SARS-CoV-2, because of its close phylogenetic resemblance to severe acute respiratory syndrome coronavirus (SARS-CoV) (Gorbalenya et al., 2020). However, SARS-CoV-2 is more transmissible than SARS-CoV and other respiratory viruses like MERS-CoV, Ebola virus, and influenza virus (Eedara et al., 2021, Xu et al., 2020, Zhou et al., 2020). SARS-CoV-2 is an enveloped, (+ss) RNA virus, mainly transmitted through inhalation of exhaled airborne droplets (Eedara et al., 2021, Yuki et al., 2020). Therefore, the respiratory tract is the primary site of infection of SARS-CoV-2, with a high density of ACE-2 glycoprotein, the receptor of the virus (Chen et al., 2020, Letko et al., 2020, Li et al., 2003, Walls et al., 2020, Zou et al., 2020). It is from the respiratory tract and lungs that the virus can gradually spread to other sites and organs (Zhang et al., 2020).
Common symptoms of COVID-19 are fatigue, fever, headache, dry cough, lymphopenia, hemoptysis, sputum production, and diarrhea (Zhu et al., 2020). In some cases, chest radiographs were found to possess an infiltrate in the upper lobe of the lung with shortness of breath and a lower level of oxygen in the blood (Phan et al., 2020). Renal tubular cells and testicular cells can be highly affected by SARS-CoV-2 because of the presence of ACE-2 on those cells (Fan et al., 2020). Anorexia, vomiting, and diarrhea are some significant features of this virus associated with the digestive system (Abd El-Aziz and Stockand, 2020).
The emergence of more transmissible and infectious variants of SARS-CoV-2 is worsening the current situation. Variants are the resultant of mutations during the replication process of a virus, and mutation is the alteration of any genetic sequence (addition/deletion/substitution) compared to the parent sequence (Ramesh et al., 2021). Currently, alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and omicron (B.1.1.529) are some of the most relevant identified variants of SARS-CoV-2 (WHO 2022b). These variants are identified in different countries and have different features. A comparison of these variants is presented in Table 1 .
Table 1.
Comparison between the most relevant SARS-CoV-2 variants (World Health Organization (WHO), 2022b; Ramesh et al., 2021).
|
SARS-CoV-2 variants | |||||
|---|---|---|---|---|---|
| WHO classification | Alpha | Beta | Gamma | Delta | Omicron |
| Pango Lineage | B.1.1.7 | B.1.351 | P.1 | B.1.617.2 | B.1.1.529 |
| Origin of detection | United Kingdom | South Africa | Brazil | India | Multiple |
| Date of designation | 18 December 2020 | 18 December 2020 | 11 January 2021 | 11 May 2021 | 26 November 2021 |
| GISAID clade | GRY | GH/501Y.V2 | GR/501Y.V3 | G/478 K.V1 | GRA |
| Nextstrain clade | 20I (V1) | 20H (V2) | 20 J (V3) | 21A, 21I, 21 J | 21 K, 21L, 21 M |
| Amino acid changes | +S: 484 K +S: 452R |
+S: L18F | +S: 681H | +S: 417 N +S: 484 K |
+S: R346K |
| Transmissibility | Higher transmission rates (∼50%). Ro = 3.5–5.2 |
Higher transmission rates (∼50%). Ro = Not available |
Transmissibility is 2.6 times higher. Ro = Not available |
Higher transmission. Ro = 5.5–6.5 | Higher transmission. Ro = Not available |
| Reinfection | Unlikely | Possible | Higher | Higher | Unknown |
| Monoclonal antibody efficacy and vaccine sera impact | Minimal/no effect | Reduced | Reduced | Reduced potentially | Unknown |
Vaccines are one of the effective approaches against contagious viral diseases although the development and the distribution process of an effective vaccine is a long process. This process can be further exacerbated because of the antigenic drift caused by mutations (Shyr et al., 2021). For example, currently available vaccines for COVID-19 are boosting the immunity against SARS-CoV-2 although the efficacy of these vaccines declines over time and differs among the existing variants stated above. The overall effectiveness of existing vaccines was found to be lesser against variants than the wild version (Charmet et al., 2021, Emary et al., 2021, Nasreen et al., 2021, Ramesh et al., 2021). From this, it is obvious to assume that, this virus will remain with us for a prolonged time. Until a safe and effective vaccine is available, efficient treatments are required to treat COVID-19.
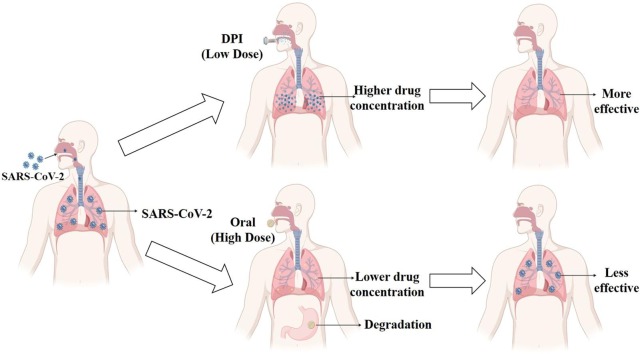
No fully effective direct-acting antiviral drugs are currently available against SARS-CoV-2 for all ages and non-hospitalized patients; instead, we rely on treatments of previous viral diseases (Dhama et al., 2020, Watkins, 2020). To date, remdesivir is the only therapeutic agent approved by the United States Food and Drug Administration (U.S. FDA) and given intravenously (U.S. Food & Drug Administration, 2020a). Molnupiravir and paxlovid are two other antiviral agents available for oral administration for using as emergency use authorization (EUA) treatment for COVID-19 by U.S. FDA developed by Merck and Pfizer, respectively. Molnupiravir is a prodrug of N-hydroxycytidine whereas paxlovid is the combination of nirmatrelvir and ritonavir (Jayk Bernal et al., 2022, Roberts et al., 2022). Most of the current antiviral/anti-SARS-CoV-2 agents are taken orally, and a little portion of the drug is reached the lung because of poor bioavailability or gastrointestinal decomposition. For that reason, inhaled treatment by pulmonary drug delivery could be considered a potential course of action to treat pulmonary diseases like COVID-19. Through pulmonary delivery, it is possible to deliver drugs to the lung directly which ensures higher drug concentration in the lung and avoids unwanted adverse effects as lower doses are required (Borghardt et al., 2018). Besides that, the selection of rightly chosen cocktail drugs that can exert synergistic activity, and formulations given via inhaled route can be a clever approach to treat this infectious disease and lower the chance of unwanted drug resistance (Shyr et al., 2021). Although pulmonary delivery has advantages, fugitive emissions during therapy (during nebulization) are the key challenge for contagious diseases managements, as it remains airborne in the indoor environment for hours and causes potential harm to other patients and physicians ( Chen et al., 2004). This is why appropriate delivery device selection and precautions are the prerequisites.
In this review, we have discussed the major limitations of currently available treatment for COVID-19 and the advantages of inhaled therapy. The scopes of inhaled combinational drugs over a single drug are discussed, which is still an untapped area for this disease. The appropriate device selection for inhalation with associated formulation challenges and storage are discussed as well.
2. Currently available treatments for COVID-19 and their limitations
Numerous drugs are now under clinical trials to test their safety and efficacy against SARS-CoV-2. Currently available treatments for COVID-19 are mainly repurposed drugs. Repurposed drugs in a pandemic situation are superior as their safety has been investigated already for other diseases, and the development of new drug(s) is a long process (Roessler et al., 2021). The development of repurposed drugs for new disease(s) requires less time and tests, so these drugs can easily enter into clinical trials (Roessler et al., 2021). Compared to new drugs, repurposed drugs are cheap and readily available. For example, the discovery of an orphan drug usually takes 10–15 years with less than a 10% success rate with around 2.5 billion dollars’ involvement whereas a repurposed drug takes 3–12 years with a 30–75% success rate and 300-million-dollar investment (Roessler et al., 2021). Again, recent advancements in repurposing modeling systems can ensure new indications and save time in the development process (Li et al., 2016). According to the U.S. FDA (until 09 May 2022) over 690 drugs are currently in the planning stages of development. The U.S. FDA has already reviewed over 460 trials and selected a few drugs for EUA, and remdesivir is the only approved antiviral agent for COVID-19 (U.S. Food Drug Administration, 2022a, U.S. Food Drug Administration, 2022b). A drug may be granted a EUA only if there is a serious life-threatening condition, there is evidence of the effectiveness of that drug and no certain alternatives are available (U.S. Food & Drug Administration, 2017). A list of approved and EUA treatments for COVID-19 by the U.S. FDA is presented in Table 2 .
Table 2.
List of U.S. FDA approved and emergency use authorization (EUA) therapeutics for COVID-19 (until 09 May 2022). (U.S. Food & Drug Administration, 2020a; U.S. Food Drug Administration, 2022a, U.S. Food Drug Administration, 2022b).
| Name and first date of issuance/approval | Approved/EUA | Manufactured/obtained by | Class/Technology | Use |
|---|---|---|---|---|
| Remdesivir (22 October 2020) | Approved | Gilead Science, USA | Antiviral | Adult and pediatric patients (at least 28 days of age with a minimum weight of 3 kg) who are mild to moderately affected and progressing to severe conditions. |
| Bebtelovimab (11 February 2022) | EUA | Eli Lilly and Company, USA | Antibodies | Adult and pediatric patients (at least 12 years and more than 40 kg) who are mild to moderately affected and progressing to severe conditions. |
| Molnupiravir (23 December 2021) | EUA | Merck, USA | Antiviral | Adult patients who are mild to moderately affected and progressing to severe conditions. |
| Nirmatrelvir co-packaged with ritonavir (22 December 2021) | EUA | Pfizer, USA | Antiviral | Same as bebtelovimab. |
| Tixagevimab and cilgavimab co-packaged (8 December 2021) | EUA | GSK, UK and Vir Biotechnology, USA | Antibodies | Same as bebtelovimab. |
| Tocilizumab (24 June 2021) | EUA | Genentech, USA | Antibodies | Hospitalized adult and pediatric patients (at least 2 years) receiving the corticosteroids (systemic) with invasive and non-invasive mechanical ventilation. |
| Sotrovimab (26 May 2021) | EUA | GSK, UK and Vir Biotechnology, USA | Antibodies | Same as bebtelovimab.1 |
| Propofol-lipuro 1% (12 March 2021) | EUA | B. Braun, Germany | General anesthetics | Patients over 16 years and need mechanical ventilation in ICU to maintain sedation via continuous infusion. |
| Bamlanivimab and etesevimab (09 February 2021) | EUA | Eli Lilly and Company, USA | Antibodies | Adult and pediatric patients who are mild to moderately affected and progressing to severe conditions.1 |
| Casirivimab and imdevimab (21 November 2020) | EUA | Regeneron Pharmaceuticals, USA | Antibodies | Same as bebtelovimab.1 |
| Baricitinib (19 November 2020) | EUA | Eli Lilly and Company, USA | Antibodies | Same as tocilizumab. |
| COVID-19 convalescent plasma (23 August 2020) | EUA | Obtained from COVID-19 patients | Biologics | Hospitalized COVID-19 patients. |
| REGIOCIT replacement solution (13 August 2020) | EUA | Baxter International Inc., USA | Electrolytes | Adult patients who need continuous renal replacement therapy in an acute care environment and if appropriate. |
| Fresenius propoven 2% (05 August 2020) | EUA | Fresenius Kabi, USA | General anesthetics | Patients who are at least 16 years of age and need mechanical ventilation in ICU. |
Currently not authorized in any U.S. regions for high-frequency omicron variant.
Besides U.S. FDA-approved and EUA treatments, many drugs have been used and still are being used for this disease. Many promising repurposed drugs that inhibit SARS-CoV-2 in laboratory cell-based studies have shown little or no efficacy in clinical studies (Reis et al., 2022). One of the critical reasons is an insufficient concentration of drug(s) in the lung, the primary infection site for COVID-19, from the administration of very high oral doses of drugs that have numerous side effects. For example, niclosamide and ivermectin showed antiviral activity in in vitro studies but both drugs have very poor absorption when given orally and thus a very little amount of drug reaches the lungs. Although niclosamide was found to be more potent compared to 3000 FDA and Investigational New Drug application (IND) -approved drugs, the dose is very high (2 g orally on day 1 followed by 500 mg twice daily for 10 days), which causes various side effects including malaise, pruritus, gastrointestinal disturbances, etc. (Jeon et al., 2020, Kumar et al., 2020). The IND approval indicates the permission for clinical studies of an experimental drug by the U.S. FDA. The so-called “magic drug” hydroxychloroquine created much craze at the beginning of the COVID-19 pandemic, but due to its high oral dose, it exerted severe side effects like liver failure, heart rhythm problems, kidney injuries, and blood as well as lymph system disorders, thus, U.S. FDA warned the use of hydroxychloroquine (U.S. Food & Drug Administration, 2020b). A guanine analog drug, favipiravir, requires high doses (1600 mg orally twice daily on day 1 and600 mg orally twice daily thereafter for 7–10 days) and it showed different pharmacokinetics in different ethnicities and rapid degradation after oral administration (Madelain et al., 2016). The only U.S. FDA-approved anti-viral drug for COVID-19 is remdesivir which when given intravenously poses side effects including liver injury, lower blood oxygen levels, allergic reaction, and breathing problems (U.S. Food & Drug Administration, 2020a). In a nutshell, high doses, severe side effects, first-pass metabolism, and poor absorption are the key limitations of currently used drugs for COVID-19. The problems associated with widely used drugs for COVID-19 are listed in Table 3 .
Table 3.
Routes of administration and limitations associated with some widely used therapeutics for COVID-19.
| Drug Name | Drug class | Route of administration | Limitations |
|---|---|---|---|
| Remdesivir | Antiviral | Intravenous | Side effects include liver injury, allergic reactions, breathing problems, blood pressure, heart rate alteration, fever, and low blood oxygen levels. |
| Favipiravir | Antiviral | Oral | High dose (1600 mg twice daily on day 1 and then 600 mg thrice daily for up to 14 days) and notable drug concentration reduction were observed. |
| Hydroxychloroquine | Antimalarial | Oral | Side effects include serious heart rhythm problems, blood and lymph system disorders, kidney injuries, and liver failure. |
| Niclosamide | Anthelmintics | Oral | High dose (2 g on day 1 followed by 500 mg twice daily for 10 days) and poor absorption and side effects including gastrointestinal disturbances, headaches, malaise, and pruritus. |
| Ivermectin | Antiparasite | Oral | Poor absorption, a very little amount of drug reaches the lungs. |
| Lopinavir/Ritonavir | Antiviral | Oral | The efficacy is still debatable and side effects include anorexia, nausea, and abdominal discomforts. |
| Ribavirin | Antiviral | Oral | High dose (1200 mg loading dose on day 1 and then 800 mg/day up to 5 days) and it showed side effects especially anemia when given in high dose. |
3. Advantages of inhaled treatment for COVID-19 based on clinical studies
The inhaled treatment for respiratory diseases is favorable as respiratory delivery can ensure a higher concentration of a drug in the lung and blood at lower doses than its oral doses meaning minimal to no side effects with better therapeutic outcomes (Khadka et al., 2021). For example, inhaled 100–200 μg of salbutamol is therapeutically equivalent to 2–4 mg of oral salbutamol, so less chance of side effects associated with this drug (Pasqua et al., 2022). Rapid onset of action is a matter of concern for severe COVID-19 patients as a sudden respiratory crisis may happen and such issues can be overcome by inhaled treatment. COVID-19 causes a series of complications that worsen the comorbid patient's conditions, especially those suffering from asthma or COPD (Leung et al., 2020). In such cases, a rapid onset of action will require more frequently than normal. Adjunct therapy as inhaled formulation can be used to lessen such symptoms which may or may not directly lower the viral loads. Like, salbutamol is given via inhaler to treat asthma attacks as it can exert effects within minutes (Pasqua et al., 2022). Inhaled treatment is non-invasive, painless, and patient-friendly (Dhand, 2007). Therefore, the members of the International Society for Aerosols in Medicine (ISAM) gave much emphasis to accelerating the process of inhaled treatment for COVID-19-affected patients (Mitchell et al., 2020). Even the remdesivir in inhaled form is now under clinical trial in the hope of a better outcome by the original manufacturer Gilead Sciences, USA (NCT04539262). Some published reports on a small group of patients showed the potential benefits of inhaled treatment although it needs further investigation in clinical trials of a larger population to have a concrete conclusion. For example, inhaled interferon beta-1a showed better outcomes with fewer side effects in phase II randomized, double-blind, and placebo-controlled studies (44% versus 22%) (Monk et al., 2021). Patients having inhaled adenosine treatment required less hospital stay compared to the patients having the control treatment (Caracciolo et al., 2021). An inhaled hydroxychloroquine can be effective in COVID-19 patients reducing side effects that are associated with oral dosage form (de Reus et al., 2020). A multicentre, noninterventional, cohort study conducted over 954 critically ill COVID-19 patients showed that inhaled corticosteroids lowered the mortality rate which was statistically significant (Al Sulaiman et al., 2022). In a randomized and open-label Phase 2 study over 61 milds to moderately affected COVID-19 patients, it was found that inhaled ciclesonide eradicated SARS-CoV-2 more compared to the standard care (Song et al., 2021). In a multicentre, open-label, multi-arm, randomized, controlled, adaptive platform trial of over 4700 participants, it was found that inhaled budesonide could improve the recovery time and lower the chance of death (Yu et al., 2021). From these investigations, it can be speculated that a better effective treatment is achievable for COVID-19 via inhaled therapy than oral therapy. The inhaled dose of anti-SARS-CoV-2 agents for COVID-19 treatment needs to be fixed based on the drug’s properties and analyzing the safety and efficacy data from preclinical and clinical studies. The aerosolization properties of the developed formulations need to be taken into consideration as well. It is easy to understand, that the deposition of drugs in the lungs is varied based on the formulations and the device used. Many researchers have reported the anticipated inhaled dose of different anti-SARS-CoV-2 agents which are mainly based on theoretical calculations with uncertainty and need to be approved by the regulatory bodies before conducting human trials. In Table 4 , we have mentioned the anticipated dose of some widely used anti-SARS-CoV-2 agents considering their reported in vitro half-maximal inhibitory concentration (IC50) values against SARS-CoV-2 (Austin and Okour, 2020; Sheahan et al., 2020, Li et al., 2022), alveolar fluid amount of 20 mL (Patton, 1996), no clearance from the lungs and different drug depositions (20–80%) in the lung (considering 20% as low and 80% as high drug deposition). A higher dose will be required for the full viral clearance. The IC50 values may also vary considering the potency of the drug used in the experiments as well as the formulation development technique. Our mentioned values are anticipated and need to be fixed before translating to animals/humans considering all the associated factors.
Table 4.
The anticipated inhaled dose of some widely used anti-SARS-CoV-2 agents considering the reported in vitro half-maximal inhibitory concentration (IC50) against SARS-CoV-2 (Austin and Okour, 2020; Sheahan et al., 2020, Li et al., 2022).
| Drug Name | IC50 (μM) | IC50 (μg/mL) | Anticipated inhaled dose (μg)* |
|---|---|---|---|
| Nirmatrelvir** | 0.18 | 0.1 | 2.5–10 |
| Niclosamide | 0.28 | 0.1 | 2.5–10 |
| Molnupiravir | 0.30 | 0.1 | 2.5–10 |
| Hydroxychloroquine | 0.72 | 0.2 | 5–20 |
| Remdesivir | 0.77 | 0.5 | 12.5–50 |
| Nitazoxanide | 2.12 | 0.7 | 17.5–70 |
| Ivermectin | 2.48 | 2.2 | 55–220 |
| Nafamostat | 22.5 | 7.8 | 195–780 |
| Favipiravir | 61.88 | 9.7 | 242.5–970 |
| Penciclovir | 95.96 | 24.3 | 607.5–2430 |
| Ribavirin | 109.5 | 26.7 | 667.5–2670 |
| *Considering total alveolar fluid of 20 mL, 20–80% of drug deposition and 50% SARS-CoV-2 inhibition in the lung ** Nirmatrelvir is the main component of Paxlovid. In Paxlovid, nirmatrelvir is co-packaged with ritonavir. | |||
4. Reformulation of anti-SARS-CoV-2 agents as inhalable form
Reformulation of the repurposed drugs which showed promising anti-SARS-CoV-2 properties and were given mainly orally or intravenously as an inhaled formulation has begun in a hope of a better outcome (Table 5 ). In this section, we have outlined the key findings of those reformulations. Further studies are required to evaluate the safety and efficacy of these formulations. All of these studies give an insight into the development feasibility of anti-SARS-CoV-2 agents as an inhalable form which will be key to developing further effective treatment for COVID-19 by conducting additional testing or adding additional agent(s). To define the aerosolization properties of inhaled formulations, few terms are commonly used. For example, emitted dose (ED) is the amount of drug that actually exits from the delivery device due to inhalation. Following an inhalation, the amount of drug having an aerodynamic particle size less than 5 µm within the ED is defined as a fine particle dose (FPD). The fine particle fraction (FPF) is FPD relative to ED. The mass median aerodynamic diameter (MMAD) indicates the diameter of which half of the particles of an aerosol by mass are larger.
Table 5.
Inhalable formulation of repurposed anti-SARS-CoV-2 agents.
| Drug Name | Formulation | Preparation technique | Excipients used | Delivery device | Ref |
|---|---|---|---|---|---|
| Remdesivir | Dry powder inhaler | Thin-film freezing | Captisol, mannitol, lactose, L-leucine | Plastiape RS00 inhaler | (Sahakijpijarn et al., 2020a) |
| Liposomal solution | Film hydration technique and probe supersonic method | SBEβ-CD, DPPC, DSPE-PEG2000, cholesterol, trehalose | Nebulizer | (Li et al., 2021) | |
| Liposomal solution | Modified hydration technique | DPPC, cholesterol, SBE-β-CD, DOPC | PARI LC PLUS nebulizer | (Vartak et al., 2021) | |
| Niclosamide | Dry powder inhaler | Thin-film freezing | Mannitol, leucine | Plastiape RS00 inhaler | (Jara et al., 2021) |
| Niclosamide-lysozyme composite | Dry powder inhaler, nasal spray, and nebulizer solution | Spray-drying, reconstitution | Sucrose, polysorbate 80, histidine, recombinant human lysozyme | Twin Caps inhaler and Aerogen Solo vibrating mesh nebulizer | (Brunaugh et al., 2021) |
| Tamibarotene | Dry powder inhaler | Spray-freeze drying | 2-hydroxypropyl-β-cyclodextrin | Breezhaler | (Liao et al., 2021) |
| Nafamostat mesylate | Inhalable microparticles | Spray-drying | Lecithin, mannitol | Plastiape RS00 inhaler | (Kang et al., 2021) |
| Triazavirin | Solution | Mixing | – | Ultrasonic nebulizer | (Valiulin et al., 2021) |
| Hydroxychloroquine sulfate | Dry powder inhaler | Air jet milling | – | Osmohaler | (Albariqi et al., 2021) |
| Isotonic solution | Mixing | – | Aerogen Solo nebulizer | (Tai et al., 2021) | |
| Ivermectin | Solution | Mixing | – | Nebulizer (Micro Cirrus, Berkshire, UK) | (Chaccour et al., 2020) |
| *SBEβ-CD = Sulfobutylether beta cyclodextrin, DPPC = Dipalmitoylphosphatidylcholine, DSPE-PEG2000 = 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol2000), DOPC = 1,2-Dioleoyl-sn-glycero-3-phosphocholine | |||||
Remdesivir, which was originally developed for treating the Ebola virus, has been repurposed for COVID-19 and is given intravenously, was reformulated as inhalable dry powder utilizing thin-film freezing (TFF) technology (Sahakijpijarn et al., 2020a). The powder produced by TFF technology is highly aersolizable with a high-surface area, thus suitable for better dissolution rate and bioavailability of poorly water-soluble drugs like remdesivir in the lungs. The dry powder prepared by TFF is mainly composed of brittle matrix and nanostructured aggregates. This nanostructured aggregates powder can ensure better drug absorption efficiency and dose uniformity in the lung than microparticles. Captisol, mannitol, lactose, and L-leucine were used in this study. Captisol was used to improve the solubility and stability of the poorly water-soluble drug, remdesivir. Mannitol, lactose, and L-leucine all were used to enhance the aerosolization property. The developed formulations were highly porous with a brittle matrix structure, and the remdesivir was in amorphous form. A Plastiape RS00 inhaler was used to deliver the dry powder. The FPF of the optimized formulation, prepared in an acetonitrile/water (50/50) co-solvent system and incorporation of L-leucine, was ∼ 93%. The dry powder was both physically and chemically stable and the in vitro aerosolization was not affected significantly after the one-month storage at 25 °C/60% RH. The in vitro dissolution study showed that the dissolution of TFF-based remdesivir was significantly higher than the unprocessed remdesivir. The prepared remdesivir dry powder showed 20 fold increase in solubility to the unprocessed remdesivir. The in vivo pharmacokinetic study conducted on Sprague-Dawley rats showed the Cmax value of 264.3 ± 88.5 ng/mL after 4 h for GS-441524, the metabolized adenosine analog of remdesivir. This study needs to be investigated further in an appropriate animal model to ensure the amount of drug achieved in the lung is sufficient to inhibit SARS-CoV-2. The overall study demonstrates the feasibility of TFF technology to produce anti-SARS-CoV-2 agents. The obtained results showed that this technique can be used to produce stable and highly aerosolizable DPI having a better dissolution rate.
On the other hand, there were two liposomal formulation studies of remdesivir for inhalation. The liposomal formulation can enhance the solubility and stability of poorly soluble drugs like remdesivir by incorporating them into the lipid bilayer and the liposome is biocompatible with the alveolar surfactants as both the components are lipid. In one study, a liposomal solution of remdesivir was developed utilizing the film hydration technique and probe supersonic method (Li et al., 2021). The used excipients were sulfobutylether beta-cyclodextrin (SBEβ-CD), dipalmitoylphosphatidylcholine (DPPC), 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol2000) (DSPE-PEG2000), cholesterol, and trehalose. SBEβ-CD was used to solubilize remdesivir. The lipids (DPPC, DSPE-PEG2000, cholesterol) were used to ensure liposomal membrane permeability and stability. DPPC was the main component in this study for better biocompatibility, as most of the lung surfactant is DPPC. The prepared liposome was within the size range of 115–130 nm. The optimized formulation was spherical with an MMAD of 4.118 µm. The FPF was 56.89% when delivered the formulation via an ultrasonic nebulizer. The formulation was physically stable for six months at 4 °C with no significant in vitro aerosolization alteration. The animal study conducted on male BALB/c mice showed better safety and higher drug accumulation in the lung for pulmonary delivery compared to delivery via injections.
In another study, Vartak et al. (Vartak et al., 2021) developed liposomal remdesivir using a modified hydration technique. The excipients used were DPPC, cholesterol, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and SBEβ-CD. The hydrodynamic size of the optimized liposome was 71.46 ± 1.35 nm which was stable after storage at 4 °C for one month. The amount of remdesivir degraded significantly when stored at 37 °C. The MMAD was 4.56 ± 0.55 µm with an FPF of 74.40 ± 2.96% when tested using a PARI LC PLUS nebulizer. The optimized DOPC (1,2-Dioleoyl-sn-glycero-3-phosphocholine,a phospholipid) containing liposomes showed greater than 90% cell viability on A549 cells at a concentration of 2.5 mg/mL of remdesivir.
Niclosamide, an anthelmintic drug that was repurposed for COVID-19 as a tablet, was reformulated for inhaled delivery for COVID-19. In one study, Jara et al. (Jara et al., 2021) developed niclosamide dry powder by TFF technology. Mannitol and L-leucine were in this study to enhance the aerosolization property of the prepared dry powder. The FPF and MMAD value for prepared dry powder was 86% and 1.11 µm respectively when tested using RS00 (Plastiape, Italy) inhaler. The tolerability and pharmacokinetic study were conducted in female Sprague-Dawley rats and Syrian hamsters respectively administering high dose of niclosamide (827.2 µg/kg) for 3 days. Although the liver, kidney, and spleen were normal, the lung tissue showed mild inflammation signs which could be attributed to the high dose of niclosamide used. The Cmax value in the hamster was measured at 159 ng/mL and 590.3 ng/mL for the niclosamide dose of 145 µg/kg and 290 µg/kg respectively. The researchers claimed that these Cmax values are sufficient to inhibit SARS-CoV-2 as the estimated IC90 value for SARS-CoV-2 is 153.1 ng/mL.
In another study, Brunaugh et al. (Brunaugh et al., 2021) developed the niclosamide-lysozyme composite to deliver via dry powder inhaler, nasal spray, and nebulizer. Niclosamide was combined with an endogenous protein lysozyme by spray drying technology to produce a dry powder. Spray-drying technique has been well explored to produce particles optimal for pulmonary delivery. Scalability, cost-effectiveness, reproducibility, and better control over particles are some of the major advantages of spray-drying. Sucrose, polysorbate 80, histidine, and recombinant human lysozyme was used in this study. Lysozyme has an anti-inflammatory effect and the inclusion of this protein can act as a carrier and also enable the reconstitution of the powder during administration. On the other hand, sucrose, polysorbate 80, and histidine were used to obtain a stable and dispersible lysozyme formulation. The geometric median diameter of the optimized formulation was below 5 µm and the fine particle dose of niclosamide was determined 136 ± 4.3 µg when 60 mg powder (0.7% niclosamide content) was aerosolized from a TwinCaps inhaler. The prepared dry powder was reconstituted in 0.45% sodium chloride solution and delivered for 2 min by an Aerogen Solo vibrating mesh nebulizer. The fine particle dose of 62.3 ± 3.7 µg was achieved after nebulization of 175 µg/mL of niclosamide.
Tamibarotene, an orally available retinoid derivative used for refractory acute promyelocytic leukemia, has been repurposed for COVID-19 and was reformulated by Liao et al. (Liao et al., 2021) as inhalable dry powder utilizing spray freeze drying (SFD) technology. SFD can produce low dense porous particles suitable for better aerosolization and drug dissolution. 2-hydroxypropyl-β-cyclodextrin was the only excipient used in this study to enhance the drug solubility. The prepared porous and spherical dry powder had the MMAD value of 1.86 ± 0.44 µm with an FPF of 65% when delivered and administered by intraperitoneal injection. The pharmacokinetic study found a higher Cmax value of tamibarotene in the lung tissue when administered intrathecally than intraperitoneal injection (94.2 ± 15.7 vs 2.6 ± 0.3 µg/mL). This study concluded that pulmonary delivery can achieve more drug concentrations in the lung than systemic delivery.
Nafamostat mesylate is an approved drug in Japan and South Korea for acute pancreatitis. This agent was repurposed for COVID-19 and given as an intravenous infusion and has been reformulated by Kang et al. (Kang et al., 2021) as inhalable microparticles by spray drying. In this study, lecithin was used as a stabilizer of nafamostat mesylate, and mannitol was used as a diluent. The powder was spherical in shape with a smooth surface. The highest FPF value of spray-dried nafamostat mesylate was obtained at 75.1 ± 5.3% with an emitted dose of 93.7 ± 2% and MMAD of 2.1 ± 0.6 µm. The device used in the study was an RS01 inhaler (Plastiape, Italy). The in vivo study was conducted in male Sprague-Dawley rats, where nafamostat mesylate (1 mg/kg) was administered both intratracheally and intravenously. After 10 min, the amount of nafamostat mesylate was found more in the bronchoalveolar lavage fluid for intratracheal administration (2.1 µg/mL) than intravenous administration (0.9 µg/mL). After 60 min, these values were 0.7 µg/mL and 0.3 µg/mL for intratracheal and intravenous administration respectively. The amount of drugs that remained in the lung tissue after 60 min were 0.4 µg/g for intravenous administration and 0.7 µg/g for intratracheal administration. This study indicates a higher drug delivery and residual time in the lung tissue for the intratracheal route than the intravenous route.
Valiulin et al. (Valiulin et al., 2021) developed the orally available antiviral agent triazavirin in inhaled form and performed the in vivo study on Outbred male mice in an inhalation chamber. The triazavirin aerosol was generated by an ultrasonic nebulizer and the aerosol flowrate in the chamber was maintained at 2.5 L/min by the chamber set-up system. The mean particle size was 560 nm with a particle size distribution of 0.003–10 µm. The in vivo study conducted on Outbred male mice (28 ± 3 g) found 2.6 µg/mL of triazavirin in blood plasma after 20 min of inhalation of 2 mg/kg of body-delivered dose.
Hydroxychloroquine sulfate, an antimalarial drug, was repurposed for COVID-19 as tablets and was reformulated as inhalable powder by Albariqi et al. (Albariqi et al., 2021) using air-jet milling technique. Milling is a simple and cost-effective technique to reduce particle size with an inhalable size range. The shape of the prepared dry powders was irregular and crystalline in nature. In vitro aerosolization of this powder from Osmohaler (Pharmaxis Ltd., Australia) at 60 L/min flow rate after storing the powders at different RH (43%, 53%, 58%, and 75%) at 25 °C for 7 days. It was found that the average median particle size (D50) was 1.73–3.44 µm. The MMAD (3.03–3.50 µm) and FPF (61.7–62.7%) values were similar when the powders were stored at 43%, 53%, and 58% RH but the MMAD (7.78 µm) was higher and FPF (33%) was lesser when stored at 75% RH.
In another study, Tai et al. (Tai et al., 2021) prepared hydroxychloroquine sulfate isotonic solution (20 mg/mL, 50 mg/mL, and 100 mg/mL) and tested for in vitro aerosolization from an Aerogen Solo nebulizer. The average MMAD values for 1 mL of 20 mg/mL, 50 mg/mL and 100 mg/mL were 3, 3.27 and 2.99 µm whereas the average FPF values were 71.8%, 67.3% and 71.3%, respectively.
Ivermectin, an antiparasitic drug, was repurposed for COVID-19 and given as tablets. Chaccour et al. (Chaccour et al., 2020) developed an inhaled nebulized form of ivermectin as a proof of concept to investigate the feasibility of delivering ivermectin to the lung. When the formulation was nebulized using a nebulizer (Micro Cirrus, Berkshire, UK) at the oxygen flow of 8 L/min, particles generated were of the size range 0.5–2.2 µm. The in vivo study was conducted in both male and female Sprague-Dawley rats (12 weeks old) for low doses (89 mg/kg) and high doses (121 mg/kg). The Cmax value in male rats was determined 86.2 ng/mL for low dose and 152 ng/mL for high dose. For female rats, the Cmax values were lower compared to the male rats with a value of 26.2 ng/mL and 51.8 ng/mL. This study concludes the feasibility of delivering ivermectin to the lungs in high concentrations via nebulization, although the safety and efficacy studies are required to come to a concrete conclusion if the achieved drug concentration is sufficient to inhibit the SARS-CoV-2.
5. Inhaled cocktail drugs as a potential treatment approach for COVID-19
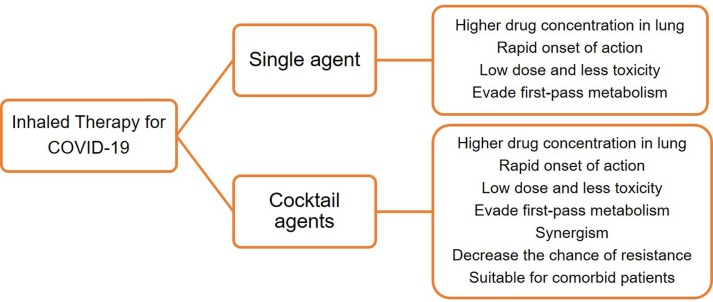
Research has progressed in developing inhaled formulations containing a single drug, as mentioned in section 4. The pulmonary delivery of selected potential cocktail drugs/antiviral agents may effectively treat COVID-19 patients compared to a single inhaled drug. The advantages of inhaled therapy for single and cocktail agents are illustrated in Fig. 1 .
Fig. 1.
Advantages of inhaled therapy (single agent and cocktail agents) for COVID-19.
While the number of combination drugs approved by the U.S. FDA has increased over the period, the combination therapy for infections and respiratory diseases has increased sharply compared to the other diseases (Das et al., 2019). For other viral diseases such as HIV/HCV-infected patients, combination therapy showed better outcomes than monotherapy (Shyr et al., 2021). COVID-19 patients experience a series of complications and comorbid patients, especially patients having asthma, COPD, or other inflammatory diseases, are more prone to COVID-19 (Leung et al., 2020). Again, patients with obesity, hypertension, liver and renal diseases, etc. suffer more than non-comorbid patients. A study over 26 patients found that arterial hypertension was the most common (65.4%), with other comorbidities like obesity (38.5%), chronic ischemic heart disease (34.6%), atrial fibrillation (26.9%), and COPD (23.1%) (Elezkurtaj et al., 2021). Another study reported that hypertension, obesity, and diabetes mellitus are the most common comorbidities in COVID-19 patients. However, COVID-19 patients having chronic kidney disease (CKD) were found to have statistically significantly higher death rates. Although obesity was more prevalent, the mortality was not significant as CKD (Ng et al., 2021). Ejaz et al. (Ejaz et al., 2020) analyzed the clinical and epidemiological data and reported that diabetes, COPD, cardiovascular disease (CVD), hypertension, HIV, and malignancies could cause potentially life-threatening conditions in COVID-19 patients. The frequency and fatality for these diseases were- obesity (48% and 68%), liver disease (43% and 29%), renal disease (9% and 26%), COPD (52% and 20%), CVD (17% and 15%), diabetes (58% and 8%), hypertension (23% and 6%), and malignancy (58% and 2%). From these studies, it is obvious that comorbidity is present, and it adversely affects COVID-19 patients. It is hard to overcome all the complications with a single drug, as a single drug cannot be effective against multiple complications. For example, remdesivir can reduce SARS-CoV-2 load but is not effective against inflammatory airway diseases like COPD or asthma. Niclosamide, another potent drug against SARS-CoV-2 showed effectiveness against inflammatory airway diseases, so it could be more effective in COVID-19 comorbid patients having asthma or COPD (Cabrita et al., 2019).
It is important to highlight that different drugs have different cytokine release inhibiting properties. Cytokine storm is the generator of a series of complications like endotheliopathy, pulmonary embolism, acute respiratory distress syndrome, and so on (Letko et al., 2020, Rothan and Byrareddy, 2020, Walls et al., 2020). While remdesivir can inhibit IL-1β, ivermectin can’t. In contrast, ivermectin can reduce the IL-1ss, IL-4, and IL-5, but remdesivir can’t (Heimfarth et al., 2020). Therefore, a cocktail formulation is needed to ensure multiple goals.
A single drug can be resistant to viruses more easily compared to a rightly chosen cocktail of drugs (Marimani et al., 2020). The resistance process happens rapidly when the viral replication is not fully restrained. One such example is the treatment for influenza. Drugs of the ‘adamantanes’ group became inactive against this disease as the influenza A virus has developed resistance against this group of drugs (Hussain et al., 2017). SARS-CoV-2 is changing its variants rapidly, a cocktail formulation will be superior to a single drug as the cocktail drugs can act on different stages of the SARS-CoV-2 life cycle. Until now, no study of drug resistance against SARS-CoV-2 is available. Hence between the two options of discovering new drugs and having a cocktail therapy to stop the resistance, the quicker approach is the latter.
The selection of drugs is a prominent issue in the cocktail formulation. If the combination is not selected wisely, it will exert an antagonistic effect and may be harmful as well (Bobrowski et al., 2021). Rightly chosen cocktail drugs can also offer synergistic activity ensuring multiple goals. A list of synergistic and antagonistic drug combinations can be found in Table 6 . The exact reasons for synergism are unknown, but possible reasons are drugs that belong to different classes or act on various stages of the virus life cycle or have an independent mechanism of action to show synergism. Remdesivir/favipiravir and ivermectin combination in an in vitro study showed synergistic activity (Jitobaom et al., 2022, Tan et al., 2021). The remdesivir and ivermectin combination was tested in RAW 264.7 murine macrophage cell line against murine coronavirus infection. Favipiravir and ivermectin combination was tested in Vero E6 cell line against SARS-CoV-2. Remdesivir/favipiravir and ivermectin cocktail exhibit synergism and one possible reason could be that their mechanisms of action are different and act at different stages of the SARS-CoV-2 life cycle. Remdesivir/favipiravir acts on RNA dependant RNA polymerases (RdRp) and ivermectin inhibits the entry of viral proteins to the nucleus by preventing the virus from binding to the importin α/β1 (Imp α/β1) heterodimer (Caly et al., 2020, Kandimalla et al., 2020). Again, the remdesivir and ebselen/disulfiram combination also showed synergistic activity against SARS-CoV-2 in the Vero cell line (Chen et al., 2021). Similarly, remdesivir and ebselen/disulfiram combination act with different mechanisms of action. For example, ebselen and disulfiram both are protease inhibitors of SARS-CoV-2 (Chen et al., 2021). A cell line study conducted on Vero E6 cells against SARS-CoV-2 showed that remdesivir and human soluble angiotensin-converting enzyme 2 (hsACE2) combination showed a better antiviral effect than the individual agent. hsACE-2 acts as an entry inhibitor for SARS-CoV-2 (Monteil et al., 2021). Niclosamide and lysozyme both showed antiviral activity against SARS-CoV-2 and the inclusion of lysozyme with niclosamide enhanced the potency of niclosamide against SARS-CoV-2 in vitro (Brunaugh et al., 2021). In a cell line-based study, Bobrowski et al. (Bobrowski et al., 2021) showed that a cocktail of nitazoxanide with remdesivir/ umifenovir/ amodiaquine could exhibit synergistic effects. These drugs have different mechanisms of action. Nitazoxanide is the entry inhibitor whereas remdesivir is the RdRp inhibitor, but umifenovir and amodiaquine are entry inhibitors. In contrast, antagonistic effects were found with remdesivir and lysosomotropic agents like hydroxychloroquine. For selecting cocktail drugs, it is always a good idea to perform in silico study first to have an idea about the effectiveness and then to perform a cell line-based study to further confirm that before proceeding to animal and clinical studies.
Table 6.
A list of drug combinations showing synergistic and antagonistic effects against SARS-CoV-2 in cell line studies.
| Drug A | Drug A mode of action | Drug B | Drug B mode of action | Effect (Drug A + Drug B) | References |
|---|---|---|---|---|---|
| Remdesivir | RdRp inhibitor | Ebselen | Protease inhibitor | Synergistic | (Chen et al., 2021) |
| Remdesivir | RdRp inhibitor | Disulfiram | Protease inhibitor | Synergistic | (Chen et al., 2021) |
| Remdesivir | RdRp inhibitor | Ivermectin | Importin α/β1 inhibitor | Synergistic | (Tan et al., 2021) |
| Remdesivir | RdRp inhibitor | Nitazoxanide | Entry inhibitor | Synergistic | (Bobrowski et al., 2021) |
| Nitazoxanide | Entry inhibitor | Umifenovir | Entry inhibitor | Synergistic | (Bobrowski et al., 2021) |
| Nitazoxanide | Entry inhibitor | Emetine dihydrochloride hydrate | Replication inhibitor | Synergistic | (Bobrowski et al., 2021) |
| Nitazoxanide | Entry inhibitor | Amodiaquine | Entry inhibitor | Synergistic | (Bobrowski et al., 2021) |
| Favipiravir | RdRp inhibitor | Ivermectin | Importin α/β1 inhibitor | Synergistic | (Jitobaom et al., 2022) |
| Otamixaban | Entry inhibitor | Camostat | TMPRSS2 inhibitor | Synergistic | (Hempel et al., 2021) |
| Otamixaban | Entry inhibitor | Nafamostat | TMPRSS2 inhibitor | Synergistic | (Hempel et al., 2021) |
| Remdesivir | RdRp inhibitor | Brequinar | Replication inhibitor | Synergistic | (Schultz et al., 2022) |
| Molnupiravir | RdRp inhibitor | Brequinar | Replication inhibitor | Synergistic | (Schultz et al., 2022) |
| Cepharanthine | Entry inhibitor | Nelfinavir | Replication inhibitor | Synergistic | (Ohashi et al., 2021) |
| Remdesivir | RdRp inhibitor | Hydroxychloroquine | Entry inhibitor | Antagonistic | (Bobrowski et al., 2021) |
| Remdesivir | RdRp inhibitor | Mefloquine | Entry inhibitor | Antagonistic | (Bobrowski et al., 2021) |
| Remdesivir | RdRp inhibitor | Amodiaquine | Entry inhibitor | Antagonistic | (Bobrowski et al., 2021) |
| *RdRp = RNA-dependent RNA polymerase, TMPRSS2 = Transmembrane protease, serine 2 | |||||
6. Selection of appropriate delivery device
The attention for inhaled treatment against SARS-CoV-2 is augmenting rapidly, although appropriate delivery devices should be addressed, and associated formulation challenges/factors should be taken under consideration before designing the formulation for pulmonary delivery.
Although there is no reliable data yet on the inhaled dose of anti-COVID drugs, it can be assumed from the oral doses that the inhaled dose of these drugs will vary from a few to many milligrams. Dry powder inhalers (DPIs), nebulizers, pressurized metered-dose inhalers (pMDls), and soft mist inhalers (SMIs) are the delivery devices for inhaled treatment. The selection of devices will depend on the dose, ease of use, product stability, and safety. These devices have different dosing capacities. For example, pMDIs and SMIs are designed to deliver low doses (from a few micrograms to a few milligrams) and the delivery efficiency of pMDIS is very low. DPIs, as well as nebulizers both, can deliver high doses (many milligrams) although nebulizers have drawbacks like poor delivery efficiency, high administration time, and not being portable. Nebulizers are mainly used for geriatric, pediatric, and hospitalized patients. Again, nebulizer formulations are less stable as they are liquid-based, and it is difficult to deliver multiple drugs having various solubilities at a time as there is a specific amount of liquid for nebulization. Being a highly transmissible infectious disease, it requires special facilities to deliver the drug for COVID-19 through nebulization to reduce its spreading (Ari, 2020). On the other hand, better product stability, delivery of poorly water-soluble drugs, delivery of combination/multiple drugs, easier administration technique (hand-breathing coordination is not required), and easy to storing and transport are some major advantages of DPIs (Lee et al., 2017, Sanders, 2011). Such advantages make DPIs an ideal dosage form for COVID-19 patients in an outpatient setting which can ensure the minimization of virus spreading (Sahakijpijarn et al., 2020a). The risk of contamination is high in nebulizers and low in DPIs (Lavorini et al., 2021). Again, if COVID-19 becomes a seasonal disease over time, DPIs will be a unique approach for the outpatients (Sun, 2020).
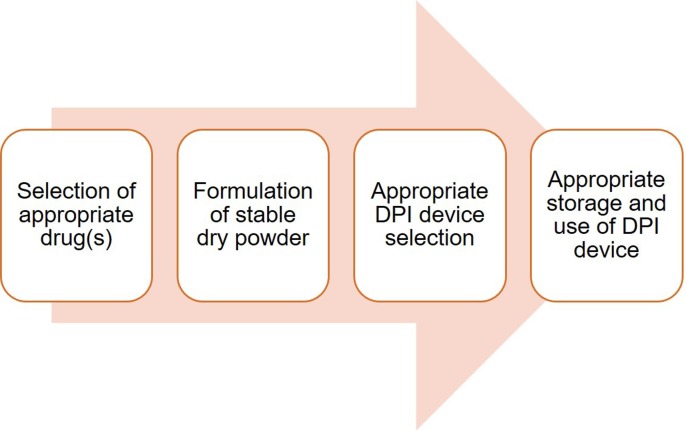
7. Key considerations for a successful DPI
The successful development of a DPI relies on different factors and so many things need to be taken into consideration. Among different factors, we have discussed some key factors such as formulation challenges, DPI device, and appropriate use as well as storage of DPI.
7.1. Formulation challenges
Various techniques have been utilized for developing DPIs, and among them, milling, spray-drying, spray freeze drying, thin-film freezing, and supercritical fluid drying are some of the key techniques. All of them have their advantages and disadvantages (Chaurasiya and Zhao, 2020). The process parameters of each technique need to be optimized for the development of a successful DPI. A suitable technique can be chosen based on the selected agent intended for pulmonary delivery. The development techniques of DPIs may vary, but the formulation challenges and considering factors are the same.
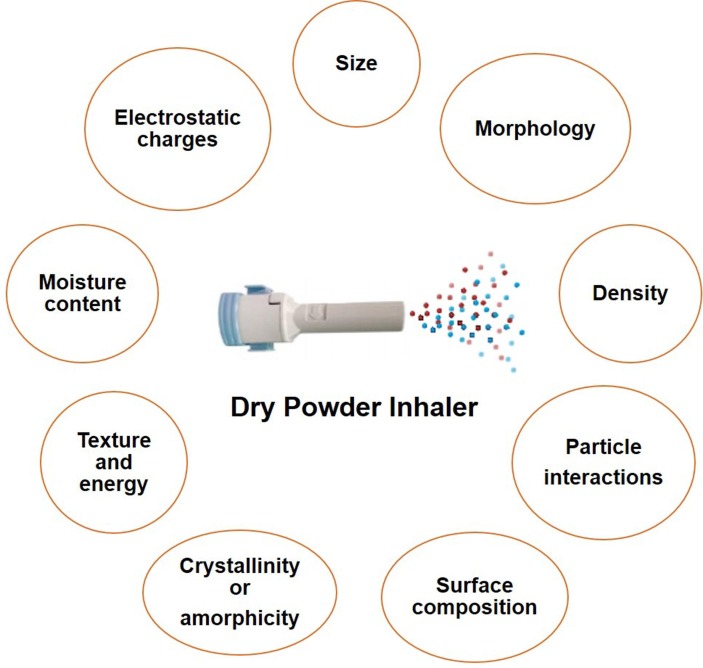
Being a highly infectious disease and from the current oral dose of previous infectious diseases such as tuberculosis, it can be assumed that the inhaled dose for COVID-19 will be high. Stable and highly aerosolizable dry powder in high doses will be required for COVID-19 patients. The aerodynamic diameter needs to be 1–5 µm for delivery to the lower respiratory tract. However, these low-micron-sized powder particles are cohesive and do not aerosolize adequately. Many factors may affect the DPIs (Fig. 2 ). Those key factors are outlined below:
Fig. 2.
Factors affecting the dry powder inhaler formulation.
Particle size: Particle size with a certain aerodynamic diameter (1–5 µm) is desired for deposition in small airways and alveolar regions. Small particles (less than 3 µm) are usually settled in the lower respiratory tract whereas large particles which are more than 5 are usually settled in the upper airways (Heyder et al., 1986). On the other hand, particles that are below 1 µm, are generally exhaled (Heyder et al., 1986).
Particle shape: It is reported that pollen-shaped particles having a petal-like surface structure have the potential to ensure higher deposition compared to others with the same aerodynamic size (Hassan and Lau, 2009). Higher de-agglomeration was observed in flake and wrinkled-shaped particles whereas particles having low contact areas show lower agglomeration (Zijlstra et al., 2004).
Density: The aerodynamic diameter within a respirable size relates with the physical diameter of about a similar range when the density of the particles is around 1 g/cc. Porous particles having low density and physical diameter above 5 µm can achieve deep lung delivery of drugs (Edwards et al., 1997). A low tapped density can reduce powder agglomeration and enhance aerosolization which is necessary for deep lung delivery. The low density of powder facilitates deep lung delivery although they are voluminous and limit the mass of powder in a single dose capsule (Momin et al., 2018).
Surface texture: Surface roughness influences the cohesive interactions between particles, which affects the aerodynamic behavior. Small-scale roughness can improve aerosolization and vice versa for large-scale roughness (Momin et al., 2018). Surface corrugation to a certain magnitude can improve aerosolization of DPIs compared to smooth surface particles. However, enhanced aerosolization for both rough as well as smooth conditions has been reported (Adi et al., 2008, Chew et al., 2005).
Surface energy and surface composition: Surface energy influences aerosolization properties a lot, and surface energy may vary in different regions of a particular surface. The low surface energy can enhance aerosolization whereas high surface energy increases agglomeration tendency and limits the aerosolization (Momin et al., 2018). Humidity, moisture, crystallinity, and surface texture, as well as the functional groups of the component, can influence the surface energy (Das et al., 2009). Surface composition affects surface energy which can influence the aerosolization and flowability of powders (Kawashima et al., 1998).
Particle interactions: Aerosolization of DPIs relies on the particle interactions (both particle–particle and particle–wall), which may be cohesive or adhesive. These forces greatly influence small particles (diameter less than 10 µm) when gravitational forces are negligible. Different factors can influence particle interactions including various forces (van der Waals, electrostatic, capillary, mechanical interlocking) as well as the morphology (size and shape), and characteristics of powder (Visser, 1995, Young et al., 2003). The higher electrostatic charge and van der Waals force reduce the aerosolization property and thus cause upper lung deposition (Momin et al., 2018). Capillary forces influence the particle interactions in hydrophilic materials rather than hydrophobic materials and decrease aerosolization by forming a solid bridge (Das et al., 2009). Particles with an irregular shape with a rough surface show higher particle interactions because of mechanical interlocking that is opposite to smooth surface particles (Momin et al., 2018). Elongated particles show low aerosolization because of their inter-particulate attractions and high contact area (Visser, 1989).
Hygroscopicity and moisture content: Hygroscopic materials absorb moisture from the environment which has an influence on the surface energy and thus impacts the particle interactions and flow properties. The aerosolization of the powder formulation is reduced at higher moisture content and thus affects the drug’s deposition in the lung (Momin et al., 2018).
Crystallinity and amorphic nature: It is reported that, compared to amorphous powder, crystalline powder shows better aerosolization as they have lower surface energy that allows for reduced particle interaction and agglomeration (Shekunov et al., 2002).
Besides those factors, post-inhalation cough after DPI usage needs to be taken into consideration to avoid the spreading of this contagious virus and patient compliance. Drugs or excipients or both can be the precursor of post-inhalation cough. The selection of drugs and excipients can be made in a manner to avoid the post-inhalation cough. Sugars (mannitol, sucrose, lactose, etc.), amino acids (L-leucine, L-isoleucine, glycine, etc.), polymers (PEG, PLGA, PVP, etc.), surfactants (DPPC, DSPC, polysorbate 80, magnesium stearate, etc.), ammonium salts (ammonium carbonate, ammonium bicarbonate) are some of the commonly used excipients in DPIs (Alhajj et al., 2021). The usage of mannitol in the DPI can induce cough and bronchospasm resulting in the spreading of the virus (Brunaugh et al., 2021, Sahakijpijarn et al., 2020b). Besides, the elevated post-inhalation cough after the usage of antiviral zanamivir has been reported (Sahakijpijarn et al., 2020b). Again, the dosage form and delivery of high doses of drugs could be the source of post-inhalation cough. The usage of a high amount of powder can irritate the lung epithelial cells resulting in the cough inducement (Geller et al., 2011, Konstan et al., 2011).
So it can be easily relatable that post-inhalation cough can be induced by various factors and is not desirable in COVID-19 patients. These factors should be taken into consideration during formulation development.
7.2. Delivery devices for DPIs
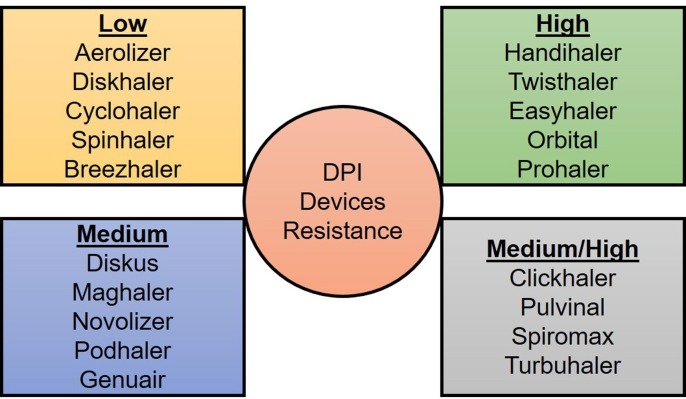
The successful deposition of dry powder in the lung is also dependent on the choice of DPI devices. As DPIs are patient-driven, the resistance of DPIs can vary the drug deposition. Different DPIs have different resistance (low/medium/high) ( Fig. 3 ) which influences the inspiratory flow rate (IFR), and as a consequence, the lung deposition can be varied (Muralidharan et al., 2015). For example, the lung deposition of some commercial DPIs after testing in healthy volunteers are as follows- novolizer (20–32%), easyhaler (19%), diskhaler (12%), turbuhaler (15–25%), etc (Laube and Dolovich, 2014). Again, it has been reported that a patient’s disease condition (asthma/COPD) may create difficulties to achieve the desired flow through the DPIs (Tantucci and Modina, 2012). The inhalation flow can be decreased in acute exacerbations and in change of the FEV1 (Forced expiratory volume in 1 s) values (Tantucci and Modina, 2012). The lungs of the COVID-19 patients are highly affected by the SARS-CoV-2 (Huang et al., 2020, Torres-Castro et al., 2021). Thus, it can affect the deposition of drugs in the respiratory tract, and such issues need to be taken under consideration when selecting the appropriate delivery device. As mentioned earlier, it is assumed that the dose will be high for COVID-19 based on the current oral dose. Again, depending on the dose (micrograms or many milligrams), DPI devices can be chosen. Examples of some devices which can deliver many milligrams of drugs include podhlaer, diskus, twincer inhaler, orbital etc. Several approved high dose DPIs are Relenza (5 mg zanamavir + 20 mg lactose/inhalation), Bronchitol (40 mg mannitol/inhalation), TOBI Podhaler (28 mg tobramycin/inhalation), Colobreathe (125 mg colistimethate sodium/inhalation) and Inavir (20–40 mg laninamivir/inhalation) (Brunaugh and Smyth, 2018). The entire dose of Bronchitol are 10 capsules and of tobramycin are 4 of 50 mg. The capsule size #0 is used for RS01 prototype device to deliver 120 mg of tobramycin with 6–8 consecutive inhalations (Buttini et al., 2018).
Fig. 3.
Some commercially available dry powder inhalers based on their resistance.
7.3. Appropriate use and storage of DPI
Appropriate use of DPIs needs to be ensured for achieving the maximum therapeutic outcome (Fig. 4 ), and thus, patient awareness is required. A study conducted on 3811 patients reported that 49–55% of patients used DPIs incorrectly (Molimard et al., 2003). A systemic review covering the 1975–2014 period (analyzing 144 articles) concluded that the inappropriate use of DPIs has not been improved during this 40 years frame (Sanchis et al., 2016). Much more inappropriate usage of DPIs have been reported (Lavorini et al., 2008, Molimard et al., 2017). These errors include incorrect dose metering, inhaler positioning, rotation sequence, mouthpiece positioning, no exhalation before activation, no forceful and deep inhalation, no breath-hold, and failure to breathe out slowly (Lavorini et al., 2008). It can be easily assumed that the therapeutic outcome will be lower than optimal. Even healthcare professionals use DPIs inappropriately (Plaza et al., 2018). Adequate training and instructions can ensure the proper use and performance of the DPIs to ensure maximum therapeutic outcomes (Chrystyn and Lavorini, 2020).
Fig. 4.
Key considerations for the development of a dry powder inhaler (DPI) and achievement of optimal therapeutic outcome.
Besides the appropriate use, proper storage of DPIs is also needed. A patient-reported survey of over 738 patients reported that 2/3 of the patients stored the DPIs sub optimally and did not check the expiry date before using. Even the patients are not aware of the healthcare providers storage of the DPIs (Norderud Laerum et al., 2016). Mishandling of DPIs can impact the functionality, and awareness of patients and healthcare providers is much needed.
8. Conclusions
Pulmonary delivery of anti-SARS-CoV-2 agents is considered an effective therapeutic approach for COVID-19, as it can ensure minimum effective drug concentration in the lungs, target site of infection, for maximum therapeutic activity with the minimal dose while minimizing adverse effects. Additional effort and resources should be dedicated to pulmonary delivery to lessen the current utmost urgency. It will also open new possibilities for treating future viral and infectious respiratory diseases. More importantly, developing effective pulmonary delivery of anti-SARS-CoV-2 medications will require determining the appropriate dose and frequency along with delivery techniques. Inhaled cocktail dry powder could be a unique approach to treat COVID-19 efficiently along with preventing the potential selection of drug-resistant SARS-CoV-2 variants. However, before administration, safety, as well as efficacy studies in clinical trials need to be considered.
CRediT authorship contribution statement
Tushar Saha: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – original draft. Miguel E. Quiñones-Mateu: Conceptualization, Investigation, Writing – review & editing, Supervision, Funding acquisition. Shyamal C. Das: Conceptualization, Methodology, Formal analysis, Investigation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Tushar Saha acknowledges the University of Otago doctoral scholarship.
Funding
This work is funded by a Laurenson Bequest Award (LA394), and the Webster Family Chair in Viral Pathogenesis, University of Otago (M.E.Q-M).
References
- Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect. Genet. Evol. 2020;83 doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adi H., Traini D., Chan H.K., Young P.M. The influence of drug morphology on aerosolisation efficiency of dry powder inhaler formulations. J. Pharm. Sci. 2008;97:2780–2788. doi: 10.1002/jps.21195. [DOI] [PubMed] [Google Scholar]
- Al Sulaiman K., Aljuhani O., Al Aamer K., Al Shaya O., Al Shaya A., Alsaeedi A.S., Alhubaishi A., Altebainawi A.F., Al Harthi A., Albelwi S., Almutairi R., Alsubaie N., Alsallum A., Korayem G.B., Alfahed A., Kensara R., Altebainawi E.F., Alenezi R.S., Alsulaiman T., Al Enazi H., Vishwakarma R., Al Dabbagh T., Bakhsh U., Al Ghamdi G. The role of inhaled corticosteroids (ICS) in critically ill patients with COVID-19: a multicentre, cohort study. J. Intensive Care Med. 2022;37:248–257. doi: 10.1177/08850666211053548. [DOI] [PubMed] [Google Scholar]
- Albariqi A.H., Chang R.Y.K., Tai W., Ke W.R., Chow M.Y.T., Tang P., Kwok P.C.L., Chan H.K. Inhalable hydroxychloroquine powders for potential treatment of COVID-19. J. Aerosol. Med. Pulm. Drug. Deliv. 2021;34:20–31. doi: 10.1089/jamp.2020.1648. [DOI] [PubMed] [Google Scholar]
- Alhajj N., O'Reilly N.J., Cathcart H. Designing enhanced spray dried particles for inhalation: a review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021;384:313–331. [Google Scholar]
- Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir. Med. 2020;167 doi: 10.1016/j.rmed.2020.105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin D., Okour M. Evaluation of potential therapeutic options for COVID-19. J. Clin. Pharmacol. 2020;60:976–977. doi: 10.1002/jcph.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowski T., Chen L., Eastman R.T., Itkin Z., Shinn P., Chen C.Z., Guo H., Zheng W., Michael S., Simeonov A., Hall M.D., Zakharov A.V., Muratov E.N. Synergistic and antagonistic drug combinations against SARS-CoV-2. Mol. Ther. 2021;29:873–885. doi: 10.1016/j.ymthe.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghardt J.M., Kloft C., Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can Respir J. 2018;2018:2732017. doi: 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunaugh A.D., Seo H., Warnken Z., Ding L., Seo S.H., Smyth H.D.C. Development and evaluation of inhalable composite niclosamide-lysozyme particles: a broad- spectrum, patient-adaptable treatment for coronavirus infections and sequalae. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0246803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunaugh A.D., Smyth H.D.C. Formulation techniques for high dose dry powders. Int. J. Pharm. 2018;547:489–498. doi: 10.1016/j.ijpharm.2018.05.036. [DOI] [PubMed] [Google Scholar]
- Buttini, F., Balducci, A., G., Colombo, G., Sonvico, F., Montanari, S., Pisi, G., Rossi, A., Colombo, P., Bettini, R., 2018. Dose administration maneuvers and patient care in tobramycin dry powder inhalation therapy. Int J Pharm. 548, 182-191. [DOI] [PubMed]
- Cabrita I., Benedetto R., Schreiber R., Kunzelmann K. Niclosamide repurposed for the treatment of inflammatory airway disease. JCI Insight. 2019;4 doi: 10.1172/jci.insight.128414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo M., Correale P., Mangano C., Foti G., Falcone C., Macheda S., Cuzzola M., Conte M., Falzea A.C., Iuliano E., Morabito A., Caraglia M., Polimeni N., Ferrarelli A., Labate D., Tescione M., Di Renzo L., Chiricolo G., Romano L., De Lorenzo A. Efficacy and effect of inhaled adenosine treatment in hospitalized COVID-19 patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.613070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaccour C., Abizanda G., Irigoyen-Barrio A., Casellas A., Aldaz A., Martinez-Galan F., Hammann F., Gil A.G. Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats. Sci. Rep. 2020;10:17073. doi: 10.1038/s41598-020-74084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmet T., Schaeffer L., Grant R., Galmiche S., Cheny O., Von Platen C., Maurizot A., Rogoff A., Omar F., David C., Septfons A., Cauchemez S., Gaymard A., Lina B., Lefrancois L.H., Enouf V., van der Werf S., Mailles A., Levy-Bruhl D., Carrat F., Fontanet A. Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: Results from a nationwide case-control study in France. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasiya B., Zhao Y.-Y. Dry powder for pulmonary delivery: a comprehensive review. Pharmaceutics. 2020;13:31. doi: 10.3390/pharmaceutics13010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Fei C.Y., Chen Y.P., Sargsyan K., Chang C.P., Yuan H.S., Lim C. Synergistic inhibition of SARS-CoV-2 replication using disulfiram/ebselen and remdesivir. ACS Pharmacol Transl Sci. 2021;4:898–907. doi: 10.1021/acsptsci.1c00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019- nCoV. Biochem. Biophys. Res. Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.C., Huang, L. M., Chan, C. C., Su, C. P., Chang, S. C., Chang, Y. Y., Chen, M. L., Hung, C. C., Chen, W. J., Lin, F. Y., Lee, Y. T., & SARS Research Group of National Taiwan University College of Medicine and National Taiwan University Hospital., 2004. SARS in hospital emergency room. Emerg. Infect. Dis. 10, 782-788. [DOI] [PMC free article] [PubMed]
- Chew N.Y.K., Tang P., Chan H.K., Raper J.A. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm. Res. 2005;22:148–152. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- Chrystyn H., Lavorini F. The dry powder inhaler features of the Easyhaler that benefit the management of patients. Expert Rev Respir Med. 2020;14:345–351. doi: 10.1080/17476348.2020.1721286. [DOI] [PubMed] [Google Scholar]
- Das P., Delost M.D., Qureshi M.H., Smith D.T., Njardarson J.T. A survey of the structures of US FDA approved combination drugs. J. Med. Chem. 2019;62:4265–4311. doi: 10.1021/acs.jmedchem.8b01610. [DOI] [PubMed] [Google Scholar]
- Das S., Larson I., Young P., Stewart P. Surface energy changes and their relationship with the dispersibility of salmeterol xinafoate powders for inhalation after storage at high RH. Eur. J. Pharm. Sci. 2009;38:347–354. doi: 10.1016/j.ejps.2009.08.007. [DOI] [PubMed] [Google Scholar]
- de Reus, Y.A., Hagedoorn, P., Sturkenboom, M.G.G., Grasmeijer, F., Bolhuis, M.S., Sibum, I., Kerstjens, H.A.M., Frijlink, H.W., Akkerman, O.W., 2020. Tolerability and Pharmacokinetic Evaluation of Inhaled Dry Powder Hydroxychloroquine in Healthy Volunteers. medRxiv. https://doi.org/10.1101/2020.12.03.20243162. [DOI] [PMC free article] [PubMed]
- Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;16:1232–1238. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R. Inhalation therapy in invasive and noninvasive mechanical ventilation. Curr Opin Crit Care. 2007;13:27–38. doi: 10.1097/MCC.0b013e328012e022. [DOI] [PubMed] [Google Scholar]
- Edwards D.A., Hanes J., Caponetti G., Hrkach J., Ben-Jebria A., Eskew M.L., Mintzes J., Deaver D., Lotan N., Langer R. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- Eedara B.B., Alabsi W., Encinas-Basurto D., Polt R., Ledford J.G., Mansour H.M. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics. 2021;13:1077. doi: 10.3390/pharmaceutics13071077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., Abosalif K.O.A., Ahmed Z., Younas S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezkurtaj S., Greuel S., Ihlow J., Michaelis E.G., Bischoff P., Kunze C.A., Sinn B.V., Gerhold M., Hauptmann K., Ingold-Heppner B., Miller F., Herbst H., Corman V.M., Martin H., Radbruch H., Heppner F.L., Horst D. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021;11:4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emary K.R.W., Golubchik T., Aley P.K., Ariani C.V., Angus B., Bibi S., Blane B., Bonsall D., Cicconi P., Charlton S., Clutterbuck E.A., Collins A.M., Cox T., Darton T.C., Dold C., Douglas A.D., Duncan C.J.A., Ewer K.J., Flaxman A.L., Faust S.N., Ferreira D.M., Feng S., Finn A., Folegatti P.M., Fuskova M., Galiza E., Goodman A.L., Green C.M., Green C.A., Greenland M., Hallis B., Heath P.T., Hay J., Hill H.C., Jenkin D., Kerridge S., Lazarus R., Libri V., Lillie P.J., Ludden C., Marchevsky N.G., Minassian A.M., McGregor A.C., Mujadidi Y.F., Phillips D.J., Plested E., Pollock K.M., Robinson H., Smith A., Song R., Snape M.D., Sutherland R.K., Thomson E.C., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Williams C.J., Hill A.V.S., Lambe T., Gilbert S.C., Voysey M., Ramasamy M.N., Pollard A.J. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Li K., Ding Y., Lu W., Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. Front. Med. (Lausanne). 2020;7 doi: 10.3389/fmed.2020.563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D.E., Weers J., Heuerding S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere technology. J. Aerosol. Med. Pulm. Drug. Deliv. 2011;24:175–182. doi: 10.1089/jamp.2010.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D., Sidorov I.A., Sola I., Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.S., Lau R.W. Effect of particle shape on dry particle inhalation: study of flowability, aerosolization, and deposition properties. AAPS PharmSciTech. 2009;10:1252–1262. doi: 10.1208/s12249-009-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimfarth L., Serafini M.R., Martins-Filho P.R., Quintans J.S.S., Quintans-Junior L.J. Drug repurposing and cytokine management in response to COVID-19: a review. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel T., Elez K., Kruger N., Raich L., Shrimp J.H., Danov O., Jonigk D., Braun A., Shen M., Hall M.D., Pohlmann S., Hoffmann M., Noe F. Synergistic inhibition of SARS-CoV-2 cell entry by otamixaban and covalent protease inhibitors: pre-clinical assessment of pharmacological and molecular properties. Chem. Sci. 2021;12:12600–12609. doi: 10.1039/d1sc01494c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder J., Gebhart J., Rudolf G., Schiller C.F., Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J. Aerosol Sci. 1986;17:811–825. [Google Scholar]
- Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., Zhou X., Liu X., Huang X., Yuan S., Chen C., Gao F., Huang J., Shan H., Liu J. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Galvin H.D., Haw T.Y., Nutsford A.N., Husain M. Drug resistance in influenza A virus: the epidemiology and management. Infect Drug Resist. 2017;10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara M.O., Warnken Z.N., Sahakijpijarn S., Moon C., Maier E.Y., Christensen D.J., Koleng J.J., Peters J.I., Hackman Maier S.D., Williams Iii R.O. Niclosamide inhalation powder made by thin-film freezing: Multi-dose tolerability and exposure in rats and pharmacokinetics in hamsters. Int. J. Pharm. 2021;603 doi: 10.1016/j.ijpharm.2021.120701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martin-Quiros A., Caraco Y., Williams-Diaz A., Brown M.L., Du J., Pedley A., Assaid C., Strizki J., Grobler J.A., Shamsuddin H.H., Tipping R., Wan H., Paschke A., Butterton J.R., Johnson M.G., De Anda C. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64:e00819–e820. doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T., Liu Z., Wang G., Guo X., Akbar Khan S., Lai C., Chen H., Huang S., Xia S., Chen B., Jia H., Chen Y., Zhou Q. Detection of COVID-19: A review of the current literature and future perspectives. Biosens. Bioelectron. 2020;166 doi: 10.1016/j.bios.2020.112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla R., John A., Abburi C., Vallamkondu J., Reddy P.H. Current status of multiple drug molecules, and vaccines: an update in SARS-CoV-2 therapeutics. Mol. Neurobiol. 2020;57:4106–4116. doi: 10.1007/s12035-020-02022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.H., Kim Y.J., Yang M.S., Shin D.H., Kim D.W., Park I.Y., Park C.W. Co- spray dried nafamostat mesylate with lecithin and mannitol as respirable microparticles for targeted pulmonary delivery: pharmacokinetics and lung distribution in rats. Pharmaceutics. 2021;13:1519. doi: 10.3390/pharmaceutics13091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y., Serigano T., Hino T., Yamamoto H., Takeuchi H. A new powder design method to improve inhalation efficiency of pranlukast hydrate dry powder aerosols by surface modification with hydroxypropylmethylcellulose phthalate nanospheres. Pharm. Res. 1998;15:1748–1752. doi: 10.1023/a:1011916930655. [DOI] [PubMed] [Google Scholar]
- Khadka P., Sinha S., Tucker I.G., Dummer J., Hill P.C., Katare R., Das S.C. Studies on the safety and the tissue distribution of inhaled high-dose amorphous and crystalline rifampicin in a rat model. Int. J. Pharm. 2021;597 doi: 10.1016/j.ijpharm.2021.120345. [DOI] [PubMed] [Google Scholar]
- Konstan M.W., Flume P.A., Kappler M., Chiron R., Higgins M., Brockhaus F., Zhang J., Angyalosi G., He E., Geller D.E. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J. Cyst. Fibros. 2011;10:54–61. doi: 10.1016/j.jcf.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Gupta N., Kodan P., Mittal A., Soneja M., Wig N. Battling COVID-19: using old weapons for a new enemy. Trop. Dis. Travel Med. Vacc. 2020;6:6. doi: 10.1186/s40794-020-00107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitobaom, K., Boonarkart, C., Manopwisedjaroen, S., Punyadee, N., Borwornpinyo, S., Thitithanyanont, A., Avirutnan, P., Auewarakul, P., 2022. Synergistic Anti-SARS- CoV-2 activity of repurposed anti-parasitic drug combinations. Research Square. https://doi.org/10.21203/rs.3.rs-1069947/v1. [DOI] [PMC free article] [PubMed]
- Laube B.L., Dolovich M.B. In: Middleton's Allergy. Adkinson N.F., Bochner B.S., Burks A.W., Busse W.W., Holgate S.T., Lemanske R.F., O'Hehir R.E., editors. Saunders; Philadelphia: 2014. Aerosols and Aerosol Drug Delivery Systems; pp. 1066–1082. [Google Scholar]
- Lavorini F., Magnan A., Dubus J.C., Voshaar T., Corbetta L., Broeders M., Dekhuijzen R., Sanchis J., Viejo J.L., Barnes P., Corrigan C., Levy M., Crompton G.K. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir. Med. 2008;102:593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Lavorini F., Usmani O.S., Dhand R. Aerosol delivery systems for treating obstructive airway diseases during the SARS-CoV-2 pandemic. Intern. Emerg. Med. 2021;16:2035–2039. doi: 10.1007/s11739-021-02812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-G., Kim D.-W., Park C.-W. Dry powder inhaler for pulmonary drug delivery: human respiratory system, approved products and therapeutic equivalence guideline. J. Pharm. Investig. 2017;48:603–616. [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J.M., Niikura M., Yang C.W.T., Sin D.D. COVID-19 and COPD. Eur. Respir. J. 2020;56:2002108. doi: 10.1183/13993003.02108-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang K., Wu D., Ren L., Chu X., Qin C., Han X., Hang T., Xu Y., Yang L., Yin L. Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19. Asian J. Pharm. Sci. 2021;16:772–783. doi: 10.1016/j.ajps.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zheng S., Chen B., Butte A.J., Swamidass S.J., Lu Z. A survey of current trends in computational drug repositioning. Brief Bioinform. 2016;17:2–12. doi: 10.1093/bib/bbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang Y., Lavrijsen M., Lamers M.M., de Vries A.C., Rottier R.J., Bruno M.J., Peppelenbosch M.P., Haagmans B.L., Pan Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q., Yuan S., Cao J., Tang K., Qiu Y., Seow H.C., Man R.C.H., Shao Z., Huang Y., Liang R., Chan J.F.W., Yuen K.Y., Lam J.K.W. Inhaled dry powder formulation of tamibarotene, a broad-spectrum antiviral against respiratory viruses including SARS-CoV-2 and influenza virus. Adv. Ther. 2021;4:2100059. [Google Scholar]
- Madelain V., Nguyen T.H., Olivo A., de Lamballerie X., Guedj J., Taburet A.M., Mentre F. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimani M., Ahmad A., Duse A. In: Combination Therapy Against Multidrug Resistance. Wani M.Y., Ahmad A., editors. Academic Press; Massachusetts: 2020. Combination therapy as an effective tool for treatment of drug-resistant viral infections; pp. 157–182. [Google Scholar]
- Mitchell J.P., Berlinski A., Canisius S., Cipolla D., Dolovich M.B., Gonda I., Hochhaus G., Kadrichu N., Lyapustina S., Mansour H.M., Darquenne C., Clark A.R., Newhouse M., Ehrmann S., Humphries R., Boushey H. Urgent appeal from international society for aerosols in medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM regulatory and standardization issues networking group. J. Aerosol. Med. Pulm. Drug. Deliv. 2020;33:235–238. doi: 10.1089/jamp.2020.1622. [DOI] [PubMed] [Google Scholar]
- Molimard M., Raherison C., Lignot S., Balestra A., Lamarque S., Chartier A., Droz- Perroteau C., Lassalle R., Moore N., Girodet P.O. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur. Respir. J. 2017;49:1601794. doi: 10.1183/13993003.01794-2016. [DOI] [PubMed] [Google Scholar]
- Molimard M., Raherison C., Lignot S., Depont F., Abouelfath A., Moore N. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J. Aerosol. Med. 2003;16:249–254. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- Momin M.A.M., Tucker I.G., Das S.C. High dose dry powder inhalers to overcome the challenges of tuberculosis treatment. Int. J. Pharm. 2018;550:398–417. doi: 10.1016/j.ijpharm.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Monk P.D., Marsden R.J., Tear V.J., Brookes J., Batten T.N., Mankowski M., Gabbay F.J., Davies D.E., Holgate S.T., Ho L.-P., Clark T., Djukanovic R., Wilkinson T.M.A., Crooks M.G., Dosanjh D.P.S., Siddiqui S., Rahman N.M., Smith J.A., Horsley A., Harrison T.W., Saralaya D., McGarvey L., Watson A., Foster E., Fleet A., Singh D., Hemmings S., Aitken S., Dudley S., Beegan R., Thompson A., Rodrigues P.M.B. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo- controlled, phase 2 trial. Lancet. Respi Med. 2021;9:196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil V., Dyczynski M., Lauschke V.M., Kwon H., Wirnsberger G., Youhanna S., Zhang H., Slutsky A.S., Hurtado Del Pozo C., Horn M., Montserrat N., Penninger J.M., Mirazimi A. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P., Hayes D., Jr., Mansour H.M. Dry powder inhalers in COPD, lung inflammation and pulmonary infections. Expert Opin Drug Deliv. 2015;12:947–962. doi: 10.1517/17425247.2015.977783. [DOI] [PubMed] [Google Scholar]
- Nasreen, S., Chung, H., He, S., Brown, K.A., Gubbay, J.B., Buchan, S.A., Fell, D.B., Austin, P.C., Schwartz, K.L., Sundaram, M.E., Calzavara, A., Chen, B., Tadrous, M., Wilson, K., Wilson, S.E., Kwong, J.C., 2021. medRxiv. https://doi.org/10.1101/2021.06.28.21259420.
- Ng, W.H., Tipih, T., Makoah, N.A., Vermeulen, J.G., Goedhals, D., Sempa, J.B., Burt, F.J., Taylor, A., Mahalingam, S., 2021. Comorbidities in SARS-CoV-2 Patients: a Systematic Review and Meta-Analysis. mBio 12, e03647-20. [DOI] [PMC free article] [PubMed]
- Norderud Laerum B., Telg G., Stratelis G. Need of education for dry powder inhaler storage and retention – a patient-reported survey. Multidiscip. Respir. Med. 2016;11:21. doi: 10.1186/s40248-016-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, H., Watashi, K., Saso, W., Shionoya, K., Iwanami, S., Hirokawa, T., Shirai, T., Kanaya, S., Ito, Y., Kim, K.S., Nomura, T., Suzuki, T., Nishioka, K., Ando, S., Ejima, K., Koizumi, Y., Tanaka, T., Aoki, S., Kuramochi, K., Suzuki, T., Hashiguchi, T., Maenaka, K., Matano, T., Muramatsu, M., Saijo, M., Aihara, K., Iwami, S., Takeda, M., McKeating, J.A., Wakita, T., 2021. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 24, 102367. [DOI] [PMC free article] [PubMed]
- Pasqua E., Hamblin N., Edwards C., Baker-Glenn C., Hurley C. Developing inhaled drugs for respiratory diseases: a medicinal chemistry perspective. Drug Discov Today. 2022;27:134–150. doi: 10.1016/j.drudis.2021.09.005. [DOI] [PubMed] [Google Scholar]
- Patton J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996;19:3–36. [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., Nguyen T.T., Cao T.M., Pham Q.D. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza V., Giner J., Rodrigo G.J., Dolovich M.B., Sanchis J. Errors in the use of inhalers by health care professionals: a systematic review. J Allergy Clin Immunol Pract. 2018;6:987–995. doi: 10.1016/j.jaip.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Ramesh S., Govindarajulu M., Parise R.S., Neel L., Shankar T., Patel S., Lowery P., Smith F., Dhanasekaran M., Moore T. Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines (Basel) 2021;9:1195. doi: 10.3390/vaccines9101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G., Silva E., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., Dos Santos C.V.Q., Campos V.H.S., Nogueira A.M.R., de Almeida A., Callegari E.D., Neto A.D.F., Savassi L.C.M., Simplicio M.I.C., Ribeiro L.B., Oliveira R., Harari O., Forrest J.I., Ruton H., Sprague S., McKay P., Guo C.M., Rowland-Yeo K., Guyatt G.H., Boulware D.R., Rayner C.R., Mills E.J. Effect of early treatment with ivermectin among patients with Covid-19. N. Engl. J. Med. 2022 doi: 10.1056/NEJMoa2115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.A., Duncan A., Cairns K.A. Pandora’s box: Paxlovid, prescribing, pharmacists and pandemic. J. Pharm. Pract. Res. 2022;52:1–4. doi: 10.1002/jppr.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler H.I., Knoers N., van Haelst M.M., van Haaften G. Drug repurposing for rare diseases. Trends Pharmacol. Sci. 2021;42:255–267. doi: 10.1016/j.tips.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakijpijarn S., Moon C., Koleng J.J., Christensen D.J., Williams Iii R.O. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12:1002. doi: 10.3390/pharmaceutics12111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakijpijarn S., Smyth H.D.C., Miller D.P., Weers J.G. Post-inhalation cough with therapeutic aerosols: formulation considerations. Adv. Drug Deliv. Rev. 2020;165–166:127–141. doi: 10.1016/j.addr.2020.05.003. [DOI] [PubMed] [Google Scholar]
- Sanchis, J., Gich, I., Pedersen, S., Aerosol Drug Management Improvement, T., 2016. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 150, 394-406. [DOI] [PubMed]
- Sanders M. In: Controlled pulmonary drug delivery. Smyth H.D.C., Hickey A.J., editors. Springer; New York, New York, NY: 2011. Pulmonary drug delivery: An historical overview; pp. 51–73. [Google Scholar]
- Schultz D.C., Johnson R.M., Ayyanathan K., Miller J., Whig K., Kamalia B., Dittmar M., Weston S., Hammond H.L., Dillen C., Ardanuy J., Taylor L., Lee J.S., Li M., Lee E., Shoffler C., Petucci C., Constant S., Ferrer M., Thaiss C.A., Frieman M.B., Cherry S. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature. 2022;604:134–140. doi: 10.1038/s41586-022-04482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T.P., Sims, A.C., Zhou, S., Graham, R.L., Pruijssers, A.J., Agostini, M.L., Leist, S.R., Schäfer, A., Dinnon III, K.H., Stevens, L.J., Chappell, J.D., Lu, X., Hughes, T.M., George, A.S., Hill, C.S., Montgomery, S.A., Brown, A.J., Bluemling, G.R., Natchus, M.G., Saindane, M., Kolykhalov., A.A., Painter, G., Harcourt, J., Tamin, A., Thornburg, N.J., Swanstrom, R., Denison, M.R., Baric, R.S., 2020. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 12, eabb5883. [DOI] [PMC free article] [PubMed]
- Shekunov B.Y., Feeley J.C., Chow A.H.L., Tong H.H.Y., York P. Physical Properties of Supercritically-Processed and Micronised Powders for Respiratory Drug Delivery. Kona. 2002;20:178–187. [Google Scholar]
- Shyr Z.A., Cheng Y.S., Lo D.C., Zheng W. Drug combination therapy for emerging viral diseases. Drug Discov Today. 2021;26:2367–2376. doi: 10.1016/j.drudis.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.Y., Yoon J.G., Seo Y.B., Lee J., Eom J.S., Lee J.S., Choi W.S., Lee E.Y., Choi Y.A., Hyun H.J., Seong H., Noh J.Y., Cheong H.J., Kim W.J. Ciclesonide inhaler treatment for mild-to-moderate COVID-19: a randomized, open-label, Phase 2 trial. J. Clin. Med. 2021;10:3545. doi: 10.3390/jcm10163545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional benefit. AAPS J. 2020;22:77. doi: 10.1208/s12248-020-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W., Chow M.Y.T., Chang R.Y.K., Tang P., Gonda I., MacArthur R.B., Chan H.K., Kwok P.C.L. Nebulised Isotonic Hydroxychloroquine Aerosols for Potential Treatment of COVID-19. Pharmaceutics. 2021;13:1260. doi: 10.3390/pharmaceutics13081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.L., Tan K.S.W., Chu J.J.H., Chow V.T. Combination treatment with remdesivir and ivermectin exerts highly synergistic and potent antiviral activity against murine coronavirus infection. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.700502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantucci C., Modina D. Lung function decline in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2012;7:95–99. doi: 10.2147/COPD.S27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., Vilaro J. Respiratory function in patients post-infection by COVID- 19: a systematic review and meta-analysis. Pulmonology. 2021;27:328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration, 2017. Emergency Use Authorization of Medical Products and Related Authorities. https://www.fda.gov/regulatory-information/search-fda- guidance-documents/emergency-use-authorization-medical-products-and-related- authorities (accessed 09 May, 2022).
- U.S. Food & Drug Administration, 2020a. FDA approves first treatment for COVID-19. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment- covid-19 (accessed 09 May, 2022).
- U.S. Food & Drug Administration, 2020b. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/drugs/drug-safety-and- availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19- outside-hospital-setting-or (accessed 09 May, 2022).
- U.S. Food & Drug Administration, 2022a. Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory- and-policy-framework/emergency-use-authorization#coviddrugs (accessed 09 May, 2022).
- U.S. Food & Drug Administration, 2022b. Coronavirus Treatment Acceleration Program (CTAP). https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus- treatment-acceleration-program-ctap (accessed 09 May, 2022).
- Valiulin S.V., Onischuk A.A., Dubtsov S.N., Baklanov A.M., An'kov S.V., Plokhotnichenko M.E., Tolstikova T.G., Dultseva G.G., Rusinov V.L., Charushin V.N., Fomin V.M. Aerosol inhalation delivery of triazavirin in mice: outlooks for advanced therapy against novel viral infections. J. Pharm. Sci. 2021;110:1316–1322. doi: 10.1016/j.xphs.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartak R., Patil S.M., Saraswat A., Patki M., Kunda N.K., Patel K. Aerosolized nanoliposomal carrier of remdesivir: an effective alternative for COVID-19 treatment in vitro. Nanomedicine. 2021;16:1187–1202. doi: 10.2217/nnm-2020-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. Van der Waals and other cohesive forces affecting powder fluidization. Powder Technol. 1989;58:1–10. [Google Scholar]
- Visser J. Particle adhesion and removal: a review. Part. Sci. Technol. 1995;13:169–196. [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368 doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2022a. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants (accessed 09 may, 2022).
- World Health Organization (WHO), 2022b. Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed 09 May, 2022).
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.-S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P.M., Price R., Tobyn M.J., Buttrum M., Dey F. Effect of humidity on aerosolization of micronized drugs. Drug Dev. Ind. Pharm. 2003;29:959–966. doi: 10.1081/ddc-120025453. [DOI] [PubMed] [Google Scholar]
- Yu L.-M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., Van Hecke O., Ogburn E., Evans P.H., Thomas N.P.B., Patel M.G., Richards D., Berry N., Detry M.A., Saunders C., Fitzgerald M., Harris V., Shanyinde M., de Lusignan S., Andersson M.I., Barnes P.J., Russell R.E.K., Nicolau D.V., Ramakrishnan S., Hobbs F.D.R., Butler C.C., Yu L.-M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., van Hecke O., Ogburn E., Evans P.H., Thomas N.P.B., Patel M.G., Richards D., Berry N., Detry M.A., Saunders C.T., Fitzgerald M., Harris V., Shanyinde M., de Lusignan S., Andersson M.I., Barnes P.J., Russell R.E.K., Nicolau D.V., Ramakrishnan S., Hobbs F.D.R., Butler C.C. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open- label, adaptive platform trial. Lancet. 2021;398:843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Geng X., Tan Y., Li Q., Xu C., Xu J., Hao L., Zeng Z., Luo X., Liu F., Wang H. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra G.S., Hinrichs W.L., de Boer A.H., Frijlink H.W. The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix. Eur. J. Pharm. Sci. 2004;23:139–149. doi: 10.1016/j.ejps.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]