Abstract
Epitranscriptomic RNA modifications can regulate fundamental biological processes, but we lack approaches to map modification sites and probe writer enzymes. Here we present a chemoproteomic strategy to characterize RNA 5-methylcytidine (m5C) dioxygenase enzymes in their native context based upon metabolic labeling and activity-based crosslinking with 5-ethynylcytidine (5-EC). We profile m5C dioxygenases in human cells including ALKBH1 and TET2 and show that ALKBH1 is the major hm5C- and f5C-forming enzyme in RNA. Further, we map ALKBH1 modification sites transcriptome-wide using 5-EC-iCLIP and ARP-based sequencing to identify ALKBH1-dependent m5C oxidation in a variety of tRNAs and mRNAs and analyze ALKBH1 substrate specificity in vitro. We also apply targeted pyridine borane-mediated sequencing to measure f5C sites on select tRNA. Finally, we show that f5C at the wobble position of tRNA-Leu-CAA plays a role in decoding Leu codons under stress. Our work provides powerful chemical approaches for studying RNA m5C dioxygenases and mapping oxidative m5C modifications and reveals the existence of novel epitranscriptomic pathways for regulating RNA function.
Subject terms: RNA, Transcriptomics
Kleiner and co-workers profile RNA 5-methylcytidine (m5C) dioxygenase enzymes using an activity-based metabolic probing strategy. They reveal ALKBH1 as the major 5-formylcytidine (f5C) writer and characterize modification sites across mRNA and tRNA.
Introduction
Chemical modifications on central biomolecules play critical roles in biology. RNA is extensively modified by a diverse collection of post-transcriptional modifications (known as the “epitranscriptome”) that can affect RNA structure, base pairing, and protein-RNA interactions. In turn, these biochemical perturbations have been shown to control RNA metabolism, splicing, protein translation, and RNA localization, thereby affecting gene expression and higher-order physiological processes1–3. Over 150 chemical marks have been identified on various types of RNA spanning all kingdoms of life4, but we still lack insight into the function and distribution of many modifications. A number of RNA modifications have also been implicated in human disease, underscoring the importance of investigating epitranscriptomic processes in biology5,6.
Studies of epitranscriptomic modifications in their native context typically require transcriptome-wide mapping together with characterization of writer, eraser, and reader proteins. These data can then motivate the generation and interrogation of functional hypotheses regarding the biological role of individual modifications. Modification mapping is a major challenge since many RNA modifications are “silent” in standard Next-Generation Sequencing (NGS) analysis and present at low stoichiometry7. Therefore, specialized workflows relying upon modification-specific antibodies or selective chemoenzymatic transformations need to be developed for each modification, and as a consequence reliable mapping data is only available for a few RNA modifications7. Further, identifying relevant modification-associated enzymes and profiling their substrates is an additional obstacle and these enzymes are typically deduced based upon screening or homology to known enzymes.
One of the more abundant modifications found in cellular RNA is 5-methylcytidine (m5C). In eukaryotes, this modification is installed by NSUN/DNMT proteins on diverse RNA species8–10, and it can affect a broad range of cellular processes such as translation, stress response, mRNA nuclear-cytoplasmic trafficking, RNA stability, splicing, and DNA damage repair11. In addition, oxidation of m5C can generate hm5C and f5C in RNA12–15. Recent work has used antibody-based sequencing to map hm5C in mRNA and tRNA16–18, but the identity of hm5C writer enzymes has not been fully elucidated and we lack nucleotide-resolution sequencing for this modification. For f5C, transcriptome-wide sequencing in mammalian systems has not been reported, but mass spectrometry has revealed sites at the wobble position of cytosolic tRNA-Leu-CAA and mitochondrial (mt)-tRNA-Met generated by the Fe(II)/α-KG-dependent dioxygenase ALKBH119,20. Further investigation into these oxidized derivatives has been hampered by the lack of tools to confidently sequence these marks and to systematically profile the writers involved in their formation.
To address this gap, our group recently developed RNA-mediated activity-based protein profiling (RNABBP)21, a reactivity-based proteomic strategy to profile RNA modifying enzymes in living cells. Here, we present an RNABBP approach to survey the human proteome for m5C oxidizing enzymes (i.e., writers of hm5C and f5C). Our strategy relies upon metabolic labeling with the m5C-mimicking warhead 5-ethynylcytidine (5-EC)22, oligo(dT)-based RNA-protein enrichment, and quantitative mass spectrometry. Our results show that ALKBH1 is the major m5C-oxidizing enzyme in HEK293T cells. Further, we use nucleoside mass spectrometry to characterize the prevalence of hm5C and f5C on human RNA and show that human mRNA contains f5C modification. Finally, we develop 5-EC-aided individual-nucleotide CLIP sequencing (iCLIP) and pyridine-borane chemical sequencing of f5C to characterize hm5C/f5C sites generated by ALKBH1 on tRNA. We validate some of these sites orthogonally with Aldehyde Reactive Probe (ARP)-based sequencing and further propose a potential role of f5C in tRNA-Leu-CAA during translation. Taken together, our work expands the generality of RNABPP and deepens our understanding of oxidative m5C modifications in the human epitranscriptome.
Results
Metabolic labeling and protein crosslinking with 5-ethynylcytidine (5-EC)
To profile m5C RNA dioxygenases using RNABPP (Fig. 1a, b), we needed a reactive probe that could be efficiently incorporated into cellular RNA and act as an m5C mimic. We chose 5-ethynylcytidine (5-EC) based on the ability of its deoxynucleoside analog (5-EdC) to covalently label TET enzymes in an activity-based manner on DNA23. Further, 5-EC has been used for RNA metabolic labeling experiments to monitor RNA transcription22, similar to the more widely used 5-ethynyluridine (5-EU) nucleoside24. Therefore, we hypothesized that metabolically incorporated 5-EC would be able to react in an activity-dependent manner with RNA m5C dioxygenases (Fig. 1b).
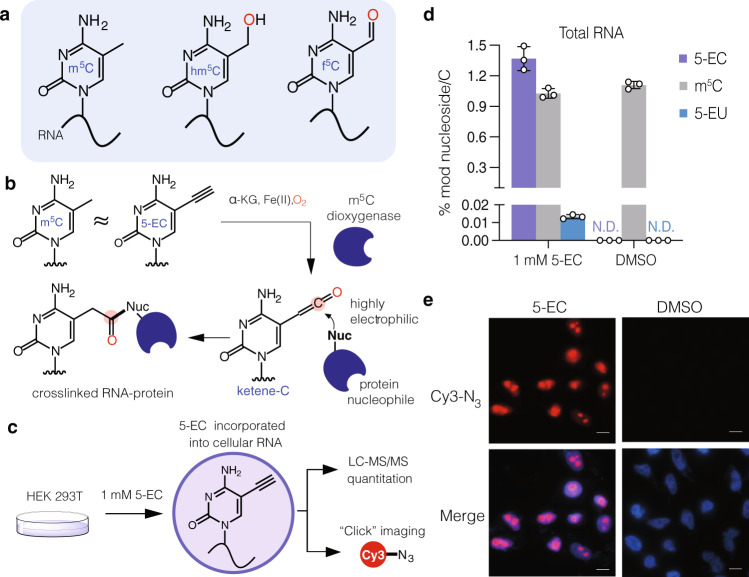
Fig. 1. Probing m5C oxidation in RNA with 5-ethynylcytidine (5-EC).
a Structure of reported m5C oxidation products in RNA. b Proposed capture of m5C dioxygenases by metabolic labeling with 5-EC. 5-EC mimics m5C in RNA and upon enzymatic oxidation generates an electrophilic ketene that can covalently trap nearby nucleophiles. Source data are provided as a Source Data file. c Strategy for metabolic labeling with 5-EC. The nucleoside is fed to mammalian cells and incorporated into cellular RNA. Incorporation can be measured by quantitative mass spectrometry or click chemistry imaging. d LC-MS/MS analysis of 5-EC, m5C, and 5-EU in total RNA of HEK293T cells after treatment with 1 mM 5-EC or DMSO for 16 h. Three independent biological replicates were analyzed. Data represent mean values ± s.d. e Fluorescence microscopy analysis of 5-EC cellular incorporation. HEK293T were treated with 1 mM 5-EC or DMSO for 16 h, fixed and labeled with Cy3-N3 before imaging by fluorescence microscopy. The experiments were repeated three times independently with similar results. Scale bar = 10 µM.
To confirm efficient metabolic labeling with 5-EC, we synthesized 5-EC (Supplementary Information), treated HEK293T cells, and measured incorporation by nucleoside LC-MS/MS and click chemistry imaging (Fig. 1c, Supplementary Figs. 1–2, Supplementary Tables 1–2). We observed levels of 1.37 ± 0.096% 5-EC/C with 1 mM 5-EC overnight treatment (Fig. 1d), a concentration that balances labeling efficiency with cytotoxicity (Supplementary Fig. 3). We also detected low levels of 5-EU (0.013 ± 0.003% of C) in total RNA from 5-EC-fed cells, likely due to deamination in cells. Levels of endogenous m5C or its oxidative derivative 5-formylcytidine (f5C) were largely unperturbed by 5-EC feeding (Fig. 1c, Supplementary Fig. 4, Supplementary Tables 2, 3). RNA labeling was also confirmed by fluorescence microscopy after CuAAC reaction with Cy3-azide, with predominant 5-EC signal in nucleoli, the site of rRNA synthesis (Fig. 1e).
Next, we evaluated the crosslinking abilities of 5-EC with the catalytic domain of TET1 (TET1-CD). TET proteins are primarily DNA 5-methylcytosine oxidative demethylases, but they can also react with RNA25,26. For example, the single TET ortholog in Drosophila (dTet) is responsible for hm5C deposition in polyadenylated RNAs16. Moreover, TET1-CD can convert m5C to hm5C in RNA in vitro, and the catalytic domains of all three TET proteins, as well as full-length TET3, can induce hm5C formation in human RNA15. Gratifyingly, in cells treated with 5-EC and expressing TET1-CD, we observed the presence of a slower migrating band consistent with RNA-protein crosslinking (Supplementary Fig. 5a). This band was absent in untreated cells expressing TET1-CD and in 5-EC-treated samples expressing a catalytically inactive mutant of TET1-CD27, consistent with mechanism-dependent crosslinking. In addition, we recovered TET1-CD after 5-EC feeding and oligo(dT)-pulldown, but not the mutant version, or non-covalently bound TET1-CD in untreated cells (Supplementary Fig. 5b). Taken together, our results show that 5-EC is efficiently incorporated into RNA and can react with Fe(II)/α-KG-dependent dioxygenases in a mechanism-based manner in cells, thereby making it suitable for RNABPP21 analysis of m5C-modifying enzymes
Proteomic profiling of 5-EC-reactive RNA modifying enzymes
After validation of 5-EC-mediated crosslinking with TET1-CD, we proceeded to investigate 5-EC-reactive proteins using our RNABPP workflow combining protein-RNA enrichment and quantitative mass spectrometry-based proteomics (Fig. 2a). We used comparative, label-free analysis to quantify protein enrichment in 5-EC treated cells versus untreated samples. After analysis of data from three independent biological replicates, we identified five proteins enriched by 5-EC treatment with statistically significant P values (<0.05). We were pleased to find that four are known or putative RNA pyrimidine modifying enzymes (Fig. 2b, Supplementary Data 1). Further, among the four pyrimidine modifying enzyme hits, the most highly enriched was ALKBH1, an Fe(II)/ α-KG-dependent dioxygenase responsible for oxidation of m5C to 5-formylcytosine (f5C) at the wobble position of mt-tRNA-Met20, and also reported to oxidize m5C at the wobble base of tRNA-Leu-CAA19. The activity of ALKBH1 on m5C sites in other RNA substrates is so far unknown. While ALKBH1 was not previously described to react with 5-EC, its identification using our method is consistent with its proposed catalytic mechanism28. We also identified m5C methyltransferases NSUN2 and NSUN5, and DUS1L, the mammalian homolog of yeast tRNA-dihydrouridine synthase (DUS) DUS1. NSUN-family m5C methyltransferases utilize a thymidylate synthase (TS)-like mechanism involving nucleophilic attack of a catalytic Cys residue on the C6 position of C to form an enzyme-bound intermediate, and a second Cys residue that mediates β-elimination after C5 methylation with SAM cofactor29. Interestingly, TS has been proposed to convert 5-ethynyl-2’-deoxyuridine into a thiol-reactive allene intermediate30. An analogous mechanism for NSUN proteins could lead to irreversible alkylation of the second Cys residue by 5-EC after rearrangement to its allene derivative (Supplementary Fig. 6). Reaction of 5-EU (generated by deamination of 5-EC in cells) with DUS1L may proceed similarly since C5-C6 reduction during dihydrouridine catalysis could also generate the proposed allene intermediate, and DUS enzymes contain a conserved catalytic Cys residue for irreversible alkylation31. Additionally, we observed enrichment, albeit below the P value threshold of 0.05, of TET2, which has been demonstrated to oxidize m5C to hm5C in RNA in vitro and in cells12–16,32, as well as MPP8, a chromatin-associated epigenetic reader protein that can interact with RNA33.
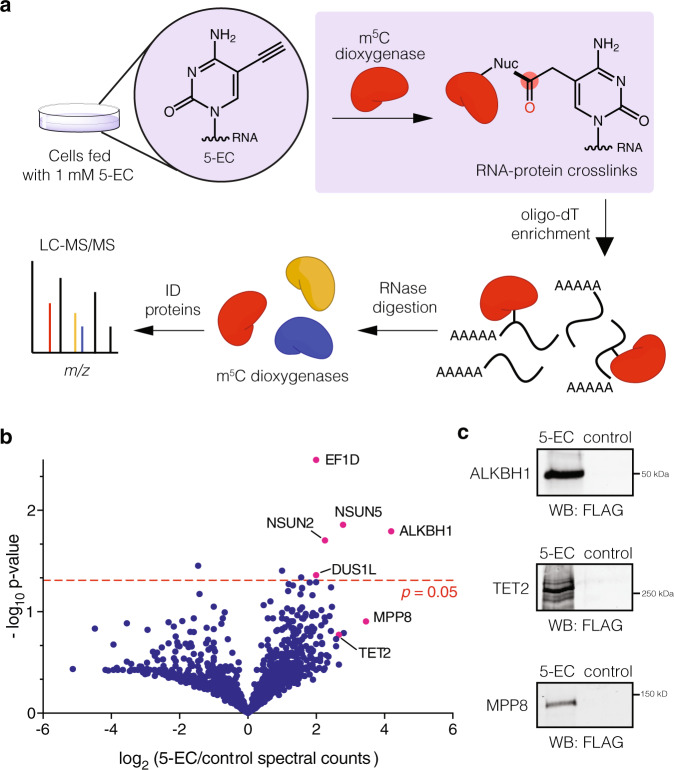
Fig. 2. Proteomic analysis of m5C dioxygenases using 5-EC RNABPP.
a 5-EC RNABPP workflow. Cells are metabolically labeled with 5-EC and crosslinked RNA-protein complexes are isolated by oligo(dT)-based enrichment. Enriched RNA-protein complexes are digested with RNase and then analyzed by mass spectrometry-based proteomics. b Volcano plot showing the enrichment of 5-EC reactive proteins. Experiment was performed independently in triplicate and protein abundance was quantified by spectral counting. Multiple student’s unpaired two-sided t-tests were used to assess statistical significance. c Western blot validation of ALKBH1, TET2, and MMP8. 293 Flp-In cells overexpressing the respective FLAG-tagged protein were treated with 1 mM 5-EC or DMSO and subjected to the RNABPP workflow before western blot analysis. Experiments were performed in triplicate with similar results. Source data are provided as a Source Data file.
To validate the enriched proteins, we generated Flp-In 293 cell lines containing ALKBH1, TET2 or MPP8 and assessed their interaction with 5-EC labeled RNA using western blot and poly(A) pulldown. We initially focused on ALKBH1 and TET2 since they have been shown to oxidize m5C, and MPP8 since its biochemical activity and interaction with RNA has been poorly characterized. Gratifyingly, we observed recovery of all three proteins only in the presence of 5-EC using the RNABPP workflow (Fig. 2c, Supplementary Figs. 7, 8). For ALKBH1, we observed a slower migrating band upon 5-EC treatment, which we assign as the putative crosslinked RNA-protein species (Supplementary Fig. 7). We could not observe a similar band for TET2, likely due to its large size (224 kDa), which makes it challenging to resolve slower migrating crosslinked bands, or due to lower overall crosslinking efficiency. Similarly, we did not observe a crosslinked species for MPP8 (Supplementary Fig. 8), although RNABPP enrichment was specific for 5-EC over feeding with the related 5-methylcytidine nucleoside. Since this protein has no reported enzymatic activity or putative enzymatic domains, we did not pursue it further.
To understand the specificity of the ALKBH1 crosslinking, we compared the recovery of ALKBH1 against that of ALKBH5, a related Fe(II)/α-KG-dependent dioxygenase that can oxidatively demethylate N6-methyladenosine (m6A) in RNA34 but with no reported activity on m5C. We observed substantially greater recovery of ALKBH1 over ALKBH5 with 5-EC (Supplementary Fig. 9), indicating that efficient crosslinking to 5-EC is not a general property of Fe(II)/α-KG-dependent dioxygenase enzymes, and likely requires specific recognition of the C5 position on cytosine. Additionally, to assess if crosslinking is mechanism-dependent, we performed in vitro crosslinking assays with purified recombinant ALKBH1 and a chemically synthesized, site-specifically 5-EC-modified oligonucleotide substrate (oligo 1) derived from the anticodon stem loop (ASL) of Mt-tRNA-Met (Supplementary Information, Supplementary Table 4). Consistent with our in-cell crosslinking results, we observed substantial amounts of a higher-running band when ALKBH1 was incubated with 5-EC oligonucleotide in the presence of required cofactors (i.e. α-KG, Fe(II), O2). (Supplementary Fig. 10). In contrast, we observed the absence of crosslinked species when the same ASL-derived oligonucleotide containing m5C (oligo 2) instead of 5-EC was substituted in the reaction. Further, crosslinking was greatly reduced in reactions with 5-EC oligo 1 when α-KG, Fe(II), or RNA were omitted, as well as in reactions containing EDTA (iron chelator) or N-oxalyl glycine (NOG, an inhibitor of α-KG-dependent enzymes). Taken together, our data establish that 5-EC is an efficient mechanism-based probe for ALKBH1 that can be applied to crosslink RNA substrates in cells through metabolic labeling or for in vitro substrate analysis using chemically synthesized oligonucleotide probes.
Contributions of ALKBH1 and TET2 to m5C oxidation
After validating interactions between ALKBH1 and TET2 with 5-EC containing RNA, we next studied the contributions of these proteins to m5C oxidation in cells. We prepared KO cells for each protein using CRISPR/Cas9 to target the corresponding gene in 293T cells (Supplementary Figs. 11–13, Supplementary Table 5). We then measured levels of m5C and reported oxidized products, hm5C, f5C, and 5-carboxycytidine (ca5C) in RNA isolated from both wild-type (WT) and KO cells by quantitative nucleoside LC-QQQ-MS (Supplementary Fig. 14, Supplementary Tables 6–10). We fractionated total RNA into small RNA and poly(A)-enriched RNA as described previously21 (Fig. 3a, Supplementary Figs. 15–18). Rigorous depletion of small RNA (which harbors high levels of many modified nucleotides) was confirmed by monitoring levels of N6-isopentenyladenosine levels (i6A), which is common to tRNA (Supplementary Fig. 19)4. We also ensured that contaminating nucleosides present in enzymatic reagents used for RNA digestion and dephosphorylation were at negligible levels (Supplementary Fig. 20).
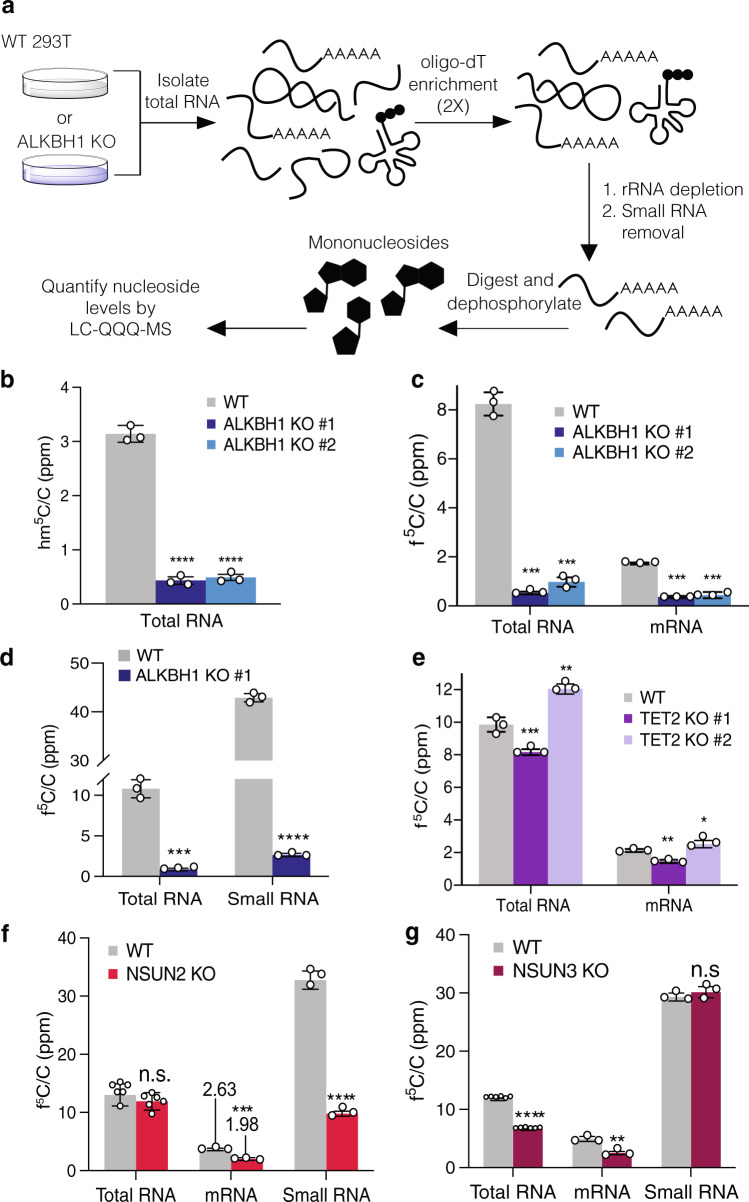
Fig. 3. ALKBH1 is the major hm5C- and f5C-forming enzyme in mRNA and small RNA.
a Strategy for mRNA isolation and LC-QQQ-MS quantification of modified nucleosides in HEK 293T wild-type (WT) and KO cells. Total RNA is isolated and subjected to two rounds of poly(A) enrichment, followed by rRNA and small RNA depletion. The mRNA is then digested to nucleosides and analyzed by LC-QQQ-MS. b Quantification of hm5C in total RNA from WT and ALKBH KO cell lines. p-values: WT vs. KO #1, p = 0.0000106; WT vs. KO #2, p = 0.0000103. c Quantification of f5C in total and mRNA from WT and ALKBH1 KO cell lines. p-values: WT vs. KO #1 in total RNA, 0.000004; in small RNA, 0.000001; WT vs. KO #2 in total RNA, 0.000007; in small RNA, 0.000036. d Quantification of f5C in small RNA from WT and ALKBH1 KO cells. p-values: WT vs KO in total RNA, p = 0.000109; in small RNA, p = 0.00000013. e Quantification of f5C in total and mRNA from WT and TET2 KO cell lines. p-values: WT vs. KO #1 in total RNA, 0.003594; in small RNA, 0.001040; WT vs. KO #2 in total RNA, 0.002192; in small RNA, 0.049386. f Quantification of f5C in total, mRNA, and small RNA from WT and NSUN2 KO cells. p-values: WT vs KO in total RNA, 0.285820; in mRNA, 0.00088; in small RNA; 0.000017. g Quantification of f5C in total, mRNA, and small RNA from WT and NSUN3 KO cells. p-values: WT vs KO in total RNA, 0.000007; in mRNA, 0.001198; in small RNA; 0.291189. Three independent biological replicates were analyzed, except in f and g, where n = 6 in total RNA measurements. Data represent mean values ± s.d. An unpaired t test (two-tailed) was used to measure the statistical significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
We were able to detect low levels (ppm range) of both hm5C and f5C in WT total RNA (Fig. 3b–g, Supplementary Fig. 21, Supplementary Tables 6–9). We could not reliably detect ca5C in total or small RNA samples (Supplementary Fig. 22, Supplementary Table 10), therefore we did not focus on this modification for further investigation. Upon ALKHB1 depletion in two independent KO cell lines, hm5C levels dropped by 84–86% in total RNA (Fig. 3b) and 87–90% in small RNA (Supplementary Fig. 21). We were not able to reliably detect hm5C above background in mRNA. Consistent with previous reports12,14, we measured f5C levels of ~10 ppm f5C/C in total RNA from WT HEK293T cells (Fig. 3c–g). In the mRNA fraction, we measured f5C/C levels of 1.7 ± 0.03 ppm, ~5-fold less than in total RNA (Fig. 3c). In contrast, f5C/C levels in small RNA (primarily composed of tRNA) were increased ~4-fold relative to total RNA to 42.9 ± 0.9 ppm (Fig. 3d). Next, we measured f5C levels in the two independent ALKBH1 KOs. In all cases, we found a major reduction of f5C generation upon knockout of ALKBH1: 88–94% reduction in total RNA, 94% decrease in small RNA, and 74–79% reduction in mRNA (Fig. 3c, d). Taken together, our analysis of oxidative m5C modification levels in bulk RNA fractions shows that ALKBH1 is the major f5C- and hm5C-forming enzyme in HEK293T cells. Further, our data suggest that ALKBH1 can install f5C on polyadenylated RNA. Notably, while bulk poly(A)RNA fractions can be contaminated with modified nucleosides from more abundant RNA species35, given the low levels of f5C in total RNA and the purity of our poly(A) fraction (Supplementary Fig. 19), we believe that the observed modification levels are unlikely to have originated from non-poly(A)RNAs. Interestingly, we find that relative levels of f5C are highest in small RNA. This is consistent with reported ALKBH1-dependent f5C sites in mt-tRNA-Met and tRNA-Leu-CAA19,20, but could also indicate the existence of other ALKBH1-dependent f5C sites on tRNA.
Subsequently, we measured the contributions of TET2 to f5C and hm5C formation. We found no statistically significant TET2-dependence of f5C levels (Fig. 3e). TET2 has been shown to oxidize m5C to hm5C in cellular RNA14–17,32,36, and indeed we observed a 33–36% decrease in hm5C levels in small RNA and a 43–74% decrease in total RNA upon TET2 KO; hm5C levels in total RNA exhibited larger variability across multiple experiments, in part due to low abundance and challenges with chromatography (Supplementary Fig. 21, Supplementary Tables 8, 9). Together, our data indicate that TET2 and ALKBH1 generate hm5C in human cells, whereas only ALKBH1 is responsible for f5C formation.
NSUN2- and NSUN3-dependent hm5C and f5C formation
The biosynthesis of hm5C and f5C on tRNA has been shown to involve the activity of at least two distinct enzymatic reactions—methylation of C to m5C, followed by oxidation of m5C20. Therefore, in order to investigate hm5C/f5C sites further, we analyzed their levels in HEK293T cells depleted of the RNA m5C methyltransferase NSUN221 or NSUN3 (Supplementary Tables 11–14). NSUN2 is the major m5C methyltransferase in human cells and is responsible for installing m5C in both mRNA37 and tRNA38,39, including methylation of C34 on tRNA-Leu-CAA, which is further oxidized by ALKBH119. NSUN3 is responsible for methylation of C34 on mt-tRNA-Met, which is converted to f5C by ALKBH1;20,40 no additional NSUN3-dependent m5C sites have been reported. Consistent with these reports9,21,38,39, we observed an 80–90% drop in m5C levels across total RNA, small RNA, and mRNA fractions from NSUN2 KO cells (Supplementary Fig. 23 Supplementary Tables 11, 12). We did not see any statistically significant decrease in m5C levels in NSUN3 KO cells suggesting a small number of substrate sites (Supplementary Fig. 23, Supplementary Tables 13, 14). In the NSUN2 KO, we observed a small but insignificant drop in f5C levels in total RNA, but a 45% decrease in f5C on poly(A) RNA and a 70% reduction in small RNA (Fig. 3f, Supplementary Tables 11, 12), indicating that most f5C sites on these substrates require NSUN2 methylation. For hm5C levels, we observed a 49–56% decrease in total RNA and 83–85% decrease on small RNA in the NSUN2 KO cells (Supplementary Fig. 21), demonstrating that the majority of hm5C oxidation occurs on NSUN2-installed m5C sites. We observed a different trend on NSUN3-dependent m5C sites: 46% decrease of f5C in total RNA and 50% decrease in mRNA upon NSUN3 KO (Fig. 3g, Supplementary Tables 13, 14), and only modest decreases in hm5C levels (Supplementary Fig. 21). Surprisingly, we detected no significant depletion in f5C levels upon NSUN3 KO in the small RNA fraction (Fig. 3g). While NSUN3 is required for f5C formation on mt-tRNA-Met20,40, our results indicate that this f5C site does not constitute a major fraction of f5C on small RNA, which is consistent with the relatively low abundance of mitochondrial tRNA species41, but also suggests the existence of additional NSUN3-independent f5C sites on small RNA. Taken together, our findings show that the majority of f5C sites on large RNA (>200 nt) are generated by ALKBH1-oxidation of NSUN3-dependent m5C residues, while the majority of f5C sites on small RNA originate from ALKBH1-oxidation of NSUN2-dependent m5C residues. Additionally, hm5C sites are generated primarily by ALKBH1-oxidation of NSUN2-dependent m5C residues. Further, we identify ALKBH1-dependent f5C sites on poly(A)RNA, broadening our understanding of the substrates of this enzyme and the distribution of f5C.
ALKBH1 regulates m1A levels on small RNA
Liu et al.42 previously reported that ALKBH1 can remove m1A on tRNA through oxidative demethylation and regulate translation. Therefore, we also investigated the role of ALKBH1 in cellular m1A levels by nucleoside LC-MS. While we observed no significant change in total m1A levels upon ALKBH1 knockout, m1A levels in small RNA increased by 68 ± 0.09% (Supplementary Fig. 24A, Supplementary Table 15), consistent with ALKBH1-mediated demethylation on a variety of tRNA substrates42. We also interrogated whether ALKBH1 could affect m1A levels in response to stress, as reported by a recent study43. Again, we observed that total m1A levels are not dynamic and are insensitive to a variety of stresses including oxidative stress, heat shock, and glucose deprivation (Supplementary Fig. 24b, Supplementary Table 15). However, m1A levels on small RNA decreased by 37–53% in WT cells under all 3 stress conditions, but were unaffected in ALKBH1 KO cells, suggesting that stress activates ALKBH1 demethylation activity towards m1A on tRNA, in line with the findings of Niizuma and co-workers43. Interestingly, we did not observe changes in overall tRNA levels upon ALKBH1 KO or stress as measured by northern blot, indicating that m1A levels are not correlated with tRNA stability (Supplementary Fig. 24c–f). Finally, bulk f5C levels on total RNA or small RNA fractions did not change upon stress induction (Supplementary Fig. 25, Supplementary Table 15), suggesting that stress-induced ALKBH1 activity is specific to certain substrates.
Mapping ALKBH1 substrates—5-EC-iCLIP
Next, we investigated RNA substrates of ALKBH1 by adapting the iCLIP sequencing method (Fig. 4a)44. We metabolically labeled RNA in cells overexpressing FLAG-tagged ALKBH1 with 5-EC and immunoprecipitated crosslinked ALKBH1-RNA complexes. After IP, anti-FLAG and streptavidin Western blot (following enzymatic biotinylation of associated RNA) indicated robust protein-RNA crosslinking (Fig. 4b, Supplementary Fig. 26). Crosslinked RNA was then fragmented, reverse transcribed, and prepared for Illumina sequencing following literature precedent (Supplementary Fig. 27)21,39,45,46. After sequencing, we performed bioinformatic analysis using the iMaps platform. Reads were de-duplicated and aligned to the genomic reference, and sites of crosslinking were identified based on reverse transcription (RT) stop signatures (Fig. 4a). Crosslinking sites were aggregated into peaks via Paraclu46, and only peaks with all of the following criteria were considered for further analysis: (a) present in two independent biological replicates, (b) displaying a cDNA score >10 and (c) absent from the no 5-EC control OR present in the non-5EC control but with a 5-EC/control score ratio >1.5 (Supplementary Data 2).
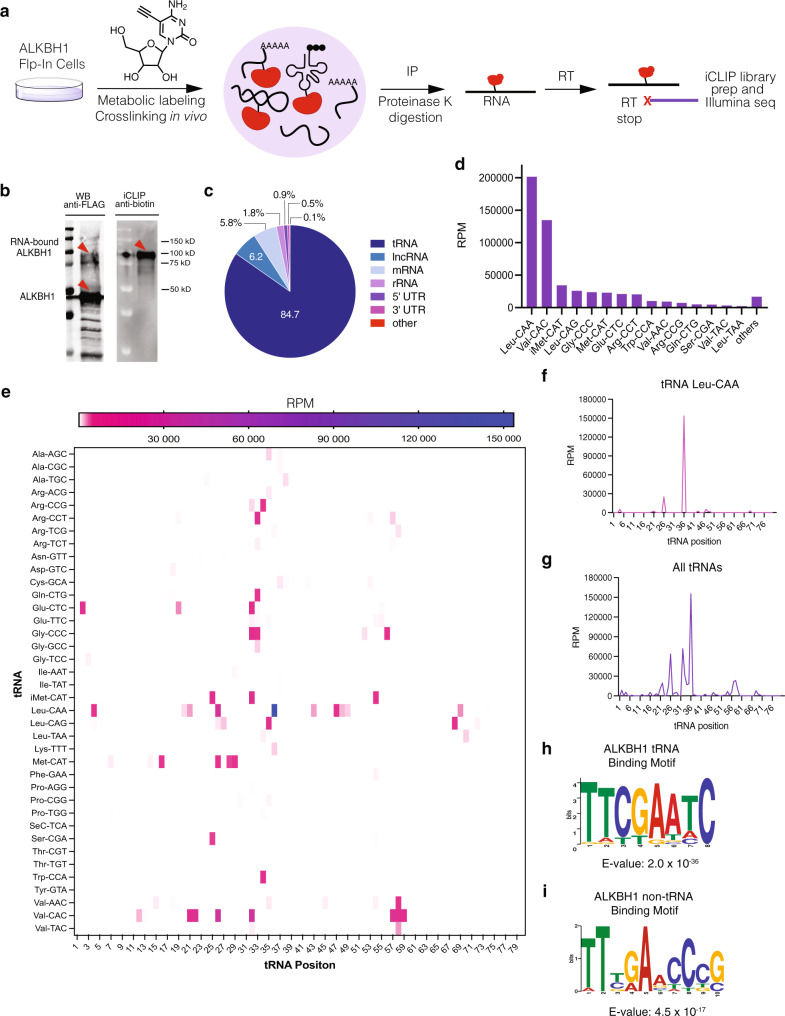
Fig. 4. 5-EC-iCLIP sequencing of ALKBH1 m5C RNA substrates.
a Strategy for 5-EC-iCLIP workflow. Cells overexpressing ALKBH1 are metabolically labeled with 5-EC and the RNA-crosslinked protein is immunoprecipitated (IP) and digested. RNA is reverse transcribed (RT) and prepared for Illumina sequencing. b Analysis of RNA-ALKBH1 crosslinking in immunoprecipitated 5-EC-iCLIP samples after biotin labeling of RNA. Experiment was repeated two times with similar results. c Distribution of uniquely mapped reads identified by 5-EC-iCLIP; lncRNA, long non-coding RNA. d Abundance of tRNA species enriched by 5-EC-iCLIP. cDNA scores from reads were normalized to reads per million (RPM). e Heatmap showing enrichment (in RPM) of all crosslink peaks in tRNA targets according to their relative position in the mature tRNAs. Peaks below 5000 RPM are not visible on color scale. f Distribution of all peaks in tRNA Leu-CAA according to their relative position in the mature tRNA. g Distribution of crosslink peaks in all tRNA targets identified by 5-EC-iCLIP according to their relative position in the mature tRNAs. h tRNA consensus motif identified by MEME from all tRNA peaks identified by 5-EC-iCLIP. i non-tRNA consensus motif identified by MEME from iCLIP non-tRNA peaks.
After removing intergenic reads, we observed the largest fraction of unique reads (84.7%) corresponding to cytosolic tRNAs, indicating that tRNA is the major substrate of ALKBH1 (Fig. 4c). In contrast, the read distribution in the control (no 5-EC treatment) resembles the normal RNA distribution in cells, with rRNA constituting 46.7% of the reads, followed by tRNA (15.3%) (Supplementary Fig. 28). He and co-workers reached a similar conclusion using UV-based CLIP analysis42. Among the 38 tRNA isodecoder families identified in our 5-EC-iCLIP experiment, cytosolic tRNA-Leu-CAA, which is known to contain ALKBH1-dependent f5C/f5Cm and hm5C/hm5Cm modifications at the wobble position, was the most abundant, encompassing 37% of the tRNA reads (Fig. 4d, e), and we observed the most abundant crosslinking peak in our iCLIP data at position 36 of tRNA-Leu-CAA (Fig. 4e, f, Supplementary Data 2). While the wobble base in this tRNA is located at residue 35, crosslinking peaks can be offset by one or two nucleotides depending on where RT stop occurs47. The presence of a crosslinking peak in our iCLIP experiment corresponding to a known ALKBH1-dependent m5C oxidation site validates our method and establishes it as an effective tool to map substrates of ALKBH1-mediated m5C oxidation.
In addition to a peak at the wobble position of tRNA-Leu-CAA, we identified 165 peaks in cytosolic tRNA (Supplementary Data 2). To visualize tRNA crosslinking, we aligned all peaks according to their relative position on the mature transcript and observed the largest accumulation of crosslinking at positions 32–36 (54.0% of all peaks) (Fig. 4e, g). 25 out of the 38 tRNA isodecoders identified displayed peaks between positions 32–36. Depending on tRNA length, positions 32–36 align with the anticodon, suggesting ALKBH1 can oxidize m5C in the anticodon or anticodon stem loop (ASL) of a number of cytosolic tRNAs. Consistent with crosslinking requiring 5-EC labeling, 21 of the identified tRNAs with crosslinks in this region have a C residue in the anticodon loop. We also observed 8 tRNAs with crosslinking peaks at positions 56–59 (11.1% of all peaks), which is likely due to the presence of a highly conserved m1A residue in the TψC -loop48,49. Rather than crosslinking to ALKBH1, this modification can induce an RT stop on its own50, thus generating an apparent crosslinking peak at that position. Another possibility is that the incorporation of 5-EC near this m1A residue could mediate crosslinking, particularly given the conservation of C at position 56 in many tRNAs. There was also an additional cluster of 10 tRNAs that displayed peaks at positions 25–28 (15.6% of all peaks). In addition to 5-EC-mediated crosslinking in this region, RT stops could be induced by the presence of a conserved N2,N2-dimethylguanosine (m22G) modification in this region4,51. Sequence motif analysis using multiple expectation maximizations for motif elicitation (MEME)52 revealed a tRNA consensus motif that matches the TψC-loop of various isoacceptors, likely due to peaks near m1A58 (Fig. 4h). A consensus motif matching the anticodon loop was not obtained, as these sequences diverge among tRNAs.
Since we initially identified ALKBH1 as an m5C dioxygenase using oligo(dT)-based enrichment and were able to quantify ALKBH1-dependent f5C in mRNA, we also analyzed CLIP peaks mapping to non-tRNA regions. We found a total of 83 peaks, with 70.0% mapping to introns of protein-coding genes (Supplementary Data 2). The remaining reads mapped to non-coding RNAs (17.1%), 5’ UTR (11.5%), 3’ UTR (0.82%), and coding regions (0.55%) (Supplementary Fig. 29). On average, peaks observed for non-tRNA were ~40-fold lower in abundance than tRNA peaks. We also performed MEME analysis on the non-tRNA reads and identified the sequence binding logo TTBRADCCCG (Fig. 4i), which shares similarities to the tRNA-binding consensus sequence (Fig. 4h).
Mapping ALKBH1 substrates—ARP sequencing
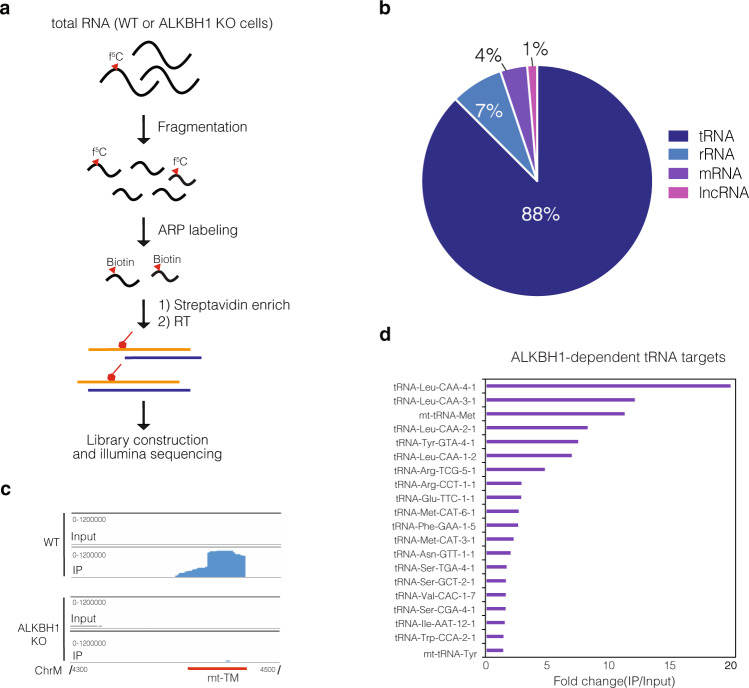
Identification of f5C in DNA has been previously achieved by pulldown with aldehyde-reactive probe (ARP; O-(biotinylcarbazoylmethyl) hydroxylamine)53. Inspired by this method, we reasoned that we could also map f5C sites within RNA by ARP-based pulldown followed by NGS (Fig. 5a). Fragmented total RNA extracted from WT or ALKBH1 KO cells was first incubated with ARP and then enriched by streptavidin pulldown followed by on-bead RT and Illumina library construction. A total RNA input library was also prepared and was used as a background control for the identification of read-enriched regions (i.e. peaks) in the pulldowns. Pulldown efficiency was ensured by adding an f5C-containing spike-in oligo prior to the IP step, which was enriched 20–40-fold in both WT and KO samples across two biological replicates (Supplementary Fig. 30a), suggesting the workflow can efficiently enrich f5C-containing RNA.
Fig. 5. Transcriptome-wide profiling of RNA f5C using Aldehyde-reactive probe (ARP)-sequencing.
a Workflow for ARP sequencing. Fragmented total RNA extracted from WT and ALKBH1 KO cells is incubated with ARP, and labeled RNA is enriched through streptavidin pulldown. Libraries are generated through RT, cDNA circulation, and PCR amplification. b Distribution of enriched ALKBH1-dependent peaks (cDNA value > 10) identified by ARP sequencing. c IGV tracks showing the reads mapped to mt-tRNA-Met in input and IP samples of WT and ALKBH1 KO cells. d Top ALKBH1-dependent f5C-containing tRNA substrates enriched by ARP pulldown. Only peaks with cDNA score >100 and present in the WT sample and absent from the ALKBH1 KO control were considered.
To define ALKBH-dependent f5C targets, we analyzed the transcriptome-wide ARP data considering only peaks with cDNA score >10 present in WT pulldown samples, and absent from the input and ALKBH1 KO controls (Supplementary Data 3). In line with the 5EC-iCLIP data, we found a majority of unique reads (88%) mapped to tRNA (Fig. 5b), with known f5C-containing substrates mt-tRNA-Met and cytosolic tRNA-Leu-CAA constituting the majority of the tRNA reads (Fig. 5c, Supplementary Data 3). Aside from these 2 targets, we also identified 32 peaks in other cytosolic tRNAs with fold-change >1.5, including 20 tRNAs with cDNA value >100 (Fig. 5d) which we considered as high-confidence f5C-containing targets. Some of these, namely tRNAs Arg-TTC, Met-CAT, Val-CAC, Ser-CGA, and Trp-CCA were also present as top enriched hits in our 5-EC-iCLIP experiment (Fig. 4d, Supplementary Fig. 30b), validating our iCLIP findings and strengthening the notion that isoacceptors other than mt-tRNA-Met and tRNA-Leu-CAA also contain f5C. We also found that 4% of total enriched peaks mapped to mRNA, suggesting again the presence of ALKBH1-dependent f5C on mRNA (Supplementary Data 3).
Measuring hm5C/f5C content in native tRNAs
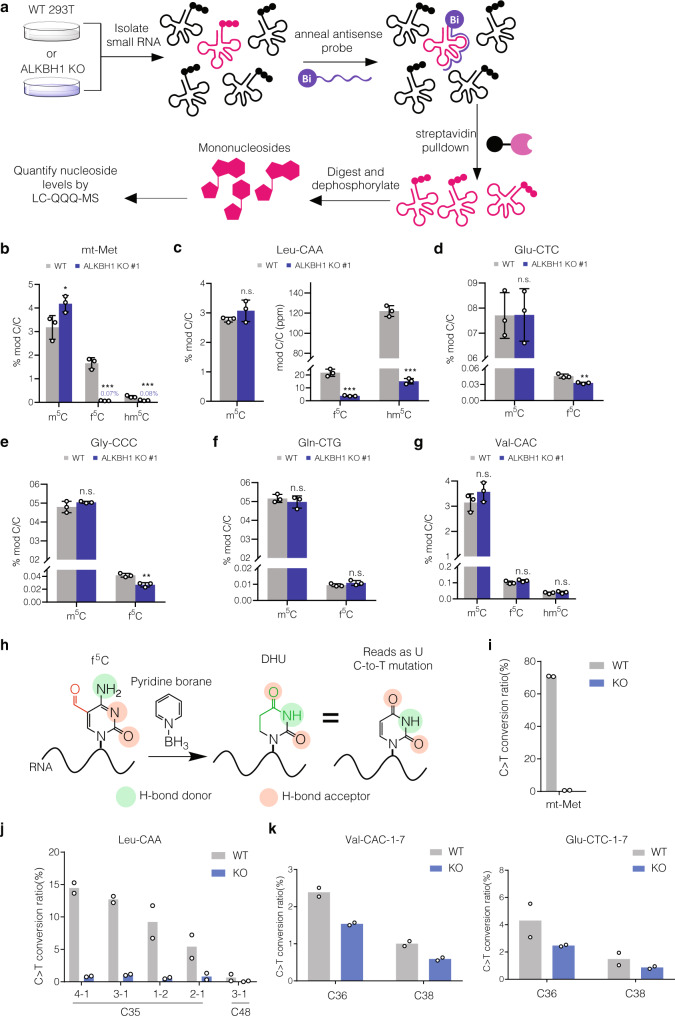
To interrogate potential ALKBH1-dependent f5C sites in tRNAs we identified through our sequencing studies, we performed antisense pulldowns42 on RNA isolated from WT or ALKBH1 KO cells followed by quantitative LC-QQQ-MS to measure m5C and its oxidized derivatives (Fig. 6a, Supplementary Figs. 31–33, Supplementary Tables 16, 17). We first analyzed mt-tRNA-Met and tRNA-Leu-CAA. We were pleased to measure hm5C and f5C levels in both tRNAs that decreased significantly in ALKBH1 KO cells (Fig. 6b, c). For mt-tRNA-Met, we observed a 96% decrease in f5C, from 1.6% to 0.07%. We also measured hm5C in this tRNA, which was reduced 65% upon ALKBH1 depletion (albeit present at much lower abundance than f5C). In contrast, tRNA-Leu-CAA showed higher hm5C levels compared to f5C, consistent with previously reported oligonucleotide mass spectrometry analysis19. We further applied this approach to four other tRNAs from our 5-EC-iCLIP data – tRNA-Glu-CTC, tRNA-Gly-CCC, tRNA-Gln-CTG, and tRNA-Val-CAC. We were able to detect f5C but not hm5C in all substrates. For both tRNA-Glu-CTC (Fig. 6d) and tRNA-Gly-CCC (Fig. 6e) we measured modest but statistically significant decreases in f5C upon ALKBH1 KO (38% reduction for tRNA-Glu-CTC and 33% reduction for tRNA-Gly-CCC). Interestingly, f5C levels in tRNA-Val-CAC and tRNA-Gln-CTG were unchanged upon ALKBH1 knockout (Fig. 6f, g).
Fig. 6. Validation of 5-EC-iCLIP tRNA hits.
a Strategy for LC-QQQ-MS quantification of modified nucleosides in individual tRNAs isolated from HEK 293T wild-type (WT) and ALKBH1 KO cells. Individual tRNAs are enriched by antisense pulldown, digested to mononucleosides and analyzed by LC-QQQ-MS. b–g Quantification of oxidized m5C products in b mt-tRNA-Met, p-values: WT vs KO for m5C, 0.026079; for f5C, 0.000132; for hm5C, 0.000244, c tRNA-Leu-CAA, p-values: WT vs KO for m5C, 0.156295; for f5C, 0.000170; for hm5C, 0.000002, d tRNA-Glu-CTC, p-values: WT vs KO for m5C, 0.975934; for f5C, 0.002584, e tRNA-Gly-CCC, p-values: WT vs KO for m5C, 0.161660; for f5C, 0.001039, f tRNA-Gln-CTG, p-values: WT vs KO for m5C, 0.365015; for f5C, 0.171454, and g tRNA-Val-CAC, p-values: WT vs KO for m5C, 0.152377; for f5C, 0.124395; for hm5C, 0.366551. h Schematic workflow for pyridine borane f5C sequencing. Pyridine borane converts f5C residues to dihydrouridine, which is read as uridine, thus generating a C-to-T signature that can be identified in sequencing (i) Validation of presence and ALKBH1-dependence of f5C at the wobble base of mt-tRNA-Met by pyridine borane sequencing. j Validation of presence and ALKBH1-dependence of f5C at the wobble base of tRNA-Leu-CAA. k Presence and ALKBH1-dependence of f5C at various positions of tRNA-Val-CAC and tRNA Glu-CTC. For (b)–(g), three independent biological replicates were analyzed. Data represent mean values ± s.d. An unpaired t-test (two-tailed) was used to measure the statistical significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. For (h)–(j), two independent biological replicates were performed and analyzed. Source data are provided as a Source Data file.
Since LC-MS analysis of bulk RNA digests does not provide information on the modification site and antisense pulldowns could still contain trace amounts of non-targeted tRNA species, we decided to pursue a complementary sequencing approach to more rigorously characterize tRNA f5C sites. In particular, Song and co-workers demonstrated that 5fC in DNA can be converted to dihydrouracil by pyridine borane treatment, resulting in C-to-T mutations upon PCR and sequencing54, and while our manuscript was in this review, this method was adapted to identify RNA f5C sites in yeast55. Similarly, we previously reported Mal-Seq56, which uses malononitrile to detect f5C in RNA through C-to-T mutations, although with lower sensitivity than the pyridine borane procedure. Therefore, we employed pyridine borane sequencing together with targeted RT-PCR analysis to study f5C sites on tRNAs of interest (Fig. 6h). We designed primers to sequence bases 19 to 50, which includes the anticodon stem loop (ASL), since our CLIP data indicated that 5-EC-induced crosslinking peaks frequently occurred in this region First, we investigated whether pyridine borane would effectively generate C-to-T mutations at f5C34 in mt-tRNA-Met, which is reported to exist at near 100% stoichiometry40. While f5C34 is sequenced almost exclusively as “C” from untreated RNA, RT-PCR on pyridine borane-treated RNA showed 71% C-to-T mutation (Fig. 6i). Further, we detected no mutations at C34 in mt-tRNA-Met in pyridine borane-treated RNA isolated from ALKBH1 KO cells, indicating that pyridine borane sequencing can specifically detect f5C in RNA (Supplementary Data 4).
Next, we chose 7 additional tRNA isoacceptors from our 5-EC-iCLIP data to analyze by pyridine-borane RT-PCR (Supplementary Data 4). In order to identify C-to-T conversion events likely to originate from f5C modification, we compared pyridine borane-induced mutations in WT and ALKBH1 KO RNA. In total, among the 8 tRNAs investigated, we identified 15 sites showing pyridine borane-induced mutations in WT RNA and lacking mutations in ALKBH1 KO RNA (Supplementary Data 4). Consistent with our CLIP data and ARP sequencing data, 4 of these sites mapped to the wobble base (C35) in tRNA-Leu-CAA isodecoders and showed mutation rates of 6–15% that were greatly reduced in the ALKBH1 KO (Fig. 6j). We should note that tRNA-Leu-CAA has been reported to contain both f5C and its 2’-O-methyl derivative f5Cm19, and we predict that pyridine borane-induced C-to-T mutations should result from both modifications. Further, since we detected only 71% C-to-T mutation at f5C34 in mt-tRNA-Met (which is reportedly modified at near 100% stoichiometry)40, the pyridine borane-induced mutation rate is likely to be an underestimate of actual modification stoichiometry. In addition, we detected ALKBH1-dependent mutations in the anticodon of tRNA-Gly-CCC, tRNA-Val-CAC and Glu-CTC at a frequency of 0.95–4.3% (Fig. 6k, Supplementary Data 4). Mutations surrounding the anticodon in the anticodon stem loop were also found in tRNA-Val-CAC, tRNA-Met-CAT, tRNA-Glu-CTC, and tRNA-Gly-CCC (Fig. 6j, k, Supplementary Data 4). Finally, we detected a small number of ALKBH1-independent f5C sites (i.e. with comparable rate of C-to-T mutation in both WT and ALKBH1 KO samples but sensitive to pyridine borane treatment) at position 25 of tRNA-Val-CAC and position 39 of tRNA-Gly-CCC, which require further investigation (Supplementary Fig. 34, Supplementary Data 4). Taken together, our data indicate the existence of f5C sites occurring in several cytosolic tRNAs.
Assessment of in vitro ALKBH1 substrate specificity
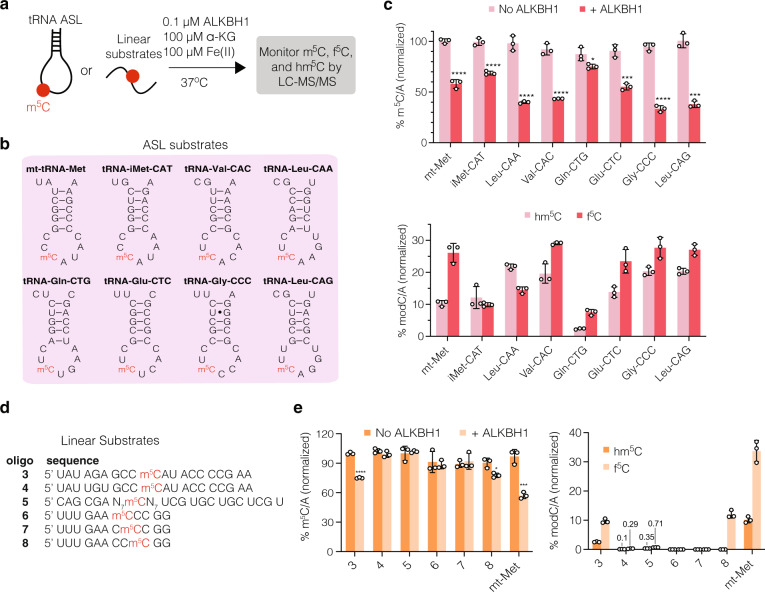
To better understand 5-EC-mediated crosslinking on diverse substrates as detected in our iCLIP experiment, we explored the in vitro activity of recombinant ALKBH1 on m5C-containing RNA oligonucleotides (Fig. 7a). We synthesized the ASLs from tRNAs Leu-CAA, iMet-CAT, Val-CAC, Gln-CTG, Glu-CTC, Gly-CCC, and Leu-CAG with m5C at the wobble position (Fig. 7b, Supplementary Table 4), given that those tRNAs showed both high levels of enrichment in the CLIP data and crosslinks near the wobble base. As a positive control, we prepared the m5C-modified ASL from mt-tRNA-Met, which has been reported to serve as an in vitro substrate for ALKBH1 oxidation20. We then setup in vitro enzyme reactions containing purified ALKBH1 with necessary cofactors and RNA substrate, and analyzed the reaction products by LC-QQQ-MS (Fig. 7c, Supplementary Fig. 35, Supplementary Tables 18–21). After incubation with ALKBH1, all synthetic m5C-containing ASL substrates showed statistically significant decreases in m5C/A levels, ranging from 12–60%, compared to the no-enzyme control. A concomitant increase in f5C/A and hm5C/A levels was also observed. Consistent with our 5-EC-iCLIP data, the most active ASLs substrates were Leu-CAA, Val-CAC, Gly-CCC, and Leu-CAG, which showed between 49–60% decrease in normalized m5C levels compared to 42% depletion for the mt-tRNA-Met ASL. Production of f5C in these tRNAs (27–29% normalized f5C) was also comparable to that in the mt-tRNA-Met control (26%), with the exception of tRNA-Leu-CAA, which showed f5C/A levels at 15%. However, tRNA-Leu-CAA displayed the highest hm5C content post-oxidation (21% normalized hm5C) among all the substates. These higher levels of hm5C compared to f5C observed in vitro are consistent with the levels of modified nucleosides measured from the native tRNA (Fig. 6c). Similar to our analysis of cellular RNA, 5-carboxycytidine (ca5C) was not reliably detected above background after ALKBH1-mediated oxidation in vitro (Supplementary Fig. 35, Supplementary Table 19), and we did not detect appreciable levels of contaminating nucleosides originating from recombinant ALKBH1 (Supplementary Fig. 20).
Fig. 7. In vitro activity of ALKBH1 on structured and linear substrates.
a Workflow to assess activity of ALKBH1 in vitro. Purified ALKBH1 is incubated with RNA substrate and cofactors at 37 °C for 30 min. The reaction is quenched, and RNA is purified, digested, and analyzed by LC-QQQ-MS. Activity was measured by quantifying the decrease of m5C and the generation of oxidized products hm5C and f5C. b Sequences of anticodon stem loops (ASLs) tested in the assay. All substrates have an m5C residue at the wobble base. c Conversion of m5C and generation of hm5C and f5C in various ASL substrates. d Sequences of linear substrates tested in the assay. e Conversion of m5C and generation of hm5C and f5C in linear substrates. For (c) and e, to account for loading differences, values were first quantified as ratios over A and then normalized to the maximum possible ratio for that substrate. Three independent replicates were performed, and data represent mean values ± s.d. An unpaired t-test (two-tailed) was used to measure the statistical significance *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. For (c), p-values (+ALKBH1 versus No enzyme) for substrate 3, 0.000006; for substrate 8, 0.0141996; for mt-Met, 0.0009131. For (e), P-values (f5C versus hm5c) for substrate 3, 0.0000927; substrate 4, 0.009919; for substrate 5, 0.0000889; for substrate 8, 0.000914; for mt-Met, 0.0004327. Source data are provided as a Source Data file.
In addition, we measured the activity of ALKBH1 on linear substrates including mutated m5C-modified ASLs from mt-tRNA-Met (3–4), a randomized 15-mer RNA library containing a single m5C site in the center (5) (Supplementary Fig. 36, Supplementary Table 4), and m5C-modified oligonucleotides matching the non-tRNA consensus sequence detected from the 5-EC-iCLIP experiment (6–8) (Fig. 7d, Supplementary Tables 20–21). While none of these linear oligonucleotides showed the same degree of m5C oxidation as the ASL substrates, we were able to detect m5C depletion and hm5C/f5C formation on substrate 3, one of the mutated mt-Met ASL substrates, as well as substrate 8, corresponding to the non-tRNA iCLIP sequence logo (Fig. 7e). The m5C random library was also subject to minimal conversion (0.35% and 0.71% hm5C/C and f5C/C normalized formation, respectively), which suggests a slight preference of ALKBH1 for some linear substrates contained within. Taken together, our in vitro activity data shows that ALKBH1 can efficiently oxidize m5C-containing stem loops taken from diverse tRNAs. Further, in select sequence contexts, ALKBH1-mediated m5C oxidation can also occur on linear substrates.
Investigating the potential function of f5C in tRNA Leu-CAA
Since our 5-EC-iCLIP data, ARP-sequencing analysis, LC-MS/MS analysis, and pyridine borane data all support the presence of f5C at the wobble position of tRNA Leu-CAA, we posited whether the modification could facilitate translation by decoding non-cognate TTA Leu codons, analogous to the proposed function of f5C on mt-tRNA-Met in decoding both AUA and AUG codons. We assessed the translation efficiency of TTG and TTA codons through an in-cell dual-luciferase assay in WT 293T or ALKBH1 KO cells. In this assay, cells are transfected with a construct containing Renilla luciferase (Rluc) linked to Firefly luciferase (Fluc). Efficient readthrough of the linker, which, in this case consists of a string of either TTG, TTA, or random codons can be quantified by an increase in Fluc signal as compared to the Rluc output. Under normal cell culture conditions, we observed no significant changes in fluorescence among different constructs or cell lines (Supplementary Fig. 37, Supplementary Table 22), indicating that translation of the dual-luciferase constructs was not affected by ALBKH1 KO. However, under glucose deprivation stress (1 mM glucose), the translation efficiency of the TTA codon linker decreased by 17.5% in ALKBH1 KO cells. In contrast, translation of the TTG linker or the random codon linker was unaffected in ALKBH1 KO cells compared to WT cells. Taken together, our data suggest that f5C at the wobble base of tRNA-Leu-CAA may aid in decoding Leu TTA codons under stress conditions.
Discussion
In this study, we develop an activity-based platform to profile RNA m5C dioxygenases in human cells. Our approach relies upon 5-EC nucleoside, which is incorporated efficiently and broadly into the transcriptome through nucleotide metabolism, where it can form covalent crosslinks with enzymes that recognize and oxidize m5C in RNA. We find that ALKBH1 is the major m5C dioxygenase in human HEK293T cells, where it is responsible for the bulk of hm5C and f5C production. Further, we use quantitative nucleoside LC-MS, modification-specific sequencing approaches, and in vitro biochemical assays to study the substrate specificity of ALKBH1 and identify the presence of f5C sites across the human transcriptome. Taken together, our work expands our knowledge of oxidative m5C modifications on RNA and provides powerful strategies to further explore these modifications and associated writer enzymes in biology.
We chose 5-EC as a mechanism-based probe for RNA m5C dioxygenases based upon the ability of the analogous deoxynucleoside to crosslink TET in vitro and in lysate23. Precedent also exists for the covalent trapping of other oxidative enzymes with terminal alkyne moieties. Stubbe and coworkers showed that thymine hydroxylase is inactivated by 5-EU57, and aryl acetylene substrates have been used as activity-based probes for cytochrome P-450 enzymes58,59. While the mechanistic basis of crosslinking has not been fully elucidated for each of these systems, the formation of a covalent adduct is likely to occur through the production of a highly reactive ketene intermediate following oxidation and concomitant 1,2 migration of the acetylenic hydrogen58. The putative ketene can then react with nearby nucleophilic residues resulting in covalent trapping of the protein60. Our work shows that 5-EC is an effective surrogate for m5C and can be applied as a general metabolic probe to study RNA m5C dioxygenases in their native context. Interestingly, despite serving as a reactive m5C surrogate, we did not observe depressed levels of m5C (and its corresponding oxidized products) in 5-EC treated samples, which suggests that this probe does not significantly affect the cellular activities of m5C-forming or m5C-oxidizing enzymes and hints to its broad applicability in other settings. Analogous approaches with alkyne-containing metabolites should be readily applicable to study diverse oxidative enzymes acting upon biopolymer and small molecule substrates, as well as to study other proposed substrates of ALKBH159,61,62.
Our work identifies new RNA m5C substrates for ALKBH1-mediated oxidation to f5C. Previous studies have reported two RNA m5C sites that are substrates for ALKBH1—m5C34 in mt-tRNA-Met (oxidized to f5C) and m5C(m)34 in cyt-tRNA-Leu-CAA (oxidized to hm5C/f5C and hm5Cm/f5Cm). Using nucleoside LC-MS analysis of fractionated RNA from WT, ALKBH1 KO, NSUN2 KO, and NSUN3 KO cells, we show that ALKBH1 installs f5C on the poly(A)RNA fraction through oxidation of NSUN2- and NSUN3-dependent m5C sites. The pathway for f5C formation on C34 of mt-tRNA-Met requires NSUN320,40 and CLIP studies have proposed that this is the only substrate for the enzyme;63 however, we observe no NSUN3-dependent depletion in f5C levels on small RNA (which contains mt-tRNA-Met), and instead observe NSUN3-dependent depletion of f5C in total RNA and poly(A)RNA, further supporting the existence of f5C (and m5C) sites catalyzed by NSUN3 and ALKBH1 beyond the canonical mt-tRNA-Met substrate. One important consideration that must be accounted for in the interpretation of low abundance hm5C and f5C sites is the possibility that these are a result of catabolism of abundant hm5C/f5C-containing RNAs followed by non-specific incorporation during transcription. While an analogous chain of events has been proposed to explain the presence of 6-methyladenine on mammalian DNA64, we do not favor this scenario since hm5C and f5C are present at low overall abundance in the transcriptome (in contrast to m6A), and it is unknown whether they are efficiently processed by pyrimidine salvage pathways.
To map ALKBH1-dependent hm5C/f5C sites at nucleotide resolution, we developed and applied multiple complementary sequencing platforms. 5-EC-iCLIP analysis and ARP-sequencing profiled ALKBH1 m5C substrates transcriptome-wide and showed that its major substrate is tRNA, which is consistent with a previous UV-CLIP study for ALKBH142, but we also found additional minor modification sites on intronic mRNA, UTR, lncRNA, and CDS regions. Indeed, the crosslinking peak at the wobble base of cyt-tRNA-Leu-CAA was the most abundant of all those identified and is likely the major ALKBH1 m5C substrate in addition to mt-tRNA-Met. The ability of ALKBH1 to act upon both nuclear/cytosolic and mitochondrial substrates is worthy of further exploration due to the primarily mitochondrial localization of the protein65. In addition, we find 5-EC-mediated crosslinking of ALKBH1 to 38 other tRNA isodecoder famlies, with the majority of peaks mapping to the ASL, and ARP-sequencing supports additional tRNA substrates beyond cyt-tRNA-Leu-CAA and mt-tRNA-Met. We interrogated the presence of f5C at these sites using targeted RT-PCR and pyridine borane-mediated f5C-to-D transformation (thereby producing characteristic C-to-U mutations upon RT)54. While not all 5-EC-iCLIP peaks in tRNA could be validated by pyridine borane sequencing, this may be due to the presence of hm5C (which does not react with pyridine borane), or due to crosslinking mediated by 5-EC incorporation at sites that lack m5C in the native system. In the latter case and supported by our comprehensive in vitro biochemical studies with recombinant ALKBH1, our analysis supports promiscuous activity of ALKBH1 on a variety of tRNA ASL sequences and even some linear m5C-containing sequences, suggesting that hm5C and f5C installation is likely to occur at these sites in systems where m5C deposition is upregulated.
We also identify TET2 as a 5-EC reactive protein, consistent with previous studies showing that TET2 converts RNA m5C in vivo and in vitro to hm5C15,23. Our data do not implicate TET2 in the formation of f5C in human total RNA, but do show that TET2 contributes to global hm5C formation (albeit to a lesser extent than ALKBH1), consistent with previous reports17,18,66. We propose that 5-EC metabolic labeling could be applied in diverse biological contexts to map the substrate of TET2 in an activity-dependent manner using 5-EC-iCLIP or analogous platforms.
Finally, our work motivates further investigation into the biological role of ALKBH1 and f5C. The presence of f5C at the wobble base of mt-tRNA-Met has been proposed to facilitate reading of unconventional mitochondrial Met codons67, and ALKBH1 KO leads to mitochondrial dysfunction40,63. The role of ALKBH1 in mediating cytoplasmic translation has been suggested to occur through demethylation of tRNA m1A5842; however, it is plausible that ALKBH1-dependent installation of f5C or related derivatives in the ASL of cytoplasmic tRNAs may also serve to tune decoding functions or to regulate tRNA structure and/or stability. Our initial studies of wobble f5C in tRNA Leu-CAA suggest a role for the modification in decoding non-cognate codons more efficiently under stress and could implicate f5C in the regulation of stress response, but more research is needed in this area. Additionally, f5C modifications in mRNA, if present at substantial stoichiometry, may modulate the expression of individual protein products. Continued work in this area will undoubtedly broaden our appreciation of epitranscriptomic processes and RNA biology.
Methods
Plasmid construction
Plasmids encoding cDNA were from the following sources: TET1 (Addgene #49792), TET1-CD H1762Y A1674A (Addgene #84479), TET2 (Addgene #41710), ALKBH1 (Genscript #OHu05179D), MPP8 (Dharmacon #MHS6278-202829247). For transfection and Flp-In cell line construction, the full sequences of TET2, ALKBH1, MPP8, TET1(CD) Mut, and the catalytic domain (CD) of TET1 (aa 1418-2136) were cloned into a modified pcDNA5/FRT/TO vector containing an N-terminal 3xFLAG tag. For protein expression, ALKBH1 was cloned into pGEX-6P-1. For the generation of KO cell lines, DNA oligos coding for guide RNA (gRNA) sequences were cloned into pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene, #42230). The oligos were phosphorylated with T4 PNK (NEB, M0201) and ligated with T4 DNA ligase (NEB, M0202) into a pX330 backbone digested with BbsI-digested (NEB, #R3539). For the dual-luciferase assay, reporter plasmids were generated by overlap PCR from pcDNA3 RLUC POLIRES FLUC (Addgene #45642) and ligation into pCDNA3.1 (Invitrogen). TTA reporter plasmid, TTG reporter plasmid, and random reporter plasmid were obtained by subcloning TTATTATTATTATTA, TTGTTGTTGTTGTTG, or AGCCCCGGGGAGCTC linkers, respectively, between the Renilla luciferase (RLuc) and Firefly luciferase (FLuc) portions.
General cell culture
Wild-type (WT) HEK 293T, and Flp-In TRex 293 cells were grown at 37 °C in a humidified atmosphere with 5% CO2 in DMEM (Life Technologies) supplemented with 10% fetal bovine serum (Atlanta), 1x penicillin-streptomycin (Gibco Life Technologies) and 2 mM l-glutamine (Life Technologies). For stress treatments when applicable, cells were heat-shocked at 42 °C for 1 h, treated for 1 h with 0.5 mM NaAsO2 (Sigma), treated for 4 h with 0.2 mM NaAsO2, or grown for 8 h in FBS- and glucose-free DMEM supplemented with 5 mM glucose (Gibco).
293 Flp-In cell line generation
To generate stable cell lines expressing 3xFLAG-tagged ALKBH1, TET2, and MPP8, Flp-In TRex 293 cells were seeded at 0.6 × 106 cells per well in six-well plates, and co-transfected with the appropriate pCDNA5/FRT/TO-3xFLAG-tagged plasmid (0.2 µg) and pOG44 plasmid (2 µg, Thermo Fisher). After selection with 100 µg/mL hygromycin B and 15 µg/mL blasticidin, colonies were expanded. To confirm expression of the desired protein, cells were induced with tetracycline (0–1 µg/mL) for 24 h. Cells were harvested and lysed in NP-40 lysis buffer [50 mM Tris HCl pH 7.5, 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 1 mM PMSF (supplemented freshly), protease inhibitor tablet (Roche, supplemented freshly)]. Protein concentration was normalized to 2 mg/mL, and proteins were separated by SDS-PAGE and analyzed by western blot with anti-FLAG M2 antibody (Sigma, #F1804, 1 µg/mL, 1:1000).
Knockout (KO) cell line generation
HEK293T WT cells (0.8 × 106) were seeded in a six-well dish. After 24 h, pX330 plasmid containing the gRNA for the target protein (2 µg) and pcDNA3-FKBP-EGFP-HOTag3 (Addgene, 200 ng) were co-transfected using Lipofectamine 2000 (Thermo Scientific). Cells were FACS-sorted 48 h after transfection. The top 95% of cells with green fluorescent protein (GFP) signals were sorted as single cells into 96-well plates. Surviving colonies were expanded to 6-well plates and then 10-cm plates. KO was confirmed by genomic PCR and western blot with mouse β-actin antibody (Cell Signaling, 1:1000, as a loading control) and either rabbit anti-ALKBH1 (Abcam #128895, 1:2000), anti-TET2 (Novus #NBP2-32104, 0.2 µg/mL) or anti-NSUN2 (Proteintech, #20853-1-AP 1:2000) antibodies.
Cell viability assay after 5-EC treatment
HEK293T WT cells were seeded in 96-well culture plates (4000 cells in 200 µl of medium per well) on day 0. On day 1, cells were treated with 5-EC in DMSO at the final concentrations of 0, 10, 50, 100, 200, 500, 1000, 2000, and 5000 µM. Cell viability was measured using the MTS assay (CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay; Promega, G5430) at 12 h, 24 h, 36 h, and 48 h post-treatment following the manufacturer’s instructions. The absorbance of each well was read at 490 nm on a Synergy H1 Microplate Reader (BioTek). All treated cells were normalized to the 0 µM sample and the curve was fitted based on a 4-parameter dose-response equation using GraphPad Prism:
| 1 |
Three independent biological replicates and three technical replicates were analyzed.
Analysis of 5-EC labeling using fluorescence microscopy
WT 293T cells were seeded on a round glass coverslip at 0.6 × 106 cells per well in a 6-well plate. Cells were allowed to grow 18–24 h and were treated with 1 mM 5-EC or vehicle for 4 h. After labeling, media were removed, and the coverslips were washed with PBS three times. Cells were fixed for 20 min at room temperature in PBS containing 3% paraformaldehyde and 2% sucrose adjusted to pH 7.3 and washed with PBS 3 times for 5 min each time. Cells were permeabilized with PBST (PBS + 0.1% Triton X-100) for 20 min at room temperature and washed 3 times with PBS for 5 min each time. Cellular RNA was labeled by Cu-assisted azide-alkyne cycloaddition (CuAAC) by incubating each coverslip upside-down (cell-side down) onto droplets (100 µL) of “click” solution (5 µM Cy3-Azide, 1 mM CuSO4, 2 mM THPTA ligand, 10 mM Na ascorbate, 1X PBS) for 2 h at room temperature in the dark. Coverslips were washed briefly 3 times with PBST and then rewashed more thoroughly 5 times with PBST for 10 min each time to remove any remaining dye. Nuclei were stained with Hoechst 33342 (Thermo, 1 ug/mL) in PBS for 5 min, and coverslips were washed with PBS twice for 5 min. Coverslips were mounted on cell-face down in ProLong Gold AntiFade Reagent (Life Technologies) atop a microscope slide and fixed in place with nail polish. Fixed cells were imaged on a Nikon Eclipse Ti microscope equipped with a 100x objective, a CMOS camera, and the NIS Elements AR software. The TRITC channel was used to excite the Cy3 dye. Images used for direct comparison were acquired using standardized illumination and exposure parameters and presented with identical LUT settings.
5-EC-mediated in vivo crosslinking of TET1(CD)
WT 293T cells were seeded at 0.6 × 106 cells/well in six-well plates and transfected with the pcDNA5-TET1(CD) plasmid (2 μg) and Lipofectamine 2000 (Invitrogen, 5 μL) following the manufacturer’s instructions. Twelve (12) h post-transfection, cells were treated with fresh media containing 5-EC or 5-EU (0.2–1 mM) for 16 h. Cells were harvested and lysed in NP-40 lysis buffer and incubated on ice for 30 min. The lysate was spun at 21,000 × g at 4 °C, normalized to 2 mg/mL protein, run on a 10% SDS-PAGE gel, and analyzed by western blot (anti-FLAG) as described above.
Poly(A) pulldown of 5-EC-treated cells for proteomics (5-EC RNABPP) or western blot
Flp-In TRex 293 cells expressing the desired protein (for western blot validations) or WT HEK 293 T cells (for proteomics experiments) (5–10 × 10 cm plates) were grown to 50% confluency, induced with tetracycline (1 µg/mL), and cultured for 24 h to ~80% confluency before overnight treatment with 1 mM 5-EC (500 mM stock in DMSO) or vehicle (or also m5C in Supplementary Fig. 8B). Cells were harvested and poly(A) pulldown was performed following published protocols with minor modifications68,69. 1 mL of oligo(dT) lysis buffer [20 mM Tris HCl pH 7.5, 500 mM LiCl, 0.5% LDS, 1 mM EDTA, 5 mM DTT (supplemented freshly), and protease inhibitor tablet] and 75 µL of equilibrated oligo(dT) beads were used per plate. Lysis, enrichment, and washes were performed at ambient temperature, and washes were performed with lysis buffer, NP-40 washing buffer (50 mM Tris HCl pH 7.5, 150 mM LiCl, 0.5% NP-40, 1 mM EDTA, 0.5 mM DTT), and NP-40 free washing buffer (just for proteomics experiments). Bead-bound RNA was eluted with oligo(dT) elution buffer [(40 µL/plate), 20 mM Tris HCl pH 7.5, 1 mM EDTA] for 5 min at 80 °C with shaking. The incubation-wash-elution procedure was repeated 2 more times, and the elutions combined and concentrated to ~75–50 µL. RNA in the elutions was digested with 25 unit/mL RNase A and 2000 unit/mL RNase T1 in 40 mM NaCl and 2 mM MgCl2 at 37 °C for 1 h. The remaining protein and the input were run on a 10% SDS-PAGE gel analyzed by western blot (anti-FLAG). Experiments were performed in triplicate.
Proteomics mass spectrometry
Sample volume was adjusted to 200 µL with 50 mM Ammonium Bicarbonate pH 8 and incubated with 5 mM TCEP at 60 °C for 10 min and then with 15 mM chloroacetamide in the dark at RT for 30 min. Proteins were digested with Trypsin Gold (Promega, 2 µg) and incubated at 37 °C for 16 h. Samples were acidified 0.2% TFA and desalted using SDB stage-tips70. Samples were dried processed for mass spectrometry on an Orbitrap Fusion Lumos (Thermo Scientific) as described previously71. Data were analyzed using Scaffold (version Scaffold 4.9, Proteome Software Inc.) to validate MS/MS-based peptide and protein identifications. Quantitation of protein abundance was done by spectral counting. Proteins were only selected for quantification if they were present in at least two replicates and not in the negative control.
Ribonucleoside quantification from biological samples by LC-QQQ-MS
Total RNA was extracted using TRIzol reagent following the manufacturer’s indications. Small RNA was isolated from total RNA using the adjusted protocol from RNA Zymo Clean and Concentrator 5 (Zymo Research). mRNA was isolated by 2 rounds of poly(A) pulldown as described above and further subjected to ribo-depletion with custom synthesized probes complementary to human rRNA72 and small RNA depletion (with Zymo Clean and Concentrator 5. RNA samples (3 µg) were digested in 20 µL with 2 units of Nuclease P1 (Wako) and final concentrations of 7 mM NaOAc and 0.4 mM ZnCl2 at 37 °C for 2 h. The digested mixture was subjected to dephosphorylation in a total volume of 30 µL with 2 units of Antarctic Phosphatase (AnP, NEB) and a final concentration of 1X AnP buffer at 37 °C for 2 h. Quantification of ribonucleosides was done an Agilent 1260 LC Infinity II system coupled to an Agilent 6470 LC/TQ module as reported previously21 and with a separation gradient from literature precedence73. Analyte detection was done by dynamic multiple reaction monitoring (DMRM) with the parameters in Table S1. Abundant nucleosides in biological samples (ACGU) were quantified in 1 ng of sample, while less abundant ones (m5C, 5-EC, hm5C, f6C, i6A) were analyzed in 100–200 ng. Commercial nucleosides were used to generate standard curves. Relative levels of m5C, 5-EC 5-EU, hm5C, f6C, and i6A were calculated by normalizing the concentration of modified nucleoside to that of the respective canonical nucleoside.
Metabolic incorporation of 5-EC was performed by feeding WT 293T cells at ~80% confluency with 1 mM 5-EC (500 mM stock) or vehicle in fresh DMEM for 16 h. RNA was processed for QQQ LC-MS/MS quantification on Agilent MassHunter Workstation Data Acquisition version 10.0 as described above using relevant standards (Supplementary Figs. 1–2). The experiment was done in triplicate.
5-EC-iCLIP
Library preparation for iCLIP was adapted from the literature45. 30 × 10 cm dish Flp-In T-Rex 293 cells expressing 3xFlag-ALKBH1 were grown to 60% confluency and treated with 1 μg/mL tetracycline. After 12 h, medium was changed to fresh medium containing 1 μg/mL tetracycline and 1 mM 5-EC and cultured for another 12 h. Cells were washed twice with cold PBS and harvested with 500 µL lysis buffer (50 mM Tris-HCL pH 7.4, 100 mM NaCl, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate). Lysates were treated with Turbo DNase (Thermo) and a low (1:200) or high (1:50) concentration of RNase I (AM2295) at 37 °C for 3 min with rotation, after which they were centrifuged at 20,000 × g for 15 min at 4 °C. The supernatant was incubated with anti-FLAG beads (200 μL Protein G beads, 15 µg anti-Flag M2 antibody) at 4 °C overnight, and the immunoprecipitated material was washed three times with high-salt buffer and then once with CutSmart buffer (NEB). The beads were treated with Quick-CIP (NEB) at 37 °C for 30 min with rotation, washed twice with high-salt buffer, and then incubated with pre-adenylated and biotin-labeled L3 linker by using T4 RNA ligase I (NEB) at 16 °C overnight. The beads were boiled and analyzed by Western blot for biotinylation with the chemiluminescent nucleic acid detection module (Thermo). The rest of the sample was gel purified based on the position of the biotin signal by using D-tube dialyzer midi (Merck-Millipore). Proteinase K-digested and purified RNA was used for reverse transcription with oligos containing randomized barcodes (UMIs) and two inversely oriented adaptor regions separated by a BamHI restriction site. cDNAs were size-purified (70–75 nt, 75–100 nt and 100–150 nt) on TBE-Urea gels and then circularized by CircLigase II (Epicentre). Circularized cDNAs were digested with BamHI and linearized cDNAs were amplified using Solexa primers and submitted for Illumina sequencing.
Bioinformatic analysis
The iCLIP and ARP pulldown sequencing data was processed using iCount Primary Analysis (Consensus mapping) pipeline on iMAPs web server (https://imaps.genialis.com/iclip). Briefly, random unique molecular identifiers were used to distinguish and discard PCR duplicates, and adaptor/barcode sequences were removed. Trimmed reads were mapped to tRNA/rRNA with STAR (v.2.7.0f). The unmapped reads were further mapped to GRCh38 with STAR (v.2.7.0f) and only uniquely mapping reads were used for further analysis. For iCLIP: iCount generated raw crosslinking sites were used for peak calling analysis by Paraclu with following parameters: Minimal sum of scores inside a cluster:10; Maximal cluster size: 6; Minimal density increase:1. For ARP pull down: peaks were called using Paraclu with default parameters. Only peaks present in two replicates and unique to the 5-EC-treated (IP sample in ARP analysis) sample or showing a fold-change of greater than 1.5 (treated versus input) were kept after intersection. Sequences 5 bases upstream and downstream were extracted from the reference and used for motif analysis by MEME (V.5.3.3) using the default parameters.
ALKBH1 expression and purification
The sequence-verified construct pGEX-6P-1-ALKBH1 was transformed into E. coli strain BL21 and expressed at 18 °C with 0.2 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 18 h. Lysis by sonication was performed in buffer containing 1X TBS, 150 mM NaCl, 5 mM EDTA, 1 mM DTT, 0.2 mg/mL lysozyme (Thermo), and 1% Triton X-100, supplemented with 1 mM PMSF, protease inhibitor tablet, and Benzonase (Sigma). The lysate was purified using Pierce glutathione agarose resin (ThermoFisher) following the manufacturer’s instructions. The GST tag from promising fractions was cleaved with PreScission protease (Sigma, 1:100 by mass of protease:sample) while simultaneously dialyzing in 1x TBS at 4 °C overnight. Cleavage was confirmed by SDS-PAGE, and the free GST tag was removed by running the sample over fresh or regenerated glutathione resin and collecting the flow-through. The protein was further fractionated on a MonoQ 5/50 GL anion exchange column (GE Healthcare) on a gradient of 50 mM to 1 M NaCl (in 50 mM Tris HCl pH 8) for 20 column volumes. The most concentrated fractions were combined dialyzed overnight at 4 °C in 50 mM Tris HCl pH 7.5 and concentrated to at least 1 mg/mL.
Oligonucleotide synthesis
Oligonucleotide synthesis was done on an ABI 394 oligonucleotide synthesizer (Applied Biosystems) using standard coupling conditions and commercial oligosynthesis reagents and phosphoramidites (Glen Research) unless otherwise noted. For synthesis of the random m5C-containing RNA library (oligo 5), a mix was created by combining each TBDMS-protected RNA phosphoramidite (i.e., A, G, C, U) in the ratios previously reported74. Oligos 3–4 and 6–8 were cleaved from the resin and 2’OH-deprotected and butanol-precipitated under standard conditions.
Oligos 1–2 were synthesized with 5-EC phosphoramidite (11, Supplementary Note) or m5C phosphoramidite (Glen Research), respectively, using ultra mild phosphoramidites (Glen Research). The final product was manually detritylated one last time with 3% dichloroacetic acid on the instrument until the orange coloration observe in the lines (from the trityl cation) disappeared. The oligos were cleaved from the resin in a solution of 3:1 NH4OH/EtOH at rt for 24 h with rotation, and the supernatant was dried on speedvac. 2’-OH deprotection was performed on the residue with 1 M TBAF on THF (1 mL) at rt for 18 h with rotation. The solution was diluted with THF (1 mL), desalted on NAP-10 columns (G&E healthcare, 2 columns per sample), eluted with 2.5 mL DEPC-H2O, and lyophilized. Incompletely lyophilized samples were precipitated with 1 mL EtOH + 100 μL 3 M NaOAc pH 5.2, dried via speedvac, resuspended in H2O, and re-lyophilized.
All oligos except for 5 were purified by reverse-phase HPLC on a Zorbax Eclipse XDB-C18 semiprep column (Agilent) using a gradient of 5 to 40% acetonitrile in 0.1 M triethylammonium acetate over 50 min or 0 to 20% acetonitrile over 30 min and characterized by ESI-TOF HRMS and MALDI-TOF MS (Supplementary Table 4). Oligo 3 was purified and characterized by denaturing urea-PAGE (Supplementary Fig. 36).
ALKBH1 activity assay on m5C-containing substrates
ALKBH1 enzymatic assays were carried out as reported previously19,20. Briefly, RNA substrate (125 pmol) was reacted with 0.1 µM ALKBH1 in a 250 µL reaction containing 20 mM HEPES pH 6.8, 5 mM MgCl2, 50 mM KCl, 1 mM DTT, 4 mM ascorbic acid, 100 µM 2-oxoglutarate, and 80 µM ammonium iron (II) sulfate. The mixture was incubated at 37 °C for the time indicated in Fig. 4C and immediately quenched by performing RNA extraction with TRIzol LS (Thermo) following the manufacturer’s directions up to the layer separation step. The aqueous layer was then transferred to a Zymo RNA Clean and Concentrator-5 spin column, and the purification was resumed following the manufacturer’s indications. The entire RNA sample was digested with Nuclease P1 and dephosphorylated with AnP as described above. Decrease of m5C and generation of hm5C and f5C were measured in 6.25 pmol of the sample by LC-QQQ-MS using commercial standards. Experiments were performed in triplicate.
In vitro ALKBH1 crosslinking assays with 5-EC oligo
Crosslinking reactions were prepared in 20 μL with the following concentrations of reagents: 50 mM tris pH 8, 50 mM KCl, 2 mM ascorbic acid, 1 mM MgCl2, 1 mM alpha-ketoglutarate, 100 μM FeCl2, 1 μM RNA oligo, and 0.2 μM recombinant ALKBH1. The reactions were incubated for 1 h at 37 °C and quenched with the addition of 1 μM 0.5 M EDTA or 10 μL 3x SDS sample buffer for Western blotting. Crosslinked protein was separated on 12% SDS-PAGE and Western-blotted for ALKBH1 as indicated above.
Antisense pulldowns and northern blotting
Specific tRNAs were enriched by antisense pulldown with biotin-labeled antisense probes in Table S16 following a modified procedure from the literature42. Small RNA was extracted from WT or ALKBH1 KO 293 T cells using the small RNA enrichment protocol from Zymo RNA Clean and Concentrator-5 (Zymo). Depending on tRNA abundance and probe efficiency, the pulldown was done on 5–25 µg of bulk small RNA. Streptavidin M-280 magnetic beads (Invitrogen) (10 µL bead per 5 µg RNA input) were used. RNA and probe-conjugated beads were incubated at 42 °C for 3 h. Elution was performed twice at 75 °C for 5 min in 10 mM Tris pH 7.5.
40–100 ng of small RNA input or pulldown elution were resolved in a 12% polyacrylamide-urea gel and analyzed by Near-IR northern blot following literature precedence with minor adjustments75. RNA was transferred overnight to a Biodyne B Nylon membrane (Thermofisher) and immobilized under a 254 nm UV lamp for 10 min. Blocking was done in pre-hybridization buffer (200 mM Na2HPO4, 7% SDS,) supplemented with 50 µg/mL of salmon sperm DNA for 2 h at 42 °C. Annealing was done in pre-hybridization buffer for 4 h at 42 °C with 50 nM of the corresponding biotinylated probe (Table S16). Co-hybridization with a FAM-labeled probe (50 nM) complementary to 5S RNA (Table S16) was performed in cases where a loading control was necessary. Secondary staining was performed with IR-800 Streptavidin dye (1:10 000, LICOR) in pre-hybridization buffer for 30 min at 30 °C.
For LC-QQQ-MS measurements, the tRNA elutions were dephosphorylated and analyzed as indicated above. Triplicates were gathered for each experiment.
Pyridine borane treatment
1 ug total RNA was incubated with 600 mM sodium acetate (pH 5.2) and 1 M pyridine borane for 16 h at 37 °C in water. The product was purified on Zymo-Spin columns. 100 ng reacted RNA was incubated with 10 μM RT primer and 1 μL 10 mM dNTP mix at 65 °C for 5 min. The reaction was reverse transcribed with Superscript II following the manufacturer’s instructions. cDNA was then PCR amplified by AccuPrime™ Pfx DNA Polymerase (Thermo). The PCR products were gel purified and submitted for high-throughput amplicon sequencing (Genewiz).
Mutation detection by amplicon sequencing
The reads were first mapped to tRNA reference sequences by STAR (Galaxy Version 2.7.8a). Only uniquely mapped reads were kept for further analysis. Pileup files were generated using Samtools mpileup (Galaxy Version 2.1.4) with default parameters. The variants were called using VarScan mpileup (Galaxy Version 2.4.3.1) with the default parameters.Mutations that were only present in WT samples but not ALKBH1 KO samples and control samples were identified as ALKBH1-dependent f5C sites.
Aldehyde reactive probe (ARP) sequencing
Total RNA extracted from WT or ALKBH1 KO cells was first treated with 2 μl DNase I (NEB) to remove DNA contamination. The total RNA was then fragmented into 150 nt fragments using RNA Fragmentation Reagents (Thermo). The incubation of fragmented RNA samples with Aldehyde Reactive Probe (ARP) was carried out with some modification from a previous report53. Briefly, 100 ng f5C containing oligo was first added into 100 μg fragmented RNA, the mix was then incubated with 2 mM ARP in 40 mM aqueous NH4OAc buffer pH 5.0 supplemented with 100 mM anisidine for 24 h at 25 °C. RNA was then purified using phenol/chloroform extraction. 200 ng RNA was taken out as input, and the rest of the samples were incubated with 50 μl pre-washed streptavidin beads in 400 μl IP buffer (200 mM NaCl, 0.4 mM EDTA, 50 mM Tris-HCl pH 7.4, and 0.1% NP-40) for 2 h at 4 °C. The beads were then washed twice with IP wash buffer (500 mM NaCl, 0.4 mM EDTA, 50 mM Tris-HCl pH 7.4, and 0.1% NP-40). The beads and input samples were treated with Quick-CIP (NEB) at 37 °C for 30 min with rotation, input samples were purified using RNA Zymo Clean kit (Zymo Research), and beads were washed twice with wash buffer. Input RNA and bead were then incubated with pre-adenylated L3 linker (rAppAGATCGGAAGAGCGGTTCAG/ddC/) by using T4 RNA ligase I (NEB) at 16 °C overnight. On-bead reverse transcription was performed using Superscript III with iCLIP RT primers (25 °C for 5 min, 42 °C for 20 min, and 50 °C for 40 min). cDNA was eluted using RNaseH and then size-purified (60–100 nt and 100–200 nt) on TBE-Urea gels. Extracted cDNA was circularized by CircLigase II (Epicentre). Circularized cDNAs were digested with BamHI and linearized cDNAs were amplified using Solexa primers and submitted for Illumina sequencing.
Luciferase translation efficiency assay
For the translation assay, 500 ng of each reporter plasmid were transfected with lipofectamine 2000 into WT HEK293T or ALKBH1 KO cells in a 24-well plate. After 4 h, cells were washed with PBS and changed to glucose deprivation medium (glucose-depleted DMEM supplemented with 10% Pen/Strep, 1 mM or 5 mM glucose) or normal medium (glucose-depleted DMEM supplemented with 10% Pen/Strep, 25 mM glucose) and grown overnight. Cells were harvested in passive lysis buffer (Promega) following the manufacturer’s protocol. The assay was performed using Dual-luciferase Reporter Assay (Promega) following instructions from the manufacturer. Luciferase signal was measured in a Synergy H1 Microplate Reader (BioTek). Fluc signal was normalized to luciferase Rluc signal to evaluate the translation efficiency of each reporter. Three independent biological replicates were analyzed and a student T-test was applied with *p < 0.05 and **p < 0.01.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank Saw Kyin and Henry Shwe at the Princeton University Mass Spectrometry and Proteomic Facility for performing proteomic analysis, and Christina DeCoste at the Princeton University Flow Cytometry Resource Facility for assistance with FACS analysis. R.E.K. acknowledges support from a National Science Foundation CAREER award (MCB-1942565), the National Institute of Health (R01 GM132189), the Sidney Kimmel Foundation and the Alfred P. Sloan Foundation. A.E.A. acknowledges the Edward C. Taylor 3rd Year Graduate Fellowship in Chemistry and an Eli Lilly-Edward C. Taylor Fellowship in Chemistry. T.W.E. acknowledges the Edward C. Taylor 3rd Year Graduate Fellowship in Chemistry. A.L. was supported by the Princeton Catalysis Initiative. All authors thank Princeton University for financial support.
Source data
Author contributions
R.E.K. conceived the study, analyzed data, prepared KO and stable cell lines, and supervised all authors. A.E.A. performed RNABPP experiments, chemical synthesis, nucleoside LC-MS, bioinformatic analysis, and in vitro enzymatic assays. A.L. performed iCLIP experiments, pyridine-borane sequencing, ARP-seq and bioinformatic analysis. X.S. prepared KO cell lines, performed nucleoside LC-MS, and performed translation assays. T.W.E. performed phosphoramidite synthesis and ALKBH1 in vitro crosslinking assays. E.M. performed phosphoramidite synthesis. R.E.K., A.E.A., and A.L. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks Bi-Feng Yuan and the other, anonymous, reviewers for their contribution to the peer review of this work.
Data availability
The data that support this study are available from the corresponding author upon reasonable request. The sequencing data generated in this study have been deposited in the GEO database under accession code GSE202815. The proteomics data used in this study are available in the ProteomeXchange database under accession code PXD029955. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: A. Emilia Arguello, Ang Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-31876-2.
References
- 1.Nachtergaele S, He C. The emerging biology of RNA post-transcriptional modifications. RNA Biol. 2017;14:156–163. doi: 10.1080/15476286.2016.1267096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiener D, Schwartz S. The epitranscriptome beyond m(6)A. Nat. Rev. Genet. 2021;22:119–131. doi: 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 3.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccaletto P, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46:D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 2019;21:552–559. doi: 10.1038/s41556-019-0319-0. [DOI] [PubMed] [Google Scholar]
- 6.Angelova MT, et al. The emerging field of epitranscriptomics in neurodevelopmental and neuronal disorders. Front Bioeng. Biotechnol. 2018;6:46. doi: 10.3389/fbioe.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Xiong X, Yi C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat. Methods. 2016;14:23–31. doi: 10.1038/nmeth.4110. [DOI] [PubMed] [Google Scholar]
- 8.Hussain S, Aleksic J, Blanco S, Dietmann S, Frye M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 2013;14:215. doi: 10.1186/gb4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auxilien S, Guerineau V, Szweykowska-Kulinska Z, Golinelli-Pimpaneau B. The human tRNA m (5) C methyltransferase Misu is multisite-specific. RNA Biol. 2012;9:1331–1338. doi: 10.4161/rna.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuorto F, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat. Struct. Mol. Biol. 2012;19:900–905. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 11.Guo G, et al. Advances in mRNA 5-methylcytosine modifications: detection, effectors, biological functions, and clinical relevance. Mol. Ther. Nucleic Acids. 2021;26:575–593. doi: 10.1016/j.omtn.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HY, Xiong J, Qi BL, Feng YQ, Yuan BF. The existence of 5-hydroxymethylcytosine and 5-formylcytosine in both DNA and RNA in mammals. Chem. Commun. (Camb.) 2016;52:737–740. doi: 10.1039/C5CC07354E. [DOI] [PubMed] [Google Scholar]
- 13.Huber SM, et al. Formation and abundance of 5-hydroxymethylcytosine in RNA. Chembiochem. 2015;16:752–755. doi: 10.1002/cbic.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W, et al. Formation and determination of the oxidation products of 5-methylcytosine in RNA. Chem. Sci. 2016;7:5495–5502. doi: 10.1039/C6SC01589A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu L, et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J. Am. Chem. Soc. 2014;136:11582–11585. doi: 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delatte B, et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 17.Lan J, et al. Functional role of Tet-mediated RNA hydroxymethylcytosine in mouse ES cells and during differentiation. Nat. Commun. 2020;11:4956. doi: 10.1038/s41467-020-18729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He C, et al. TET2 chemically modifies tRNAs and regulates tRNA fragment levels. Nat. Struct. Mol. Biol. 2021;28:62–70. doi: 10.1038/s41594-020-00526-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawarada L, et al. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haag S, et al. NSUN3 and ABH1 modify the wobble position of mt-tRNAMet to expand codon recognition in mitochondrial translation. EMBO J. 2016;35:2104–2119. doi: 10.15252/embj.201694885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai, W. et al. Activity-based RNA-modifying enzyme probing reveals DUS3L-mediated dihydrouridylation. Nat. Chem. Biol.17, 1178–1187 (2021). [DOI] [PMC free article] [PubMed]
- 22.Qu D, et al. 5-Ethynylcytidine as a new agent for detecting RNA synthesis in live cells by “click” chemistry. Anal. Biochem. 2013;434:128–135. doi: 10.1016/j.ab.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Ghanty U, DeNizio JE, Liu MY, Kohli RM. Exploiting substrate promiscuity to develop activity-based probes for ten-eleven translocation family enzymes. J. Am. Chem. Soc. 2018;140:17329–17332. doi: 10.1021/jacs.8b04722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jao CY, Salic A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc. Natl Acad. Sci. USA. 2008;105:15779–15784. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Outeiral V, de la Parte C, Fidalgo M, Guallar D. The complexity of TET2 functions in pluripotency and development. Front Cell Dev. Biol. 2020;8:630754. doi: 10.3389/fcell.2020.630754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeNizio JE, Liu MY, Leddin EM, Cisneros GA, Kohli RM. Selectivity and promiscuity in TET-mediated oxidation of 5-methylcytosine in DNA and RNA. Biochemistry. 2019;58:411–421. doi: 10.1021/acs.biochem.8b00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XS, et al. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–247 e17. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loenarz C, Schofield CJ. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem. Sci. 2011;36:7–18. doi: 10.1016/j.tibs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Santi DV. m5C RNA and m5C DNA methyl transferases use different cysteine residues as catalysts. Proc. Natl Acad. Sci. USA. 2000;97:8263–8265. doi: 10.1073/pnas.97.15.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr PJ, Robins MJ, Santi DV. Reaction of 5-ethynyl-2’-deoxyuridylate with thiols and thymidylate synthetase. Biochemistry. 1983;22:1696–1703. doi: 10.1021/bi00276a027. [DOI] [PubMed] [Google Scholar]
- 31.Rider LW, Ottosen MB, Gattis SG, Palfey BA. Mechanism of dihydrouridine synthase 2 from yeast and the importance of modifications for efficient tRNA reduction. J. Biol. Chem. 2009;284:10324–10333. doi: 10.1074/jbc.M806137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Q, et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature. 2018;554:123–127. doi: 10.1038/nature25434. [DOI] [PubMed] [Google Scholar]
- 33.Chang Y, et al. MPP8 mediates the interactions between DNA methyltransferase Dnmt3a and H3K9 methyltransferase GLP/G9a. Nat. Commun. 2011;2:533. doi: 10.1038/ncomms1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser S, et al. Strategies to avoid artifacts in mass spectrometry-based epitranscriptome analyses. Angew. Chem. Int. Ed. Engl. 2021;60:23885–23893. doi: 10.1002/anie.202106215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y, et al. Permethylation of ribonucleosides provides enhanced mass spectrometry quantification of post-transcriptional RNA modifications. Anal. Chem. 2022;94:7246–7254. doi: 10.1021/acs.analchem.2c00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinoda S, et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8734–8745. doi: 10.1093/nar/gkz575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoddami V, Cairns BR. Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nat. Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano S, et al. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met) Nat. Chem. Biol. 2016;12:546–551. doi: 10.1038/nchembio.2099. [DOI] [PubMed] [Google Scholar]
- 41.Behrens A, Rodschinka G, Nedialkova DD. High-resolution quantitative profiling of tRNA abundance and modification status in eukaryotes by mim-tRNAseq. Mol. Cell. 2021;81:1802–1815 e7. doi: 10.1016/j.molcel.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:1897. doi: 10.1016/j.cell.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 43.Rashad S, et al. The stress specific impact of ALKBH1 on tRNA cleavage and tiRNA generation. RNA Biol. 2020;17:1092–1103. doi: 10.1080/15476286.2020.1779492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konig J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 2010;17:909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huppertz I, et al. iCLIP: protein-RNA interactions at nucleotide resolution. Methods. 2014;65:274–287. doi: 10.1016/j.ymeth.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frith MC, et al. A code for transcription initiation in mammalian genomes. Genome Res. 2008;18:1–12. doi: 10.1101/gr.6831208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Nostrand EL, Shishkin AA, Pratt GA, Nguyen TB, Yeo GW. Variation in single-nucleotide sensitivity of eCLIP derived from reverse transcription conditions. Methods. 2017;126:29–37. doi: 10.1016/j.ymeth.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chujo T, Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18:2269–2276. doi: 10.1261/rna.035600.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozanick S, Krecic A, Andersland J, Anderson JT. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA. 2005;11:1281–1290. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hauenschild R, et al. The reverse transcription signature of N-1-methyladenosine in RNA-Seq is sequence dependent. Nucleic Acids Res. 2015;43:9950–9964. doi: 10.1093/nar/gkv895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Straby KB. The human tRNA(m(2)(2)G(26))dimethyltransferase: functional expression and characterization of a cloned hTRM1 gene. Nucleic Acids Res. 2000;28:3445–3451. doi: 10.1093/nar/28.18.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey TL, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raiber EA, et al. Genome-wide distribution of 5-formylcytosine in embryonic stem cells is associated with transcription and depends on thymine DNA glycosylase. Genome Biol. 2012;13:R69. doi: 10.1186/gb-2012-13-8-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, et al. Bisulfite-free direct detection of 5-methylcytosine and 5-hydroxymethylcytosine at base resolution. Nat. Biotechnol. 2019;37:424–429. doi: 10.1038/s41587-019-0041-2. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, et al. Single-BAse Resolution Mapping Reveals Distinct 5-formylcytidine in Saccharomyces cerevisiae mRNAs. ACS Chem. Biol. 2022;17:77–84. doi: 10.1021/acschembio.1c00633. [DOI] [PubMed] [Google Scholar]
- 56.Li A, Sun X, Arguello AE, Kleiner RE. Chemical method to sequence 5-formylcytosine on RNA. ACS Chem. Biol. 2022;17:503–508. doi: 10.1021/acschembio.1c00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thornburg LD, Stubbe J. Mechanism-based inactivation of thymine hydroxylase, an alpha-ketoglutarate-dependent dioxygenase, by 5-ethynyluracil. Biochemistry. 1993;32:14034–14042. doi: 10.1021/bi00213a037. [DOI] [PubMed] [Google Scholar]
- 58.Ortiz de Montellano PR, Komives EA. Branchpoint for heme alkylation and metabolite formation in the oxidation of arylacetylenes by cytochrome P-450. J. Biol. Chem. 1985;260:3330–3336. doi: 10.1016/S0021-9258(19)83624-4. [DOI] [PubMed] [Google Scholar]
- 59.Wright AT, Cravatt BF. Chemical proteomic probes for profiling cytochrome p450 activities and drug interactions in vivo. Chem. Biol. 2007;14:1043–1051. doi: 10.1016/j.chembiol.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gan LS, Acebo AL, Alworth WL. 1-Ethynylpyrene, a suicide inhibitor of cytochrome P-450 dependent benzo[a]pyrene hydroxylase activity in liver microsomes. Biochemistry. 1984;23:3827–3836. doi: 10.1021/bi00312a006. [DOI] [PubMed] [Google Scholar]
- 61.Wright AT, Song JD, Cravatt BF. A suite of activity-based probes for human cytochrome P450 enzymes. J. Am. Chem. Soc. 2009;131:10692–10700. doi: 10.1021/ja9037609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuerst R, Breinbauer R. Activity-based protein profiling (ABPP) of oxidoreductases. Chembiochem. 2021;22:630–638. doi: 10.1002/cbic.202000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Haute L, et al. Deficient methylation and formylation of mt-tRNA(Met) wobble cytosine in a patient carrying mutations in NSUN3. Nat. Commun. 2016;7:12039. doi: 10.1038/ncomms12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musheev MU, Baumgartner A, Krebs L, Niehrs C. The origin of genomic N(6)-methyl-deoxyadenosine in mammalian cells. Nat. Chem. Biol. 2020;16:630–634. doi: 10.1038/s41589-020-0504-2. [DOI] [PubMed] [Google Scholar]
- 65.Wagner, A. et al. Mitochondrial Alkbh1 localizes to mtRNA granules and its knockdown induces the mitochondrial UPR in humans and C. elegans. J. Cell Sci.132, jcs223891 (2019). [DOI] [PubMed]
- 66.Shen H, et al. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J. Biol. Chem. 2021;296:100087. doi: 10.1074/jbc.RA120.014226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bilbille Y, et al. The human mitochondrial tRNAMet: structure/function relationship of a unique modification in the decoding of unconventional codons. J. Mol. Biol. 2011;406:257–274. doi: 10.1016/j.jmb.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castello A, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 69.Baltz AG, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 71.Arguello AE, DeLiberto AN, Kleiner RE. RNA chemical proteomics reveals the N(6)-methyladenosine (m(6)A)-regulated protein-RNA interactome. J. Am. Chem. Soc. 2017;139:17249–17252. doi: 10.1021/jacs.7b09213. [DOI] [PubMed] [Google Scholar]
- 72.Adiconis X, et al. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nat. Methods. 2013;10:623–629. doi: 10.1038/nmeth.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su D, et al. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc. 2014;9:828–841. doi: 10.1038/nprot.2014.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arguello AE, Leach RW, Kleiner RE. In vitro selection with a site-specifically modified rna library reveals the binding preferences of N(6)-methyladenosine reader proteins. Biochemistry. 2019;58:3386–3395. doi: 10.1021/acs.biochem.9b00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller BR, Wei T, Fields CJ, Sheng P, Xie M. Near-infrared fluorescent northern blot. RNA. 2018;24:1871–1877. doi: 10.1261/rna.068213.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support this study are available from the corresponding author upon reasonable request. The sequencing data generated in this study have been deposited in the GEO database under accession code GSE202815. The proteomics data used in this study are available in the ProteomeXchange database under accession code PXD029955. Source data are provided with this paper.