Abstract
Background:
Survival rates following in-hospital cardiac arrest (IHCA) are lower during nights and weekends (off-hours), as compared to daytime on weekdays (on-hours). Telemedicine Critical Care (TCC) may provide clinical support to improve IHCA outcomes, particularly during off-hours.
Objective:
To evaluate the association between hospital availability of TCC and IHCA survival.
Methods:
We identified 44,585 adults at 280 U.S. hospitals in the Get With The Guidelines® - Resuscitation registry who suffered IHCA in an Intensive Care Unit (ICU) or hospital ward between July 2017 and December 2019. We used 2-level hierarchical multivariable logistic regression to investigate whether TCC availability was associated with better survival, overall, and during on-hours (Monday–Friday 7:00 a.m.−10:59 p.m.) vs. off-hours (Monday–Friday 11:00 p.m.−6:59 a.m., and Saturday-Sunday, all day, and US national holidays).
Results:
14,373 (32.2%) participants suffered IHCA at hospitals with TCC, and 27,032 (60.6%) occurred in an ICU. There was no difference between TCC and non-TCC hospitals in acute resuscitation survival rate or survival to discharge rates for either IHCA occurring in the ICU (acute survival odds ratio [OR] 1.02, 95% CI 0.92–1.15; survival to discharge OR 0.94 [0.83–1.07]) or outside of the ICU (acute survival OR 1.03 [0.91–1.17]; survival to discharge OR 0.99 [0.86–1.12]. Timing of cardiac arrest did not modify the association between TCC availability and acute resuscitation survival (P = .37 for interaction) or survival to discharge (P = .39 for interaction).
Conclusions:
Hospital availability of TCC was not associated with improved outcomes for in-hospital cardiac arrest.
Keywords: Cardiopulmonary arrest, Cardiopulmonary resuscitation, Telehealth, Telemedicine, Critical Care, Tele-ICU, Tele-Critical Care
INTRODUCTION
In-hospital cardiac arrest (IHCA) affects approximately 300,000 patients annually in the United States.1 Survival after IHCA is dependent on early recognition, prompt initiation of resuscitation protocols, and high-quality post-resuscitation care. Patient outcomes following IHCA remain extremely poor, with survival to discharge rates of 20% nationwide.2 Further, survival rates are lower for the > 50% of IHCA occurring during nights and weekends,3,4 when the ability of hospital staff to recognize deteriorating patients, prevent IHCA, or quickly initiate resuscitation protocols may be reduced.5,6
Telemedicine Critical Care (TCC) combines audiovisual technologies with electronic medical records access to enable the remote provision of critical care.7 This has the potential to improve patient outcomes by assisting bedside caregivers in detecting clinical instability and in executing care plans. Additionally, TCC improves access to trained intensive care physicians,8 whose presence in an intensive care unit (ICU) has been associated with improved survival.9
Thus far, studies evaluating the effect of TCC on broad ICU outcomes have shown mixed results.10–18 To our knowledge, the association between TCC availability and improved outcomes for specific critical care syndromes, such as IHCA, has not been investigated. We hypothesized that a care delivery model which can derive operational intelligence and support from TCC could impact outcomes by enabling earlier recognition of clinical deterioration, and more efficient activation of clinical responses. Characterizing the association between TCC and IHCA outcomes could have implications on its continued nationwide rollout, as well as on quality efforts targeted at improving resuscitation care.
Therefore, the specific objectives of this study were to investigate (1) whether hospital availability of TCC was associated with better survival outcomes for IHCA, and (2) whether timing of the cardiac arrest modified the strength of any associations between TCC availability and IHCA survival outcomes. We hypothesized that hospital availability of TCC would be associated with better IHCA survival outcomes, and that this association would be stronger for IHCA events that occur during night-time or weekends when hospitals are understaffed.
To address this knowledge gap, we used data from the Get With The Guidelines® -Resuscitation (GWTG-R) registry — a large national database of IHCA events — linked with the American Hospital Association (AHA) Annual survey, which provided data on hospital availability of TCC.
METHODS
Data Sources:
The GWTG-R is a prospective, voluntary, multi-site registry of IHCA resuscitation events.19 Multiple case-finding approaches ensure that all cases within a hospital are captured.20,21 Data collection is based on the Utstein template for uniform reporting guidelines and is standardized across participating sites.20,22 Data completeness and accuracy is ensured using a rigorous training and certification process, the use of standardized software, internal data checks, and periodic re-abstraction.
The AHA survey dataset is derived from voluntarily completed annual surveys of all AHA-registered U.S. hospitals. Information on TCC availability was included in the AHA data starting in fiscal year 2018 (July 2017 – June 2018) through a survey question on availability of “eICU” as a hospital facility or service. Similar to prior studies,23,24 we categorized a hospital as having TCC (hereafter referred to as TCC hospitals) if they responded yes for that specific year (eTable 1). We linked the GWTG-R data to the survey data using AHA hospital identifiers. This study was deemed exempt by the Human Subjects Protection Office at the Washington University School of Medicine.
Study Population:
We identified 70,881 patients 18 years or older with an index pulseless IHCA between July 1, 2017, and December 31, 2019. We excluded arrests at hospitals that did not respond to the AHA surveys or had missing information on TCC availability; at hospitals with less than 10 cardiac arrests over the study period; that occurred outside of an ICU or hospital ward (e.g., emergency room and operating room); and in patients with an implantable cardioverter-defibrillator. Additionally, we excluded patients with missing information related to arrest time or survival. The final study sample was comprised of 44,585 arrests occurring in the ICU or general inpatient unit at 280 participating hospitals (Figure 1). TCC as currently implemented in most US hospitals,23 typically targets beds within the physical confines of an ICU. However, to enable us to evaluate the robustness of any findings of improved survival among TCC hospitals, we a priori included arrests outside of the ICU as “controls”.
Figure 1: Derivation of the Study Cohort.
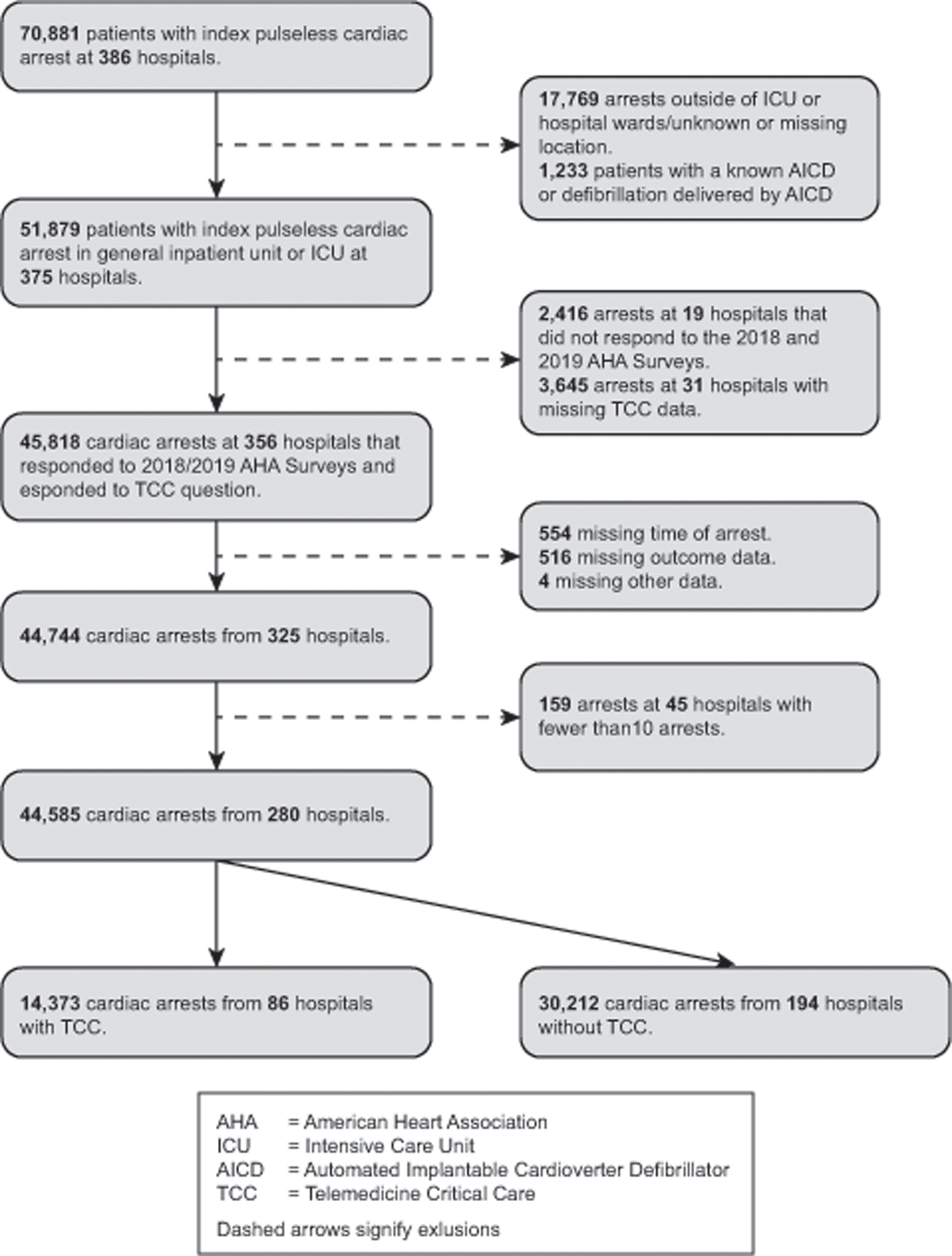
Study Variables and Outcomes:
Our primary exposure variable was TCC availability at GWTG-R participant hospitals for 2018 and 2019. We assumed that hospitals with TCC availability during 2019 had it for the entire 2019 calendar year (i.e., inclusive of July–December 2019). The primary outcome variable was acute resuscitation survival (defined as return of spontaneous circulation [ROSC] for at least 20 contiguous minutes at any time after the initial pulseless arrest). Our secondary outcome was survival to hospital discharge.
Patient-level data were obtained from the GWTG-R and included demographics (age, sex, race), co-morbidities and pre-existing medical conditions (current or prior myocardial infarction, current or prior heart failure, diabetes mellitus, hypotension, metabolic and electrolyte abnormalities, respiratory failure, renal insufficiency, hepatic insufficiency, metastatic or hematologic malignancy, septicaemia, pneumonia, major trauma, acute stroke, baseline depression in neurological function), cardiac arrest characteristics (initial arrest rhythm [asystole, pulseless electrical activity, ventricular fibrillation, pulseless ventricular tachycardia], hospital location of cardiac arrest [intensive care unit, non-intensive care unit (monitored ward, non-monitored ward)], time of arrest [on-hours: 7.00 a.m.–10:59 p.m., Monday–Friday; or off-hours:11.00 p.m.–6.59 a.m., Monday–Friday, or anytime on weekends and US national holidays], whether the event was witnessed, use of hospital-wide cardiac arrest alert) and interventions in place prior to time of cardiac arrest (assisted or mechanical ventilation, vasoactive agent, anti-arrhythmic, pulmonary artery catheter, and renal dialysis). We also obtained data on rates of delayed defibrillation (> 2minutes) for shockable IHCA and delayed (> 5 minutes) first dose of adrenaline (epinephrine) for non-shockable IHCA, time to ROSC, and total duration of cardiopulmonary resuscitation (CPR). Hospital-level variables included geographic location, hospital setting, ownership, bed size, teaching status, physician services (adult cardiology, cardiac surgery, interventional cardiac catheterization, hospitalist, intensivist), staffing, and intensive care beds. The rate of missing data was < 1% for all variables, except race and for hospital ownership type. Unknown or missing data on race were treated as separate “Unknown” categories. Missing values for hospital ownership type were imputed using multiple imputation.
Statistical Analysis:
Comparisons of patient and cardiac arrest characteristics between TCC and non-TCC hospitals were performed using t-tests for continuous variables and chi square tests for categorical variables. Due to large sample sizes, we used standardized difference (SD) in covariate means to compare hospital groups with and without TCC.25 A SD of > 10% suggests a covariate imbalance.
Next, we calculated unadjusted proportions of acute resuscitation survival and survival to discharge between TCC and non-TCC hospitals. We then estimated a multivariable generalized linear mixed regression model with binomial distribution, with hospital sites included as random effects, and patient- and hospital-level covariates as fixed effects. We used the variance components covariance structure for random hospital site effect in the model. We calculated risk-adjusted survival proportions, accounting for confounding by patient and cardiac arrest characteristics and clustering of patients within hospitals. The model included a categorical variable for calendar year, the location (ICU/non-ICU) and timing (on-hours/off-hours) of arrest, and the interaction terms for arrest location (TCC*location) and timing (TCC*time of arrest). Hospital-level variables that were not statistically significant were excluded from the final models. We reported survival outcomes primarily for arrests that occurred in the ICU.
Sensitivity and Exploratory Analyses:
To ensure that survival estimates were not biased by inclusion of cardiac arrests that occurred in the first half of fiscal year 2020, we evaluated the robustness of our primary analysis by excluding arrests that occurred between July and December 2019. To test the hypothesis that TCC may have the greatest benefits among smaller, less resourced hospitals and among patient subgroups, we performed 3 exploratory analyses: i) stratified by hospital size, (ii) among the subgroup of patients with non-shockable rhythms, and iii) among the subgroup of patients who survived acute resuscitation, to evaluate post resuscitation survival.
All analyses were performed using SAS version 9.4 for Windows (SAS Institute Inc. Cary, NC) and R version 4.0. All hypothesis tests were 2-sided with a significance threshold level of 0.05.
RESULTS
Of 44,585 study participants, 14,373 (32.2%) suffered IHCA at a TCC hospital. The mean age was 65.6 years; 41.2% were female and 22.5% were black. Most cardiac arrest events were witnessed (86.5%), associated with use of hospital wide cardiac arrest alert (80.8%), and occurred in an intensive care setting (60.6 %). Cardiac arrests occurred equally during on-hours vs. off-hours (53% vs. 47%). Ventricular fibrillation and pulseless ventricular tachycardia (shockable IHCA) accounted for 14.5% of all cardiac arrests. Other than a higher proportion of patients at TCC hospitals experiencing delayed defibrillation for shockable rhythms (31.1 vs. 26.3%) and having a pulmonary catheter in place prior to cardiac arrest (16.5 vs. 9.9%) compared to non-TCC hospitals, standardized differences in baseline and cardiac arrest characteristics between both patient groups were small (less than 10%, Table).
Table:
Patient Baseline and Cardiac Arrest Characteristics
| Demographic characteristics | Overall (N = 44585) | Telemedicine Critical Care (N = 14373) | No Telemedicine Critical Care (N = 30212) | Standardized Differences |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 65.6 (15.2) | 65.5 (15.4) | 65.6 (15.1) | 0.01 |
| Sex | 0.01 | |||
| Female | 18361 (41.2) | 5873 (40.9) | 12488 (41.3) | |
| Male | 26224 (58.8) | 8500 (59.1) | 17724 (58.7) | |
| Race | 0.14 | |||
| White | 29992 (67.3) | 9235 (64.3) | 20757 (68.7) | |
| Black/African American | 10050 (22.5) | 3450 (24.0) | 6600 (21.8) | |
| Other | 1395 (3.1) | 380 (2.6) | 1015 (3.4) | |
| Unknown | 3148 (7.1) | 1308 (9.1) | 1840 (6.1) | |
| Cardiac Arrest Characteristics | ||||
| Initial Arrest rhythm | 0.04 | |||
| Asystole | 10072 (22.6) | 3161 (22.0) | 6911 (22.9) | |
| Pulseless electrical activity | 25054 (56.2) | 8037 (55.9) | 17017 (56.3) | |
| Pulseless ventricular tachycardia | 3192 (7.2) | 1048 (7.3) | 2144 (7.1) | |
| Ventricular fibrillation | 3239 (7.3) | 1071 (7.5) | 2168 (7.2) | |
| Unknown | 3028 (6.8) | 1056 (7.3) | 1972 (6.5) | |
| Location of cardiac arrest | 0.05 | |||
| Intensive care unit | 27032 (60.6) | 8742 (60.8) | 18290 (60.5) | |
| Monitored unit | 8239 (18.5) | 2795 (19.4) | 5444 (18.0) | |
| Non-monitored unit | 9314 (20.9) | 2836 (19.7) | 6478 (21.4) | |
| Time of Arrest | 0.01 | |||
| On-hours | 20955 (47.0) | 6800 (47.3) | 14155 (46.9) | |
| Off-hours | 23630 (53.0) | 7573 (52.7) | 16057 (53.1) | |
| Event observation | ||||
| Witnessed | 38573 (86.5) | 12261 (85.3) | 26312 (87.1) | 0.05 |
| Response to event | ||||
| Use of hospital wide cardiac arrest alert | 36031 (80.8) | 11210 (78.0) | 24821 (82.2) | 0.10 |
| Delayed defibrillationa | 1270 (27.8) | 459 (31.1) | 811 (26.3) | 0.11 |
| Delayed first dose of adrenalineb | 426 (7.6) | 163 (9.1) | 263 (6.9) | 0.08 |
| Time to ROSCc,d | 7 (4,12) | 7 (4,12) | 7 (4.12) | .01 |
| CPR Durationc,e | 12, (5,25) | 13 (6,26) | 12 (5,25) | .01 |
| Pre-existing medical conditions: | ||||
| Current myocardial infarction | 6293 (14.1) | 1683 (11.7) | 4610 (15.3) | 0.10 |
| Prior myocardial infarction | 6733 (15.1) | 2183 (15.2) | 4550 (15.1) | 0.00 |
| Current heart failure | 7186 (16.1) | 2162 (15.0) | 5024 (16.6) | 0.04 |
| Prior heart failure | 11376 (25.5) | 3738 (26.0) | 7638 (25.3) | 0.02 |
| Hypotension | 14123 (31.7) | 4408 (30.7) | 9715 (32.2) | 0.03 |
| Metabolic or electrolyte abnormality | 13438 (30.1) | 4349 (30.3) | 9089 (30.1) | 0.00 |
| Respiratory failure | 22537 (50.5) | 6900 (48.0) | 15637 (51.8) | 0.08 |
| Renal insufficiency | 17913 (40.2) | 5334 (37.1) | 12579 (41.6) | 0.09 |
| Hepatic insufficiency | 4905 (11.0) | 1541 (10.7) | 3364 (11.1) | 0.01 |
| Septicaemia | 3904 (8.8) | 1176 (8.2) | 2728 (9.0) | 0.03 |
| Pneumonia | 7224 (16.2) | 2131 (14.8) | 5093 (16.9) | 0.06 |
| Diabetes mellitus | 16638 (37.3) | 5394 (37.5) | 11244 (37.2) | 0.01 |
| Acute Stroke | 1935 (4.3) | 709 (4.9) | 1226 (4.1) | 0.04 |
| Baseline depression in CNS function | 3525 (7.9) | 1062 (7.4) | 2463 (8.2) | 0.03 |
| Major trauma | 1989 (4.5) | 553 (3.8) | 1436 (4.8) | 0.05 |
| Metastatic/hematologic malignancy | 5509 (12.4) | 1817 (12.6) | 3692 (12.2) | 0.01 |
| Interventions prior to arrest | ||||
| Assisted or mechanical ventilation | 21764 (48.8) | 6882 (47.9) | 14882 (49.3) | 0.03 |
| Vasoactive agent | 14496 (32.5) | 4636 (32.3) | 9860 (32.6) | 0.01 |
| Intravenous anti-arrhythmic therapy | 2 (0.0) | 0 (0.0) | 2 (0.0) | 0.01 |
| Pulmonary artery catheter | 5369 (12.0) | 2369 (16.5) | 3000 (9.9) | 0.19 |
| Dialysis | 1809 (4.1) | 503 (3.5) | 1306 (4.3) | 0.04 |
Values are n (%), except for Age, mean (SD)
CNS – central nervous system; ROSC – return of spontaneous circulation
time to defibrillation >2 minutes for shockable rhythms. N = 4562 for comparisons
time to first dose of adrenaline > 5 minutes for non-shockable rhythms. N = 5624 for comparisons
Minutes, median (interquartile range)
N = 32,284 (excludes 690 arrests with ROSC but missing time of ROSC)
N = 73, 923 (excludes 662 arrests where CPR duration could not be determined)
Among 280 GWTG-R participant hospitals, 86 (30.7%) had TCC capability, almost all (99.3%) were located in non-rural areas, approximately three-quarters (75.7%) were non-profit hospitals, the majority were teaching hospitals (81.1%), and had adult cardiology (95.7%), adult cardiac surgery (82.1%), adult interventional cardiac catheterization (93.6%), hospitalist (90.7%), and intensivist (84.6%) services. The hospitals were equally distributed across the geographic regions of the U.S. Compared to non-TCC hospitals, TCC hospitals were more likely to be large (> 500 total beds) and to have adult cardiac surgery, intensivist, and resident physician services (eTable 2). Compared to respondent hospitals, cardiac arrests at non-respondent hospitals were more likely to occur in the ICU or other monitored unit, to be witnessed, and to be associated with the use of hospital wide cardiac arrest alerts (eTable 3).
Acute Resuscitation Survival
Compared to non-TCC hospitals, patients suffering IHCA in an ICU at TCC hospitals had significantly higher unadjusted acute resuscitation survival (76.0% vs. 74.0%, SD=2.0, eTable 4). However, after adjusting for hospital, patient, and cardiac arrest characteristics, there was no significant difference in acute resuscitation survival rates (odds ratio [OR] 1.02, 95% confidence interval [CI] 0.91–1.15, Figure 2). Similarly, there was no difference in acute resuscitation survival rates for ICU arrests during on-hours (OR 1.05 [0.92–1.21]) or off hours (OR 1.00 [0.88–1.13]).
Figure 2: Survival Rates following In-Hospital Cardiac Arrest.
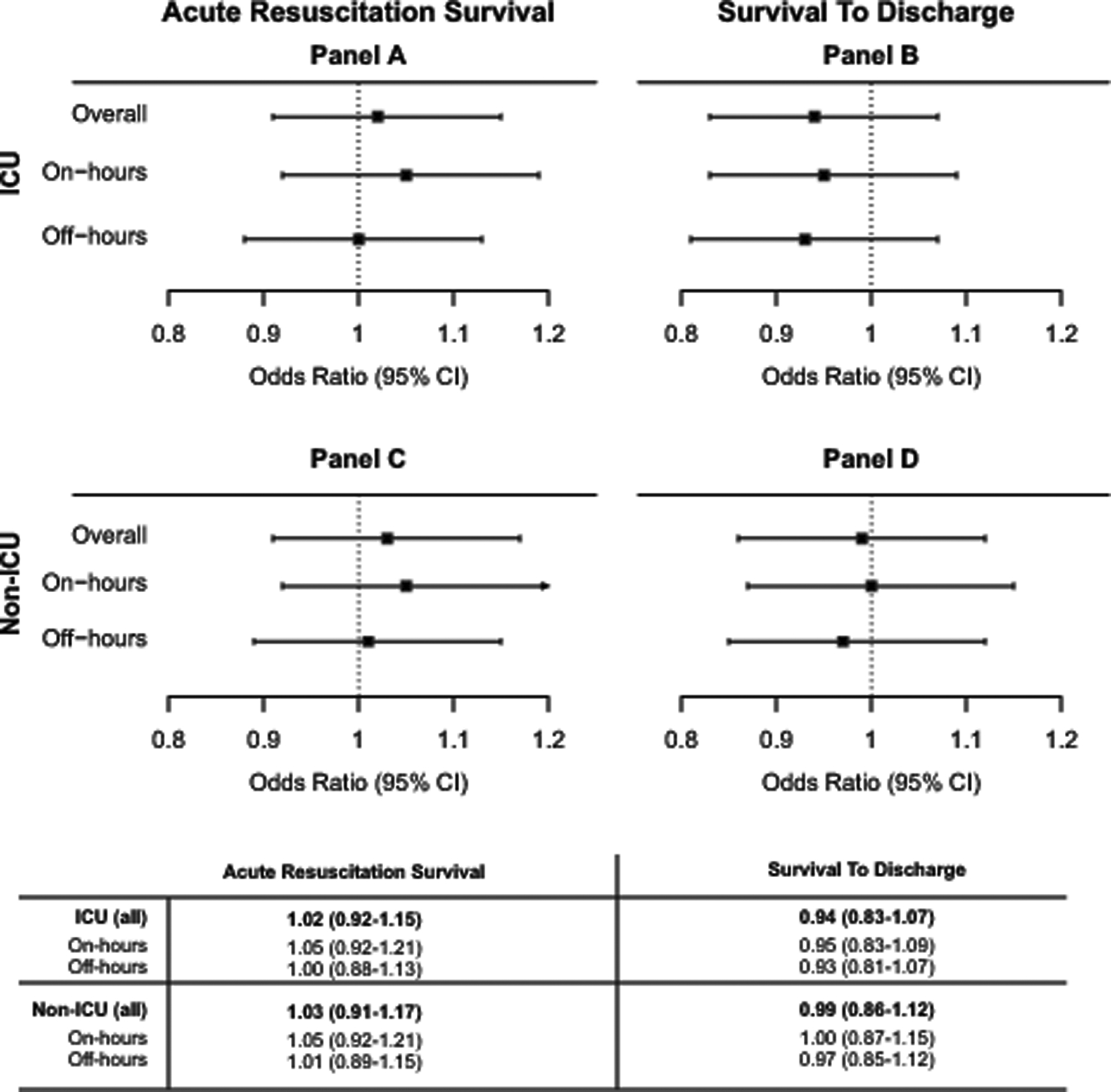
Odds Ratios and 95% Confidence Intervals showing differences between TCC and non-TCC hospitals overall, and during on- or off-hours, for (A&C) Acute Resuscitation Survival rate, and (B&D) Survival to Discharge rate, for IHCA occurring in either (A&B) ICU, or (C&D) non-ICU locations.
We found marked variation in the association of TCC with adjusted acute survival across individual hospitals (Figure 3A). This association was however not modified by any cardiac arrest or hospital characteristic, including whether the event was witnessed, the activation of a hospital wide response for the arrest, hospital ownership type, bed size, and teaching status (P > 0.05 for all interactions). Sensitivity analyses that excluded cardiac arrests between July and December 2019 yielded similar results, as did the exploratory analysis stratified by hospital size and among patient subgroup with non-shockable arrest rhythms (eTables 5,6,7).
Figure 3: Hospital-level Variation in Survival rates.
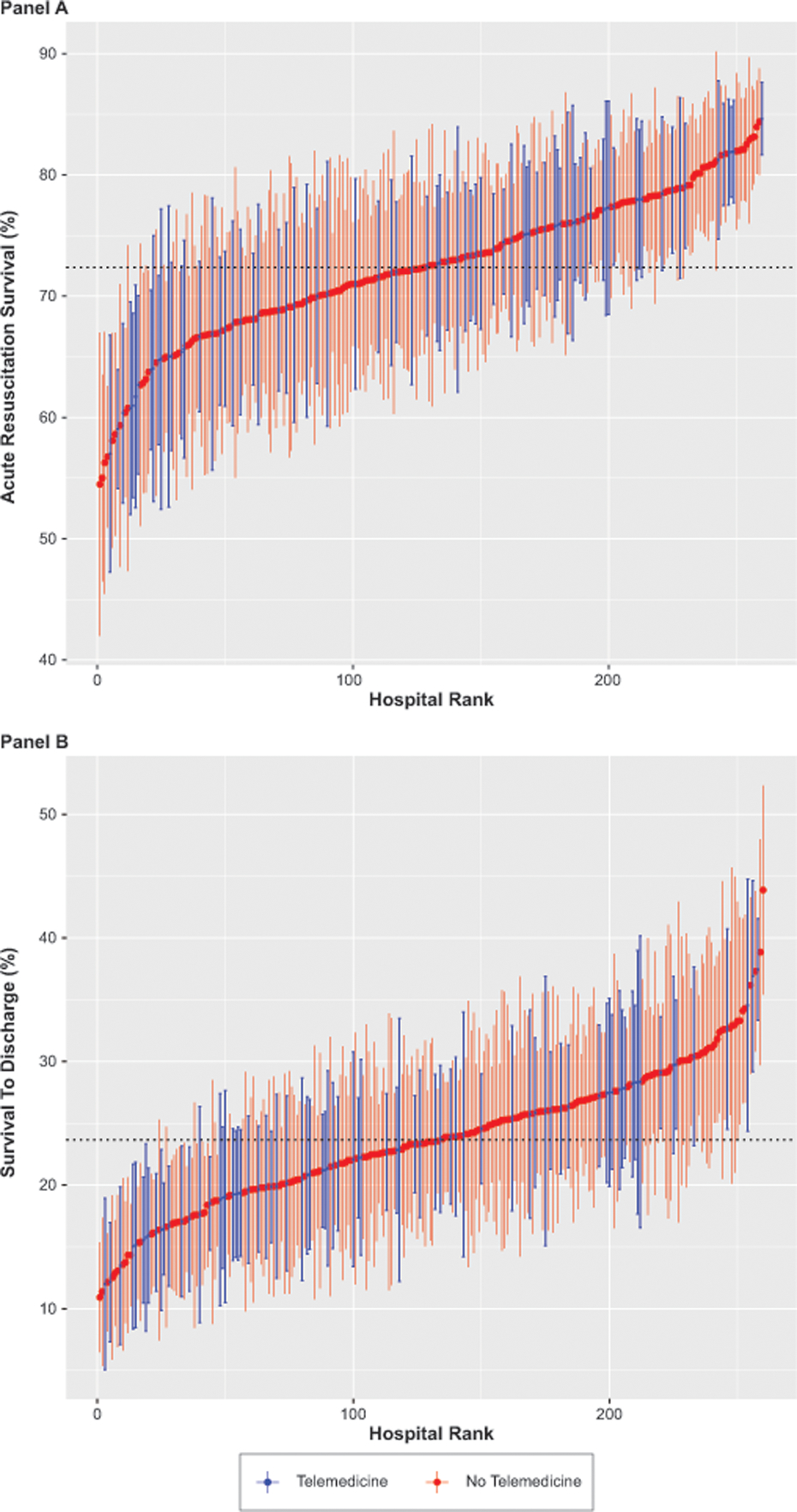
This figure shows the (A) Risk-Adjusted Acute Resuscitation Survival rates, and (B) Survival to Discharge rates for individual TCC (blue) or non-TCC (red) hospitals. Error lines indicate 95% confidence intervals. The dotted horizontal line indicates mean survival rate.
Survival to Discharge
Compared to non-TCC hospitals, TCC hospitals had significantly higher survival to discharge for IHCA occurring in ICU locations in unadjusted analysis (21.7% vs. 20.8%, SD = 0.8, eTable 4) but not after risk adjustment (OR 0.94 [0.83–1.07]). There was also no difference in survival to discharge rates for ICU arrests during on-hours (OR 0.95 [0.83–1.09]) vs. off hours (OR 0.93 [0.81–1.07], Figure 2). The association of TCC with discharge survival also varied widely across individual hospitals (Figure 3B). Exploratory analysis of discharge survival among patients who survived acute resuscitation yielded similar results (eTable 8).
Cardiac Arrests Outcomes in Non-ICU Locations
Unadjusted acute resuscitation survival and survival to discharge rates for arrests occurring outside of the ICU were significantly higher at TCC hospitals, in comparison to non-TCC hospitals (74.2% vs. 72.6%, SD=1.6; 28.7% vs. 27.5%, SD=1.1 respectively, eTable 4), but not in adjusted analysis, (Acute resuscitation OR 1.03 [0.91–1.17]; Survival to discharge OR 0.99 [0.86–1.12], Figure 2). There was also no difference in outcomes for non-ICU arrests during on-hours vs. off hours at TCC hospitals compared to non-TCC hospitals (Acute resuscitation OR on-hours: 1.05 [0.92–1.21]; off-hours 1.01 [0.89–1.13], Survival to discharge OR on-hours: 1.00 [0.87 – 1.15]; off-hours 0.97 [0.85 – 1.12], Figure 2).
DISCUSSION:
In this study of > 40,000 patients experiencing IHCA at 280 hospitals, availability of TCC was associated with both a higher unadjusted acute resuscitation survival rate and survival to discharge rate among cardiac arrests that occurred in an ICU. However, these associations were small and were not statistically significant in risk-adjusted analyses, regardless of the timing of arrest.
We had hypothesized that TCC availability would improve IHCA outcomes. Many factors contribute to poor IHCA survival during nights and weekends, including changes in hospital staffing patterns,26–29 and the impact of shift work, particularly during nighttime, on the performance of skilled activities, such as CPR.30–33 These factors, which can potentially be mitigated by TCC, likely affect the ability of hospital staff to recognize deteriorating patients and prevent IHCA, or quickly initiate resuscitation following IHCA, all of which may impact survival.3
Despite these putative mechanisms, our study failed to demonstrate a clear association between TCC availability and improved IHCA survival outcomes. One possibility is that the beneficial effects of TCC occur upstream of IHCA. TCC may reduce the occurrence of IHCA among monitored ICU patients.34 It is therefore possible that IHCAs taking place at TCC hospitals are mostly unpreventable, perhaps due to a sicker patient cohort, whose care is already maximized in the ICU, and whose care trajectories leading to IHCA could not be further modified by TCC. Our study found that 67% more patients experiencing IHCA at TCC hospitals had pulmonary artery catheters placed, supporting the idea that IHCAs at TCC hospitals may have occurred in sicker patients.
Another possibility lies in the variable nature of TCC coverage models, and in the manner of remote TCC provider involvement during IHCA. Coverage models range from continuous surveillance requiring direct observation through fixed-installation video cameras, to a consultation service model, using mobile telemedicine carts that are brought to the patient on an as-needed basis.35–37 The detection of patient deterioration or of active IHCA is more likely with continuous monitoring. Our study found a higher proportion of patients with delayed defibrillation shocks at TCC hospitals compared to non-TCC hospitals. Although noteworthy, this finding cannot be meaningfully interpreted without data on the type of TCC monitoring in place. Also, the active role of TCC providers during CPR for IHCA varies from de facto team leaders to “copilots” who assist the bedside code team leaders.38 Whether or not TCC consultation during IHCA improves CPR quality remains a subject of research interest.39
These differences likely explain the mixed results that have been observed in other studies evaluating the effectiveness of TCC.17,18 One of these, a large national study using administrative data, reported a small overall reduction in mortality, but also found wide variation in TCC effect across adopting hospitals.17 It is therefore possible that TCC implementation characteristics may directly influence the overall effectiveness of TCC as an intervention, and its effectiveness for specific critical care syndromes, like IHCA.40,41
Finally, the efficacy of TCC may depend not only on its implementation, but also on the broader context into which it is added. TCC may be particularly useful in improving outcomes at small rural hospitals that lack critical care expertise, or urban hospitals experiencing temporary surges, such as during the COVID-19 pandemic, but may not further improve outcomes in already-fully-staffed hospitals, such as those participating in GWTG-R. However, rural and community access hospitals—arguably the most likely to benefit— remain the least likely to have implemented this technology.23 An improved understanding of how and where to optimally implement TCC interventions is crucial for continued adoption in rural and other hospitals for whom costs remain a significant barrier to implementation.36,42 Our study highlights the need to further investigate the implementation and contextual characteristics of TCC programs to better drive policy-relevant research in this field43 and the importance of resuscitation training, regardless of TCC availability.
Our study has several limitations. Firstly, our study design does not support conclusions about causal links between TCC availability and IHCA outcomes. This is especially relevant due to the potential of TCC to reduce the incidence of IHCA. The GWTG-R registry does not capture data on the total number of hospitalizations, which are necessary to determine incidence of IHCA. Secondly, because our datasets lacked information on TCC program characteristics, we are unable to adjust for different TCC models of care or to perform pre- and post-implementation comparisons to determine if TCC led to improvement in IHCA outcomes over time. It is therefore possible that TCC hospitals originally had significantly poorer outcomes than non-TCC hospitals, which improved after TCC adoption. Thirdly, we evaluated TCC as a hospital-level intervention. At a patient-level, we do not know whether or to what extent TCC was involved in resuscitation or post-resuscitation care. We also do not have data on the response times or the leaders of the cardiac arrest response teams — critical components of quality CPR. Fourthly, our study may not generalize beyond GWTG-R hospitals who having made a commitment to data collection and quality improvement, are not representative of all hospitals nationwide. Finally, not all hospitals in the GWTG-R registry responded to the AHA annual surveys, which introduces the possibility of non-response bias, especially given the differences in arrest characteristics between AHA survey-respondent and non-respondent hospitals among the GWTG-R hospitals.
CONCLUSIONS:
In risk-adjusted analyses, we did not find an association between hospital TCC availability and outcomes for IHCA in the ICU, either overall or within the subset of IHCA occurring during off-hours. Future studies are needed to address the impact of specific TCC organizational and implementation characteristics on IHCA prevention and outcomes, and contextual factors that might impact TCC effectiveness, as it relates to IHCA.
Supplementary Material
ACKNOWLEDGMENTS
The GWTG programs are provided by the American Heart Association. Hospitals participating in the registry submit clinical information regarding the medical history, hospital care, and outcomes of consecutive patients hospitalized for cardiac arrest using an online, interactive case report form and Patient Management Tool™ (IQVIA, Parsippany, New Jersey). IQVIA serves as the data collection (through their Patient Management Tool) and coordination center for the American Heart Association/American Stroke Association GWTG programs. The University of Pennsylvania serves as the data analytic center and has an agreement to prepare the data for research purposes.
We thank Scott Appel of the Biostatistics Analysis Center at the University of Pennsylvania for technical assistance with data set linkages.
We thank RJ Waken, PhD, for technical assistance with data visualization productions.
The manuscript was edited by the Scientific Editing Service of the Institute of Clinical and Translational Sciences at Washington University, which is supported by an NIH Clinical and Translational Science Award (UL1 TR002345).
FUNDING SOURCES
Financial Support: This project was supported by (i) the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), under award # KL2 TR002346; (ii) the Center for Administrative Data research, which is supported in part by the Washington University Institute of Clinical and Translational Sciences CTSA grant #UL1 TR002345 from the NCATS of the NIH, and grant #R24 HS19455 awarded by the Agency for Healthcare Research and Quality (AHRQ).
Dr. Ofoma is supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) award # KL2 TR002346
Dr. Marin Kollef is supported by the Barnes-Jewish Hospital Foundation
Dr. Karen Joynt Maddox is supported by the National Heart, Lung, and Blood Institute (R01HL143421) and National Institute on Aging (R01AG060935, R01AG063759, and R21AG065526).
Footnotes
CONFLICTS OF INTEREST
Dr. Joynt Maddox serves on the Health Policy Advisory Council for the Centene Corporation (St. Louis, MO). All other authors have declared no conflict of interest
All GWTG participating institutions were required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule.
CRediT Author Statement:
Ofoma: Conceptualization, Methodology, Writing – Original Draft, Funding Acquisition
Drewry: Writing – Review and Editing
Maddox: Conceptualization, Methodology, Writing – Review and Editing
Boyle: Writing – Review and Editing
Deych: Methodology, Formal Analysis, Writing – Review and Editing
Kollef: Conceptualization, Writing – Review and Editing
Girotra: Conceptualization, Methodology, Writing – Review and Editing
Joynt Maddox: Conceptualization, Methodology, Writing – Review and Editing
GWTG-R Investigators: Resources, Data Curation, Writing-Review and Editing
REFERENCES
- 1.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. Annual Incidence of Adult and Pediatric In-Hospital Cardiac Arrest in the United States. Circulation Cardiovascular quality and outcomes. 2019;12(7):e005580. [PMC free article] [PubMed] [Google Scholar]
- 2.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in-hospital cardiac arrest. The New England journal of medicine. 2012;367(20):1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ofoma UR, Basnet S, Berger A, Kirchner HL, Girotra S, American Heart Association Get With the Guidelines - Resuscitation I. Trends in Survival After In-Hospital Cardiac Arrest During Nights and Weekends. J Am Coll Cardiol. 2018;71(4):402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. Jama. 2008;299(7):785–792. [DOI] [PubMed] [Google Scholar]
- 5.Brady WJ, Gurka KK, Mehring B, Peberdy MA, O’Connor RE, American Heart Association’s Get with the Guidelines I. In-hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82(7):845–852. [DOI] [PubMed] [Google Scholar]
- 6.Morrison LJ, Neumar RW, Zimmerman JL, et al. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation. 2013;127(14):1538–1563. [DOI] [PubMed] [Google Scholar]
- 7.Herasevich V, Subramanian S. Tele-ICU Technologies. Crit Care Clin. 2019;35(3):427–438. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181(11):1164–1169. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox ME, Chong CA, Niven DJ, et al. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Critical care medicine. 2013;41(10):2253–2274. [DOI] [PubMed] [Google Scholar]
- 10.McCambridge M, Jones K, Paxton H, Baker K, Sussman EJ, Etchason J. Association of health information technology and teleintensivist coverage with decreased mortality and ventilator use in critically ill patients. Archives of internal medicine. 2010;170(7):648–653. [DOI] [PubMed] [Google Scholar]
- 11.Morrison JL, Cai Q, Davis N, et al. Clinical and economic outcomes of the electronic intensive care unit: results from two community hospitals. Critical care medicine. 2010;38(1):2–8. [DOI] [PubMed] [Google Scholar]
- 12.Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. Jama. 2011;305(21):2175–2183. [DOI] [PubMed] [Google Scholar]
- 13.Kohl BA, Fortino-Mullen M, Praestgaard A, Hanson CW, Dimartino J, Ochroch EA. The effect of ICU telemedicine on mortality and length of stay. J Telemed Telecare. 2012;18(5):282–286. [DOI] [PubMed] [Google Scholar]
- 14.Willmitch B, Golembeski S, Kim SS, Nelson LD, Gidel L. Clinical outcomes after telemedicine intensive care unit implementation. Critical care medicine. 2012;40(2):450–454. [DOI] [PubMed] [Google Scholar]
- 15.Lilly CM, McLaughlin JM, Zhao H, et al. A multicenter study of ICU telemedicine reengineering of adult critical care. Chest. 2014;145(3):500–507. [DOI] [PubMed] [Google Scholar]
- 16.Nassar BS, Vaughan-Sarrazin MS, Jiang L, Reisinger HS, Bonello R, Cram P. Impact of an intensive care unit telemedicine program on patient outcomes in an integrated health care system. JAMA Intern Med. 2014;174(7):1160–1167. [DOI] [PubMed] [Google Scholar]
- 17.Kahn JM, Le TQ, Barnato AE, et al. ICU Telemedicine and Critical Care Mortality: A National Effectiveness Study. Medical care. 2016;54(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox ME, Adhikari NK. The effect of telemedicine in critically ill patients: systematic review and meta-analysis. Critical care. 2012;16(4):R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. [DOI] [PubMed] [Google Scholar]
- 20.Cummins RO, Chamberlain D, Hazinski MF, et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital ‘Utstein style’. American Heart Association. Circulation. 1997;95(8):2213–2239. [DOI] [PubMed] [Google Scholar]
- 21.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA : the journal of the American Medical Association. 2006;295(1):50–57. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation. 2004;63(3):233–249. [DOI] [PubMed] [Google Scholar]
- 23.Ofoma UR, Maddox TM, Perera C, et al. Characteristics of U.S. Acute Care Hospitals That Have Implemented Telemedicine Critical Care. Crit Care Explor. 2021;3(7):e0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams D, Lawrence J, Hong YR, Winn A. Tele-ICUs for COVID-19: A Look at National Prevalence and Characteristics of Hospitals Providing Teleintensive Care. Journal of Rural Health. 2021;37(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009;38(6):1228–1234. [Google Scholar]
- 26.Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. The New England journal of medicine. 2011;364(11):1037–1045. [DOI] [PubMed] [Google Scholar]
- 27.Neuraz A, Guerin C, Payet C, et al. Patient Mortality Is Associated With Staff Resources and Workload in the ICU: A Multicenter Observational Study. Critical care medicine. 2015;43(8):1587–1594. [DOI] [PubMed] [Google Scholar]
- 28.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA : the journal of the American Medical Association. 2002;288(17):2151–2162. [DOI] [PubMed] [Google Scholar]
- 29.Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. The New England journal of medicine. 2012;366(22):2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barger LK, Ayas NT, Cade BE, et al. Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS medicine. 2006;3(12):e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn G Circadian rhythm, shift work, and emergency medicine. Annals of emergency medicine. 2001;37(1):88–98. [DOI] [PubMed] [Google Scholar]
- 32.Rollinson DC, Rathlev NK, Moss M, et al. The effects of consecutive night shifts on neuropsychological performance of interns in the emergency department: a pilot study. Annals of emergency medicine. 2003;41(3):400–406. [DOI] [PubMed] [Google Scholar]
- 33.Scott LD, Rogers AE, Hwang WT, Zhang Y. Effects of critical care nurses’ work hours on vigilance and patients’ safety. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2006;15(1):30–37. [PubMed] [Google Scholar]
- 34.Shaffer JP, Johnson JW, Kaszuba F, Breslow MJ. REMOTE ICU MANAGEMENT IMPROVES OUTCOMES IN PATIENTS WITH CARDIOPULMONARY ARREST.: 18. Crit Care Med. 2005;33(12):A5. [Google Scholar]
- 35.Caples SM. Intensive Care Unit Telemedicine Care Models. Crit Care Clin. 2019;35(3):479–482. [DOI] [PubMed] [Google Scholar]
- 36.Lee JT, Kerlin MP. ICU Telemedicine and the Value of Qualitative Research for Organizational Innovation. Am J Respir Crit Care Med. 2019;199(8):935–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilly CM, Fisher KA, Ries M, et al. A national ICU telemedicine survey: validation and results. Chest. 2012;142(1):40–47. [DOI] [PubMed] [Google Scholar]
- 38.Peltan ID, Poll JB, Guidry D, Brown SM, Beninati W. Acceptability and Perceived Utility of Telemedical Consultation during Cardiac Arrest Resuscitation. A Multicenter Survey. Annals of the American Thoracic Society. 2020;17(3):321–328. [DOI] [PubMed] [Google Scholar]
- 39.Peltan ID, Guidry D, Brown K, Kumar N, Beninati W, Brown SM. Telemedical Intensivist Consultation During In-Hospital Cardiac Arrest Resuscitation: A Simulation-Based, Randomized Controlled Trial. Chest. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahn JM, Rak KJ, Kuza CC, et al. Determinants of Intensive Care Unit Telemedicine Effectiveness. An Ethnographic Study. Am J Respir Crit Care Med. 2019;199(8):970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalvelage C, Rademacher S, Dohmen S, Marx G, Benstoem C. Decision-Making Authority During Tele-ICU Care Reduces Mortality and Length of Stay-A Systematic Review and Meta-Analysis. Critical care medicine. 2021;49(7):1169–1181. [DOI] [PubMed] [Google Scholar]
- 42.Kumar G, Falk DM, Bonello RS, Kahn JM, Perencevich E, Cram P. The costs of critical care telemedicine programs: a systematic review and analysis. Chest. 2013;143(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahn JM, Hill NS, Lilly CM, et al. The research agenda in ICU telemedicine: a statement from the Critical Care Societies Collaborative. Chest. 2011;140(1):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


