Abstract
Senescence is a multi-functional cell fate, characterized by an irreversible cell-cycle arrest and a pro-inflammatory phenotype, commonly known as the Senescence-Associated secretory Phenotype (SASP). Emerging evidence indicates that accumulation of senescent cells in multiple tissues, drives tissue dysfunction and several age-related conditions. This has spurred the academic community and industry to identify new therapeutic interventions targeting this process.
Mitochondrial dysfunction is an often-unappreciated hallmark of cellular senescence which plays important roles not only in the senescence growth arrest but also in the development of the SASP and resistance to cell-death. Here, we review the evidence that supports a role for mitochondria in the development of senescence and describe the underlying mechanisms. Finally, we propose that a detailed road map of mitochondrial biology in senescence will be crucial to guide the future development of senotherapies.
Keywords: Mitochondria, senescence, SASP, aging
Graphical Abstract
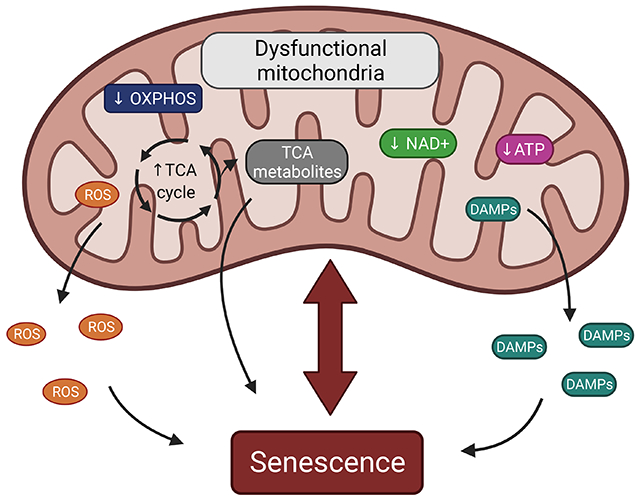
Mitochondria play a central role in the development of cellular senescence. Senescence is characterized by several mitochondrial functional changes such as a decrease in OXPHOS, reduced levels of NAD+ and ATP and accumulation of TCA cycle metabolites, DAMPs, and ROS. Here, we provide an overview of the recent findings demonstrating how these mitochondrial changes can contribute to the senescence-associated growth arrest and the SASP.
1-. Introduction
Cellular senescence is a process that imposes a permanent proliferative arrest on cells in response to various stressors. It is thought to have evolved as a tumor suppressive mechanism; however, recent data indicates that it may play other important physiological roles in embryonic development, wound healing, and tissue repair [1]. It is a complex phenotype, involving kinetic alterations in virtually all aspects of the cell’s biology. These manifestations of the senescent phenotype are highly dependent on the cell-type, inducing stimuli and physiological context. This phenotypic and temporal heterogeneity makes it nearly impossible for the scientific community to find specific markers that are universal to all types of senescent cells [2].
While initially thought to be a process that exclusively occurs in mitotic cells, recent data indicates that many post-mitotic cells can activate a senescent program and exhibit many senescent markers common to proliferative cells [3].
Multiple studies have shown that senescent cells accumulate during aging in multiple tissues and in age-related diseases. To investigate whether senescence plays a causal role in aging, mouse models were developed which allow the specific elimination of senescent cells [4,5]. These models allowed the demonstration that clearance of senescent cells alleviates the onset of several pathologies in aging mice, supporting the hypothesis that accumulation of senescent cells is a driver of age-associated tissue dysfunction [6-9].
Senescent cells can impact on age-related tissue dysfunction in a cell-autonomous fashion by limiting the regenerative potential of tissues. However, senescent cells can secrete high levels of pro-inflammatory cytokines, chemokines, and extra-cellular matrix degrading proteins, which are collectively known as the senescence-associated secretory phenotype (SASP) [10]. The discovery of the SASP proposed a possible mechanism to explain why senescent cells, which are thought to accumulate in relatively low numbers in tissues during aging, have such large detrimental effects on tissue function and induce systemic effects. The SASP, when chronic, can transmit senescence to otherwise healthy cells [11,12], it can be pro-fibrotic [9,13], it can recruit and activate immune cells contributing to chronic inflammation [14,15] and can also impair the function of stem and progenitor cells [16]. Another important characteristic of senescence is their resistance to cell-death, thought to the result of up-regulation of several cell survival mechanisms, including anti-apoptotic pathways [7,17,18]. This combined with an age-dependent decline in the ability of the immune system to clear senescent cells [19], may contribute to their persistence in tissues during aging.
Senescence can be induced by several stimuli such as telomere dysfunction, DNA damaging agents, oncogene activation, mitochondrial dysfunction, chromatin modifications amongst others. Two main pathways are thought to be engaged in the senescence-associated growth arrest: the p53/p21 pathway, mostly triggered by a DNA damage response which results in the stabilization of p53 and upregulation of its transcriptional target p21 (a cyclin-dependent kinase inhibitor) and the p16Ink4a pathway which can act as a second barrier by preventing CDK4- and CDK6-mediated inactivation of RB (retinoblastoma protein) to block cell cycle progression [1].
Senescence is also characterized by dramatic changes in mitochondrial mass, dynamics, structure, and function. These changes are tightly interconnected to other features of cellular senescence and play an important role in both the cell-cycle arrest and the regulation of the SASP. Here we will review the literature indicating aspects of mitochondrial biology which are altered in senescent cells and how they impact on the senescence phenotype. Finally, we will discuss how targeting mitochondrial function may be a powerful strategy to counteract senescence during aging and age-related disease.
2-. Mitochondrial phenotype in senescent cells
2.1. Mitochondrial dynamics, quality control and content in cellular senescence
The textbook representation of a mitochondrion is usually that of a static and isolated organelle. Advances in live-cell microscopy have revealed that mitochondria are in fact highly dynamic organelles, forming a densely interconnected network and frequently undergoing fusion and fission events. These events are thought to be essential for the maintenance of mitochondrial function, quality control and mitochondrial inheritance [20].
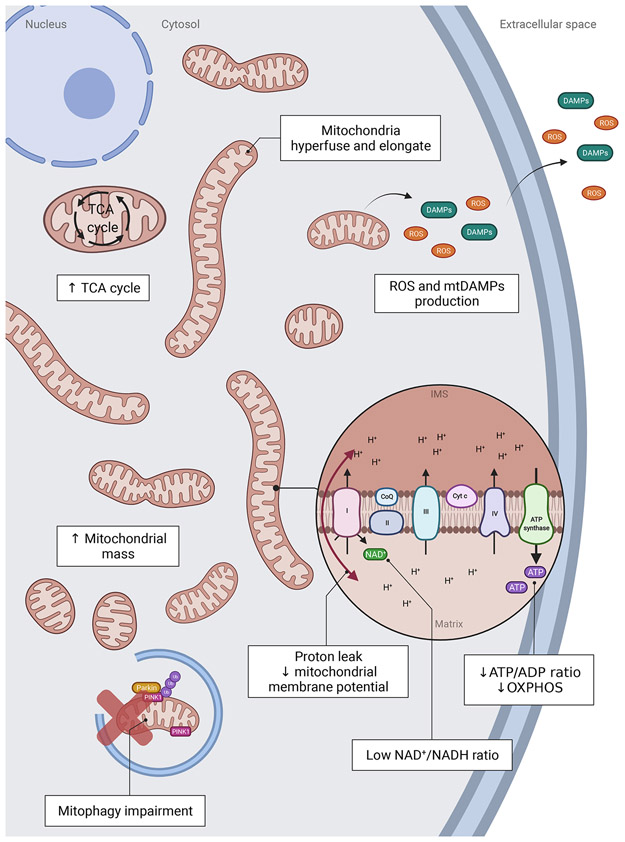
Numerous studies indicate that cellular senescence is characterized by significant changes in mitochondrial dynamics and organization. It is generally believed that damaged mitochondria can fuse with healthy ones to dilute and rearrange the matrix or use fission to separate and subsequently degrade damaged mitochondria. Thus, to ensure healthy cellular function, the balance between fission and fusion must be tightly controlled [21]. Data indicate that senescence is associated with impaired mitochondrial dynamics. In different models of cellular senescence, mitochondria are found to be elongated, enlarged and hyperfused [22-26]. During senescence, an elongated and hyperfused mitochondrial network has been linked with reduced expression of the protein FIS1, a protein which participates in mitochondrial fission by recruiting DRP1 into mitochondria [27](Fig.1). Consistently, knock-down of FIS1 has been shown to lead to increased Reactive Oxygen Species (ROS) production and induce cellular senescence. In contrast, overexpression of FIS1 was shown to prevent mitochondrial elongation and reverse the senescent phenotype [27]. This work suggests that mitochondrial fission plays a protective role against cellular senescence. Consistent with this idea, Park and colleagues demonstrated that depletion of MARCH5, a mitochondrial E3 ubiquitin ligase that regulates mitofusin-1, leads mitochondrial elongation and cellular senescence via inhibition of the activity of the fission protein DRP1 [28].
Figure 1: Mitochondria in senescent cells exhibit several changes in terms of function, structure, and dynamics.
Senescent cells show increased mitochondrial protein leak and decreased mitochondrial membrane potential as well as decreased ATP/ADP and NAD+/NADH ratios. Mitochondrial mass is increased as well as the abundance of TCA cycle metabolites, and mitochondria become hyperfused and elongated. Mitophagy is decreased, potentially inhibiting the cell’s ability to eliminate dysfunctional mitochondria. Mitochondria produce excessive amounts of ROS and DAMPs.
The hyperfused mitochondrial network observed during senescence could be a protective mechanism contributing to apoptosis resistance [29]. In fact, studies demonstrate that senescent cells are protected from both intrinsic and extrinsic pro-apoptotic signals, likely due to the increased expression of anti-apoptotic proteins of the Bcl-2 family, which control the outer mitochondrial membrane permeability and the release of pro-apoptotic molecules such as cytochrome c by inhibiting BAX and BAK macropore formation [17,30]. Apoptosis resistance is recognized as one of the hallmarks of senescence and one of the targets for the development of interventions capable of specifically killing senescent cells, commonly named senolytic [19,31]. Apoptosis is closely linked with mitochondrial dynamics. During apoptosis, DRP1, a protein which regulates mitochondrial fission, has been shown to translocate from the cytosol to mitochondria, resulting in loss of mitochondrial membrane potential and cytochrome c release [32,33]. In contrast, OPA1, a profusion protein, has been shown to protect against apoptosis by preventing cytochrome c release [34]. Finally, decreasing fission by deletion of phosphoglycerate mutase 5 (PGAM5), a phosphatase which normally dephosphorylates Drp1 and induces fission, has been shown to induce cellular senescence in vitro and in vivo. This deletion leads to senescence by an increase of mTOR pathway and IRF/IFNβ signaling [35].
However, whether mitochondrial hyperfusion contributes to protection against apoptosis in senescent cells remains to be investigated.
Another mechanism that could explain mitochondrial phenotypes exhibited by senescent cells is impaired mitophagy (Fig.1). Mitophagy refers to the selective autophagic degradation of damaged or dysfunctional mitochondria and plays an important role in mitochondria quality control and homeostasis. Mitochondria are enclosed by autophagosomes which fuse with lysosomes where they are eventually degraded [36]. Mitophagy is a complex process involving the activation of numerous pathways under different conditions. One of the most studied is the PINK1-Parkin pathway. When mitochondria are damaged or dysfunctional, the sentinel protein PTEN-induced kinase 1 (PINK1) accumulates in their outer membrane. This accumulation leads to the recruitment of the E3 ubiquitin ligase Parkin from the cytosol which then ubiquitinates numerous proteins localized on the outer mitochondrial membrane. This ubiquitination mediates the autophagosome formation and the lysosomal elimination of dysfunctional mitochondria [37,38]. Several studies indicate that senescent cells have impaired mitophagy and importantly that defects in mitophagy may play a role in the induction of cellular senescence [39-41]. For example, Ahmad and colleagues showed that exposure to cigarette smoke (CS), a stressor known to induce cellular senescence [42], leads to reduced Parkin translocation to damage mitochondria. This results in perinuclear accumulation of damaged mitochondria and is associated with the induction of senescence [43]. Using a similar model of CS-induced senescence, Araya and colleagues showed that while Parkin knock-down resulted in impaired mitophagy and induction of senescence, its overexpression was sufficient to induce mitophagy and attenuate cellular senescence [44]. Mechanistically, it has been suggested that during senescence, accumulation of the protein p53 in the cytosol leads to the sequestration of Parkin, inhibiting its translocation to the mitochondria. This process has been shown to maintain or reinforce the senescence phenotype [43,45,46].
Another important feature of cellular senescence is an increase in mitochondrial content (Fig.1). This has been shown to occur regardless of the inducing stimuli, whether senescence is induced by oncogenes [47], genotoxic stress [48], oxidative stress or extensive replication [24,25]. While the majority of these observations were performed in fibroblasts, increased mitochondrial content during senescence has also been shown in other cell-types such as adipose-derived mesenchymal stromal cells [49,50], cardiomyoblasts [46], hepatocytes [51], pancreatic β-cells [52] and others. This phenotype is however not universal to all types of cells. For instance, T CD8 cells display lower mitochondrial content compared to T CD4, which is associated with a faster acquisition of an immuno-senescent profile [53]. Cardiomyocytes isolated from aged mice show increased expression of senescence-associated markers, but decreased mRNA expression of mitochondrial genes [9]. Thus, similarly to other features of senescence, data supports that the senescence-associated mitochondrial phenotype is cell-type specific.
2.2. Mitochondrial metabolism and oxidative phosphorylation during cellular senescence
Mitochondria are the cellular main source of energy through the generation of ATP. To produce ATP, mitochondria use sugars, amino acids, and fatty acids as fuels. These nutrients are degraded and metabolized by the tricarboxylic acid (TCA) cycle, which leads to the production of 3 molecules of NADH and 1 molecule of FADH2 [54]. These byproducts are used secondarily by the electron transport chain (ETC) to create an electrochemical gradient characterized by an accumulation of protons (H+) in the mitochondrial intermembrane space, which will ultimately result in the generation of ATP by the enzyme ATP synthase. The process of ATP production requires the presence of oxygen and is generally called oxidative phosphorylation (OXPHOS). These two cell machineries, TCA cycle and OXPHOS are therefore tightly connected [55].
Senescent cells have been shown to be less efficient in producing ATP [56,57], leading to a bioenergetic imbalance with an increase in AMP/ATP ratio. This drop of ATP production could be explained, in senescent cells, by a decrease in the efficiency of OXPHOS (characterized by less H+ in the intermembrane space) associated with a reduction of mitochondrial membrane potential (Fig.1). Consistently, a decrease in mitochondrial membrane potential has been described in the context of replicative senescence [25,58], stress-induced senescence [48] and oncogene-induced senescence (OIS) [47]. This decrease in mitochondrial membrane potential was associated with increased production of ROS, suggesting that mitochondria in senescent cells are dysfunctional [25,59]. Interestingly, mitochondrial uncoupling using carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP) was show to induce premature senescence in human fibroblasts [60].
As previously mentioned, many types of senescent cells show increased mitochondrial mass and this is reflected by a significantly higher rate of oxygen consumption per cell. However, despite respiring more due to having more mitochondria, data indicates that senescent cells experience a shift towards glycolysis [39]. This phenomenon was already described several decades ago when Bittles and Harper reported that replicatively senescent cells exhibit a highly significant increase in glucose uptake and a corresponding increase in lactate production [61]. More recently, similar observations were made using more sophisticated analyses such as metabolomics or measures of mitochondrial oxygen flux and extracellular acidification rates in different senescent cell-types [39,62,63]. It should be noted however that this metabolic shift is not universal for all type of cells and senescent stimuli. For instance, in the context of oncogene induced senescence (OIS), no changes in glucose uptake were observed, but there was an increase in oxygen consumption rate and TCA activity, linked to an increase in pyruvate dehydrogenase activity [64]. Additionally, enhanced glycolysis via overexpression of glycolytic enzymes phosphoglycerate mutase or glucosephosphate isomerase was shown to bypass both replicative and oncogene-induced senescence [65]. Furthermore, replicative senescent human mammary epithelial cells did not show an overall glycolytic shift, with no changes in glucose consumption or lactate secretion [57]. This highlights the need to avoid overgeneralizations when it comes to metabolism during senescence, as it may be vary according to cell-type and physiological context.
Several studies have shown that senescent cells show an increase in TCA cycle metabolites [62,64,66,67] (Fig.1) and the TCA cycle and OXPHOS are tightly connected and in constant feedback with each other as reviewed here [55].
Moreover, different TCA metabolites have been shown to play numerous roles in diverse functions such as lymphangiogenesis, stem cell function, immune modulation, tumorigenesis, thermogenesis but also in chromatin modifications, DNA methylation and post-translational protein modifications [55]. Given that senescence is characterized by epigenetic and chromatin modifications, it is likely that modulation of these metabolites could have an impact on the senescence phenotype.
3-. Mitochondria as inducers of senescence
As highlighted before, mitochondria are complex organelles where multiple biochemical reactions occur, and which play a role in various cellular functions. While previous data indicates that multiple aspects of mitochondrial biology are altered in cellular senescence, it is challenging to ascertain which factors, if any, contribute causally to the induction of the senescent phenotype.
To begin to address this question, our group first proposed a proof-of-principle experiment, in which we generated human fibroblasts which contained no mitochondria. This was based on prior observations that damaged mitochondria can be removed via the PINK-1/Parkin mitophagy pathway [36]. We found that ectopic expression of the ubiquitin E3 ligase Parkin combined with short-term treatment with mitochondrial uncoupler FCCP, leads to widespread mitophagy resulting in the generation of cells with virtually no mitochondria [68]. Using this tool, we found that removing mitochondria in different models of cellular senescence, attenuated their pro-inflammatory phenotype, however, cells did not resume the cell-cycle [51]. This work suggested that the SASP is dependent on mitochondria, however, the underlying mechanisms are not yet elucidated.
In this section of the review, we will summarize what is known about potential mechanisms by which mitochondria may contribute to the SASP during senescence. We will also review evidence that changes in mitochondria may contribute to the senescence-associated cell-cycle arrest.
3.1. Mitochondrial ROS as a driver of senescence
ROS is a general term used to describe several reactive molecules which are partially reduced metabolites of oxygen metabolism. These molecules can be generated by various enzymatic and metabolic reactions, including mitochondrial respiration [69]. Indeed, mitochondria have been found to be one of the major producers of ROS in the cell, notably during mitochondrial respiration where the leakage of electrons along the electron transport chain (ETC) produces the free radical superoxide anion (O2-•) [70,71]. Superoxide anions are converted in hydrogen peroxide (H2O2) by antioxidant enzyme superoxide dismutase (SOD) and finally transform in H2O by catalase [72]. H2O2 can be then also be partially reduced and converted to another, more toxic free radical, the hydroxyl radical (•OH), by the Fenton reaction [70].
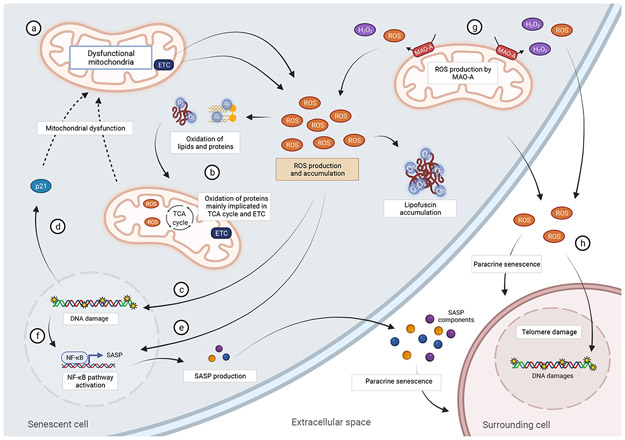
As mentioned above, senescent cells contain dysfunctional mitochondria, and several studies indicate that they produce excessive ROS [25,48]. ROS has been shown to contribute to damage to several macromolecules including proteins, lipids, and DNA (Fig.2). Importantly in the context of cellular senescence, mitochondrial ROS has been shown to induce DNA damage and accelerate telomere-induced senescence. Telomeres are genomic regions rich in guanine residues which are particularly susceptible to oxidative modifications and when exposed to mild oxidative stress have been shown to accumulate single-stranded breaks [73]. Upon cell division, these single-stranded breaks, result in accelerated telomere shortening and contribute to cellular senescence [74]. Consistent with a role for ROS in the process, interventions known to reduce mitochondrial ROS, have been shown to decelerate telomere shortening and extend replicative lifespan [25,75].
Figure 2: Mitochondrial ROS play important roles in senescence.
Dysfunctional mitochondria in senescent cells produce high ROS which can lead to the oxidation of proteins, lipids, and DNA. High ROS can induce both single- and double-stranded breaks in the genome, particularly at telomere regions, leading to senescence. High ROS production can activate NF-κB (a major regulator of the SASP) directly or via induction of a DNA damage Response. ROS can play important roles in spreading senescence to surrounding, otherwise healthy cells, via the induction of a DNA damage response.
Acute exposure to pro-oxidant agents also leads to premature senescence, however, this process was traditionally viewed as occurring independently of telomere shortening. More recent studies have indicated that acute exposure to pro-oxidant or DNA damaging agents can lead to DNA double stranded breaks (DSBs) at telomere regions, which occur independently of telomere length and cannot be rescued by telomerase [76,77]. In contrast to DSBs induced in non-telomeric regions, telomere damage elicits a persistent DDR (DNA Damage Response) which is thought to enforce checkpoints that maintain the senescence cell-cycle arrest. This occurs because telomere-binding proteins, such as TRF2, inhibit DNA repair [76-78] potentially to avoid chromosomal fusions and maintain the linear structure of chromosomes. Thus, as genomes are constantly exposed to endogenous or exogenous damage throughout the life of a cell, when damage occurs at telomeres, it will not be efficiently repaired, resulting in accumulation of telomere damage, and eventually leading to cellular senescence. Consistent with a role for ROS in mechanisms mediating telomere maintenance, recent studies have shown that oxidative modifications at telomeres can accelerate telomere dysfunction [79], impair telomerase activity [80] and disrupt the binding of telomere-associated proteins TRF1 and TRF2 which are important for telomere stability [81].
ROS produced by senescent cells can have both autocrine and paracrine effects (Fig.2). Mitochondrial ROS produced by senescent cells have been shown to contribute to the maintenance of cellular senescence, via a positive self-amplifying loop, which constantly generate DNA damage [48]. ROS produced by senescent cells have also been shown to induce a DNA damage response in neighboring cells via gap junctions [82]. Senescent melanocytes have been shown to induce telomere damage to surrounding keratinocytes via ROS signaling, potentially contributing to epidermal atrophy during aging [83].
Mitochondrial ROS in senescence does not affect solely DNA damage but can also contribute to oxidative modifications to other macromolecules such as proteins and lipids. Recently, Hamon and colleagues have used metabolomic and proteomic approaches to analyze oxidative modifications that occur during replicative senescence. They observed that numerous proteins are found carbonylated, 4-Hydroxynonenal (HNE)-modified (an aldehyde produced during lipid peroxidation) or advance glycation end product (AGE)-modified, with half of them localized to the mitochondria. Interestingly, these oxidized proteins were implicated in metabolic processes such as the tricarboxylic acid (TCA) cycle, oxidative phosphorylation and fatty acid β-oxidation [84]. Lipofuscin, a nondegradable aggregate of oxidized lipids, proteins, oligosaccharides and transition metals which accumulate in lysosomes, has been shown to accumulate in senescent cells [85] and has recently been proposed as a marker of senescent cells [86]. Interestingly, treatment with synthetic lipofuscin can by itself induce senescence [85], suggesting that lipofuscin accumulation can play a causal role in the process.
ROS in senescent cells do not necessarily originate from mitochondria. For instance, endothelial cell senescence was shown to be induced by endogenous NADPH oxidase NOX4 [87], which in turn can contribute to mitochondrial dysfunction by inactivating complex I of the mitochondria [88]. NOX4 has also been shown to play a role in OIS [89], suggesting that ROS produced independently of mitochondria plays a role in senescence.
ROS has also been shown to regulate the SASP (Fig.2). Studies show that ROS can activate NF-κB (a major regulator of the SASP) [90] or lead to its activation indirectly via ROS-dependent induction of DNA damage and a DDR [48]. Inhibition of ATM (the main initiator of the DDR) has been shown to decrease the SASP [91]. Mechanistically, ATM has been shown to activate NEMO, IKK complex and nuclear translocation of NF-κB [92].
While considerable data support a role for mitochondrial ROS as a driver of senescence in culture, less is known in vivo, however, mouse models of mitochondrial dysfunction and enhanced oxidative stress show increased senescence in different tissues.
For instance, cardiomyocyte-specific overexpression of the mitochondrial enzyme monoamine oxydase A (MAO-A), which has been shown to increase during cardiac aging [46,93], leads to increased ROS and mitochondrial dysfunction [94]. Cardiomyocytes from TgMAO-A mice show increased telomere dysfunction and markers of cellular senescence, which can be rescued by treatment with antioxidant N-acetyl cysteine (NAC) [9]. More recently, it was shown that MAO-A driven cardiomyocyte senescence also induces paracrine senescence to cardiac stromal cells [95].
Additionally, deficiency of antioxidant enzyme mitochondrial superoxide dismutase 2 (SOD2), has been shown to induce cellular senescence in mouse skin [96] and result in an age-dependent accumulation of telomere associated foci (TAF) in cardiomyocytes [9]. Selective SOD2 deficiency in connective tissue was shown to induce accelerated aging in different tissues, such as muscle, bones and skin, associated with increased oxidative damage and induction of cellular senescence [97]. Moreover, deletion of the antioxidant enzyme cytosolic superoxide dismutase 1 (SOD1) was associated with increased oxidative stress and expression of senescence and SASP markers in kidney [98]. Finally, mice with reduced expression of DNA repair enzyme ERCC1-XPF endonuclease show increased levels of ROS and accumulate senescent cells in different tissues. Importantly, chronic treatment with a mitochondrial-targeted free radical scavenger was shown to attenuate oxidative damage and the accumulation of senescence cells, thus supporting a causal link between mitochondrial ROS and induction of cellular senescence in vivo [99]. Similarly, accelerated aging mouse model nfkb1−/− showed increased ROS and telomere dysfunction, which could be significantly reduced by treatment with antioxidant BHA [100].
All together, these studies support a central role for mitochondrial ROS in the induction and maintenance of senescence both in vivo and in vitro.
3.2. The role of mitochondrial metabolites in senescence
As previously discussed, senescent cells experience a bioenergetic imbalance characterized by an increase in AMP/ATP and ADP/ATP ratios [101]. Evidence suggests that these changes can contribute causally to cellular senescence. Increase of these ratios can lead to the activation of the AMP-activated protein kinase (AMPK), a central mediator of cellular metabolism, which has been shown to play a role in senescence [56,102]. AMPK activation can lead to the direct phosphorylation and activation of p53 [103,104], down regulation of proliferative genes, such as cyclin A, or reduction of retinoblastoma protein (RB) phosphorylation, all of which have been shown to contribute to the senescence growth arrest [102]. AMPK activation can also increase the nuclear presence of RNA stabilizing factor human antigen R (HuR) protein, resulting in increased p16 expression but also inhibiting the stabilization of mRNAs encoding cyclins, leading to a senescent phenotype [101,105].
The nicotinamide adenine dinucleotide (NAD) has been shown to play important roles in metabolism and cellular senescence. This coenzyme can be found in 2 forms: the oxidized NAD+ and the reduced NADH. Senescent cells are characterized by a decrease in the NAD+/NADH ratio [106]. Nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme for NAD+ salvage from nicotinamide, has been shown to decrease in senescent cells [107,108]. Importantly, decreased NAMPT activity induces senescence whereas its overexpression delays senescence induction [109]. Knock-down of the cytosolic malate dehydrogenase (MDH1), an enzyme that catalyzes the reversible reduction of oxaloacetate to malate, has been shown to a reduce NAD+/NADH ratios, activate AMPK, and induce cellular senescence [110]. Low NAD+/NADH levels have also been implicated in the regulation of the SASP. Wiley and colleagues described that mitochondrial dysfunction induced senescence resulted in an atypical SASP with no induction of commonly expressed SASP factors IL-1α, IL-1β, IL-6 and IL-8. This observation was confirmed in vivo in a progeroid mouse model (Polg mutator mouse) characterized by accumulation of mtDNA mutations and mitochondrial dysfunction in various tissues [111]. NAD seems to play different roles in the regulation of the SASP depending on the inducing stimuli. For instance, levels of NAD+, NAD+/NADH ratios and NAMPT expression differ significantly between replicative and oncogene-induced senescence (OIS) and this seems to impact on the extend of SASP expression [108]. In contrast to replicative senescence where NAMPT and NAD+/NADH ratios decline, OIS is characterized by increased expression of NAMPT enzyme and higher NAD+/NADH ratios. Nacarelli and colleagues propose that during OIS, NAMPT promotes the SASP through NAD+-mediated suppression of AMPK kinase, which suppresses the p53-mediated inhibition of p38 MAPK to enhance NF-κB activity [108].
Recent evidence suggests other mechanistic links between NAD metabolism and the regulation of cellular senescence. It has been observed that NADase CD38 (cluster of differentiation 38) activity is increased during aging and it was suggested that this ecto-enzyme is responsible for the age-dependent decline in NAD+ [112-114]. Recently, it was shown that senescent cells can stimulate the expression of CD38 in non-senescent cells, especially in immune cells, via the SASP [112,114,115]. This led to the hypothesis that accumulation of senescent cells and the SASP may contribute to the age-dependent decline in NAD+ levels via up-regulation of CD38 in non-senescent cells. Furthermore, CD38 has also been shown, in vivo, to be an inducer of mitochondrial dysfunction, which could potentially contribute to further induction of senescence [115]. Interestingly, pharmacological inhibition of CD38 was shown not only to prevent the decline in NAD+ levels during aging but also reduce telomere-associated DNA damage, a major driver of senescence [116].
As previously discussed, senescence is characterized by significant changes in the TCA cycle and several of its pathways and metabolites have been implicated in the regulation of senescence. Supraphysiological concentrations of pyruvate, which enters the mitochondrial matrix and is converted into Acetyl-CoA, have been shown to accelerate replicative senescence in vitro [117]. Enhanced usage of pyruvate via the enzyme pyruvate dehydrogenase (PDH), which links glycolysis to the TCA cycle, plays a key role in oncogene-induced senescence [64]. Acetyl-CoA production from acetate has been shown to accelerate chronological aging in yeast and induce senescence in human endothelial cells [118]. Acetyl-CoA can also be used in lipid synthesis and in fact, it has been shown that fatty acid synthase (FASN) which combines acetyl-CoA with malonyl-CoA to produce long-chain fatty acids plays a role in the initial stages of the senescence program [119].
Jiang and colleagues demonstrated a role for malic enzymes ME1 and ME2, two TCA cycle enzymes which recycle malate into pyruvate, in cellular senescence. Authors showed that p53 represses ME1 and ME2 expression, which reciprocally activate p53 through MDM2 and AMPK in a feed-forward manner [104].
Fumarate, a precursor to malate in the TCA cycle, has also been implicated in senescence. Accumulation of fumarate was shown to lead to the formation of succunicGSH, a covalent adduct between fumarate and glutathione, increasing oxidative stress and inducing senescence in epithelial cells and fibroblasts [120].
Fumarylacetoacetate hydrolase (FAH) domain containing protein 1 (FAHD1) is a mitochondrial metabolic enzyme which can hydrolyze acylpyruvates and decarboxylase oxaloacetate, a TCA cycle metabolite. Downregulation of this enzyme was shown to inhibit mitochondrial function and include premature senescence in human endothelial cells [121].
Glutamine metabolism and consumption has also been shown to be increase in senescence [122,123]. This pathway feeds the TCA cycle by increasing the levels of α-ketoglutarate by glutaminase and glutamate dehydrogenase activity [124]. Intriguingly, on one hand, pharmacological inhibition of glutaminolysis has been found to induce premature senescence in HUVECS [122] and, on the other hand, has been identified as a new senolytic treatment, improving health span, when administered to aged mice [123].
Interestingly, TCA cycle metabolites can also have effects that counteract senescence. For instance, α-ketoglutarate has been shown to act as a senomorphic by reducing the SASP in senescents fibroblasts and extend healthspan and lifespan in aged mice [125].
While the role of TCA cycle intermediates in non-metabolic signaling functions has been extensively studied in the context of cancer and inflammation [126], their role in senescence remains poorly understood. Given the interlink between senescence, cancer, and inflammation, understanding their role in senescence may lead to the identification of new therapeutic approaches to counteract aging and age-related diseases.
3.3. The role of mitochondrial DAMPs and mitochondrial derived peptides in senescence
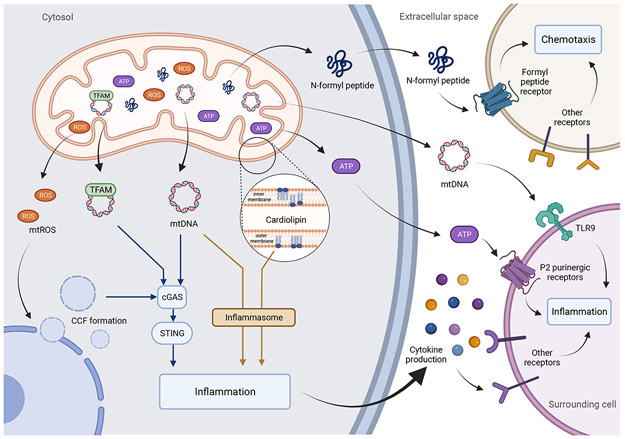
As a response to stress, cells can release endogenous components to the extracellular space to trigger an immune response which are collectively called the damage-associated molecular patterns (DAMPs). DAMPs are recognized by pattern-recognition receptors (PPR) often localized in immune cells, but also found in non-immunes cells, and promote inflammatory responses [127]. An emerging concept is that inflammation during aging and age-related diseases may arise because of the innate immune system identifying damaged components derived from mitochondria. Indeed, mitochondrial components such as cardiolipin, mitochondrial transcription factor A (TFAM), N-formyl peptide, ATP, succinate, cytochrome c and mitochondrial DNA (mtDNA) have been identified as a subclass of DAMPs, commonly referred to as mitochondrial DAMPs [128,129] (Fig.3). Whether these mitochondrial DAMPs play a role in senescence is still relatively understudied.
Figure 3: Mitochondria are a major source of DAMPs.
TFAM, mtDNA, cardiolipin, ATP and N-formyl peptides can be released from mitochondria into the cytosol or the extracellular space and engage pro-inflammatory pathways. Mitochondrial ROS has been shown to contribute to the nuclear release into the cytosol of CCF during senescence which can be recognized by DNA sensor cGAS and activate the STING.
Mitochondrial dysfunction during senescence has been shown to contribute to the generation of cytosolic chromatin fragments (CCFs) which trigger the SASP though activation of cGAS-STING pathway (reviewed in [130]). It has been suggested that during senescence, mitochondrial ROS activate the stress-activated kinases JNK1/2, which interacts with the CCF suppressor 53BP1, leading to CCF formation [131]. CCF once in the cytosol are recognized by DNA sensor Cyclic GMP-AMP synthase (cGAS) which has been shown to be a major driver of the SASP [130]. Consistent with this idea, wide-spread clearance of mitochondria or suppression of mitochondrial ROS was shown to prevent formation of CCF and inhibit the SASP [51,131].
Mitochondrial DNA (mtDNA) is present at thousands of copies per cell and has been shown to be a potent inducer of inflammation once outside the mitochondrial matrix via different PPRs, including TLR9, cGAS-STING and the inflammasome [132]. Studies have shown that circulating cell-free mtDNA increases in the plasma during aging and in the context of several age-related diseases, leading to the hypothesis that it is a major contributor to inflammation [133]. A recent study has implicated senescent cells as major sources of cell-free mtDNA [134]. This study showed that elimination of senescent cells by senolytic drugs significantly reduced circulating cell-free mtDNA in aged mice. Additionally, senolytic treatment improved the survival of mice receiving transplants from aged mice [134].
The inflammasome has also been demonstrated to be activated in senescent cells in vitro and in vivo [11]. This pathway is mainly initiated by activation of the cytoplasmic NLR family pyrin domain containing 3 (NLRP3) by DAMPs, which by oligomerization forms the NLRP3 inflammasome and activates caspase-1. Once activated, caspase-1 matures pro-IL1-β and pro-IL18 into IL-1β and IL-18 respectively [135]. Interestingly, other mitochondrial components have been shown to activate the inflammasome such as mitochondrial ROS and cardiolipin [136,137]. Cardiolipin is a glycerophospholipid found exclusively in the inner mitochondrial membrane, but upon mitochondrial stress, can be exposed in the outer membrane where it can activate different pathways such as autophagy, apoptosis but also the inflammasome [137,138]. Importantly, it has been shown that senescent cells accumulate cardiolipin, and that cardiolipin can, in turn, also induce premature senescence in human fibroblast in vitro [139].
Mitochondrial derived peptides humanin and MOTS-c were also shown to be elevated in senescent cells. Interestingly, their administration to senescent cells resulted in higher mitochondrial respiration and a modest increase in selected components of the SASP [140].
The mitochondrial transcription factor A (TFAM), a member of HMGB family and essential for maintenance of mtDNA, has also be found to be immunostimulatory. For example, absence of TFAM can cause aberrant mtDNA packaging and mtDNA release into the cytosol where it activates the cGAS-STING pathway and inflammation [141]. Moreover, specific deletion of TFAM in T cells was shown to induce premature senescence in different tissues triggered by an increase of pro-inflammatory cytokines [142]. TFAM can also by itself or synergistically with other DAMPs, as N-formyl peptide, to induce an inflammatory response in immune cells [143,144].
4-. Mitochondrial therapy- keeping senescence at bay
As we described in the previous sections, mitochondria are key regulators of the senescent phenotype by contributing to both the cell cycle arrest and the SASP. Another aspect we have not yet discussed is that mitochondria may hold the key to the survival of senescent cells, because they are major regulators of apoptosis. Mitochondrial outer membrane permeabilization (MOMP) is often essential for apoptosis and is regulated by interactions between pro- and anti-apoptotic members of the Bcl-2 protein family [145].
Currently, there is great enthusiasm regarding the development of pharmacological interventions targeting senescent cells, given their pivotal roles in aging and age-related diseases [146]. Two classes of senotherapies are currently being investigated: “senolytic” therapies that specifically induce cell-death of senescent cells and “senomorphic” therapies that suppress the SASP without affecting cell viability. We argue that obtaining a detailed road map to mitochondrial biology in senescence will be essential to guide the development of both classes of therapies for the following reasons:
First, many of the senolytic drugs being currently developed exert their effects by targeting anti-apoptotic proteins which are located at mitochondria and prevent mitochondrial-driven apoptosis. Senolytic drugs such as ABT-263 (navitoclax) and others in this family exert their senolytic effect by targeting the anti-apoptotic B-cell lymphoma 2 (BCL-2) family of proteins located in mitochondria [17,18]. More recently, mitochondria-targeted tamoxifen (MitoTam) was shown to induce apoptosis of senescent cells and reduce the expression of senescent markers p21 and p16 in kidney and lung of aged mice [147]. Furthermore, as we previously described mitochondrial apoptosis depends on processes such as mitochondrial fusion and fission and mitophagy both of which are altered in senescent cells.
Secondly, data supports that mitochondria play an essential role in the development of the SASP [51,131]. They are major sources of DAMPs and metabolites which have been shown to engage pro-inflammatory pathways. Senomorphic drugs such rapamycin and metformin which have been shown to suppress the SASP [148-150], have also been shown to improve mitochondrial function [51,151,152]. Therefore, uncovering the underlying mechanisms mediating mitochondrial dysfunction during senescence, will be important in the developing new therapeutic avenues targeting the SASP.
Given the wide-ranging alterations in mitochondria in senescent cells and their key role in regulating cell-death and inflammation, we hypothesize that screening for compounds targeting different aspects of mitochondrial biology may lead to the identification new classes of senolytic and senomorphic drugs.
Conclusions and future perspectives
Evidence in the literature is clear in highlighting that senescent cells show significant alterations in mitochondrial function and metabolism. However, many of the reported observations are limited to a relatively small number of cell-types and may not be universal to all subtypes of senescent cells. In fact, as we discussed in this review, several mitochondrial and metabolic parameters differ depending on the senescent cell-type and stressor. Thus, a more in-depth characterization of mitochondrial function in different senescent cell-types is warranted.
Furthermore, while data indicates that mitochondria become dysfunctional in senescent cells cultured in vitro, very little is known about mitochondrial function, structure and dynamics in senescent cells present in tissues in vivo. To investigate this further, methods that allow the isolation of senescent live cells from different tissues need to be developed so that these cells can be further characterized ex vivo. While this may be currently feasible using mouse models expressing reporters of senescence-associated genes, isolation of senescent cells in human tissues will present further challenges.
Another important question in the field, is how much senescent cells contribute to the overall mitochondrial dysfunction observed in tissues during aging. Studies are consistent in showing that mitochondria isolated from aged tissues show decreased respiratory coupling and increased ROS production. The extent to which senescent cells contribute to these phenotypes is not yet known.
Acknowledgements:
Work in JFP lab is funded by NIH grants 1R01AG068048-01; 1UG3CA268103-01 and P01 AG062413 and the Ted Nash long life foundation. Images in this review were created with BioRender.com.
Abbreviations
- AMPK
5' AMP-activated protein kinase
- ATP
Adenosine triphosphate
- ADP
Adenosine diphosphate
- BCL-2
B-cell lymphoma 2
- CCF
cytoplasmic chromatin fragment
- CD38
cluster of differentiation 38
- cGAS
cyclic GMP-AMP synthase
- DAMPs
damage-associated molecular patterns
- DDR
DNA damage response
- DSBs
DNA double stranded breaks
- ETC
electron transport chain
- H+
protons
- IL
interleukin
- MAO-A
Monamine Oxidase A
- MOMP
mitochondrial outer membrane permeabilisation
- MitoTAM
mitochondrial targeted tamoxifen
- MOTS-c
mitochondrial open reading frame of the 12S rRNA-c
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- OIS
oncogene-induced senescence
- OXPHOS
oxidative phosphorylation
- PINK1
PTEN induced kinase 1
- RB
retinoblastoma protein
- ROS
reactive oxygen species
- SASP
senescence associated secretory phenotype
- SOD
superoxide dismutase
- STING
stimulator of interferon genes
- TAF
telomere associated foci
- TCA
tricarboxylic acid
- TFAM
mitochondrial transcription factor A
- TLR9
toll-like receptor-9
Footnotes
Conflicts of Interest: authors declare that they have no conflicts of interest
References
- 1.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M & Demaria M (2019) Cellular Senescence: Defining a Path Forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- 2.Ogrodnik M, Salmonowicz H, Jurk D & Passos JF (2019) Expansion and Cell-Cycle Arrest: Common Denominators of Cellular Senescence. Trends Biochem Sci 44, 996–1008. [DOI] [PubMed] [Google Scholar]
- 3.von Zglinicki T, Wan T & Miwa S (2021) Senescence in Post-Mitotic Cells: A Driver of Aging? Antioxid Redox Signal 34, 308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL & van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, VanSteeg H, Dollé MET, Hoeijmakers JHJ, deBruin A, Hara E & Campisi J (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J,A. Saltness R, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD & van Deursen JM (2016) Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ & Kirkland JL (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL & Jurk D (2021) Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD & Passos JF (2019) Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38, e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppé J-P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez P-Y & Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L & Gil J (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGPP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T & Kirkland JL (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H & LeBrasseur NK (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8, 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagnado A, Leslie J, Ruchaud-Sparagano M-H, Victorelli S, Hirsova P, Ogrodnik M, Collins AL, Vizioli MG, Habiballa L, Saretzki G, Evans SA, Salmonowicz H, Hruby A, Geh D, Pavelko KD, Dolan D, Reeves HL, Grellscheid S, Wilson CH, Pandanaboyana S, Doolittle M, von Zglinicki T, Oakley F, Gallage S, Wilson CL, Birch J, Carroll B, Chapman J, Heikenwalder M, Neretti N, Khosla S, Masuda CA, Tchkonia T, Kirkland JL, Jurk D, Mann DA & Passos JF (2021) Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J 40, e106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martini H, Iacovoni JS, Maggiorani D, Dutaur M, Marsal DJ, Roncalli J, Itier R, Dambrin C, Pizzinat N, Mialet-Perez J, Cussac D, Parini A, Lefevre L & Douin-Echinard V (2019) Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90 + subset. Aging Cell 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL & Jurk D (2019) Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab 29, 1061–1077.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I & Krizhanovsky V (2016) Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD & Kirkland JL (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J, Tsoory M, Schirmbeck R, Amit I, Geiger H & Krizhanovsky V (2018) Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 9, 5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11, 872–84. [DOI] [PubMed] [Google Scholar]
- 21.Sebastián D, Palacín M & Zorzano A (2017) Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol Med 23, 201–215. [DOI] [PubMed] [Google Scholar]
- 22.Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC & Yoon G (2006) Formation of elongated giant mitochondria in DFO-induced cellular senescence: Involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol 209, 468–480. [DOI] [PubMed] [Google Scholar]
- 23.Chapman J, Fielder E & Passos JF (2019) Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 593, 1566–1579. [DOI] [PubMed] [Google Scholar]
- 24.Lee HC, Yin PH, Chi CW & Wei YH (2002) Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci 9, 517–526. [DOI] [PubMed] [Google Scholar]
- 25.Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TBL & von Zglinicki T (2007) Mitochondrial Dysfunction Accounts for the Stochastic Heterogeneity in Telomere-Dependent Senescence. PLoS Biol 5, e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J & Jendrach M (2010) Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci 123, 917–926. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ & Cho H (2007) Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem 282, 22977–22983. [DOI] [PubMed] [Google Scholar]
- 28.Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ & Cho H (2010) Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci 123, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youle RJ & van der Bliek AM (2012) Mitochondrial Fission, Fusion, and Stress. Science (80- ) 337, 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cory S, Huang DCS & Adams JM (2003) The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 22, 8590–8607. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Segura A, Nehme J & Demaria M (2018) Hallmarks of Cellular Senescence. Trends Cell Biol 28, 436–453. [DOI] [PubMed] [Google Scholar]
- 32.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL & Youle RJ (2001) The Role of Dynamin-Related Protein 1, a Mediator of Mitochondrial Fission, in Apoptosis. Dev Cell 1, 515–525. [DOI] [PubMed] [Google Scholar]
- 33.Estaquier J & Arnoult D (2007) Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ 14, 1086–94. [DOI] [PubMed] [Google Scholar]
- 34.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV., Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B & Scorrano L (2006) OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell 126, 177–189. [DOI] [PubMed] [Google Scholar]
- 35.Yu B, Ma J, Li J, Wang D, Wang Z & Wang S (2020) Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics. Nat Commun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narendra D, Tanaka A, Suen D-F & Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann A, Madreiter-Sokolowski C, Stryeck S & Abdellatif M (2021) Targeting the Mitochondria-Proteostasis Axis to Delay Aging. Front Cell Dev Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekine S & Youle RJ (2018) PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol 16, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korolchuk VI, Miwa S, Carroll B & von Zglinicki T (2017) Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 21, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalle Pezze P, Nelson G, Otten EG, Korolchuk VI, Kirkwood TBL, von Zglinicki T & Shanley DP (2014) Dynamic modelling of pathways to cellular senescence reveals strategies for targeted interventions. PLoS Comput Biol 10, e1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M & Muñoz-Cánoves P (2016) Autophagy maintains stemness by preventing senescence. Nature 529, 37–42. [DOI] [PubMed] [Google Scholar]
- 42.Birch J, Anderson RK, Correia-Melo C, Jurk D, Hewitt G, Marques FM, Green NJ, Moisey E, Birrell MA, Belvisi MG, Black F, Taylor JJ, Fisher AJ, De Soyza A & Passos JF (2015) DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 309, L1124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H & Rahman I (2015) Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J 29, 2912–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araya J, Tsubouchi K, Sato N, Ito S, Minagawa S, Hara H, Hosaka Y, Ichikawa A, Saito N, Kadota T, Yoshida M, Fujita Y, Utsumi H, Kobayashi K, Yanagisawa H, Hashimoto M, Wakui H, Ishikawa T, Numata T, Kaneko Y, Asano H, Yamashita M, Odaka M, Morikawa T, Nishimura SL, Nakayama K & Kuwano K (2019) PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy 15, 510–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T & Matoba S (2013) Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun 4, 2308. [DOI] [PubMed] [Google Scholar]
- 46.Manzella N, Santin Y, Maggiorani D, Martini H, Douin-Echinard V, Passos JF, Lezoualc’h F, Binda C, Parini A & Mialet-Perez J (2018) Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell, e12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moiseeva O, Bourdeau V, Roux A, Deschênes-Simard X & Ferbeyre G (2009) Mitochondrial Dysfunction Contributes to Oncogene-Induced Senescence. Mol Cell Biol 29, 4495–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, Saretzki G, Rudolph KL, Kirkwood TBL & von Zglinicki T (2010) Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janikiewicz J, Szymański J, Malinska D, Patalas-Krawczyk P, Michalska B, Duszyński J, Giorgi C, Bonora M, Dobrzyn A & Wieckowski MR (2018) Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis 9, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stab BR, Martinez L, Grismaldo A, Lerma A, Gutiérrez ML, Barrera LA, Sutachan JJ & Albarracín SL (2016) Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front Aging Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correia-Melo C, Marques FDM, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A, Rushton MD, Charles M, Jurk D, Tait SWG, Czapiewski R, Greaves L, Nelson G, Bohlooly-Y M, Rodriguez-Cuenca S, Vidal-Puig A, Mann D, Saretzki G, Quarato G, Green DR, Adams PD, von Zglinicki T, Korolchuk VI & Passos JF (2016) Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 35, 724–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helman A, Klochendler A, Azazmeh N, Gabai Y, Horwitz E, Anzi S, Swisa A, Condiotti R, Granit RZ, Nevo Y, Fixler Y, Shreibman D, Zamir A, Tornovsky-Babeay S, Dai C, Glaser B, Powers AC, Shapiro AMJ, Magnuson MA, Dor Y & Ben-Porath I (2016) p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med 22, 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Callender LA, Carroll EC, Bober EA, Akbar AN, Solito E & Henson SM (2020) Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 19, e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spinelli JB & Haigis MC (2018) The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 20, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Reyes I & Chandel NS (2020) Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zwerschke W, Mazurek S, Stöckl P, Hütter E, Eigenbrodt E & Jansen-Dürr P (2003) Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J 376, 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delfarah A, Parrish S, Junge JA, Yang J, Seo F, Li S, Mac J, Wang P, Fraser SE & Graham NA (2019) Inhibition of nucleotide synthesis promotes replicative senescence of human mammary epithelial cells. J Biol Chem 294, 10564–10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutter E, Renner K, Pfister G, Stöckl P, Jansen-Dürr P & Gnaiger E (2004) Senescence-associated changes in respiration and oxidative phosphorylation in primary human fibroblasts. Biochem J 380, 919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stöckl P, Hütter E, Zwerschke W & Jansen-Dürr P (2006) Sustained inhibition of oxidative phosphorylation impairs cell proliferation and induces premature senescence in human fibroblasts. Exp Gerontol 41, 674–682. [DOI] [PubMed] [Google Scholar]
- 60.Stöckl P, Zankl C, Hütter E, Unterluggauer H, Laun P, Heeren G, Bogengruber E, Herndler-Brandstetter D, Breitenbach M & Jansen-Dürr P (2007) Partial uncoupling of oxidative phosphorylation induces premature senescence in human fibroblasts and yeast mother cells. Free Radic Biol Med 43, 947–958. [DOI] [PubMed] [Google Scholar]
- 61.Bittles AH & Harper N (1984) Increased glycolysis in ageing cultured human diploid fibroblasts. Biosci Rep 4, 751–756. [DOI] [PubMed] [Google Scholar]
- 62.James EL, Michalek RD, Pitiyage GN, de Castro AM, Vignola KS, Jones J, Mohney RP, Karoly ED, Prime SS & Parkinson EK (2015) Senescent Human Fibroblasts Show Increased Glycolysis and Redox Homeostasis with Extracellular Metabolomes That Overlap with Those of Irreparable DNA Damage, Aging, and Disease. J Proteome Res 14, 1854–1871. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Rebollo E, Franzen J, Goetzke R, Hollmann J, Ostrowska A, Oliverio M, Sieben T, Rath B, Kornfeld JW & Wagner W (2020) Senescence-Associated Metabolomic Phenotype in Primary and iPSC-Derived Mesenchymal Stromal Cells. Stem Cell Reports 14, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, MacKay G, Van Der Burg SH, Verdegaal EME, Cascante M, Shlomi T, Gottlieb E & Peeper DS (2013) A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112. [DOI] [PubMed] [Google Scholar]
- 65.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A & Beach D (2005) Glycolytic enzymes can modulate cellular life span. Cancer Res 65, 177–185. [PubMed] [Google Scholar]
- 66.Sabbatinelli J, Prattichizzo F, Olivieri F, Procopio AD, Rippo MR & Giuliani A (2019) Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front Physiol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dörr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Däbritz JHM, Lisec J, Lenze D, Gerhardt A, Schleicher K, Kratzat S, Purfürst B, Walenta S, Mueller-Klieser W, Gräler M, Hummel M, Keller U, Buck AK, Dörken B, Willmitzer L, Reimann M, Kempa S, Lee S & Schmitt CA (2013) Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425. [DOI] [PubMed] [Google Scholar]
- 68.Correia-Melo C, Ichim G, Tait SWG & Passos JF (2017) Depletion of mitochondria in mammalian cells through enforced mitophagy. Nat Protoc 12, 183–194. [DOI] [PubMed] [Google Scholar]
- 69.Santos AL, Sinha S & Lindner AB (2018) The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxid Med Cell Longev 2018, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Almeida AJPO , Ribeiro TP, de Medeiros IA, Porto S & de Medeiros IA (2017) Aging: Molecular Pathways and Implications on the Cardiovascular System. Oxid Med Cell Longev 2017, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petersen S, Saretzki G & von Zglinicki T (1998) Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res 239, 152–60. [DOI] [PubMed] [Google Scholar]
- 74.von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27, 339–44. [DOI] [PubMed] [Google Scholar]
- 75.Saretzki G, Murphy MP & von Zglinicki T (2003) MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell 2, 141–143. [DOI] [PubMed] [Google Scholar]
- 76.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, Herbig U, Longhese MP & d’Adda di Fagagna F (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hewitt G, Jurk D, Marques FDM, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J & Passos JF (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bae NS & Baumann P (2007) A RAP1/TRF2 Complex Inhibits Nonhomologous End-Joining at Human Telomeric DNA Ends. Mol Cell 26, 323–334. [DOI] [PubMed] [Google Scholar]
- 79.Fouquerel E, Barnes RP, Uttam S, Watkins SC, Bruchez MP & Opresko PL (2019) Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol Cell 75, 117–130.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fouquerel E, Lormand J, Bose A, Lee HT, Kim GS, Li J, Sobol RW, Freudenthal BD, Myong S & Opresko PL (2016) Oxidative guanine base damage regulates human telomerase activity. Nat Struct Mol Biol 23, 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Opresko PL, Fan J, Danzy S, Wilson DM & Bohr VA (2005) Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res 33, 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C & von Zglinicki T (2012) A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 11, 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Victorelli S, Lagnado A, Halim J, Moore W, Talbot D, Barrett K, Chapman J, Birch J, Ogrodnik M, Meves A, Pawlikowski JS, Jurk D, Adams PD, Heemst D, Beekman M, Slagboom PE, Gunn DA & Passos JF (2019) Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J 38, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamon MP, Ahmed EK, Baraibar MA & Friguet B (2020) Proteome Oxidative Modifications and Impairment of Specific Metabolic Pathways During Cellular Senescence and Aging. Proteomics 20, 1–10. [DOI] [PubMed] [Google Scholar]
- 85.von Zglinicki T, Nilsson E, Döcke WD & Brunk UT (1995) Lipofuscin accumulation and ageing of fibroblasts. Gerontology 41 Suppl 2, 95–108. [DOI] [PubMed] [Google Scholar]
- 86.Evangelou K, Lougiakis N, Rizou SV., Kotsinas A, Kletsas D, Muñoz-Espín D, Kastrinakis NG, Pouli N, Marakos P, Townsend P, Serrano M, Bartek J & Gorgoulis VG (2017) Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 16, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lener B, Kozieł R, Pircher H, Hütter E, Greussing R, Herndler-Brandstetter D, Hermann M, Unterluggauer H & Jansen-Dürr P (2009) The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem J 423, 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kozieł R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA & Jansen-Dürr P (2013) Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J 452, 231–9. [DOI] [PubMed] [Google Scholar]
- 89.Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, Talbot M, Dardalhon M, Al Ghuzlan A, Bidart J-M, Schlumberger M & Dupuy C (2012) ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene 31, 1117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nelson G, Kucheryavenko O, Wordsworth J & von Zglinicki T (2018) The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech Ageing Dev 170, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodier F, Coppé J, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR & Campisi J (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11, 973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Osorio FG, Bárcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JMP & López-Otín C (2012) Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev 26, 2311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A & Francés B (2003) Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol 284, H1460–H1467. [DOI] [PubMed] [Google Scholar]
- 94.Villeneuve C, Guilbeau-Frugier C, Sicard P, Lairez O, Ordener C, Duparc T, De Paulis D, Couderc B, Spreux-Varoquaux O, Tortosa F, Garnier A, Knauf C, Valet P, Borchi E, Nediani C, Gharib A, Ovize M, Delisle M-B, Parini A & Mialet-Perez J (2013) p53-PGC-1α Pathway Mediates Oxidative Mitochondrial Damage and Cardiomyocyte Necrosis Induced by Monoamine Oxidase-A Upregulation: Role in Chronic Left Ventricular Dysfunction in Mice. Antioxid Redox Signal 18, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martini H, Lefevre L, Sayir S, Itier R, Maggiorani D, Dutaur M, Marsal DJ, Roncalli J, Pizzinat N, Cussac D, Parini A, Mialet-Perez J & Douin-Echinard V (2021) Selective cardiomyocyte oxidative stress leads to bystander senescence of cardiac stromal cells. Int J Mol Sci 22, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Velarde MC, Flynn JM, Day NU, Melov S & Campisi J (2012) Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY) 4, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Treiber N, Maity P, Singh K, Kohn M, Keist AF, Ferchiu F, Sante L, Frese S, Bloch W, Kreppel F, Kochanek S, Sindrilaru A, Iben S, Högel J, Ohnmacht M, Claes LE, Ignatius A, Chung JH, Lee MJ, Kamenisch Y, Berneburg M, Nikolaus T, Braunstein K, Sperfeld A-D, Ludolph AC, Briviba K, Wlaschek M & Scharffetter-Kochanek K (2011) Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell 10, 239–254. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H & Richardson A (2017) A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1− mice is correlated to increased cellular senescence. Redox Biol 11, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robinson AR, Yousefzadeh MJ, Rozgaja TA, Wang J, Li X, Tilstra JS, Feldman CH, Gregg SQ, Johnson CH, Skoda EM, Frantz M-C, Bell-Temin H, Pope-Varsalona H, Gurkar AU, Nasto LA, Robinson RAS, Fuhrmann-Stroissnigg H, Czerwinska J, McGowan SJ, Cantu-Medellin N, Harris JB, Maniar S, Ross MA, Trussoni CE, LaRusso NF, Cifuentes-Pagano E, Pagano PJ, Tudek B, Vo NV., Rigatti LH, Opresko PL, Stolz DB, Watkins SC, Burd CE, Croix CM St., Siuzdak G, Yates NA, Robbins PD, Wang Y, Wipf P, Kelley EE & Niedernhofer LJ (2018) Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol 17, 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SLF, Fullard N, Nelson G, Mann J, Van De Sluis B, Mann DA & Von Zglinicki T (2014) Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W, Yang X, López de Silanes I, Carling D & Gorospe M (2003) Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem 278, 27016–23. [DOI] [PubMed] [Google Scholar]
- 102.Peyton KJ, Liu XM, Yu Y, Yates B & Durante W (2012) Activation of AMP-activated protein kinase inhibits the proliferation of human endothelial cells. J Pharmacol Exp Ther 342, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ & Thompson CB (2005) AMP-Activated Protein Kinase Induces a p53-Dependent Metabolic Checkpoint. Mol Cell 18, 283–293. [DOI] [PubMed] [Google Scholar]
- 104.Jiang P, Du W, Mancuso A, Wellen KE & Yang X (2013) Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang N, Yi J, Gaier Guo †, Liu X, Shang Y, Tong T, Cui Q, Zhan M, Gorospe M & Wang W (2010) HuR Uses AUF1 as a Cofactor To Promote p16 INK4 mRNA Decay. Mol Cell Biol 30, 3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khaidizar FD, Bessho Y & Nakahata Y (2021) Nicotinamide Phosphoribosyltransferase as a Key Molecule of the Aging/Senescence Process. Int J Mol Sci 22, 3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pi C, Ma C, Wang H, Sun H, Yu X, Gao X, Yang Y, Sun Y, Zhang H, Shi Y, Li Y, Li Y & He X (2021) MiR-34a suppression targets Nampt to ameliorate bone marrow mesenchymal stem cell senescence by regulating NAD+-Sirt1 pathway. Stem Cell Res Ther 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV., Schultz D, ichi Noma K, Baur JA, Schug Z, Tang HY, Speicher DW, David G & Zhang R (2019) NAD + metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol 21, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP & Pickering JG (2007) Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem 282, 10841–5. [DOI] [PubMed] [Google Scholar]
- 110.Lee S-M, Dho SH, Ju S-K, Maeng J-S, Kim J-Y & Kwon K-S (2012) Cytosolic malate dehydrogenase regulates senescence in human fibroblasts. Biogerontology 13, 525–36. [DOI] [PubMed] [Google Scholar]
- 111.Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E & Campisi J (2016) Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chini CCS, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, Gomez LS, Hogan KA, Tarragó MG, Puranik AS, Agorrody G, Thompson KL, Dang K, Clarke S, Childs BG, Kanamori KS, Witte MA, Vidal P, Kirkland AL, De Cecco M, Chellappa K, McReynolds MR, Jankowski C, Tchkonia T, Kirkland JL, Sedivy JM, van Deursen JM, Baker DJ, van Schooten W, Rabinowitz JD, Baur JA & Chini EN (2020) CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat Metab 2, 1284–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chini C, Hogan KA, Warner GM, Tarragó MG, Peclat TR, Tchkonia T, Kirkland JL & Chini E (2019) The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem Biophys Res Commun 513, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, Schmidt MS, Heckenbach I, Kwok R, Wiley CD, Wong HS, Gibbs E, Iyer SS, Basisty N, Wu Q, Kim IJ, Silva E, Vitangcol K, Shin KO, Lee YM, Riley R, Ben-Sahra I, Ott M, Schilling B, Scheibye-Knudsen M, Ishihara K, Quake SR, Newman J, Brenner C, Campisi J & Verdin E (2020) Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat Metab 2, 1265–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A & Chini EN (2016) CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab 23, 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tarragó MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D, Cho DS, Boslett JJ, Miller JD, Zweier JL, Passos JF, Doles JD, Becherer DJ & Chini EN (2018) A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Metabolic Dysfunction by Reversing Tissue NAD+ Decline. Cell Metab 27, 1081–1095.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu D & Finkel T (2002) A role for mitochondria as potential regulators of cellular life span. Biochem Biophys Res Commun 294, 245–248. [DOI] [PubMed] [Google Scholar]
- 118.Chen W, Yu X, Wu Y, Tang J, Yu Q, Lv X, Zha Z, Hu B, Li X, Chen J, Ma L, Workman JL & Li S (2021) The SESAME complex regulates cell senescence through the generation of acetyl-CoA. Nat Metab 3, 983–1000. [DOI] [PubMed] [Google Scholar]
- 119.Fafián-Labora J, Carpintero-Fernández P, Jordan SJD, Shikh-Bahaei T, Abdullah SM, Mahenthiran M, Rodríguez-Navarro JA, Niklison-Chirou MV & O’Loghlen A (2019) FASN activity is important for the initial stages of the induction of senescence. Cell Death Dis 10, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng L, Cardaci S, Jerby L, MacKenzie ED, Sciacovelli M, Johnson TI, Gaude E, King A, Leach JDG, Edrada-Ebel R, Hedley A, Morrice NA, Kalna G, Blyth K, Ruppin E, Frezza C & Gottlieb E (2015) Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun 6, 6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petit M, Koziel R, Etemad S, Pircher H & Jansen-Dürr P (2017) Depletion of oxaloacetate decarboxylase FAHD1 inhibits mitochondrial electron transport and induces cellular senescence in human endothelial cells. Exp Gerontol 92, 7–12. [DOI] [PubMed] [Google Scholar]
- 122.Unterluggauer H, Mazurek S, Lener B, Hütter E, Eigenbrodt E, Zwerschke W & Jansen-Dürr P (2008) Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology 9, 247–259. [DOI] [PubMed] [Google Scholar]
- 123.Johmura Y, Yamanaka T, Omori S, Wang T-W, Sugiura Y, Matsumoto M, Suzuki N, Kumamoto S, Yamaguchi K, Hatakeyama S, Takami T, Yamaguchi R, Shimizu E, Ikeda K, Okahashi N, Mikawa R, Suematsu M, Arita M, Sugimoto M, Nakayama KI, Furukawa Y, Imoto S & Nakanishi M (2021) Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science (80- ) 371, 265–270. [DOI] [PubMed] [Google Scholar]
- 124.Yoo HC, Yu YC, Sung Y & Han JM (2020) Glutamine reliance in cell metabolism. Exp Mol Med 52, 1496–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Asadi Shahmirzadi A, Edgar D, Liao CY, Hsu YM, Lucanic M, Asadi Shahmirzadi A, Wiley CD, Gan G, Kim DE, Kasler HG, Kuehnemann C, Kaplowitz B, Bhaumik D, Riley RR, Kennedy BK & Lithgow GJ (2020) Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab 32, 447–456.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O’Neill LA & Mills EL (2019) Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat Metab 1, 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gong T, Liu L, Jiang W & Zhou R (2020) DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 20, 95–112. [DOI] [PubMed] [Google Scholar]
- 128.Nakahira K, Hisata S & Choi AMK (2015) The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxidants Redox Signal 23, 1329–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grazioli S & Pugin J (2018) Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front Immunol 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller KN, Victorelli SG, Salmonowicz H, Dasgupta N, Liu T, Passos JF & Adams PD (2021) Cytoplasmic DNA: sources, sensing, and role in aging and disease. Cell 184, 5506–5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, Perez-Garcia A, Kiourtis C, Dasgupta N, Lei X, Kruger PJ, Nixon C, Clark W, Jurk D, Bird TG, Passos JF, Berger SL, Dou Z & Adams PD (2020) Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev 34, 428–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Riley JS & Tait SW (2020) Mitochondrial DNA in inflammation and immunity. EMBO Rep 21, e49799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C & Cossarizza A (2014) Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging.” Eur J Immunol 44, 1552–1562. [DOI] [PubMed] [Google Scholar]
- 134.Iske J, Seyda M, Heinbokel T, Maenosono R, Minami K, Nian Y, Quante M, Falk CS, Azuma H, Martin F, Passos JF, Niemann CU, Tchkonia T, Kirkland JL, Elkhal A & Tullius SG (2020) Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swanson K V, Deng M & Ting JPY (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou R, Yazdi AS, Menu P & Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–226. [DOI] [PubMed] [Google Scholar]
- 137.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL & Sutterwala FS (2013) Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation. Immunity 39, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dudek J (2017) Role of Cardiolipin in Mitochondrial Signaling Pathways. Front Cell Dev Biol 5, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arivazhagan P, Mizutani E, Fujii M & Ayusawa D (2004) Cardiolipin induces premature senescence in normal human fibroblasts. Biochem Biophys Res Commun 323, 739–742. [DOI] [PubMed] [Google Scholar]
- 140.Kim SJ, Mehta HH, Wan J, Kuehnemann C, Chen J, Hu JF, Hoffman AR & Cohen P (2018) Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 10, 1239–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A & Shadel GS (2015) Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Desdín-Micó G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabandé-Rodríguez E, Blanco EM, Alfranca A, Cussó L, Desco M, Ibañez B, Gortazar AR, Fernández-Marcos P, Navarro MN, Hernaez B, Alcamí A, Baixauli F & Mittelbrunn M (2020) T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chaung WW, Wu R, Ji Y, Dong W & Wang P (2012) Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med 30, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Crouser ED, Shao G, Julian MW, Macre JE, Shadel GS, Tridandapani S, Huang Q & Wewers MD (2009) Monocyte activation by necrotic cells is promoted by mitochondrial proteins and formyl peptide receptors. Crit Care Med 37, 2000–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tait SWG & Green DR (2010) Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11, 621–632. [DOI] [PubMed] [Google Scholar]
- 146.Robbins PD, Jurk D, Khosla S, Kirkland JL, Lebrasseur NK, Miller JD, Passos JF, Pignolo RJ, Tchkonia T & Niedernhofer LJ (2021) Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu Rev Pharmacol Toxicol 61, 779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hubackova S, Davidova E, Rohlenova K, Stursa J, Werner L, Andera L, Dong LF, Terp MG, Hodny Z, Ditzel HJ, Rohlena J & Neuzil J (2019) Selective elimination of senescent cells by mitochondrial targeting is regulated by ANT2. Cell Death Differ 26, 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laberge R-M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez P-Y, Benz CC, Kapahi P, Nelson PS & Campisi J (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17, 1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN & Ferbeyre G (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12, 489–498. [DOI] [PubMed] [Google Scholar]
- 150.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, Georgilis A, Montoya A, Wolter K, Dharmalingam G, Faull P, Carroll T, Martínez-Barbera JP, Cutillas P, Reisinger F, Heikenwalder M, Miller RA, Withers D, Zender L, Thomas GJ & Gil J (2015) mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17, 1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Correia-Melo C, Birch J, Fielder E, Rahmatika D, Taylor J, Chapman J, Lagnado A, Carroll BM, Miwa S, Richardson G, Jurk D, Oakley F, Mann J, Mann DA, Korolchuk VI & Passos JF (2019) Rapamycin improves healthspan but not inflammaging in nfκb1 −/− mice. Aging Cell 18, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Algire C, Moiseeva O, Deschênes-Simard X, Amrein L, Petruccelli L, Birman E, Viollet B, Ferbeyre G & Pollak MN (2012) Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res 5, 536–543. [DOI] [PubMed] [Google Scholar]