Abstract
Gibberella zeae, a major cause of cereal scab, may be divided into two chemotypes based on production of the trichothecenes deoxynivalenol (DON) and nivalenol (NIV). We cloned and sequenced the gene cluster for trichothecene biosynthesis from each chemotype. G. zeae H-11 is a DON producer isolated from corn, and G. zeae 88-1 is a NIV producer from barley. We sequenced a 23-kb gene cluster from H-11 and a 26-kb cluster from 88-1, along with the unlinked Tri101 genes. Each gene cluster contained 10 Tri gene homologues in the same order and transcriptional directions as those of Fusarium sporotrichioides. Between H-11 and 88-1 all of the Tri homologues except Tri7 were conserved, with identities ranging from 88 to 98% and 82 to 99% at the nucleotide and amino acid levels, respectively. The Tri7 sequences were only 80% identical at the nucleotide level. We aligned the Tri7 genes and found that the Tri7 open reading frame of H-11 carried several mutations and an insertion containing 10 copies of an 11-bp tandem repeat. The Tri7 gene from 88-1 carried neither the repeat nor the mutations. We assayed 100 G. zeae isolates of both chemotypes by PCR amplification with a primer pair derived from the Tri7 gene and could differentiate the chemotypes by polyacrylamide gel electrophoresis. The PCR-based method developed in this study should provide a simple and reliable diagnostic tool for differentiating the two chemotypes of G. zeae.
Gibberella zeae (Schwein.) Petch (anamorph: Fusarium graminearum Schwabe) is an important pathogen of cereal crops in many areas of the world. The fungus causes head and seedling blight of small grains, such as wheat and barley; ear and stalk rot of corn; and stem rot of carnation (7, 20, 28). Head blight and ear rot reduce the yield of grain, and harvested grain is often contaminated with mycotoxins, such as trichothecenes and zearalenone (23). The fungus produces 8-ketotrichothecenes, including deoxynivalenol (DON), 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, nivalenol (NIV), and 4-acetylnivalenol (4-ANIV), as well as an estrogenic mycotoxin, zearalenone (27, 35). Of these 8-ketotrichothecenes, DON and NIV are frequently found in cereals harvested in Korea and Japan (17, 38, 40). Compared to DON, NIV is present at higher levels in cereals and exhibits greater toxicity (33). Therefore, NIV is of greater concern in these countries.
Based on 8-ketotrichothecene production, Ichinoe et al. (15) divided G. zeae into two chemotypes groups. The DON chemotype produces DON and acetyl-DON, and the NIV chemotype produces NIV and 4-ANIV. These chemotypes appear to differ in geographic distribution. The NIV chemotype has been reported in several countries of Africa, Asia, and Europe (10, 14, 22, 35, 37), but it has not been reported in North America (1, 27). Since the NIV chemotype is of great concern in the countries where it has appeared, an efficient system for differentiating DON and NIV chemotypes is desired. Recently O'Donnell et al. (30) divided global population of G. zeae into seven biogeographically structured lineages based on phylogenetic analysis using six gene genealogies. The two chemotypes, however, do not appear to correlate with the global phylogenetic structure of G. zeae.
Although the geographic distribution of DON and NIV chemotypes has been well studied, little is known about the genetic basis of 8-ketotrichothecene production by these chemotypes. However, the molecular genetics of the production of T-2 toxin by Fusarium sporotrichioides has been studied intensively, using various mutant strains blocked at specific steps in the trichothecene pathway (9, 13). Many trichothecene biosynthesis genes are localized in a gene cluster comprising at least 10 genes. These genes include those encoding trichodiene synthetase (Tri5) (11), P450 oxygenases (Tri4 and Tri11) (2, 12), acetyltransferase (Tri3) (25), a transcription factor (Tri6) (32), a toxin efflux pump (Tri12) (3), and several unidentified hypothetical proteins (Tri7, Tri8, Tri9, and Tri10) (13, 26). Another acetyltransferase (Tri101) (19) is unlinked to the cluster. Homologues of Tri5, Tri6, Tri11, Tri12, and Tri101 have been previously found in G. zeae (18, 24, 31, 39), and all of the genes exhibited high degrees of sequence homology to those of F. sporotrichioides. A homologue of the F. sporotrichioides Tri12 was designated Tri102 in G. zeae (39).
In this study we built on the F. sporotrichioides results to analyze the trichothecene biosynthesis gene clusters in the DON and NIV chemotypes of G. zeae. The objectives of the study were to identify genetic differences between the trichothecene biosynthetic pathways of the two chemotypes, and to develop a rapid method for G. zeae chemotype identification based on PCR analysis.
MATERIALS AND METHODS
Strains, media, and culture conditions.
G. zeae strains H-11 (a DON producer) and 88-1 (a NIV producer) were isolated from Korean corn and barley, respectively, and used for sequence analysis. For PCR assays, 100 field isolates of G. zeae were used. Of these isolates, 25 DON producers were from the United States. The remaining 25 DON and 50 NIV producers were from a previous study of Korean strains (35). Fungi were cultured on potato dextrose agar (Difco Laboratories, Detroit, Mich.) and preserved as 25% glycerol stock cultures frozen at −80°C. To isolate genomic DNA, fungal conidia were inoculated into 100 ml of complete liquid medium (8) at 106 per ml. Cultures were incubated in 250-ml Erlenmyer flasks at 25°C for 48 h on a rotary shaker (200 rpm), after which mycelia were harvested and lyophilized. Escherichia coli strains were grown on Luria-Bertani agar or liquid medium supplemented with ampicillin (75 μg/ml).
DNA manipulations and PCR conditions.
Fungal genomic DNA was prepared as previously described (16). E. coli colonies carrying recombinant plasmids were screened by a single-tube mini-prep method (21). For sequencing, plasmids were purified from 5-ml E. coli cultures using a Qiagen kit (Qiagen Inc., Valencia, Calif.). Standard procedures were used for restriction endonuclease digestions, ligations, and agarose or polyacrylamide gel electrophoresis (34).
PCR primers used for Tri gene amplification in this study are listed in Table 1. They were synthesized by the Bioneer oligonucleotide synthesis facility (Bioneer Corporation, Chungwon, Korea), dissolved at 100 μM in sterilized water, and stored at −20°C. For PCRs, about 50 ng of genomic DNA was used as a template in a 50-μl reaction mixture containing Ex Taq PCR buffer, which contains 2 mM MgCl2 (TaKaRa Biomedicals, Shiga, Japan), deoxynucleoside triphosphate mixture (0.2 mM each), a 2 μM concentration of each primer, and 1.25 U of Ex Taq (TaKaRa Biomedicals). PCRs were performed in a thermal cycler (MJ Research, Inc., Waltham, Mass.) set to the following: denaturation at 94°C for 2 min; 30 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 2 min 30 s; and a final extension step at 72°C for 10 min. After PCR was completed, PCR mixtures were stored at 4°C.
TABLE 1.
Primers used for PCR-based cloning
| Primer no. | Primer designation | Sequence (5′ to 3′)b | Position (bp)c |
|---|---|---|---|
| 1 | Tri5/p1 | AGATCGTACAGCTAAATTCAGAGC | 15500–15523 |
| 2 | Tri5/p2 | CCACTAGCTCAATTGAACTTAGGA | 17393–17370 |
| 3 | Tri6/p1 | TGGAGNGCNTTNCCNCTNTTTGA | 12663–12685 |
| 4 | Tri6/p2 | TTGTGTATNCGNCTATAGTGATC | 13276–13254 |
| 5 | Tri4/p2 | TCNGTYTTNCCNGGDATNGC | 9757–9776 |
| 5′ | Tri4/2p2 | GTCGATGTCGGCTTTAGTGGTGTC | 9810–9833 |
| 6 | Tri4/p1 | GGNCCNTAYYTNGARTTYTA | 11293–11274 |
| 6′ | Tri4/2p1 | AGCCTTAGTCAATATCCCCATCAG | 11431–11409 |
| 7 | Tri3/p1 | CAGCGCTATACAGATGGAAGTC | 6823–6844 |
| 8 | Tri3/p2 | TCGGTATAGTTTGCATCATTGTAG | 8487–8464 |
| 9 | Tri7/p1 | CGCATCGAAAGTGGAAGGTT | 4618–4637 |
| 9′ | Tri7/p5 | TGCCTCTCCTGCCATCGTC | 4750–4768 |
| 10 | Tri7/p2 | ATGAACCAAAATGATACCCAGATG | 5823–5800 |
| 11 | Tri11/p2 | ACATTNAGNGGNGGCTTGTTCC | 21419–21440 |
| 12 | Tri11/p1 | ATGTTTCAATATTCNTTNTGGCC | 23140–23118 |
| 13 | Tri102/p2 | TGGTGNGGNACATGATCNGTNAGG | 23890–23913 |
| 14 | Tri102/p1 | GACCTNGAATCNCAGCCNGACGAC | 25661–25638 |
| 15 | Itri5n/p1 | ACTTGACTAGGTAGCCACGGTGTTTTATTC | 15723–15694 |
| 16 | Itri5n/p2 | GAAAAGGTCAAGCATCAGGATACAGAGGA | 17204–17232 |
| 17 | Itri65/p1 | GAAGTCAAAGTCGAAGCAAACAAGTGG | 12878–12852 |
| 18 | Itri65/p2 | GCCAAGCAAATGCCCGTATCCCAGTT | 12971–12996 |
| 19 | Itri7n/p1 | GAAACCGCGATGACCGATACTGCTGCTAAG | 4837–4808 |
| 20 | Itri7n/p2 | GCCACAAAGCAAGACGGAGACGAAGGAG | 5744–5771 |
| 21 | Itri11n/p2 | TAGCCGCAATTGGTACCAGGATAGCAG | 21481–21455 |
| 22 | Itri11n/p1 | CCGCAGGGGATGGAAAAAGAGGTTGTA | 22979–23005 |
| 23 | I2tri5/p2 | GCGATGCTGCCAGGCTCAAGGTAAAG | 14905–14880 |
| 24 | I2tri5/p1 | AACTAATTGTCTGTAACCTGAGCCTGTAACCA | 17553–17584 |
| 25 | Itri4nd/p2 | CYGATTGGAGGAGAACACTTGATAACATTT | 9936–9907 |
| 26 | Itri4n/p1 | GCTTTGGTCCCGGGATCTTTCGCAGTG | 11305–11331 |
| 26′ | Itri4d/p1 | GGACGATGGGGCCTTGAGGATGTGATG | 8425–8451d |
| 27 | Itri8/p1 | TTAATTTAGGCGAACAAGAGACGGAAGACAGT | 1191–1160 |
| 28 | Itri8/p2 | AGTATCGGTCATCGCGGTTTCATTCTGG | 4817–4844 |
| 29 | Itri102n/p5 | ATTTTGCCGGCTTTGTCTGCATCCTTCTC | 24716–24688 |
| 30 | Itri102nd/p1 | TGAAGAACAAAGTAGGTAGYGCAGACATT | 25510–25538 |
| 31 | Tri6nd/p2 | CACGACCAGGAAGGACAACAATG | 13197–13219 |
| 32 | Tri6nd/p1 | CTTGGCGGGATCGGGAGAC | 12713–12695 |
| 33 | Tri5n/p1 | GGGGAGTTGTTAGGCTTGGATTTG | 14358–14335 |
| 34 | Itri3nd/p1 | TCCGAAGAGAAGTAGCGATGAARAGAT | 6857–6931 |
| 35 | Itri3d/p2 | TCCGTTGAGAGCATAGACTTTGTTGTGAA | 8322–8350 |
| 36 | Itri53/p3 | TGTATCCTGATGCTTGACCTTTTC | 17227–17204 |
| 37 | Itri53/p2 | CTTGCAGAACTGAACAGGCGACGGTATG | 18282–18309 |
| 38 | Tri8/delp2 | ACAGCGTGGACACAGCAACCCTTACTT | 4203–4229 |
| 39 | I53n/np3 | TCGGCAACATGGCAACGGACTT | 20553–20574 |
| 40 | I11n/np4 | GCTTTTCGCGCTGTTGCCGTAGG | 20764–20742 |
| 41 | 8n/p2 | AGATGAATTACCAGGCAGATGTCCAGAAGTCG | 2567–2598 |
| 42 | 7d/p1 | AGGCCGTGAAAATGAACCGCTTGATGTA | 1963–1936d |
| 43 | 5d/np1 | CACTACGAAACTGCCGGCCATCACATA | 12866–12840d |
| 44 | 7d/np1 | TGTGCCGTGAGCCCCAGAATGAAGC | 2271–2247d |
| 45 | I7d/p1 | ACGGTGTACGAGTGGTTTGTGTCCTATTCA | 2363–2392d |
| 46 | Itri102d/p4 | TATCCCTCTTTCTCCTTGGTGACTACTGG | 21988–22016d |
| 47 | Tri5d/p4 | TGGTTTGGGTCATTGTGTTGGGGCTTCT | 14063–14090d |
| 48 | I11d/np4 | TGCTTTCATTAATTTTCGCTTTTC | 17990–17967d |
| 48′ | I11d/np2 | CTTGCAACTTTAACGTGTCCAGAT | 20533–20556d |
| 49 | I4d/np3 | ATGGCAAGTTATGGGGTCAGC | 9187–9207d |
| 50 | Itri102nd/p2 | GCTAATGTCTACTACTTCGCTATGGCACTGG | 21122–21092d |
Amplification of Tri gene clusters.
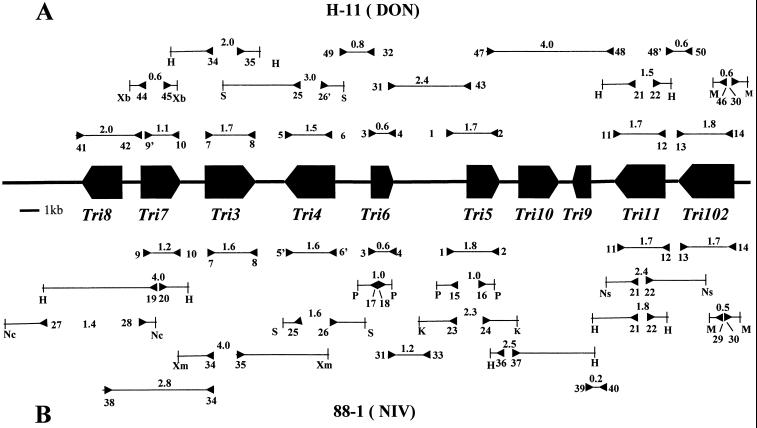
We amplified Tri genes from both strains (Fig. 1) by using several sets of degenerate primers that were based on the known sequences of Tri genes from G. zeae and F. sporotrichioides. Most of the degenerate primer pairs successfully amplified fragments of the expected sizes from the two strains. When necessary, primers were redesigned according to the consensus sequences of already-acquired Tri genes (e.g., primer pairs 5-6 and 5′-6′ in Table 1 and Fig. 1). The PCR product sequences were used to design specific primers for amplifying regions flanking the amplified Tri genes. A PCR was performed using two primers, each derived from different Tri genes based on the assumption that the order of the Tri genes in the G. zeae and F. sporotrichioides clusters is the same. For example, PCR using primers 31 and 43 successfully amplified the region between Tri6 and Tri5 from H-11 (Fig. 1). When this strategy failed, inverse PCR using primers derived from the known sequences was performed as previously described (29, 41). These PCR strategies yielded various sizes of DNA fragments sufficient for construction of contigs for both strains.
FIG. 1.
PCR strategies for amplification of trichothecene biosynthesis gene clusters from G. zeae strains H-11 (A) and 88-1 (B). Block arrows indicate the locations of Tri ORFs and their transcription directions within the gene cluster. The connecting thick lines represent noncoding DNA regions. The inverted arrowheads indicate PCR primer locations and orientations. Numbers above the thin lines bounded by inverted arrowheads indicate the approximate sizes (in kilobases) of amplified PCR products. Numbers next to or below the inverted arrowheads indicate the primers used (see Table 1 for primer names and sequences), and the letters indicate restriction enzyme sites: H, HindIII; K, KpnI; M, MseI; Nc, NcoI; Ns, NsiI; P, PstI; S, SpeI; Xa, XbaI; Xm, XmnI. Note that Tri101 is not part of the cluster and is not shown here.
Cloning and sequencing.
The amplified PCR products were analyzed by agarose gel electrophoresis. PCR products of the expected sizes were cloned into pCR2.1TOPO using the TOPO TA cloning kit (Invitrogen, San Diego, Calif.). Sequencing of the inserts in pCR2.1 TOPO was initiated with M13 reverse and forward primers and then extended using specific primers corresponding to the newly sequenced regions. DNA sequencing was done at the National Instrumentation Center for Environmental Management, Seoul National University, Suwon, Korea, using an ABI377 automated DNA sequencer (Applied Biosystems Inc., Foster City, Calif.). Primers for sequencing were designed using the PrimerSelect program (DNASTAR, Inc., Madison, Wis.). Sequences were assembled using the SeqMan program (DNASTAR, Inc.) and analyzed with the MegAlign and MapDraw programs (DNASTAR, Inc.). BLAST (4) searches were done against the NCBI and GenBank databases.
PCR assays.
To amplify the inserted region of Tri7 from 50 DON- and 50 NIV-producing isolates, we designed a specific set of primers. The sequence of primer GzTri7/f1 (forward) is 5′-GGCTTTACGACTCCTCAACAATGG-3′, and the sequence of primer GzTri7/r1 (reverse) is 5′-AGAGCCCTGCGAAAG(C/T)ACTGGTGC-3′. PCRs were performed in a thermal cycler set to the following: denaturation at 94°C for 2 min; 30 cycles of 94°C for 1 min, 60°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 10 min. After PCR was completed, PCR mixtures were stored at 4°C. PCR products were electrophoresed on 5% polyacrylamide gels; the products of the expected size (∼160 bp) were purified using a QIAquick PCR purification kit (Qiagen Inc.) and directly sequenced using primers GzTri7/f1 and GzTri7/r1.
Nucleotide sequence accession numbers.
The sequences of the trichothecene biosynthesis gene clusters we obtained from G. zeae 88-1 and H-11 have been deposited in GenBank under accession numbers AF336365 and AF336366, respectively.
RESULTS
Structural organization of trichothecene biosynthesis gene clusters from DON and NIV chemotypes.
Construction of contigs using the sequenced PCR fragments yielded 26-and 23-kb gene clusters from G. zeae 88-1 and H-11, respectively. Each cluster carried 8 open reading frames (ORFs), all of which were readily identified by sequence comparisons with current databases. Two additional ORFs (designated Tri9 and Tri10) were found between Tri5 and Tri11 as those in the trichothecene gene cluster of F. sporotrichioides (Fig. 1). The order and transcription directions of the ORFs are identical for the two G. zeae chemotypes and F. sporotrichioides.
Comparative sequence analysis.
We compared the sizes of the ORFs, putative introns, and adjacent noncoding DNA regions (Table 2) and the percent identities of the ORFs and their flanking sequences (Table 3) in the two G. zeae Tri clusters and other strains of G. zeae and F. sporotrichioides. All of the Tri genes except Tri7 exhibited significant conservation between the two strains, ranging from 88% (Tri8) to 98% (Tri101) identity at the nucleotide level and from 82% (Tri8) to 99% (Tri101) at the amino acid level. The flanking regions exhibited less nucleotide sequence identity than the ORFs, ranging from 57% (region between Tri3 and Tri4) to 89% (region between Tri5 and Tri11). In particular, strain H-11 lacked a 226-bp stretch in the noncoding region between Tri3 and Tri4 that was present in strain 88-1 (data not shown), giving this region the lowest percent identity overall.
TABLE 2.
Sizes of Tri ORFs, introns, and noncoding regions in trichothecene biosynthesis gene clusters of G. zeae and F. sporotrichioides
| Region(s) | No. of nucleotides (bp)/amino acids
|
Putative intron(s) (bp)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| 88-1 | H-11 | G. zeaeb | F. sporotrichioidesb | 88-1 | H-11 | G. zeaeb | F. sporotrichioidesb | |
| 8 | 1,335/445 | 1,338/446 | NAc | 1,344/447 | 0 | 0 | NA | 0 |
| 8–7 | 701/—d | 681/— | ||||||
| 7 | 1,377/442 | 1,444/431 | NA | 1,250/398 | 51 | 151 | NA | 53 |
| 7–3 | 799/— | 812/— | ||||||
| 3 | 1,763/512 | 1,768/512 | NA | 1,783/513 | 55, 49, 58, 65 | 56, 49, 58, 69 | NA | 56, 61, 59, 65e |
| 3–4 | 1,165/— | 946/— | ||||||
| 4 | 1,742/521 | 1,742/521 | NA | 1,743/520 | 58, 52, 69 | 58, 52, 69 | NA | 58, 52, 70e |
| 4–6 | 1,171/— | 1,168/— | ||||||
| 6 | 657/219 | 657/219 | 657/218 | 654/217 | 0 | 0 | 0 | 0 |
| 6–5 | 2,935/— | 2,886/— | ||||||
| 5 | 1,180/376 | 1,187/376 | 1,187/375 | 1,185/374 | 52 | 59 | 59 | 60 |
| 11 | 1,741/493 | 1,741/493 | 1,741/492 | 1,722/492 | 57, 54, 78, 73 | 57, 54, 78, 73 | 57, 55, 82, 68 | 49, 54, 76, 64 |
| 11–102 | 649/— | 632/— | ||||||
| 102 | 1,905/591 | 1,902/590 | 1,906/598 | 1,902/598 | 56, 76 | 56, 76 | 60, 76 | 56, 49e |
| 101 | 1,202/400f | 1,356/452 | 1,356/451 | 1,380/459 | 0 | 0 | 0 | 0 |
The order of introns is 5′ to 3′ in each ORF.
Sequences of noncoding DNA regions are not compared here. Refer to Table 3 for accession numbers of each sequence.
NA, not available.
—, no translation product from noncoding DNA regions.
Confirmed by cDNA sequencing.
Putative translation product from an incomplete ORF.
TABLE 3.
Sequence homology of G. zeae and F. sporotrichioides Tri ORFs and noncoding regionsa
| Region(s) | % Nucleotide (% amino acid) identity betweenb:
|
||||
|---|---|---|---|---|---|
| 88-1 and H-11 | 88-1 and F. sporotrichioides | H-11 and F. sporotrichioides | 88-1 and G. zeae | H-11 and G. zeae | |
| 8 | 88 (82) | 81 (77) | 81 (75) | —c | — |
| 8–7 | 68 | — | — | — | — |
| 7 | 80d | 73 (72) | 64d | — | — |
| 7–3 | 86 | — | — | — | — |
| 3 | 93 (96) | 83 (85) | 82 (84) | — | — |
| 3–4 | 57 | — | — | — | — |
| 4 | 96 (96) | 83 (85) | 84 (88) | — | — |
| 4–6 | 88 | — | — | — | — |
| 6 | 94 (95) | 86 (86) | 85 (85) | 95 (95) | 96 (96) |
| 6–5 | 82 | — | — | — | — |
| 5 | 94 (97) | 84 (90) | 85 (91) | 94 (97) | 100 (100) |
| 5–11 | 89 | — | — | — | — |
| 11 | 94 (97) | 84 (91) | 84 (91) | 91 (94) | 92 (94) |
| 11–102 | 81 | — | — | — | — |
| 102 | 92 (89) | 79 (75) | 76 (74) | 90 (85) | 90 (85) |
| 101 | 98 (99) | 80 (78) | 80 (77) | 99 (100) | 99 (99) |
Homology is presented as percent identity at both the nucleotide and amino acid (in parentheses) levels for Tri genes and at the nucleotide level only for noncoding DNA regions. Percent identities were calculated from pairwise comparison of the sequences using the Martinez/Needleman-Wunsch method (for nucleotides) and the Lipman-Pearson alignment (for amino acids) in the DNASTAR program.
88-1 is the NIV chemotype, and H-11 is the DON chemotype. G. zeae represents the sequences of other G. zeae strains from previous studies; GenBank accession numbers are AB017495 (Tri6), U22464 (Tri5), AB024617 (Tri11 and 102), and AB011417 (Tri101). The sequence of Tri5 in G. zeae is from a North American strain, and the other sequences are from a Japanese strain. F. sporotrichioides GenBank accession numbers are U22463 (Tri8, Tri7, and Tri3), U22462 (Tri4), U22150 (Tri6), M27246 (Tri5), AF011355 (Tri11 and Tri12), and AF127176 (Tri101).
—, not determined.
Homology at the amino acid level was not determined because the Tri7 gene of H-11 was defective.
The gene with the highest identity for the two chemotypes was Tri101, which is not part of the Tri gene cluster. The nucleotide and amino acid sequence identities were 98 and 99%, respectively. In addition, several Tri genes from other G. zeae strains, including Tri5, Tri6, Tri11, Tri102, and Tri101 (18, 24, 31, 39), were compared to the corresponding Tri ORFs of H-11 and 88-1 (Table 3). These genes were also highly similar to those of both chemotypes at both the nucleotide and amino acid levels. In contrast, the Tri genes of F. sporotrichioides were less similar to those of the G. zeae chemotypes, ranging from 76 to 86% identity for nucleotide sequences and from 74 to 91% identity for amino acid sequences.
Alignment of Tri7 ORFs from G. zeae DON and NIV chemotypes and F. sporotrichioides.
Unlike the other Tri ORFs, the Tri7 ORFs from the two G. zeae chemotypes and from F. sporotrichioides exhibited striking differences. First, the nucleotide sequence of the H-11 Tri7 ORF exhibited only 80% identity to that of 88-1 and 64% identity to that of F. sporotrichioides (Table 3). This level of conservation was significantly lower than that found for the other Tri ORFs. Second, alignment of the nucleotide sequences of these three Tri7 ORFs revealed several alterations present only in H-11 (data not shown). For example, a substitution in a potential start codon in H-11 appeared to have occurred, causing a deficient translation start signal (by comparison with F. sporotrichioides). The H-11 Tri7 nucleotide sequence also appeared to have had several deletions, an addition, or other substitutions, resulting in frame shifts that created an internal stop in the putative Tri7 amino acid sequence. Due to these features, comparisons of the H-11 Tri7 amino acid sequence with those of 88-1 and F. sporotrichioides could not be made. Finally, the most substantial differences between the H-11 Tri7 gene and the other two Tri7 genes were found within a putative intron sequence. The Tri7 ORFs of 88-1 and F. sporotrichioides were interrupted by a putative intron at the same position, identified from the consensus signals for intron splicing (5′-GTAAT∼TAG-3′). The corresponding region of H-11 was, however, much longer (151 bp) than those of 88-1 and F. sporotrichioides (51 bp each). This size difference was due to the presence of 10 tandem repeats of an 11-bp nucleotide stretch (CACAATATTAG) within that region of the H-11 Tri7 sequence. These repeats were not present in either 88-1 or F. sporotrichioides.
Amplification of the inserted regions of Tri7 genes from field isolates.
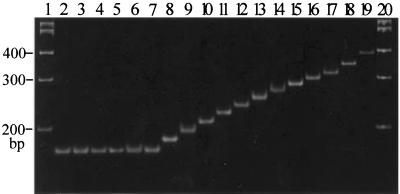
Selected regions spanning the Tri7 insertion from G. zeae field isolates were PCR amplified using primers GzTri7/f1 and GzTri7/r1. Amplification yielded products of various sizes from DON chemotypes but yielded products of only one size (∼160 bp) from all NIV chemotypes tested (Fig. 2). The amplified DON chemotype products were of distinctly lower mobility than the NIV chemotype products on 5% polyacrylamide gels. Direct sequencing of the PCR products revealed that the actual sizes of the products from DON chemotypes varied, ranging from 173 to 327 bp, depending on the number of 11-bp repeats within each sequence, whereas the products from NIV chemotypes were identical in size (161 bp) due to a lack of the repeat. The inserted repeats in the Tri7 sequences varied from 2 to 16 copies for the 50 DON-producing isolates (data not shown). This variation was not, however, related to their geographical origins.
FIG. 2.
PCR amplification patterns of the inserted regions of Tri7 from DON- or NIV-producing isolates of G. zeae on a 5% polyacrylamide gel. Lanes 1 and 20, 100-bp DNA ladder; lane 2, 88-1 (NIV chemotype); lanes 3 to 7, Korean NIV chemotypes. Lanes 8 to 19 show DON chemotypes with various numbers of repeats (in parentheses): lane 8, Korean (2); lane 9, United States (3); lane 10, Korean (4); lane 11, United States (5); lane 12, Korean (6); lane 13, Korean (7); lane 14, United States (8); lane 15, United States (9); lane 16, Korean (10); lane 17, United States (11); lane 18, United States (13); lane 19, United States (16)
DISCUSSION
We determined the sequence of the trichothecene biosynthesis gene cluster from DON and NIV-producing G. zeae isolates. We used these sequences to identify potential changes that might be responsible for differences in 8-ketotrichothecene biosynthesis.
Based on sequence differences, we think that the Tri7 gene may be one of the elements responsible for the difference in trichothecene production between the two chemotypes, as either no Tri7 protein or a truncated version is synthesized in H-11. These features are conserved in all of the DON isolates tested (data not shown), suggesting that the Tri7 gene is nonfunctional in all DON chemotypes. To confirm the role of Tri7 protein in G. zeae trichothecene biosynthesis, functional studies are needed. Previous experiments suggest that Tri7 protein is required for acetylation of the hydroxyl group at C-4 of T-2 toxin produced by F. sporotrichioides (13, 26). If the function of Tri7 in G. zeae is the same as in F. sporotrichioides, Tri7 protein is not required for DON production but is required for acetylation of the hydroxyl group at C-4 of NIV to convert to 4-ANIV; NIV chemotypes usually produce both NIV and 4-ANIV. Recently, an additional homologue of oxygenase (TriD) was found immediately upstream of Tri102 in both F. sporotrichioides and a DON-producing strain of G. zeae. However, sequence comparison showed that the TriD gene of the DON chemotype was defective (6). If TriD is functional in the NIV chemotypes as in F. sporotrichioides, both TriD and Tri7 would be determinants for biosynthesis of NIV and 4-ANIV in G. zeae.
The presence of different numbers of repeats within the Tri7 gene sequences of DON isolates suggests certain possibilities. The defective Tri7 gene in DON chemotypes of G. zeae may no longer be under selection pressure. This lack of selection would allow mutations to accumulate. Another possible mechanism for the variable number of repeats among the DON isolates is unequal crossing over during sexual recombination (5). To test this possibility, it is necessary to evaluate ascospore progeny from G. zeae DON chemotypes and see if the number of repeats present in the progeny is the same as in the parents. The Tri7 gene sequence could also be used as a marker to assess genetic diversity among DON isolates as well as to distinguish the chemotypes of G. zeae.
O'Donnell et al. (30) estimated global genetic diversity of G. zeae using a molecular phylogenetic analysis with multiple-gene genealogies. They discovered seven lineages within 37 isolates of G. zeae from a worldwide collection and demonstrated that the trichothecene chemotypes were not lineage specific. However, no Tri7 polymorphism was found in the Korean NIV-producing isolates in this study. These results are consistent with the hypothesis that there is a NIV chemotype-specific lineage in Korea, most likely lineage 6. In addition, the two Korean G. zeae chemotypes appear to be different lineages; 88-1 and H-11 were identified as lineages 6 and 7, respectively, based on sequence comparisons of Tri101. It is unclear whether the other Korean DON-producing isolates are lineage 7, as found for North American isolates. To confirm these results further studies are needed to determine the lineage(s) of Korean G. zeae isolates.
Differentiating chemotypes of G. zeae isolates is of great practical importance in several Asian countries, including Korea and Japan, where both chemotypes are common (15, 35, 36). Such a technique would also be useful in plant quarantine for North American countries, including the United States and Canada, where no NIV chemotypes have been detected (1, 27). The use of a PCR assay with Tri7 primers would avoid the difficulties that arise from time-consuming and laborious chemical analyses of toxins. In this study, we demonstrated that a PCR assay with Tri7 primers provides a rapid and reliable method for differentiating the two chemotypes of G. zeae from Korea and North America. If this PCR assay works on isolates from other locations, then it may be globally applicable. This assay also could be applicable for evaluating contamination of cereal samples with either DON- or NIV-producing isolates of G. zeae. This method, which is based on the size variation of PCR products, should be more reliable than other PCR-based detection systems that are based on the absence or presence of a PCR band and which may yield false-negative results. In addition, our studies of the nucleotide sequences of the Tri genes from the two chemotypes will allow development of a DNA probe hybridizing to different restriction fragment(s) of genomic DNA from each chemotype.
The sequence similarity in other Tri genes and noncoding regions between the two chemotypes of G. zeae most likely reflects their evolutionary divergence. However, the conservation of mutations, except for the number of repeats, within the Tri7 genes of DON isolates from both Korea and the United States leads us to question whether all DON isolates in different lineages have a common ancestry. Future studies are needed to determine if the Tri7 polymorphisms found in this study also are found in the other lineages.
ACKNOWLEDGMENTS
This study was supported by special research funds from the Korean Ministry of Agriculture and Forestry for 2000–2001 and by a postdoctoral fellowship to T.L. and graduate fellowships to D.-W.O., J.L., and H.-S.K. from the Korean Ministry of Education through the Brain Korea 21 Project.
We thank Robert L. Bowden of the Department of Plant Pathology, Kansas State University, Manhattan, for providing G. zeae strains for this study.
REFERENCES
- 1.Abbas H K, Mirocha C J, Kommedahl T, Vesonder R F, Golinski P. Production of trichothecene and non-trichothecene mycotoxins by Fusarium species isolated from maize in Minnesota. Mycopathologia. 1989;108:55–58. doi: 10.1007/BF00436784. [DOI] [PubMed] [Google Scholar]
- 2.Alexander N J, Hohn T M, McCormick S P. The TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl Environ Microbiol. 1988;64:221–225. doi: 10.1128/aem.64.1.221-225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander N J, McCormick S P, Hohn T M. TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: gene isolation and expression in yeast. Mol Gen Genet. 1999;261:977–984. doi: 10.1007/s004380051046. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Bowden R L, Leslie J F. Sexual recombination in Gibberella zeae. Phytopathology. 1999;89:182–188. doi: 10.1094/PHYTO.1999.89.2.182. [DOI] [PubMed] [Google Scholar]
- 6.Brown D W, McCormick S P, Alexander N J, Proctor R H, Desjardins A E. A genomic and biochemical approach to trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet Newsl. 2001;48(Suppl.):165. doi: 10.1006/fgbi.2001.1256. [DOI] [PubMed] [Google Scholar]
- 7.Cook R J. Fusarium diseases of wheat and other small grains in North America. In: Nelson P E, Toussoun T A, editors. Fusarium diseases, biology, and taxonomy. University Park: The Pennsylvania State University Press; 1981. pp. 39–52. [Google Scholar]
- 8.Correll J C, Klittich C J R, Leslic J F. Nitrate nonutilizing mutants and their use in vegetative compatibility tests. Phytopathology. 1987;77:1640–1646. [Google Scholar]
- 9.Desjardins A E, Hohn T M, McCormick S P. Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiol Rev. 1993;57:595–604. doi: 10.1128/mr.57.3.595-604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desjardins A E, Manadhar H K, Plattner R D, Maragos C M, Shrestha K, McCormick S P. Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J Agric Food Chem. 2000;48:1377–1383. doi: 10.1021/jf991022b. [DOI] [PubMed] [Google Scholar]
- 11.Hohn T M, Beremand P D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989;79:131–138. doi: 10.1016/0378-1119(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 12.Hohn T M, Desjardins A E, McCormick S P. The Tri4 gene of Fusarium sporotrichioides encodes a cytochrome P450 monooxygenase involved in trichothecene biosynthesis. Mol Gen Genet. 1995;248:95–102. doi: 10.1007/BF02456618. [DOI] [PubMed] [Google Scholar]
- 13.Hohn T M, McCormick S P, Alexander N J, Desjardins A E, Proctor R H. Function and biosynthesis of trichothecenes produced by Fusarium species. In: Kohmoto K, Yoder O C, editors. Molecular genetics of host-specific toxins in plant diseases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 17–24. [Google Scholar]
- 14.Ichinoe M, Amano R, Morooka N, Yoshizawa T, Suzuki T, Kurisu M. Geographic difference of toxigenic fungi of Fusarium species. Proc Jpn Assoc Mycotoxicol. 1980;11:20–22. [Google Scholar]
- 15.Ichinoe M, Kurata H, Sugiura Y, Ueno Y. Chemotaxonomy of Gibberella zeae with special reference to production of trichothecenes and zearalenone. Appl Environ Microbiol. 1983;46:1364–1369. doi: 10.1128/aem.46.6.1364-1369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerenyi Z, Zeller K, Hornok L, Leslie J F. Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl Environ Microbiol. 1999;65:4071–4076. doi: 10.1128/aem.65.9.4071-4076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J-C, Kang H-J, Lee D-H, Lee Y-W, Yoshizawa T. Natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in barley and corn in Korea. Appl Environ Microbiol. 1993;59:3798–3802. doi: 10.1128/aem.59.11.3798-3802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura M, Kaneko I, Komiyama M, Takatsuki A, Koshino H, Yoneyama K, Yamaguchi I. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J Biol Chem. 1998;273:1654–1661. doi: 10.1074/jbc.273.3.1654. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M, Matsumoto G, Shingu Y, Yoneyama K, Yamaguchi I. The mystery of the trichothecene 3-O-acetyltransferase gene. Analysis of the region around Tri101 and characterization of its homologue from Fusarium sporotrichioides. FEBS Lett. 1998;435:163–168. doi: 10.1016/s0014-5793(98)01061-8. [DOI] [PubMed] [Google Scholar]
- 20.Kommedahl T, Windels C E. Root-, stalk-, and ear-infecting Fusarium species on corn in the USA. In: Nelson P E, Toussoun T A, Cook R J, editors. Fusarium diseases, biology, and taxonomy. University Park: The Pennsylvania State University Press; 1981. pp. 94–103. [Google Scholar]
- 21.Liu Z, Mishra N C. A single-tube method for plasmid mini-prep from large numbers of clones for direct screening by size or restriction digestion. BioTechniques. 1995;18:214–217. [PubMed] [Google Scholar]
- 22.Logrieco A, Bottalico A, Altomare C. Chemotaxonomic observation on zearalenone and trichothecene production by Gibberella zeae from cereals in southern Italy. Mycologia. 1988;80:892–895. doi: 10.1007/BF03192015. [DOI] [PubMed] [Google Scholar]
- 23.Marasas W F O, Nelson P E, Toussoun T A. Toxigenic Fusarium species: identity and mycotoxicology. University Park: The Pennsylvania State University Press; 1984. [Google Scholar]
- 24.Matsumoto G, Wuchiyama J, Shingu Y, Kimura M, Yoneyama K, Yamaguchi I. The trichothecene biosynthesis regulating gene from the type B producer Fusarium strains: sequence of Tri6 and its expression in Escherichia coli. Biosci Biotechnol Biochem. 1999;63:2001–2004. doi: 10.1271/bbb.63.2001. [DOI] [PubMed] [Google Scholar]
- 25.McCormick S P, Hohn T M, Desjardins A E. Isolation and characterization of Tri3, a gene encoding 15-O-acetyltransferase from Fusarium sporotrichioides. Appl Environ Microbiol. 1996;62:353–359. doi: 10.1128/aem.62.2.353-359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick S P, Hohn T M, Desjardins A E, Proctor R H, Alexander N J. Role of toxins in plant microbial interactions. In: Romeo J T, Downum K R, Verpoorte R, editors. Recent advances in phytochemistry. 32. Phytochemical signals and plant-microbe interactions. New York, n.y.: Plenum Press; 1998. pp. 17–30. [Google Scholar]
- 27.Mirocha C J, Abbas H K, Windels C E, Xie W. Variation in deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, and zearalenone production by Fusarium graminearum isolates. Appl Environ Microbiol. 1989;55:1315–1316. doi: 10.1128/aem.55.5.1315-1316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson P E, Pennypacker B W, Toussoun T A, Horst R K. Fusarium stub dicback of carnation. Phytopathology. 1975;65:575–581. [Google Scholar]
- 29.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–624. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell K, Kistler H C, Tacke B K, Casper H H. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc Natl Acad Sci USA. 2000;97:7905–7910. doi: 10.1073/pnas.130193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor R H, Hohn T M, McCormick S P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant-Microbe Interact. 1995;8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 32.Proctor R H, Hohn T M, McCormick S P, Desjardins A E. Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl Environ Microbiol. 1995;61:1923–1930. doi: 10.1128/aem.61.5.1923-1930.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu J C, Ohtsubo K, Izumiyama N, Nakamura K, Tanaka T, Yamamura H, Ueno Y. The acute and chronic toxicities of nivalenol in mice. Fund Appl Toxicol. 1988;11:38–47. doi: 10.1016/0272-0590(88)90268-0. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Seo J-A, Kim J-C, Lee D-H, Lee Y-W. Variation in 8-ketotrichothecenes and zearalenone production by Fusarium graminearum isolates from corn and barley in Korea. Mycopathologia. 1996;134:31–37. doi: 10.1007/BF00437050. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura Y, Watanabe Y, Tanaka T, Yamamoto S, Ueno Y. Occurrence of Gibberella zeae strains that produce both nivalenol and deoxynivalenol. Appl Environ Microbiol. 1990;56:3047–3051. doi: 10.1128/aem.56.10.3047-3051.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sydenham E W, Marasas W F O, Thiel P G, Shephard G S, Nieuwenhuis J J. Production of mycotoxins by selected Fusarium graminearum and F. crookwellense isolates. Food Addit Contam. 1991;8:31–41. doi: 10.1080/02652039109373953. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka T, Hasegawa A, Yamamoto S, Lee U S, Sugiura Y, Ueno Y. Worldwide contamination of cereals by the Fusarium mycotoxins nivalenol, deoxynivalenol and zearalenone. I. Survey of 19 countries. J Agric Food Chem. 1988;36:979–983. [Google Scholar]
- 39.Wuchiyama J, Kimura M, Yamaguchi I. A trichothecene efflux pump encoded by Tri102 in the biosynthesis gene cluster of Fusarium graminearum. J Antibiot. 2000;53:196–200. doi: 10.7164/antibiotics.53.196. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizawa T, Jin Y Z. Natural occurrence of acetylated derivatives of deoxynivalenol and nivalenol in wheat and barley in Japan. Food Addit Contam. 1995;12:689–694. doi: 10.1080/02652039509374358. [DOI] [PubMed] [Google Scholar]
- 41.Yun S-H, Arie T, Kaneko I, Yoder O C, Turgeon B G. Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet Biol. 2000;31:7–20. doi: 10.1006/fgbi.2000.1226. [DOI] [PubMed] [Google Scholar]