Abstract
The enzyme xanthine oxidoreductase (XOR) catalyzes the synthesis of uric acid (UA) from hypoxanthine and xanthine, which are products of purine metabolism starting from ribose-5-phosphate. Several studies suggested a relationship between hyperuricemia and hepatic steatosis; however, few previous studies have directly examined the relationship between XOR activity and hepatic steatosis. A total of 223 subjects with one or more cardiovascular risk factors were enrolled. The liver-to-spleen (L/S) ratio on computed tomography and the hepatic steatosis index (HSI) were used to assess hepatic steatosis. We used a newly developed highly sensitive assay based on [13C2, 15N2] xanthine and liquid chromatography/triple quadrupole mass spectrometry to measure plasma XOR activity. Subjects with the L/S ratio of < 1.1 and the HSI of < 36 had increased XOR activity and serum UA levels. Independent of insulin resistance and serum UA levels, multivariate logistic regression analysis revealed that plasma XOR activity was associated with the risk of hepatic steatosis as assessed by the L/S ratio and HSI. According to the findings of this study, plasma XOR activity is associated with hepatic steatosis independent of insulin resistance and serum UA levels.
Subject terms: Endocrinology, Gastroenterology
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of fatty accumulation in the liver on imaging or histology, excluding fatty liver secondary to alcohol, drugs, or genetic disorders1,2. NAFLD is a major cause of liver disease, and its prevalence is reported to be increasing1–3; NAFLD has been reported to be a risk factor for not only hepatic disease-related mortality but also cardiovascular disease4–7.
Imaging techniques, such as abdominal ultrasonography, computed tomography (CT), and magnetic resonance imaging, are useful in evaluating hepatic steatosis. Abdominal ultrasound is the recommended screening test for NAFLD, but CT has also been used to evaluate hepatic steatosis. The CT value of the liver decreases with the degree of fat deposition, and by measuring the ratio of the CT values in the liver to those in the spleen [the liver-to-spleen (L/S) ratio], it is possible to calculate the fat content of the liver8–12. In addition to imaging tests, the hepatic steatosis index (HSI) has been useful as screening indices for hepatic steatosis13.
Metabolic syndrome and type 2 diabetes mellitus, which are associated with insulin resistance, are known to be risk factors for the development of NAFLD14–16. In addition, hyperuricemia has been reported to be a risk factor for metabolic syndrome and NAFLD17,18. Xanthine oxidoreductase (XOR) is an enzyme regulating the synthesis of uric acid (UA) and generation of reactive oxygen species (ROS)19,20. XOR activity is associated with insulin resistance21 and is elevated in metabolic syndrome and type 2 diabetes22,23. In addition, previous studies demonstrated that XOR activity was significantly increased in a mouse model of NAFLD and that fatty liver induced by a high-fat diet was suppressed by administration of XOR inhibitors24,25.
It is assumed that XOR activity is associated with hepatic steatosis in humans. However, in humans, XOR activity is extremely low compared with that in rodents; this makes accurate measurement difficult26–28. Therefore, a novel human plasma XOR activity assay has been developed using a combination of liquid chromatography (LC) and triple quadrupole mass spectrometry (TQMS) to detect [13C2, 15N2] UA using [13C2, 15N2] xanthine as a substrate26–28.
The aim of this study was to clarify the relationship between XOR activity accurately evaluated by this novel method and hepatic steatosis assessed by the L/S ratio and HSI in humans.
Results
Characteristics of the study participants
Results are given as the median (interquartile range), unless otherwise stated. The characteristics of the participants are presented in Table 1. A total of 223 subjects were included (142 females and 81 males). The age was 66 (53–73) years; the body mass index (BMI) was 23.1 (21.2–26.4) kg/m2; the abdominal circumference (AC) assessed using CT was 83.9 (77.0–91.8) cm; the subcutaneous fat area (SFA) was 162.7 (108.1–221.8) cm2; and the visceral fat area (VFA) was 86.1 (53.1–119.0) cm2. The XOR activity was 42.7 (25.3–78.6) pmol/h/mL; the serum UA was 5.3 (4.5–6.2) mg/dL; the urinary UA to creatinine ratio (UACR) was 0.46 (0.40–0.55); the L/S ratio was 1.3 (1.2–1.5); and the HSI was 32.6 (29.3–37.9).
Table 1.
Participant characteristics.
| N (female:male) | 223 (142:81) |
|---|---|
| Age (years) | 66 (53–73) |
| BMI (kg/m2) | 23.1 (21.2–26.4) |
| Abdominal circumference (cm) | 83.9 (77.0–90.8) |
| Subcutaneous fat area (cm2) | 162.7 (108.1–221.8) |
| Visceral fat area (cm2) | 86.1 (53.1–119.0) |
| HbA1c (%) | 5.8 (5.6–6.2) |
| HOMA–R | 1.4 (0.9–2.2) |
| T-Chol (mg/dL) | 194.0 (174.0–215.0) |
| TG (mg/dL) | 108.0 (77.0–154.5) |
| HDL-Chol (mg/dL) | 57.0 (48.5–70.0) |
| XOR activity (pmol/h/mL) | 42.7 (25.3–78.6) |
| UA (mg/dL) | 5.3 (4.5–6.2) |
| UACR | 0.46 (0.40–0.55) |
| L/S ratio | 1.3 (1.2–1.5) |
| HSI | 32.6 (29.3–37.9) |
| AST (U/L) | 20.0 (16.0–25.0) |
| ALT (U/L) | 18.0 (13.0–26.0) |
| γ-GTP | 21.0 (15.8–32.0) |
| Hypertension | 171 (76.7%) |
| Diabetes | 51 (22.9%) |
| Dyslipidemia | 145 (65.0%) |
The results are presented as median (interquartile range). BMI, body mass index; HbA1c, hemoglobin A1c; HOMA-R, homeostasis model assessment ratio; T-Chol, total-cholesterol; TG, triglycerides; HDL-Chol, high-density lipoprotein-cholesterol; XOR, xanthine oxidoreductase; UA, uric acid; UACR, urine uric acid to creatinine ratio; L/S, liver-to-spleen; HSI, hepatic steatosis index; AST, aspartate transaminase; ALT, alanine transaminase: γ-GTP, γ-glutamyl transpeptidas.
Differences in patient background categorized by the L/S ratio and HSI
According to the previous reports11,12, the participants were divided into three groups based on their L/S ratios, < 1.1, 1.1–1.296, and > 1.296, and each parameter was compared (Table 2). As the L/S ratio decreased, the proportion of males significantly increased (P = 0.004), as did BMI, AC, SFA, VFA, and HOMA-R (all P < 0.001). Furthermore, as the L/S ratio decreased, plasma XOR activity and serum UA levels significantly increased (P < 0.001 for both).
Table 2.
Differences in clinical parameters based on liver-to-spleen (L/S) ratio.
| L/S ratio Variables |
> 1.296 (N = 126) |
1.1–1.296 (N = 61) |
< 1.1 (N = 36) |
P for trend |
|---|---|---|---|---|
| Female:Male | 90:36 | 35:26 | 17:19 | 0.004 |
| Age (years) | 68 (59–75) | 60 (49–70) | 61 (52–71) | 0.001 |
| BMI (kg/m2) | 22.3 (20.7–24.4) | 24.6 (22.1–27.8) | 26.2 (24.3–29.5) | < 0.001 |
| AC (cm) | 80.9 (73.9–86.3) | 87.4 (80.2–94.7) | 88.1 (85.3–99.5) | < 0.001 |
| SFA (cm2) | 144.4 (102.1–195.9) | 181.0 (113.4–232.1) | 184.4 (128.9–270.3) | < 0.001 |
| VFA (cm2) | 76.9 (47.0–99.2) | 88.2 (50.3–128.3) | 119.0 (98.4–156.1) | < 0.001 |
| HbA1c (%) | 5.8 (5.5–6.0) | 5.8 (5.5–6.3) | 5.9 (5.7–6.8) | 0.164 |
| HOMA-R | 1.2 (0.8–1.8) | 1.7 (1.1–2.2) | 2.6 (1.6–3.5) | < 0.001 |
| T-Chol (mg/dL) | 192.0 (175.0–215.8) | 194.0 (171.0–211.0) | 198.0 (172.0–216.3) | 0.557 |
| TG (mg/dL) | 96.5 (71.3–144.3) | 111.0 (80.0–150.0) | 160.0 (120.0–211.0) | < 0.001 |
| HDL–Chol (mg/dL) | 61.0 (52.0–74.0) | 57.0 (45.0–66.0) | 49.0 (42.8–57.3) | < 0.001 |
|
XOR activity (pmol/h/mL) |
32.2 (22.8–52.8) | 59.1 (25.9–92.8) | 82.4 (61.5–137.5) | < 0.001 |
| UA (mg/dL) | 5.0 (4.2–6.0) | 5.5 (4.8–6.4) | 5.6 (5.0–6.5) | < 0.001 |
| UACR | 0.47 (0.41–0.55) | 0.45 (0.37–0.54) | 0.46 (0.40–0.52) | 0.101 |
| HSI | 30.7 (28.3–33.7) | 34.5 (31.2–39.2) | 38.1 (35.3–42.0) | < 0.001 |
| AST (U/L) | 19.0 (15.0–23.0) | 20.0 (16.0–24.0) | 26.0 (20.8–31.5) | < 0.001 |
| ALT (U/L) | 16.0 (12.0–19.8) | 20.0 (13.0–30.0) | 34.0 (24.8–42.0) | < 0.001 |
| γ-GTP (U/L) | 19.0 (14.0–25.8) | 24.0 (16.5–32.0) | 36.0 (25.5–68.5) | < 0.001 |
| Hypertension | 89 | 53 | 29 | 0.031 |
| Diabetes | 24 | 17 | 10 | 0.137 |
| Dyslipidemia | 81 | 37 | 27 | 0.523 |
Clinical parameters and the proportion of comorbidities among the three groups were examined using Jonckheere–Terpstra test or Cochran–Armitage test.
BMI, body mass index; AC, abdominal circumference; SFA, subcutaneous fat area; visceral fat area, VFA; HbA1c, hemoglobin A1c; HOMA-R, homeostasis model assessment ratio; T-Chol, total-cholesterol; TG, triglycerides; HDL-Chol, high-density lipoprotein-cholesterol; UA, uric acid; UACR, urine uric acid to creatinine ratio; HSI, hepatic steatosis index; AST, aspartate transaminase; ALT, alanine transaminase: γ-GTP, γ-glutamyl transpeptidas.
According to the previous method13, the participants were also divided into three groups based on their HSI, < 30.0, 30.0–36.0, and > 36.0, and each parameter was compared (Table 3). The proportion of males significantly increased with increasing HSI (P = 0.009), as did BMI, AC, SFA, VFA, and HOMA-R (all P < 0.001). Furthermore, with increasing HSI, plasma XOR activity and serum UA levels significantly increased (both P < 0.001).
Table 3.
Differences in clinical parameters based on hepatic steatosis index (HSI).
| HSI Variables |
< 30.0 (N = 68) |
30.0 – 36.0 (N = 84) |
> 36.0 (N = 71) |
P for trend |
|---|---|---|---|---|
| Female:male | 51:17 | 53:31 | 38:33 | 0.009 |
| Age (years) | 70 (64–76) | 67 (54–73) | 56 (47–67) | < 0.001 |
| BMI (kg/m2) | 20.5 (18.9–21.5) | 23.1 (22.3–24.6) | 28.5 (26.1–31.6) | < 0.001 |
| AC (cm) | 74.2 (70.5–80.6) | 84.0 (79.7–87.7) | 94.6 (88.5–101.9) | < 0.001 |
| SFA (cm2) | 105.0 (75.9–145.5) | 163.9 (122.6–208.7) | 230.1 (180.7–336.1) | < 0.001 |
| VFA (cm2) | 57.5 (30.4–86.3) | 82.1 (57.3–108.1) | 123.2 (85.9–163.0) | < 0.001 |
| HbA1c (%) | 5.7 (5.6–5.9) | 5.7 (5.5–6.2) | 5.9 (5.6–7.0) | 0.015 |
| HOMA-R | 0.9 (0.6–1.5) | 1.3 (1.0–2.0) | 2.2 (1.4–2.8) | < 0.001 |
| T-Chol (mg/dL) | 193.0 (176.5–214.3) | 194.5 (172.8–216.3) | 194.0 (173.5–214.5) | 0.585 |
| TG (mg/dL) | 84.5 (62.5–118.0) | 107.0 (78.5–151.3) | 128.0 (101.0–210.0) | < 0.001 |
| HDL–Chol (mg/dL) | 66.0 (54.8–78.3) | 58.5 (51.5–70.5) | 49.0 (43.0–57.5) | < 0.001 |
|
XOR activity (pmol/h/mL) |
27.1 (19.2–43.0) | 39.7 (25.6–73.6) | 79.2 (47.2–127.0) | < 0.001 |
| UA (mg/dL) | 5.0 (4.1–5.4) | 5.1 (4.4–6.3) | 5.8 (5.2–6.5) | < 0.001 |
| UACR | 0.47 (0.42–0.57) | 0.46 (0.40–0.55) | 0.46 (0.38–0.53) | 0.125 |
| L/S ratio | 1.4 (1.3–1.5) | 1.3 (1.2–1.5) | 1.2 (1.0–1.3) | < 0.001 |
| AST (U/L) | 19.5 (16.0–22.0) | 20.0 (16.0–23.0) | 24.0 (18.0–28.0) | 0.002 |
| ALT (U/L) | 13.0 (9.0–17.0) | 18.0 (13.8–24.0) | 30.0 (20.5–39.5) | < 0.001 |
| γ-GTP (U/L) | 16.0 (12.0–24.0) | 21.0 (16.0–31.3) | 26.0 (21.0–43.0) | < 0.001 |
| Hypertension | 46 | 65 | 60 | 0.019 |
| Diabetes | 8 | 21 | 22 | 0.007 |
| Dyslipidemia | 35 | 33 | 40 | 0.633 |
Clinical parameters and the proportion of comorbidities among the three groups were examined using Jonckheere–Terpstra test or Cochran–Armitage test.
BMI, body mass index; AC, abdominal circumference; SFA, subcutaneous fat area; visceral fat area, VFA; HbA1c, hemoglobin A1c; HOMA-R, homeostasis model assessment ratio; T-Chol, total-cholesterol; TG, triglycerides; HDL-Chol, high-density lipoprotein-cholesterol; XOR, xanthine oxidoreductase; UACR, urine uric acid to creatinine ratio; L/S, liver-to-spleen; AST, aspartate transaminase; ALT, alanine transaminase.
Assessment of liver fibrosis progression
In patients with the L/S ratio of < 1.1, the NAFLD fibrosis score (NFS) and the Fibrosis-4 (FIB-4) index were calculated to predict the progression of liver fibrosis1,29–32. In these subjects, the NFS was −2.086 (−2.836 to −0.532) with an NFS of > 0.676 observed in only three subjects, while the FIB-4 index was 1.09 (0.74–1.56) with an FIB-4 index of ≥ 2.67 observed in only four subjects.
Association of the XOR activity and UA levels with hepatic steatosis
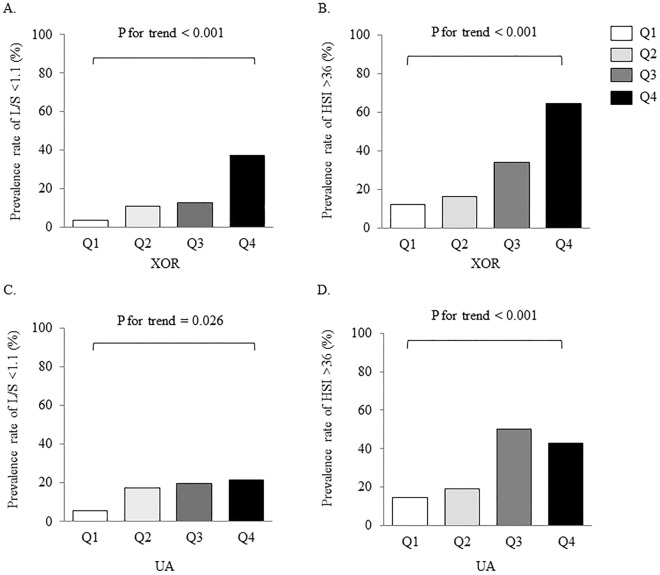
Participants were divided into quartiles based on their XOR activity. The proportion of each XOR activity with an L/S ratio of < 1.1 and an HSI of > 36.0 is shown in Fig. 1. The proportions of subjects with the L/S ratio of < 1.1 and the HSI of > 36.0 significantly increased with increasing XOR activity (both P < 0.001). Serum UA levels were used to divide participants into quartiles. Similar to XOR activity, increasing UA levels increased the proportion of subjects with the L/S ratio of < 1.1 (P = 0.026) and the HSI of > 36.0 (P < 0.001).
Figure 1.
Comparisons of indices of hepatic steatosis among quadrants of xanthine oxidoreductase (XOR) activity or uric acid (UA) levels. (A) Plasma XOR activity and prevalence rate of liver-to-spleen (L/S) ratio < 1.1, (B) Serum UA levels and prevalence rate of L/S ratio < 1.1, (C) XOR and prevalence rate of hepatic steatosis index (HSI) > 36.0, (D) UA levels and prevalence rate of HSI > 36.0. The proportion of hepatic steatosis between quartiles was examined using the Cochran–Armitage test. Abbreviations: Q, quadrant.
An ordinal logistic regression analysis using the L/S ratio as the objective variable and UA levels as the explanatory variable revealed that UA levels had a crude odds ratio (OR) of 1.454 [95% confidence interval (CI): 1.159–1.824, P < 0.001] (Table 4). A similar analysis revealed that a crude OR for XOR activity per 10 pmol/h/mL of 1.080 (95% CI: 1.039–1.123, P < 0.001). Thus, both UA levels and XOR activity were associated with a lower L/S ratio. Next, since hepatic steatosis, hyperuricemia, and XOR activity were all associated with insulin resistance, we ran a logistic regression analysis in Model 1 with UA levels, XOR activity, and HOMA-R as explanatory variables. Results showed that XOR activity and HOMA-R were associated with a lower L/S ratio, with an OR for XOR activity per 10 pmol/h/mL of 1.052 (95% CI: 1.013–1.093, P = 0.009) and an OR for HOMA-R of 1.593 (95% CI: 1.230–2.062, P < 0.001), but UA levels were not, with an OR of 1.228. Furthermore, because NAFLD is known to be associated with obesity, hypertension, dyslipidemia, and diabetes mellitus1,3,14, we performed a logistic regression analysis in Model 3 that adjusted for age, sex, and the presence of hypertension, dyslipidemia, and diabetes mellitus. The results indicated that XOR activity was associated with a lower L/S ratio, with an OR for XOR activity per 10 pmol/h/mL of 1.047 (95% CI: 1.009–1.086, P = 0.016) independent of HOMA-R and serum UA levels.
Table 4.
Logistic regression analyses of the factors associated with liver-to-spleen (L/S) ratio.
| L/S ratio | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 (n = 216) |
Model 2 (n = 216) |
Model 3 (n = 216) |
||||||
| Crude OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P | |
| UA |
1.454 (1.159–1.824) |
< 0.001 |
1.228 (0.960–1.570) |
0.102 |
1.128 (0.875–1.454) |
0.354 |
1.047 (0.800–1.370) |
0.737 |
|
XOR activity per 10 pmol/h/mL |
1.080 (1.039–1.123) |
< 0.001 |
1.052 (1.013–1.093) |
0.009 |
1.047 (1.009–1.086) |
0.016 |
1.047 (1.009–1.086) |
0.016 |
| HOMA-R |
1.836 (1.437–2.347) |
< 0.001 |
1.593 (1.230–2.062) |
< 0.001 |
1.411 (1.070–1.860) |
0.015 |
1.407 (1.050–1.884) |
0.022 |
| BMI |
1.190 (1.117–1.268) |
< 0.001 |
1.102 (1.022–1.188) |
0.012 |
1.087 (1.006–1.174) |
0.034 | ||
In model 1, an ordinal logistic regression analysis was performed with L/S ratio as the objective variable and serum uric acid (UA) levels, plasma xanthine oxidoreductase (XOR) activity, and homeostasis model assessment ratio (HOMA-R) as explanatory variables. In Model 2, body mass index (BMI) was added as an explanatory variable. In Model 3, logistic regression analysis was performed adjusting for age, gender, and presence of hypertension, dyslipidemia, and diabetes mellitus.
OR, odds ratio; CI, confidence interval.
Table 5 shows the results of HSI as a dependent factor. Independent of HOMA-R and serum UA levels, XOR activity was associated with increased HSI, with an OR for XOR activity per 10 pmol/h/mL of 1.158 (95% CI: 1.085–1.237, P < 0.001).
Table 5.
Logistic regression analyses of the factors associated with hepatic steatosis index (HSI).
| HSI | ||||
|---|---|---|---|---|
| Model 1 (n = 216) | ||||
| Crude OR (95% CI) |
P | OR (95% CI) |
P | |
| UA |
1.583 (1.276–1.964) |
< 0.001 |
1.298 (1.018–1.654) |
0.035 |
|
XOR activity per 10 pmol/h/mL |
1.227 (1.144–1.315) |
< 0.001 |
1.158 (1.085–1.237) |
< 0.001 |
| HOMA-R |
2.413 (1.795–3.245) |
< 0.001 |
1.878 (1.379–2.559) |
< 0.001 |
In model 1, an ordinal logistic regression analysis was performed with HSI as the objective variable and serum uric acid (UA) levels, plasma xanthine oxidoreductase (XOR) activity, and homeostasis model assessment ratio (HOMA-R) as explanatory variables.
OR, odds ratio; CI, confidence interval.
Discussion
XOR is an enzyme that controls the synthesis of UA. XOR has also been reported to cause vascular endothelial dysfunction through adipogenesis and ROS production33–36. Furthermore, XOR activity has been reported to induce hepatic steatosis via ROS production and activation of the c-Jun N-terminal kinase37. In animal experiments, it has been reported that inhibiting XOR can suppress hepatic steatosis24,25. Therefore, it is assumed that XOR activity and hepatic steatosis are related in humans; however, measuring XOR activity in humans has proven difficult. XOR activity was accurately measured in this study using LC/TQMS, and it was found that XOR activity is associated with hepatic steatosis in humans.
UA itself has been reported to induce hepatic lipid accumulation by inducing mitochondrial oxidative stress and insulin resistance38,39. In fact, it has been reported that hyperuricemia is associated with the development and progression of NAFLD17,18,40. According to the findings of this study, plasma XOR activity was more useful than serum UA levels as an explanatory variable for the lower L/S ratio. However, because this study primarily aimed to investigate the relationship between XOR activity and hepatic steatosis, we excluded patients taking XOR inhibitors, which could interfere with measuring plasma XOR activity. In fact, there were only 17 (7.6%) patients with untreated hyperuricemia (serum UA ≥ 7.0 mg/dL) in this study. Therefore, further studies including patients with hyperuricemia may be necessary to clarify the relationship between UA levels or XOR activity and hepatic steatosis.
Insulin resistance causes hepatic steatosis via various mechanisms, including promoting the transfer of free fatty acids from adipose tissue to the liver, and is a major risk factor for the development of NAFLD41–44. Furthermore, insulin resistance raises the risk of hyperuricemia by increasing not only XOR activity but also UA reabsorption21,45. On the other hand, a study using mice with genetically disrupted hepatocyte XOR found that suppressing hyperuricemia through hepatic XOR inhibition did not improve systemic metabolic abnormalities, including insulin resistance46. However, that paper did not directly evaluate the relationship between XOR activity or UA levels and hepatic steatosis46. It is important to note that the findings of this study show that XOR activity is associated with hepatic steatosis independent of insulin resistance. Long-term prospective studies are needed, however, to better understand the relationship between XOR activity and hepatic steatosis.
In this study, the HSI and L/S ratios were also used to assess hepatic steatosis. According to a case–control study conducted in Korea, NAFLD can be excluded with a negative likelihood ratio of 0.186 for HSI < 30.0 and detected with a positive likelihood ratio of 6.069 for HSI > 36.013. Furthermore, in a study of Western subjects, the L/S ratio was found to have a sensitivity of 93% for detecting moderate to severe fatty liver9. A study using liver transplant donors in Japan found that the L/S ratio > 1.1 was sufficient to rule out moderate or severe fatty liver12. Furthermore, when liver biopsies were performed to histologically evaluate the liver and compared with the L/S ratio, it was reported that the L/S ratio cutoff value for detecting clinically problematic fatty liver was 1.1, and the L/S ratio assuming the absence of hepatic steatosis was higher than 1.29611. However, it has been reported that using the L/S ratio to assess mild fatty liver is difficult9. Therefore, further investigation, including echography and liver biopsy, is considered necessary to accurately evaluate hepatic steatosis.
In this study, we used the NFS and the FIB-4 index to evaluate the progression of hepatic fibrosis1,29–32, but only three patients with an L/S ratio < 1.1 had a high NFS, and only four patients had a high FIB-4 index, and no significant relationship between hepatic fibrosis and XOR activity was found. Due to the high invasiveness of liver biopsy, it was not performed in this study. However, a prospective study with liver biopsy is required to thoroughly investigate the relationship between NAFLD onset and progression and XOR activity.
The limitation of this study is that it was a single-center, cross-sectional analysis. In this study, we used the L/S ratio and HSI as indices of hepatic steatosis and NFS and the FIB-4 index as indices of hepatic fibrosis progression; however, a liver biopsy is still necessary to diagnose fatty liver, and further studies are required. Additionally, long-term prospective studies including patients with hyperuricemia are needed to better understand the relationship between XOR activity or serum UA levels and hepatic steatosis.
In conclusion, the findings of this study show that plasma XOR activity is associated with hepatic steatosis independent of insulin resistance and serum UA levels. Thus, XOR activity may be involved in hepatic steatosis in humans.
Methods
Study design and participants.
This cross-sectional analysis was conducted as part of the Hyogo Sleep Cardio-Autonomic Atherosclerosis (HSCAA) study47,48. The HSCAA study is a single-center cohort study that aims to investigate the interrelationships among sleep disorders, autonomic neuropathy, metabolic diseases, and atherosclerotic diseases47,48. The HSCAA study included patients aged 20 years and older with one or more cardiovascular risk factors (obesity, smoking, cardiovascular event history, hypertension, dyslipidemia, diabetes mellitus, chronic kidney disease) and being treated at the Hyogo College of Medicine Hospital.
Since we started XOR measurements from 2018 for the subjects who were registered or followed in the HSCAA study, this cross-sectional study included 310 patients, from January 2018 to July 2021, who consented to abdominal CT examinations. In the end, 223 patients were analyzed in the present study after excluding 87 with alcoholic habits (> 30 g/day for males and > 20 g/day for females), autoimmune hepatitis, viral hepatitis, or under treatment with XOR inhibitors.
The HSCAA study has been approved by the Ethics Committee of Hyogo College of Medicine Hospital (Approval No. 2351). Written informed consent was obtained from all subjects and the study was conducted in full accordance with the Declaration of Helsinki. The present study protocol was approved by the Ethics Committee of Hyogo College of Medicine Hospital (Approval No. 3601) and performed with an opt-out option, as explained in instructions posted on the website of the hospital. All methods in our study were performed in accordance with the relevant guidelines and regulations.
Visceral fat area and subcutaneous fat area
CT was performed using SIEMENS SOMATOM Definition AS + or SOMATOM Definition H (Siemens Healthcare GmbH, Erlangen, Germany) with 10 mm slices. We evaluated the visceral fat area (VFA), subcutaneous fat area (SFA), and waist circumference using Ziostation 2 (AMIN Ltd., Tokyo, Japan). The AC was measured at the umbilical height.
Hepatic steatosis and liver fibrosis
The L/S ratio and HSI were used to evaluate hepatic steatosis. Hepatic and splenic attenuation values were measured on non-contrast-CT scans using four circular region-of-interest (ROI) cursors in the liver and two in the spleen. In the liver, four ROIs were located in each of the right lobe and the left lobe. All measurements were manually obtained in regions of uniform parenchymal attenuation, with care being taken to avoid vessels, artifacts, and other areas that might have spuriously increased or decreased measurements. The calculation of the L/S ratio was as follow: L/S ratio = (Average attenuation value of the liver) / (Average attenuation value of the spleen)9–12. HSI was calculated from ALT, AST, BMI, sex, and the presence of diabetes mellitus13.
In addition, the NAFLD fibrosis score (NFS) and the Fibrosis-4 (FIB-4) index were calculated to predict the progression of liver fibrosis in patients with the L/S ratio of < 1.11,29–32. NFS was calculated from age, BMI, AST, ALT, the presence of glucose intolerance, platelet count, and albumin31,32. It has been reported that by applying the high cutoff score (NFS > 0.676), the presence of advanced fibrosis could be diagnosed with high accuracy31,32. The FIB-4 index was calculated from age, AST, ALT, and platelet count. It has been reported that its cutoff value < 1.45 can exclude hepatic fibrosis, and its cutoff value > 2.67 can predict hepatic fibrosis30.
Plasma XOR activity measurement
The assay protocol of XOR activity in humans was reported previously26–28. In brief, 100 μL of plasma samples (purified by Sephadex G25 resin) were mixed with a Tris buffer (pH 8.5) containing [13C2,15N2] xanthine as a substrate, NAD+, and [13C3,15N3] UA as an internal standard. The mixtures were incubated at 37 °C for 90 min, mixed with 500 µL of methanol, and centrifuged at 2000× g for 15 min at 4 °C. The supernatants were transferred to new tubes and dried using a centrifugal evaporator. The residues were reconstituted with 150 μL of distilled water, filtered through an ultrafiltration membrane, and measured using LC/TQMS. LC/TQMS comprised a Nano Space SI-2 LC system (Shiseido Co., Ltd., Tokyo, Japan) and a TSQ Triple Quadrupole LC–MS system (ThermoFisher Scientific GmbH, Bremen, Germany) equipped with an ESI interface. Calibration standard samples of [13C2,15N2] UA were also measured, and the amounts of production were quantitated from the calibration curve. XOR activities were expressed in pmol/mL/h26–28.
Other parameters
At the same time as that for the CT scan, blood samples were taken for AST, ALT, UA, fasting blood glucose, immunoreactive insulin, total cholesterol (T-Chol), high-density lipoprotein cholesterol (HDL-Chol), and TG. In addition, serum UA levels were measured using the uricase/peroxidase technique with an autoanalyzer (Pureauto S UA Sekisui Medical, Ltd., Tokyo, Japan). Height, weight, and blood pressure were also measured.
Type 2 diabetes was diagnosed based on results showing fasting plasma glucose ≥ 126 mg/dL, causal plasma glucose ≥ 200 mg/dL, or 2-h plasma glucose ≥ 200 mg/dL during a 75-g oral glucose tolerance test, or previous therapy for diabetes49. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or taking treatment for hypertension. We defined dyslipidemia as the presence of LDL-C ≥ 140 mg/dL, HDL-C ≤ 40 mg/dL, TG level ≥ 150 mg/dL, or taking treatment for dyslipidemia.
Statistical analysis
The results were presented as median (interquartile range), unless otherwise stated. We used the Jonckheere-Terpstra test to compare the trend of data between three or more groups. The Cochran–Armitage test was used for the trend of the ratio between three or more groups.
Hepatic steatosis was graded as follows: with hepatic steatosis (L/S ratio < 1.1)11,12, without hepatic steatosis (L/S ratio > 1.296), and intermediate (L/S ratio = 1.1–1.296)11. In model 1, an ordinal logistic regression analysis was performed with L/S ratio as the objective variable and serum UA levels, plasma XOR activity, and HOMA-R as explanatory variables. In Model 2, BMI was added as an explanatory variable. In Model 3, we used an ordinal logistic regression analysis, and the L/S ratio was used as the objective variable; UA, XOR activity, and the HOMA-R were used as the explanatory variables, adjusted for age, sex, and components for Japanese diagnostic criteria of metabolic syndrome (AC, blood pressure, plasma glucose, HDL, and TG).
HSI of > 36.0, < 30, and 30 − 36 were defined as high, low, and intermediate, respectively, based on the previous report13. Then, an ordinal logistic regression analysis was performed with HSI as the objective variables and UA, XOR, and HOMA-R as explanatory variables.
Statistical analyses were conducted using the BellCurve software version 2.15 (Social Survey Research Information Co., Ltd., Tokyo, Japan), with P < 0.05 indicating statistical significance.
Acknowledgements
The authors thank the subjects who participated in this study and all the physicians and the co-medical staff at Hyogo college medicine hospital for their support in collecting the data.
Author contributions
C.Y, Y.K., T.T., and H.K. were engaged in the preparation of the study protocol, the analysis of data, laboratory tests, and the preparation of this article. T.M and T.N were engaged in laboratory test. K.O., M.O., A.Mo., A.Mi., M.K.-H., K.K.-H., M.K., K.K., and T.S. were engaged in the data collection. All authors listed have contributed to the work and approved the final version.
Funding
The present study was supported in part by Japan Society for The Promotion of Science KAKENHI Grant Number: 21K10511.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
Although Takayo Murase and Takashi Nakamura are affiliated with Sanwa Kagaku Kenkyusho Co. Ltd., their involvment did not alter our adherence regarding sharing of data and materials. The other authors have no conflicts of interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tokushige K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J. Gastroenterol. 2021;56:951–963. doi: 10.1007/s00535-021-01796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi Y, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 5.Adams LA, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 7.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 8.Ricci C, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J. Hepatol. 1997;27:108–113. doi: 10.1016/S0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 9.Saadeh S, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, et al. Macrovesicular hepatic steatosis in living liver donors: Use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 11.Kan H, et al. Non-invasive assessment of liver steatosis in non-alcoholic fatty liver disease. Hepatol. Res. 2014;44:E420–E427. doi: 10.1111/hepr.12330. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki M, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.TP.0000140499.23683.0D. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N. Engl. J. Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 15.NICE guideline. Nonalcoholic fatty liver disease (NAFLD): assessment and management. https://www.nice.org.uk/guidance/ng49/chapter/Recommendations. Accessed July 1, 2021.
- 16.Younossi ZM, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, et al. Hyperuricemia precedes non-alcoholic fatty liver disease with abdominal obesity moderating this unidirectional relationship: three longitudinal analyses. Atherosclerosis. 2020;311:44–51. doi: 10.1016/j.atherosclerosis.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Yuan H, et al. Serum uric acid levels and risk of metabolic syndrome: A dose-response meta-analysis of prospective studies. J. Clin. Endocrinol. Metab. 2015;100:4198–4207. doi: 10.1210/jc.2015-2527. [DOI] [PubMed] [Google Scholar]
- 19.Meneshian A, Bulkley GB. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- 20.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004;555(Pt 3):589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washio KW, et al. Xanthine oxidoreductase activity is correlated with insulin resistance and subclinical inflammation in young humans. Metabolism. 2017;70:51–56. doi: 10.1016/j.metabol.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Battelli MG, Bortolotti M, Polito L, Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. BBA –Mol. Basis Disease. 2018;1864:2557–2565. doi: 10.1016/j.bbadis.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kuppusamy UR, Indran M, Rokiah P. Glycaemic control in relation to xanthine oxidase and antioxidant indices in Malaysian Type 2 diabetes patients. Diabet. Med. 2005;22:1343–1346. doi: 10.1111/j.1464-5491.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, et al. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: One stone hits two birds. J. Hepatol. 2015;62:1412–1419. doi: 10.1016/j.jhep.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Nakatsu Y, et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G42–51. doi: 10.1152/ajpgi.00443.2014. [DOI] [PubMed] [Google Scholar]
- 26.Murase T, et al. Xanthine oxidoreductase activity assay in tissues using stable isotope-labeled substrate and liquid chromatography high-resolution mass spectrometry. J. Chromatogr. B. 2016;1008:189–197. doi: 10.1016/j.jchromb.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Murase T, Oka M, Nampei M, Miyachi A, Nakamura T. A highly sensitive assay for xanthine oxidoreductase activity using a combination of [13C2,15N2] xanthine and liquid chromatography/triple quadrupole mass spectrometry. J. Labelled Comp. Radiopharm. 2016;59:214–220. doi: 10.1002/jlcr.3390. [DOI] [PubMed] [Google Scholar]
- 28.Murase T, Nampei M, Oka M, Miyachi A, Nakamura T. A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope-labeled xanthine and LC/TQMS. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2016;1039:51–58. doi: 10.1016/j.jchromb.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Angulo A, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:847–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 30.Shah AG, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring system can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 32.Sumida Y, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung KJ, et al. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 2007;5:115–128. doi: 10.1016/j.cmet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 34.George J, Carr E, Davies J, Belch JJF, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 35.Washio K, et al. Xanthine oxidoreductase activity correlates with vascular endothelial dysfunction in patients with type 1 diabetes. Acta Diabetol. 2020;57:31–39. doi: 10.1007/s00592-019-01362-1. [DOI] [PubMed] [Google Scholar]
- 36.Furuhashi M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020;319:E827–E834. doi: 10.1152/ajpendo.00378.2020. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa T, et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci. Rep. 2020;10:815. doi: 10.1038/s41598-020-57784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 39.Lanaspa MA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong S, Song J, Wang L, Zhang S, Wang Y. Hyperuricemia and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016;28:132–138. doi: 10.1097/MEG.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 41.Reccia I, et al. Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism. 2017;72:94–108. doi: 10.1016/j.metabol.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ—Linking NAFLD and insulin resistance. Endocr. Rev. 2019;40:1367–1393. doi: 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- 43.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 2019;70:711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 44.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 45.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–3011. doi: 10.1001/jama.1991.03470210076036. [DOI] [PubMed] [Google Scholar]
- 46.Harmon DB, et al. Hepatocyte-specific ablation or whole-body inhibition of xanthine oxidoreductase in mice corrects obesity-induced systemic hyperuricemia without improving metabolic abnormalities. Diabetes. 2019;68:1221–1229. doi: 10.2337/db18-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadoya M, et al. Plasma brain-derived neurotrophic factor and reverse dipping pattern of nocturnal blood pressure in patients with cardiovascular risk factors. PLoS ONE. 2014;9:e105977. doi: 10.1371/journal.pone.0105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadoya M, et al. Serum Macro TSH Level is Associated with Sleep Quality in Patients with Cardiovascular Risks - HSCAA Study. Sci. Rep. 2017;7:44387. doi: 10.1038/srep44387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haneda M, et al. Japanese Clinical Practice Guideline for Diabetes 2016. J. Diabet. Investig. 2018;9:657–697. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.