Abstract
High dietary salt (NaCl) increases blood pressure (BP) and can adversely impact multiple target organs including the vasculature, heart, kidneys, brain, autonomic nervous system, skin, eyes, and bone. However, patients with orthostatic disorders are told to increase their NaCl intake to help alleviate symptoms. While there is evidence to support the short-term benefits of increasing NaCl intake in these patients, there are few studies assessing the benefits and side effects of long-term high dietary NaCl. The evidence reviewed suggests that high NaCl can adversely impact multiple target organs, often independent of BP. However, few of these studies have been performed in patients with orthostatic disorders. We conclude that the recommendation to increase dietary NaCl in patients with orthostatic disorders should be done with care, keeping in mind the adverse impact on dietary NaCl in people without orthostatic disorders. Modest, rather than robust, increases in NaCl intake may be sufficient to alleviate symptoms but also minimize any long-term negative effects.
Keywords: Dietary salt, sodium recommendations, blood pressure variability, target organ damage, orthostatic disorders
1. INTRODUCTION
Historically, high dietary salt (NaCl) has been shown to increase blood pressure (BP) in those who are salt-sensitive (Grillo et al., 2019). Moreover, high dietary salt can modestly increase plasma osmolality and serum sodium. For example, an increase of sodium from a low sodium diet of 460 mg of sodium per day to a high sodium diet of 6,900 mg of sodium per day increased serum sodium from 138.6 to 140.2 mOsm/kg H2O and increased plasma osmolality from 286.4 to 288.5 mmol/L in healthy adults, respectively (Ramick et al., 2019). High dietary salt can adversely impact multiple target organs, sometimes independent of changes in BP (Farquhar et al., 2015; Robinson et al., 2019). Decreasing habitual dietary salt is a common dietary recommendation for the general population and is endorsed by the American Heart Association (AHA) (Lloyd-Jones et al., 2010) and the most recent U.S. Dietary Guidelines for Americans (U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition., 2020). Salt reduction decreases BP and may prevent the development of hypertension (Grillo et al., 2019; He et al., 2013). Nonetheless, average intake of salt among the U.S. population is estimated to be ~8,700 mg of salt per day (~3,400 mg of sodium) (Fig 1) (Quader et al., 2017). In spite of some controversial findings associating a low sodium diet with increased morbidity and mortality (García-Ortiz et al., 2012; O’Donnell et al., 2011), there is a broad consensus that 8,700 mg of salt per day (3,400 mg of sodium) is too high, and public health would be improved and healthcare costs reduced if this value were decreased (Benjamin et al., 2019; Bibbins-Domingo et al., 2010; Cappuccio et al., 2011; Webb et al., 2017).
Figure 1.
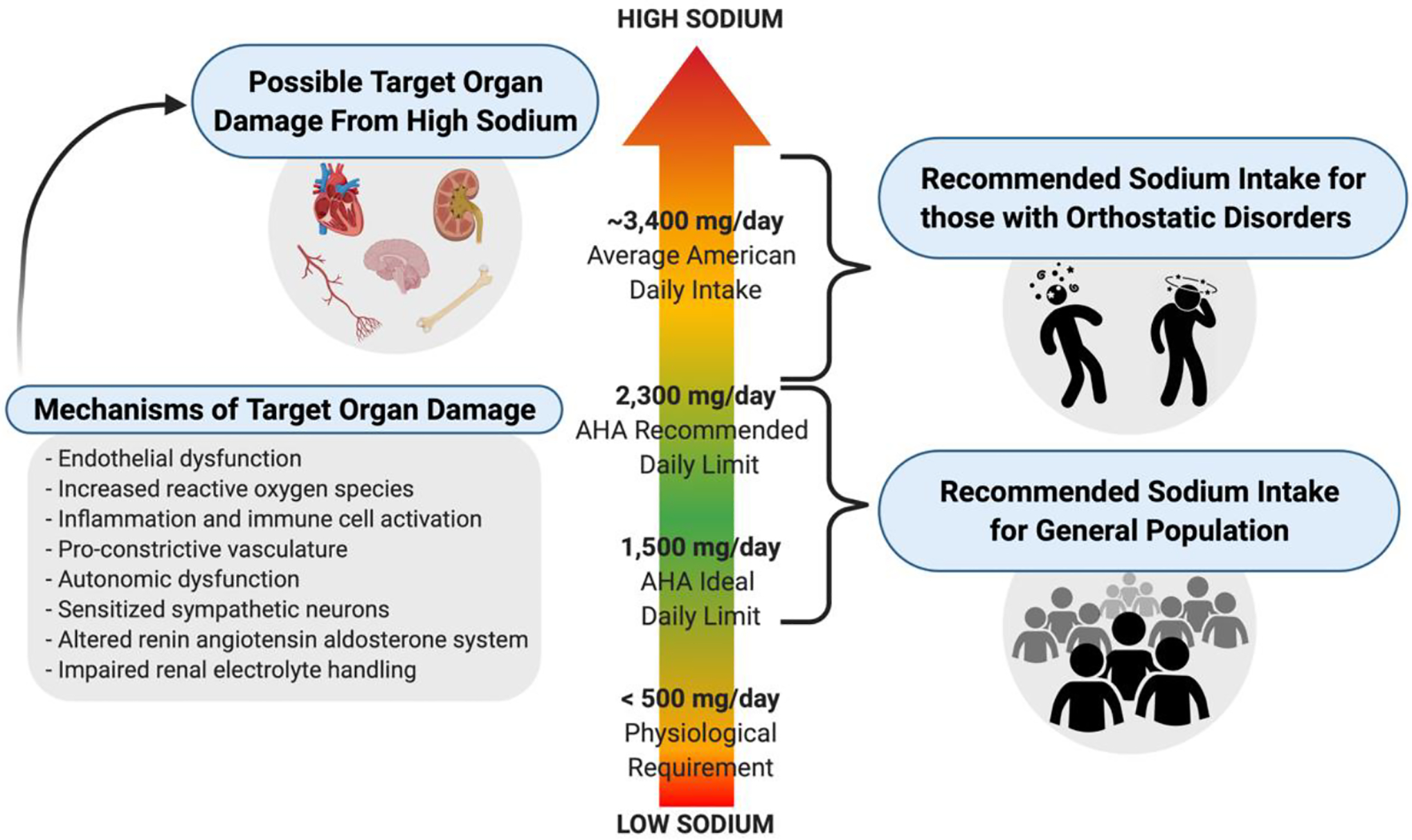
Dietary sodium recommendations and possible target organ damage. AHA’s recommended daily sodium limit for the general population is less than 2,300 mg of sodium per day (i.e., less than 6,000 mg of salt per day), whereas clinicians often recommend those with orthostatic disorders to consume greater than 2,300 mg of sodium per day. Possible target organ damage of consuming a diet high in sodium is thought to occur through BP independent mechanisms. Created with BioRender.com
AHA proposed an attainable goal to decrease sodium intake to less than ~6,000 mg of salt per day (2,300 mg of sodium). However, more than 6,000 mg of salt per day (2,300 mg of sodium) is routinely prescribed for patients with orthostatic disorders like orthostatic hypotension (OH), postural tachycardia syndrome (POTS), idiopathic orthostatic tachycardia, and syncope (Fig 1). They are told to increase their salt intake, to help combat their symptoms. The American Society of Hypertension recommendations vary from 6,000 – 10,000 mg of salt per day (2,400 – 4,000 mg of sodium) (Loughlin et al., 2020; Shibao et al., 2013) and others have suggested 10,000 – 20,000 mg of salt per day (4,000 – 8,000 mg of sodium) (Figueroa et al., 2010) for patients with OH. Salt recommendations from the Canadian Cardiovascular Society are 10,000 mg of salt per day (4,000 mg of sodium) (Raj et al., 2020) and a Heart Rhythm Society Expert Consensus Statement recommended 10,000 – 12,000 mg of salt per day (4,000 – 4,800 mg of sodium) (Sheldon et al., 2015) for patients with POTS. Based upon the broad range of recommendations, sodium recommendations appear to not be different between POTS and OH. It appears that increasing dietary salt in patients with orthostatic disorders may similarly increase plasma sodium compared to healthy controls (Garland et al., 2021), however, randomized studies assessing the efficacy and long-term side effects of these recommendations are lacking (Loughlin et al., 2020). Some recommend increasing dietary salt if urinary sodium excretion is less than 170 mmol/24 hours (~3,900 mg of sodium/24 hours) and this is typically done by adding 1,000 – 2,000 mg of sodium to the diet three times per day (El-Sayed and Hainsworth, 1996; Lanier et al., 2011; Loughlin et al., 2020; Low and Singer, 2008). One study showed that in patients with posturally related syncope who excreted less than ~3,900 mg of sodium per day, two months of ~2,400 mg of sodium supplementation per day improved orthostatic intolerance, peripheral vascular control, and cerebral autoregulation (Claydon and Hainsworth, 2004).
A recent systematic review and meta-analysis by Loughlin et al. (2020) showed that, in patients with orthostatic intolerance syndromes, increased salt in the diet improved orthostatic symptoms (mean follow-up of 44 days), decreased orthostatic tachycardia (−4 bpm), increased time to pre-syncope (+1.57min), and increased head up tilt or standing systolic BP (12 mmHg), but only resulted in a small increase in seated or supine systolic BP (1 mmHg) compared to controls. Therefore, in this patient group, a high salt diet seems to affect mainly the orthostatic BP. The only short term side effects reported included nausea and intolerance to salt with little effect on supine BP (Loughlin et al., 2020).
While there is evidence to support the short-term benefits of increasing daily salt intake in patients suffering from orthostatic disorders to alleviate symptoms (Loughlin et al., 2020), there is a paucity of research on the long-term effects of this practice. In this article we will review the evidence demonstrating that high dietary salt can adversely impact multiple target organs, independent of BP (Fig 1). For consistency and clarity, we will refer to sodium in mg (1mmol/L of sodium = 23mg of sodium). This review, and the literature as a whole, does not generally include those with orthostatic disorders. However, the findings from this review may inform recommendations in these patients and advocate future research on the long-term effect of high dietary salt in those with orthostatic disorders.
2. DIETARY SALT AND BLOOD PRESSURE VARIABILITY
Very short-term (beat-to-beat) and short-term (over 24hr period) spontaneous fluctuations in BP is termed BP variability (BPV) (Parati et al., 2013). Augmented BPV is associated with elevated left ventricular (LV) mass index (Sega et al., 2002), cardiac and cerebrovascular events (Verdecchia et al., 2007), the severity and rate of progression of end-organ damage (Mancia and Parati, 2003; Parati et al., 1987; Sarafidis et al., 2018), and cardiovascular (CV) events and mortality (Stevens et al., 2016). Mechanisms contributing to BPV include modulation of central and reflex autonomic function, emotional and behavior fluctuations, and changes in humoral systems (Parati et al., 2013). Since dietary salt has been shown to exaggerate sympathetic activity responses (Simmonds et al., 2014), it is possible that the high salt-induced sympathetic activity may act on BPV in this pathway.
Data from salt-resistant, normotensive rodents suggest that excess dietary salt intake increases BPV (Simmonds et al., 2014). However, data in humans are conflicting. One study found that high habitual dietary salt intake positively correlated with 24hr BPV and independently predicted 24hr BPV in hypertensives across the lifespan, independent of resting BP (Ozkayar et al., 2016). Conversely, our lab recently demonstrated that 10 days of a high dietary sodium intake (~7,000 mg/day) did not increase beat-to-beat BPV or 24hr BPV compared with medium (~2,300 mg/day) or low (~1,000 mg/day) dietary sodium intake in young salt-resistant normotensives (Migdal et al., 2020a). The differences in these studies may reside in the study population (older hypertensives vs. younger normotensives, respectively). As such, it is important to consider study population and length of dietary intervention (habitual vs. controlled feeding) when interpreting results. More studies are needed to test the reproducibility of these prior findings and to evaluate if these results stand true in other diverse populations such as patients with orthostatic disorders.
To that effect, orthostatic hypotension is associated with increased day-to-day BPV in an elderly population-based sample (Cremer et al., 2020). Also, increasing severity of orthostatic intolerance symptoms in patients with orthostatic intolerance is associated with greater BPV (Sunwoo et al., 2017). To the best of our knowledge, to date there have not been any published studies that have investigated the effect of sodium supplementation on BPV in patients with orthostatic disorders. Dietary sodium or urinary excretion of sodium were not reported in the two aforementioned studies in patients with orthostatic hypotension (Cremer et al., 2020) and intolerance (Sunwoo et al., 2017). It is possible that sodium supplementation could augment BPV in patients with orthostatic disorders, although this has not been tested.
3. DIETARY SALT AND TARGET ORGAN EFFECTS
3.1. Vasculature
3.1.1. Arterial Stiffness
Carotid-femoral pulse wave velocity (PWV) is the gold-standard measure of central arterial stiffness (Laurent et al., 2006) and is a strong predictor of CV events and mortality (Vlachopoulos et al., 2010). Arterial stiffness (PWV) is increased in patients with OH (Cremer et al., 2020; Sung et al., 2014). To the best of our knowledge there have not been any studies that investigated the effect of sodium supplementation on arterial stiffness in patients with orthostatic disorders. Nonetheless, our lab demonstrated that 7 days of high dietary sodium (6,900 mg/day) increased PWV in young and middle-aged normotensive adults when compared to a low sodium diet (460 mg/day), however, there was no effect when covaried for mean arterial pressure (Muth et al., 2017). This suggests that the increase in PWV was dependent on blood pressure. Interestingly, another set of studies provided key evidence that high dietary salt is associated with higher PWV, independent of BP (Avolio et al., 1986, 1985). Lastly, a systematic review of clinical trials demonstrated that average reduction in sodium intake of ~2,000 mg/day was associated with a 2.84% reduction in PWV and may be, at least in part, independent of changes in BP (D’Elia et al., 2018).
3.1.2. Endothelial Function
Endothelial dysfunction is widely accepted as an antecedent of CV disease (CVD) (Anderson et al., 2011; Gokce et al., 2002; Perticone et al., 2001), therefore assessments of endothelial function can be used as an important early detection tool of vascular dysfunction. Flow-mediated dilation (FMD) and cutaneous vascular conductance are two primary macro and microvascular, respectively, non-invasive measurements of endothelial function in humans (Brunt and Minson, 2011; Holowatz et al., 2008; Thijssen et al., 2019). Assessments of macro- and microvascular function have been shown to be largely mediated by nitric oxide (NO) (Green et al., 2014; Patik et al., 2021). Growing evidence suggests that high salt decreases NO bioavailability by increasing reactive oxygen species (ROS) (Greaney et al., 2012; Ramick et al., 2019), largely superoxide (O2−), and by decreasing endogenous antioxidant activity (Guers et al., 2019). Specifically, high salt increases O2− by increasing NADPH oxidase and scavenging NO to form peroxynitrate, which oxidizes the eNOS cofactor tetrahydrobiopterin, resulting in less NO production and more O2− production (Patik et al., 2021). Indeed, local infusion of ascorbic acid (non-specific ROS scavenger), Tempol (O2− scavenger), or apocynin (NADPH oxidase inhibitor) restored microvascular function in response to local heating in subjects consuming a 7-day high sodium (6,900 mg/day) diet (Ramick et al., 2019). High salt has also been shown to decrease endogenous antioxidant activity by decreasing superoxide dismutase. For example, superoxide dismutase expression decreased in rats fed a high salt diet when compared to a normal salt diet (Guers et al., 2019). Collectively, the increased ROS production and decreased antioxidant activity promotes a pro-oxidative stress environment.
Cell culture studies have shown that hypernatremia causes stiffening, shortening, and degradation of the endothelial glycocalyx (Martin et al., 2018; Oberleithner et al., 2011; Schierke et al., 2017). Damage to the glycocalyx, a protective barrier between the lumen and endothelial cells (Möckl, 2020), could facilitate sodium entry into the endothelial cells, thereby contributing to salt-induced endothelial dysfunction. Also, high sodium conditions may increase inflammation and immune cell response (Hucke et al., 2016; Kleinewietfeld et al., 2013; Wenzel et al., 2019; Zhang et al., 2015), which have been associated with decreased endothelial function (Bhagat and Vallance, 1997; Kovacs et al., 2006; Zhang, 2008). Other potent vasoconstrictors may play a role in endothelial function with high salt. Indeed, a reduction in endothelin-1 with sodium restriction has been suggested to mediate improvements in endothelial and vascular smooth muscle function (Dickinson et al., 2014). Lastly, high salt intake has been shown to increase copeptin, which suggests arginine vasopressin may be increased during sodium loading (Tasevska et al., 2014). Together, these endogenous pro-constrictor agents that are increased during a high salt diet could potentially increase vascular tone and stiffness and decrease endothelial function. For a more thorough evaluation of the mechanisms by which dietary salt contributes to endothelial dysfunction, we direct the reader to a recently published review by our lab group (Patik et al., 2021).
Numerous studies have demonstrated that high dietary salt decreases FMD when compared to a lower salt diet in healthy adults (Cavka et al., 2016; DuPont et al., 2013; Lennon-Edwards et al., 2014; Matthews et al., 2015). Importantly, some of these studies determined that this effect occurred in salt-resistant adults, suggesting the effect of high dietary salt on endothelial function is independent of BP (DuPont et al., 2013; Matthews et al., 2015). Specifically, a 7-day high sodium (6,900 mg/day) diet reduced brachial artery FMD, compared to a low sodium diet (460 mg/day), with no difference in the reduction of FMD between salt-sensitive and salt-resistant adults (Matthews et al., 2015). In a sub-set of subjects, we demonstrated salt-resistant adults on the high sodium diet, had augmented vascular tone and attenuated low-flow mediated constriction (Shenouda et al., 2020), an emerging complimentary measure of FMD used to assess endothelial function (Gori et al., 2016, 2008). High dietary salt has also been shown to decrease measures of microvascular function when compared to a lower salt diet (Eisenach et al., 2012; Tzemos et al., 2008) and in some cases this effect appears to be independent of BP (Barić et al., 2019; Greaney et al., 2012; Ramick et al., 2019). For example, our lab demonstrated that cutaneous vascular conductance was decreased during local heating when subjects were on a 7-day high sodium (~8,000 mg/day) diet compared to a low sodium (460 mg/day) diet and that this was independent of BP (Greaney et al., 2012). Similarly, some have shown that lowering salt intake improved endothelial function independent of changes in BP (Dickinson et al., 2014; Jablonski et al., 2013), while the evidence of the effect of salt intake on endothelium independent vasodilation is conflicting (Dickinson et al., 2014; DuPont et al., 2013).
Patients with POTS demonstrate impaired endothelial function as shown by decreased FMD when compared to healthy controls in some (Chopoorian et al., 2021), but not others (Liao et al., 2010; Smith et al., 2021). Recently, Smith et al. (2021) examined the effect of a high sodium diet on cardiovascular health. Interestingly, they showed that a 6-day high sodium (6,900 mg/day) diet in female patients with POTS did not worsen endothelial function when compared to a low sodium (230 mg/day) diet (Smith et al., 2021). They suggest this finding may be because high dietary sodium has been shown to have a greater effect on FMD in men compared to women (Lennon-Edwards et al., 2014) but this has not been demonstrated in other studies. It is also possible no change in FMD may be a floor effect of the influence of high sodium on endothelial function in patients with orthostatic disorders, as one study showed patients with POTS have impaired FMD compared to healthy controls (Chopoorian et al., 2021). However, Smith et al. (2021) did not show differences in FMD between patients with POTS and healthy controls. As evident of the paucity of research, more investigations need to occur to 1) definitively determine endothelial function in patients with orthostatic disorders compared to healthy controls; and 2) investigate by what mechanisms endothelial function may be impacted by sodium supplementation in patients with orthostatic disorders.
3.2. Renal
The renin angiotensin aldosterone system (RAAS) is critical in the chronic maintenance of BP through neurohumoral alterations which affect vascular tone as well as sodium and water retention. Briefly, the macula densa cells are activated in response to a decreased sodium load in the distal convoluted tubule. The juxtaglomerular cells secrete prorenin and then activate renin. Renin then cleaves angiotensinogen, produced by the liver, into angiotensin I. Angiotensin I is converted to angiotensin II by angiotensin converting enzyme. Angiotensin II binds to angiotensin II type 1 and/or type 2 receptors which can be found in the kidneys, adrenal cortex and medulla, systemic vasculature, cardiac cells, and brain. In short, when acting through angiotensin type I receptors, Ang II increases renal absorption of sodium in the proximal tubules, stimulates the release of aldosterone from the adrenal cortex which increases sodium reabsorption in the distal tubule and collecting duct, vasoconstricts systemic arterioles, stimulates thirst and water intake via the hypothalamus, and stimulates the release of vasopressin from the posterior pituitary which increase water reabsorption in the kidney by inserting aquaporin channels at the collecting duct. As a result of these actions, vascular tone, sodium and water retention are increased. Together these actions maintain blood volume and BP. Conversely, when sodium intake is high the RAAS is suppressed to prevent sodium retention, volume expansion, and an increase in blood pressure. However, when the RAAS is not suppressed in the presence of a high sodium diet, BP can increase in those who are “salt-sensitive” via vasoconstriction as well as sodium and fluid retention, causing an expansion of extracellular fluid volume. High dietary salt can also have deleterious effects on the kidneys through mechanisms independent of BP. Some rodent studies suggest that the intra-renal renin angiotensin aldosterone system is involved in the adverse effects of dietary salt on the kidney, independent of BP (Susic et al., 2011; Thomson et al., 2006; Varagic et al., 2008). For example, angiotensin II type 1 receptor blockade in hypertensive rats on a high salt diet ameliorated sodium-induced renal damage, kidney mass index, and proteinuria, despite no change in BP (Varagic et al., 2008). Another study showed increased NADPH activity and pro-oxidative stress mRNA expression in rats fed high salt (Kitiyakara et al., 2003). Lastly, others found that a high salt diet induced renal hypertrophy and collagen deposition in normotensive and hypertensive rats, possibly via TGF-B1 dependent pathways, suggesting the effect of high salt on renal structural changes is independent of BP (Yu et al., 1998).
Overtime, high dietary sodium may be associated with development of chronic kidney disease (CKD). Indeed, high salt intake is associated with biomarkers of early-stage renal damage, namely urinary vanin-1, neutrophil gelatinase-associated lipocalin, kidney injury molecule (Hosohata, 2017; Hosohata et al., 2016). If a high salt diet persists, this can lead to impairments in renal function and may ultimately progress to CKD. A systematic review found that a high sodium diet (>4,600 mg/day) is associated with adverse renal outcomes like proteinuria and declines in estimated glomerular filtration rate (eGFR) (Smyth et al., 2014). Specifically, high sodium intake was associated with declining eGFR in people without CKD (Lin et al., 2010) and increased risk of end stage renal disease in patients with CKD (Vegter et al., 2012). Indeed, Smyth et al. (2014) found that hypertensives consuming a higher salt diet over 10-years had a more rapid decline in eGFR compared to those consuming a lower salt diet (Ohta et al., 2013). A separate review found that when the average dietary sodium excretion was reduced from ~4,100 mg/day to ~2,400 mg/day, proteinuria and albuminuria decreased in people with CKD (Garofalo et al., 2018). This also held true in those without CKD when dietary sodium excretion was lower (~2,000 vs. ~3,900 mg/day) (P. A. Swift et al., 2005).
As expected, patients with POTS on a fixed dietary sodium intake (~3,400 mg/day) for 3 days had a lower plasma volume compared to healthy controls (Raj et al., 2005). However, the patients with POTS lacked a higher plasma renin activity and had a paradoxical lower concentration of aldosterone compared to healthy controls (Raj et al., 2005). This anomaly of the RAAS in patients with POTS may be contributing toward low water retention resulting in low blood volume and displaying of orthostatic intolerance symptoms. Another study showed that plasma volume was increased when patients with POTS consumed high dietary sodium (6,900 mg/day) for 6 days compared to a sodium consumption recommended by AHA for healthy adults (2,300 mg/day) (Garland et al., 2021). Furthermore, there was a compensatory reduction in plasma renin activity and aldosterone during the high compared to lower sodium condition (Garland et al., 2021). Lastly, more information is needed on the effects of a high sodium condition on renal function in patients with orthostatic disorders.
3.3. Cardiac
Rodent studies document an adverse effect of high sodium on pathological LV remodeling that can lead to varying degrees and types of LV dysfunction (Doi et al., 2000; Inoko et al., 1994; Klotz et al., 2006; Varagic et al., 2008; Yu et al., 1998). Rodent and cell studies suggest that sodium-induced LV hypertrophy and stiffening may be due to cellular hypertrophy in the vascular smooth muscle cells and myocardial cells, increased fibrosis, collagen expression, and TGF-B1 (Gu et al., 1998; Yu et al., 1998). In humans, high salt has been shown to be associated with increased congestive heart failure (He et al., 2002), coronary events and cardiovascular mortality independent of BP (Tuomilehto et al., 2001). Additionally, high sodium is associated with LV mass and thickness (Du Cailar et al., 2010; Haring et al., 2015; van der Westhuizen et al., 2019), in some cases even when controlled for BP (Jin et al., 2009; Rodriguez et al., 2011). Interestingly, one of these studies (Du Cailar et al., 2010) found that after a 3 year follow up period, LV mass continued to increase in hypertensive adults, regardless of sodium excretion, in those who had high plasma aldosterone concentrations. As such, it has been postulated that a high sodium intake may be a crucial contributing factor toward the development of LV hypertrophy in states of elevated mineralocorticoids and RAAS activity (Burnier et al., 2007). Consistent with this conclusion, both severe 12-week and moderate 12-month dietary salt restriction reduced LV mass, however, there was minimal improvement in LV function (Ferrara et al., 1984; Jula and Karanko, 1994). The mechanisms at play here are unclear but may include a reduction in preload and afterload. Regarding cardiac function, 14 days (Musiari et al., 1999) and as short as 5 days (Tzemos et al., 2008) of high dietary salt supplementation impaired LV diastolic function and this may be independent of LV mass (Langenfeld et al., 1998). However, these findings do not appear to be independent of high salt-induced BP in both normotensives (Tzemos et al., 2008) and hypertensives (Langenfeld et al., 1998), and may be due to increased LV stiffness, which can precede changes in LV function.
Another possible explanation of compromised LV structure and function induced by high salt may be due to augmented pressure wave reflection which can increase pulsatile load on the left ventricle (Chirinos and Segers, 2010a, 2010b). A 7-day high sodium (6,900 mg/day) diet has been shown to increase arterial pressure wave reflection compared to low sodium (460 mg/day) (Muth et al., 2017) and in another study this was independent of brachial BP (Starmans-Kool et al., 2011). When wave reflections return to the heart earlier and in greater magnitude, mid-to-late systolic afterload is increased. Over the course of years, central hemodynamic profiles like this can have deleterious effects on left ventricular structure and function, leading to increased LV mass or heart failure (Chirinos et al., 2012; Hashimoto et al., 2008; Zamani et al., 2015). Future studies need to determine whether reducing wave reflections via a low salt diet leads to improvements in LV structure or function.
Pressure wave reflections are increased in patients with OH (Sung et al., 2014). Additionally, OH is independently associated with higher risk of CVD outcomes in patients with CKD (Rouabhi et al., 2021). Separately, OH also predicts adverse cardiac changes such as LV hypertrophy (Magnusson et al., 2016) and is associated with incident heart failure with the exclusion of other co-morbidities (Jones et al., 2012). Yet, the acute or chronic effect of sodium supplementation on cardiac health in patients with orthostatic disorders is not known. Therefore, researchers should focus future investigations on the effect of sodium supplementation on cardiac structure and function as well as pressure wave reflections in patients with orthostatic disorders.
3.4. Cerebrovascular
High dietary salt also has deleterious effects on the cerebrovasculature. High dietary salt is associated with increased risk of stroke independent of BP (Perry and Beevers, 1992), and increase stroke related deaths (Li et al., 2012). Multiple rodent studies have demonstrated that cerebral autoregulation and reactivity are impaired with a high salt intake (Allen et al., 2019; Durand and Lombard, 2013; Lombard et al., 2003), some independent of BP (Cosic et al., 2016; Matic et al., 2018). The suggested mechanisms of impaired cerebrovascular function in rodent studies during a high salt diet revolve around reduced cerebral NO bioavailability from increased oxidative stress, specifically superoxide, and high salt-induced angiotensin II suppression which may decrease superoxide dismutase in the cerebral arteries (Allen et al., 2019; Durand and Lombard, 2013; Matic et al., 2018), with potentially a minor role of increased inflammation (Cosic et al., 2016). Longitudinal data indicates that impaired cerebrovascular reactivity to hypercapnia is a predictor of stroke in humans (Markus and Cullinane, 2001; Silvestrini et al., 2000). Unfortunately, to the best of our knowledge there have not been any chronic or habitual studies that assessed cerebral autoregulation or reactivity in humans on a high dietary salt diet. Yet, our lab recently demonstrated that an acute high sodium meal (1,495 mg) did not impair cerebrovascular reactivity during a hyper and hypocapnia perturbation in healthy young adults compared to an acute low sodium meal (138mg) (Migdal et al., 2020b).
OH is predictive of ischemic stroke (Eigenbrodt et al., 2000), and OH is present in ~¼ of patients with a stroke (Phipps et al., 2012). In response to a normocapnic head up tilt test, cerebral autoregulation is impaired in patients with POTS as demonstrated by decreased cerebral blood flow with greater variability compared to healthy controls (Ocon et al., 2009). Indeed two months of ~2,400 mg of sodium supplementation per day improved cerebral autoregulation among orthostatic intolerance and other symptoms (Claydon and Hainsworth, 2004) in patients with posturally related syncope who excreted less than ~3,900 mg of sodium per day. This study shows the benefit of a two-month sodium supplementation, however, the effect of longer term (years) sodium interventions in patients with POTS and OH are unknown.
3.5. Autonomic Function
Increased sympathetic outflow is related to poor CV, renal, and metabolic outcomes (Charkoudian and Wallin, 2014; Fisher et al., 2009). Acute intracerebroventricular and intravenous infusions of sodium increase sympathetic outflow in rodents (Kinsman et al., 2017; Stocker et al., 2015) and humans (Farquhar et al., 2006). Studies in hypertensives showed that extremely low dietary sodium intake (≤500mg/day) increased sympathetic outflow (Abrahão et al., 2002; Anderson et al., 1989; Grassi et al., 1997). However, consuming an extremely low sodium diet (≤500mg/day) is not representative of the population at large and is far below AHA’s ideal sodium intake of 1,500mg/day. Considering this, our lab showed that a more realistic 10-day reduction in sodium of 2,300mg/day to 1,000mg/day did not affect basal sympathetic outflow (Babcock et al., 2019). Interestingly, we reported that neurovascular transduction, which is the change in beat-by-beat blood flow and BP in response from muscle sympathetic nerve activity bursts, decreased on the low salt diet (Babcock et al., 2019). More studies are needed to investigate if a high salt diet that is representative of the population’s daily sodium intake (~3,400mg/day) alters sympathetic outflow when compared to a recommended salt diet (AHA’s recommended sodium limit of 2,300mg/day). Rodent and cell studies have identified specialized sodium chloride sensing neurons in the circumventricular organs (which lack a complete blood brain barrier), such as the organum vasculosum laminae terminalis and subfornical organ (Kinsman et al., 2017; Shi et al., 2007; Stocker et al., 2013). These neurons can sense increased serum and cerebrospinal fluid sodium but what is less clear is which sodium-sensing channel initiates these responses. Sodium-sensing channels that receive the most attention include furosemide-sensitive channels such as NKCC2 (Konopacka et al., 2015), benzamil-sensitive channels such as ENaC (Abrams and Osborn, 2008; Nishimura et al., 1998), and Nax-positive glial cells (Noda and Hiyama, 2015; Nomura et al., 2019). Nonetheless, when these sodium sensitive neurons are stimulated, they send a signal to the hypothalamic paraventricular nucleus onto the rostral ventrolateral medulla which ultimately controls sympathetic outflow to target organs that act on BP regulation (Stocker et al., 2013). Furthermore, these sodium sensing areas of the brain are responsible for initiating the release of arginine vasopressin, which acts a systemic vasoconstrictor (Henderson and Byron, 2007), in the presence of hypernatremia (Tasevska et al., 2014), which may be potentially through NKCC2 mechanisms (Konopacka et al., 2015). The effect of high dietary salt on sympathetic outflow may in part depend on central sodium sensing. Though central sodium sensing has been investigated in rodents (Kinsman et al., 2017; Shi et al., 2007; Stocker et al., 2013), human studies are lacking. Future studies should further investigate central sodium sensing in humans to determine the link between high salt and sympathetic activity in humans.
Although resting supine MSNA does not appear to be different between patients with POTS and healthy controls, patients with POTS demonstrated an exaggerated sympathetic outflow responses with orthostasis and baroreflex challenges (Bonyhay and Freeman, 2004; N. M. Swift et al., 2005). In contrast, hypotensive episodes are suggested to be closely related to a lack of sympathetic outflow (Mano and Iwase, 2003). For example, there is diminished sympathetic outflow during a head-up tilt test in healthy adults after 14-day head-down bed rest induced OH (Kamiya et al., 2003). However, studies of sympathetic outflow at rest and orthostasis are lacking in patients with chronic OH. Nonetheless, it appears as if the etiology of orthostatic disorder (POTS vs. OH) may determine the sympathetic outflow response. Norepinephrine, a catecholamine marker of sympathetic activity, is elevated in patients with POTS and a 6-day high sodium (6,900 mg/day) diet appears to reduce norepinephrine (Garland et al., 2021). Yet it is unknown what the effect sodium supplementation has directly on sympathetic outflow. Future investigations should determine 1) if sodium supplementation alters resting sympathetic outflow in patients with POTS and OH like seen in healthy adults; and 2) if improvements in heart rate and blood pressure to orthostatic stresses from sodium supplementation are influenced by changes in sympathetic outflow in POTS and OH.
3.6. Skin, Eyes, and Bone
3.6.1. Skin
Sodium loading has been shown to increase non-osmotic storage of sodium without commensurate water retention. As explained in reviews (Selvarajah et al., 2018; Wiig et al., 2018), sodium ‘leakage’ into the skin has been suggested to be primarily caused by paracellular diffusion and vascular damage of the glycocalyx and endothelium. Then sodium, and not water, binds to glycosaminoglycans, but when the sodium binding capacity of glycosaminoglycans is exceeded, interstitial hypertonicity develops (Titze et al., 2004, 2003). Macrophages are then signaled and lead to a cascade of events that secretes vascular endothelial growth factor (VEGF)-C to cause lymphangiogenesis (MacHnik et al., 2010, 2009). The enhanced lymphatic system allows for sodium to return back into the blood to be excreted through the kidneys. These mechanisms allow the skin to serve as a primary storage compartment of excess sodium and may play a key function in acute buffering volume and BP changes with high salt intake (Selvarajah et al., 2018; Wiig et al., 2018). Interestingly, one study showed that skin sodium to potassium ratio increased with 7 days of dietary sodium loading (4,600 mg/day pill supplement) in healthy humans and this may be influenced by sex (Selvarajah et al., 2017). Specifically, men experienced a significant increase in skin Na:K and no change in BP and weight. Conversely women did not have an increase in skin Na:K but did have an increase in BP and weight. Though why these sex differences exist is speculative, this along with animal studies (Titze et al., 2003) suggests the skin may play a key role in retaining sodium in effort to buffer blood volume and pressure with high salt intake. Sodium deposition in the skin appears to be more prevalent in populations that have salt-sensitive BP and is associated with hypertension and age (Kopp et al., 2013), hyperlipidemia (Crescenzi et al., 2018), diabetics on hemodialysis (Kopp et al., 2018), and LV hypertrophy in those with CKD unaffected by BP (Schneider et al., 2017). It has been suggested that chronic high skin sodium may impair VEGF-C induced lymphangiogenesis and this may reduce efflux of sodium from the skin to the systemic circulation and attenuate the vasodilatory response to salt loading (Selvarajah et al., 2018). Additional studies are needed to determine if the mechanisms of short-term accumulation of skin sodium differs from long term accumulation and whether decreasing skin sodium accumulation is effective in those with co-morbidities.
3.6.2. Bone
Several cross-sectional studies showed that elevated sodium excretion was associated with lower bone mineral density (BMD) in Asian women, but not men (Cao et al., 2017; Kim et al., 2015; Park et al., 2016). Additionally, a meta-analysis found that high dietary sodium, but not sodium excretion, increased the risk of osteoporosis, but not decreased BMD, in a more heterogenous sample of studies (Fatahi et al., 2018). Conversely, a prospective observational study of women in the United States demonstrated that sodium intake was not associated with BMD at common sites of bone degradation nor was associated with incident fractures (Carbone et al., 2016). These conflicting results may be dependent on the method of sodium assessment (intake vs. excretion), subject population, ethnicity, skin pigmentation, sex, and age (pre- vs. post-menopausal) (Fatahi et al., 2018). The mechanisms by which sodium may influence bone are unclear. One suggested mechanism is that excessive sodium intake may increase bone resorption biomarkers and decrease parathyroid hormone secretion (Cappuccio, 2013; Fatahi et al., 2018; Goulding, 1981; Goulding and Gold, 1986; Lin et al., 2003). Together these alterations may play a key role in decreasing calcium reabsorption, osteoblast differentiation and proper bone formation (Cappuccio, 2013; Fatahi et al., 2018; Parfitt, 1976; Sellmeyer et al., 2008). Future mechanistic studies are needed to further elucidate the effect of dietary salt on bone health.
3.6.3. Eyes
Observational studies in humans suggest that high salt is related to cataracts (Bae et al., 2015; Cumming et al., 2000; Tavani et al., 1996) and sodium restriction in rats decreases cataracts (Rodriguez-Sargent et al., 1989). The mechanisms of cataractogenesis due to high sodium intake is unclear but may be due to a rise in the aqueous humor and lenticular sodium concentrations causing an expansion of extracellular fluid volume and development of lens opacities (Bae et al., 2015; Mirsamadi et al., 2012). Salt loading has also been shown to damage the eyes by causing neuronal retinal edema (Qin et al., 2009), increased retinal venular but decreased retinal arteriole tortuosity (Wenstedt et al., 2021), and can aggravate neuronal retinal degeneration induced by ischemia-reperfusion injury (Li et al., 2019). These results are suggested to be through increased VEGF, inflammatory cytokines, and reduced bioavailability of NO (Li et al., 2019; Wenstedt et al., 2021). Interestingly, compared to a normal salt diet, a low salt diet in Sprague Dawley rats reduced oxygen-induced retinopathy in part by reducing neovascularization, VEGF, inflammatory cytokines, and mRNA of mineralocorticoid and angiotensin type 1 receptors (Deliyanti et al., 2014).
4. PERSPECTIVES
Increasing salt and fluid intake is one of the first non-pharmacologic changes introduced when managing orthostatic disorders (Arnold and Raj, 2017; Palma and Kaufmann, 2020; Stewart et al., 2018). The potential adverse effects of a high salt diet underscore the need to use salt judiciously in patients with orthostatic disorders. Caution should be exercised when interpreting the limited research as many unknowns remain regarding long-term effects of a high salt diet on other measures of cardiovascular health and target organ damage in patients with orthostatic disorders. Furthermore, questions persist concerning when to intervene and appropriate treatment. Systematic reviews and health organizations (Lanier et al., 2011; Loughlin et al., 2020; Low and Singer, 2008) suggest that patients with sodium excretions of <170 mmol/24 hours (~3,900 mg of sodium/24 hours) should supplement with 1,000 – 2,000 mg of sodium 3 times/day. This frequently echoed recommendation comes from a seminal paper that had a limited sample size (El-Sayed and Hainsworth, 1996), showing that 15 of 21 subjects given an additional 120 mmol/day (~2,800 mg/day) of sodium for 8 months improved orthostatic tolerance and expanded plasma volume. Further, these patients that responded to dietary sodium supplementation had an initial daily sodium excretion of <170mmol/day (~3,900 mg/day). Similarly, in pediatric and adolescent patients with POTS, sodium excretion of <124 mmol/24 hours seemed to be an indicator of the effectiveness of sodium supplementation (40–60 mg/kg per day for 1 month) (Zhang et al., 2012). As such, clinicians should consider utilizing 24-hour urinary sodium analyses for patients to better gauge which patients are viable candidates for the recommended sodium supplementation. Further, clinicians should weigh the potential risks and benefits of prescribing sodium to patients whose sodium excretion exceeds 170 mmol/24 hours (for adults) or perhaps 124 mmol/24 hours (for children) and consider alternative modes of treatment. The role of patient demographics (i.e., age, sex, pregnancy, ethnicity, and body mass) and method of salt supplementation should also be considered when prescribing dietary salt. For instance, prescribing patients to consume a diet higher in salt may promote an increased intake of salty processed foods that are calorie-dense and nutrient-deficient, which may lead to other comorbidities. On the other hand, pill capsules may cause GI distress and plasma volume loss via vomiting and dehydration (Fu and Levine, 2018). One solution to this may be the use of enteric coated capsules that do not release the sodium until it leaves the stomach and enters the small intestine. There is a clear need for future research to compare the effectiveness, dietary tolerance, and cardiovascular outcomes of different methods of salt supplementation.
5. CONCLUSION
The literature suggests high dietary sodium has deleterious effects on blood pressure and major organ systems. This is concerning given the high habitual dietary sodium intake in the U.S. The effect of high dietary sodium in patients with orthostatic disorders is less studied although high dietary sodium is routinely prescribed to these patients. As such, more research is needed to investigate these areas. We suggest that the recommendation to increase dietary salt in patients with orthostatic disorders should be done with care. Modest, rather than robust, increases in salt intake may be sufficient to alleviate symptoms but also minimize any long-term negative effects.
ACKNOWLEDGEMENTS
We would like to thank Darshil Patel, Ronald McMillan, and Nathan Romberger for their assistance in gathering references and proofreading this review article.
FUNDING
Some of the authors have been supported by the following grants which have also contributed to some of the original work cited here: NIH P20 GM113125; NIH R01 HL128388; NIH R01 HL104106.
ABBREVIATIONS
- AHA
American heart association
- BMD
Bone mineral density
- BP
Blood pressure
- BPV
Blood pressure variability
- CKD
Chronic kidney disease
- CV
Cardiovascular
- CVD
Cardiovascular disease
- eGFR
Estimated glomerular filtration rate
- ENaC
Epithelial sodium channel
- eNOS
Endothelial nitric oxide synthase
- FMD
Flow-mediated dilation
- fMRI
Functional magnetic resonance imaging
- OH
Orthostatic hypotension
- LV
Left ventricle
- mRNA
Messenger ribonucleic acid
- NaCl
Salt
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NKCC2
Na-K-2Cl co-transporter
- NO
Nitric oxide
- O2−
Superoxide
- POTS
Postural orthostatic tachycardia syndrome
- PWV
Pulse wave velocity
- ROS
Reactive oxygen species
- TGF-B1
Transforming growth factor beta 1
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrahão SB, Tinucci T, Santello JL, Mion D, 2002. Salt supresses baseline muscle sympathetic nerve activity in salt-sensitive and salt-resistant hypertensives. J. Hum. Hypertens 16, 843–850. 10.1038/sj.jhh.1001492 [DOI] [PubMed] [Google Scholar]
- Abrams J, Osborn JW, 2008. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin. Exp. Pharmacol. Physiol 35, 687–694. 10.1111/j.1440-1681.2008.04929.x.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LA, Schmidt JR, Thompson CT, Carlson BE, Beard DA, Lombard JH, 2019. High salt diet impairs cerebral blood flow regulation via salt-induced angiotensin II suppression. Microcirculation 26, e12518. 10.1111/micc.12518.HIGH [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL, 1989. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14, 177–183. 10.1161/01.HYP.14.2.177 [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM, 2011. Microvascular function predicts cardiovascular events in primary prevention: Long-term results from the firefighters and their endothelium (FATE) study. Circulation 123, 163–169. 10.1161/CIRCULATIONAHA.110.953653 [DOI] [PubMed] [Google Scholar]
- Arnold AC, Raj SR, 2017. Orthostatic Hypotension: A Practical Approach to Investigation and Management. Can. J. Cardiol 33, 1725–1728. 10.1016/j.cjca.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O’Rourke MF, 1986. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis 6, 166–169. 10.1161/01.atv.6.2.166 [DOI] [PubMed] [Google Scholar]
- Avolio AP, Fa-quan D, Wei-qiang LI, Yao-fei L, Zhen-dong H, Lian-fen X, ORourke MF, 1985. Effects of ageing on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 71, 202–210. [DOI] [PubMed] [Google Scholar]
- Babcock MC, Robinson AT, Migdal KU, Watso JC, Wenner MM, Stocker SD, Farquhar WB, 2019. Reducing dietary sodium to 1000 mg per day reduces neurovascular transduction without stimulating sympathetic outflow. Hypertension 73, 587–593. 10.1161/HYPERTENSIONAHA.118.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Shin DS, Lee SC, Hwang IC, 2015. Sodium intake and socioeconomic status as risk factors for development of age-related cataracts: The Korea national health and nutrition examination survey. PLoS One 10, 1–11. 10.1371/journal.pone.0136218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barić L, Drenjančević I, Matić A, Stupin M, Kolar L, Mihaljević Z, Lenasi H, Šerić V, Stupin A, 2019. Seven-Day Salt Loading Impairs Microvascular Endothelium-Dependent Vasodilation without Changes in Blood Pressure, Body Composition and Fluid Status in Healthy Young Humans. Kidney Blood Press. Res 44, 835–847. 10.1159/000501747 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, 2019. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Bhagat K, Vallance P, 1997. Inflammatory Cytokines Impair Endothelium-Dependent Dilatation in Human Veins In Vivo. Circulation 96, 3042–3047. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L, 2010. Projected effect of dietary salt reductions on future cardiovascular disease. Obstet. Gynecol. Surv 65, 441–442. 10.1097/OGX.0b013e3181e5f253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonyhay I, Freeman R, 2004. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110, 3193–3198. 10.1161/01.CIR.0000147280.90339.E9 [DOI] [PubMed] [Google Scholar]
- Brunt VE, Minson CT, 2011. Cutaneous thermal hyperemia: More than skin deep. J. Appl. Physiol 111, 5–7. 10.1152/japplphysiol.00544.2011 [DOI] [PubMed] [Google Scholar]
- Burnier M, Phan O, Wang Q, 2007. High salt intake: A cause of blood pressure-independent left ventricular hypertrophy? Nephrol. Dial. Transplant 22, 2426–2429. 10.1093/ndt/gfm321 [DOI] [PubMed] [Google Scholar]
- Cao WT, He J, Chen GD, Wang C, Qiu R, Chen YM, 2017. The association between urinary sodium to potassium ratio and bone density in middle-aged Chinese adults. Osteoporos. Int 28, 1077–1086. 10.1007/s00198-016-3835-9 [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, 2013. Cardiovascular and other effects of salt consumption. Kidney Int. Suppl 3, 312–315. 10.1038/kisup.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Capewell S, Lincoln P, McPherson K, 2011. Policy options to reduce population salt intake. BMJ 343. 10.1136/bmj.d4995 [DOI] [PubMed] [Google Scholar]
- Carbone L, Johnson KC, Huang Y, Pettinger M, Thomas F, Cauley J, Crandall C, Tinker L, LeBoff MS, Wactawski-Wende J, Bethel M, Li W, Prentice R, 2016. Sodium intake and osteoporosis. Findings from the Women’s Health Initiative. J. Clin. Endocrinol. Metab 101, 1414–1421. 10.1210/jc.2015-4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavka A, Jukic I, Ali M, Goslawski M, Bian JT, Wang E, Drenjancevic I, Phillips SA, 2016. Short-term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J. Hypertens 34, 676–684. 10.1097/HJH.0000000000000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Wallin BG, 2014. Sympathetic neural activity to the cardiovascular system: Integrator of systemic physiology and interindividual characteristics. Compr. Physiol 4, 827–850. 10.1002/cphy.c130038 [DOI] [PubMed] [Google Scholar]
- Chirinos JA, Kips JG, Jacobs DR, Brumback L, Duprez DA, Kronmal RA, Bluemke DA, Townsend RR, Vermeersch S, Segers P, 2012. Arterial Wave Reflection and Incident Cardiovascular Events and Heart Failure: The Multiethnic Study of Atherosclerosis. Biophys. Chem 257, 2432–2437. 10.1016/j.immuni.2010.12.017.Two-stage [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos JA, Segers P, 2010a. Noninvasive evaluation of left ventricular afterload: Part 1: Pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension 56, 555–562. 10.1161/HYPERTENSIONAHA.110.157321 [DOI] [PubMed] [Google Scholar]
- Chirinos JA, Segers P, 2010b. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension 56, 563–570. 10.1161/HYPERTENSIONAHA.110.157339 [DOI] [PubMed] [Google Scholar]
- Chopoorian AH, Wahba A, Celedonio J, Nwazue V, Smith EC, Garland EM, Paranjape S, Okamoto LE, Black BK, Biaggioni I, Raj SR, Gamboa A, 2021. Impaired Endothelial Function in Patients With Postural Tachycardia Syndrome. Hypertension 77, 1001–1009. 10.1161/hypertensionaha.120.16238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon VE, Hainsworth R, 2004. Salt Supplementation Improves Orthostatic Cerebral and Peripheral Vascular Control in Patients with Syncope. Hypertension 43, 809–813. 10.1161/01.HYP.0000122269.05049.e7 [DOI] [PubMed] [Google Scholar]
- Cosic A, Jukic I, Stupin A, Mihalj M, Mihaljevic Z, Novak S, Vukovic R, Drenjancevic I, 2016. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol 594, 4917–4931. 10.1113/JP272297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer A, Boutouyrie P, Laurent S, Gosse P, Tzourio C, 2020. Orthostatic hypotension: a marker of blood pressure variability and arterial stiffness: a cross- sectional study on an elderly population: the 3-City study. J. Hypertens 38, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Crescenzi R, Marton A, Donahue PMC, Mahany HB, Lants SK, Wang P, Beckman JA, Donahue MJ, Titze J, 2018. Tissue sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema. Obes. (Silver Spring) 26, 310–317. 10.1038/s41395-018-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming RG, Mitchell P, Smith W, 2000. Dietary sodium intake and cataract: The Blue Mountains Eye Study. Am. J. Epidemiol 151, 624–626. 10.1093/oxfordjournals.aje.a010251 [DOI] [PubMed] [Google Scholar]
- D’Elia L, Galletti F, La Fata E, Sabino P, Strazzullo P, 2018. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens 36, 734–743. 10.1097/HJH.0000000000001604 [DOI] [PubMed] [Google Scholar]
- Deliyanti D, Armani R, Casely D, Figgett WA, Agrotis A, Wilkinson-Berka JL, 2014. Retinal vasculopathy is reduced by dietary salt restriction: Involvement of glia, ENaCα, and the renin-angiotensin-aldosterone system. Arterioscler. Thromb. Vasc. Biol 34, 2033–2041. 10.1161/ATVBAHA.114.303792 [DOI] [PubMed] [Google Scholar]
- Dickinson KM, Clifton PM, Keogh JB, 2014. A reduction of 3g/day from a usual 9g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised cross_over study in normotensive overweight and obese subjects. Atherosclerosis 233, 32–38. 10.1016/j.atherosclerosis.2013.11.078 [DOI] [PubMed] [Google Scholar]
- Doi R, Masuyama T, Yamamoto K, Doi Y, Mano T, Sakata Y, Ono K, Kuzuya T, Hirota S, Koyama T, Miwa T, Hori M, 2000. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J. Hypertens 18, 111–120. [DOI] [PubMed] [Google Scholar]
- Du Cailar G, Fesler P, Ribstein J, Mimran A, 2010. Dietary sodium, aldosterone, and left ventricular mass changes during long-term inhibition of the renin-angiotensin system. Hypertension 56, 865–870. 10.1161/HYPERTENSIONAHA.110.159277 [DOI] [PubMed] [Google Scholar]
- DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG, 2013. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31, 530–536. 10.1097/HJH.0b013e32835c6ca8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand MJ, Lombard JH, 2013. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. Am. J. Hypertens 26, 739–747. 10.1093/ajh/hpt015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D, 2000. Orthostatic hypotension as a risk factor for stroke: The Atherosclerosis Risk in Communities (ARIC) study, 1987–1996. Stroke 31, 2307–2313. 10.1161/01.STR.31.10.2307 [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT, 2012. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J. Appl. Physiol 112, 1049–1053. 10.1152/japplphysiol.01197.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed H, Hainsworth R, 1996. Salt supplement increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart 75, 134–140. 10.1136/hrt.75.2.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS, 2015. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol 65, 1042–1050. 10.1016/j.jacc.2014.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME, 2006. Sympathetic neural responses to increased osmolality in humans. Am. J. Physiol. - Hear. Circ. Physiol 291, 2181–2186. 10.1152/ajpheart.00191.2006 [DOI] [PubMed] [Google Scholar]
- Fatahi S, Namazi N, Larijani B, Azadbakht L, 2018. The Association of Dietary and Urinary Sodium With Bone Mineral Density and Risk of Osteoporosis: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr 37, 522–532. 10.1080/07315724.2018.1431161 [DOI] [PubMed] [Google Scholar]
- Ferrara AL, De Simone G, Pasanisi F, Mancini Marcello, Mancini Mario, 1984. Left ventricular mass reduction during salt depletion in arterial hypertension. Hypertension 6, 755–759. 10.1161/01.hyp.6.5.755 [DOI] [PubMed] [Google Scholar]
- Figueroa JJ, Basford JR, Low PA, 2010. Preventing and treating orthostatic hypotension: As easy as A, B, C. Cleve. Clin. J. Med 77, 298–306. 10.3949/ccjm.77a.09118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ, 2009. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. Basic Clin 148, 5–15. 10.1016/j.autneu.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Levine BD, 2018. Exercise and non-pharmacological treatment of POTS. Auton. Neurosci. Basic Clin 215, 20–27. 10.1016/j.autneu.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ortiz L, Recio-Rodríguez JI, Rodríguez-Sánchez E, Patino-Alonso MC, Agudo-Conde C, Rodríguez-Martín C, Castaño-Sánchez C, Runkle I, Gómez-Marcos MA, 2012. Sodium and potassium intake present a J-shaped relationship with arterial stiffness and carotid intima-media thickness. Atherosclerosis 225, 497–503. 10.1016/j.atherosclerosis.2012.09.038 [DOI] [PubMed] [Google Scholar]
- Garland EM, Gamboa A, Nwazue VC, Celedonio JE, Paranjape SY, Black BK, Okamoto LE, Shibao CA, Biaggioni I, Robertson D, Diedrich A, Dupont WD, Raj SR, 2021. Effect of High Dietary Sodium Intake in Patients With Postural Tachycardia Syndrome. J. Am. Coll. Cardiol 77, 2174–2184. 10.1016/j.jacc.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo C, Borrelli S, Provenzano M, De Stefano T, Vita C, Chiodini P, Minutolo R, De Nicola L, Conte G, 2018. Dietary salt restriction in chronic kidney disease: A meta-analysis of randomized clinical trials. Nutrients 10, 1–15. 10.3390/nu10060732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce N, Keaney JF, Hunter LM, Watkins MT, Menzoian JO, Vita JA, 2002. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 105, 1567–1572. 10.1161/01.CIR.0000012543.55874.47 [DOI] [PubMed] [Google Scholar]
- Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD, 2008. Conduit Artery Constriction Mediated by Low Flow. A Novel Noninvasive Method for the Assessment of Vascular Function. J. Am. Coll. Cardiol 51, 1953–1958. 10.1016/j.jacc.2008.01.049 [DOI] [PubMed] [Google Scholar]
- Gori T, von Henning U, Muxel S, Schaefer S, Fasola F, Vosseler M, Schnorbus B, Binder H, Parker JD, Munzel T, 2016. Both Flow-Mediated Dilation and Constriction are associated with changes in blood flow and shear stress: Two complementary perspectives on endothelial function. Clin Hemorheol Microcirc 64, 255–266. [DOI] [PubMed] [Google Scholar]
- Goulding A, 1981. Fasting urinary sodium/creatinine in relation to calcium/creatinine and hydroxyproline/creatinine in a general population of women. N. Z. Med. J 93, 294–297. [PubMed] [Google Scholar]
- Goulding A, Gold E, 1986. Effects of dietary sodium chloride loading on parathyroid function, 1,25-dihydroxyvitamin d, calcium balance, and bone metabolism in female rats during chronic prednisolone administration. Endocrinology 119, 2148–2154. 10.1210/endo-119-5-2148 [DOI] [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Lanfrachni A, Bolla G, Mancia G, 1997. Baroreflex Impairment by Low Sodium Diet in Mild or Moderate Essential Hypertension. Hypertension 29, 802–807. [DOI] [PubMed] [Google Scholar]
- Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB, 2012. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590, 5519–5528. 10.1113/jphysiol.2012.236992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ, 2014. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension 63, 376–382. 10.1161/HYPERTENSIONAHA.113.02044 [DOI] [PubMed] [Google Scholar]
- Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G, 2019. Sodium intake and hypertension. Nutrients 11, 1–16. 10.3390/nu11091970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Anand V, Shek EW, Moore MC, Brady AL, Kelly WC, Adair TH, 1998. Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension 31, 1083–1087. 10.1161/01.HYP.31.5.1083 [DOI] [PubMed] [Google Scholar]
- Guers JJ, Kasecky-Lardner L, Farquhar WB, Edwards DG, Lennon SL, 2019. Voluntary wheel running prevents salt-induced endothelial dysfunction: Role of oxidative stress. J. Appl. Physiol 126, 502–510. 10.1152/japplphysiol.00421.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring B, Wang W, Lee ET, Jhamnani S, Howard BV, Devereux RB, 2015. Effect of dietary sodium and potassium intake on left ventricular diastolic function and mass in adults ≤40 years (from the Strong Heart Study). Am. J. Cardiol 115, 1244–1248. 10.1016/j.amjcard.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto J, Nichols WW, O’Rourke MF, Imai Y, 2008. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: Influence of arterial wave reflection. Am. J. Hypertens 21, 329–333. 10.1038/ajh.2007.49 [DOI] [PubMed] [Google Scholar]
- He FJ, Li J, MacGregor GA, 2013. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 346. 10.1136/bmj.f1325 [DOI] [PubMed] [Google Scholar]
- He J, Ogden L, Bazzano LA, Vupputuri S, Loria C, Whelton PK, 2002. Dietary sodium intake and incidence of congestive heart failure in overweight U.S. men and women. Am. J. Hypertens 162, 1619–1624. 10.1016/s0895-7061(00)00316-2 [DOI] [PubMed] [Google Scholar]
- Henderson KK, Byron KL, 2007. Vasopressin-induced vasoconstriction: Two concentration-dependent signaling pathways. J. Appl. Physiol 102, 1402–1409. 10.1152/japplphysiol.00825.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL, 2008. The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol 105, 370–372. 10.1152/japplphysiol.00165.2008 [DOI] [PubMed] [Google Scholar]
- Hosohata K, 2017. Biomarkers for chronic kidney disease associated with high salt intake. Int. J. Mol. Sci 18. 10.3390/ijms18102080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosohata K, Yoshioka D, Tanaka A, Ando H, Fujimura A, 2016. Early urinary biomarkers for renal tubular damage in spontaneously hypertensive rats on a high salt intake. Hypertens. Res 39, 19–26. 10.1038/hr.2015.103 [DOI] [PubMed] [Google Scholar]
- Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, Ehling P, Meuth SG, Roth J, Kuhlmann T, Wiendl H, Klotz L, 2016. Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J. Autoimmun 67, 90–101. 10.1016/j.jaut.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Inoko M, Kihara Y, Morii I, Fujiwara H, Sasayama S, 1994. Transition from compensatory hypertrophy to dilated failing left ventricles in Dahl salt-sensitive rats. Am. J. Physiol. - Hear. Circ. Physiol 267, H2471–H2482. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR, 2013. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J. Am. Coll. Cardiol 61, 335–343. 10.1016/j.jacc.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kuznetsova T, Maillard M, Richart T, Thijs L, Bochud M, Herregods MC, Burnier M, Fagard R, Staessen JA, 2009. Independent relations of left ventricular structure with the 24-hour urinary excretion of sodium and aldosterone. Hypertension 54, 489–495. 10.1161/HYPERTENSIONAHA.109.130492 [DOI] [PubMed] [Google Scholar]
- Jones CD, Loehr L, Franceschini N, Rosamond WD, Chang PP, Shahar E, Couper DJ, Rose KM, 2012. Orthostatic hypotension as a risk factor for incident heart failure: The atherosclerosis risk in communities study. Hypertension 59, 913–918. 10.1161/HYPERTENSIONAHA.111.188151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jula AM, Karanko HM, 1994. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation 89, 1023–1031. 10.1161/01.CIR.89.3.1023 [DOI] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Fu Q, Iwase S, Hayano J, Kawada T, Mano T, Sunagawa K, 2003. Pathophysiology of orthostatic hypotension after bed rest: Paradoxical sympathetic withdrawal. Am. J. Physiol. - Hear. Circ. Physiol 285, 5–7. 10.1152/ajpheart.00965.2002 [DOI] [PubMed] [Google Scholar]
- Kim SW, Jeon JH, Choi YK, Lee WK, Hwang IR, Kim JG, Lee IK, Park KG, 2015. Association of urinary sodium/creatinine ratio with bone mineral density in postmenopausal women: KNHANES 2008–2011. Endocrine 49, 791–799. 10.1007/s12020-015-0532-y [DOI] [PubMed] [Google Scholar]
- Kinsman BJ, Simmonds SS, Browning KN, Stocker SD, 2017. The Organum Vasculosum of the Lamina Terminalis Detects NaCl to Elevate Sympathetic Nerve Activity and Blood Pressure. Hypertension 69, 163–170. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS, 2003. Salt Intake, Oxidative Stress, and Renal Expression of NADPH Oxidase and Superoxide Dismutase. J. Am. Soc. Nephrol 14, 2775–2782. 10.1097/01.ASN.0000092145.90389.65 [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA, 2013. Sodium chloride drives autoimmune disease by the induction of pathogenic TH 17 cells. Nature 496, 518–522. 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D, 2006. Development of heart failure in chronic hypertensive Dahl rats: Focus on heart failure with preserved ejection fraction. Hypertension 47, 901–911. 10.1161/01.HYP.0000215579.81408.8e [DOI] [PubMed] [Google Scholar]
- Konopacka A, Qiu J, Yao ST, Greenwood MP, Greenwood M, Lancaster T, Inoue W, De Mecawi AS, Vechiato FMV, De Lima JBM, Coletti R, Hoe SZ, Martin A, Lee J, Joseph M, Hindmarch C, Paton J, Antunes-Rodrigues J, Bains J, Murphy D, 2015. Osmoregulation requires brain expression of the renal Na-K-2Cl cotransporter NKCC2. J. Neurosci 35, 5144–5155. 10.1523/JNEUROSCI.4121-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J, 2013. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61, 635–640. 10.1161/HYPERTENSIONAHA.111.00566 [DOI] [PubMed] [Google Scholar]
- Kopp C, Linz P, Maier C, Wabel P, Hammon M, Nagel AM, Rosenhauer D, Horn S, Uder M, Luft FC, Titze J, Dahlmann A, 2018. Elevated tissue sodium deposition in patients with type 2 diabetes on hemodialysis detected by 23Na magnetic resonance imaging. Kidney Int 93, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Toth J, Tarjan J, Koller A, 2006. Correlation of flow mediated dilation with inflammatory markers in patients with impaired cardiac function. Beneficial effects of inhibition of ACE. Eur. J. Heart Fail 10.1016/j.ejheart.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Langenfeld MRW, Schobel H, Veelken R, Weihprecht H, Schmieder RE, 1998. Impact of dietary sodium intake on left ventricular diastolic filling in early essential hypertension. Eur. Heart J 19, 951–958. 10.1053/euhj.1997.0854 [DOI] [PubMed] [Google Scholar]
- Lanier JB, Mote MB, Clay EC, 2011. Evaluation and management of orthostatic hypotension. Am. Fam. Physician 84, 527–536. [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, 2006. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J 27, 2588–2605. 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- Lennon-Edwards S, Ramick MG, Matthews EL, Brian MS, Farquhar WB, Edwards DG, 2014. Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutr Metab Cardiovasc Dis 24, 990–995. 10.1016/j.numecd.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fang W, Hu F, Zhou X, Cheng Y, Jiang C, 2019. A high-salt diet aggravates retinal ischaemia/reperfusion injury. Exp. Eye Res 188, 107784. 10.1016/j.exer.2019.107784 [DOI] [PubMed] [Google Scholar]
- Li XY, Cai XL, Da Bian P, Hu LR, 2012. High salt intake and stroke: Meta-analysis of the epidemiologic evidence. CNS Neurosci. Ther 18, 691–701. 10.1111/j.1755-5949.2012.00355.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Chen S, Liu X, Zhang Q, Ai Y, Wang Y, Jin H, Tang C, Du J, 2010. Flow-mediated vasodilation and endothelium function in children with postural orthostatic tachycardia syndrome. Am. J. Cardiol 106, 378–382. 10.1016/j.amjcard.2010.03.034 [DOI] [PubMed] [Google Scholar]
- Lin J, Hu FB, Curhan GC, 2010. Associations of diet with albuminuria and kidney function decline. Clin. J. Am. Soc. Nephrol 5, 836–843. 10.2215/CJN.08001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D, Svetkey LP, 2003. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J. Nutr 133, 3130–3136. 10.1093/jn/133.10.3130 [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, 2010. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation 121, 586–613. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- Lombard JH, Sylvester FA, Phillips SA, Frisbee JC, 2003. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am. J. Physiol. - Hear. Circ. Physiol 284, 1124–1133. 10.1152/ajpheart.00835.2002 [DOI] [PubMed] [Google Scholar]
- Loughlin EA, Judge CS, Gorey SE, Costello MM, Murphy RP, Waters RF, Hughes DS, Kenny RA, O’Donnell MJ, Canavan MD, 2020. Increased Salt Intake for Orthostatic Intolerance Syndromes: A Systematic Review and Meta-Analysis. Am. J. Med 133, 1471–1478.e4. 10.1016/j.amjmed.2020.05.028 [DOI] [PubMed] [Google Scholar]
- Low PA, Singer W, 2008. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol 5, 451–458. 10.1016/j.jamda.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHnik A, Dahlmann A, Kopp C, Goss J, Wagner H, Van Rooijen N, Eckardt KU, Müller DN, Park JK, Luft FC, Kerjaschki D, Titze J, 2010. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor c expression and induces salt-sensitive hypertension in rats. Hypertension 55, 755–761. 10.1161/HYPERTENSIONAHA.109.143339 [DOI] [PubMed] [Google Scholar]
- MacHnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, MacHura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, Van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J, 2009. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med 15, 545–552. 10.1038/nm.1960 [DOI] [PubMed] [Google Scholar]
- Magnusson M, Holm H, Bachus E, Nilsson P, Leosdottir M, Melander O, Jujic A, Fedorowski A, 2016. Orthostatic hypotension and cardiac changes after long-term follow-up. Am. J. Hypertens 29, 847–852. 10.1093/ajh/hpv187 [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, 2003. The role of blood pressure variability in end-organ damage. J. Hypertens 21, S17–23. [DOI] [PubMed] [Google Scholar]
- Mano T, Iwase S, 2003. Sympathetic nerve activity in hypotension and orthostatic intolerance. Acta Physiol. Scand 177, 359–365. 10.1046/j.1365-201X.2003.01081.x [DOI] [PubMed] [Google Scholar]
- Markus H, Cullinane M, 2001. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124, 457–467. 10.1093/brain/124.3.457 [DOI] [PubMed] [Google Scholar]
- Martin JV, Liberati DM, Diebel LN, 2018. Excess sodium is deleterious on endothelial and glycocalyx barrier function: A microfluidic study. J. Trauma Acute Care Surg 85, 128–134. 10.1097/TA.0000000000001892 [DOI] [PubMed] [Google Scholar]
- Matic A, Jukic I, Stupin A, Baric L, Mihaljevic Z, Unfirer S, Tartaro Bujak I, Mihaljevic B, Lombard JH, Drenjancevic I, 2018. High salt intake shifts the mechanisms of flow-induced dilation in the middle cerebral arteries of Sprague-Dawley rats. Am. J. Physiol. Heart Circ. Physiol 315, H718–H730. 10.1152/ajpheart.00097.2018 [DOI] [PubMed] [Google Scholar]
- Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB, 2015. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J. Appl. Physiol 118, 1510–1515. 10.1152/japplphysiol.00023.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdal KU, Babcock MC, Robinson AT, Watso JC, Wenner MM, Stocker SD, Farquhar WB, 2020a. The Impact of High Dietary Sodium Consumption on Blood Pressure Variability in Healthy, Young Adults. Am. J. Hypertens 33, 422–429. 10.1093/ajh/hpaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdal KU, Robinson AT, Watso JC, Babcock MC, Lennon SL, Martens CR, Serrador JM, Farquhar WB, 2020b. A high salt meal does not impair cerebrovascular reactivity in healthy young adults. Physiol. Rep 8, 1–13. 10.14814/phy2.14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsamadi M, Nourmohammadi I, Imamian M, 2012. Comparative study of serum Na + and K + levels in senile cataract patients and normal individuals. Int. J. Med. Sci 1, 165–169. 10.7150/ijms.1.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möckl L, 2020. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol 8, 1–14. 10.3389/fcell.2020.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiari LL, Ceriati R, Taliani U, Montesi MC, Novarini A, 1999. Early abnormalities in left ventricular diastolic function of sodium-sensitive hypertensive patients. J. Hum. Hypertens 13, 711–716. 10.1038/sj.jhh.1000893 [DOI] [PubMed] [Google Scholar]
- Muth BJ, Brian MS, Chirinos JA, Lennon SL, Farquhar WB, Edwards DG, 2017. Central systolic blood pressure and aortic stiffness response to dietary sodium in young and middle-aged adults. J. Am. Soc. Hypertens 11, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M, 1998. Benzamil blockade of brain Na+ channels averts Na+-induced hypertension in rats. Am. J. Physiol. - Regul. Integr. Comp. Physiol 274, 635–644. 10.1152/ajpregu.1998.274.3.r635 [DOI] [PubMed] [Google Scholar]
- Noda M, Hiyama TY, 2015. Sodium sensing in the brain. Pflugers Arch. Eur. J. Physiol 467, 465–474. 10.1007/s00424-014-1662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi Kenta, Kobayashi Kazuto, Kuwaki T, Takahashi K, Matsui S, Noda M, 2019. [Na +] Increases in Body Fluids Sensed by Central Na x Induce Sympathetically Mediated Blood Pressure Elevations via H + -Dependent Activation of ASIC1a. Neuron 101, 60–75.e6. 10.1016/j.neuron.2018.11.017 [DOI] [PubMed] [Google Scholar]
- O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE, 2011. Urinary Sodium and Potassium Excretion and Risk of Cardiovascular Events. JAMA 306, 2229–38. [DOI] [PubMed] [Google Scholar]
- Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K, 2011. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. Eur. J. Physiol 462, 519–528. 10.1007/s00424-011-0999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM, 2009. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am. J. Physiol. - Hear. Circ. Physiol 297, 664–673. 10.1152/ajpheart.00138.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Tsuchihashi T, Kiyohara K, Oniki H, 2013. High salt intake promotes a decline in renal function in hypertensive patients: A 10-year observational study. Hypertens. Res 36, 172–176. 10.1038/hr.2012.155 [DOI] [PubMed] [Google Scholar]
- Ozkayar N, Dede F, Ates I, Akyel F, Yildirim T, Altun B, 2016. The relationship between dietary salt intake and ambulatory blood pressure variability in non-diabetic hypertensive patients. Nefrologia 36, 694–700. 10.1016/j.nefro.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Palma JA, Kaufmann H, 2020. Management of Orthostatic Hypotension, CONTINUUM Lifelong Learning in Neurology 10.1212/CON.0000000000000816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Ochoa JE, Lombardi C, Bilo G, 2013. Assessment and management of blood-pressure variability. Nat. Rev. Cardiol 10, 143–155. [DOI] [PubMed] [Google Scholar]
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G, 1987. Relationship of 24-Hour Blood Pressure Mean and Variability to Severity of Target Organ Damage in Hypertension. J. Hypertens 5, 93–98. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, 1976. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological a. Metab. Clin. Exp 25, 809–844. [DOI] [PubMed] [Google Scholar]
- Park Y, Kwon SJ, Ha YC, 2016. Association between Urinary Sodium Excretion and Bone Health in Male and Female Adults. Ann. Nutr. Metab 68, 189–196. [DOI] [PubMed] [Google Scholar]
- Patik JC, Lennon SL, Farquhar WB, Edwards DG, 2021. Endothelial Function and Potential Countermeasures. Nutrients 13, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry IJ, Beevers DG, 1992. Salt intake and stroke: a possible direct effect. J. Hum. Hypertens 6, 23–25. [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G, 2001. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104, 191–196. 10.1161/01.CIR.104.2.191 [DOI] [PubMed] [Google Scholar]
- Phipps MS, Schmid AA, Kapoor JR, Peixoto AJ, Williams LS, Bravata DM, 2012. Orthostatic hypotension among outpatients with ischemic stroke. J. Neurol. Sci 314, 62–65. 10.1016/j.jns.2011.10.031 [DOI] [PubMed] [Google Scholar]
- Qin Y, Fan J, Ye X, Xu G, Liu W, Da C, 2009. High salt loading alters the expression and localization of glial aquaporins in rat retina. Exp. Eye Res 89, 88–94. 10.1016/j.exer.2009.02.017 [DOI] [PubMed] [Google Scholar]
- Quader ZS, Zhao L, Gillespie C, Cogswell ME, Terry AL, Moshfegh A, Rhodes D, 2017. Sodium Intake Among Persons Aged ≥2 Years — United States, 2013–2014. MMWR Morb Mortal Wkly 66, 324–238. 10.15585/mmwr.mm6612a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D, 2005. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 111, 1574–1582. 10.1161/01.CIR.0000160356.97313.5D [DOI] [PubMed] [Google Scholar]
- Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, Thibodeau-Jarry N, Sheldon RS, 2020. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can. J. Cardiol 36, 357–372. 10.1016/j.cjca.2019.12.024 [DOI] [PubMed] [Google Scholar]
- Ramick MG, Brian MS, Matthews EL, Patik JC, Seals DR, Lennon SL, Farquhar WB, Edwards DG, 2019. Apocynin and tempol ameliorate dietary sodium-induced declines in cutaneous microvascular function in salt-resistant humans. Am. J. Physiol. - Hear. Circ. Physiol 317, H97–H103. 10.1152/ajpheart.00786.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AT, Edwards DG, Farquhar WB, 2019. The Influence of Dietary Salt Beyond Blood Pressure. Curr. Hypertens. Rep 21. 10.1007/s11906-019-0948-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sargent C, Berrios G, Irrizarry JE, Estape ES, Cangiano JL, Martinez-Maldonado M, 1989. Prevention and reversal of cataracts in genetically hypertensive rats through sodium restriction. Investig. Ophthalmol. Vis. Sci 30, 2356–2360. [PubMed] [Google Scholar]
- Rodriguez CJ, Bibbins-Domingo K, Jin Z, Daviglus ML, Goff DC, Jacobs DR, 2011. Association of sodium and potassium intake with left ventricular mass: Coronary artery risk development in young adults. Hypertension 58, 410–416. 10.1161/HYPERTENSIONAHA.110.168054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouabhi M, Durieux J, Al-Kindi S, Cohen JB, Townsend RR, Rahman M, 2021. Orthostatic Hypertension and Hypotension and Outcomes in CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study. Kidney Med 3, 206–215.e1. 10.1016/j.xkme.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafidis PA, Ruilope LM, Loutradis C, Gorostidi M, de la Sierra A, de le Cruz JJ, Vinyoles E, Divison-Garrote JA, Segura J, Banegas JR, 2018. Blood pressure variability increases with advancing chronic kidney disease stage: a cross- sectional analysis of 16?546 hypertensive patients. J. Hypertens 36, 1076–1085. [DOI] [PubMed] [Google Scholar]
- Schierke F, Wyrwoll MJ, Wisdorf M, Niedzielski L, Maase M, Ruck T, Meuth SG, Kusche-Vihrog K, 2017. Nanomechanics of the endothelial glycocalyx contribute to Na+ - Induced vascular inflammation. Sci. Rep 7, 1–11. 10.1038/srep46476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, Schlieper G, Saritas T, Floege J, Schmid M, Birukov A, Dahlmann A, Linz P, Janka R, Uder M, Schmieder RE, Titze JM, Eckardt KU, 2017. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J. Am. Soc. Nephrol 28, 1867–1876. 10.1681/ASN.2016060662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, Ferrario M, Mancia G, 2002. Blood pressure variability and organ damage in a general population: Results from the PAMELA study. Hypertension 39, 710–714. 10.1161/hy0202.104376 [DOI] [PubMed] [Google Scholar]
- Sellmeyer DE, Schloetter M, S, D.E.D.E., Sebastian A, 2008. Excretion and bone resorption induced by a high sodium chloride diet. J. Clin. Endocrinol. Metab 87, 2008–2012. [DOI] [PubMed] [Google Scholar]
- Selvarajah V, Connolly K, McEniery C, Wilkinson I, 2018. Skin Sodium and Hypertension: a Paradigm Shift? Curr. Hypertens. Rep 20. 10.1007/s11906-018-0892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM, Wilkinson IB, 2017. Novel Mechanism for Buffering Dietary Salt in Humans: Effects of Salt Loading on Skin Sodium, Vascular Endothelial Growth Factor C, and Blood Pressure. Hypertension 70, 930–937. 10.1161/HYPERTENSIONAHA.117.10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K, 2015. 2015 Heart Rhythm Society Expert Consensus Statement on the Diagnosis and Treatment of Postural Tachycardia Syndrome, Inappropriate Sinus Tachycardia, and Vasovagal Syncope. Hear. Rhythm 12, e41–e63. 10.1016/j.hrthm.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenouda N, Ramick MG, Lennon SL, Farquhar WB, Edwards DG, 2020. High dietary sodium augments vascular tone and attenuates low-flow mediated constriction in salt-resistant adults. Eur. J. Appl. Physiol 120, 1383–1389. 10.1007/s00421-020-04370-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Stocker SD, Toney GM, 2007. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am. J. Physiol. - Regul. Integr. Comp. Physiol 293, 2279–2289. 10.1152/ajpregu.00160.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao C, Lipsitz LA, Biaggioni I, 2013. ASH Position Paper: Evaluation and Treatment of Orthostatic Hypotension. J. Clin. Hypertens 15, 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Caltagirone C, 2000. Impaired Cerebral Vasoreactivity and Risk of Stroke in Patients With Asymptomatic Carotid Artery Stenosis. J. Am. Hear. Assoc 283, 2122–27. [DOI] [PubMed] [Google Scholar]
- Simmonds SS, Lay J, Stocker SD, 2014. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64, 583–589. 10.1161/HYPERTENSIONAHA.114.03250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EC, Celedonio J, Nwazue VC, Garland EM, Paranjape SY, Chopoorian AH, Wahba A, Biaggioni I, Black B, Shibao CA, Diedrich A, Okamoto LE, Raj SR, Gamboa A, 2021. High-sodium diet does not worsen endothelial function in female patients with postural tachycardia syndrome. Clin. Auton. Res 10.1007/s10286-021-00772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth A, O’Donnell MJ, Yusuf S, Clase CM, Teo KK, Canavan M, Reddan DN, Mann JFE, 2014. Sodium intake and renal outcomes: A systematic review. Am. J. Hypertens 27, 1277–1284. 10.1093/ajh/hpt294 [DOI] [PubMed] [Google Scholar]
- Starmans-Kool MJ, Stanton AV, Xu YY, McG Thom SA, Parker KH, Hughes AD, 2011. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J. Appl. Physiol 110, 468–471. 10.1152/japplphysiol.00917.2010 [DOI] [PubMed] [Google Scholar]
- Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ, 2016. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Boris JR, Chelimsky G, Fischer PR, Fortunato JE, Grubb BP, Heyer GL, Jarjour IT, Medow MS, Numan MT, Pianosi PT, Singer W, Tarbell S, Chelimsky TC, 2018. Pediatric disorders of orthostatic intolerance. Pediatrics 141. 10.1542/peds.2017-1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB, 2015. Cerebrospinal Fluid Hypernatremia Elevates Sympathetic Nerve Activity and Blood Pressure via the Rostral Ventrolateral Medulla. Hypertension 66, 1184–1190. 10.1161/HYPERTENSIONAHA.115.05936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Monahan KD, Browning KN, 2013. Neurogenic and Sympathoexcitatory Actions of NaCl in Hypertension. Curr. Hypertens. Rep 15, 538–546. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SH, Chen ZY, Tseng TW, Lu DY, Yu WC, Cheng HM, Chen CH, 2014. Wave reflections, arterial stiffness, and orthostatic hypotension. Am. J. Hypertens 27, 1446–1455. 10.1093/ajh/hpu063 [DOI] [PubMed] [Google Scholar]
- Sunwoo JS, Yang TW, Kim DY, Lim JA, Kim TJ, Byun JI, Moon J, Lee ST, Jung KH, Park K Il, Jung KY, Kim M, Lee SK, Chu K, 2017. Association of blood pressure variability with orthostatic intolerance symptoms. PLoS One 12, 1–13. 10.1371/journal.pone.0179132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG, 2011. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J. Hypertens 29, 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift NM, Charkoudian N, Dotson RM, Suarez GA, Low PA, 2005. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am. J. Physiol. - Hear. Circ. Physiol 289, H1226–H1233. 10.1152/ajpheart.01243.2004 [DOI] [PubMed] [Google Scholar]
- Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA, 2005. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: A randomized control trial. Hypertension 46, 308–312. 10.1161/01.HYP.0000172662.12480.7f [DOI] [PubMed] [Google Scholar]
- Tasevska I, Enhörning S, Burri P, Melander O, 2014. High salt intake increases copeptin but salt sensitivity is associated with fluid induced reduction of copeptin in women. Int. J. Hypertens 2014. 10.1155/2014/641587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavani A, Negri E, La Vecchia C, 1996. Food and nutrient intake and risk of cataract. Ann. Epidemiol 6, 41–46. 10.1016/1047-2797(95)00099-2 [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Bruno RM, Van Mil ACCM, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L, 2019. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J 40, 2534–2547. 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V, 2006. An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J. Clin. Invest 116, 1110–1116. 10.1172/JCI26092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF, 2003. Osmotically inactive skin Na+ storage in rats. Am. J. Physiol. - Ren. Physiol 285, 1108–1117. 10.1152/ajprenal.00200.2003 [DOI] [PubMed] [Google Scholar]
- Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, Dietsch P, Hilgers KF, 2004. Glycosaminoglycan polymerization may enable osmotically inactive Na + storage in the skin. Am. J. Physiol. - Hear. Circ. Physiol 287, 203–208. 10.1152/ajpheart.01237.2003 [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A, 2001. Urinary sodium excretion and cardiovascular mortality in Finland: A prospective study. Lancet 357, 848–851. 10.1016/S0140-6736(00)04199-4 [DOI] [PubMed] [Google Scholar]
- Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM, 2008. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51, 1525–1530. 10.1161/HYPERTENSIONAHA.108.109868 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025 9th Edition., 2020. 10.1016/B978-0-323-60984-5.00062-7 [DOI]
- van der Westhuizen B, Schutte AE, Gafane-Matemane LF, Kruger R, 2019. Left ventricular mass independently associates with 24-hour sodium excretion in young masked hypertensive adults: The African-PREDICT study. Int. J. Cardiol 276, 218–223. [DOI] [PubMed] [Google Scholar]
- Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, López B, Díez J, 2008. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am. J. Physiol. - Hear. Circ. Physiol 294, 853–858. 10.1152/ajpheart.00737.2007 [DOI] [PubMed] [Google Scholar]
- Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P, 2012. Sodium intake, ACE inhibition, and progression to ESRD. J. Am. Soc. Nephrol 23, 165–173. 10.1681/ASN.2011040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G, 2007. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am. J. Hypertens 20, 154–161. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C, 2010. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol 55, 1318–1327. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Webb M, Fahimi S, Singh GM, Khatibzadeh S, Micha R, Powles J, Mozaffarian D, 2017. Cost effectiveness of a government supported policy strategy to decrease sodium intake: Global analysis across 183 nations. BMJ 356. 10.1136/bmj.i6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstedt EFE, Beugelink L, Schrooten EM, Rademaker E, Rorije NMG, Wouda RD, Schlingemann RO, Wong TY, Vogt L, 2021. High-salt intake affects retinal vascular tortuosity in healthy males: an exploratory randomized cross-over trial. Sci. Rep 11, 1–8. 10.1038/s41598-020-79753-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel UO, Bode M, Kurts C, Ehmke H, 2019. Salt, inflammation, IL-17 and hypertension. Br. J. Pharmacol 176, 1853–1863. 10.1111/bph.14359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Luft FC, Titze JM, 2018. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol 222, 1–15. 10.1111/apha.13006 [DOI] [PubMed] [Google Scholar]
- Yu HCM, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI, 1998. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98, 2621–2628. 10.1161/01.CIR.98.23.2621 [DOI] [PubMed] [Google Scholar]
- Zamani P, Bluemke DA, Jacobs DR, Duprez DA, Kronmal RA, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, Segers P, Hannan P, Chirinos JA, 2015. Resistive and Pulsatile Arterial Load as Predictors of Left Ventricular: Mass and Geometry: The Multiethnic Study of Athersclerosis. Hypertension 65, 85–92. 10.1002/humu.21020.Gene [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, 2008. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol 103, 398–406. 10.1007/s00395-008-0733-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liao Y, Tang C, Du J, Jin H, 2012. Twenty-four-hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J. Pediatr 161, 281–284. 10.1016/j.jpeds.2012.01.054 [DOI] [PubMed] [Google Scholar]
- Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu Xu, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu Xiaolong, Yi X, Liu Xiaojing, Zhang L, Duan SZ, 2015. High salt primes a specific activation state of macrophages, M(Na). Cell Res 25, 893–910. 10.1038/cr.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]


