Abstract
Free-living Naegleria fowleri amoebae cause primary amoebic meningoencephalitis (PAM). Because of the apparent conflict between their ubiquity and the rarity of cases observed, we sought to develop a model characterizing the risk of PAM after swimming as a function of the concentration of N. fowleri. The probability of death from PAM as a function of the number of amoebae inhaled is modeled according to results obtained from animals infected with amoeba strains. The calculation of the probability of inhaling one or more amoebae while swimming is based on a double hypothesis: that the distribution of amoebae in the water follows a Poisson distribution and that the mean quantity of water inhaled while swimming is 10 ml. The risk of PAM for a given concentration of amoebae is then obtained by summing the following products: the probability of inhaling n amoebae × the probability of PAM associated with inhaling these n amoebae. We chose the lognormal model to assess the risk of PAM because it yielded the best analysis of the studentized residuals. Nonetheless, the levels of risk thereby obtained cannot be applied to humans without correction, because they are substantially greater than those indicated by available epidemiologic data. The curve was thus adjusted by a factor calculated with the least-squares method. This provides the PAM risk in humans as a function of the N. fowleri concentration in the river. For example, the risk is 8.5 × 10−8 at a concentration of 10 N. fowleri amoebae per liter.
Free-living amoebae are ubiquitous protozoa that have been isolated from most regions of the world. One species, Naegleria fowleri, is potentially pathogenic in humans, causing fatal primary amoebic meningoencephalitis (PAM) (5). It grows preferentially in warm water (25 to 44°C) and has been isolated from both natural and artificial sources (2, 12–14, 16, 20, 43, 44).
The first case of PAM was described in 1965 (23). Human infection by N. fowleri occurs by the nasal route, after contact between water containing the amoebae and the nasal mucosa. After possible local multiplication, amoebae cross the nasal mucosa and the cribriform plate of the ethmoid bone and invade the brain, where they cause hemorrhage, inflammation, and extensive necrosis. Death usually occurs 10 to 14 days after exposure (35). More than 180 cases have now been reported throughout the world.
Most of the cases have been reported in the United States, Australia, and the Czech Republic (respectively, 81, 19, and 18 cases). They most often involve children in good health or young adults (i) who had been swimming in heated pools or thermal waters (Czech Republic, Belgium, or New Zealand [7, 8, 29, 30, 36]) or in ponds, lakes, or rivers (North America [1, 10, 17, 19, 21]), or (ii) who were infected as a result of using domestic water colonized by these amoebae (Australia [4, 5]).
There are few available data from humans on which we can base a quantitative assessment of the risk of PAM associated with swimming or other recreational activity carried out in water containing N. fowleri. Until now, the only estimate, which is based on data from Florida (43), reported a risk of 7 cases per billion swimming episodes in water for which no precise N. fowleri concentration has been measured (but up to 40 N. fowleri amoebae/liter).
These data are insufficient for a precise estimate of the PAM risk to swimmers as a function of the N. fowleri concentration in the water. In the field of chemical carcinogenesis, when no human data are available, the risks associated with exposure to low doses are usually assessed by modeling experimental results from animal exposure to high doses and then extrapolating these results first to low doses and then to humans. This procedure assumes that there is no threshold below which exposure is not associated with an increase in risk. In microbiology, the same procedure was developed by Haas (26) in 1983 but has had only a limited development since then (24, 25, 27, 28, 40), probably because of methodological problems associated with work on microorganisms, compared with chemical or physical agents.
The goal of this study is to assess the risk of PAM for swimmers potentially exposed to N. fowleri.
MATERIALS AND METHODS
The risk for humans of contracting PAM while swimming in fresh water results from the occurrence of both of the two following independent events: first, the risk of exposure to n N. fowleri amoebae when swimming in water with a concentration, c, of N. fowleri, and, second, the risk of developing PAM after inhaling a number n of N. fowleri amoebae.
Animal experiments.
To model this risk, we used a mouse model and carried out experimental inoculation of mice with nine different strains of N. fowleri isolated from five different thermally polluted watercourses in France. The concentration of N. fowleri in water was determined by incubating replicate samples at 44°C on non-nutrient agar plates spread with Escherichia coli according to the following procedure: filtration of 10 × 100 ml, 30 × 10 ml and direct deposit of 20 × 1 ml, 20 × 0.1 ml; the results were expressed as the most probable number (MPN) per liter after recording the number of positive plates for each replicate. After isolation and identification by isoenzyme typing (37), the N. fowleri strains were axenized on serum-caseine-glucose-yeast extract medium at 37°C. For inoculation, the amoebae were recovered by gentle agitation followed by 800 × g centrifugation of the culture medium. After decanting the supernatant, the amoebae were resuspended and the concentration of the N. fowleri suspension was determined by at least three separate cell counts with a Thoma hemocytometer. The nasal instillation was performed on groups of at least 10 1-month-old Swiss OF1 female mice, which were first anesthetized by intraperitoneal injection of a mixture of ketamine, diazepam, and atropine; 17 μl of the amoebic suspension was deposited intranasally in mice lying in the dorsal decubitus position. The initial inoculum ranged from 4.8 × 104 to 1.9 × 103 amoebae per mouse, depending on the strain tested. For two strains (D98.2.1.h10. and SL98.2.1.f17.), we carried out successive 10-fold dilutions of the initial suspension in order to establish dose-response curves for groups of 20 mice.
The animals were observed daily, for a period of at least 21 days, in order to monitor them for the appearance of clinical signs characteristic of PAM.
We also integrated into these results previous published data from three other experiments with mice (3, 31, 44). We used these experimental data to model the probability of death from PAM as a function of the number of amoebae inhaled.
Modeling the risk of PAM from animal data.
Evidence that no single model is specifically adapted to microbiological experimentation can be found in the fact that so many models have been proposed (26). To determine which was most appropriate to these observations, we tested five that are frequently used for similar studies: the fractional (derived from a logistic model), lognormal, Weibull, Beta, and uniform distribution models. Two estimators were used to determine the models' parameters: maximum likelihood and least squares. We studied the validity of each model with a Kolmogorov-Smirnov test and an analysis of the studentized residuals.
Calculation of risk associated with one swimming episode.
In assuming that distribution of amoebae in the water follows a Poisson distribution, the probability of inhaling n amoebae (Pinh) as a function of their concentration in the water is as follows:
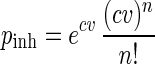 |
where c is the concentration of amoebae per liter of water, v is the volume of water inhaled, expressed in liters, and n is the number of amoebae inhaled.
We assumed that the mean quantity of water inhaled by the nose during a swimming episode is 10 ml. This volume corresponds to the amount prescribed for nasal irrigation (5 ml per nostril), which is representative of a prolonged swim with the head underwater.
The risk r of death from PAM during one exposure to water containing N. fowleri is then
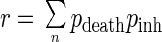 |
where pdeath is the probability of death from PAM when n amoebae are inhaled, calculated by the preceding modeling process from the animal experimental data.
This first step produced a risk curve with no correction factor for extrapolation to humans. We then compared the data with the global risk estimate that Wellings based on human epidemiologic data. We estimated the curve for the maximum risk of human death from PAM by adjusting the first curve to the risk observed in Florida (43), choosing the lowest concentration observed (set at 1 N. fowleri per liter, the detection limit of the MPN methods [44]). The curve corresponding to the minimum risk was obtained by adjusting the first curve to the risk observed in Florida, choosing the highest concentration (40 N. fowleri amoebae/liter). The adjustment factors of the curve were calculated by the least-squares method.
RESULTS
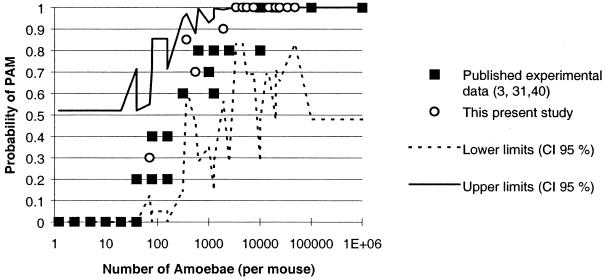
Inoculation of mice with strains of N. fowleri isolated from watercourses in France provided results similar to those of previously published animal experiments. A significant relation between mouse mortality and infecting dose was observed (Table 1). Additionally, the mean time to death increased with decreasing amoeba doses. Our experiments were performed with 10 to 20 mice per dose, more than are usually used in this kind of experiment (compare with 5 mice per dose [3, 44] and 10 mice per dose [31]). This is not, however, a large number from a statistical viewpoint, and thus the percentages obtained vary substantially, as shown by the wide 95% confidence interval of mortality in the sample population, estimated from charts (41) and based on the observed frequencies of PAM in the mice (Fig. 1).
TABLE 1.
Mortality observed in mice after intranasal instillation of different N. fowleri strainsa
| Strain | Isolation (day/mo/yr) | Initial N. fowleri concn in water (NPP/liter) | Dose/mouse | No. of mice | Mortality (%) | MTDb ± SD |
|---|---|---|---|---|---|---|
| D98.2.1.h10 | 14/05/98 | 871 | 47,600 | 20 | 100 | 6.9 ± 0.71 |
| D98.1.3.f13∗ | 05/03/98 | 95 | 34,000 | 13 | 100 | 5.3 ± 0.75 |
| G98.1.1.h1 | 12/03/98 | 440 | 24,000 | 9 | 100 | 5.4 ± 0.52 |
| G98.1.2.e23 | 12/03/98 | 34 | 20,000 | 11 | 100 | 5.3 ± 0.46 |
| G98.2.3.f8a | 06/05/98 | 588 | 14,700 | 10 | 100 | 5.2 ± 0.42 |
| D98.2.1.h10 | 14/05/98 | 871 | 13,300 | 10 | 100 | 5.9 ± 0.56 |
| S-L98.2.1.e10a | 08/09/98 | 46.5 | 7,500 | 10 | 100 | 7.5 ± 0.97 |
| Chi98.2.1.d8a | 08/09/98 | 45.6 | 5,500 | 10 | 100 | 76 ± 1.26 |
| Cat98.1.2.d6b | 24/08/98 | 0.5 | 5,500 | 10 | 100 | 9.4 ± 1.26 |
| D98.2.1.h10 | 14/05/98 | 871 | 4,570 | 20 | 100 | 11.7 ± 5.56 |
| S-L98.2.1.f17 | 08/09/98 | 46.5 | 3,400 | 20 | 100 | 7.6 ± 1.04 |
| S-L98.2.1.f17 | 08/09/98 | 46.5 | 1,930 | 10 | 90 | 7.55 ± 1.42 |
| D98.2.1.h10 | 14/05/98 | 871 | 550 | 20 | 70 | 16.3 ± 7.36 |
| S-L98.2.1.f17 | 08/09/98 | 46.5 | 370 | 20 | 85 | 9.3 ± 2.03 |
| S-L98.2.1.f17 | 08/09/98 | 46.5 | 70 | 20 | 30 | 11.5 ± 2.26 |
Italics indicate initial experiments effected without successive dilutions.
MTD, mean time to death (in days).
FIG. 1.
Probability of PAM in mice (with 95% confidence interval [CI]) as a function of amoebic dose instilled.
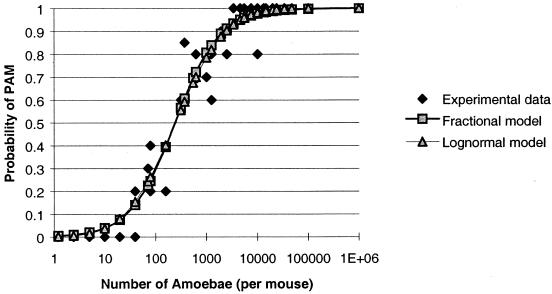
All the experimental results were used for the modeling. The best results were obtained with the maximum-likelihood method in the fractional and lognormal models. The Weibull, Beta, and uniform models were not considered valid by the Kolmogorov-Smirnov test. Figure 2 reports for both the retained models the risk of PAM in mice as a function of the number of amoebae inhaled. Because these results were similar, we used the lognormal model, which gave the best coefficient of correlation (0.96) and the best analysis of residuals.
FIG. 2.
Modeling of the probability of PAM in mice as a function of amoebic number with two mathematical models.
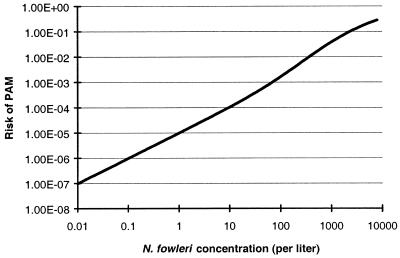
The risk of PAM during a human swimming episode was calculated as described above. Figure 3 shows the risk of PAM calculated as a function of the concentration of amoebae in the water. This first curve contains no correction factor for the extrapolation from mice to humans.
FIG. 3.
Modeling the risk of PAM, as a function of amoebic concentration in water without any correction factor for extrapolation from mice to humans.
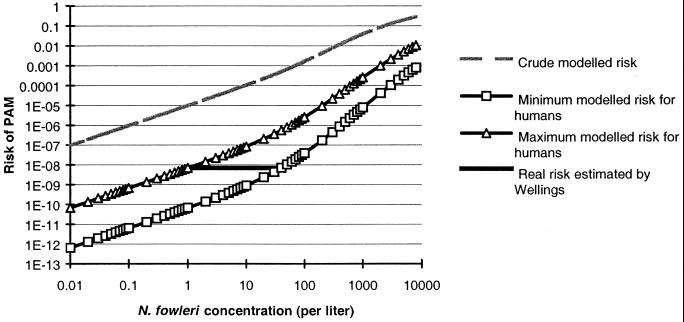
The values of the maximum and minimum extrapolation factors were found to be 20 and 88, respectively. A realistic estimation of the PAM risk as a function of the N. fowleri concentration should be located between these two curves, which correspond to the minimum and maximum estimation of risk in humans (Fig. 4).
FIG. 4.
Risk of PAM in humans by swimming as a function of amoebic concentration in water.
In the process of risk assessment, the precautionary principle is routinely applied by selecting the highest risk estimates in cases of uncertainty. Accordingly, when we choose the “maximum” model, the risk of PAM associated with swimming once in water containing a concentration of 10 N. fowleri amoebae per liter is 8.5 × 10−8. For 100 N. fowleri per liter, it reaches 2.5 × 10−6, and for 1,000 N. fowleri amoebae per liter, it reaches 2.6 × 10−4.
DISCUSSION
This article reports for the first time a quantitative estimate of the risk of PAM associated with swimming, as a function of the N. fowleri concentration in the water. This estimate required that we make several assumptions, clearly stated, about the values of the parameters involved in calculating this risk.
The first curve, which was based on animal data, was adjusted according to the published estimate based on human epidemiologic data (43). Two principal reasons justify this.
First, several articles lead us to conclude that humans are more resistant to MAP than mice are. For example, no case of PAM associated with N. australiensis or N. italica has so far been described among humans, although these species are pathogenic for mice (15). Moreover, while many studies (6, 9, 18, 34, 39) have shown the presence of anti-N. fowleri antibodies in humans, these have not been found in laboratory animals; this may explain the greater resistance among humans. Antibodies are important because they may immobilize the amoebae and slow their migration. Slower migration might make effective treatment possible if the infection is diagnosed in time. Antibodies may also enhance complement lysis of amoebae (42).
Second, the data collected in southern Australia about infection through a public water system confirm our estimate: 10 PAM cases (of the 19 reported for the entire continent) and a number of exposures of the order of 3 billion (1.5 million inhabitants, for the region of Adelaide, over a 15-year period, taking at least 100 baths or showers, with water inhalation, per year). The concentrations measured varied from 1 to 400 amoebae per liter.
We have bounded the risk interval observed in Florida by minimum and maximum N. fowleri concentrations. Although Wellings found that many samples of large volumes of water were negative for this amoeba, we chose a lower limit of 1 amoeba per liter, because it corresponds to the threshold detection limit for the MPN methods routinely used. Furthermore, the exposures, or bathing episodes, are essentially linked to the warmest periods, that is, to the season when amoeba concentrations are highest. Finally, for low concentrations, the yields of detection methods are low (38), so that concentrations that do not take the method's yield into account are underestimated.
When we consider the construction of the model itself, we note that the risks calculated for the low concentrations are based primarily on the estimated risk associated with the inhalation of a single amoeba. Can one N. fowleri alone cause PAM? Ferrante (22) considers that significant doses of amoebae must be inhaled to cause the disease. The inhalation of sediment particles that serve as a support for the attachment and growth of amoebae may fulfill this condition. The formation of such microaggregates, made up of several dozen or even several hundred amoebae, is observed on floating particles even in axenic cultures and can occur fortuitously under natural conditions, especially in the absence of current. The inhalation of these particles, after they have become suspended in the water, may be associated with particular sporting activities (diving, underwater swimming, etc.) and might thus directly provide the minimum infectious dose that Ferrante considers to exist. By supposing that a single amoeba can cause the illness, the model tends to overestimate the risk, as indeed the procedures for risk assessment call for in the presence of uncertainty. We must nonetheless bear in mind that the model was then adjusted on the basis of human data from Florida, and that the Australian data, based on slightly different exposure processes, yield a risk estimate concordant with ours.
Finally, the risk of death from PAM has been modeled from animal data. Although the three studies previously published yielded similar results, they were based on a very small number of mice (5 to 10), which resulted in a wide confidence interval. Moreover, the strains of N. fowleri used for these nasal instillations had been passed previously through the brains of mice, a treatment known to increase the virulence of this amoeba (33). In swimming episodes, the public is exposed to environmental or wild amoebae, which may be less pathogenic. Conversely, it has been established that the virulence of trophozoites diminishes with time, as subculturing takes place in axenic cultures (11). For this reason, we tried to follow a procedure similar to the natural circumstances of infection to assess the virulence of the strains from different French water sources; that is, mice were exposed to amoebae as soon as they were axenized, after isolation and identification. Under these conditions, the results we obtained with groups of 20 mice per dose are completely equivalent to those of previous studies, but their 95% confidence interval is narrower (Table 1 and Fig. 1). The study by John and Nussbaum (32), who exposed mice by making them swim in water containing different concentrations of N. fowleri for varying durations, confirms our results.
Conclusion.
The extrapolation of animal observations at high doses to low doses by modeling is an essential chain in the procedure of health risk assessment, now commonly used in the domain of cancer. Microbiology reasons more often in terms of a minimal infecting dose, but it is not certain that this concept has any reality for many microorganisms. Thus, if we accept that one N. fowleri can, after local multiplication, cause PAM, this type of extrapolation is justified.
In addition, the decision to adjust the animal experimental curve to make it correspond to the available human data is clearly appropriate, for the initial animals results were much greater than the apparent risk to humans. From the point of view of risk management, it appears appropriate to choose to model the maximum human risk.
Based upon this quantitative risk assessment, the French health authorities have set a maximum level of 100 N. fowleri amoebae per liter, not to be exceeded in watercourses where human exposure is possible.
REFERENCES
- 1.Barnett N D P, Kaplan A M, Hopkin R J, Saubolle M A, Rudinsky M F. Primary amoebic meningoencephalitis with Naegleria fowleri: clinical review. Pediatr Neurol. 1996;15:230–234. doi: 10.1016/s0887-8994(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 2.Brown T J, Cursons R T, Keys E A, Marks M, Miles M. The occurrence and distribution of pathogenic free-living amoebae in thermal areas of North Island of New Zealand. NZ J Mar Freshwater Res. 1983;17:59–69. [Google Scholar]
- 3.Carter R F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis and of the experimental pathological changes induced by it. J Pathol. 1970;100:217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- 4.Carter R F. Primary amoebic meningoencephalitis. Trans R Soc Trop Med Hyg. 1972;66:193–213. doi: 10.1016/0035-9203(72)90147-2. [DOI] [PubMed] [Google Scholar]
- 5.Carter R F. Primary amoebic meningoencephalitis: clinical pathological and epidemiological features of six fatal cases. J Pathol Bacteriol. 1968;96:1–25. doi: 10.1002/path.1700960102. [DOI] [PubMed] [Google Scholar]
- 6.Cerva L. Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. J Hyg Epidemiol Microbiol Immunol. 1989;33:99–103. [PubMed] [Google Scholar]
- 7.Cerva L. Amoebic meningoencephalitis: sixteen fatalities. Science. 1968;160(823):92. doi: 10.1126/science.160.3823.92. [DOI] [PubMed] [Google Scholar]
- 8.Cerva L. Studies of limax amoebae in a swimming pool. Hydrobiologia. 1971;38:141–161. [Google Scholar]
- 9.Cursons R T, Brown T J, Keys E A, Moriarty K M, Till D. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun. 1980;29:401–407. doi: 10.1128/iai.29.2.401-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darby C P, Conradi S E, Holbrook T W, Chatellier C. Primary amebic meningoencephalitis. Am J Dis Child. 1979;133:1025–1027. doi: 10.1001/archpedi.1979.02130100049010. [DOI] [PubMed] [Google Scholar]
- 11.De Jonckheere J F. Differences in virulence of Naegleria fowleri. Pathologie Biologie. 1979;27:453–458. [PubMed] [Google Scholar]
- 12.De Jonckheere J F. Occurrence of Naegleria and Acanthamoeba in aquaria. Appl Environ Microbiol. 1979;38:590–593. doi: 10.1128/aem.38.4.590-593.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Jonckheere J F. Pathogenic and nonpathogenic Acanthamoeba sp. in thermally polluted discharges and surface waters. J Protozool. 1981;28:56–59. doi: 10.1111/j.1550-7408.1981.tb02804.x. [DOI] [PubMed] [Google Scholar]
- 14.De Jonckheere J F. Studies on pathogenic free-living amoebae in swimming pools. Bull Inst Pasteur. 1979;77:385–392. [Google Scholar]
- 15.De Jonckheere J F, Aerts M, Martinez A J. Naegleria australiensis: experimental meningoencephalitis in mice. Trans R Soc Trop Med Hyg. 1983;77:712–716. doi: 10.1016/0035-9203(83)90212-2. [DOI] [PubMed] [Google Scholar]
- 16.De Jonckheere J F, Van De Voorde H. The distribution of N. fowleri in man-made thermal waters. Am J Trop Med Hyg. 1977;76:10–15. doi: 10.4269/ajtmh.1977.26.10. [DOI] [PubMed] [Google Scholar]
- 17.DeNapoli T S, Robinson J R, Rutman J Y, Rhodes M M. Primary amoebic meningoencephalitis after swimming in the Rio Grande. Tex Med. 1996;92(10):59–63. [PubMed] [Google Scholar]
- 18.Dubray B L, Wilhelm W E, Jennings B R. Serology of Naegleria fowleri and Naegleria lovaniensis in a hospital survey. J Protozool. 1987;34:322–327. doi: 10.1111/j.1550-7408.1987.tb03183.x. [DOI] [PubMed] [Google Scholar]
- 19.Duma R J. The epidemiology of naturally acquired human, waterborne meningoencephalitis due to thermophilic, pathogenic Naegleria. In: Gerhold R, editor. Proceedings of Symposium of Microbiology of Power Plant Thermal Effluents. Iowa City: University of Iowa Press; 1978. pp. 107–116. [Google Scholar]
- 20.Duma R J. Study of pathogenic free-living amebas in fresh-water lakes in Virginia. U.S. Washington, D.C.: Environmental Protection Agency; 1981. [Google Scholar]
- 21.Duma R J, Ferrell H W, Nelson E C, Jones M M. Primary amoebic meningoencephalitis. N Engl J Med. 1969;281:1315–1323. doi: 10.1056/NEJM196912112812401. [DOI] [PubMed] [Google Scholar]
- 22.Ferrante A. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 1991;13:31–47. doi: 10.1111/j.1365-3024.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 23.Fowler M, Carter R F. Acute pyogenic meningitis probably due to Acanthamoeba sp. A preliminary report. BMJ. 1965;2(5464):740–742. doi: 10.1136/bmj.2.5464.734-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerba C P, Rose J B, Haas C N, Crabtree K D. Waterborne rotavirus: a risk assessment. Water Res. 1996;30:2929–2940. [Google Scholar]
- 25.Gibson C, III, Haas C, Rose J. Risk assessment of waterborne protozoa: current status and future trends. Parasitology. 1998;117:S205–212. doi: 10.1017/s0031182099004746. [DOI] [PubMed] [Google Scholar]
- 26.Haas C N. Estimation of risk due to low doses of microorganisms: a comparison of alternative methodologies. Am J Epidemiol. 1983;118:573–582. doi: 10.1093/oxfordjournals.aje.a113662. [DOI] [PubMed] [Google Scholar]
- 27.Haas C N, Crockett C S, Rose J B, Gerba C P, Fazil A M. Assessing the risk posed by oocysts in drinking water. J Am Water Works Assoc. 1996;88:131–136. [Google Scholar]
- 28.Haas C N, Rose J B, Gerba C, Regli S. Risk assessment of virus in drinking water. Risk Anal. 1993;13:545–552. doi: 10.1111/j.1539-6924.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 29.Hermanne J. Primary amoebic meningo-encephalitis. Clinical aspects. Ann Soc Belg Med Trop. 1974;54:287–295. [PubMed] [Google Scholar]
- 30.Jadin J B. Primary amoebic meningo-encephalitis and the pathogenicity of water-borne amoebas. Bull Acad R Med Belg. 1974;126:439–466. [PubMed] [Google Scholar]
- 31.John D T, Hoppe K L. Susceptibility of wild mammals to infection with Naegleria fowleri. J Parasitol. 1990;76:865–868. [PubMed] [Google Scholar]
- 32.John D T, Nussbaum S L. Naegleria fowleri infection acquired by mice through swimming in amoebae-contaminated water. J Parasitol. 1983;69(5):871–874. [PubMed] [Google Scholar]
- 33.Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988;52:114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marciano-Cabral F, Cline M L, Bradley S G. Specificity of antibodies from human sera for Naegleria species. J Clin Microbiol. 1987;25:692–697. doi: 10.1128/jcm.25.4.692-697.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez A J, Visvesvara G S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:538–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicoll A M. Fatal primary amoebic meningoencephalitis. N Z Med J. 1973;78(496):108–112. [PubMed] [Google Scholar]
- 37.Pernin P, Grelaud G. Application of isoenzymatic typing to the identification of nonaxenic strains of Naegleria (Protozoa, Rhizopoda) Parasitol Res. 1989;75:595–598. doi: 10.1007/BF00930954. [DOI] [PubMed] [Google Scholar]
- 38.Pernin P, Pélandakis M, Rouby Y, Faure A, Siclet F. Comparative recoveries of Naegleria fowleri amobae from seeded river water by filtration and centrifugation. Appl Environ Microbiol. 1998;64:955–959. doi: 10.1128/aem.64.3.955-959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reilly M F, Marciano-Cabral F, Bradley D W, Bradley S G. Agglutination of Naegleria fowleri and Naegleria gruberi by antibodies in human serum. J Clin Microbiol. 1983;17:576–581. doi: 10.1128/jcm.17.4.576-581.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose J B, Gerba C P. Use of risk assessment for development of microbial standards. Water Sci Technol. 1991;24:29–34. [Google Scholar]
- 41.Saporta G. Probabilités, analyses des données et statistiques. France: Technip; 1990. [Google Scholar]
- 42.Toney D M, Marciano-Cabral F. Membrane vesiculation of Naegleria fowleri amoebae as a mechanism for resisting complement damage. J Immunol. 1994;152:2952–2959. [PubMed] [Google Scholar]
- 43.Wellings F M, Amuso P T, Chang S L, Lewis A L. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977;34:661–667. doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellings F M, Amuso P T, Lewis A I, Farmelo M J, Moody D J, Osikowicz C L. Pathogenic Naegleria: distribution in nature. U.S. Cincinnati, Ohio: Environmental Protection Agency; 1979. [Google Scholar]