Summary
Background
Cervical cancer screening coverage is a key monitoring indicator of the WHO cervical cancer elimination plan. We present global, regional, and national cervical screening coverage estimates against the backdrop of the 70% coverage target set by WHO.
Methods
In this review and synthetic analysis, we searched scientific literature, government websites, and official documentation to identify official national recommendations and coverage data for cervical cancer screening for the 194 WHO member states and eight associated countries and territories published from database inception until Oct 30, 2020, supplemented with a formal WHO country consultation from Nov 27, 2020, to Feb 12, 2021. We extracted data on the year of introduction of recommendations, the existence of individual invitation to participate, financing of screening tests, primary screening and triage tests used, recommended ages and screening intervals, use of self-sampling, and use of screen-and-treat approaches. We also collected coverage data, either administrative or survey-based, as disaggregated as possible by age and for any available screening interval. According to data completeness and representativeness, different statistical models were developed to produce national age-specific coverages by screening interval, which were transformed into single-age datapoints. Missing data were imputed. Estimates were applied to the 2019 population and aggregated by region and income level.
Findings
We identified recommendations for cervical screening in 139 (69%) of 202 countries and territories. Cytology was the primary screening test in 109 (78%) of 139 countries. 48 (35%) of 139 countries recommended primary HPV-based screening. Visual inspection with acetic acid was the most recommended test in resource-limited settings. Estimated worldwide coverage in women aged 30–49 years in 2019 was 15% in the previous year, 28% in the previous 3 years, and 32% in the previous 5 years, and 36% ever in lifetime. An estimated 1·6 billion (67%) of 2·3 billion women aged 20–70 years, including 662 million (64%) of 1·0 billion women aged 30–49 years, had never been screened for cervical cancer. 133 million (84%) of 158 million women aged 30–49 years living in high-income countries had been screened ever in lifetime, compared with 194 million (48%) of 404 million women in upper-middle-income countries, 34 million (9%) of 397 million women in lower-middle-income countries, and 8 million (11%) of 74 million in low-income countries.
Interpretation
Two in three women aged 30–49 years have never been screened for cervical cancer. Roll-out of screening is very low in low-income and middle-income countries, where the burden of disease is highest. The priority of the WHO elimination campaign should be to increase both screening coverage and treatment of detected lesions; however, expanding the efforts of surveillance systems in both coverage and quality control are major challenges to achieving the WHO elimination target.
Funding
Instituto de Salud Carlos III, European Regional Development Fund, Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia, and Horizon 2020.
Translations
For the French, Spanish translations of the abstract see Supplementary Materials section.
Introduction
Cervical cancer is a fully preventable disease, but remains the main cause of cancer death in women in 36 low-income and middle-income countries (LMICs).1, 2 In November, 2020, WHO launched a global initiative to eliminate cervical cancer as a public health problem. WHO proposes a global elimination threshold of four cases per 100 000 women-years and the implementation of a triple intervention strategy, consisting of vaccinating at least 90% of girls against human papillomaviruses (HPV) by the age of 15 years, screening 70% of women using a high-performance test by 35 years of age and again by 45 years of age, and treating at least 90% of identified precancerous lesions and invasive cancers.3
Research in context.
Evidence before this study
In November, 2020, WHO launched a global initiative to eliminate cervical cancer as a public health problem during the 21st century. Robust surveillance and monitoring systems should be implemented at the national or subnational level as part of the elimination strategy. Despite the long existence of cervical cancer screening programmes, sustainable and comparable coverage estimates are not yet available. Many countries produce screening coverage statistics from administrative data or representative surveys, but it is difficult to make comparisons due to differences in programme delivery strategy, screening ages, and screening intervals.
Added value of this study
We developed methods to present baseline estimates of global cervical cancer screening coverage for 2019 (before the COVID-19 pandemic). We have adapted a previously validated methodological approach to produce global human papillomavirus (HPV) vaccination coverage estimates. The chosen methodology allows comparability of the estimates despite the heterogeneity of screening policies and variability of available coverage data within countries. This work includes a systematic review of official cervical cancer screening recommendations and coverages worldwide, supplemented with a formal WHO country consultation and the estimation of individual 2019 country coverages using a stepwise algorithm to impute missing data that allows the calculation of standardised global estimates.
Implications of all the available evidence
Having standardised information on coverage of different screening strategies worldwide allows for a comprehensive evaluation of the strategy-based effect on cervical cancer burden. By 2020, the extent of coverage and organisation of cervical screening varied widely across the world. There were no official screening recommendations in 63 countries. 48 countries (mainly high-income and upper-middle-income) had adopted or are transitioning to HPV-based primary screening. Despite the many available screening modalities, we estimated that, globally, 64% of women aged 30–49 years have never been screened for cervical cancer, representing 662 million women in the target age group of the WHO elimination campaign. Unequal distribution exists by income level, with coverages 7 times higher or more in high-income than in low-income and lower-middle-income countries, highlighting substantial inequities in cancer burden and prevention. Our estimations emphasise that we are still a long way from achieving the WHO target of 70% screening coverage of women aged 30–49 years with a high-performance test, especially in regions of the world with the greatest burden of disease. Scaling up cervical screening in these regions is a major challenge that must be taken on in order to achieve the WHO elimination target.
The proven superiority of HPV testing4, 5 has led WHO to recommend primary HPV-based screening6, 7, 8 and, consequently, many programmes are transitioning from cytology.8, 9 However, there are substantial barriers for adoption and sustainable scale-up of HPV-based screening including stakeholders’ beliefs, resource constraints, and poor availability of affordable, clinically-validated HPV tests.10, 11, 12 Implementation of robust surveillance and monitoring systems are key to identifying gaps and progressing towards cervical cancer elimination.3 Screening coverage is one of the core indicators reflecting the capacity to provide testing for primary screening at a country level.
We present the status of cervical cancer screening programmes worldwide, including the adoption of HPV-based strategies, and the methods and results for the first edition of WHO coverage estimates of cervical cancer screening. We view the data presented as the baseline from which to monitor and evaluate the effect of forthcoming interventions as part of the elimination strategy, and to be analysed and discussed against the backdrop of the 70% screening coverage target of women aged 35–45 years.
Methods
Data sources
In this review and synthetic analysis, we searched scientific literature, government websites, and official documentation to identify official national recommendations and coverage data for cervical cancer screening for the 194 WHO member states and eight associated countries and territories (American Samoa, Bermuda, French Polynesia, Greenland, Hong Kong, Palestine, Puerto Rico, and Tokelau), published from database inception until Oct 30, 2020. For each country, the search strategy included academic and official channels for information on cancer control plans, screening policies, and coverage statistics (eg, health departments and national epidemiological institutions), followed by a systematic search in PubMed. Search terms, translations, and eligibility criteria are in appendix 3 (p 3). 11 professional translators assisted investigators in the search and the interpretation of information in local languages. References of included publications were reviewed to identify additional sources. We also included recognised international data sources: the USAID Demographic and Health Surveys (DHS) Program, WHO World Health Surveys, and WHO STEPwise Approach to Noncommunicable Diseases (NCD) Risk Factor Surveillance (STEPS) surveys.13 Retrieved information was cross-checked and supplemented with official responses to WHO NCD Country Capacity Survey 2019 and unpublished WHO STEPwise approach to surveillance (STEPS) survey data.13, 14
Eligibility criteria included sources that described in detail national official cervical cancer screening recommendations (either as a law or governmental regulation, decision, directive, or recommendation). Countries with no identifiable official recommendations were considered to have no screening programmes. To characterise screening programmes, we retrieved information on the year of introduction, the existence of individual invitation to participate, financing of screening tests, primary screening and triage tests used, recommended ages and screening intervals, use of self-sampling, and use of screen-and-treat approaches. Eligible coverage data could be derived from administrative or survey data, with no restrictions on the year of collection, but had to meet quality and representativeness criteria for inclusion. The criteria for data representativeness were based on the absence of major changes in the screening program, in the healthcare system, or in the income-level status of the country. Only national, population-based screening data representative of the country's situation in 2019 entered the final database (appendix 3 p 3). Data were extracted by six independent investigators, including BS, MP, and RM, with discrepancies resolved by forced consensus. This study complies with the GATHER recommendations.15
Methods of estimation and statistical analysis
We searched official screening recommendations for each country and age-specific coverage for any of the following screening intervals: previous 1 year, previous 2 years, previous 3 years, previous 5 years, and ever in lifetime. We extracted coverage by age and any available screening interval. Most coverages were reported aggregated by age groups of 5, 10, or more than 10 years. Although for many countries we collected coverage data from many different sources, we generally selected coverage data (representative for 2019) from one single source. When multiple sources were available, we prioritised administrative data in countries with organised programmes and accurate registries, and survey data in countries with opportunistic screening or with no centralised registries. We also prioritised the most recent data and the most disaggregated data by age groups when more than one representative estimation was available for a given country (eg, if coverage data was available for 2019 for the age groups 30–39 years, 40–49 years, and 30–49 years, the first two groups were selected). Coverages were transformed into single-age datapoints by assigning the same coverage to all ages in the reported age group and applying corrections as appropriate (appendix 3 pp 4–7, 16–19).
Missing data treatment included the development of a multi-step algorithm using different statistical techniques (appendix 3 pp 8–10) based on the closest available data (appendix 3 pp 11–14, 20–26, 50–51). Iteratively and in this order, the following procedures were applied whenever possible: linear interpolation between screening intervals, multiple imputations (40) per missing datapoint using the predictive mean matching method, last observation carried forward, or next observation carried backward techniques, or the use of a ponderation rate based on coverage from countries with the same income and the same age-related screening recommendations. Covariates included in the final model are in appendix 3 (pp 8–9). For each single-age datapoint imputation, it was verified that no coverage exceeded that of its next upper screening interval, and when necessary, coverage was recalculated. Country-specific estimates for the 202 countries and territories were computed from the estimation of the number of screened women for each age group, screening interval, and country as numerator and the UN populations as denominator. Bootstrap 95% CIs were calculated using the percentile method with 3000 bootstrap replications using R (version 3.6.1).16
Country-specific estimates were aggregated by age group according to different geographical and income groups: five regions and 22 subregions using the UN classification system, eight subregions of UN Sustainable Development Goals classification, six WHO regions, and by income level using the 2019 World Bank's classification. Following WHO's quality standards for data publication, an official consultation round with WHO member states and associated countries was done from Nov 27, 2020, to Feb 12, 2021, to review, comment on, and provide insight on the estimates. Countries were presented with draft estimates and sources of data. 83 countries responded to the country consultation, resulting in an update of screening policies in 33 countries and coverages in 42 countries (appendix 3 p 15). Coverage estimates before and after consultation were similar, except for Latin America and the Caribbean, for which post-consultation estimates were up to 20% lower than pre-consultation estimates. These differences were explained by an update of the coverage data in Brazil and Colombia, switching from using very high coverage data from surveys to using lower coverage data from administrative sources.
To assess and validate the methodology to treat missing data, we did an exhaustive sensitivity analysis, simulations, and an evaluation of the effect of imputations in the final estimations (appendix pp 27, 28, 52–64). To approximate the incremental needs in screening capacity required to achieve the WHO elimination target of 70% of women screened with a high-performance test by 35 years of age and again by 45 years of age, we produced an incremental factor that was calculated by dividing the 70% target coverage by the estimated country coverage in the previous 5 years in women aged 35–49 years. The factor was produced only for countries with coverage below the elimination target. We also estimated the minimum number of women aged 35–49 years to be screened in 5 years to meet the 70% target, applying the 70% coverage to the corresponding UN female population in 2019.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
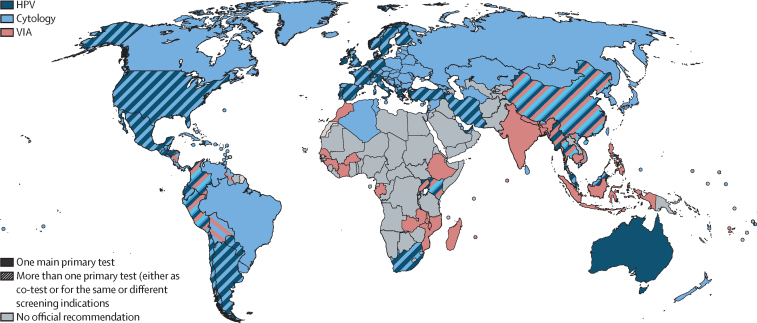
Through our search, we identified official cervical cancer screening recommendations in 139 (69%) of 202 countries and age-specific coverage data for at least one screening interval in 164 (81%) of 202 countries (table 1; figure 1; appendix 3 pp 29–49). All 139 countries with documented official recommendations for cervical cancer screening reported publicly funded primary screening tests. 56 (88%) of 64 high-income countries and 83 (60%) of 138 LMICs had screening recommendations, corresponding to 18 (33%) of 54 countries in Africa, 33 (69%) of 48 countries in Asia, 41 (95%) of 43 countries in Europe, 11 (58%) of 19 countries in Oceania, and 36 (95%) of 38 countries in the Americas. Recommendations had been recently introduced or changed in the last 5 years in 54 (39%) of 139 countries and in the last 10 years in 84 (60%) countries. Only 40 (29%) of 139 countries sent women individual screening invitations. Most (55 [40%] of 139) countries recommended beginning screening between the ages of 25 and 29 years, and 91 (65%) recommended ending screening between the ages of 60 and 69 years. Ten (7%) of 139 countries followed WHO recommendation to prioritise screening in women aged 30–49 years and women from LMICs (mainly from Asia [six countries] and Africa [three countries]). 46 (33%) of 139 countries recommended screening in women younger than 25 years. 48 (35%) of 139 countries recommended HPV-based screening, but most (at least 21 countries) were still transitioning from cytology to HPV as the main test. HPV testing was mainly recommended in women 30 years and older in 5-year intervals, although eight countries recommended it at frequencies lower than 5 years, and eight countries recommended it in women younger than 30 years. Visual inspection with acetic acid (VIA) was generally used in women aged 30–49 years in 3–5-year intervals. Cytology was recommended across all age ranges, usually in 3-year intervals, but 15 countries recommended it every 1 or 2 years. In 52 (37%) of 139 countries, more than one screening test was recommended, either interchangeably (in 28 countries) or recommended differently according to age or setting (in underserved populations). HPV testing was introduced as a triage test (and not recommended for primary use) for atypical squamous cells of undetermined significance or other indications in 18 countries. Six countries (Belarus, Belgium, Canada, Japan, New Zealand, and Trinidad and Tobago) announced plans in 2019 for introducing HPV-based screening by 2024. Combined or alone, cytology was still the most used screening test, with 109 (78%) of 139 countries recommending it for at least one indication. In resource-constrained settings, the most common screening approach was the VIA test. VIA was the primary test in 41 (29%) of 139 countries (in 9 low-income and 31 middle-income countries), and in 21 (51%) of 41 countries was the only nationally recommended test. The screen-and-treat approach was recommended in 31 (76%) of 41 countries using VIA as the primary test.
Table 1.
Main characteristics of cervical cancer screening in 139 countries with documented official recommendations
| World (N=139)* |
Countries by income |
||||||
|---|---|---|---|---|---|---|---|
| High (n=56)* | Upper middle (n=46) | Lower middle (n=25) | Low (n=12) | ||||
| Screening invitations sent to individuals | 40 (29%)† | 30 (54%)† | 9 (20%) | 1 (4%) | 0 | ||
| Year of introduction of current recommendationsठ| |||||||
| 2016–20 | 54 (39%) | 23 (41%) | 19 (41%) | 8 (32%) | 4 (33%) | ||
| 2011–15 | 30 (22%) | 10 (18%) | 13 (28%) | 5 (20%) | 2 (17%) | ||
| 2010 and earlier | 36 (26%) | 14 (25%) | 8 (17%) | 10 (40%) | 4 (33%) | ||
| Recommended age to begin screening, years | |||||||
| 24 or younger | 46 (33%) | 28 (50%) | 13 (28%) | 5 (20%) | 0 | ||
| 25–29 | 55 (40%) | 23 (41%) | 10 (22%) | 18 (72%) | 4 (33%) | ||
| 30–34 | 31 (22%) | 5 (9%) | 11 (24%) | 9 (36%) | 6 (50%) | ||
| 35–39 | 6 (4%) | 0 | 3 (7%) | 1 (4%) | 2 (17%) | ||
| 40 or older | 1 (1%) | 0 | 1 (2%) | 0 | 0 | ||
| Recommended age to end screening, years | |||||||
| 49 or younger | 18 (13%) | 1 (2%) | 6 (13%) | 5 (20%) | 6 (50%) | ||
| 50–59 | 16 (12%) | 4 (7%) | 5 (11%) | 4 (16%) | 3 (25%) | ||
| 60–64 | 45 (32%) | 16 (29%) | 20 (43%) | 7 (28%) | 2 (17%) | ||
| 65–69 | 46 (33%) | 26 (46%) | 12 (26%) | 7 (28%) | 1 (8%) | ||
| 70 or older | 14 (10%) | 9 (16%) | 3 (7%) | 2 (8%) | 0 | ||
| Cytology-based screening¶ | 109 (78%) | 53 (95%) | 41 (89%) | 13 (52%) | 2 (17%) | ||
| Recommended ages and interval for cytology-based screening§ | |||||||
| Age 29 years and younger | 88/109 (81%) | 49/53 (92%) | 29/41 (71%) | 10/13 (77%) | 0 | ||
| Every 1–2 years | 13/88 (15%) | 8/49 (16%) | 4/29 (14%) | 1/10 (10%) | 0 | ||
| Every 3 years | 67/88 (76%) | 38/49 (78%) | 22/29 (76%) | 7/10 (70%) | 0 | ||
| Every 4 years or more | 5/88 (6%) | 1/49 (2%) | 2/29 (7%) | 2/10 (20%) | 0 | ||
| Age 30–49 years | 98/109 (90%) | 47/53 (89%) | 37/41 (90%) | 12/13 (92%) | 2/2 (100%) | ||
| Every 1–2 years | 14/98 (14%) | 8/47 (17%) | 4/37 (11%) | 1/12 (8%) | 1/2 (50%) | ||
| Every 3 years | 65/98 (66%) | 31/47 (66%) | 26/37 (70%) | 8/12 (67%) | 0 | ||
| Every 4 years or more | 13/98 (13%) | 5/47 (11%) | 6/37 (16%) | 2/12 (17%) | 0 | ||
| Age 50 years and older | 90/109 (83%) | 44/53 (83%) | 34/41 (83%) | 11/13 (85%) | 1/2 (50%) | ||
| Every 1–2 years | 14/90 (16%) | 7/44 (16%) | 5/34 (15%) | 1/11 (9%) | 1/1 (100%) | ||
| Every 3 years | 58/90 (64%) | 27/44 (61%) | 23/34 (68%) | 8/11 (73%) | 0 | ||
| Every 4 years or more | 14/90 (16%) | 8/44 (18%) | 5/33 (15%) | 1/11 (9%) | 0 | ||
| Recommended triage test for cytology-based screening | |||||||
| HPV test | 34/109 (31%) | 27/53 (51%) | 7/41 (17%) | 0 | 0 | ||
| HPV-based screening¶‖ | 48 (35%) | 25 (45%) | 16 (35%) | 4 (16%) | 3 (25%) | ||
| Recommended ages and interval for HPV screening§ | |||||||
| Age 29 years and younger | 8/48 (17%) | 7/25 (28%) | 1/16 (6%) | 0 | 0 | ||
| Every 3–4 years | 3/8 (38%) | 3/7 (43%) | 0 | 0 | 0 | ||
| Every 5 years | 4/8 (50%) | 4/7 (57%) | 0 | 0 | 0 | ||
| Every 5 years or more | 1/8 (13%) | 0 | 1/1 (100%) | 0 | 0 | ||
| Age 30–49 years | 47/48 (98%) | 24/25 (96%) | 16/16 (100%) | 4/4 (100%) | 3/3 (100%) | ||
| Every 3–4 years | 6/47 (13%) | 5/24 (21%) | 1/16 (6%) | 0 | 0 | ||
| Every 5 years | 37/47 (79%) | 19/24 (79%) | 13/16 (81%) | 4/4 (100%) | 1/3 (33%) | ||
| Every 5 years or more | 3/47 (6%) | 0 | 2/16 (13%) | 0 | 1/3 (33%) | ||
| Age 50 years and older | 43/48 (90%) | 25/25 (100%) | 14/16 (88%) | 3/4 (75%) | 1/3 (33%) | ||
| Every 3–4 years | 4/43 (9%) | 3/25 (12%) | 1/14 (7%) | 0 | 0 | ||
| Every 5 years | 37/43 (86%) | 21/25 (84%) | 12/14 (86%) | 3/3 (100%) | 1/1 (100%) | ||
| Every 5 years or more | 2/43 (5%) | 1/25 (4%) | 1/14 (7%) | 0 | 0 | ||
| Recommended triage test for primary HPV-based screening | |||||||
| Cytology | 18/48 (38%) | 12/25 (48%) | 6/16 (38%) | 0 | 0 | ||
| Cytology or HPV genotyping | 6/48 (13%) | 2/25 (8%) | 4/16 (25%) | 0 | 0 | ||
| Cytology or VIA | 1/48 (2%) | 0 | 1/16 (6%) | 0 | 0 | ||
| HPV genotyping | 2/48 (4%) | 1/25 (4%) | 0 | 0 | 1/3 (33%) | ||
| VIA | 2/48 (4%) | 0 | 0 | 2/4 (50%) | 0 | ||
| Screen and treat strategy | 4/48 (4%) | 0 | 0 | 2/4 (50%) | 0 | ||
| VIA as primary screening test¶ | 41 (29%) | 1 (2%) | 13 (28%) | 18 (72%) | 9 (75%) | ||
| Recommended ages and interval for VIA screening§ | |||||||
| Age 29 years and younger | 17/41 (41%) | 0 | 5/13 (38%) | 8/18 (44%) | 4/9 (44%) | ||
| Every 1–2 years | 1/17 (6%) | 0 | 1/5 (20%) | 0 | 0 | ||
| Every 3 years | 11/17 (65%) | 0 | 4/5 (80%) | 5/8 (63%) | 2/4 (50%) | ||
| Every 4 years or more | 2/17 (12%) | 0 | 0 | 2/8 (25%) | 0 | ||
| Age 30–49 years | 38/41 (93%) | 0 | 13/13 (100%) | 16/18 (89%) | 9/9 (100%) | ||
| Every 1–2 years | 3/38 (8%) | 0 | 2/13 (15%) | 1/16 (6%) | 0 | ||
| Every 3 years | 17/38 (45%) | 0 | 8/13 (62%) | 7/16 (44%) | 2/9 (22%) | ||
| Every 4 years or more | 14/38 (37%) | 0 | 3/13 (23%) | 7/16 (44%) | 4/9 (44%) | ||
| Age 50 years and older | 18/41 (44%) | 0 | 5/13 (38%) | 9/18 (50%) | 4/9 (44%) | ||
| Every 1–2 years | 0 | 0 | 0 | 0 | 0 | ||
| Every 3 years | 9/18 (50%) | 0 | 3/5 (60%) | 5/9 (56%) | 1/4 (25%) | ||
| Every 4 years or more | 6/18 (33%) | 0 | 2/5 (40%) | 3/9 (33%) | 1/4 (25%) | ||
| Underserved populations | 4/41 (10%) | 1/1 (100%) | 2/13 (15%) | 1/18 (6%) | 0 | ||
| Screen and treat strategy with VIA | 31/41 (76%) | 0 | 7/13 (54%) | 16/18 (89%) | 8/9 (89%) | ||
HPV=Human papillomaviruses. VIA=visual inspection with acetic acid.
Partial implementation in United Arab Emirates (Abu-Dabi).
Variability among country regions in Belgium, Canada, and Spain. Organised programmes in small regions in Greece not included.
Including introduction of modifications in the recommended primary tests, modifications to ages to start and end screening, and modifications to screening interval.
No information was available about the year of introduction of current recommendations in 19 countries (Bosnia and Herzegovina, Cyprus, Monaco, Guinea, Antigua and Barbuda, The Bahamas, Bermuda, Dominican Republic, Grenada, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Trinidad and Tobago, Venezuela, Cook Islands, Vanuatu, Bahrain, North Korea, and Timor-Leste), about the recommended screening interval for cytological screening in seven countries (Albania, Cyprus, Dominica, Cook Islands, Vanuatu, Iran, and Syria), and about the recommended screening interval for VIA screening in six countries (Guinea, Madagascar, Mozambique, Bolivia, Panama, and Timor-Leste).
Combined with other main screening tests or alone.
Including countries that are transitioning to HPV as the main test. Not including countries that reported plans in 2019 for introduction of HPV-based screening by 2024 (Canada, New Zealand, Belgium, Belarus, Japan, and Trinidad and Tobago).
Figure 1.
Official recommended tests for primary cervical cancer screening
The solid pattern indicates the recommendation of one of the tests (either cytology, HPV, or VIA). The striped pattern indicates the coexistence of more than one test, which can have the same indication or be used for different indications (eg, different tests are indicated at different ages, or in different settings or outreach). HPV=human papillomaviruses. VIA=visual inspection with acetic acid.
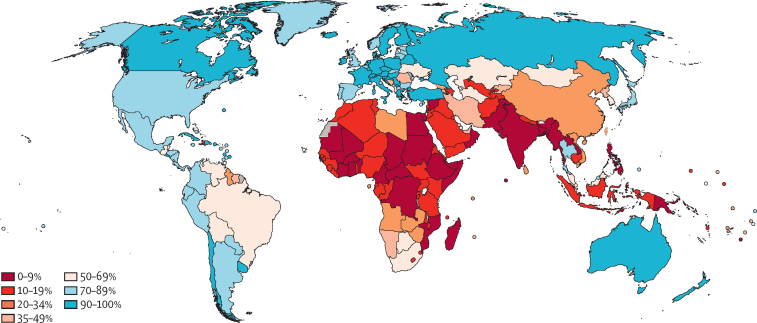
We estimate that, globally, 370 million (36%) of 1 billion women aged 30–49 years have been screened for cervical cancer ever in lifetime; 160 million (15%) in the previous year, 292 million (28%) in the previous 3 years, and 330 million (32%) in the previous 5 years (table 2). High-income countries are estimated to have at least 3 times higher coverages for testing women in the previous year, 3 years, and 5 years, and ever in a lifetime than LMICs. Within LMICs, upper-middle-income countries had coverages ranging from 19% in the previous year to 48% ever in lifetime, compared with coverage from 4% in the previous year to 9% ever in lifetime in lower-middle-income countries and from 3% to 11% in low-income countries (table 2). SDG regions of Europe and North America (88%, ever-in-lifetime coverage), Latin America and the Caribbean (73%, ever-in-lifetime coverage), and Australia and New Zealand (95%, ever-in-lifetime coverage) presented the highest coverage estimates. Lifetime coverage of 70% or higher in women aged 30–49 years was observed in 75 (37%) of 202 countries, none of which were low-income countries (figure 2). Coverage estimates for women aged 25–65 years are in appendix 3 (pp 65–66).
Table 2.
Estimates of cervical cancer screening coverage in women aged 30–49 years in 2019
|
Screening in the previous year |
Screening in the previous 3 years |
Screening in the previous 5 years |
Screening ever in lifetime |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of screened women in millions, N (95% CI) | Coverage, % (95% CI) | Number of screened women in millions, N (95% CI) | Coverage, % (95% CI) | Number of screened women in millions, N (95% CI) | Coverage, % (95% CI) | Number of screened women in millions, N (95% CI) | Coverage, % (95% CI) | |||
| Global screening coverage | 159·6 (142·0–179·0) | 15% (14–17) | 292·4 (259·9–327·4) | 28% (25–32) | 329·8 (295·0–367·2) | 32% (29–36) | 369·7 (332·2–409·9) | 36% (32–40) | ||
| Coverage by country income level | ||||||||||
| High income | 66·8 (57·1–77·3) | 42% (36–49) | 110·9 (95·7–127·5) | 70% (61–81) | 121·2 (104·8–139·0) | 77% (66–88) | 132·6 (114·8–151·8) | 84% (73–96) | ||
| LMICs | 92·8 (78·1–109·6) | 11% (9–13) | 181·5 (153·3–213·3) | 21% (18–24) | 208·6 (178·2–242·8) | 24% (20–28) | 237·1 (204·2–274·1) | 27% (23–31) | ||
| Upper-middle-income | 76·2 (61·9–92·5) | 19% (15–23) | 151·6 (124·8–183·2) | 38% (31–45) | 172·6 (143·7–206·1) | 43% (36–51) | 194·4 (163·0–230·3) | 48% (40–57) | ||
| Lower-middle-income | 14·8 (12·5–17·2) | 4% (3–4) | 25·0 (22·0–28·1) | 6% (6–7) | 29·5 (26·1–33·2) | 7% (7–8) | 34·4 (30·4–38·7) | 9% (8–10) | ||
| Low-income | 1·9 (1·5–2·4) | 3% (2–3) | 4·9 (4·2–5·7) | 7% (6–8) | 6·5 (5·7–7·5) | 9% (8–10) | 8·2 (7·2–9·4) | 11% (10–13) | ||
| Coverage by SDG regions and subregions | ||||||||||
| Sub-Saharan Africa | 4·3 (3·3–5·6) | 4% (3–5) | 9·6 (8·0–11·4) | 9% (7–11) | 12·8 (10·7–15·1) | 12% (10–14) | 15·9 (13·4–18·7) | 15% (12–17) | ||
| Eastern Africa | 1·2 (0·9–1·5) | 3% (2–3) | 2·7 (2·3–3·1) | 6% (5–7) | 3·8 (3·3–4·3) | 9% % (7–10) | 4·8 (4·2–5·5) | 11% (10–13) | ||
| Middle Africa | 0·3 (0·2–0·5) | 2% (1–3) | 1·2 (0·9–1·6) | 7% (5–9) | 1·6 (1·2–2·1) | 10% (7–13) | 2·0 (1·5–2·6) | 12% (9–16) | ||
| Southern Africa | 2·0 (1·2–3·0) | 22% (13–32) | 3·2 (2·0–4·4) | 34% (21–47) | 4·0 (2·5–5·5) | 42% (27–58) | 4·7 (3·0–6·6) | 50% (32–70) | ||
| Western Africa | 0·8 (0·5–1·4) | 2% (1–4) | 2·6 (1·7–3·8) | 7% (4–10) | 3·5 (2·3–5·1) | 9% (6–13) | 4·4 (2·9–6·4) | 11% (7–16) | ||
| Northern Africa and Western Asia | 5·6 (4·8–6·6) | 8% (7–10) | 11·6 (9·2–14·2) | 17% (14–21) | 16·5 (12·4–20·9) | 24% (18–31) | 19·1 (14·3–24·2) | 28% (21–36) | ||
| Northern Africa | 1·5 (1·1–1·8) | 5% (4–6) | 2·4 (2·0–2·8) | 8% (6–9) | 2·6 (2·2–3·0) | 8% (7–9) | 2·8 (2·3–3·2) | 9% (7–10) | ||
| Western Asia | 4·1 (3·3–5·0) | 11% (9–14) | 9·1 (6·7–11·6) | 25% (19–32) | 13·8 (9·8–18·1) | 38% (27–50) | 16·1 (11·5–21·1) | 45% (32–59) | ||
| Central and Southern Asia | 10·2 (7·8–12·9) | 4% (3–5) | 14·2 (11·6–17·1) | 5% (4–6) | 16·4 (13·6–19·5) | 6% (5–7) | 18·7 (15·6–22·0) | 7% (6–8) | ||
| Central Asia | 0·8 (0·6–1·1) | 8% (6–11) | 1·9 (1·6–2·2) | 19% (16–22) | 2·9 (2·4–3·5) | 29% (24–35) | 3·4 (2·8–4·0) | 34% (28–40) | ||
| Southern Asia | 9·4 (7·0–11·9) | 4% (3–5) | 12·3 (9·7–14·9) | 5% (4–6) | 13·5 (10·7–16·3) | 5% (4–6) | 15·3 (12·2–18·3) | 6% (5–7) | ||
| Eastern and South-Eastern Asia | 44·4 (31·4–59·6) | 13% (9–17) | 91·7 (67·2–120·0) | 27% (20–35) | 100·5 (74·6–130·2) | 29% (22–38) | 109·4 (82·0–140·8) | 32% (24–41) | ||
| Eastern Asia | 38·3 (26·2–51·9) | 15% (11–21) | 77·7 (54·9–101·9) | 31% (22–41) | 82·3 (58·3–107·8) | 33% (24–44) | 87·8 (62·5–114·7) | 36% (25–46) | ||
| South-Eastern Asia | 6·1 (4·8–7·6) | 6% (5–8) | 14·0 (11·6–16·6) | 15% (12–18) | 18·2 (15·0–21·6) | 19% (16–23) | 21·6 (17·8–25·6) | 23% (19–27) | ||
| Latin America and Caribbean | 27·2 (23·4–31·1) | 29% (25–34) | 50·0 (42·8–57·5) | 54% (46–62) | 56·5 (48·2–65·1) | 61% (52–71) | 67·8 (57·1–79·1) | 74% (62–86) | ||
| Caribbean | 2·0 (1·6–2·4) | 36% (28–43) | 3·2 (2·6–3·9) | 58% (47–70) | 3·6 (2·9–4·3) | 64% (52–77) | 3·9 (3·2–4·6) | 70% (56–83) | ||
| Central America | 9·7 (7·0–12·5) | 39% (28–50) | 16·6 (11·8–21·7) | 67% (48–87) | 18·8 (13·4–24·7) | 76% (54–99) | 21·4 (15·2–28·0) | 86% (61–100) | ||
| South America | 15·5 (13·4–17·8) | 25% (22–29) | 30·2 (25·7–35·0) | 49% (42–57) | 34·1 (28·8–39·8) | 55% (47–64) | 42·5 (34·8–51·4) | 69% (56–83) | ||
| Oceania* | 32·2k(21·6k–46·3k) | 2% (2–3) | 84·6k(70·1k–100·3k) | 6% (5–7) | 110·9k(92·6k–130·1k) | 8% (7–9) | 147·8k (121·7k–176·5k) | 11% (9–13) | ||
| Melanesia | 16·4k (6·9k–29·7k) | 1% (0·5–2) | 47·8k (38·3k–59·0k) | 4% (3–5) | 65·7k (54·2k–78·0k) | 5% (4–6) | 94·1k (76·1k–114·5k) | 7% (6–9) | ||
| Micronesia | 5·1k (4·1k–6·2k) | 13% (10–15) | 8·5k (7·3k–9·8k) | 21% (18–24) | 10·1k (8·9k–11·5k) | 25% (22–28) | 11·8k (10·3k–13·4k) | 29% (25–33) | ||
| Polynesia | 10·7k (7·7k–13·9k) | 13% (10–17) | 28·2k (20·4k–36·5k) | 35% (25–45) | 35·1k (25·3k–45·7k) | 43% (31–56) | 41·8k (30·1k–54·9k) | 51% (37–67) | ||
| Australia and New Zealand | 1·1 (0·9–1·3) | 27% (21–33) | 2·9 (2·3–3·5) | 71% (56–86) | 3·5 (2·8–4·2) | 85% (67–100) | 3·9 (3·1–4·8) | 96% (76–100) | ||
| Europe and Northern America | 66·7 (57·1–77·2) | 44% (38–51) | 112·3 (96·8–129·2) | 74% (64–85) | 123·5 (106·6–141·6) | 81% (70–93) | 134·9 (116·7–154·4) | 89% (77–100) | ||
| Eastern Europe | 17·6 (13·9–21·8) | 39% (31–49) | 31·2 (24·7–38·3) | 70% (55–86) | 34·7 (27·4–42·6) | 78% (61–95) | 38·2 (30·1–46·9) | 85% (67–100) | ||
| Northern Europe | 4·7 (3·5–6·1) | 34% (25–44) | 9·7 (7·2–12·2) | 70% (52–88) | 11·1 (8·4–14·0) | 80% (61–100) | 12·7 (9·6–15·9) | 91% (69–100) | ||
| Southern Europe | 9·4 (7·4–11·7) | 44% (34–54) | 16·2 (12·9–19·9) | 75% (60–92) | 17·8 (14·2–21·7) | 83% (66–100) | 19·2 (15·4–23·4) | 89% (71–100) | ||
| Western Europe | 12·9 (10·2–15·5) | 52% (41–62) | 19·0 (15·4–22·5) | 76% (62–91) | 21·0 (17·1–24·9) | 85% (69–100) | 23·4 (19·1–27·5) | 94% (77–100) | ||
| Northern America | 22·1 (14·9–29·8) | 47% (32–63) | 36·4 (24·8–48·5) | 77% (53–100) | 39·0 (26·6–52·1) | 83% (57–100) | 41·7 (28·5–55·7) | 89% (61–100) | ||
| Coverage by WHO region | ||||||||||
| African region | 5·0 (3·9–6·4) | 4% (3–6) | 10·4 (8·7–12·2) | 9% (8–11) | 13·6 (11·5–15·9) | 12% (10–14) | 16·8 (14·2–19·6) | 15% (13–17) | ||
| European region | 48·0 (42·2–54·2) | 37% (32–41) | 84·9 (75·4–94·7) | 65% (58–72) | 98·7 (87·9–109·6) | 75% (67–84) | 109·6 (97·8–121·7) | 84% (75–93) | ||
| Eastern Mediterranean region | 6·8 (5·0–9·0) | 8% (6–10) | 9·8 (7·6–12·4) | 11% (9–14) | 11·3 (8·9–14·1) | 13% (10–16) | 13·1 (10·3–16·2) | 15% (12–18) | ||
| Region of the Americas | 49·3 (40·4–59·1) | 35% (29–42) | 86·4 (71·6–102·5) | 62% (51–74) | 95·5 (79·4–113·0) | 69% (57–81) | 109·4 (91·1–129·1) | 79% (65–93) | ||
| South-East Asia region | 8·7 (6·9–10·6) | 3% (3–4) | 15·4 (12·9–18·1) | 6% (5–7) | 19·0 (15·9–22·4) | 7% (6–8) | 22·1 (18·4–25·9) | 8% (7–9) | ||
| Western Pacific region | 41·8 (28·7–56·8) | 15% (10–20) | 85·5 (60·7–113·0) | 30% (21–40) | 91·7 (65·5–120·8) | 32% (23–42) | 98·7 (71·1–129·4) | 35% (25–45) | ||
SDG=UN Sustainable Development Goals.
Excluding Australia and New Zealand.
Figure 2.
Ever in lifetime cervical cancer screening coverage in women aged 30–49 years in 2019 by country
Table 3 illustrates the need to increase screening capacity to reach the cervical cancer elimination target. 5-year screening coverage in women aged 35–49 years was less than 70% in 138 countries; 20 (32%) of 62 high-income countries (excluding Niue and Cook Islands), 43 (72%) of 60 upper-middle-income countries, 44 (94%) of 47 lower-middle-income countries, and all 31 (100%) low-income countries. All but one (North Korea) low-income countries had less than 21% coverage. All but eight (Guinea, Tajikistan, Tanzania, Togo, Rwanda, Afghanistan, Malawi, and North Korea) would need to increase their screening capacity 7 times or more to reach the elimination target. 37 lower-middle-income and 22 upper-middle-income countries would have to double their capacity at least, and only six high-income countries, mainly from the Middle East and North Africa region, would have to increase their capacity by that amount.
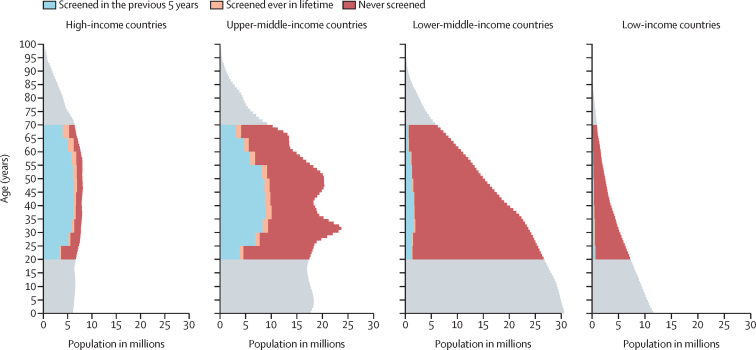
Globally, an estimated 1·6 billion (67%) of 2·3 billion women aged 20–70 years had never been screened for cervical cancer, including 521 million (57%) of 909 million women in upper-middle-income countries, 804 million (92%) of 872 million women in lower-middle-income countries, and 152 million (90%) of 169 million women in low-income countries (figure 3). To assess the potential effect of our imputation system, we did a comprehensive sensitivity analysis of our missing data treatment (appendix 3 pp 27–28, 52–64). Validation tests showed high performance of the model in predicting coverages in the absence of original data. We ran 50 simulations in which we drew a random sample of 200 data points to impute. Results showed a correlation index of 0·89 (appendix 3 p 27). We removed all data for each country individually, imputed the data, and then compared the original with the imputed. We obtained almost identical global and regional coverage estimates, except when the most populated countries were imputed, such as China, India, Brazil, or the USA (appendix 3 p 28). A sensitivity analysis changing the steps of the missing imputation algorithm was done. Global, regional, and income-level estimations differed by 1–2%, except when linear interpolation was not used, in which estimations differed by up to 10% (appendix 3 pp 59–61). Finally, we considered different coverage scenarios ranging from 0–100% for countries without data. Global estimates differed by 4–7% with the most extreme scenarios (appendix 3 pp 62–64).
Figure 3.
Female population pyramid by cervical cancer screening status and income level in 2019
Number of women are from the 2019 UN population estimates. Countries are grouped according to the 2019 World Bank's classification.
Discussion
This study expands knowledge accumulated in WHO STEPS surveillance framework and, to our knowledge provides for the first time global, regional, and national estimates of cervical cancer screening coverage as the baseline for WHO strategy to eliminate cervical cancer as a public health problem. Despite effective screening modalities and WHO recommendations to prioritise cervical cancer screening in women aged 30–49 years, two in three women in this age range have never been screened for cervical cancer, and there are large differences in the rates of screening across global regions. In 2019, 84% (95% CI 73–96) of women aged 30–49 years living in high income countries and 48% (40–57) living in upper-middle-income countries had been screened ever in lifetime, compared with 9% (8–10) of women in this age group in lower-middle-income countries and 11% (10–13%) in low-income countries. Additionally, 19 (61%) of 31 low-income countries and 23 (44%) of 52 lower-middle-income countries still do not have official recommendations for cervical cancer screening.
Increasing cervical screening coverage involves more than merely increasing screening participation. Some countries will need to build their screening infrastructure from scratch; others require expanding the screening capacity up to 70 times (table 3). Challenges and barriers include scaling up laboratory resources, securing trained personnel to ensure adequate diagnosis, managing women who have positive results, establishing monitoring systems and quality assurance measures, and maintaining government implications to support the programme.18 However, in the short term, until HPV-vaccinated cohorts reach older ages (≥25 years), screening and treating cervical lesions is the primary strategy to reduce cervical cancer incidence and mortality.3, 19, 20
Table 3.
Incremental factor and number of women needing to be screened to meet the 70% screening coverage target of women aged 35–45 years, in countries with estimated coverage less than 70%
|
Screening coverage in the previous 5 years |
Incremental needs needed to meet the target of 70% screening coverage |
|||
|---|---|---|---|---|
| % | Number of women aged 35–49 screened | Incremental factor | Minimum number of women aged 35–49 years to be screened in 5 years | |
| Low-income countries | ||||
| Benin | 0·6% | 4702 | 116·7 | 561 803 |
| Somalia | 2% | 17 984 | 35·0 | 605 116 |
| Mozambique | 3% | 60 643 | 23·3 | 1 416 946 |
| Ethiopia | 3% | 259 706 | 23·3 | 5 194 122 |
| Mali | 5% | 62 951 | 14·0 | 851 451 |
| Madagascar | 5% | 99 377 | 14·0 | 1 337 771 |
| Chad | 5% | 47 133 | 14·0 | 626 126 |
| Syria | 5% | 77 844 | 14·0 | 1 124 475 |
| Burkina Faso | 6% | 78 264 | 11·7 | 926 691 |
| Nepal | 6% | 174 543 | 11·7 | 1 987 180 |
| Central African Republic | 7% | 19 066 | 10·0 | 197 315 |
| Yemen | 8% | 164 759 | 8·8 | 1 369 503 |
| South Sudan | 8% | 56 591 | 8·8 | 514 427 |
| DR Congo | 8% | 403 613 | 8·8 | 3 766 799 |
| Niger | 8% | 103 307 | 8·8 | 904 329 |
| Burundi | 8% | 56 004 | 8·8 | 474 662 |
| Eritrea | 8% | 19 604 | 8·8 | 163 009 |
| Uganda | 8% | 220 445 | 8·8 | 1 872 034 |
| Haiti | 9% | 84 785 | 7·8 | 675 706 |
| Guinea-Bissau | 9% | 12 110 | 7·8 | 95 778 |
| Sierra Leone | 9% | 47 670 | 7·8 | 379 398 |
| The Gambia | 9% | 13 204 | 7·8 | 104 927 |
| Liberia | 10% | 34 050 | 7·0 | 250 258 |
| Tajikistan | 11% | 82 788 | 6·4 | 506 381 |
| Guinea | 11% | 88 825 | 6·4 | 586 307 |
| Togo | 11% | 64 484 | 6·4 | 424 696 |
| Tanzania | 11% | 435 839 | 6·4 | 2 734 106 |
| Rwanda | 12% | 117 537 | 5·8 | 666 629 |
| Malawi | 15% | 183 682 | 4·7 | 839 585 |
| Afghanistan | 15% | 349 264 | 4·7 | 1 621 711 |
| North Korea | 36% | 994 884 | 1·9 | 1 949 250 |
| Lower-middle-income countries | ||||
| Pakistan | 1% | 219 239 | 70·0 | 11 699 835 |
| Sudan | 1% | 40 810 | 70·0 | 2 148 730 |
| Timor-Leste | 1% | 891 | 70·0 | 57 262 |
| Côte d'Ivoire | 2% | 29 938 | 35·5 | 1 182 076 |
| Philippines | 2% | 167 014 | 35·5 | 6 692 791 |
| Egypt | 2% | 175 714 | 35·0 | 6 091 946 |
| India | 2% | 3 181 677 | 35·0 | 90 283 238 |
| Papua New Guinea | 3% | 24 360 | 23·3 | 509 639 |
| Ghana | 3% | 77 809 | 23·3 | 1 670 438 |
| Laos | 4% | 22 622 | 17·5 | 430 590 |
| Myanmar | 4% | 208 598 | 17·5 | 4 040 871 |
| Mauritania | 6% | 18 875 | 11·7 | 237 486 |
| Djibouti | 6% | 5 054 | 11·7 | 63 280 |
| Cameroon | 6% | 100 952 | 11·7 | 1 236 689 |
| Bangladesh | 6% | 1 017 679 | 11·7 | 11 524 547 |
| Senegal | 9% | 105 150 | 7·8 | 836 781 |
| Vanuatu | 9% | 1959 | 7·8 | 15 958 |
| Indonesia | 10% | 2 790 965 | 7·0 | 20 350 786 |
| Nigeria | 11% | 1 534 149 | 6·4 | 9 524 026 |
| Comoros | 11% | 6 733 | 6·4 | 43 907 |
| Federated States of Micronesia | 11% | 1 021 | 6·4 | 6291 |
| Uzbekistan | 12% | 373 145 | 5·8 | 2 229 676 |
| Solomon Islands | 13% | 6 875 | 5·4 | 37 850 |
| Kenya | 13% | 544 403 | 5·4 | 2 841 315 |
| Cambodia | 14% | 209 838 | 5·0 | 1 071 677 |
| Congo | 15% | 61 477 | 4·7 | 295 344 |
| Kiribati | 15% | 1 485 | 4·7 | 6725 |
| Lesotho | 15% | 25 352 | 4·7 | 118 211 |
| Palestine | 15% | 54 538 | 4·7 | 247 233 |
| Eswatini | 17% | 16 087 | 4·1 | 67 596 |
| Zambia | 17% | 201 960 | 4·1 | 822 093 |
| Morocco | 18% | 672 050 | 3·9 | 2 600 807 |
| Tunisia | 19% | 248 244 | 3·7 | 902 657 |
| Angola | 20% | 401 694 | 3·5 | 1 409 907 |
| Zimbabwe | 20% | 227 139 | 3·5 | 786 877 |
| São Tomé and Príncipe | 21% | 3 219 | 3·3 | 10 660 |
| Vietnam | 26% | 2 759 030 | 2·7 | 7 329 834 |
| Kyrgyzstan | 32% | 177 116 | 2·2 | 388 056 |
| Bhutan | 41% | 26 982 | 1·7 | 46 329 |
| Cape Verde | 43% | 21 599 | 1·6 | 34 821 |
| Bolivia | 52% | 506 674 | 1·3 | 686 445 |
| Mongolia | 52% | 174 376 | 1·3 | 233 462 |
| Ukraine | 57% | 2 898 181 | 1·2 | 3 535 175 |
| Honduras | 67% | 572 506 | 1·0 | 598 034 |
| Upper-middle-income countries | ||||
| Tonga | 5% | 470 | 14·0 | 6042 |
| Maldives | 6% | 2309 | 11·7 | 28 989 |
| Gabon | 9% | 15 921 | 7·8 | 121 377 |
| Azerbaijan | 11% | 112 818 | 6·4 | 733 620 |
| Iraq | 11% | 344 551 | 6·4 | 2 158 960 |
| Jordan | 12% | 107 345 | 5·8 | 618 538 |
| Equatorial Guinea | 12% | 10 152 | 5·8 | 57 945 |
| Algeria | 15% | 669 494 | 4·7 | 3 046 145 |
| Fiji | 16% | 13 752 | 4·4 | 58 480 |
| Lebanon | 18% | 121 892 | 3·9 | 479 593 |
| Libya | 19% | 148 869 | 3·7 | 550 198 |
| Guyana | 19% | 13 696 | 3·7 | 49 534 |
| Georgia | 21% | 86 137 | 3·3 | 283 774 |
| Tuvalu | 23% | 191 | 3·0 | 585 |
| Mauritius | 26% | 35 152 | 2·7 | 93 443 |
| Sri Lanka | 30% | 684 872 | 2·3 | 1 586 565 |
| Bosnia and Herzegovina | 30% | 101 810 | 2·3 | 238 539 |
| Samoa | 30% | 4 399 | 2·3 | 10 118 |
| Namibia | 31% | 62 153 | 2·3 | 139 412 |
| Suriname | 33% | 18 268 | 2·1 | 39 340 |
| China | 33% | 52 106 588 | 2·1 | 109 290 354 |
| Armenia | 37% | 114 682 | 1·9 | 218 739 |
| Marshall Islands | 37% | 2 464 | 1·9 | 4611 |
| Montenegro | 39% | 25 387 | 1·8 | 45 602 |
| Romania | 39% | 830 959 | 1·8 | 1 478 369 |
| Botswana | 40% | 89 867 | 1·8 | 157 723 |
| Brazil | 42% | 9 854 929 | 1·7 | 16 397 169 |
| South Africa | 44% | 2 528 569 | 1·6 | 4 041 092 |
| Venezuela | 46% | 1 304 621 | 1·5 | 1 992 926 |
| Iran | 46% | 4 435 063 | 1·5 | 6 683 514 |
| Belize | 49% | 17 814 | 1·4 | 25 215 |
| Nauru | 51% | 488 | 1·4 | 674 |
| American Samoa | 51% | 2 170 | 1·4 | 2926 |
| Malaysia | 52% | 1 614 886 | 1·3 | 2 169 340 |
| Turkmenistan | 55% | 307 277 | 1·3 | 392 533 |
| Ecuador | 56% | 914 438 | 1·3 | 1 146 263 |
| Albania | 58% | 142 405 | 1·2 | 172 621 |
| Kazakhstan | 59% | 1 063 767 | 1·2 | 1 262 097 |
| Guatemala | 60% | 888 608 | 1·2 | 1 031 414 |
| Serbia | 66% | 603 785 | 1·1 | 639 744 |
| North Macedonia | 67% | 150 621 | 1·0 | 158 142 |
| Thailand | 68% | 5 546 781 | 1·0 | 5 743 708 |
| Bulgaria | 68% | 512 875 | 1·0 | 524 291 |
| High-income countries | ||||
| Oman | 8% | 26 395 | 8·8 | 234 032 |
| Saudi Arabia | 15% | 514 562 | 4·7 | 2 447 166 |
| Kuwait | 17% | 88 668 | 4·1 | 362 023 |
| Seychelles | 27% | 2 801 | 2·6 | 7253 |
| Bahrain | 28% | 37 223 | 2·5 | 92 957 |
| Brunei | 32% | 15 777 | 2·2 | 34 952 |
| United Arab Emirates | 36% | 285 021 | 1·9 | 547 515 |
| Qatar | 41% | 74 491 | 1·7 | 125 987 |
| Hong Kong | 44% | 447 943 | 1·6 | 712 672 |
| Estonia | 57% | 75 790 | 1·2 | 93 810 |
| Latvia | 58% | 110 560 | 1·2 | 133 858 |
| Trinidad and Tobago | 60% | 92 825 | 1·2 | 109 103 |
| French Polynesia | 60% | 17 920 | 1·2 | 20 942 |
| Greenland | 60% | 2 674 | 1·2 | 3100 |
| Israel | 61% | 485 141 | 1·1 | 554 130 |
| Japan | 62% | 8 117 259 | 1·1 | 9 128 555 |
| San Marino | 66% | 2 451 | 1·1 | 2617 |
| Bahamas | 66% | 28 970 | 1·1 | 30 724 |
| South Korea | 68% | 4 045 242 | 1·0 | 4 154 903 |
| Singapore | 68% | 467 024 | 1·0 | 478 694 |
Niue and Cook Islands not included because 2019 UN population data were not available. The number of women aged 35–49 years screened in the last 5 years and the minimum number of women aged 35–49 years to be screened in 5 years to meet the 70% target were calculated using the 2019 UN population estimates.17
Coverage is not always associated with effective screening. Testing is imperative, but for screening to be effective, it needs to be followed by adequate diagnosis, follow-up, and management of positive results. Another crucial factor is the tests used; HPV-based screening adoption outside high-income countries has been constrained by economic factors and competing health priorities. In central and eastern Europe, screening is cytology-based except in Montenegro and Albania, where HPV-based screening has recently been introduced. Despite high coverage, central and eastern Europe have the highest cervical cancer burden in Europe (age-standardised incidence 14·5 per 100 000 women).21 Screening is mainly opportunistic in countries in central and eastern Europe, with many tests done outside the organised programme and a high proportion of cervical cancers diagnosed at late stages, highlighting the urgent need to scale up from opportunistic to organised, population-based HPV screening programmes.22, 23 Other European regions with established and successful prevention programmes are already moving towards population-based HPV screening.7 Most Latin American countries also suffer from a high burden of cervical cancer (age-standardised incidence >13·5 per 100 000 women) despite long-term screening programmes and moderate-to-high screening coverage.21 Although the region has a wide range of testing strategies, in areas in which screening coverage is not low, screening quality is poor and the adherence to follow-up and management of positive results is difficult because of multiple, context-dependent issues (eg, accessibility and poverty).24, 25
Our estimates also show that in some countries, mainly in Latin America and Europe, the screening coverage in 2019 was greater than 50%. Most of these countries have opportunistic screening, and official recommendations still include annual cytology. Countries in the Middle East and North Africa region have the lowest cervical cancer rates and low screening coverages; still, implementation of cervical cancer screening programmes should be prioritised in these countries, not only because of expected increases in cervical cancer burden secondary to changes in sexual behaviour, but also because most cervical cancer cases are diagnosed at late stages, and therefore followed by low survival and high mortality rates.26
This work supports the findings of a recent increase in screen-and-treat strategies, mainly in lower-middle-income and low-income countries that use VIA-based screening. VIA was proposed as a low-cost, easy-to-implement test, but the poor repeatability and low accuracy to detect precancer prevent its recommendation as a primary screening test.6, 7 Unfortunately, the switch to HPV testing in these settings remains unaffordable and unsustainable in the absence of funding. Only eight lower-middle-income and low-income countries (El Salvador, Guatemala, Haiti, Honduras, Kenya, Myanmar, Rwanda, and Uganda) recommend HPV screening. Accurate, robust, user-friendly, and affordable assays are a prerequisite for successful implementation of HPV-based screening.11
The COVID-19 pandemic might have worsened existing inequalities in screen coverage; it has temporarily disrupted cervical cancer prevention activities in many countries, depleted resources, and, consequently, halted the introduction of HPV testing in the short and medium terms. However, the pandemic's negative effect might also create opportunities for more efficient prevention, including the extension of HPV testing with self-sampling or the introduction of innovative digital, mobile, and artificial intelligence technologies to assist in the delivery of cervical screening.27, 28, 29, 30, 31
Our estimates show higher disparities in coverage than a previous analysis based on 2003 WHO population-based surveys.32 Our coverage estimates are generally lower than those observed in a 2020 study on the analysis of surveys following the WHO STEPwise approach, carried out from 2005–18.33 There are several possible explanations for these discrepancies. Although in the 2020 study33 the analysis was based on 55 LMICs, we included all countries and territories using a methodology providing conservative coverage estimates for countries with no official screening coverage data. Most of these countries are African countries without screening recommendations and registries, and consequently have lower expected coverages than other countries. Further, in addition to survey data, our data sources included administrative data that might have decreased coverage estimates. Further, we added additional new data. Finally, regarding India specifically, a country that substantially contributed to our estimates due to its large population, ever-in-lifetime coverage was reported to be lower than 3% for women aged 30–49 years in the 2017–18 NCD monitoring survey compared with the 25–30% estimate from the 2015–16 DHS survey.33 This discrepancy could be attributed to question bias; the 2015–16 DHS survey question was “Have you ever undergone a cervix examination?”, which does not necessarily indicate that the woman has received a screening test during the pelvic examination.
There are some limitations to our methodological approach. First, we might have failed to identify relevant data published in local languages or data that was not yet published. However, the large number of sources assessed, the technical assistance of professional translators and local experts, and WHO country consultation ensure that the data collection process was systematic and thorough. Second, coverage data derived from surveys or administrative data are subject to their own biases. Administrative data are subject to numerator and denominator biases (eg, underreporting or no reporting of tests done outside the organised programme and collection of numerators and denominators from different sources), might be difficult to access, and often do not document the quality of the data. Survey data allow for estimating screening coverage in countries without screening registries and include screening tests done outside the organised screening programme. The main disadvantage of screening data is that results could be inappropriately generalised beyond the survey population.34 If more than one source per country was identified, we prioritised administrative data in countries with organised and accurate programmes. In contrast, we prioritised survey data in countries with opportunistic screening or when screening outside the organised programme was frequent and not registered centrally. Third, the main challenge was the treatment of missing data, for which we applied multiple methodologies (appendix 3 pp 8–14, 50–51). Only six countries had complete data for all ages and screening intervals, and in the 38 countries without information, coverage was estimated through data modelling from other countries. A comprehensive validation analysis to test the treatment of the missing data supported the model's high performance in predicting coverages in the absence of original data. Additionally, the effect of political instability, wars, immigration, or any other factor that could affect screening coverage has only been considered by including a covariate in the predictive mean matching model that categorises countries in fragile and conflict-affected situations. Finally, our coverage estimates refer to 2019 and therefore do not reflect recent changes in cervical screening programmes. Our estimates represent the first edition of WHO cervical cancer screening estimates. Future updates are planned to monitor and evaluate the effect of the interventions and activities implemented as part of the cervical cancer elimination strategy. In conclusion, this work presents the first global estimates of cervical cancer screening coverage, answering essential questions for global health governance, and monitoring the cervical cancer elimination campaign.
Our results show that most adult women in the world have never had the opportunity to be screened for cervical cancer and about one-third of women aged 30–49 years have been screened ever in their lifetime, highlighting that there is still a long way to reaching the WHO target of screening 70% of women twice during the ages of 35–45 years with a high-performance test, especially in LMICs. To eliminate cervical cancer as a substantial public health problem, it is essential to improve access to cervical cancer prevention and treatment worldwide, particularly in low-income and lower-middle-income countries, which often have coverage levels below 10% and are at the highest burden of disease.
Data sharing
The study's findings are supported by data available in public online repositories and data available upon request from the data provider. A detailed table of data sources can be found in the appendix. Produced estimates are published on WHO Global Health Observatory Data Repository (https://www.who.int/data/gho). Upon request, computer code is available in the IDIBELL repository (https://repository.idibell.cat).
Declaration of interests
The Cancer Epidemiology Research Programme (with which LB, BS, ER, and LA are affiliated) has received sponsorship for grants from Merck Sharp & Dohme and HPV test kits at no cost from Roche for research purposes. All other authors report no competing interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of WHO. Authors who are identified as personnel of the International Agency for Research on Cancer, WHO, are responsible alone for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer, WHO.
Acknowledgments
Acknowledgments
This work was supported by a grant from the Instituto de Salud Carlos III (Spanish Government) through the projects PI18/01137 (co-funded by European Regional Development Fund, a way to build Europe) and CIBERESP CB06/02/0073, and the Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia grants to support the activities of research groups (SGR 2017–2019; grant numbers 2017SGR1718 and 2017SGR1085). The project has also received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement number 847845. We thank the CERCA Programme and Generalitat de Catalunya for institutional support.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
LB conceptualised the project. LB, BS, and ER designed the study and planned the analysis. BS designed the data-extraction form, did the literature search, and collected the data. RM contributed to the collection of data from the Latin American region; MP contributed to the collection of data from the central and eastern Europe region. ER did the formal statistical analysis. LB and BS supervised the statistical analyses. LB, BS, LMR, and MC did WHO country consultation. BS, ER, RM, MP, MC, LMR, and NB cross-checked data. LB, BS, and ER prepared the tables and figures and wrote the manuscript. All authors contributed to data interpretation, critically revised subsequent drafts, and read and approved the submitted version. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccarella S, Lortet-Tieulent J, Saracci R, Conway DI, Straif K, Wild CP. International Agency for Research on Cancer; Lyon, France: 2019. Reducing social inequalities in cancer: evidence and priorities for research. [PubMed] [Google Scholar]
- 3.WHO Global strategy to accelerate the elimination of cervical cancer as a public health problem. Nov 17, 2020. https://www.who.int/publications-detail-redirect/9789240014107
- 4.Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 6.Bouvard V, Wentzensen N, Mackie A, et al. The IARC perspective on cervical cancer screening. N Engl J Med. 2021;385:1908–1918. doi: 10.1056/NEJMsr2030640. [DOI] [PubMed] [Google Scholar]
- 7.IARC Cervical cancer screening. IARC Handbooks of Cancer Prevention Volume 18. 2022. https://publications.iarc.fr/604
- 8.WHO Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. July 6, 2021. https://www.who.int/publications/i/item/9789240030824
- 9.Maver PJ, Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2020;26:579–583. doi: 10.1016/j.cmi.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Gravitt PE, Silver MI, Hussey HM, et al. Achieving equity in cervical cancer screening in low- and middle-income countries (LMICs): Strengthening health systems using a systems thinking approach. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2020.106322. [DOI] [PubMed] [Google Scholar]
- 11.Arbyn M, Simon M, Peeters E, et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin Microbiol Infect. 2021;27:1083–1095. doi: 10.1016/j.cmi.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Poljak M, Oštrbenk Valenčak A, Gimpelj Domjanič G, Xu L, Arbyn M. Commercially available molecular tests for human papillomaviruses: a global overview. Clin Microbiol Infect. 2020;26:1144–1150. doi: 10.1016/j.cmi.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 13.WHO NCD Microdata repository. https://extranet.who.int/ncdsmicrodata/index.php/home#index.html
- 14.WHO Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2019 global survey. March 17, 2020. https://www.who.int/publications-detail-redirect/ncd-ccs-2019
- 15.Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388:e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 16.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: a language and environment for statistical computing. [Google Scholar]
- 17.United Nations World population prospects. 2019. https://population.un.org/wpp/Download/Standard/Population/
- 18.Holme F, Kapambwe S, Nessa A, Basu P, Murillo R, Jeronimo J. Scaling up proven innovative cervical cancer screening strategies: challenges and opportunities in implementation at the population level in low- and lower-middle-income countries. Int J Gynaecol Obstet. 2017;138(suppl 1):63–68. doi: 10.1002/ijgo.12185. [DOI] [PubMed] [Google Scholar]
- 19.Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:575–590. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Cancer today. https://gco.iarc.fr/today
- 22.Poljak M, Seme K, Maver PJ, et al. Human papillomavirus prevalence and type-distribution, crvical cancer screening practices and current status of vaccination implementation in central and eastern Europe. Vaccine. 2013;31(suppl 7):H59–H70. doi: 10.1016/j.vaccine.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Ryzhov A, Corbex M, Piñeros M, et al. Comparison of breast cancer and cervical cancer stage distributions in ten newly independent states of the former Soviet Union: a population-based study. Lancet Oncol. 2021;22:361–369. doi: 10.1016/S1470-2045(20)30674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14:391–436. doi: 10.1016/S1470-2045(13)70048-2. [DOI] [PubMed] [Google Scholar]
- 25.Lopez MS, Baker ES, Maza M, et al. Cervical cancer prevention and treatment in Latin America. J Surg Oncol. 2017;115:615–618. doi: 10.1002/jso.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosch FX, Seoud M, Garland SM, Albero G, Lamontagne DS. Comprehensive control of HPV infections and related diseases in the extended Middle East and north Africa Region. Vaccine. 2013;31(suppl 6):G1–82. doi: 10.1016/j.vaccine.2012.06.098. [DOI] [PubMed] [Google Scholar]
- 27.Woo YL, Gravitt P, Khor SK, Ng CW, Saville M. Accelerating action on cervical screening in lower- and middle-income countries (LMICs) post COVID-19 era. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2020.106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Rapid assessment of service delivery for NCDs during the COVID-19 pandemic. May 29, 2020. https://www.who.int/publications/m/item/rapid-assessment-of-service-delivery-for-ncds-during-the-covid-19-pandemic
- 29.Rabeneck L, Saraiya M. COVID-19 and the disruption of cancer screening programs: key lessons for the recovery. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentzensen N, Clarke MA, Perkins RB. Impact of COVID-19 on cervical cancer screening: challenges and opportunities to improving resilience and reduce disparities. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbyn M, Bruni L, Kelly D, et al. Tackling cervical cancer in Europe amidst the COVID-19 pandemic. Lancet Public Health. 2020;5:e425. doi: 10.1016/S2468-2667(20)30122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemp JM, De Neve J-W, Bussmann H, et al. Lifetime prevalence of cervical cancer screening in 55 low- and middle-income countries. JAMA. 2020;324:1532–1542. doi: 10.1001/jama.2020.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard M, Agarwal G, Lytwyn A. Accuracy of self-reports of Pap and mammography screening compared to medical record: a meta-analysis. Cancer Causes Control. 2009;20:1–13. doi: 10.1007/s10552-008-9228-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study's findings are supported by data available in public online repositories and data available upon request from the data provider. A detailed table of data sources can be found in the appendix. Produced estimates are published on WHO Global Health Observatory Data Repository (https://www.who.int/data/gho). Upon request, computer code is available in the IDIBELL repository (https://repository.idibell.cat).