Abstract
Plant health is strongly impacted by beneficial and pathogenic plant microbes, which are themselves structured by resource inputs. Organic fertilizer inputs may thus offer a means of steering soil-borne microbes, thereby affecting plant health. Concurrently, soil microbes are subject to top-down control by predators, particularly protists. However, little is known regarding the impact of microbiome predators on plant health-influencing microbes and the interactive links to plant health. Here, we aimed to decipher the importance of predator-prey interactions in influencing plant health. To achieve this goal, we investigated soil and root-associated microbiomes (bacteria, fungi and protists) over nine years of banana planting under conventional and organic fertilization regimes differing in Fusarium wilt disease incidence. We found that the reduced disease incidence and improved yield associated with organic fertilization could be best explained by higher abundances of protists and pathogen-suppressive bacteria (e.g. Bacillus spp.). The pathogen-suppressive actions of predatory protists and Bacillus spp. were mainly determined by their interactions that increased the relative abundance of secondary metabolite Q genes (e.g. nonribosomal peptide synthetase gene) within the microbiome. In a subsequent microcosm assay, we tested the interactions between predatory protists and pathogen-suppressive Bacillus spp. that showed strong improvements in plant defense. Our study shows how protistan predators stimulate disease-suppressive bacteria in the plant microbiome, ultimately enhancing plant health and yield. Thus, we suggest a new biological model useful for improving sustainable agricultural practices that is based on complex interactions between different domains of life.
Subject terms: Microbial ecology, Microbiome
Introduction
Healthy soil is the basis for agricultural production [1], providing essential resources for human wellbeing, such as food, feed and fibre [2]. The demand of the rapidly growing human population for food further stresses the critical role of soil in food production [3]. Conventional agriculture plays an important role in meeting the food demands of a growing human population [4], but largely depends on synthetic fertilizers, pesticides and herbicides, resulting in severe environmental pollution and, in the long run, reduced ecosystem functioning [5, 6]. Organic farming provides a potentially more sustainable alternative to conventional farming [7] by enhancing soil biodiversity and beneficial ecological interactions, thereby reducing negative environmental impacts compared with conventional management [8, 9].
Common to agricultural practices is the need to reduce soil-borne pathogens that pose a major threat to food production [10, 11]. Organic agricultural practices rely on enhancing the soil and root-associated beneficial microbiome, and the ecological interactions therein, thereby avoiding the adverse side effects of pesticides on the environment and human health caused by conventional agricultural practices [12–14]. Several microbial genera (e.g. Bacillus and Pseudomonas) are involved in disease suppression, making them the basis of biocontrol in organic agriculture [15, 16]. Through these and other functions, such as shaping the microbiome through versatile secondary metabolites [17–19], these microbial taxa play major roles in soil system functioning and have therefore been termed keystone species [20–22]. Microbial secondary metabolites have many important ecological roles [23]. For instance, they fight against other microbial competitors [24, 25] and act as signaling molecules in inter- and intraspecies interactions [26]. Nonribosomal peptides are among the microbial secondary metabolites produced by the keystone species mentioned above [27–29]. Antimicrobial nonribosomal peptides, such as the antibiotics vancomycin, gramicidin and lipopeptides (surfactin, iturin A and bacillomycin), can be used as microbicides to inhibit plant pathogens [30–33].
Soil protists might indirectly affect pathogens and plant health by altering the soil microbiome composition and thereby secondary metabolite production [34–36]. Indeed, protists represent the main predators of soil bacteria and fungi [37]. Predatory protists in the plant rhizosphere have also been reported to positively link with plant-beneficial microbes that improve plant performance [34, 38]. Through the production of secondary metabolites, these microbes not only suppress soil-borne pathogens [15], but also defend themselves against predation by protists [39]. However, since plant-beneficial bacteria have been shown to produce metabolites that, in addition to suppress soil-borne pathogens, also defend themselves against protistan predation, it is worth disentangling the interactions between plant-beneficial bacteria and their protistan predators.
In this study, we used Illumina amplicon sequencing and qPCR to investigate the diversity and community composition of bacteria, fungi and protists as well as the abundance of the major crop pathogen Fusarium oxysporum (F. oxysporum) in the bulk soil, rhizosphere and endosphere of banana plants, in fields treated with chemical and organic fertilizers. In addition, we performed Illumina metagenomic sequencing to explore the functional potential of the microbiome and differences between treatments of selected rhizosphere samples. These analyses sought to identify microbiome components and functions linked with disease suppression and plant health. We performed follow-up greenhouse experiments to validate the observed importance of potential plant health-influencing interactions between protists and their microbial prey, with special attention to microbial functions related to disease suppression. We hypothesized that organic management enhances predator-prey interactions as compared to conventional management, thereby resulting in healthier plants via the improved functional capabilities of the microbiome.
Results
Disease incidence, banana yield and pathogen density
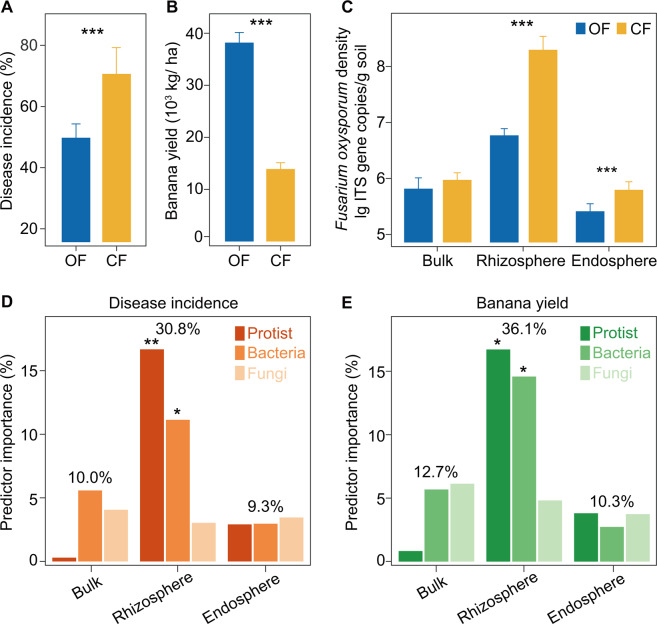
The organic fertilizer treatment significantly reduced Fusarium wilt disease incidence and increased banana yield compared with the chemical fertilizer treatment (Student’s t test: p < 0.05; Fig. 1A, B). F. oxysporum density was marginally affected by the different farming systems in bulk soils (Student’s t test: p = 0.059), and significant differences were observed between treatments in the rhizosphere and endosphere (Student’s t test: p < 0.05; Fig. 1C). With respect to habitat compartment, random forest analysis indicated that F. oxysporum density in the rhizosphere had the strongest predictive importance for Fusarium wilt disease incidence and banana yield (p < 0.05; Supplementary Fig. 1).
Fig. 1. Effects and underlying drivers of organic and chemical fertilizer treatments on Fusarium wilt disease incidence and banana yield.
A Disease incidences of banana Fusarium wilt in organic and chemical fertilizer treatments. B Banana yield in organic and chemical fertilizer treatments. C Fusarium oxysporum density in organic and chemical fertilizer treatments in the three compartments. D, E The random forest mean predictor importance (% increase of the MSE) of bacterial, fungal and protistan community composition for banana Fusarium wilt disease incidence (D) and banana yield (E) in the three compartments. In panels A, B and C, asterisks indicate significant differences as defined by the Student’s t test (***p < 0.001). OF Organic fertilizer treatment, CF Chemical fertilizer treatment. In panels D, E, to estimate the importance of microbial predictors, we used the percentage increases in the MSE (mean squared error). Significance levels of each predictor are represented by *p < 0.05 or **p < 0.01.
Microbial diversity and community composition and underlying drivers of disease incidence and banana yield
Compartment and fertilization were the main drivers of the community composition of all microbial groups (bacteria, fungi and protists; PERMANOVA: p < 0.05; Supplementary Table 1 and Supplementary Fig. 2), but did not alter their alpha diversity (two-way ANOVA: p > 0.05; Supplementary Table 2). Subsequent analyses therefore focused on changes in microbial community composition.
With respect to habitat compartment, random forest analysis indicated that the microbial community composition in the rhizosphere was the strongest predictor of disease incidence and banana yield (disease incidence: 30.8% increase of the mean squared error (MSE); banana yield: 36.1% increase of the MSE; Fig. 1D, E). In particular, protistan and bacterial community compositions had a high level of explanatory power with respect to disease incidence and banana yield (p < 0.05), with protistan communities most strongly predicting disease incidence (16.7% increase of the MSE) and banana yield (16.7% increase of the MSE; Fig. 1D, E). In contrast, bulk protistan community composition was the weakest predictor of disease incidence and banana yield (random forest analysis; disease incidence: 0.3% increase of the MSE; banana yield: 0.8% increase of the MSE; Fig. 1D, E). Given these results, we focused subsequent analyses on bacterial and protistan communities in the rhizosphere compartment.
Protistan and bacterial taxonomic and functional composition and links with pathogen density
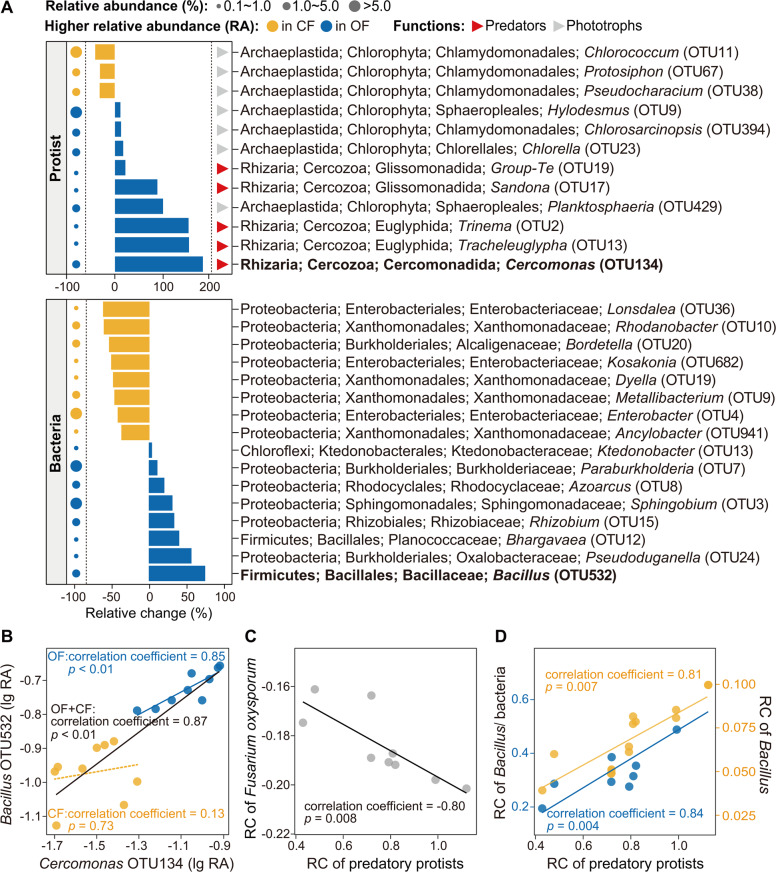
Linear discriminant analysis Effect Size (LEfSe) of the rhizosphere communities indicated that the relative abundances of nine protistan OTUs and eight bacterial OTUs were significantly higher in the organic than in the chemical fertilizer treatment (p < 0.05; Fig. 2A). Also, the relative abundances of three protistan OTUs and eight bacterial OTUs were significantly higher in the chemical than in the organic fertilizer treatment (LEfSe analysis: p < 0.05; Fig. 2A). In particular, the predatory protist Cercomonas (Rhizaria; Cercozoa; Cercomonadida: increased of 182% in the organic fertilizer treatment) and the bacterial taxon Bacillus (Firmicutes; Bacillales; Bacillaceae: increased of 75% in the organic fertilizer treatment; Fig. 2A). Spearman’s correlation analysis showed that the increase of the relative changes of Cercomonas and Bacillus were correlated with the relative decrease of disease incidence and the relative increase of banana yield (p < 0.05; Supplementary Table 3). Multi-bipartite model analysis of the food web was used to further identify potential links between protists, bacteria and F. oxysporum. This analysis revealed that Cercomonas was positively correlated with Bacillus in the organic fertilizer treatment and overall (organic fertilizer + chemical fertilizer) (Spearman’s correlation: p < 0.05), but not in the chemical fertilizer treatment (Spearman’s correlation: p > 0.05; Fig. 2B and Supplementary Fig. 3). Moreover, multi-bipartite model analysis indicated that Bacillus was negatively correlated with F. oxysporum in the organic fertilizer treatment and overall (organic fertilizer + chemical fertilizer) (Spearman’s correlation: p < 0.05; Supplementary Fig. 3). Furthermore, for the protistan functional groups, the organic fertilizer treatment increased the relative abundance of predatory protists (increased of 76.4% in the organic fertilizer treatment; mainly caused by cercozoan Cercomonadida and Glissomonadida) and decreased the relative abundance of phototrophs (decreased of 16.9% in the organic fertilizer treatment; mainly caused by chorophyte Chlamydomonadales) and plant pathogens (decreased of 39.0% in the organic fertilizer treatment; mainly caused by oomycetan Peronosporales) compared with the chemical fertilizer treatment (Student’s t test: p < 0.05; Supplementary Fig. 4 and Supplementary Table 4). Spearman’s correlation analysis showed that the increase of the relative change of predatory protistan relative abundance was correlated with the relative decrease of F. oxysporum density, and the relative increase of Bacillus density and the ratio of Bacillus density to total bacteria density (p < 0.05; Supplementary Table 5, Fig. 2C, D).
Fig. 2. Comparison of protistan and bacterial community composition and correlations of key protistan and bacterial OTU, predatory protists and Fusarium oxysporum density, and predatory protists and Bacillus/ bacteria.
A The effects of different fertilization treatments on the relative abundance of protistan and bacterial OTUs in the rhizosphere. B Correlations between key indicator protistan and bacterial OTUs of different fertilization treatments in the rhizosphere multi-bipartite model of the food web. C, D Correlations between the relative change of the relative abundance of protistan functional groups and the relative change of Fusarium oxysporum density (C), Bacillus density and the ratio of Bacillus density to total bacteria density (D) in the rhizosphere. In panels A, B, the relative abundances of protistan and bacterial OTUs were significantly higher in the organic and chemical fertilizer treatments based on linear discriminant analysis (higher relative abundance in the organic fertilizer treatment: LDA score > 3.0 and p < 0.05; higher relative abundance in the chemical fertilizer treatment: LDA score < −3.0 and p < 0.05). OF Organic fertilizer treatment; CF Chemical fertilizer treatment. Circles are proportional to the average relative abundance of each OTU. RA Relative abundance, relative change = (OF-CF)/CF. In panels C, D, RC = relative change ((OF-CF)/CF); Bacillus/ bacteria = the ratio of Bacillus density to total bacteria density.
Microbial functional genes and their potential interactions with pathogen density
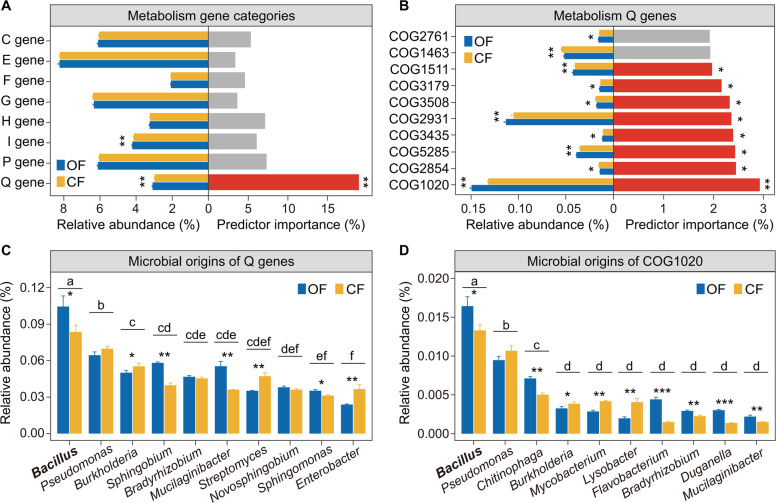
Random forest analysis indicated that Q (secondary metabolite biosynthesis) genes had the strongest predictive importance for F. oxysporum density (p < 0.05, 19.0% increase of the MSE; Fig. 3A) which decreased with the increase of the relative abundance of Q genes (Fig. 3A and Fig. 1C). Moreover, the relative abundance of Q genes was higher in the organic than in the chemical fertilizer treatment (Student’s t test: p < 0.05; Fig. 3A). Furthermore, random forest analysis showed that COG1020 (nonribosomal peptide synthetase gene) was the strongest predictor of F. oxysporum density (p < 0.05, 2.9% increase of the MSE) among all the Q gene categories (Fig. 3B). The relative abundance of COG1020 was higher in the organic than in the chemical fertilizer treatment (Student’s t test: p < 0.05, Fig. 3B). Moreover, ANOVA showed that the relative abundances of Q genes and COG1020 derived from Bacillus populations were significantly higher than those derived from other microbial populations across treatments (Tukey’s HSD test: p < 0.05; Fig. 3C, D). They were higher in the organic fertilizer treatment compared with the chemical fertilizer treatment (Student’s t test: p < 0.05; Fig. 3C, D).
Fig. 3. Microbial functional genes and their potential interactions with pathogen density.
A The random forest mean predictor importance (% increase of the MSE) of the metabolism gene categories for Fusarium oxysporum density and the relative abundance of the metabolism gene categories in different fertilization treatments. B The top 10 most important random forest mean predictors (% increase of the MSE) of the Q gene category of Fusarium oxysporum density and the relative abundance of these genes in different fertilization treatments. C The top 10 microbial origins of metabolism Q genes. D The top 10 microbial origins of COG1020. In panels A, B, asterisks indicate significant differences of relative abundances as defined by the Student’s t test (*indicates p < 0.05, **indicates p < 0.01); for the random forest analysis, to estimate the importance of microbial predictors, we used the percentage increases in the MSE (mean squared error). In panels C, D, bars with different letters indicate significant differences as defined by one-way ANOVA with Tukey’s HSD test (p < 0.05). Asterisks indicate significant differences as defined by the Student’s t test (*indicates p < 0.05, **indicates p < 0.01, ***indicates p < 0.001). OF Organic fertilizer treatment; CF Chemical fertilizer treatment.
Pathogen suppression capability of predatory protists and their potential interactions with Bacillus isolates
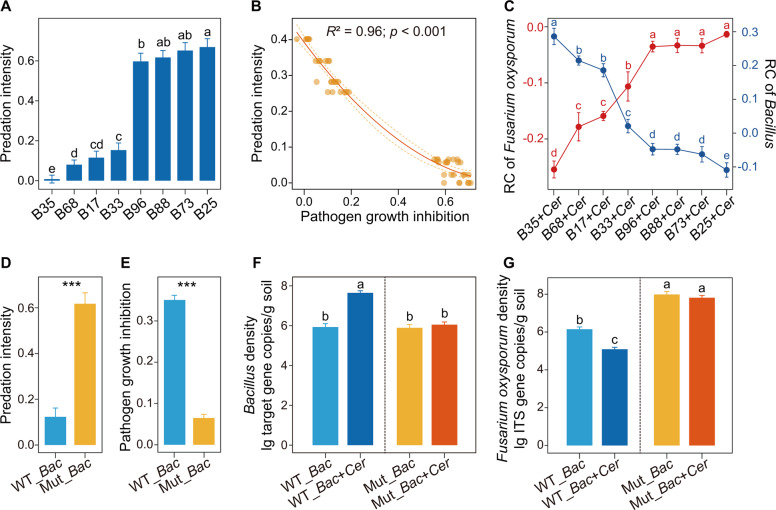
In the first part of our greenhouse experiment, ANOVA showed a clear suppression effect on F. oxysporum for the Cer (102) (Cer: Cercomonas lenta (C. lenta); 102 cells/g dry soil; decrease of 71.4%) and Cer (103) treatments (103 cells/g dry soil; decrease of 85.0%) compared with the control in nonsterilized soils (Tukey’s HSD test: p < 0.05; Fig. 4A), but no clear suppression for the Cer (101) treatment (101 cells/g dry soil; Tukey’s HSD test: p > 0.05; Fig. 4A). When sterilized soils were used in the greenhouse experiment, no significant difference in F. oxysporum density was observed between the S_F. oxysporum and S_F. oxysporum + C. lenta treatments (Student’s t test: p > 0.05; Supplementary Fig. 5A). ANOVA indicated that the Cer (102) (102 cells/g dry soil) and Cer (103) treatments (103 cells/g dry soil) also significantly increased the ratio of Bacillus density to total bacteria density and nonribosomal peptide synthetase (NRPS) gene abundance compared with the control in nonsterilized soils (Tukey’s HSD test: p < 0.05, Fig. 4B, C and Supplementary Fig. 6A, B).
Fig. 4. Pathogen suppression capability of predatory protists and their potential interactions with Bacillus and NRPS gene.
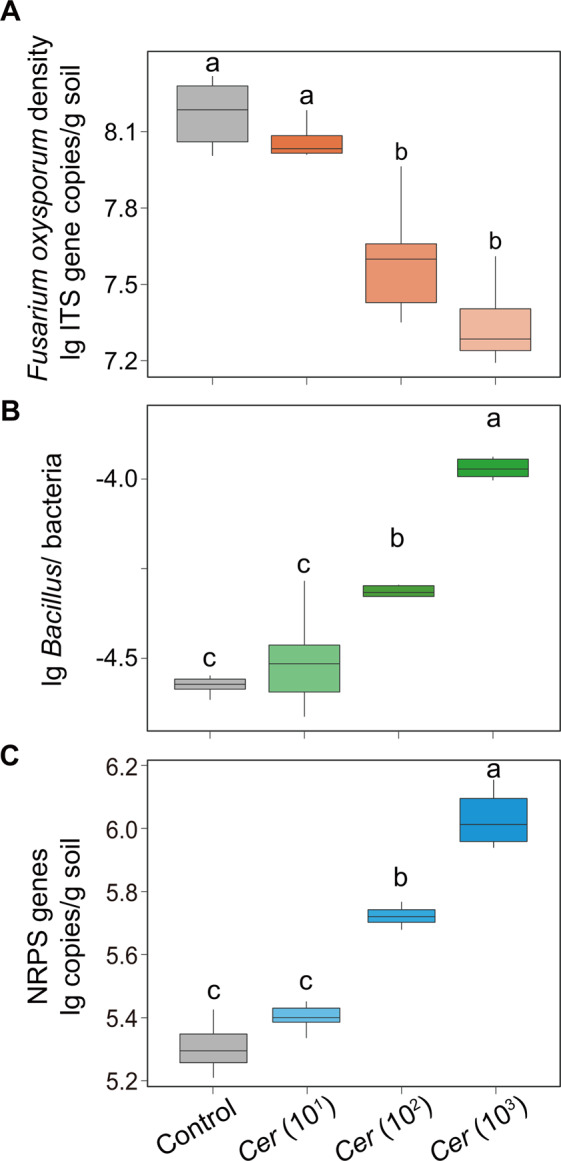
A The effects of different concentrations of predatory protists on Fusarium oxysporum density. B The effects of different concentrations of predatory protists on the ratio of Bacillus density to total bacteria density. C The effects of different concentrations of predatory protists on the abundance of nonribosomal peptide synthetase (NRPS) gene. In panels A–C, bars with different letters indicate significant differences as defined by one-way ANOVA with Tukey’s HSD test (p < 0.05). In the control, no protists were added. Cer (101): Cercomonas lenta strain ECO-P-01 (1.0 × 101 cells g−1 dry soil); Cer (102): Cercomonas lenta strain ECO-P-01 (1.0 × 102 cells g−1 dry soil); Cer (103): Cercomonas lenta strain ECO-P-01 (1.0 × 103 cells g−1 dry soil). Bacillus/ bacteria = the ratio of Bacillus density to total bacteria density.
Furthermore, indigenous Bacillus strains were isolated for follow-up greenhouse experiments. Phylogenetic analysis showed that Bacillus isolate B35 (B. amyloliquefaciens) had 100% sequence identity with the Bacillus OTU (bacterial OTU532; see above Fig. 2). In addition, ANOVA indicated that Bacillus isolate B35 also displayed the strongest ability to inhibit F. oxysporum growth and form biofilms (Tukey’s HSD test: p < 0.05; Supplementary Fig. 7). For the second part of the greenhouse experiment using sterilized soils, ANOVA showed that the predatory protist (C. lenta) had the lowest predation intensity on Bacillus B35 within Bacillus isolates (Tukey’s HSD test: p < 0.05; Fig. 5A and Supplementary Fig. 5B), which was in line with a laboratory experiment using Page’s amoeba saline (PAS) (Supplementary Fig. 5D, E). Random forest and regression analysis indicated that the F. oxysporum growth inhibition ability of Bacillus isolates significantly influenced the predation intensity of the predatory protist (C. lenta) on Bacillus isolates (p < 0.05; Supplementary Fig. 5C, Fig. 5B).
Fig. 5. Interactions between the pathogen, the bacterial isolates and the predator in the greenhouse experiment.
In panels A, C, F and G, bars with different letters indicate significant differences as defined by one-way ANOVA with Tukey’s HSD test (p < 0.05). In panels D, E, asterisks indicate significant differences as defined by the Student’s t test (***p < 0.001). Relative change = (X-control)/control, X = Bacillus + Cercomonas lenta, control = Bacillus only. B Bacillus isolate; Cer: Cercomonas lenta strain ECO-P-01. WT_Bac: wild type Bacillus strain. Mut_Bac: mutant Bacillus strain (disrupted in the bacillomycin D pathway) [54]. Predation intensity = (Yc–Yp)/Yc, where Yc is the Bacillus density in the control, and Yp is the Bacillus density in the Bacillus + Cercomonas lenta treatment [51].
In the third part of the greenhouse experiment, ANOVA showed significant differences in the density of Bacillus after co-inoculation of each of the Bacillus isolates with the predatory protist (C. lenta) (Tukey’s HSD test: p < 0.05; Supplementary Fig. 6C), while no significant differences were detected between the control treatments (the same bacteria but without the protist) (Tukey’s HSD test: p > 0.05; Supplementary Fig. 6C). Additionally, the Bacillus (relative change) increase in density was correlated with the decrease of F. oxysporum (relative change) (regression analysis: p < 0.05, R2 = 0.85; Supplementary Table 6, Fig. 5C and Supplementary Fig. 6D).
In the fourth part of the greenhouse experiment, we found that the predation intensity of C. lenta on Mut_Bac (Mut_Bac: mutant Bacillus strain; disrupted in the bacillomycin D pathway) was higher compared with WT_Bac (WT_Bac: wild type Bacillus strain; Student’s t test: p < 0.05; Fig. 5D and Supplementary Fig. 6E), and the F. oxysporum growth inhibition of Mut_Bac was lower compared with WT_Bac (Student’s t test: p < 0.05; Fig. 5E). ANOVA showed that WT_Bac + Cer treatment significantly increased the Bacillus density when compared with WT_Bac treatment (Tukey’s HSD test: p < 0.05), while no significant differences were observed among the WT_Bac, Mut_Bac and Mut_Bac + Cer treatments in nonsterilized soils (Tukey’s HSD test: p > 0.05; Fig. 5F). Moreover, ANOVA indicated that the WT_Bac and WT_Bac + Cer treatments significantly decreased the F. oxysporum density compared with the Mut_Bac and Mut_Bac + Cer treatments in nonsterilized soils (Tukey’s HSD test: p < 0.05; Fig. 5G). Furthermore, ANOVA showed that the WT_Bac + Cer treatment significantly decreased the F. oxysporum density compared with WT_Bac treatment (Tukey’s HSD test: p < 0.05), while no significant difference was detected between the Mut_Bac and Mut_Bac + Cer treatments in nonsterilized soils (Tukey’s HSD test: p > 0.05; Fig. 5G).
Discussion
We here demonstrate that predator-prey interactions stimulated by organic fertilizer inputs change microbiome functioning and improve plant health, which were overall in line with our hypotheses.
More specifically, we show that organic fertilization supports a disease suppressive and consequently plant health-increasing soil microbiome, which supports former findings [11, 40, 41]. The increases of particularly predatory protists is also in line with previous studies and can be explained by increased prey availability and physicochemical niche space [42–44]. The observed positive effects of organic fertilization were not equally important for all soil microbes in all plant and soil habitats. In fact, we found that especially protists and bacteria in the rhizosphere, rather than bulk or endosphere microbes determine disease incidence and plant yield. There is a common notion that the rhizosphere is the main area where soil-borne pathogens compete with beneficial microbiota [45, 46], but interestingly the tightly plant-associated root endosphere, where many mutualists and pathogens directly invade the plant [47], seems of less direct importance in controlling plant health. This suggests that the determination of the composition of mutualistic and pathogenic microbes and their function is mainly determined in the rhizosphere, which we here show is determined by protists. Whether protists are also of key importance to shape the plant-beneficial rhizosphere microbiome beyond the system of banana with Fusarium wilt disease represents a key field for future research. Yet, previous studies showing that the importance of protistan communities in predicting tomato health in presence of pathogenic Ralstonia solanacearum [34] supports this hypothesis.
Notably, in our study we identified precise protistan predatory taxa and their importance in regulating bacteria with potential to suppress pathogens. Our findings confirm the pivotal role of rhizosphere Cercozoa, a major group of protists in soil [48, 49] and key microbiome links in agricultural systems [50], for plant health. We show that the disease-suppressive function of Cercozoa is likely linked to increases of disease-suppressive bacteria, especially Bacillus. In our greenhouse study we could show that Cercozoa enhanced Bacillus likely by preying preferentially on other bacteria which led to increased production of secondary compounds that reduced pathogen abundances. This adds to our recent finding that cercozoan taxa increased cucumber yield effectively via their specific enhancement of plant-beneficial microorganisms (in that study Trichoderma) [43]. Also the idea that protists selectively prey [38, 51] and commonly enhance secondary metabolite-producing bacteria [39] is supported in our study. This suggests that effects of predatory protists on disease suppression might be a general phenomenon in soils, making protists keystone species that are worth exploring as biocontrol agents to increase sustainable soil management.
Prominently, we found that the pathogen population density was negatively affected by the secondary metabolite biosynthesis gene abundance, particularly non-ribosomal peptide syntheses (NRPS) genes. Microbial secondary metabolite biosynthesis function has been widely shown to be closely linked with plant pathogen suppression [23, 33, 34]. Previously, plenty of Bacillus species (e.g. B. amyloliquefaciens, B. subtilis and B. velezensis) were reported to produce antimicrobial compounds (e.g., iturin and bacillomycin) [52–54], which provide them with a competing advantage against plant pathogens [55, 56]. Here, we show the strong influence of protists in inducing higher expression levels of these genes, suggesting that these secondary genes have evolved to combat predation. In fact, antimicrobial compounds can reduce predation, such as by inducing encystation or paralysis of protist predators [38, 39]. We further confirmed the effectiveness of these defense compounds since the Bacillus mutants deficient in producing bacillomycin D (regulated by NRPS genes) were more preyed on by protists than wild type strains. Thus, we propose that the NRPS gene-induced pathogen suppression represents a plant-beneficial side effect of bacterial anti-predator defence.
Conclusions
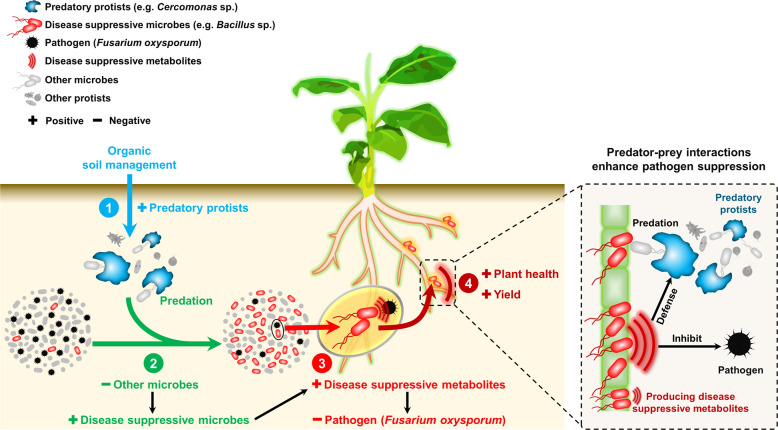
We demonstrate that organic fertilization can stimulate predatory interactions that catalyze keystone interactions between protistan predators and pathogen-antagonistic bacteria that together can reduce pathogenic fungi and thereby increase plant health and productivity. We have summarized the results of our experiments in a conceptual model (Fig. 6). Future transcriptomic and proteomic approaches will delineate the exact nature of the molecular dialogue between protists and bacteria and how their combined action serves to confer disease suppression. Together, our work shows the potential to improve current biocontrol practices based on the application of Bacillus by adding protists to enhance secondary metabolite production. As such, we envision that our results provide a novel viewpoint to manipulate the soil microbiome by a combined focus on microbial predators and pathogen suppressors, which together can help increase the disease-suppressive functions of agroecosystems in a sustainable way.
Fig. 6. Conceptual model.
Conceptual model depicting the mechanisms illustrating how selective grazing by protists on rhizosphere bacteria favours pathogen-antagonistic bacteria, ultimately inhibiting plant pathogens.
Materials and methods
Site description and experimental design
The experimental site was located in Jianfeng town, Ledong County (18°38´N, 108°45´E), one of the most important banana production areas in Hainan Province, China. This region has a tropical monsoon climate with an average annual temperature and precipitation of 24 °C and 1150 mm, respectively. The soil has been classified as a sandy loam that developed from dry red soil. The field experiment was performed with organic and conventional long-term treatments over nine successive years from 2011 to 2019. Briefly, two treatments were used in this study as follows: organic fertilizer treatment (OF, soil amended with chicken manure organic fertilizer) and chemical fertilizer treatment (CF, soil amended with chemical fertilizer). Detailed information about the kinds and amounts of these composts and chemical fertilizers is listed in the supplementary material (Supplementary Table 7). Organic and chemical fertilizers were applied annually in the spring one week before sowing crops. Fertilizer was spread uniformly and then evenly distributed over the soil surface and mixed with the soil layer to a depth of 25–30 cm by turning the soil manually. All other farm operations were managed using traditional farming methods. The detailed fertilization scheme and field management have been described in a previous study [57].
Disease incidence and banana yield determination
Banana wilt disease was identified based on the appearance of typical wilt symptoms, including brown discolouration of vascular tissues, pseudostem splitting, leaf yellowing and plant death [58]. The disease incidence was calculated as the percentage of infected plants and determined until the incidence stabilized. All mature banana fruits for each treatment of the year were collected and weighed to calculate the banana yield and the results were expressed as kg/ha.
Soil sampling and DNA extraction
Bulk soil, rhizosphere soil and banana endosphere samples were collected from 9 individual plants per field site in 2019 from the long-term field experiment described above. Bulk soil samples were collected after removing banana plants from the field and then collecting soil randomly from cores to a depth of 10 cm from each replicate. Rhizosphere soil and root samples were obtained as described in previous research [59]. Briefly, the plant roots were shaken vigorously to separate soil that was not tightly adhered to the roots, leaving approximately 1 mm of soil still attached to the roots. We placed the roots with soil still attached in a sterile flask with 50 ml of sterile phosphate-buffered saline (pbs) solution and then vigorously stirred the roots with sterile forceps, cleaning all the soil from the root surfaces. The soil suspension was centrifuged at 8000 rpm for 20 min, and the pellet soil was defined as rhizosphere soil. Plant roots that were recovered were vortexed in consecutive washes of sterile pbs solution, and tightly adhering microbes at the root surface were removed. The roots were sonicated three times at 50–60 Hz for 30 s in sterile pbs solution to remove all rhizoplane soils. The sonication procedure strips the rhizoplane microbes from the root surface as well as portions of the rhizodermis. The remaining roots were placed in Falcon tubes for endosphere DNA extraction. All soil and root samples were stored at −80 °C prior to DNA extraction.
For each soil sample (36 soil samples in total: 18 bulk and 18 rhizosphere soil samples), total genomic DNA was extracted from 0.5 g soil using the PowerSoil DNA Isolation Kit (Mo Bio Laboratories Inc., USA) according to the manufacturer’s instructions. For each root sample (18 root samples), genomic DNA extraction was performed using the Plant DNA Maxi Kit (OMEGA Inc., USA) following the manufacturer’s instructions. We used a NanoDrop ND2000 (Thermo Scientific, Waltham, MA, USA) spectrophotometer to measure the genomic DNA concentration and purity to meet the quantitative real-time PCR amplification (qPCR) assay and Illumina sequencing requirements.
Real-Time PCR assay, amplicon sequencing and metagenome sequencing
Abundances, expressed as copy numbers, of bacteria, F. oxysporum, Bacillus and NRPS genes were determined using the bacterial primers Eub338F/Eub518R [60], F. oxysporum specific primers FOF1/FOR1 [61], Bacillus specific primers Bs16S1/Bs16SR [62] and NRPS gene specific primers A3F/A7R [63], respectively (Supplementary Table 8), following established protocols [16, 28, 64] (Supplementary Table 8) on a qTOWER Real-Time PCR System (Analytik Jena, Germany). The assay for total bacteria, F. oxysporum and Bacillus was performed in a 20 µl reaction mixture containing 10 µl SYBR Premix Ex Taq (2×), 0.8 µl of each primer (10 µmol/L), 0.4 µl ROX Reference Dye II, 2 µl of template DNA and 6 µl of sterile water. The assay for the NRPS genes was performed in a 20 µl reaction mixture containing 10 µl SYBR Premix Ex Taq (2×), 0.4 µl of each primer (20 µmol/L), 0.4 µl ROX Reference Dye II, 1 µl of template DNA and 7.8 µl of sterile water. Standard curves were generated using established protocols [16, 28, 64]. The results were expressed as log10 values (target copy number g−1 dry soil) for further statistical analyses.
Bacterial-, fungal- and eukaryote-wide primer sets were used for high-throughput MiSeq sequencing (Illumina): 520 F and 802 R to amplify prokaryotic 16 S rRNA gene V4 regions [65], ITS1F and ITS2 to amplify fungal ITS1 regions [66, 67], and V4_1f and TAReukREV3 to amplify eukaryotic 18 S rRNA gene V4 regions [68]. While there are no true ‘universal’ primers to target all protistan taxa, we used the broadly targeted primer set (V4_1f and TAReukREV3), which has been used in previous studies [68–70] to explore protistan communities. Bacterial, fungal and eukaryotic sequencing libraries were constructed according to previously described protocols [34, 71]. Paired-end sequencing was performed on an MiSeq platform (Illumina) at Personal Biotechnology Co., Ltd. (Shanghai, China). Furthermore, we performed metagenomic sequencing to detect potential functional microbiome differences between treatments. As only rhizosphere protistan and bacterial community compositions showed significant contributions to plant disease and yield, we focused subsequent metagenomic sequencing analyses on rhizosphere soil samples. Three of nine replicates of each treatment (6 samples in total: 2 treatments × 3 replicates) were randomly selected for metagenomic sequencing analyses. Sequencing libraries were generated using the NEB Next Ultra DNA Library Prep Kit for Illumina (New England Biolabs, MA, USA) following the manufacturer’s recommendations, and index codes were added. The library quality was assessed on the Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY) and Agilent 4200 (Agilent, Santa Clara, CA) system. Metagenomic shotgun sequencing libraries were prepared and sequenced at Magigene Biotechnology Co., Ltd. (Guangzhou, China). All the raw sequence data were deposited in the NCBI Sequence Read Archive (SRA) under BioProject PRJNA737165 (Illumina sequencing of amplicons) and PRJNA736854 (Illumina sequencing of metagenomes).
Bioinformatics analyses
Bacterial, fungal and eukaryotic raw sequences were split according to their unique barcodes. Furthermore, adaptors and primer sequences were trimmed using cutadapt (https://github.com/marcelm/cutadapt). Subsequently, the trimmed bacterial, fungal and eukaryotic sequences were processed with the UPARSE pipeline according to previously established protocols [34, 71]. Briefly, bacterial sequences with expected errors > 1.0 or a length shorter than 200 bp, fungal sequences with expected errors > 1.0 or a length shorter than 200 bp and eukaryotic sequences with expected errors > 1.0 or a length shorter than 350 bp were removed. After discarding singletons, the remaining sequences were assigned to OTUs at a 97% similarity threshold, followed by the removal of chimaeras using UCHIME [72]. Finally, bacterial and fungal OTUs were classified using the RDP classifier (Version: 11.5) against the RDP Bacterial 16 S rRNA gene database and the UNITE Fungal ITS database, respectively [73]. Eukaryotic OTUs were classified against the Protist Ribosomal Reference database (PR2) [74]. To obtain the protistan OTU table, we discarded OTUs assigned as Rhodophyta, Streptophyta, Metazoa, Fungi and unclassified Opisthokonta sequences. We further assigned taxonomic protistan OTUs into different functional groups according to their feeding mode [49, 75], i.e., predators, parasites, phototrophs, plant pathogens and saprotrophs.
For meta-genomic sequencing, the raw data were trimmed using Trimmomatic to remove adapter sequences and quality-filtered using fastq_quality_filter from the FASTX toolkit with default settings. Filtered reads were assembled with MEGAHIT (https://github.com/voutcn/megahit). CD-HIT (Version: 4.7) was adopted to remove redundancy and obtain the unique initial gene catalogue. The clean data of each sample was mapped to the initial gene directory using BBMAP (http://jgi.doe.gov/data-and-tools/bbtools). We also obtained the number of reads to which genes mapped in each sample. Based on the number of mapped reads and the gene length, the relative abundance information for each gene in each sample was calculated using the following formula: G = (r/L) × (1/(∑r/L)), where r represents the read number aligning to the gene, and L represents the length of the gene. DIAMOND (https://github.com/bbuchfink/diamond) was used to blast the genes against the microbial sequences, all of which were extracted from the NR (non-redundant) database of NCBI (National Center for Biotechnology Information). We used MEGAN (https://softwareab.informatik.unituebingen.de/download/megan6) to obtain the taxon annotation information for the sequences by the LCA algorithm. The table containing the relative abundance information of each gene in each taxonomy hierarchy (kingdom, phylum, class, order, family, genus, species) was obtained based on the LCA annotation result and the gene relative abundance table. Functional annotation of the genes was performed in eggNOG [76]. The relative abundance of each functional hierarchy equals the sum of the relative abundance of genes annotated to that functional level. The relative abundance of a taxon in one functional hierarchy equals the sum of the relative abundance of genes annotated to the taxon in this functional hierarchy. To focus on potentially functional activities of the microbiome, microbial metabolism genes (eight general categories: [Q] secondary metabolite biosynthesis, [P] inorganic ion transport and metabolism, [I] lipid transport and metabolism, [H] coenzyme transport and metabolism, [G] carbohydrate transport and metabolism, [F] nucleotide transport and metabolism, [E] amino acid transport and metabolism and [C] energy production and conversion) and their corresponding taxonomy annotations were extracted for subsequent analyses.
Multi-bipartite model of the food web analyses
As only rhizosphere protistan and bacterial community compositions significantly contributed to explaining plant disease and yield, we used the multi-bipartite model of the food web to examine potential links among protists, bacteria and pathogenic F. oxysporum in the rhizosphere. We selected abundant (average relative abundance > 0.1%) protistan and bacterial OTUs and the density of F. oxysporum for model construction. The links among protists, bacteria and F. oxysporum were calculated by Spearman’s correlation analysis. A pairwise Spearman’s correlation coefficient was calculated with the “corr.test” function in the package “psych” [34, 77] in R (version 3.4.4). The p values were adjusted using the false discovery rate method [78]. Spearman’s correlation coefficient higher than 0.6 (or lower than −0.6) and a p value < 0.05 were selected for the multi-bipartite model of the food web construction, and the multi-bipartite model of the food web was visualized in Cytoscape (version 3.5.1)
Bacillus strain isolation and assays of Fusarium inhibition, biofilm formation and cell size
Given the role of the Bacillus genus in disease suppression [79, 80] and the links among Bacillus, predatory protists and F. oxysporum in our analyses (see results), we isolated Bacillus strains from the rhizosphere soil collected from the field experiment. In brief, 5 g of soil was suspended in a 250 ml Erlenmeyer flask containing 45 ml of sterile distilled water. After stirring at 180 rpm for 40 min, the Erlenmeyer flask containing the soil suspension was placed in an 80 °C hot water bath for 30 min. Serial dilutions were then made from the soil suspension and spread onto plates containing NA medium amended with cycloheximide (100 μg ml−1) to prevent fungal growth, and plates were incubated at 37 °C for 48 h. The suspected Bacillus colonies were preliminarily identified on the basis of morphology and Gram staining. Bacillus colonies were then purified by streaking with NA medium amended with cycloheximide (100 μg ml−1) and identified based on the full-length 16 S rRNA gene sequence, as described previously [81]. Eight different species of Bacillus strains were selected for greenhouse experiments (Supplementary Table 9) from a collection of 110 isolated Bacillus strains isolated from rhizosphere soil amended with organic fertilizer and examined by full-length 16 S rRNA gene sequence analysis. The ability of Bacillus isolates to inhibit the growth of F. oxysporum was tested using a dual culture assay, and biofilm formation and cell size were tested as previously described [80, 82, 83].
Greenhouse and laboratory experimental systems and setup
-
Greenhouse experiments
Greenhouse experiments were conducted to test the effects of predatory protists, the interactions of predatory protists and Bacillus isolates and the interactions of predatory protists and a mutant Bacillus strain (disrupted in the bacillomycin D pathway) [54] on pathogen inhibition in the rhizosphere. Soils for the greenhouse experiment were collected from the chemical fertilizer treatment at the abovementioned long-term field experiment site and passed through a 2 mm sieve to ensure homogenization. One part of the soil was used for nonsterilized soil treatments, and the other part of the soil was used for sterilized soil treatments. For the latter, the soil was sterilized by Co75 γ-ray irradiation (65 KGy) at Nanjing Xiyue Technology Co., Ltd, Nanjing, China. Greenhouse experiments were performed using a randomized complete block design with six replicates for each treatment, and each replicate was run in polypropylene pots filled with 1 kg dry soil. Per pot, one sterile banana plant seedling (Musa AAA Cavendish cv. Brazil) was planted. The pot experiments were run in a greenhouse (daytime: 16 h and average 30 °C, night: 8 h and average 26 °C, all-day average humidity of 75%) located at Nanjing Agriculture University with periodic randomization throughout the experiment. The detailed process and inoculation treatments for the greenhouse experiment are shown in Supplementary Fig. 8 and Supplementary Methods. The protist strain (C. lenta ECO-P-01) used for experimentation has been isolated and identified previously [84]. Six weeks after inoculation, rhizosphere samples of each banana plant in nonsterilized and sterilized soil treatments were collected, and total genomic DNA of each rhizosphere sample was extracted as described above. The abundances of F. oxysporum, Bacillus, total bacteria and the NRPS genes in rhizosphere samples were determined using qPCR according to the above described methods.
-
Laboratory experiments
Laboratory co-culture experiments were conducted to verify the intensity of protistan predation of the different Bacillus isolates that were used in the greenhouse experiments and were set up in 96-well microtiter plates. The detailed inoculation treatments of the laboratory experiments were shown as follows: 1) Control, each Bacillus strain (1 × 105 cells per ml) was inoculated in 200 μl Page’s amoeba saline (PAS) (per well), 2) Co-culture treatment, each Bacillus strain (1 × 105 cells per ml) and the predatory C. lenta strain ECO-P-01 (1 × 103 cells per ml) were inoculated in 200 μl Page’s amoeba saline (PAS) (per well). All the plates were sealed with parafilm and placed in a shaking incubator (20 °C) under agitation (100 rpm) in the dark. After 1 week, a standard 10-fold dilution plating assay was used to count the number of Bacillus using selective media, as described in previous research [16].
Statistical analyses
The α-diversity of bacterial, fungal and protistan communities was estimated using the nonparametric Shannon index. A principal coordinate analysis (PCoA) based on Bray–Curtis distance metrics was used to explore the differences in bacterial, fungal and protistan community compositions. The α-diversity and PCoA of bacterial, fungal and protistan communities were calculated using MOTHUR. Two-way ANOVA was performed to assess the effects of the compartment and fertilization pattern on the diversity (Shannon index) of distinct microbial groups using SPSS v20.0 (SPSS Inc. USA). Permutational multivariate analysis of variance (PERMANOVA) [85] was performed to assess the effects of the compartment and fertilization regime on the community composition of distinct microbial groups using the adonis function with 999 permutations in the “vegan” package [86] in R (version 3.4.3). Random forest (RF) analysis [87] was performed to disentangle the main microbial predictors of disease incidence and banana yield with the “randomForest” package [88] in R (version 3.4.4). In these RFs, we selected the structure (PCoA1) of bacteria, fungi and protists as microbial predictors and calculated their effect on disease incidence and banana yield. To estimate the importance of microbial predictors, we used the percentage increases in the MSE (mean squared error) of variables [87]: higher MSE% values imply more important variables [87]. The significance of each microbial predictor in the random forest analysis was assessed with the “rfPermute” package [89] in R (version 3.4.4). Testing of the linear discriminant analysis Effect Size (LEfSe) was performed to identify significant differences in abundant protistan and bacterial taxa (average relative abundance > 0.1%) between fertilization regimes [90]. The Kruskal–Wallis (KW) sum-rank test was used in the LEfSe analysis to detect the features with significantly different abundances between assigned classes, and linear discriminant analysis (LDA) was then performed to estimate the effect size of each differentially abundant taxon. The alpha value employed for the factorial Kruskal–Wallis test was 0.05, and the threshold employed on the logarithmic LDA score for discriminative features was 3.0 (higher relative abundance in the organic fertilizer treatment: LDA score > 3.0 and p < 0.05; higher relative abundance in the chemical fertilizer treatment: LDA score < −3.0 and p < 0.05). LEfSe analysis was performed using the Huttenhower lab Galaxy server (http://huttenhower.sph.harvard.edu/galaxy) [90]. The relative change in each microbial index in the organic fertilizer treatment relative to the chemical fertilizer treatment was calculated using the following formula: (OF-CF)/CF, with OF representing the value of the microbial index in the organic fertilizer samples and CF representing those in the chemical fertilizer samples [91]. In the greenhouse experiments, the formula for the relative change was (X-control)/control, where X indicates the value of the microbial index in the samples subjected to the co-inoculation treatment of Bacillus isolates with predatory protist, and the control indicates the value of the microbial index in the samples subjected to only Bacillus inoculation treatment [91]. The formula for the predation intensity of predatory protists on Bacillus was (Yc - Yp)/Yc, where Yc is the Bacillus density in the only Bacillus inoculation treatment (control treatment) in sterilized soils or Page’s amoeba saline (PAS), and Yp is the Bacillus density in the co-inoculation of Bacillus and predatory protists treatment (test treatment) in sterilized soils or Page’s amoeba saline (PAS) [51]. Spearman’s correlation analysis was performed using the “corr.test” function in the package “psych” [34, 77] in R (version 3.4.4). Regression analysis, the Student’s t test and one-way ANOVA with Tukey’s HSD test were performed in SPSS v20.0 (SPSS Inc. USA). A normal distribution was tested using the Shapiro–Wilk test, and nonnormal data were log10 transformed [92].
Supplementary information
Acknowledgements
This study was funded by the National Natural Science Foundation of China (42090065, 31972509, 41867006 and 32102475), the Fundamental Research Funds for the Central Universities (KYXK202009), the China Postdoctoral Science Foundation (2021TQ0156 and 2021M691613), the 111 project (B12009), and the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD). Stefan Geisen was supported by an NWO-VENI grant from the Netherlands Organisation for Scientific Research (016.Veni.181.078).
Author contributions
SG, CT, AJ, WX, ZX, ZG, RL, QS, GAK and SG developed the ideas and designed the experimental plans. SG, CT, ZW, ZS, BW, SL, RL and YR performed the experiments. SG, CT, WX, RL and SG analyzed the data. SG, CT, AJ, RL, QS, GAK and SG participated in the completion of the manuscript.
Data availability
All raw 16 S rRNA, ITS and 18 S rRNA gene sequences are available at the NCBI Sequence Read Archive (SRA) under the accession number BioProject PRJNA737165. The raw data of metagenomics-derived gene catalogues are publicly available under the accession number BioProject PRJNA736854.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sai Guo, Chengyuan Tao.
Contributor Information
Rong Li, Email: lirong@njau.edu.cn.
Qirong Shen, Email: shenqirong@njau.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-022-01244-5.
References
- 1.Amundson R, Berhe AA, Hopmans JW, Olson C, Sztein AE, Sparks DL. Soil and human security in the 21st century. Science. 2015;348:1261071. doi: 10.1126/science.1261071. [DOI] [PubMed] [Google Scholar]
- 2.Borrelli P, Robinson DA, Fleischer LR, Lugato E, Ballabio C, Alewell C, et al. An assessment of the global impact of 21st century land use change on soil erosion. Nat Commun. 2017;8:2013. doi: 10.1038/s41467-017-02142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho FP. Pesticides, environment, and food safety. Food Energy Secur. 2017;6:48–60. doi: 10.1002/fes3.108. [DOI] [Google Scholar]
- 4.Santos VB, Araújo ASF, Leite LFC, Nunes LAPL, Melo WJ. Soil microbial biomass and organic matter fractions during transition from conventional to organic farming systems. Geoderma. 2012;170:227–31. doi: 10.1016/j.geoderma.2011.11.007. [DOI] [Google Scholar]
- 5.Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–4. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 6.Tu C, Louws FJ, Creamer NG, Paul Mueller J, Brownie C, Fager K, et al. Responses of soil microbial biomass and N availability to transition strategies from conventional to organic farming systems. Agric Ecosyst Environ. 2006;113:206–15. doi: 10.1016/j.agee.2005.09.013. [DOI] [Google Scholar]
- 7.Blundell R, Schmidt JE, Igwe A, Cheung AL, Vannette RL, Gaudin ACM, et al. Organic management promotes natural pest control through altered plant resistance to insects. Nat Plants. 2020;6:483–91. doi: 10.1038/s41477-020-0656-9. [DOI] [PubMed] [Google Scholar]
- 8.Verbruggen E, Röling WFM, Gamper HA, Kowalchuk GA, Verhoef HA, van der Heijden MGA. Positive effects of organic farming on below-ground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. N. Phytol. 2010;186:968–79. doi: 10.1111/j.1469-8137.2010.03230.x. [DOI] [PubMed] [Google Scholar]
- 9.Lupatini M, Korthals GW, de Hollander M, Janssens TKS, Kuramae EE. Soil microbiome is more heterogeneous in organic than in conventional farming system. Front Microbiol. 2017;7:2064. doi: 10.3389/fmicb.2016.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H, Zhang D, Ren L, Song Z, Li Q, Wu J, et al. Bio-activation of soil with beneficial microbes after soil fumigation reduces soil-borne pathogens and increases tomato yield. Environ Pollut. 2021;283:117160. doi: 10.1016/j.envpol.2021.117160. [DOI] [PubMed] [Google Scholar]
- 11.Shahi DK, Kachhap S, Kumar A, Agarwal BK. Organic agriculture for plant disease management. In: Singh KP, Jahagirdar S, Sarma BK. (eds). Emerging Trends in Plant Pathology. 2021. Springer, Singapore, pp 643–62.
- 12.Francioli D, Schulz E, Lentendu G, Wubet T, Buscot F, Reitz T. Mineral vs organic amendments: microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front Microbiol. 2016;7:1446. doi: 10.3389/fmicb.2016.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Barrios A, Sahib MR, DeBolt S. “I’ve got the magic in me”: the microbiome of conventional vs organic production systems. In: Singh DP, Singh HB, Prabha R. (eds). Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 1: Fundamental Mechanisms, Methods and Functions. 2017. Springer, Singapore, pp 85–95.
- 14.Chowdhury SP, Babin D, Sandmann M, Jacquiod S, Sommermann L, Sørensen SJ, et al. Effect of long-term organic and mineral fertilization strategies on rhizosphere microbiota assemblage and performance of lettuce. Environ Microbiol. 2019;21:2426–39. doi: 10.1111/1462-2920.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller DM. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology. 2007;97:250–6. doi: 10.1094/PHYTO-97-2-0250. [DOI] [PubMed] [Google Scholar]
- 16.Tao C, Li R, Xiong W, Shen Z, Liu S, Wang B, et al. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome. 2020;8:137. doi: 10.1186/s40168-020-00892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM. Phenazine antibiotics produced by fluorescent Pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J. 2009;3:977–91. doi: 10.1038/ismej.2009.33. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Zhao M, Li R, Huang Q, Rensing C, Shen Q. Lipopeptides produced by B. amyloliquefaciens NJN-6 altered the soil fungal community and non-ribosomal peptides genes harboring microbial community. Appl Soil Ecol. 2017;117–8:96–105. doi: 10.1016/j.apsoil.2017.05.002. [DOI] [Google Scholar]
- 19.Kiesewalter HT, Lozano-Andrade CN, Strube ML, Kovács ÁT. Secondary metabolites of Bacillus subtilis impact the assembly of soil-derived semisynthetic bacterial communities. Beilstein J Org Chem. 2020;16:2983–98. doi: 10.3762/bjoc.16.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee S, Schlaeppi K, van der Heijden MGA. Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol. 2018;16:567–76. doi: 10.1038/s41579-018-0024-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Han X, Yan J, Zou W, Wang E, Lu X, et al. Keystone microbiomes revealed by 14 years of field restoration of the degraded agricultural soil under distinct vegetation scenarios. Front Microbiol. 2020;11:1915. doi: 10.3389/fmicb.2020.01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang X, Cai X, Zhou Y, Han X, Zhang C-S, Ilyas N, et al. Pseudomonas inoculation stimulates endophytic Azospira population and induces systemic resistance to bacterial wilt. Front Plant Sci. 2021;12:1964. doi: 10.3389/fpls.2021.738611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyc O, Song C, Dickschat JS, Vos M, Garbeva P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017;25:280–92. doi: 10.1016/j.tim.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Cornforth DM, Foster KR. Competition sensing: the social side of bacterial stress responses. Nat Rev Microbiol. 2013;11:285–93. doi: 10.1038/nrmicro2977. [DOI] [PubMed] [Google Scholar]
- 25.Berg G, Mahnert A, Moissl-Eichinger C. Beneficial effects of plant-associated microbes on indoor microbiomes and human health? Front Microbiol. 2014;5:15. doi: 10.3389/fmicb.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straight PD, Willey JM, Kolter R. Interactions between Streptomyces coelicolor and Bacillus subtilis: Role of surfactants in raising aerial structures. J Bacteriol. 2006;188:4918–25. doi: 10.1128/JB.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González O, Ortíz-Castro R, Díaz-Pérez C, Díaz-Pérez AL, Magaña-Dueñas V, López-Bucio J, et al. Non-ribosomal peptide synthases from Pseudomonas aeruginosa play a role in cyclodipeptide biosynthesis, quorum-sensing regulation, and root development in a plant host. Micro Ecol. 2017;73:616–29. doi: 10.1007/s00248-016-0896-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Yuan J, Zhang R, Dong M, Deng X, Zhu C, et al. Microflora that harbor the NRPS gene are responsible for Fusarium wilt disease-suppressive soil. Appl Soil Ecol. 2018;132:83–90. doi: 10.1016/j.apsoil.2018.08.022. [DOI] [Google Scholar]
- 29.Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol. 2019;10:302. doi: 10.3389/fmicb.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tambadou F, Lanneluc I, Sablé S, Klein GL, Doghri I, Sopéna V, et al. Novel nonribosomal peptide synthetase (NRPS) genes sequenced from intertidal mudflat bacteria. FEMS Microbiol Lett. 2014;357:123–30. doi: 10.1111/1574-6968.12532. [DOI] [PubMed] [Google Scholar]
- 31.Prieto C. Characterization of nonribosomal peptide synthetases with NRPSsp. In: Evans BS. (ed). Nonribosomal Peptide and Polyketide Biosynthesis: Methods and Protocols. 2016. Springer, New York, NY, pp 273–8. [DOI] [PubMed]
- 32.Yuan J, Ruan Y, Wang B, Zhang J, Waseem R, Huang Q, et al. Plant growth-promoting rhizobacteria strain Bacillus amyloliquefaciens NJN-6-enriched bio-organic fertilizer suppressed Fusarium wilt and promoted the growth of banana plants. J Agric Food Chem. 2013;61:3774–80. doi: 10.1021/jf400038z. [DOI] [PubMed] [Google Scholar]
- 33.Yuan J, Li B, Zhang N, Waseem R, Shen Q, Huang Q. Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J Agric Food Chem. 2012;60:2976–81. doi: 10.1021/jf204868z. [DOI] [PubMed] [Google Scholar]
- 34.Xiong W, Song Y, Yang K, Gu Y, Wei Z, Kowalchuk GA, et al. Rhizosphere protists are key determinants of plant health. Microbiome. 2020;8:27. doi: 10.1186/s40168-020-00799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakur MP, Geisen S. Trophic regulations of the soil microbiome. Trends Microbiol. 2019;27:771–80. doi: 10.1016/j.tim.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Müller MS, Scheu S, Jousset A. Protozoa drive the dynamics of culturable biocontrol bacterial communities. PLOS ONE. 2013;8:e66200. doi: 10.1371/journal.pone.0066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geisen S, Mitchell EAD, Adl S, Bonkowski M, Dunthorn M, Ekelund F, et al. Soil protists: A fertile frontier in soil biology research. FEMS Microbiol Rev. 2018;42:293–323. doi: 10.1093/femsre/fuy006. [DOI] [PubMed] [Google Scholar]
- 38.Gao Z, Karlsson I, Geisen S, Kowalchuk G, Jousset A. Protists: puppet masters of the rhizosphere microbiome. Trends Plant Sci. 2019;24:165–76. doi: 10.1016/j.tplants.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Jousset A, Lara E, Wall LG, Valverde C. Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl Environ Microbiol. 2006;72:7083–90. doi: 10.1128/AEM.00557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Xiong W, Zhang R, Hang X, Wang D, Li R, et al. Continuous application of different organic additives can suppress tomato disease by inducing the healthy rhizospheric microbiota through alterations to the bulk soil microflora. Plant Soil. 2018;423:229–40. doi: 10.1007/s11104-017-3504-6. [DOI] [Google Scholar]
- 41.Chen D, Wang X, Zhang W, Zhou Z, Ding C, Liao Y, et al. Persistent organic fertilization reinforces soil-borne disease suppressiveness of rhizosphere bacterial community. Plant Soil. 2020;452:313–28. doi: 10.1007/s11104-020-04576-3. [DOI] [Google Scholar]
- 42.Müller JP, Hauzy C, Hulot FD. Ingredients for protist coexistence: Competition, endosymbiosis and a pinch of biochemical interactions. J Anim Ecol. 2012;81:222–32. doi: 10.1111/j.1365-2656.2011.01894.x. [DOI] [PubMed] [Google Scholar]
- 43.Guo S, Xiong W, Hang X, Gao Z, Jiao Z, Liu H, et al. Protists as main indicators and determinants of plant performance. Microbiome. 2021;9:64. doi: 10.1186/s40168-021-01025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren F, Sun N, Xu M, Zhang X, Wu L, Xu M. Changes in soil microbial biomass with manure application in cropping systems: a meta-analysis. Soil Tillage Res. 2019;194:104291. doi: 10.1016/j.still.2019.06.008. [DOI] [Google Scholar]
- 45.Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–86. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 2009;321:341–61. doi: 10.1007/s11104-008-9568-6. [DOI] [Google Scholar]
- 47.Compant S, Cambon MC, Vacher C, Mitter B, Samad A, Sessitsch A. The plant endosphere world – bacterial life within plants. Environ Microbiol. 2021;23:1812–29. doi: 10.1111/1462-2920.15240. [DOI] [PubMed] [Google Scholar]
- 48.Oliverio AM, Geisen S, Delgado-Baquerizo M, Maestre FT, Turner BL, Fierer N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci Adv. 2020;6:eaax8787. doi: 10.1126/sciadv.aax8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumack K, Fiore-Donno AM, Bass D, Bonkowski M. Making sense of environmental sequencing data: ecologically important functional traits of the protistan groups Cercozoa and Endomyxa (Rhizaria) Mol Ecol Resour. 2020;20:398–403. doi: 10.1111/1755-0998.13112. [DOI] [PubMed] [Google Scholar]
- 50.Romdhane S, Spor A, Banerjee S, Breuil M-C, Bru D, Chabbi A, et al. Land-use intensification differentially affects bacterial, fungal and protist communities and decreases microbiome network complexity. Environ Microbiome. 2022;17:1. doi: 10.1186/s40793-021-00396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jousset A, Rochat L, Péchy-Tarr M, Keel C, Scheu S, Bonkowski M. Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J. 2009;3:666–74. doi: 10.1038/ismej.2009.26. [DOI] [PubMed] [Google Scholar]
- 52.Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL. Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem. 2002;34:955–63. doi: 10.1016/S0038-0717(02)00027-5. [DOI] [Google Scholar]
- 53.Romero D, de Vicente A, Rakotoaly RH, Dufour SE, Veening J-W, Arrebola E, et al. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant Microbe Interact. 2007;20:430–40. doi: 10.1094/MPMI-20-4-0430. [DOI] [PubMed] [Google Scholar]
- 54.Xu Z, Mandic-Mulec I, Zhang H, Liu Y, Sun X, Feng H, et al. Antibiotic bacillomycin D affects iron acquisition and biofilm formation in Bacillus velezensis through a Btr-mediated FeuABC-dependent pathway. Cell Rep. 2019;29:1192–1202.e5. doi: 10.1016/j.celrep.2019.09.061. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Wei Z, Tan S, Mei X, Shen Q, Xu Y. Suppression of bacterial wilt of tomato by bioorganic fertilizer made from the antibacterial compound producing strain Bacillus amyloliquefaciens HR62. J Agric Food Chem. 2014;62:10708–16. doi: 10.1021/jf503136a. [DOI] [PubMed] [Google Scholar]
- 56.Wang B, Shen Z, Zhang F, Raza W, Yuan J, Huang R, et al. Bacillus amyloliquefaciens strain W19 can promote growth and yield and suppress Fusarium wilt in banana under greenhouse and field conditions. Pedosphere. 2016;26:733–44. doi: 10.1016/S1002-0160(15)60083-2. [DOI] [Google Scholar]
- 57.Shen Z, Ruan Y, Chao X, Zhang J, Li R, Shen Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol Fertil Soils. 2015;51:553–62. doi: 10.1007/s00374-015-1002-7. [DOI] [Google Scholar]
- 58.Jeger MJ, Eden-Green S, Thresh JM, Johanson A, Waller JM, Brown AE. Banana diseases. In: Gowen S. (ed). Bananas and Plantains. 1995. Springer Netherlands, Dordrecht, pp 317–81.
- 59.Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci. 2015;112:E911–20. doi: 10.1073/pnas.1423603112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fierer N, Jackson JA, Vilgalys R, Jackson RB. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol. 2005;71:4117–20. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiménez-Fernández D, Montes-Borrego M, Navas-Cortés JA, Jiménez-Díaz RM, Landa BB. Identification and quantification of Fusarium oxysporum in planta and soil by means of an improved specific and quantitative PCR assay. Appl Soil Ecol. 2010;46:372–82. doi: 10.1016/j.apsoil.2010.10.001. [DOI] [Google Scholar]
- 62.Mori K, Iriye R, Hirata M, Takamizawa K. Quantification of Bacillus species in a wastewater treatment system by the molecular analyses. Biotechnol Bioprocess Eng. 2004;9:482–9. doi: 10.1007/BF02933490. [DOI] [Google Scholar]
- 63.Ayuso-Sacido A, Genilloud O. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Micro Ecol. 2005;49:10–24. doi: 10.1007/s00248-004-0249-6. [DOI] [PubMed] [Google Scholar]
- 64.Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R, et al. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol Biochem. 2017;104:39–48. doi: 10.1016/j.soilbio.2016.10.008. [DOI] [Google Scholar]
- 65.Claesson MJ, O’Sullivan O, Wang Q, Nikkilä J, Marchesi JR, Smidt H, et al. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLOS ONE. 2009;4:e6669. doi: 10.1371/journal.pone.0006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds). PCR Protocols. 1990. Academic Press, San Diego, pp 315–22.
- 67.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–8. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 68.Bass D, Silberman JD, Brown MW, Pearce RA, Tice AK, Jousset A, et al. Coprophilic amoebae and flagellates, including Guttulinopsis, Rosculus and Helkesimastix, characterise a divergent and diverse rhizarian radiation and contribute to a large diversity of faecal-associated protists. Environ Microbiol. 2016;18:1604–19. doi: 10.1111/1462-2920.13235. [DOI] [PubMed] [Google Scholar]
- 69.Geisen S, Vaulot D, Mahé F, Lara E, Vargas C de, Bass D. A user guide to environmental protistology: primers, metabarcoding, sequencing, and analyses. BioRxiv 2019;850610:1–34.
- 70.Xiong W, Jousset A, Li R, Delgado-Baquerizo M, Bahram M, Logares R, et al. A global overview of the trophic structure within microbiomes across ecosystems. Environ Int. 2021;151:106438. doi: 10.1016/j.envint.2021.106438. [DOI] [PubMed] [Google Scholar]
- 71.Xiong W, Li R, Ren Y, Liu C, Zhao Q, Wu H, et al. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol Biochem. 2017;107:198–207. doi: 10.1016/j.soilbio.2017.01.010. [DOI] [Google Scholar]
- 72.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guillou L, Bachar D, Audic S, Bass D, Berney C, Bittner L, et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013;41:D597–D604. doi: 10.1093/nar/gks1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong W, Li R, Guo S, Karlsson I, Jiao Z, Xun W, et al. Microbial amendments alter protist communities within the soil microbiome. Soil Biol Biochem. 2019;135:379–82. doi: 10.1016/j.soilbio.2019.05.025. [DOI] [Google Scholar]
- 76.Huerta-Cepas J, Szklarczyk D, Forslund K, Cook H, Heller D, Walter MC, et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016;44:D286–93. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Revelle W, Revelle MW. Package ‘psych’. Compr R Arch Netw. 2015;337:338. [Google Scholar]
- 78.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- 79.Bargabus RL, Zidack NK, Sherwood JE, Jacobsen BJ. Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides, biological control agent. Physiol Mol Plant Pathol. 2002;61:289–98. doi: 10.1006/pmpp.2003.0443. [DOI] [Google Scholar]
- 80.Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004;134:307–19. doi: 10.1104/pp.103.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJJ, Vicente Ade, Bloemberg G. Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol. 2007;103:1950–9. doi: 10.1111/j.1365-2672.2007.03433.x. [DOI] [PubMed] [Google Scholar]
- 82.Aneja KR. Experiments in microbiology, plant pathology and biotechnology. 2007. New Age International, New Delhi.
- 83.Mela F, Fritsche K, de Boer W, van Veen JA, de Graaff LH, van den Berg M, et al. Dual transcriptional profiling of a bacterial/fungal confrontation: Collimonas fungivorans versus Aspergillus niger. ISME J. 2011;5:1494–504. doi: 10.1038/ismej.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao Z. Soil protists: From traits to ecological functions. 2020. Utrecht University.
- 85.Anderson MJ. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. 2017. American Cancer Society, pp 1–15.
- 86.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, et al. Package ‘vegan’. Community Ecol Package Version. 2013;2:1–295. [Google Scholar]
- 87.Breiman L. Random forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 88.Liaw A, Wiener M. Classification and regression by randomForest. R N. 2002;23:18–22. [Google Scholar]
- 89.Archer E. rfPermute: Estimate permutation p-values for random forest importance metrics. R Package Version 20 2016.
- 90.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oguntunde PG, Fosu M, Ajayi AE, van de Giesen N. Effects of charcoal production on maize yield, chemical properties and texture of soil. Biol Fertil Soils. 2004;39:295–9. doi: 10.1007/s00374-003-0707-1. [DOI] [Google Scholar]
- 92.Mcdonald JH. Handbook of biological statistics. 2009. Baltimore: sparky house publishing, Baltimore.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw 16 S rRNA, ITS and 18 S rRNA gene sequences are available at the NCBI Sequence Read Archive (SRA) under the accession number BioProject PRJNA737165. The raw data of metagenomics-derived gene catalogues are publicly available under the accession number BioProject PRJNA736854.