Despite the absence of a licensed TB vaccine that affords better protective efficacy than the century-old attenuated live vaccine (Mycobacterium bovis bacille Calmette-Guérin),1 the global incidence of TB has remained fairly stable (at around 130/100,000 population) over the past decade.2,3 Nevertheless, the goal of the End TB Strategy is to reduce the annual number of TB-related deaths from 1.8 million to 450,000 between 2015 and 2025.4 It is extremely worrying that 1.5 million people died from TB in 2020, and that trends since the onset of the COVID-19 pandemic suggest that TB mortality is increasing.3
Prevention is a critical component of global TB control. Although infection control measures can decrease airborne transmission of M. tuberculosis, these measures are often difficult to implement and to sustain in high-burden countries with crowded living conditions and many people living in poverty. In contrast, evidence suggests that TB preventive therapy can be effectively delivered in high-burden settings.5 To circumvent the expense of testing individuals to detect an M. tuberculosis-specific immune response in the absence of active disease (Mtb-sir-nodis),6 a more precise acronym than latent infection with M. tuberculosis (LTBI),7 TB control programs provide preventive therapy to children and adults who are close contacts of patients with pulmonary TB and people living with HIV. Other populations that can benefit from TB preventive therapy include immunocompromised individuals (e.g., those who receive treatments with tumor necrosis factor [TNF]-antagonists or Janus-kinase inhibitors), and recent migrants from high-incidence countries to low-incidence settings. Although the overall numbers of patients belonging to these groups among the total burden of TB worldwide is less substantial, these populations are often tested for Mtb-sir-nodis.
After witnessing the effect that isoniazid (INH) had on treatment outcomes for children with TB, Dr E Lincoln (Head of the Children’s Chest Clinic at Bellevue Hospital in New York from 1922 to 1956), noted in 1954: “The use of isoniazid will probably have to be considered for every child with primary TB and probably also for children with known recent conversion of the tuberculin skin test even when roentgenograms are normal. The duration for therapy would be for a year, in order to cover the period during which meningitis would be most likely to develop”.8 In the years following the sentinel work of Dr Lincoln, the duration of preventive therapy steadily decreased.9,10 In 1965, the American Thoracic Society first recommended 12–18 months of INH monotherapy for TB prevention.11 Subsequently, the recommended duration of INH preventive therapy decreased to 12 months in 1974,12 and to 6–12 months in 1986.13 Over the past 2 decades, preventive treatment options have expanded to include 6–9 months INH monotherapy; or 4 months of rifampicin (RIF) monotherapy in 2000;14 3 months of combined, weekly INH and rifapentine (RPT) in 2011;15 3 months of combined, daily INH and RIF;16 and finally in 2020, one month of combined, daily INH and RPT (Figure).17 This decrease in time needed for the comparative regimens to prevent the development of TB is likely a result of the different pharmacological actions of the drugs included in the respective preventive regimens. As INH inhibits cell wall synthesis, it is only effective against dividing cells. During the initial phase of TB treatment, when many bacteria are dividing, INH is highly effective at reducing the number of viable bacilli. However, at later stages of TB treatment, or in the treatment of Mtb-sir-nodis, the number of replicating bacteria is low and the number of viable bacteria that are in a non-replicating state is relatively high; thus, INH has limited action during the continuation phase of TB treatment or against Mtb-sir-nodis. It is unlikely that the addition of other drugs that inhibit cell wall synthesis or cell division will be able to substantially shorten the duration of preventive therapy while maintaining high efficacy.
Figure.
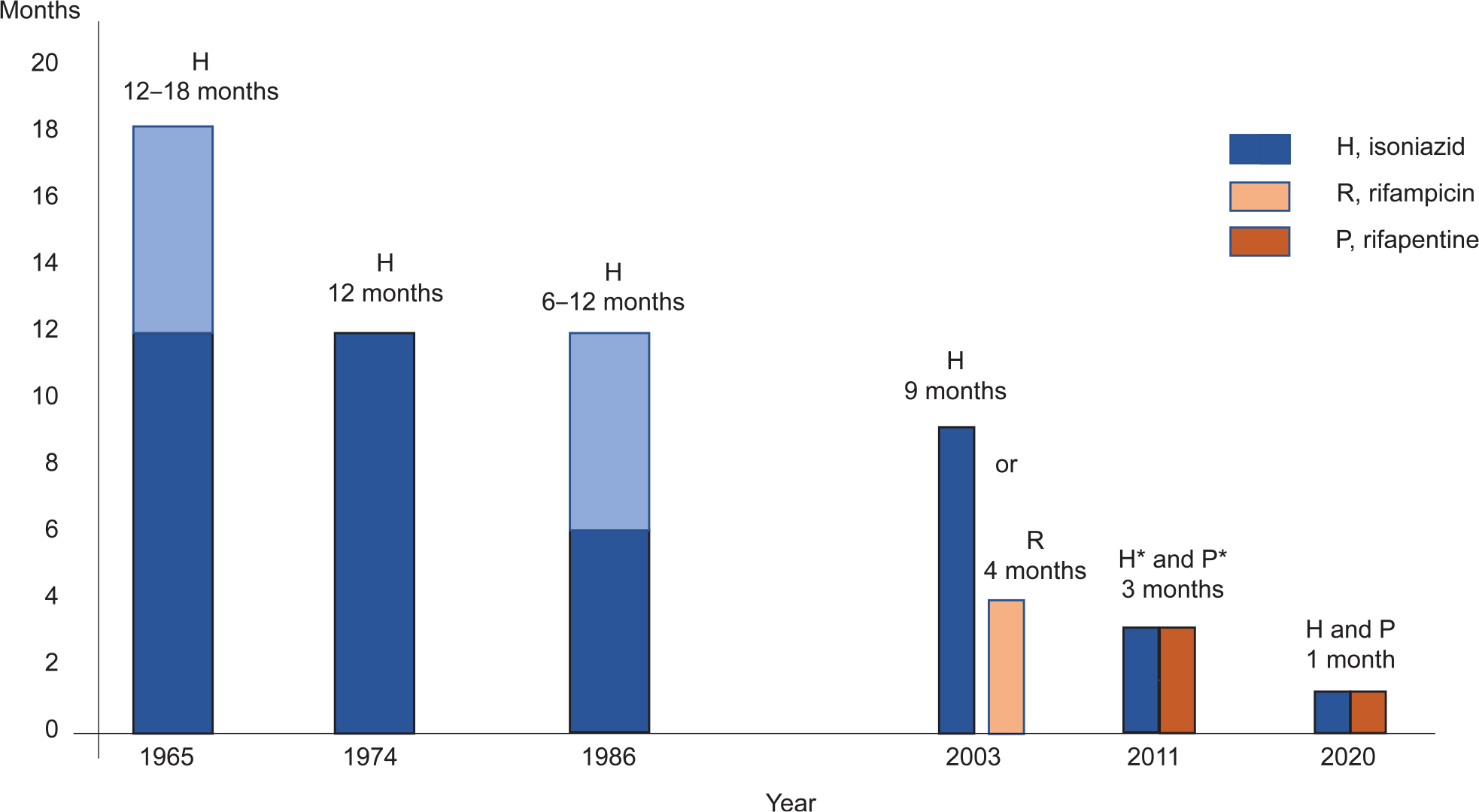
Changes in the recommendation for the duration of preventive therapy between 1965 and 2020. Daily intake of medicines with the exception of *once weekly therapy.
However, rifamycins inhibit mycobacterial RNApolymerase and are active when bacteria are not replicating. Not surprisingly, shorter durations of daily treatment with rifamycins can achieve the same protective efficacy in the treatment of Mtb-sir-nodis as longer duration treatment with INH. At present, it is unclear if other medicines that act independently of cell division, such as inhibitors of RNA-protein translation (e.g., oxazolidinones or aminoglycosides) or respiratory chain blockers (e.g., bedaquiline or clofazimine), are suitable for inclusion in TB preventive therapy regimens. In two recent large multicenter open-label trials, one in children <18 years of age,18 and another in adults with Mtb-sir-nodis,19 treatment with 4 months of RIF was not inferior to treatment with 9 months of INH. In both studies, the RIF regimen was associated with higher completion rates than the INH regimen. In this issue of the Journal, new evidence is provided by a study conducted between 2011 and 2017 in children and adults in Indonesia,20 where the current estimated TB incidence is 316 (288–345)/100,000 population.3 In 156 children and 860 adults with Mtb-sir-nodis, participants were randomized to receive either 4 months of daily RIF or 9 months of daily INH for the prevention of TB. The shorter preventive treatment regimen resulted in a higher rate of treatment completion, lower rates of Grade 3–5 adverse events and was less expensive. In contrast to previous studies, treatment with RIF over 4 months was more effective at TB prevention than treatment with INH over 9 months. Overall, the number of individuals who developed TB (one in the RIF arm and four in the INH arm) was low; hence, it must be assumed that the difference in efficacy of the two regimens are adherence-related.
In most high-burden settings, 6 months’ duration of anti-TB therapy is the current standard for the treatment of drug-susceptible TB. In addition, the WHO currently recommends an additional 6–9 months of INH preventive therapy after completion of anti-TB therapy.17 In our view, a rifamycin-based regimen of shorter duration is preferable if accessible and risk of adverse events or drug–drug interactions are minimal. At present, the WHO suggests a variety of options for the prevention of TB in settings where incidence of multidrug-resistant TB (MDR-TB) is low (Table). Evidence increasingly demonstrates that the duration of preventive therapy can be dramatically reduced with the addition of a long acting rifamycin, RPT, while maintaining efficacy. One month of daily therapy with RPT + INH (28 doses) or 3 months of once weekly therapy with RPT + INH (12 doses) are the most attractive options. Although the evidence demonstrating the benefit of the shortest preventive therapy option (i.e., 1-month daily INH and RPT), has been primarily derived from studies of people living with HIV,21 the WHO has extended this recommendation also to people free of HIV on the basis of plausibility. Unfortunately, RPT is not available in many countries. Where RPT is not available, daily treatment with RIF in monotherapy for 4 months or with RIF + INH for 3 months are the next best options, when drug resistance is not a concern.17 Due to the wide heterogeneity of data, a comprehensive appraisal of the cost-effectiveness of Mtb-sir-nodis management stratified by population group, and type of regimen or intervention is still not available.17 Nevertheless, despite ongoing challenges to improve universal access to RPT, and a lack of robust cost-effectiveness data to guide population specific preventive therapy guidelines,17 the era of INH monotherapy as the preferred option for the prevention of TB should be over.
Table.
Recommended therapies and dosages for TB preventive therapies, adapted from17
| Regimen | Dose by weight band | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 month of daily INH + RPT (1HP): 28 doses | Age ≥13 years (regardless of weight band) | |||||
| INH 300 mg/day | ||||||
| RPT 600 mg/day | ||||||
| 3 months of weekly INH + RPT (3HP): 12 doses | Age 2–14 years | |||||
| Medicines/formulations | 10–15 kg | 16–23 kg | 24–30 kg | 31–34 kg | >34 kg | |
| INH 100 mg* | 3 | 5 | 6 | 7 | 7 | |
| RPT 150 mg | 2 | 3 | 4 | 5 | 5 | |
| Age >14 years | ||||||
| Medicines/formulations | 30–35 kg | 36–45 kg | 46–55 kg | 56–70 kg | >70 kg | |
| INH 300 mg | 3 | 3 | 3 | 3 | 3 | |
| RPT 150 mg | 6 | 6 | 6 | 6 | 6 | |
| *300 mg can be used to reduce the pill burden | ||||||
| 3 months of daily INH + RIF (3HR): ∼90 doses | INH: | |||||
| Age ≥10 years: 5 mg/kg/day | ||||||
| Age <10 years: 10 mg/kg/day (range 7–15 mg) | ||||||
| RIF: | ||||||
| Age ≥10 years: 10 mg/kg/day | ||||||
| Age <10 years: 15 mg/kg/day (range 10–20 mg) | ||||||
| 4 months of daily RIF (4R): ∼120 doses | Age ≥10 years: 10 mg/kg/day | |||||
| Age <10 years: 15 mg/kg/day (range 10–20 mg) | ||||||
| 6 or 9 months of daily INH therapy (6H, 9H): ∼180 or 270 doses | Age ≥10 years: 5 mg/kg/day | |||||
| Age <10 years: 10 mg/kg/day (range 7–15 mg) | ||||||
INH = isoniazid; RPT = rifapentine; RIF = rifampicin.
Preventive therapy in 2022 is still far from perfect. Important questions remain, such as the ideal preventive therapy regimen for individuals with Mtb-sir-nodis in settings with a high burden of MDR-TB. In these settings, (including many countries of the former Soviet Union), clinical trials are urgently needed to evaluate the effectiveness of novel drugs for the prevention of TB. In order to have an impact on the global burden of TB, short-duration and effective preventive treatments must be widely implemented. Substantial achievements have been made since the days of Dr Lincoln’s first insights, but we should not be satisfied yet. It would be wonderful if TB could be effectively prevented with just a few days of treatment, with minimal to no adverse events. In this context, a quote by Bo Jackson should influence all our work on TB:
“Set your goals high, and don’t stop till you get there!”
References
- 1.Lange C, et al. 100 years of Mycobacterium bovis bacille Calmette-Guerin. Lancet Infect Dis 2021. Sep 7; doi: 10.1016/S1473-3099(21)00403-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2011. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 3.World Health Organization. Global tuberculosis report, 2021. Geneva, Switzerland: WHO, 2021. [Google Scholar]
- 4.World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 5.Adjobimey M, et al. Implementation of isoniazid preventive therapy in children aged under 5 years exposed to tuberculosis in Benin. Int J Tuberc Lung Dis 2016; 20(8): 1055–1059. [DOI] [PubMed] [Google Scholar]
- 6.Mandalakas AM, et al. Tuberculosis prevention in children: a prospective community-based study in South Africa. Eur Respir J. 2021. Apr 22;57(4):2003028. doi: 10.1183/13993003.03028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack U, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009; 33: 956–973. [DOI] [PubMed] [Google Scholar]
- 8.Lincoln EM. The effect of antimicrobial therapy on the prognosis of primary tuberculosis in children. Am Rev Tuberc 1954; 69: 682–689. [DOI] [PubMed] [Google Scholar]
- 9.Salazar-Austin N, et al. Seventy years of tuberculosis prevention: efficacy, effectiveness, toxicity, durability, and duration. Am J Epidemiol 2019; 188(12): 2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis 1999; 3(10): 847–850. [PubMed] [Google Scholar]
- 11.Runyon EH. Preventive Treatment in tuberculosis: a statement by the Committee on Therapy, American Thoracic Society. Am Rev Respir Dis 1965; 91: 297–298. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Preventive therapy of tuberculous infection. Am Rev Respir Dis 1974; 110(3): 371–374. [PubMed] [Google Scholar]
- 13.American Thoracic Society & Centers for Disease Control. Medical Section of the American Lung Association: treatment of tuberculosis and tuberculosis infection in adults and children. Am Rev Respir Dis 1986; 134(2): 355–363. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society (ATS) & the Centers for Disease Control and Prevention (CDC). Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000; 161(4 Pt 2): S221–S247. [DOI] [PubMed] [Google Scholar]
- 15.Sterling TR, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365(23): 2155–2166. [DOI] [PubMed] [Google Scholar]
- 16.Spyridis NP, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis 2007; 45(6): 715–722. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva Switzerland:WHO, 2020. [PubMed] [Google Scholar]
- 18.Diallo T, et al. Safety and side effects of rifampin versus isoniazid in children. N Engl J Med 2018; 379(5): 454–463. [DOI] [PubMed] [Google Scholar]
- 19.Menzies D, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018; 379(5): 440–453. [DOI] [PubMed] [Google Scholar]
- 20.Apriani L, et al. Experience with 4 months rifampin and 9 months isoniazid for TB preventive treatment in Indonesia. Int J Tuberc Lung Dis 2022; 26: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swindells S, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019; 380(11): 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]


