Abstract
Ammonia oxidation in laboratory liquid batch cultures of autotrophic ammonia oxidizers rarely occurs at pH values less than 7, due to ionization of ammonia and the requirement for ammonium transport rather than diffusion of ammonia. Nevertheless, there is strong evidence for autotrophic nitrification in acid soils, which may be carried out by ammonia oxidizers capable of using urea as a source of ammonia. To determine the mechanism of urea-linked ammonia oxidation, a ureolytic autotrophic ammonia oxidizer, Nitrosospira sp. strain NPAV, was grown in liquid batch culture at a range of pH values with either ammonium or urea as the sole nitrogen source. Growth and nitrite production from ammonium did not occur at pH values below 7. Growth on urea occurred at pH values in the range 4 to 7.5 but ceased when urea hydrolysis was complete, even though ammonia, released during urea hydrolysis, remained in the medium. The results support a mechanism whereby urea enters the cells by diffusion and intracellular urea hydrolysis and ammonia oxidation occur independently of extracellular pH in the range 4 to 7.5. A proportion of the ammonia produced during this process diffuses from the cell and is not subsequently available for growth if the extracellular pH is less than 7. Ureolysis therefore provides a mechanism for nitrification in acid soils, but a proportion of the ammonium produced is likely to be released from the cell and may be used by other soil organisms.
Growth of pure cultures of autotrophic ammonia-oxidizing bacteria in liquid culture is optimal within the pH range 7.0 to 8.5 and typically does not occur below a pH of 6.5 (3). Nitrification has, however, been reported in acid soils at pH values as low as 3.7 (5, 9, 11, 25, 26, 34, 35, 37). Although this may be due, in some cases, to heterotrophic nitrification (1, 22) there is strong evidence for autotrophic nitrification in acid soils (19, 38). Autotrophic ammonia oxidizers have been isolated from and enumerated in acid soils (7, 13, 16, 20, 26) and 16S rRNA gene sequences of ammonia oxidizers are frequently detected (6, 33), with evidence for changes in relative abundance of different sequence clusters in soils of different pH (32). However, pure cultures often fail to nitrify at the bulk pH value of the soil from which they were isolated (1). Pure cultures of acidophilic nitrite oxidizers have been isolated (8, 14), but there is only a single report of slow-growing acidophilic ammonia oxidizers from fertilized acid tea soils, capable of growing at low pH in mineral medium containing 1,000 mg of NH4+-N liter−1 (15). Acid-sensitive ammonia oxidizers may be responsible for nitrification in acid soils, since fertilization can lead to an increase in the number of autotrophic nitrifiers (23). However, this increase in number is often associated with the addition of compounds that may increase pH, such as urea or carbonate, and is consistent with the view that autotrophic nitrification may be restricted to microsites of alkaline pH (29). The localized pH inhibition of autotrophic nitrification may also be relieved through the buffering capacity of the soil in which the oxidizers are found (4), but in laboratory culture, growth at low pH has only been observed in biofilms (3, 17) or agglomerates (10).
An alternate explanation for nitrification in acid soils is the ability of many ammonia oxidizers to hydrolyze urea (1, 21, 36). Urea has been found to stimulate autotrophic nitrification in Dutch acid heathland and forest soils (9, 11), and stimulation was shown to be independent of an increase in pH. The isolation of a ureolytic ammonia oxidizer belonging to the genus Nitrosospira from this soil, and its subsequent growth in liquid medium containing urea with an acidophilic nitrite oxidizer, resulted in autotrophic nitrification at pH 4.5 (8). Growth of this strain on ammonium sulfate was not observed below pH 5.5.
The mechanism for acidophilic growth of ureolytic ammonia oxidizers is not clear. A wide range of nonnitrifying bacteria possess urease activity (24), and ammonia released by such organisms will provide a substrate for ammonia oxidizers and will also, potentially, increase local pH. Similarly, ureolytic ammonia oxidizers would have to compete with heterotrophs for the ammonia released into the environment. This suggests that ammonia produced from urea must be retained within the cell to provide an ecological advantage.
The aim of this study was to assess urea hydrolysis followed by ammonia oxidation as a mechanism for autotrophic nitrification in acid soil. This was achieved by determining the influence of pH on the kinetics of urea hydrolysis and ammonia oxidation in liquid culture by a ureolytic strain of Nitrosospira that is unable to grow on ammonium at pH values less than 7.
MATERIALS AND METHODS
Bacterial strains and cultural conditions.
A ureolytic autotrophic ammonia oxidizer, Nitrosospira sp. strain NPAV, originally isolated from soil, was supplied by E. Schmidt, University of Minnesota. Routine growth and maintenance were carried out as described by Keen and Prosser (18) in Skinner and Walker (31) (SW) medium as modified by Powell and Prosser (27) and containing 50 μg NH4+-N ml−1 as ammonium sulfate. An acidophilic nitrite oxidizer, Nitrobacter sp. NHBI, was supplied by W. de Boer, Institute for Ecological Research, Heteren, The Netherlands. This strain was subcultured as for Nitrosospira sp. strain NPAV but in Schmidt and Belser (30) (SB) medium containing 50 μg NO2−-N ml−1 and adjusted to pH 7 by addition of 10 M NaOH prior to autoclaving. The absence of contamination by heterotrophs was checked by plating on nutrient agar (Oxoid) and incubation at 25°C for at least 21 days. Cultures were also examined microscopically for the presence of contaminants. Growth was assessed as the disappearance of ammonia and production of acid and nitrite (Nitrosospira) and as disappearance of nitrite (Nitrobacter) using spot tests for ammonia (Nessler's reagent) and nitrite (Griess llosvay's reagents 1 and 2). Acid production was detected by inclusion of the pH indicator phenol red in media.
Influence of pH on growth on ammonia and urea in poorly buffered medium.
The effect of pH on growth of Nitrosospira sp. strain NPAV was determined in batch cultures prepared using 250-ml Erlenmeyer flasks containing 100 ml of SB medium with either 23.3 μg of NH4+-N ml−1 or 23.3 μg of urea-N ml−1. After autoclaving at 121°C for 20 min, the pH of the medium was adjusted to 4, 5, 6, 7, or 7.5 by aseptic addition of sterile 1 M NaOH or 1 M H2SO4, as appropriate. Medium was then inoculated with 3 ml of a stationary-phase culture of Nitrosospira sp. strain NPAV, grown in the same medium at pH 7. Cocultures of Nitrosospira sp. strain NPAV and Nitrobacter sp. strain NHB1 were also prepared by inoculation of 3 ml of stationary phase cultures of each strain.
Cultures were incubated at 25°C in the dark without shaking, although a number of control cultures were incubated shaken to check for oxygen limitation. No difference was observed in growth kinetics of shaken or static cultures under these conditions. Samples (1 to 2 ml) were removed for measurement of pH (Russell pH electrode) and assessment of growth and were preserved at 4°C following addition of 10 μl of a 1% (wt/vol) solution of potassium ethyl xanthate, an inhibitor of both ammonia- and nitrite-oxidizing bacteria. Growth was assessed by analysis for urea, ammonia, nitrite, and nitrate, as required, using an RFA Autoanalyser system (Alpkem Corporation, Clackamas, Oreg.). Cultures were checked for purity at 1- to 2-day intervals as described above.
Growth on urea and ammonium at constant pH.
Combined growth of Nitrosospira sp. strain NPAV and Nitrobacter sp. strain NHB1 under controlled pH conditions was studied in a modular fermentor (Gallenkamp, Loughborough, United Kingdom) consisting of a 1.3-liter-glass vessel with an operating volume of 1 liter. The pH of the medium was controlled by automated addition of 0.02 M NaOH or 0.1 M H2SO4. Silicone rubber tubing was used throughout, and the vessel was covered with aluminium foil to prevent photoinhibition. The culture vessel and components were autoclaved at 121°C for 20 min with SB medium containing 23.3 μg of NH4+-N ml−1 or nitrogen-free medium, after which filter-sterilized urea was dispensed by passage through a 0.2-μm-pore-size Millipore filter to give a final concentration of 23.3 μg of urea-N ml−1. Agitation was achieved using a magnetic impeller (120 rpm), and the temperature was maintained at 25°C. Filter-sterilized, humidified air was delivered by a HyFlo model C air pump (Medcalf Bros. Ltd., Potters Bar, England) at a rate of 500 ml min−1. Medium was inoculated with 27 ml of stationary phase cultures of Nitrosospira sp. strain NPAV and Nitrobacter sp. strain NHB1, and purity and growth were assessed in samples as described above.
Growth of Nitrosospira in medium containing ammonia and urea was also assessed in SW medium buffered with 50 mM 2(n-morpholino)ethane sulphonic acid (MES) (Sigma) and adjusted to pH 6.2 with 10 M NaOH and concentrated HCI prior to autoclaving. Specific rates of product formation were calculated by linear regression of linear regions of semilogarithmic plots of product concentration versus time. All experiments and treatments were repeated at least three times. Means and standard errors were calculated from triplicates. Graphical data are from a single replicate typical of a particular treatment.
RESULTS
Growth on ammonia in poorly buffered medium.
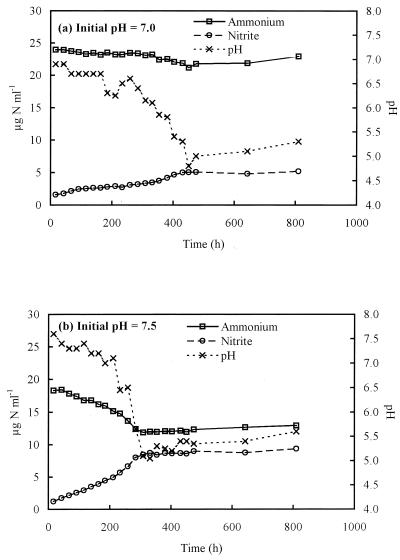
Batch growth of Nitrosospira sp. strain NPAV occurred in SB medium containing ammonium at initial pH values of 7 and 7.5 (Fig. 1) but not at pH 6 or lower. SB medium is poorly buffered, and complete conversion of ammonium to nitrite was prevented by production of nitrous acid and consequent pH reduction to <6. Variation in the lag phase was seen between replicates, but when growth commenced, rates of change in ammonia, nitrite, and pH were similar in triplicate cultures. Initial ammonium concentration was lower in medium adjusted to the higher initial pH value, due to greater volatilization of ammonia prior to inoculation. Ammonium limitation will not have occurred, however, as concentrations were always significantly greater than reported saturation constants for ammonia oxidizers (28). Growth from initial pH values of 7.5 and 7 led to production of similar amounts of nitrite (5.31 and 5.35 μg NO2−-N ml−1, respectively [Table 1]) and the final pH values were not significantly different (Student's t test, P = 0.10). The pH of the medium fell below the minimum pH for growth and rose slightly following cessation of nitrite oxidation.
FIG. 1.
Changes in ammonium and nitrite concentrations and pH during growth of Nitrosospira sp. strain NPAV in liquid batch culture on poorly buffered medium containing ammonium, at initial pH values of 7 (a) and 7.5 (b).
TABLE 1.
Summary of data on Nitrosospira sp. strain NPAV from this studya
| Substrate | Inoculum | Initial pH | Final concn of ammonium (μg of N ml−1) | Final concn of nitrite (monoculture) or nitrite + nitrate (coculture) (μg of N ml−1) | Specific rate of product formation (h−1) | Final pH |
|---|---|---|---|---|---|---|
| Ammonia | Monoculture | 6.0 | No growth | No growth | NA | NA |
| Ammonia | Monoculture | 7.0 | 22.7 (0.27) | 5.31 A (0.36) | 0.0012 A (0.0004) | 5.17 (0.07) |
| Ammonia | Monoculture | 7.5 | 16.5 (1.80) | 5.35 A (2.03) | 0.0028 A (0.0010) | 6.57 (0.48) |
| Ammonia | Coculture | 6.0 | No growth | No growth | NA | NA |
| Ammonia | Coculture | 7.0 | 21.8 (0.28) | 2.01 (0.24) | 0.0068 A (0.0023) | 4.37 A (0.27) |
| Ammonia | Coculture | 7.5 | 11.1 (1.01) | 7.48 (0.96) | 0.0104 A (0.0013) | 4.37 A (0.22) |
| Urea | Monoculture | 4.0 | 16.4 A (0.13) | 13.5 A (0.16) | 0.021 A (0.00029) | 5.43 AB (0.03) |
| Urea | Monoculture | 5.0 | 15.8 AB (0.66) | 15.0 B (0.59) | 0.022 A (0.00023) | 5.57 CD (0.03) |
| Urea | Monoculture | 6.0 | 14.5 B (0.38) | 13.4 A (0.47) | 0.022 A (0.00015) | 5.50 ACF (0.06) |
| Urea | Monoculture | 7.0 | 9.38 C (0.70) | 16.1 B (1.01) | 0.033 (0.00035) | 5.73 E (0.03) |
| Urea | Monoculture | 7.5 | 9.60 C (0.36) | 21.2 (0.16) | 0.014 (0.00811) | 5.67 BDEF (0.07) |
| Urea | Coculture | 6.0 | 16.1 A (0.3) | 14.4 (0.53) | 0.030 A (0.0054) | 4.47 (0.03) |
| Urea | Coculture | 7.0 | 11.8 AB (2.6) | 16.2 (2.71) | 0.009 (0.0018) | 5.90 A (0.65) |
| Urea | Coculture | 7.5 | 10.2 B (0.2) | 19.1 (0.15) | 0.027 A (0.0064) | 5.00 A (0.10) |
| Urea (MES buffer) | Coculture | 6.2 | 1.18 (0.17) | 28.2 (0.35) | 0.0172 A (0.0005) | 6.2 |
| Ammonia + urea (MES buffer) | Coculture | 6.2 | 22.3 (0.89) | 33.9 (0.32) | 0.0195 A (0.0008) | 6.2 |
Final product concentrations, specific product formation rates, and final pH values during growth of Nitrosopira sp. strain NPAV in monoculture or in coculture with an acidophilic nitrite oxidizer, Nitrobacter sp. strain NHB1, during batch growth in buffered or unbuffered media at a range of initial pH values. Values with identical letters beside them did not vary significant (P > 0.05) with pH within a particular treatment. Values in parentheses are standard errors.
NA, not available.
To determine whether growth was inhibited by accumulation of nitrite, the above experiments were repeated with a coculture of Nitrosospira sp. strain NPAV and Nitrobacter sp. strain NHB1, an acidophilic nitrite oxidizer. Growth of the coculture occurred only at initial pH values of 7 and 7.5. At pH 7, no nitrite was detected and nitrate was the sole oxidation product. At pH 7.5, nitrite accumulated during the first 260 h before decreasing below the level of detection following conversion to nitrate. There was no evidence of nitrite toxicity. At pH 7, nitrite produced by monocultures of Nitrosospira was greater than nitrite and nitrate produced during coculture with Nitrobacter (Student's t test, P = 0.003), while at pH 7.5, final product concentrations were not significantly different (P = 0.42) (Table 1). Despite this, the final pH values were significantly lower in cocultures of Nitrosospira and Nitrobacter, possibly due to greater ionization of nitric acid in the weakly buffered medium.
Growth on urea in poorly buffered medium.
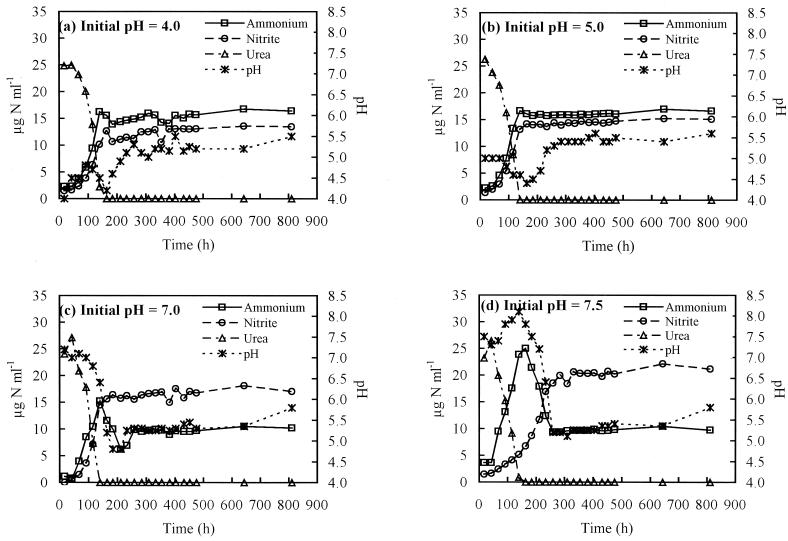
Growth of Nitrosospira sp. strain NPAV in SB medium containing urea occurred at all pH values tested in the range 4 to 7.5. No urea hydrolysis was detected in uninoculated controls at the same initial pH values. Lag phases prior to growth were much shorter than those observed during growth on ammonium and were often not detectable. In addition, standard errors associated with specific rates of product formation during growth on urea were much lower than those during growth on ammonium (Table 1). Following inoculation of medium at an initial pH value of 4, urea hydrolysis was accompanied by an exponential increase in ammonium and nitrite concentrations (Fig. 2a), but no further increases were observed following completion of urea hydrolysis at approximately 180 h. The pH increased to 4.7 during initial production of ammonium from urea and decreased to 4.2 as ammonia oxidation proceeded. Following cessation of urea hydrolysis and ammonia oxidation, the pH increased to a final value of 5.4. Thus, ammonia oxidation to nitrite occurred at pH values below 4.7 but ceased when urea was fully utilized, even though ammonium was present in the medium. The final ammonium and nitrite concentrations were 16.4 and 13.5 μg of N ml−1, respectively, representing conversion of approximately 45% of supplied urea-N to nitrite-N. The total final N concentration was greater than that initially supplied due to water loss during autoclaving and to evaporation during long-term incubation.
FIG. 2.
Changes in urea, ammonium, and nitrite concentrations and pH during growth of Nitrosospira sp. strain NPAV in liquid batch culture on poorly buffered medium containing urea, at initial pH values of 4 (a), 5 (b), 7 (c), and 7.5 (d).
At an initial pH of 5, urea hydrolysis was again associated with initial exponential increases in ammonia and nitrite concentrations (Fig. 2b). Ammonia oxidation to nitrite ceased when urea was fully utilized and the final ammonium concentration was similar to that following growth at pH 4 (Table 1). The pH did not increase during initial production of ammonium from urea but decreased during nitrite formation, as observed above. Following completion of urea hydrolysis, the pH increased to a final value of 5.5, and approximately 49% of urea-N supplied was converted to nitrite-N. Similar changes in urea, ammonium, and nitrite concentrations and in pH were seen during growth from an initial pH value of 6 (data not shown), and final nitrite concentrations and pH values were similar to those in cultures with initial pH values of 4 and 5 (Table 1).
Growth in medium at an initial pH of 7 showed different behavior because of the ability of Nitrosospira to oxidize ammonium directly at this pH. Urea hydrolysis was accompanied, as at pH values 4 to 6, by increases in ammonium and nitrite concentrations (Fig. 2c). Ammonia concentration decreased, however, for a short period after exhaustion of urea and led to further increase in nitrite concentration. This was accompanied by a reduction in pH, which eventually inhibited ammonia oxidation and nitrite production. The final ammonium concentration was lower than that at acid pH values. The final nitrite concentration, although greater than those at initial pH values of 4 and 6, was not significantly different from that at pH 5 (Table 1), and approximately 62% of urea-N was converted to nitrite-N. The final pH was similar to those in cultures grown from lower initial pH values.
Growth from an initial pH of 7.5 led to ammonia production at the same rate as at lower initial pH values, but subsequent conversion to nitrite was slower (Fig. 2d). Significant ammonia oxidation occurred after exhaustion of urea, and final ammonia and nitrite concentrations were lower and higher, respectively, than those at acidic initial pH values. Reduction in pH eventually inhibited ammonia oxidation, and the final pH of 5.67 was not significantly different from that at lower initial pH values (Table 1).
Despite variation in pH during growth, nitrite concentration increased exponentially over significant periods during growth on urea, enabling calculation of specific rates of nitrite production at different initial pH values (Table 1). Under these conditions, the specific rate of nitrite production is equivalent to specific growth rate during exponential growth (27). Specific rates of nitrite production from urea showed little variation with initial pH values in the range 4 to 6, reflecting the fact that most nitrite production occurred when the pH was approximately 5.5 in these cultures. The rate increased in cultures with an initial pH of 7, but the lowest value was obtained for cultures at pH 7.5, reflecting the low rate of ammonium reduction referred to above. At pH values greater than or equal to 7, the nitrite oxidation rate reflects both rates of urea hydrolysis and oxidation of extracellular ammonium. At all initial pH values studied, final nitrite concentrations were greater than those in cultures grown on ammonium. Growth in the presence of Nitrobacter sp. strain NHB1 did not significantly affect product formation (Table 1), suggesting a lack of nitrite inhibition.
Growth on urea and ammonium at constant pH.
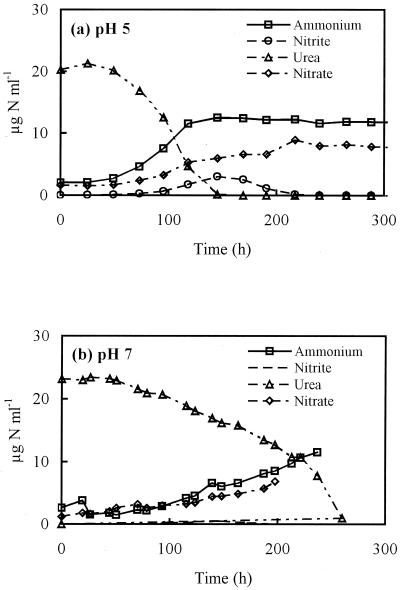
Growth on Nitrosospira sp. strain NPAV in coculture with Nitrobacter sp. strain NHB1 was studied in a pH-controlled fermentor at pH values of 5, 7, and 8, with urea as the substrate, and at 5, 6, 7, and 7.5, with ammonium as the substrate. At pH 5, changes in urea, ammonium, and nitrite concentrations were similar to those in unbuffered medium (Fig. 3). Urea hydrolysis was completed within 150 h and was accompained by oxidation of ammonium, which ceased when urea was fully converted. A lag in the growth of Nitrobacter led to an accumulation of nitrite, which was subsequently oxidized completely to nitrate. At pH 7, growth on urea was followed by growth on ammonium (Fig. 3b). No growth occurred on urea at pH 8 during incubation for 450 h, but urea hydrolysis and ammonia oxidation commenced immediately when the pH control was switched off and the pH was slightly reduced. With ammonium as a substrate, nitrite was produced exponentially at pH 7 and 7.5, but no growth occurred at pH 6 or lower.
FIG. 3.
Changes in urea, ammonium, nitrite, and nitrate concentrations during growth of Nitrosospira sp. strain NPAV and Nitrobacter sp. strain NHB1 in liquid batch culture on urea in a pH-controlled fermentor at pH values of 5 (a) and 7 (b).
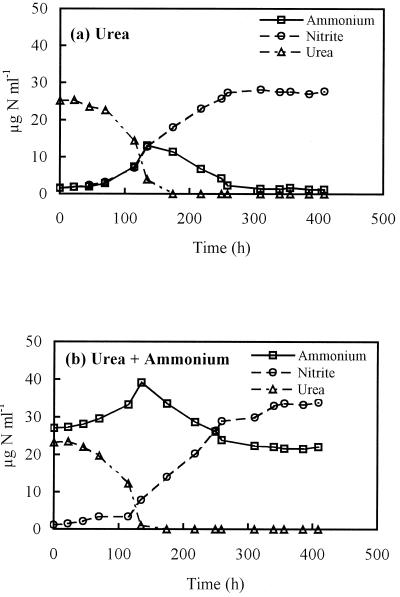
Growth was assessed in buffered medium at pH 6.2 on urea (Fig. 4a) and medium containing urea and ammonium (Fig. 4b). In both cases, growth continued on ammonium after the urea had been utilized but was eventually inhibited, presumably by the low pH. Growth in this medium on ammonium alone was not possible. Thus, cells actively growing on urea could oxidize free ammonium after complete urea hydrolysis at a pH that was inhibitory for growth on ammonium. While final nitrite concentrations were significantly different, specific nitrite production rates were not significantly different in the presence of urea and ammonium during exponential product formation (Table 1).
FIG. 4.
Changes in urea, ammonium, and nitrite concentrations during growth of Nitrosospira sp. strain NPAV in liquid batch culture on buffered medium at pH 6.2 containing urea (a) or urea and ammonium (b).
DISCUSSION
This study provides a further demonstration of the inability of pure cultures of ammonia-oxidizing bacteria to grow on ammonium at low pH in liquid batch culture. Growth of Nitrosospira sp. strain NPAV was possible at pH 7 but not at pH 6.2 or lower. The most likely mechanism for inhibition of growth is ionization of NH3 to NH4+ at low pH and either the lack of an active ammonium transport system or insufficient energy for active transport. An alternative explanation is inhibition by nitrite, but the pH minimum for growth was not altered in this study by coculture with the acidophilic nitrite oxidizer, Nitrobacter sp. strain NHB1, which removed nitrite at all pH values tested. Although ammonia oxidation was eventually inhibited by acidification of the medium, the final pH was lower than the pH minimum for batch growth, suggesting that actively growing cells may be capable of ammonia oxidation at lower pH values than stationary-phase cells. Further evidence for this came from growth in buffered medium at pH 6.2, in which nitrite production continued for some time after complete urea hydrolysis. In this case, nitrite formation was not exponential without urea, and growth was dependent on the presence of urea, as nitrite was not produced with ammonium as the sole, nitrogen source. In unbuffered medium there was no ammonium-dependent growth below pH 7. The ability of actively growing cells to grow at pH values lower than stationary-phase cells has also been observed for Nitrobacter in nitrite-limited continuous culture (18) and may be of significance in natural environments in which the activity of ammonia oxidizers is maintained by low but continuous rates of substrate supply. The ability of this strain to hydrolyze urea did, however, enable significant growth at pH values as low as 4 both in the presence and absence of Nitrobacter sp. strain NHB1.
The changes in urea, ammonia, and nitrite concentrations during growth at low pH suggest a mechanism for urea-linked ammonia oxidation. Urea hydrolysis led to the simultaneous appearance of ammonia and nitrite in the growth medium. Ammonia concentration increased at a greater initial rate than nitrite concentration, but increases in both were exponential and are therefore likely to be linked to growth. Urea hydrolysis and nitrite formation were tightly linked. In unbuffered medium at pH values of 6 and below, nitrite production ceased when urea hydrolysis was completed, even though there was sufficient ammonia present in the medium for growth. This suggests the following mechanism for acidophilic growth on urea. The preferred form for uptake of ammonium is NH3, which can diffuse passively across the cell membrane. As pH decreases, NH3 ionizes to form NH4+, which requires active transport. There is limited evidence for active transport of NH4+ by ammonia oxidizers (12), but even if it were possible, active transport would require energy which the cell may not be able to supply at a low pH. Evidence of urea transport in other microorganisms suggests uptake by simple diffusion (24). Urea can therefore be taken up by cells at a low pH, through diffusion, and is then hydrolyzed intracellularly to ammonia. A proportion of this ammonia diffuses into the medium, but some remains in the cell and is oxidized to nitrite, which then also diffuses into the medium. The ammonia released into the medium ionizes and is no longer available for uptake and oxidation by the cell and accumulates. Growth and nitrite production therefore cease when urea hydrolysis is completed.
A further influence of urea hydrolysis is in increasing pH through release of ammonia. In our liquid culture experiments, the ammonia produced was not sufficient to raise the pH above 7, even when the initial pH of the medium was 6. This was, in part, due to acidification of the medium by nitrous acid produced during ammonia oxidation. In contrast, cultures at initial pH values of 7 and 7.5 showed increases in pH value, which could be attributed to urea hydrolysis. Alkanization by release of ammonia may contribute to ammonia oxidation in acid soils and may provide microenvironments favorable for nitrification (29). The difficulties in growing Nitrosospira on urea at a pH above 7.5 in buffered or pH-controlled media suggested that urea may be inhibitory to growth under these conditions. Rates of urea hydrolysis were similar at initial pH values of 4.5, and 6 and were similar to those of ammonia oxidation, suggesting that urea hydrolysis in these situations may not limit ammonia oxidation.
This study therefore demonstrates the ability of ureolytic autotrophic ammonia oxidizers to contribute to nitrification at pH values as low as 4 when supplied with urea, without the requirement for surface growth or microenvironments of higher pH. This may be of importance for nitrogen cycling in acid soils in which urea is produced, although there is strong competition from other ureolytic microorganisms for urea. The study also demonstrates that urea-linked ammonia oxidation at acid pH values results in diffusion of non-oxidized ammonia from the cell which cannot be utilized further by autotrophic ammonia oxidizers and which may provide a supply of ammonium to other soil organisms.
ACKNOWLEDGMENT
Simon Burton acknowledges receipt of a Natural Environment Research Council Postgraduate Studentship.
REFERENCES
- 1.Adams J A. Identification of heterotrophic nitrification in strongly acid larch humus. Soil Biol Biochem. 1986;18:339–341. [Google Scholar]
- 2.Allison S M, Prosser J I. Urease activity in neutrophilic autotrophic ammonia-oxidising bacteria isolated from acid soils. Soil Biol Biochem. 1991;23:45–51. [Google Scholar]
- 3.Allison S M, Prosser J I. Ammonia oxidation at low pH by attached populations of nitrifying bacteria. Soil Biol Biochem. 1993;25:935–941. [Google Scholar]
- 4.Bazin M J, Rutili A, Gaines A, Lynch J. Humic acid relieves pH-inhibition of nitrification in continuous flow columns. FEMS Microbiol Ecol. 1991;85:9–14. [Google Scholar]
- 5.Boswell J G. The microbiology of acid soils. New Phytol. 1955;54:311–319. [Google Scholar]
- 6.Bruns M A, Stephen J R, Kowalchuk G A, Prosser J I, Paul E A. Comparative diversity of ammonia oxidizer 16S rRNA gene sequences in never-tilled, tilled, and successional soils, Appl. Environ Microbiol. 1999;65:2994–3000. doi: 10.1128/aem.65.7.2994-3000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer W, Duyts H, Laanbroek H J. Autotrophic nitrification in a fertilized heath soil. Soil Biol Biochem. 1988;20:845–850. [Google Scholar]
- 8.de Boer W, Laanbroek H J. Ureolytic nitrification at low pH by Nitrosospira spec. Arch Microbiol. 1989;152:178–181. [Google Scholar]
- 9.de Boer W, Duyts H, Laanbroek H J. Urea stimulated autotrophic nitrification in suspensions of fertilised, acid heath soil. Soil Biol Biochem. 1989;21:349–354. [Google Scholar]
- 10.de Boer W, Klein Gunnewiek P J A, Veenhuis M, Bock E, Laanbroek H J. Nitrification at low pH by aggregated chemolithotrophic bacteria. Appl Environ Microbiol. 1991;57:3600–3604. doi: 10.1128/aem.57.12.3600-3604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer W, Tietema A, Klein Gunnewiek P J A, Laanbroek H J. The autotrophic ammonium-oxidizing community in a nitrogen-saturated acid forest soil in relation to pH-dependent nitrifying activity. Soil Biol Biochem. 1992;24:229–234. [Google Scholar]
- 12.Glover H E. The relationship between inorganic nitrogen oxidation and organic carbon production in batch and chemostat cultures of marine nitrifying bacteria. Arch Microbiol. 1985;142:45–50. [Google Scholar]
- 13.Hankinson T R, Schmidt E L. Examination of an acid forest soil for ammonia-and nitrite-oxidizing autotrophic bacteria. Can J Microbiol. 1984;30:1125–1132. [Google Scholar]
- 14.Hankison T R, Schmidt E L. An acidophilic and a neutrophilic Nitrobacter strain isolated from the numerically predominant nitrite-oxidising population of an acid forest soil. Appl Environ Microbiol. 1988;54:1536–1540. doi: 10.1128/aem.54.6.1536-1540.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayatsu M. The lowest limit of pH for nitrification in tea soil and isolation of an acidophilic ammonia oxidising bacterium. Soil Sci Plant Nutro. 1993;39:219–226. [Google Scholar]
- 16.Jiang Q Q, Bakken L R. Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol Ecol. 1999;30:171–186. doi: 10.1111/j.1574-6941.1999.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 17.Keen G A, Prosser J I. Interrelationship between pH and surface growth of Nitrobacter. Soil Biol Biochem. 1987;19:665–672. [Google Scholar]
- 18.Keen G A, Prosser J I. Steady state and transient growth of autotrophic nitrifying bacteria. Arch Microbiol. 1987;147:73–79. [Google Scholar]
- 19.Killham K. Heterotrophic nitrification. In: Prosser J I, editor. Nitrification. Society for General Microbiology special publication 20. Oxford, United Kingdom: IRL Press; 1986. pp. 117–126. [Google Scholar]
- 20.Klemedtsson L, Jiang Q Q, Kasimir-Klemedtsson Å, Bakken L R. Autotrophic ammonium-oxidising bacteria in Swedish mor humus. Soil Biol Biochem. 1999;31:839–847. [Google Scholar]
- 21.Koops H-P, Böttcher B, Möller U C, Pommerening-Röser A, Stehr G. Classification of eight new species of ammonia-oxidising bacteria: Nitrosomonas communis sp. nov., Nitrosomonas ureae sp. nov., Nitrosomonas aestuarii sp. nov., Nitrosomonas marina sp. nov., Nitrosomonas nitrosa sp. nov., Nitrosomonas eutropha sp. nov., Nitrosomonas oligotropha sp. nov. and Nitrosomonas halophila sp. nov. J Gen Microbiol. 1991;137:1689–1699. [Google Scholar]
- 22.Lang E, Jagnow G. Fungi of a forest soil nitrifying at low pH values. FEMS Microbiol Ecol. 1986;38:257–265. [Google Scholar]
- 23.Martikainen P J. Nitrification in forest soil of different pH as affected by urea, ammonium sulphate and potassium sulphate. Soil Biol Biochem. 1985;17:363–367. [Google Scholar]
- 24.Mobely H L T, Hausinger R P. Microbial ureases: significance, regulation, and molecular characterisation. Microbiol Rev. 1989;53:85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen C. Nitrogen cycling in the soil. Nature (London) 1929;123:144. [Google Scholar]
- 26.Pennington P I, Ellis R C. Autotrophic and heterotrophic nitrification in acidic forest and native grassland soils. Soil Biol Biochem. 1993;25:1399–1408. [Google Scholar]
- 27.Powell S J, Prosser J I. Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture. Appl Environ Microbiol. 1986;52:782–787. doi: 10.1128/aem.52.4.782-787.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prosser J I. Autotrophic nitrification in bacteria. Adv Microbiol Physiol. 1989;30:125–181. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt E L. Nitrification in soil. In: Stevenson F L, editor. Nitrogen in agricultural soils: agronomy 22. Madison, Wis: American Society for Agronomy; 1982. pp. 253–288. [Google Scholar]
- 30.Schmidt E L, Belser L W. Nitrifying bacteria. In: Page A L, Miller R H, Keeney D R, editors. Methods of soil analysis agronomy 9. Madison, Wis: American Society for Agronomy; 1982. pp. 1027–1042. [Google Scholar]
- 31.Skinner F A, Walker N. Growth of Nitrosomonas europaea in batch and continuous culture. Arch Microbiol. 1961;38:339–349. [Google Scholar]
- 32.Stephen J R, Kowalchuk G A, Bruns M-A V, McCaig A E, Phillips C J, Embley T M, Prosser J I. Analysis of β-subgroup ammonia oxidizer populations in soil by DGGE analysis and hierarchical phylogenetic probing. Appl Environ Microbiol. 1998;64:2958–2965. doi: 10.1128/aem.64.8.2958-2965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rDNA sequences related to β-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:3922–3928. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troelstra A, De Boer W, Riemer L, Verstraten J M. Nitrate production in nitrogen-saturated acid forest soils: vertical distribution and characteristics. Soil Biol Biochem. 1992;24:235–240. [Google Scholar]
- 35.Walker N, Wickramasinghe K N. Nitrification and autotrophic nitrifying bacteria in acid tea soils. Soil Biol Biochem. 1979;11:231–236. [Google Scholar]
- 36.Watson S W. Taxonomic considerations of the family Nitrobacteraceae Buchanan: requests for opinions. Int J Syst Bacteriol. 1971;21:254–270. [Google Scholar]
- 37.Weber D F, Gainey P L. Relative sensitivity of nitrifying organisms to hydrogen ions in soils and in solution. Soil Science. 1962;94:138–145. [Google Scholar]
- 38.Weier K L, Gilliam J W. Effect of acidity of nitrogen mineralization in Atlantic coastal plain soils. Soil Sci Soc Am J. 1986;50:1210–1214. [Google Scholar]