Abstract
Objective
Evidence on simultaneous changes in body mass index (BMI) and cognitive decline, which better reflect the natural course of both health phenomena, is limited.
Methods
We capitalized on longitudinal data from 15,977 initially non‐demented elderly from the Alzheimer's Disease Centers followed for 5 years on average. Changes in BMI were defined as (1) last minus first BMI, (2) mean of all follow‐up BMIs minus first BMI, and (3) standard deviation of BMI change from baseline and all follow‐up visits (representing variability).
Results
Participants with significant changes in BMI (increase or decrease of ≥5%), or who had greater variability in BMI, had faster cognitive decline. This pattern was consistent irrespective of normal (BMI < 25; N = 5747), overweight (25 ≤ BMI < 30; N = 6302), or obese (BMI ≥ 30; N = 3928) BMI at baseline.
Conclusions
Stability in BMI predicts better cognitive trajectories suggesting clinical value in tracking BMI change, which is simple to measure, and may point to individuals whose cognition is declining.
Keywords: Cognitive decline, body mass index, older adults, stability
1. INTRODUCTION
Progressive loss of cognition is common in older adults and its prevention and treatment is a major public health priority. The aging research community has successfully identified diverse risk factors for late‐life cognitive impairment. Consistent evidence associates body mass index (BMI), the most common measure of global adiposity, in midlife, with poor cognitive outcomes in late life. 1 , 2 , 3 Individuals who were obese in midlife have a 2‐ to 3‐fold higher dementia risk in old age compared to individuals who were non‐obese. 4 We and others showed more cognitive impairment and dementia with greater BMI in old age, 5 , 6 but some have found no associations, or even lower dementia risk in obese older adults. 2 , 4 , 7 In addition, losing weight has also been associated with higher dementia risk. 8
Despite the relevance of BMI to the risk of incident cognitive impairment and the subtle but consistent decrease in BMI that accompanies old age, 9 there is limited evidence on the simultaneous changes in BMI and cognitive decline, 10 , 11 which better reflect the natural course of both health phenomena. There is evidence indicating that BMI stability over time is associated with less frailty, disability, and mortality in late life. 12 , 13 However, whether BMI stability is associated with better cognitive trajectories is essentially unknown. To address this gap in knowledge, we exploited longitudinal clinical data from ≈16,000 initially non‐demented older adults participating in the Alzheimer's Disease (AD) Centers (ADCs) across the United States to examine associations of changes in BMI over time with changes in cognition, investigate whether both increases and decreases of BMI are related to cognitive decline, and if these associations are consistent in those who have normal, overweight or obese BMI at baseline.
2. METHODS
2.1. Data source and sample derivation
Data are drawn from the National Alzheimer's Coordinating Center (NACC), which maintains the Uniform Data Set (UDS) of participants enrolled in ADCs funded by the National Institute on Aging (NIA). 14 , 15 , 16 , 17 The study included all participants who were enrolled in NACC‐UDS between September 2005 (UDS start date) and the December 2019 data freeze (N = 42,022). To be eligible for the current study, at baseline, participants had to be ≥60 years of age, without a diagnosis of dementia as determined by clinician consensus, have a Clinical Dementia Rating (CDR) of 0 or 0.5, and have at least one annual follow‐up visit. The sample selection steps are presented in Figure 1. Recruitment methods vary between ADCs; therefore NACC‐UDS should not be considered a population‐based sample. Written informed consent was provided by all participants and their informants and approved by local institutional review boards (IRBs). Research using the NACC database was approved by the University of Washington IRB.
FIGURE 1.
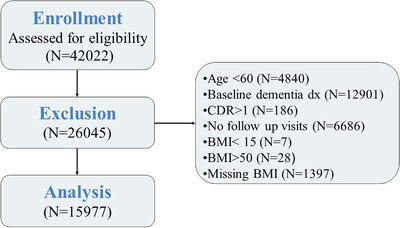
Sample selection steps. Abbreviation: dx, Diagnosis; CDR, Clinical Dementia Rating Scale; BMI, body mass index.
2.2. Measures
2.2.1. Global cognition and cognitive domains
All participants were administered a standardized neuropsychological battery at each study visit. We categorized the neuropsychological tests into the following domains 18 : memory (Wechsler Memory Scale ‐ Revised [WMS]‐R Logical Memory Immediate and Delayed Recall), attention/working memory (WMS‐R Digit Span forward and backward, Trail Making Test [TMT] Part A), language (category fluency [mean of animals and vegetables], Short Form of the Boston Naming Test [BNT]), and executive function (TMT Part B). 18 For all neuropsychological tests except TMT A and B, higher scores indicate better cognition. TMT A and B scores were reversed so that higher scores also indicate better cognition. Cognitive composite scores were computed using previously published methods. 19 First, a baseline z‐score for each test was computed. A baseline composite domain was constructed by averaging the z‐scores for each test in that domain, and a baseline global cognitive z‐score was calculated by averaging the z‐scores from all tests. Missing values for individual tests were allowed. However, the domain score was set to missing if more than half of the tests in that domain were missing. The global score was set to missing if more than half of all tests were missing. Requiring complete data on all tests did not change study results. Mean and standard deviation (SD) from the baseline scores were used to construct corresponding z‐scores for each domain and global cognition.
RESEARCH IN CONTEXT
Systematic review: Literature searches using PubMed were conducted to ascertain original and review articles, using keywords including “BMI,” “adiposity,” “trajectories,” and “cognitive decline.” These articles suggested an association of baseline BMI with cognitive decline, with some suggesting that in old age, higher BMI may be a protective factor. PubMed search did not identify studies reporting on the long‐term simultaneous changes in BMI and cognitive decline.
Interpretation: Our study provides new evidence that greater changes in BMI over time are associated with a faster rate of cognitive decline, suggesting that in initially non‐demented older adults, stability in BMI predicts a better cognitive trajectory.
Future directions: These results suggest a potential clinical value in tracking BMI change, a simple, non‐invasive measure, as it may point to individuals whose cognition is declining. Further work is needed to identify mechanisms underlying departures from stable BMI in old age and their contribution to cognitive decline.
2.2.2. BMI
BMI was calculated based on measured weight and height (weight in kilograms [kg] divided by squared height in centimeters) at baseline and at each follow‐up visit. For descriptive analyses, each participant's baseline BMI (BMIbl) was categorized into three groups based on the World Health Organization (WHO) classification 20 of underweight or normal (<25 kg/m2), overweight (25 ≤ BMI <30 kg/m2), and obese (≥30 kg/m2). Few participants were underweight (<18.5 kg/m2) and therefore were combined with the normal weight group. BMI outside the range of 15 to 50 kg/m2 were treated as biologically unlikely and were dropped.
2.2.3. Change in BMI
Change in BMI was measured in three ways: (1) difference between BMI at last visit and BMIbl (∆BMIlast‐bl) as commonly used in clinical trials for weight loss, 21 , 22 (2) difference between mean BMI across all follow‐up visits and BMIbl (∆BMImean‐bl). For (1) and (2), changes in BMI were categorized as significant increase (≥5% increase), stable (<5% change), or significant decrease in BMI (≥5% decrease), as a 5% change is defined as clinically significant by the UUS) Food and Drug Administration (FDA). 23 Finally, (3) we also constructed a variable for within‐person variability, that is, SD of BMI change from baseline and all follow‐up visits (∆BMI‐SD), categorized into quartiles 1
2.2.4. Demographics and clinical characteristics
Demographic characteristics included age, sex, race (White, Black, vs. Other), ethnicity (Hispanic/Latino vs. Other), years of education, and years of follow‐up. Cardiovascular risk factors included history of diabetes, hypertension, and hypercholesterolemia, ascertained by self‐report or clinician assessment. Baseline diagnosis included cognitively normal (CN) and mild cognitive impairment (MCI). Participants’ function was measured using the CDR scale, 24 , 25 based on reports by the participant and study partner, and the Functional Assessment Questionnaire (FAQ) reported from study partners interviews. 26 , 27 Depressive symptoms were measured using the 15‐item Geriatric Depression Scale (GDS‐15). 28 , 29 Number of medications a participant is taking was used as a proxy for overall health status. 30 Apolipoprotein E (APOE) genotype for participants who are willing to provide samples was reported by the ADCs. We constructed an indicator variable for any APOE e4 allele and an indicator for missing APOE information. Except for APOE, missing values were relatively rare (from no missing values for age, sex, education CDR, and diagnosis to a maximum of 3.3% missing values for the FAQ). Because results were unchanged when we applied mean substitution to missing values, observations with missing values were dropped in the present analyses.
2.2.5. Statistical analyses
Baseline characteristics were reported as N (percent) or mean (SD) as appropriate by BMIbl groups. Our primary outcome measure was change in global cognition from baseline (∆zcog). The main independent variables in the analyses were time, change in the respective BMI measures (∆BMIlast‐bl, ∆BMImean‐bl, and ∆BMI‐SD), and their interaction with time. All models were adjusted for covariates that have been associated with cognitive decline and dementia including age, sex, race/ethnicity, years of education, years of follow‐up, baseline diagnosis (CN vs. MCI), FAQ, GDS, APOE genotype, number of medications (reflecting co‐morbidities), and indicators for history of diabetes, hypertension, and hypercholesterolemia. All models were estimated using linear mixed models (LMMs) and included participant level random intercept and linear slope to allow participants to differ in their overall rate of change over time. A random effect for ADC was also included. Changes in the cognitive domain of memory, attention/working memory, language, and executive functions were examined separately. Finally, because BMI changes in old age differ in men and women, 31 , 32 we tested the interaction of each of the BMI measures with sex on cognitive decline. All analyses were performed using Stata 13.0. 33
3. RESULTS
3.1. Baseline sample characteristics
Table 1 describes the characteristics of the sample at baseline. Participants were ≈74 years of age, included a majority of women (59%) and White participants (81%), and had an average of 15.6 ± 3.6 years of education; 15.8% were MCI, with average Mini‐Mental Status Exam (MMSE) score of 28.4 ± 2.0, consistent with a sample that does not have dementia baseline. FAQ was 1.2 ± 2.8, reflecting little impairment in activities of daily living; GDS was 1.6 ± 2.2, indicating relatively little clinical depression. About 13% had diabetes, 52% had hypertension, and 53% had high cholesterol. Of those who agreed to have APOE genotyping (83% of the sample), 29% had at least one APOE ε4 allele. Between‐group differences were statistically significant at P < .01 for all variables except for proportion of participants with missing APOE status (P = .37), years of follow‐up (P = .40), CDR score (P = .10), and FAQ score (P = .11). Finally, on average, participants had 5.2 ± 3.1 in‐person visits (initial visit + 4 annual follow‐up visits) and there was no difference in attrition rate between baseline BMI groups.
TABLE 1.
Baseline characteristics by baseline BMI group
| Variables | Total Sample | Normal (BMI < 25 kg/m2) | Overweight (25 ≤ BMI < 30 kg/m2) | Obese (BMI ≥30 kg/m2) | |
|---|---|---|---|---|---|
| N (%) | 15,977 | 5,747 (36) | 6,302 (39.4) | 3,928 (24.6) | |
| Age, mean (SD) | 73.5 (7.6) | 74.5 (8.0) | 73.8 (7.5) | 71.7 (6.9) | |
| Female (%) | 59.2 | 66.8 | 50.6 | 62.0 | |
| Hispanic (%) | 6.9 | 5.0 | 7.4 | 9.0 | |
| White (%) | 80.9 | 86.1 | 82.7 | 70.5 | |
| Black (%) | 14.7 | 7.9 | 13.8 | 26.0 | |
| Other (%) | 4.4 | 6.0 | 3.5 | 3.5 | |
| Years of education, mean (SD) | 15.6 (3.6) | 16.1 (3.0) | 15.7 (3.2) | 14.9 (3.4) | |
| Years of follow‐up, mean (SD) | 5.2 (3.1) | 5.3 (3.1) | 5.2 (3.1) | 5.1 (3.0) | |
| BMI, mean (SD) | 27.2 (5.0) | 22.5 (1.8) | 27.2 (1.4) | 34.1 (3.8) | |
| MMSE (range 0 to 30), mean (SD) | 28.4 (2.0) | 28.4 (2.0) | 28.4 (2.0) | 28.3 (2.0) | |
| FAQ (range 0 to 30), mean (SD) | 1.2 (2.8) | 1.3 (2.9) | 1.3 (2.9) | 1.2 (2.8) | |
| Hypertension (%) | 52.1 | 39.3 | 53.4 | 68.7 | |
| Hyperlipidemia (%) | 53.4 | 44.8 | 56.4 | 61.3 | |
| Diabetes (%) | 12.8 | 6.4 | 11.6 | 23.9 | |
| GDS (range 0 to 15), mean (SD) | 1.6 (2.2) | 1.5 (2.1) | 1.6 (2.1) | 1.8 (2.3) | |
| APOE ε4 (%) | 28.8 | 30.3 | 28.8 | 26.5 | |
| Number of medications, mean (SD) | 5.7 (4.1) | 5.1 (5.8) | 5.7 (4.1) | 6.6 (4.5) | |
| MCI (%) | 15.8 | 16.0 | 15.3 | 16.3 | |
| CDR at baseline (%) | 0 | 62.1 | 61.7 | 60.9 | 64.5 |
| 0.5 | 37.9 | 38.3 | 39.1 | 35.5 |
Abbreviation: SD, standard deviation; BMI, body mass index; MMSE, Mini Mental State Exam; FAQ, Functional Activities Questionnaire; GDS, Geriatric Depression Scale; APOE, Apolipoprotein E; MCI, mild cognitive impairment; CDR, Clinical Dementia Rating scale.
Thirty‐six percent of the participants had normal weight, 39.4% were overweight, and 24.6% were obese, which is consistent with the reported rates of overweight and obesity in this age group in the general US population. 34 As shown in Table 2, 26.7% of the sample decreased from the first to the last BMI measure by 5% or more (in initially normal, overweight, and obese group, 20.9%, 27.1%, and 34.6%, respectively), 55.8% remained stable in BMI over time (<5% BMI change; in initially normal, overweight, and obese group, 57.7%, 56.7%, and 51.8%, respectively), and 17.5% increased 5% or more in BMI (in initially normal, overweight, and obese group, 21.5%, 16.3%, and 13.6%, respectively).
TABLE 2.
Change in BMI between baseline and last visit: total sample and by baseline BMI group
| Total sample | Normal (BMI < 25 kg/m2) | Overweight (25 ≤ BMI < 30 kg/m2) | Obese(BMI ≥30 kg/m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Mean change (SD) | N | % | Mean change (SD) | N | % | Mean change (SD) | N | % | Mean change (SD) | |
| Significant decrease, change in BMI ≥5% | 4,200 | 26.7 | −3.12 (1.93) | 1,179 | 20.8 | −2.23 (1.10) | 1,680 | 27.1 | −2.95 (1.59) | 1,341 | 34.6 | −4.13 (1.93) |
| Stable, change in BMI <5% | 8,775 | 55.8 | −0.04 (0.72) | 3,262 | 57.7 | −0.01 (0.59) | 3,510 | 56.6 | −0.03 (0.72) | 2,003 | 51.8 | −0.11 (0.90) |
| Significant increase, change in BMI ≥5% | 2,752 | 17.5 | 2.63 (1.51) | 1,215 | 21.5 | 2.38 (1.43) | 1,010 | 16.3 | 2.65 (1.50) | 527 | 13.6 | 3.18 (1.59) |
Abbreviation: BMI, body mass index; SD, standard deviation.
3.2. Associations of changes in BMI with changes in global cognition
For descriptive purposes, unadjusted means (SD) of change in global cognition and in specific cognitive domain scores for each of the three BMI change measures are shown in Table S1. There were significant group differences in change in global cognition and in the specific cognitive domains (all P < .01) for each of the BMI change measures such that the stable group/s had less cognitive decline.
LMMs were applied to test the associations of ∆zcog with the three BMI change measures and are presented in Table 3. Greater changes in ∆BMIlast‐bl and in ∆BMImean‐bl (both increases and decreases of 5% or more in BMI) were associated with a faster rate of cognitive decline (Table 3; Figure 2A and 2B, respectively). Similarly, the interaction of ∆BMI‐SD (categorized into quartiles) with time on global cognition was significant—greater SD (representing greater changes in BMI over time, both increase and decrease)—was associated with a faster rate of global cognitive decline (Table 3; Figure 2C).
TABLE 3.
Associations of changes in BMI with changes in global cognition and cognitive domains
| Global cognition | Episodic memory | Attention/working memory | Language | Executive functions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variables | Coef. (SE) | P‐value | Coef. (SE) | P‐value | Coef. (SE) | P‐value | Coef. (SE) | P‐value | Coef. (SE) | P‐value | |
|
Last BMI – Baseline BMI (∆BMIlast‐bl, reference group: BMI change < 5%) |
Time | −0.041 | <.001 | −0.001 | .739 | −0.046 | <.001 | −0.059 | <.001 | −0.073 | <.001 |
| (0.002) | (0.003) | (0.002) | (0.002) | (0.003) | |||||||
| Decrease ≥5% | 0.030 | .001 | 0.041 | .017 | −0.012 | .292 | 0.039 | .001 | −0.017 | .323 | |
| (0.009) | (0.017) | (0.011) | (0.012) | (0.017) | |||||||
| Increase ≥5% | 0.029 | .005 | −0.012 | .541 | 0.016 | .205 | 0.025 | .076 | −0.032 | .109 | |
| (0.010) | (0.020) | (0.013) | (0.014) | (0.020) | |||||||
| Decrease ≥5%∙ time | −0.026 | <.001 | −0.033 | <.001 | −0.014 | <.001 | −0.027 | <.001 | −0.163 | <.001 | |
| (0.0030) | (0.004) | (0.003) | (0.004) | (0.004) | |||||||
| Increase ≥5%∙ time | −0.025 | <.001 | −0.024 | <.001 | −0.017 | <.001 | −0.036 | <.001 | −0.010 | .051 | |
| (0.003) | (0.005) | (0.003) | (0.004) | (0.005) | |||||||
|
Average BMI – Baseline BMI (∆BMImean‐bl, reference group: BMI change < 5%) |
Time | −0.046 | <.001 | −0.006 | .013 | −0.049 | <.001 | −0.066 | <.001 | −0.076 | <.001 |
| (0.002) | (0.003) | (0.002) | (0.002) | (0.003) | |||||||
| Decrease ≥5% | 0.029 | .003 | 0.074 | <.001 | −0.016 | .177 | 0.034 | .011 | −0.016 | .402 | |
| (0.010) | (0.019) | (0.012) | (0.013) | (0.019) | |||||||
| Increase ≥5% | 0.026 | .025 | 0.018 | .405 | 0.028 | .047 | 0.010 | .522 | −0.003 | .881 | |
| (0.012) | (0.022) | (0.014) | (0.016) | (0.023) | |||||||
| Decrease ≥5%∙ time | −0.273 | <.001 | −0.040 | <.001 | −0.015 | <.001 | −0.028 | <.001 | −0.018 | <.001 | |
| (0.003) | (0.005) | (0.003) | (0.004) | (0.004) | |||||||
| Increase ≥5%∙ time | −0.017 | <.001 | −0.017 | .003 | −0.015 | <.001 | −0.013 | .007 | −0.008 | .147 | |
| (0.004) | (0.006) | (0.004) | (0.005) | (0.006) | |||||||
|
Quartiles of SD of ∆BMI (∆BMI‐SD, reference group: lowest quartile of SD) |
Time | −0.040 | <.001 | 0.006 | .166 | −0.044 | <.001 | −0.059 | <.001 | −0.067 | <.001 |
| (0.003) | (0.004) | (0.003) | (0.003) | (0.004) | |||||||
| Quartile 2 | 0.019 | .099 | 0.032 | .163 | 0.022 | .121 | 0.005 | .778 | 0.013 | .548 | |
| (0.012) | (0.023) | (0.015) | (0.016) | (0.022) | |||||||
| Quartile 3 | 0.008 | .496 | 0.017 | .447 | 0.009 | .531 | 0.003 | .872 | 0.029 | .193 | |
| (0.012) | (0.023) | (0.015) | (0.016) | (0.022) | |||||||
| Quartile 4 | 0.031 | .008 | 0.011 | .622 | 0.023 | .121 | 0.018 | .249 | 0.039 | .086 | |
| (0.012) | (0.023) | (0.015) | (0.016) | (0.023) | |||||||
| Quartile 2∙ time | −0.007 | .069 | −0.016 | .003 | −0.005 | .144 | −0.007 | .125 | −0.003 | .508 | |
| (0.004) | (0.006) | (0.004) | (0.005) | (0.005) | |||||||
| Quartile 3∙ time | −0.015 | <.001 | 0.028 | <.001 | −0.011 | .002 | −0.016 | .001 | −0.017 | .001 | |
| (0.004) | (0.006) | (0.004) | (0.005) | (0.005) | |||||||
| Quartile 4∙ time | −0.027 | <.001 | −0.030 | <.001 | −0.022 | <.001 | −0.026 | <.001 | −0.031 | <.001 | |
| (0.004) | (0.006) | (0.004) | (0.005) | (0.005) | |||||||
Note: Covariates included age, sex, race/ethnicity, years of education, years of follow‐up, baseline diagnosis (CN vs. MCI), FAQ, GDS, APOE genotype, number of medications (reflecting comorbidities), and indicators for history of diabetes, hypertension, hypercholesterolemia.
Abbreviation: BMI, body mass index; Coef, Coefficient; SE, standard error.
FIGURE 2.
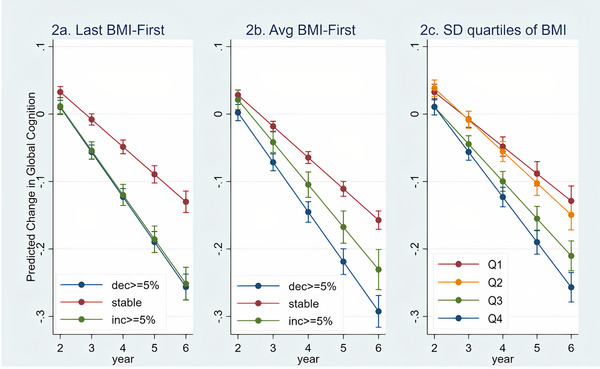
Associations of changes in BMI (last minus first BMI, average BMI at follow‐up minus first BMI, quartiles of standard deviation) with decline in global cognition. Abbreviation: BMI, Body Mass Index; Avg, Average; SD, standard deviation; decr, decrease; inc, increase.
To exemplify the differences in rate of decline according to the estimations for ∆BMIlast‐bl, we calculated the proportion of cognitive decline in ≥5% change in BMI compared to the stable BMI group. The predicted decline in the global cognition z‐score for the stable group averaged 0.16 (see Table S1) over the period of the study. In contrast, in the group with ≥5% decrease in BMI, the predicted decline in the global cognition z‐score was 0.27 and 0.26 points for those with a ≥5% increase in BMI. This represents a 64% and 62%, respectively, faster decline in global cognition in the groups with changes in BMI compared to the stable group.
3.3. Associations of changes in BMI with changes in specific cognitive domains
We repeated analyses using the three BMI change variables for the specific cognitive domains (Table 3). Both declines and increases of 5% or more from first to last BMI, and from mean BMI to first BMI, compared to stable BMI (<5% change) were associated with faster decline in all cognitive domains; for executive functions, those whose BMI increased did not reach statistical significance. When comparing the highest quartiles of SD to the lowest SD quartile, the second quartile was associated only with a faster rate of episodic memory decline. The third and fourth quartiles, representing greater variability, were associated with a faster rate of decline in all cognitive domains (Table 3).
3.4. Associations of BMI changes with cognitive decline stratified by baseline BMI
To explore whether the pattern of association of greater BMI change over time with faster cognitive decline is specific to a particular baseline BMI group (for example, participants who were obese), we repeated all analyses stratifying by baseline BMI group. Overall, results remained the same, that is, in each of the BMI groups, greater changes (either increases or decreases) of BMI over time were associated with a faster rate of cognitive decline (Table S2 and Figure S1‐S3 ).
We performed a sensitivity analysis using cut‐off points of increases and decreases of 10% or more in BMI for ∆BMIlast‐bl and ∆BMImean‐bl. Results were essentially unchanged (Table S3). Finally, none of the interactions of the BMI measures with sex on cognitive decline were significant.
4. DISCUSSION
This study followed ≈16,000 well‐characterized community‐dwelling older adults without dementia at baseline for an average of 5 years and provides new evidence that greater increases, decreases, or variability in BMI over time are associated with an accelerated rate of cognitive decline after adjusting for a broad range of potential sociodemographic, clinical, and functional confounders. Furthermore, this pattern is consistent, irrespective of whether a person has normal, overweight, or obese BMI at baseline. As shown in Figure 2, all groups had some degree of decline including the group with stable BMI. However, the rate of decline in the non‐stable groups was over 60% faster than the rate of decline in the stable BMI group. These results suggest a potential clinical value in tracking BMI in annual medical visits, which is simple, non‐expensive, non‐invasive, and quick to measure, as it may point to individuals whose cognition is declining and allow for early intervention. Further work is needed to identify the biological causes underlying departures from stable BMI in old age and their contribution to cognitive decline.
The current study extends prior work in several important ways. First, we assessed changes in BMI over time, measured in several different ways, and their associations with cognitive decline. Second, the large sample size and the relatively long follow‐up permitted investigation of the association of both increase and decrease in BMI with cognitive decline. An increase and decrease of 5% or more in BMI was used, as it is well‐accepted as clinically significant. 23 Third, because obese individuals have a greater range for BMI decline while normal‐weight individuals have a greater range for BMI increase, we examined whether the associations of changes in BMI and cognitive decline differ by baseline BMI category (based on the World Health Organization [WHO] BMI categorization), and showed that these associations are consistent regardless of BMI category. These results indicate that in old age, the stability of BMI may confer protection against cognitive decline, irrespective of the initial BMI of the individual. Finally, analyses of the individual cognitive abilities used to construct global cognition, showed that changes in BMI over time were associated significantly with accelerated decline in episodic memory, attention/working memory, and language but not with executive function, suggesting that changes in BMI may be associated differentially with some dimensions of cognitive abilities more than others.
We and others have shown that the negative effects of a higher BMI on brain and cognition persist through late‐life. 32 , 35 However, there is scarce research on changes in BMI in old age; most studies provide evidence suggesting that decreasing BMI is associated with poor health outcomes including congestive heart failure and mortality. 36 , 37 Our study showed that a decline in BMI over time was associated with accelerated cognitive decline. Consistent with our results, a 1‐unit annual decline in BMI was associated with about a 35% increase in the risk of AD compared with a person experiencing no change in BMI in older adults, 10 suggesting that loss of BMI may reflect pathologic processes that contribute to the subsequent development of AD. Furthermore, weight loss during 1 year was associated with a faster clinical progression of MCI. 11 Although decline in BMI may represent a true risk factor for cognitive decline, it is also possible that it represents reverse causality, that is, that brain changes related to dementia affect BMI earlier than cognition. 32 Of interest, polymorphisms in FTO, PPARG, PPARA, and APOE genes, 38 implicated in AD, 39 , 40 are significant predictors of BMI variability, 41 suggesting a common cause. An alternative explanation is that both BMI variability and cognitive decline are manifestations of an underlying morbidity and are not causally related.
It is important to note that in studies examining associations of change in BMI with health outcomes, including cognitive impairment, the comparison is typically to individuals who had a stable BMI. Because in our study we showed that both decrease and increase in BMI over time were associated with accelerated cognitive decline, irrespective of the BMI at baseline, it is plausible that conceptually, in old age, BMI stability over time is associated with reduced risk of poor cognitive outcomes. The mechanisms underlying the potential health benefits of BMI stability are unknown. Aging leads to numerous physiological, endocrine, and metabolic changes, which manifest in changes in body composition, reflected in loss of bone, loss of muscle mass and strength, and increased body fat and fat redistribution. 9 , 42 , 43 These changes are accompanied by an increased low‐grade chronic inflammation, 44 , 45 , 46 which has been consistently associated with poor health outcomes 47 , 48 and with poor cognitive outcomes and incident dementia. 49 , 50 Indeed, BMI stability over time is associated with fewer comorbidities in late life. 12 , 13 Our results suggest that BMI stability is also associated with a healthier cognitive trajectory.
Biological homeostasis is the ability of the “normal” body to maintain equilibrium, balancing multiple factors—including blood pressure, cholesterol, as well as body weight—at stable levels despite temporary changes in life conditions. 51 The progressive homeostatic imbalance that occurs with aging 52 , 53 , 54 may be at least in part responsible for the change in BMI observed among older adults, 55 , 56 and facilitates deleterious processes that cause a myriad of cellular dysfunctions, including neuronal death and neurodegeneration. 52 , 53 Stability in BMI over time may represent less departure from homeostasis, possibly conferring the preservation of neuronal health. Of interest, in the stratified analysis, among those who had initially normal BMI, the rate of decline in cognition was nominally faster than among participants with baseline overweight or obesity, despite the lack of difference in numerous baseline medical and sociodemographic characteristics. We speculate that this may point to an adaptive process of the overweight and obese individuals to their higher BMI, reaching a new homeostatic balance, 57 , 58 , 59 and conferring a degree of protection against cognitive decline.
The study has limitations. The NACC data set is based on a convenience sample of participants approaching the ADCs in the United States, rather than a community‐based sample. Furthermore, the sample has 80% White participants with underrepresentation of ethnic minorities. Thus caution must be applied when generalizing the results to the whole population of older adults and the results should be replicated in more diverse cohorts. The cohort includes significant majority of women, which in some studies have shown different trajectories of BMI than men. However, the interaction of BMI changes with sex was tested, and sex did not modify the associations we have found. The cardiovascular risk factors are self‐reported rather than directly measured. The prevalence of diabetes and hypertension in our sample is less than that reported in national statistics (Table 1. 12.8% vs. 26.8% for diabetes 60 and 52.1% vs. 70% for hypertension 61 ), whereas hyperlipidemia percentage was close to national statistics (53.4% vs. 48% 62 ). Overall there is likelihood that some of the confounding factors were underreported in our sample. Our results remained robust after adjusting for cardiovascular risk factors and diseases, and for depression and Activities of Daily Living (ADL), but other factors such as cancer may have affected BMI. Increased adiposity has been consistently associated with higher levels of chronic inflammation markers, which in turn have been related to AD. 63 However, inflammatory markers are not available in the NACC data set. Because the study investigated changes in BMI over time, which may be related to increasing morbidity, we repeated the analyses replacing baseline number of medications by changes in number of medications at last follow‐up compared to first follow‐up, such that a greater number represented an increase in number of medications, suggesting growing morbidity. However, adjusting for this covariate did not change any of the results (data not shown). It is important to note that BMI may not be an optimal measure of adiposity in older adults due to redistribution of body fat with age, 64 , 65 , 66 , 67 indicating the need for more direct measures of adiposity in studies of older adults, such as regional fat measures, and fat‐related secreted factors. Finally, overall, the rates of cognitive decline found in this study are relatively small, possibly because participants with dementia at baseline were excluded. Yet, the clinical hallmark of AD is the progressive loss of cognitive function. A long‐term extrapolation of a greater decline in cognition of over 60% in the groups with BMI change compared to the group with stable BMI may represent a progressive disease process.
Confidence in our findings is enhanced by several factors. Body weight and height were directly measured (rather than self reported) at every visit. A large number of male and female participants were examined annually for up to 14 years, with structured, validated, and harmonized clinical measures of cognition permitting investigation of global cognition as well as specific cognitive domains.
In conclusion, we found that stability in BMI over time, in contrast to increases or decreases in BMI, is associated with a slower rate of cognitive decline, both in global cognition and specific cognitive domains. Disentangling the biological pathways underlying different trajectories of BMI in old age, and their contribution to brain health and disease, is necessary to develop potential therapies.
CONFLICTS OF INTEREST
Hung‐Mo Lin reports consulting fees in the past 36 months from Edwards and DSMB participation for “Comprehensive Home‐based Self‐Management Support for COPD Patients” (NHLBI 1 R34 HL143747‐01), where no payment was made. Mary Sano reports consulting fees in the past 36 months from vTv, F. Hoffman Laroche, BioXcel, Avenir, Biogen Idec, Eisai, Genentech, Minerva, Karuna, Novartis, Novo Nordisk, and Hospital for Special Surgery; in addition to DSMB participation for Syneos. All other authors have nothing to disclose.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
National Institute on Aging (NIA) P50 AG005138 AG016976 P30 AG066514 for Mary Sano, and R01 AG034087, R01 AG053446, R01 AG051545, R01 AG061093, and AG043878 for Michal Schnaider Beeri. Mary Sano and Carolyn W. Zhu also are supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Beeri MS, Tirosh A, Lin H‐M, et al. Stability in BMI over time is associated with a better cognitive trajectory in older adults. Alzheimer's Dement. 2022;18:2131–2139. 10.1002/alz.12525
REFERENCES
- 1. Ravona‐Springer R, Schnaider‐Beeri M, Goldbourt U. Body weight variability in midlife and risk for dementia in old age. Neurology. 2013;80(18):1677‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid‐life and late life for the development of dementia: a systematic review and meta‐analysis of longitudinal studies. Age Ageing. 2016;45(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 3. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556‐1560. [DOI] [PubMed] [Google Scholar]
- 4. Wotton CJ, Goldacre MJ. Age at obesity and association with subsequent dementia: record linkage study. Postgrad Med J. 2014;90(1068):547‐551. [DOI] [PubMed] [Google Scholar]
- 5. West RK, Ravona‐Springer R, Heymann A, et al. Waist circumference is correlated with poorer cognition in elderly type 2 diabetes women. Alzheimers Dement. 2016;12(8):925‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18‐year follow‐up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524‐1528. [DOI] [PubMed] [Google Scholar]
- 7. Singh‐Manoux A, Dugravot A, Shipley M, et al. Obesity trajectories and risk of dementia: 28 years of follow‐up in the Whitehall II Study. Alzheimers Dement. 2018;14(2):178‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart R, Masaki K, Xue QL, et al. A 32‐year prospective study of change in body weight and incident dementia: the Honolulu‐Asia aging study. Arch Neurol. 2005;62(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 9. JafariNasabian P, Inglis JE, Reilly W, Kelly OJ, Ilich JZ. Aging human body: changes in bone, muscle and body fat with consequent changes in nutrient intake. J Endocrinol. 2017;234(1): R37‐R51. [DOI] [PubMed] [Google Scholar]
- 10. Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892‐897. [DOI] [PubMed] [Google Scholar]
- 11. Besser LM, Gill DP, Monsell SE, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28(1):36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strandberg TE, Stenholm S, Strandberg AY, Salomaa VV, Pitkala KH, Tilvis RS. The “obesity paradox,” frailty, disability, and mortality in older men: a prospective, longitudinal cohort study. Am J Epidemiol. 2013;178(9):1452‐1460. [DOI] [PubMed] [Google Scholar]
- 13. Li ZB, Wang ML, Dong SY, et al. Effects of body mass index and weight change on mortality in older men with impaired glucose regulation. Exp Gerontol. 2017;89:87‐92. [DOI] [PubMed] [Google Scholar]
- 14. Morris JC, Weintraub S, Chui HC, et al. The uniform data set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐216. [DOI] [PubMed] [Google Scholar]
- 15. Beekly DL, Ramos EM, Lee WW, et al, Centers NIAAsD . The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249‐258. [DOI] [PubMed] [Google Scholar]
- 16. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Besser L, Kukull W, Knopman DS, et al. Neuropsychology Work Group D, clinical core leaders of the National Institute on Aging‐funded USAsDC. Version 3 of the National Alzheimer's Coordinating Center's uniform data set. Alzheimer Dis Assoc Disord. 2018;32(4):351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sano M, Zhu CW, Grossman H, Schimming C. Longitudinal cognitive profiles in diabetes: results from the National Alzheimer's Coordinating Center's uniform data. J Am Geriatr Soc. 2017;65(10):2198‐2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beeri MS, Ravona‐Springer R, Moshier E, et al. The Israel diabetes and cognitive decline (IDCD) study: design and baseline characteristics. Alzheimers Dement. 2014;10(6):769‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1‐452. [PubMed] [Google Scholar]
- 21. Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low‐fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71(2):379‐388. [DOI] [PubMed] [Google Scholar]
- 22. Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: cENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137(11):1143‐1157. [DOI] [PubMed] [Google Scholar]
- 23. Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long‐term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005;29(10):1153‐1167. [DOI] [PubMed] [Google Scholar]
- 24. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. BrJPsychiatry. 1982;140:566‐572. [DOI] [PubMed] [Google Scholar]
- 25. Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's Disease cooperative study experience. Neurology. 1997;48(6):1508‐1510. [DOI] [PubMed] [Google Scholar]
- 26. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 27. Zhu CW, Grossman HT, Sano M. Why do they just sit? Apathy as a core symptom of Alzheimer disease. Am J Geriatr Psychiatry. 2019;27(4):395‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 29. Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15‐item geriatric depression scale in functionally impaired, cognitively intact, community‐dwelling elderly primary care patients. J Am Geriatr Soc. 2005;53(9):1570‐1576. [DOI] [PubMed] [Google Scholar]
- 30. Subramaniam H. Co‐morbidities in dementia: time to focus more on assessing and managing co‐morbidities. Age Ageing. 2019;48(3):314‐315. [DOI] [PubMed] [Google Scholar]
- 31. Joo SH, Yun SH, Kang DW, Hahn CT, Lim HK, Lee CU. Body mass index in mild cognitive impairment according to age, sex, cognitive intervention, and hypertension and risk of progression to Alzheimer's disease. Front Psychiatry. 2018;9:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karlsson IK, Gatz M, Arpawong TE, Dahl Aslan AK, Reynolds CA. The dynamic association between body mass index and cognition from midlife through late‐life, and the effect of sex and genetic influences. Sci Rep. 2021;11(1):7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stata Statistical Software: Release 16 [computer program]. Version 11. StataCorp LLC.; 2019. [Google Scholar]
- 34. https://www.cdc.gov/nchs/hus/contents2018.htm#Table_026
- 35. West RK, Livny A, Ravona‐Springer R, et al. Higher BMI is associated with smaller regional brain volume in older adults with type 2 diabetes. Diabetologia. 2020;63(11):2446‐2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang YF, Tang Z, Guo J, et al. BMI and BMI changes to all‐cause mortality among the elderly in Beijing: a 20‐year cohort study. Biomed Environ Sci. 2017;30(2):79‐87. [DOI] [PubMed] [Google Scholar]
- 37. Keller HH, Ostbye T. Body mass index (BMI), BMI change and mortality in community‐dwelling seniors without dementia. J Nutr Health Aging. 2005;9(5):316‐320. [PubMed] [Google Scholar]
- 38. Zhang H, Zheng W, Hua L, et al. Interaction between PPAR gamma and SORL1 gene with Late‐Onset Alzheimer's disease in Chinese Han population. Oncotarget. 2017;8(29):48313‐48320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barker W, Harwood D, Duara R, et al. The APOE‐epsilon4 allele and Alzheimer disease among African Americans, Hispanics, and whites. JAMA. 1998;280(19):1661‐1662. [DOI] [PubMed] [Google Scholar]
- 40. Li H, Ren Y, Mao K, et al. FTO is involved in Alzheimer's disease by targeting TSC1‐mTOR‐Tau signaling. Biochem Biophys Res Commun. 2018;498(1):234‐239. [DOI] [PubMed] [Google Scholar]
- 41. Goni L, Garcia‐Granero M, Milagro FI, Cuervo M, Martinez JA. Phenotype and genotype predictors of BMI variability among European adults. Nutr Diabetes. 2018;8(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: european consensus on definition and diagnosis report of the European working group on sarcopenia in older people A. Age Ageing. 2010;39(4):412‐423. J. Cruz‐Gentoft et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X‐Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9): e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immunity & Ageing. 2005;2(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation—results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study–. Am J Clin Nutr. 2005;82(2):428‐434. [DOI] [PubMed] [Google Scholar]
- 46. Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102(3):919‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wiebe N, Stenvinkel P, Tonelli M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw Open. 2019;2(8): e1910456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Gonzalo‐Calvo D, de Luxan‐Delgado B, Martinez‐Camblor P, et al. Chronic inflammation as predictor of 1‐year hospitalization and mortality in elderly population. Eur J Clin Invest. 2012;42(10):1037‐1046. [DOI] [PubMed] [Google Scholar]
- 49. Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for health care practice and research. The Journal of Neuroscience Nursing. 2012;44(4):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Therapeutic advances in chronic disease. 2011;2(3):175‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pomatto LCD, Davies KJA. The role of declining adaptive homeostasis in ageing. J Physiol. 2017;595(24):7275‐7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morawe T, Hiebel C, Kern A, Behl C. Protein Homeostasis, Aging and Alzheimer's Disease. MolNeurobiol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol. 2010;190(5):719‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muller AWJ. Aging is an adaptation that selects in animals against disruption of homeostasis. Med Hypotheses. 2018;119:68‐78. [DOI] [PubMed] [Google Scholar]
- 55. Di Francesco V, Fantin F, Omizzolo F, et al. The anorexia of aging. Dig Dis. 2007;25(2):129‐137. [DOI] [PubMed] [Google Scholar]
- 56. Chapman IM. Endocrinology of anorexia of ageing. Best Pract Res Clin Endocrinol Metab. 2004;18(3):437‐452. [DOI] [PubMed] [Google Scholar]
- 57. West RK, Beeri MS, Schmeidler J, et al. Homocysteine and cognitive function in very elderly nondemented subjects. Am J Geriatr Psychiatry. 2011;19(7):673‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. West R, Beeri MS, Schmeidler J, et al. Better memory functioning associated with higher total and low‐density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. AmJGeriatrPsychiatry. 2008;16(9):781‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schmeidler J, Mastrogiacomo CN, Beeri MS, Rosendorff C, Silverman JM. Distinct age‐related associations for body mass index and cognition in cognitively healthy very old veterans. Int Psychogeriatr. 2019;31(6):895‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Statistics about Diabetes. Vol https://www.diabetes.org/resources/statistics/statistics‐about‐diabetes#:~:text=Prevalence%20in%20seniors%3A%20The%20percentage,diagnosed%20with%20diabetes%20every%20year; 2018.
- 61. Mozaffarian D, Benjamin EJ, Go AS, et al, American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29‐322. [DOI] [PubMed] [Google Scholar]
- 62. Health disparities in the medicare population: hyperlipidemia. Vol https://www.cms.gov/About‐CMS/Agency‐Information/OMH/Downloads/Data‐Snapshot‐Hyperlipidemia‐06‐2016.pdf; 2016.
- 63. Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13(9):913‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Letra L, Santana I, Seica R. Obesity as a risk factor for Alzheimer's disease: the role of adipocytokines. Metab Brain Dis. 2014;29(3):563‐568. [DOI] [PubMed] [Google Scholar]
- 65. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2(3):141‐147. [DOI] [PubMed] [Google Scholar]
- 66. Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuk JL, Saunders TJ, Davidson LE, Ross R. Age‐related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339‐348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


