Abstract
Background
The Tpeak‐end(Tp‐e) has not been compared in all 12 ECG leads in healthy adults to determine if the Tp‐e varies across leads. If there is variation, it remains uncertain, which lead(s) are preferred for recording in order to capture the maximal Tp‐e value.
Objective
The purpose of the current study was to determine the optimal leads, if any, to capture the maximal Tp‐e interval in healthy young adults.
Methods
In 88 healthy adults (ages 21–38 years), including derivation (n = 21), validation (n = 20), and smoker/vaper (n = 47) cohorts, the Tp‐e was measured using commercial computer software (LabChart Pro 8 with ECG module, ADInstruments) in all 12 leads at rest and following a provocative maneuver, abrupt standing. Tp‐e was compared to determine which lead(s) most frequently captured the maximal Tp‐e interval.
Results
In the rest and abrupt standing positions, the Tp‐e was not uniform among the 12 leads; the maximal Tp‐e was most frequently captured in the precordial leads. At rest, grouping leads V2–V4 resulted in detection of the maximum Tp‐e in 85.7% of participants (CI 70.7, 99.9%) versus all other leads (p < .001). Upon abrupt standing, grouping leads V2‐V6 together, resulted in detection of the maximum Tp‐e 85.0% of participants (CI 69.4, 99.9% versus all other leads; p < .001). These findings were confirmed in the validation cohort, and extended to the smoking/vaping cohort.
Conclusion
If only a subset of ECG leads will be recorded or analyzed for the Tp‐e interval, selection of the precordial leads is preferred since these leads are most likely to capture the maximal Tp‐e value.
Keywords: sudden death, Tp‐e/QT, Tp‐e/QTc, Tpeak‐end, ventricular repolarization
1. INTRODUCTION
Sudden cardiac death is a leading cause of death in the United States, and thus clinical risk markers and indices to identify increased sudden death risk have been sought (Deyell et al., 2015). The electrocardiogram (ECG) is a powerful noninvasive tool to detect abnormalities in cardiac electrical activity that might be predictive of increased risk for sudden arrhythmic death (Rautaharju et al., 2009). Traditionally, abnormal ventricular repolarization estimated by prolongation of the QT interval is considered a marker for increased risk of lethal ventricular arrhythmias. However, since the QT interval includes both ventricular depolarization and repolarization, subtle but clinically meaningful changes in repolarization may be obscured (Antzelevitch, 2007; Dobson et al., 2013). Further, the QT interval may lack sufficient sensitivity and precision, since even minor increases in the QT interval, which do not exceed the normal range, may portend an increased risk for sudden death in specific populations (Deyell et al., 2015; Ahnve, 1985). As an alternative, the interval from the peak of the T wave to the end of the T wave, the Tpeak‐Tend (Tp‐e) interval, which does not include ventricular depolarization, has been proposed as a better predictor of ventricular arrhythmias and sudden arrhythmic death than the QT interval or the QT interval corrected for heart rate (Bazett's, QTc) (Antzelevitch et al., 2017; Tse et al., 2017).
Although Tp‐e prolongation has been reported in a wide range of cohorts with increased sudden arrhythmic death risk (Tse et al., 2017; Takenaka et al., 2003; Shimizu et al., 2002; Castro Hevia et al., 2006; Maury et al., 2015; Panikkath et al., 2011; Lubinski et al., 1998; Bachmann et al., 2016), this interval is not universally accepted as a marker of pro‐arrhythmia (Malik et al., 2019; Malik et al., 2018). Controversy surrounds the very basic electrophysiological question of what the Tp‐e actually represents. That prolongation in the Tp‐e represents transmural, global (apical‐basal), or even right–left ventricular heterogeneity of repolarization has each been argued and supported by data (Antzelevitch, 2001; Patel et al., 2009; Janse et al., 2012; Srinivasan et al., 2019). Further underlying the controversy is the lack of uniformity in the measurement of the Tp‐e (Malik et al., 2019). Very fundamentally, judgments of relative risk for lethal ventricular arrhythmia risk may be based on Tp‐e measurements from only a subset of ECG leads. However, it remains uncertain that leads are most sensitive to detect Tp‐e prolongation at rest. Surprisingly, the sources commonly referenced as evidence for specific lead selection have not systematically compared the Tp‐e interval among the 12 ECG leads (Castro Hevia et al., 2006; Haarmark et al., 2010). In fact, we have not found any publications comparing the Tp‐e interval in all 12 leads in healthy adults. Additionally, just as the QT interval may require interventions to unmask abnormal repolarization (Viskin et al., 2010; Wong et al., 2010), interventions to unmask Tp‐e prolongation have also been recommended (Takenaka et al., 2003; Markiewicz‐Loskot et al., 2020), but again, which leads are most sensitive to detect these changes remains unstudied and uncertain.
In a retrospective analysis of ECG recordings from only two leads, we recently reported that acutely smoking a tobacco cigarette significantly increased the Tp‐e interval more than vaping an electronic cigarette (Ip et al., 2020). Before embarking on a large, prospective study of the impact of vaping and smoking on the Tp‐e interval, given the lack of consensus on lead selection for Tp‐e measurement, we felt that it was first critical to determine systematically which leads, if any, were most sensitive, thus preferable, to detect the longest Tp‐e interval at rest and during an evocative maneuver. In the current study we compared the frequency of the maximal Tp‐e at rest and during abrupt standing among the 12‐ECG leads in healthy young adults, then validated our findings in an independent cohort, and finally extended these findings to a cohort of smokers and vapers.
2. MATERIAL AND METHODS
2.1. Human subjects
2.1.1. Derivation and validation cohorts
Healthy participants between the ages 21–45 years meeting the following criteria were eligible for enrollment in the derivation and validation cohorts: 1) non‐obese (<30 kg/m2 BMI), 2) no known health problems, including asthma, diabetes, heart disease, hypertension, or hyperlipidemia, 3) not pregnant (urine pregnancy test administered on the day of the study), 4) not competitive (non‐inter‐collegiate) athletes, and 5) not taking prescription medications regularly (besides oral contraceptives). Finally, participants were screened through a questionnaire to ensure that they did not smoke, use illicit drugs regularly, or drink >2 alcoholic drinks per day. Plasma nicotine and urinary drug tests were administered at the start of the session to detect surreptitious use of nicotine products and drugs.
2.1.2. Smoking/vaping cohort
Otherwise healthy participants who met the above criteria, but who had either smoked tobacco cigarettes and/or vaped electronic cigarettes for at least 1 year were eligible for the smoking/vaping cohort.
The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written informed consent was obtained from each participant. The research reported in this paper adhered to the guidelines set forth by the Declaration of Helsinki.
2.1.3. ECG recording technique
The skin on the chest was cleaned with alcohol wipes, and then ten electrodes (Covidien™ or Kendall ™) were placed on the chest according to standard ECG protocol. Recording electrodes were 3.0 cm in diameter, foam silver‐silver chloride conductors with adhesive hydrogel. The ECG was recorded with digital recording software: LabChart Pro 8 with ECG module (ADInstruments, 1000 Hz sampling frequency). Recordings were optimized to minimize noise and artifacts.
2.1.4. Experimental session
All study participants were instructed to abstain from caffeine and exercise for at least 12 h prior to their study session. Study participants in the smoking/vaping cohort were instructed to refrain from using their tobacco product on the day of the study. Participants were situated in a quiet, temperature‐controlled (21°C) room in the Human Physiology Laboratory located in the UCLA Clinical and Translational Research Center. The participants were placed in the supine position in a reclining chair with a footrest, and ECG electrodes were positioned. After a short rest period, the 12‐lead ECG was recorded continuously for 5 min. Participants were instructed to remain still and avoid speaking for the duration of the ECG recording. The use of a digital device during the recording was not allowed. Talking was minimized by research staff during data acquisition. At the end of the 5‐min recording period, the footrest was quickly lowered and the participant was instructed to stand up and remain still. The ECG recoding was continued for 30 s, capturing the peak heart rate following standing.
2.1.5. ECG recording analysis
Twelve‐lead ECG recordings were analyzed using commercially available software (LabChart Pro 8 with ECG module, ADInstruments). All beats were averaged via block averaging, for each of the twelve leads, resulting in one PQRST complex per lead. Three to four hundred beats were averaged in the 5‐min rest‐supine recording, and four to eight beats at peak heart rate were averaged in the brief standing recording. The ECG Analysis Module software automatically identified the beginning of the QRS complex, the peak of the T wave, and the end of the T wave; cursors were placed on each auto‐identified point and placement was over read by at least one investigator (I.R. or J.M.) to ensure accuracy. For our intervals of interest, Tp‐e and QT, the software designated the Tp‐e interval as the peak of the T wave to the end of the T wave and the QT interval as the onset of the QRS complex to the end of the T wave. The end of the T wave was precisely and automatically identified by the software at the intersection of the tangent to the T wave's downslope with the isoelectric line (Panikkath et al., 2011). For negative T waves, Tp‐e was measured as the interval from the nadir of the T wave to the end of the T wave (Antzelevitch, 2007). Leads in which T waves were low amplitude (<1.5 mm) or flattened were not included in the analysis (Rautaharju et al., 2009). U waves were not included in the Tp‐e interval (Panikkath et al., 2011). QTc was calculated using Bazett's formula (Rautaharju et al., 2009).
2.2. Statistical analysis
Primary outcomes were Tp‐e, Tp‐e/QT, and Tp‐e/QTc. For a given ECG outcome, (Tp‐e, Tp‐e/QT, Tp‐e/QTc,) and position (supine‐rest or standing), the lead with the maximum outcome value across the 12 leads was identified for each subject. Since there were no ties, only one of the 12 leads has the maximum value for a given ECG outcome, position and subject. Thus, the total number of subjects with a maximum at each lead could be tabulated, and the distribution of each subject's maximums across the 12 leads was determined. Leads were then grouped into two categories such that leads where the maximum was most frequently located were combined into one category and leads where the maximum was infrequently found were combined into the other category. Since repolarization may be longer in women than men (Rautaharju et al., 2009), the distribution of maximal Tp‐e was also compared between the sexes. A McNemar test (2 × 2) was used to compute p values for comparing the percent of subjects with maxima in the two categories between outcomes. A chi‐square test was used to compute p values for comparing the distribution across the 12 leads to a uniform distribution. A similar chi‐square test was used to compare the percent of leads grouped into two categories versus the uniform 50% in each category.
For each ECG outcome, means were compared across leads using a one‐way repeated measure (mixed) model after confirming that the outcome followed a normal distribution. The mixed model is used since observations across lead or across position (supine, standing) are on the same subjects and are non‐independent. p values <.05 were considered significant.
3. RESULTS
3.1. Study population
The study population consisted of 88 participants; baseline characteristics of the derivation (n = 21), validation (n = 20), and smoker/vaper (n = 47) cohorts are displayed in Table 1. There were no differences.
TABLE 1.
Baseline characteristics
| Cohort | Derivation | Validation | Smoker/vaper | p‐value |
|---|---|---|---|---|
| Sample size | n = 21 | n = 20 | n = 47 | |
| Age, years | 24.7 ± 5.5 | 24.2 ± 3.0 | 23.5 ± 2.79 | .78 |
| Sex (M/F) | 10/11 | 10/10 | 19/28 | .72 |
| BMI (kg/m2) | 22.1 ± 3.29 | 22.6 ± 3.04 | 22.4 ± 2.74 | .82 |
| Highest level of education | ||||
| Bachelors | 18 | 14 | 43 | .07 |
| Postgraduate | 3 | 6 | 4 | |
Note Values are given as number or mean ± SD.
BMI indicates body mass index.
3.2. Derivation cohort
In our 21 participants enrolled in our derivation cohort, we first determined the distribution among the 12 leads of the longest Tp‐e, Tp‐e/QT, and Tp‐e/QTc, reasoning that the lead(s) in which the maximal Tp‐e, Tp‐e/QT, and Tp‐e/QTc occurred would be preferable for future investigations of abnormal repolarization.
3.3. ECG leads for rest‐supine Tp‐e, Tp‐e/QT and Tp‐e/QTc
In the rest‐supine position, the mean values of the Tp‐e length, and Tp‐e/QT and Tp‐e/QTc ratios were not uniform among the 12 leads (Figure 1). Furthermore, the frequency of the maximal value for each of these primary outcomes in each participant varied according to lead (Table 2). The maximal values for Tp‐e, Tp‐e/QT, and Tp‐e/QTc were most frequently located in the precordial leads, specifically lead V3, followed by V4 and then V2. When these three leads were grouped together, the maximum Tp‐e value was localized to one of these three leads in 85.7% of participants (CI 70.7, 99.9%) versus all other leads, specifically the limb leads and leads V1,V5, and V6 (p < .001) (Figure 2a). The maximum Tp‐e/QT ratio was localized to one of these three leads in 85.7% of participants (CI 70.7, 99.9%) versus other leads (p < .001) (Figure 2b). The maximum value for Tp‐e/QTc ratio was localized to one of these three leads in 85.7% of participants (CI 70.7, 99.9%) versus all other leads (p < .001) (Figure 2c). The lead location of the maximal Tp‐e value was not impacted by sex (female versus male: 81.0 versus 90.0%, p = .66).
FIGURE 1.
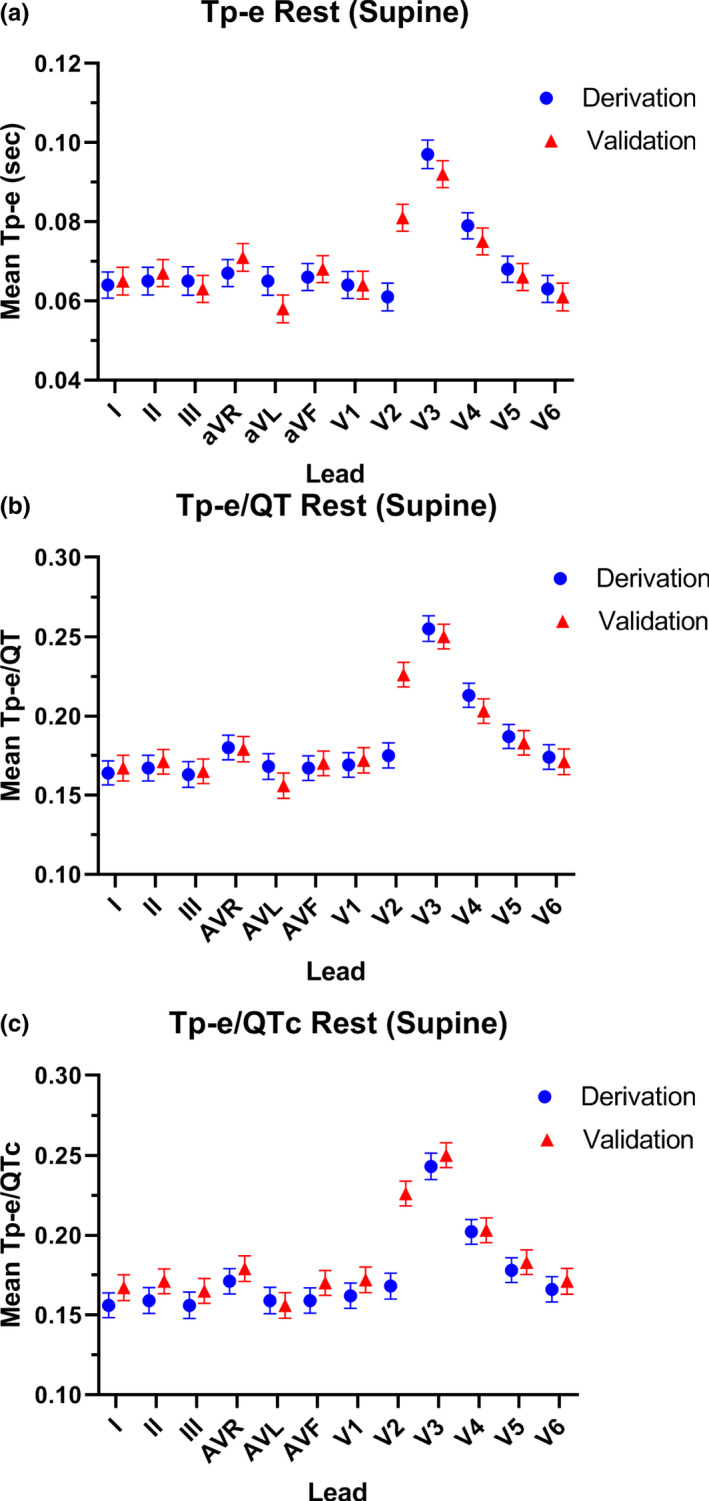
Mean length of each ECG parameter in each lead in the derivation and validation cohorts at rest. In the rest‐supine position, the mean values of the Tp‐e length (a), and Tp‐e/QT ratio (b), and Tp‐e/QTc ratio (c) were not uniform among the 12 leads in the derivation cohort (blue circles) or the validation cohort (red triangles)
TABLE 2.
Lead location of maximal ECG parameter (%)
| Outcome | Cohort | n | I | II | III | aVR | aVL | aVF | V1 | V2 | V3 | V4 | V5 | V6 | p‐value a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest/supine | |||||||||||||||
| Tp‐e | Derivation | n = 21 | 0.0% | 0.0% | 4.8% | 0.0% | 9.5% | 0.0% | 0.0% | 4.8% | 57.1% | 23.8% | 0.0% | 0.0% | <.0001 |
| Validation | n = 20 | 0.0% | 0.0% | 0.0% | 0.0% | 5.0% | 5.0% | 5.0% | 20.0% | 60.0% | 5.0% | 0.0% | 0.0% | <.0001 | |
| Tp‐e/QT | Derivation | n = 21 | 0.0% | 0.0% | 4.8% | 0.0% | 9.5% | 0.0% | 0.0% | 9.5% | 52.4% | 23.8% | 0.0% | 0.0% | <.0001 |
| Validation | n = 20 | 0.0% | 0.0% | 0.0% | 0.0% | 5.0% | 0.0% | 5.0% | 20.0% | 70.0% | 0.0% | 0.0% | 0.0% | <.0001 | |
| Tp‐e/QTc | Derivation | n = 21 | 0.0% | 0.0% | 4.8% | 0.0% | 9.5% | 0.0% | 0.0% | 9.5% | 52.4% | 23.8% | 23.8% | 0.0% | <.0001 |
| Validation | n = 20 | 0.0% | 0.0% | 0.0% | 0.0% | 5.0% | 0.0% | 5.0% | 20.0% | 70.0% | 0.0% | 0.0% | 0.0% | <.0001 | |
| Standing | |||||||||||||||
| Tp‐e | Derivation | n = 21 | 0.0% | 0.0% | 5.0% | 0.0% | 5.0% | 0.0% | 5.0% | 0.0% | 40.0% | 30.0% | 10.0% | 5.0% | <.0001 |
| Validation | n = 20 | 5.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 5.3% | 21.1% | 36.8% | 21.1% | 15.8% | 0.0% | .0003 | |
| Tp‐e/QT | Derivation | n = 21 | 0.0% | 0.0% | 5.0% | 0.0% | 0.0% | 0.0% | 5.0% | 0.0% | 45.0% | 30.0% | 10.0% | 5.0% | <.0001 |
| Validation | n = 20 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 5.3% | 21.1% | 36.8% | 21.1% | 15.8% | 0.0% | <.0001 | |
| Tp‐e/QTc | Derivation | n = 21 | 0.0% | 0.0% | 5.0% | 0.0% | 0.0% | 0.0% | 5.0% | 0.0% | 45.0% | 30.0% | 10.0% | 5.0% | <.0001 |
| Validation | n = 20 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 5.3% | 21.1% | 42.1% | 21.1% | 10.5% | 0.0% | <.0001 | |
p‐value indicates distribution compared to uniform.
FIGURE 2.
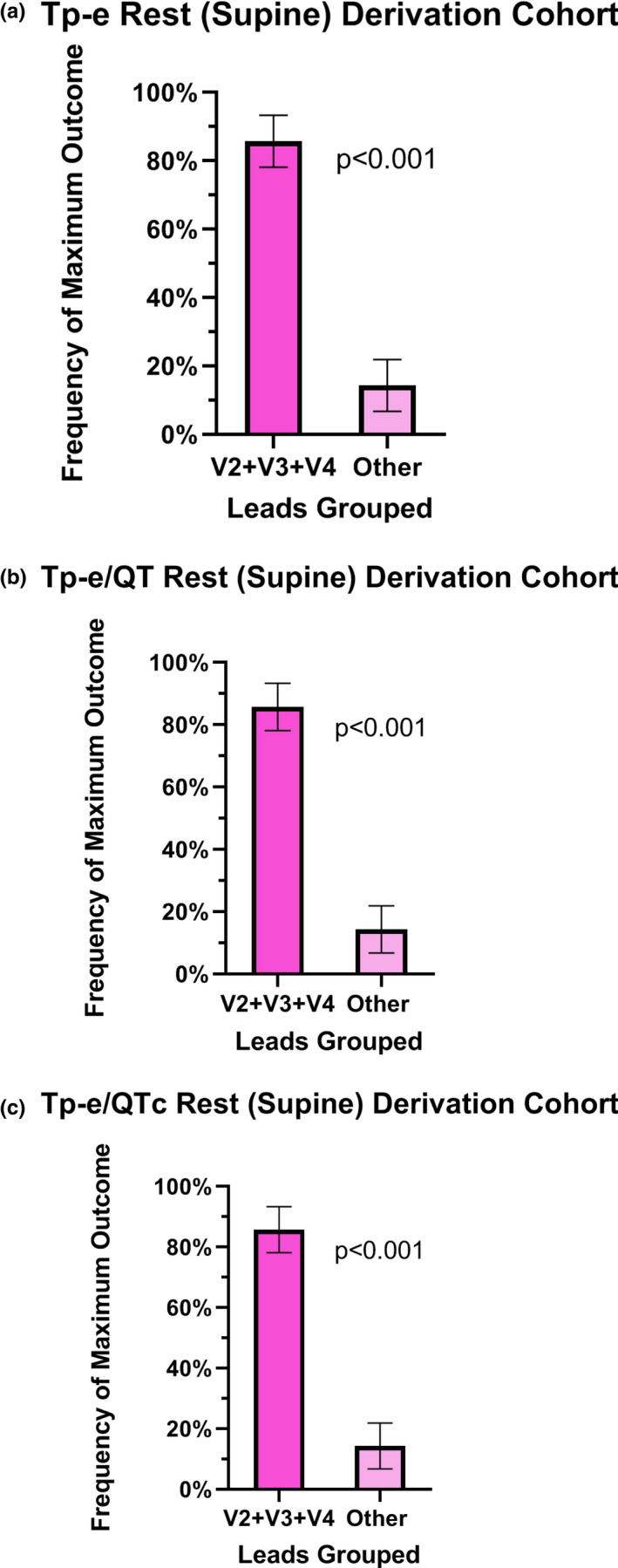
Lead location of the maximal ECG parameter at rest in the derivation cohort. When V2–V4 leads were grouped together, the maximum Tp‐e value was localized to one of these three leads in 85.7% of participants (CI 70.7, 99.9%) versus all other leads (p < .001) (a). The maximum Tp‐e/QT ratio was localized to one of these three leads in 85.7% of participants (CI 70.7, 99.9%) versus other leads (p < .001) (b). The maximum value for Tp‐e/QTc ratio was localized to one of these three leads in 85.7% of participants (CI 70.7, 99.9%) versus all other leads (p < .001) (c)
3.4. ECG leads for standing Tp‐e, Tp‐e/QT and Tp‐e/QTc
With standing, the heart rate increased from 67.5 ± 12.0 bpm to a peak of 97.5 ± 10.8 bpm (p < .001). During this increased heart rate, the averaged values of the Tp‐e interval, Tp‐e/QT and Tp‐e/QTc ratios in individual leads are shown in Figure 3 and were not uniform among the 12 leads. Similar to the supine‐rest recordings, the frequency of the maximal value for each of these primary outcomes in each participant varied according to lead (Table 2). However, when the same 3 leads (V2‐V4) in which the maximal Tp‐e, Tp‐e/QT, and Tp‐e/QTc were localized during supine‐rest were then grouped during standing, capture of the maximal value for each outcome was comparatively poor (Tp‐e 70.0% CI 49.9, 90.1%, Tp‐e/QT 75.0% CI 56.0, 94.0% and Tp‐e/QTc 75.0% CI 56.0, 94.0%). When V5 and V6 were added to the grouped leads, the maximum Tp‐e value was localized to one of these five leads in 85.0% of participants (CI 69.4, 99.9% versus all other leads; p < .001) (Figure 4a). The maximum Tp‐e/QT ratio was localized to one of these five leads in 90.0% of participants (CI 76.9, 99.9% versus other leads; p < .001) (Figure 4b), and the maximum Tp‐e/QTc ratio was localized to one of these five leads in 90.0% of participants (CI 76.9, 99.9% versus other leads; p < .001 (Figure 4c).
FIGURE 3.
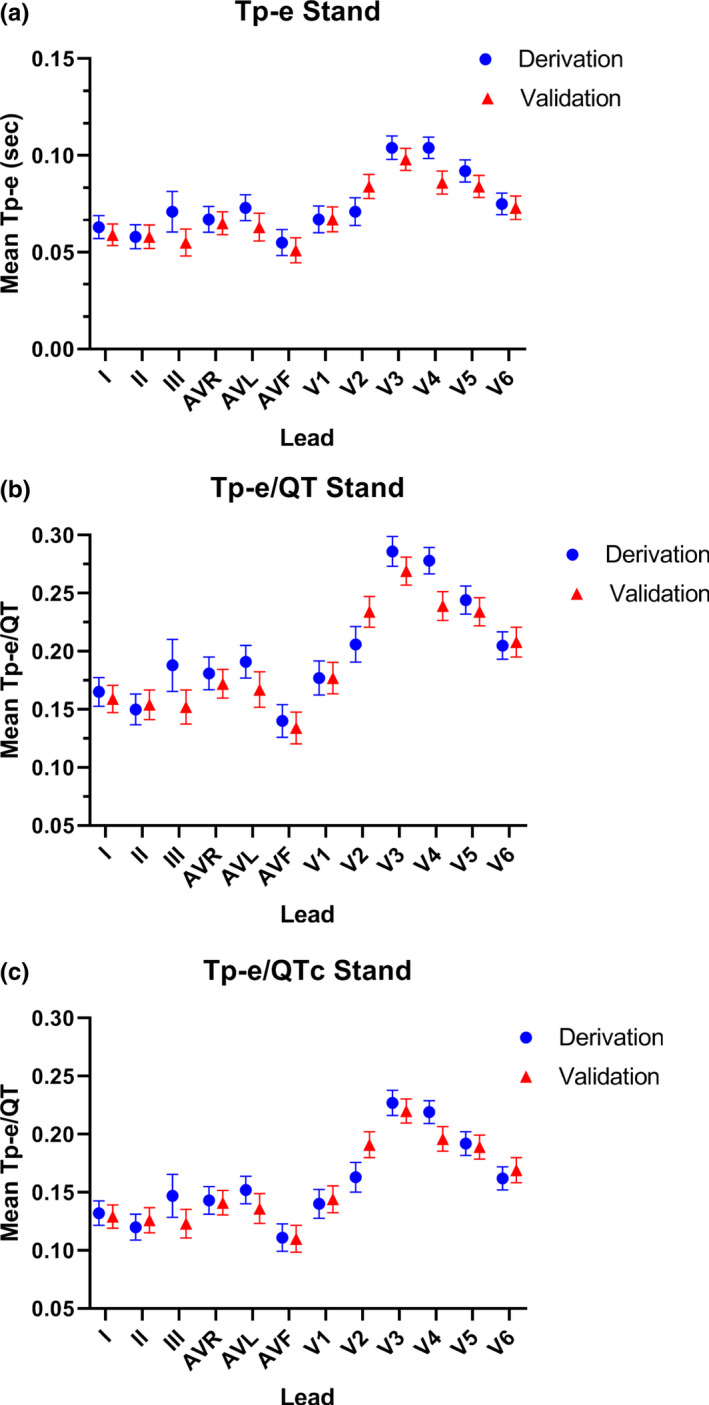
Mean length of each ECG parameter in each lead in the derivation and validation cohorts upon standing. In the rest‐supine position, the mean values of the Tp‐e length (a), and Tp‐e/QT ratio (b), and Tp‐e/QTc ratio (c) were not uniform among the 12 leads in the derivation cohort (blue circles) or the validation cohort (red triangles)
FIGURE 4.
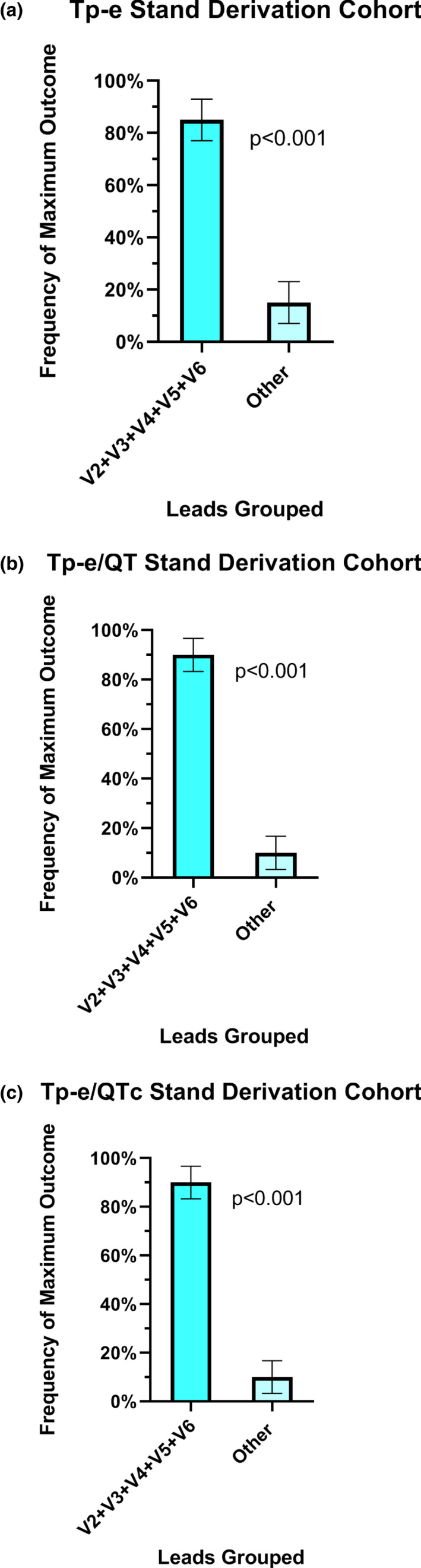
Lead location of the maximal ECG parameter upon standing in the derivation cohort. When V2‐V6 leads were grouped together, the maximum Tp‐e value was localized to one of these five leads in 85.0% of participants (CI 69.4, 99.9% versus all other leads; p < .001) (a). The maximum Tp‐e/QT ratio was localized to one of these five leads in 90.0% of participants (CI 76.9,99.9% versus other leads; p < .001) (b), and the maximum Tp‐e/QTc ratio was localized to one of these five leads in 90.0% of participants (CI 76.9,99.9% versus other leads; p < .001) (c)
3.5. Validation cohort
We next sought to confirm our findings in an independent, validation cohort (n = 20). Similar to findings in the derivation cohort, at supine‐rest, the mean values of the Tp‐e length, and Tp‐e/QT and Tp‐e/QTc ratios were not uniform among the 12 leads (Figure 1). At supine‐rest, the frequency of the maximal value for each of these primary outcomes in each participant varied according to lead (Table 2), and when V2‐V4 were grouped, the maximal value for each ECG outcome was similarly captured, (Tp‐e 85.0% CI 69.4, 99.9%, versus derivation cohort p = .94; Tp‐e/QT 90.0% CI 76.9, 99.9%, versus derivation cohort p = .67; Tp‐e/QTc 90.0% CI 76.9, 99.9%, versus derivation cohort p = .67). Similarly, during standing, the location of the maximal Tp‐e, Tp‐e/QT, and Tp‐e/QTc varied according to lead (Table 2). When V2‐V6 were grouped, the maximal value for each ECG outcome was similarly captured, (Tp‐e 89.5% CI 75.7, 99.9%, versus derivation cohort p = .67; Tp‐e/QT 94.7%, CI 84.7, 99.9%, versus derivation cohort p = .58; Tp‐e/QTc 94.7%, CI 84.7, 99.9%, versus derivation cohort p = .58).
3.6. Smoking/vaping cohort
We next determined if the location of the maximal values for Tp‐e, Tp‐e/QT, and Tp‐e/QTc could be extended to our cohort that included smokers and vapers (n = 47). To increase the power of this analysis, the derivation and validation cohorts, which were not different from each other, were combined, (“derivation/validation cohort”).
At supine‐rest in the smoking/vaping cohort, the frequency of the maximal value for each of these primary outcomes in each participant varied according to lead (Table 3). When the V2‐V4 leads were grouped together, the maximum Tp‐e value was localized to one of these three leads in 78.7% (CI 67.0, 90.4%) of participants and was not different from the derivation/validation cohort (p = .58). Similarly, the maximum Tp‐e/QT ratio was localized to one of these three leads in 83.0% (CI 72.2, 93.7%) of participants, and was not different from the derivation/validation cohort (p = .56). The maximum Tp‐e/QTc ratio was localized to one of these three leads in 80.9% (CI 69.6, 92.1%) of participants, and was not different from the derivation/validation cohort (p = .40).
TABLE 3.
Lead location of maximal ECG parameter (%)
| Outcome | Cohort | n | I | II | III | aVR | aVL | aVF | V1 | V2 | V3 | V4 | V5 | V6 | p‐value a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest/supine | |||||||||||||||
| Tp‐e | Derivation/validation | n = 41 | 0.0% | 0.0% | 2.4% | 0.0% | 7.3% | 2.4% | 2.4% | 12.2% | 58.5% | 14.6% | 0.0% | 0.0% | <.0001 |
| Smoker/vaper | n = 47 | 2.1% | 2.1% | 4.3% | 2.1% | 6.4% | 0.0% | 4.3% | 21.3% | 40.4% | 10.0% | 0.0% | 0.0% | <.0001 | |
| Tp‐e/QT | Derivation/validation | n = 41 | 0.0% | 0.0% | 2.4% | 0.0% | 7.3% | 0.0% | 2.4% | 14.6% | 61.0% | 12.2% | 0.0% | 0.0% | <.0001 |
| Smoker/vaper | n = 47 | 0.0% | 0.0% | 2.1% | 2.1% | 6.4% | 2.1% | 2.1% | 23.4% | 48.9% | 10.6% | 2.15 | 0.0% | <.0001 | |
| Tp‐e/QTc | Derivation/validation | n = 41 | 0.0% | 0.0% | 2.4% | 0.0% | 7.3% | 0.0% | 2.4% | 14.6% | 61.0% | 12.2% | 0.0% | 0.0% | <.0001 |
| Smoker/vaper | n = 47 | 0.0% | 0.0% | 2.1% | 4.3% | 6.4% | 2.1% | 2.1% | 23.4% | 44.7% | 12.8% | 2.1% | 0.0% | <.0001 | |
| Standing | |||||||||||||||
| Tp‐e | Derivation/validation | n = 41 | 2.6% | 0.0% | 2.6% | 0.0% | 5.1% | 0.0% | 5.1% | 7.7% | 33.3% | 28.2% | 12.8% | 2.6% | .0000 |
| Smoker/vaper | n = 47 | 2.2% | 8.7% | 2.2% | 4.3% | 6.5% | 2.2% | 0.0% | 13.3% | 15.2% | 26.1% | 13.0% | 8.7% | .0018 | |
| Tp‐e/QT | Derivation/validation | n = 41 | 0.0% | 0.0% | 2.6% | 0.0% | 2.6% | 0.0% | 5.15 | 10.3% | 35.9% | 28.2% | 12.8% | 2.6% | .0000 |
| Smoker/vaper | n = 47 | 0.0% | 8.7% | 2.2% | 0.0% | 4.3% | 2.2% | 0.0% | 13.0% | 13.0% | 26.1% | 17.4% | 13.0% | <.0002 | |
| Tp‐e/QTc | Derivation/validation | n = 41 | 0.0% | 0.0% | 2.6% | 0.0% | 2.6% | 0.0% | 5.1% | 10.3% | 38.5% | 28.2% | 10.3% | 2.6% | .0000 |
| Smoker/vaper | n = 47 | 0.0% | 8.7% | 2.2% | 0.0% | 4.3% | 2.2% | 0.0% | 13.0% | 13.0% | 26.1% | 17.4% | 13.0% | <.0002 | |
p‐value indicates distribution compared to uniform.
Finally, during standing, the location of the maximal Tp‐e, Tp‐e/QT, and Tp‐e/QTc in the smoking/vaping cohort varied according to lead (Table 3). When the V2‐V6 leads were grouped, the maximum Tp‐e value was localized to one of these five leads in 73.9% (CI 61.2, 86.6%) of participants, and was not different from the derivation/validation cohort (p = .29). The maximum Tp‐e/QT ratio was localized to one of these five leads in 82.6% (CI 71.7, 93.6%) of participants, and was not different from the derivation/validation cohort (p = .37). The maximum Tp‐e/QTc ratio was localized to one of these five leads in 82.6% (CI 71.7, 93.6%) of participants, and was not different from the derivation/validation cohort, (p = .37).
4. DISCUSSION
This is the first study, to our knowledge, to compare ventricular repolarization simultaneously recorded in all 12 ECG leads in healthy adults in order to provide a basis for optimal lead selection for Tp‐e recordings. We found that the length of the Tp‐e interval varies significantly among the 12 leads, and that the maximal Tp‐e interval was not uniformly distributed. To a high degree of significance, the maximal Tp‐e interval was most often detected in the precordial leads, specifically in leads V2, V3, and V4. Furthermore, these findings were validated in an independent cohort. Interestingly, in our participants in the derivation and validation cohorts at supine‐rest, the maximal Tp‐e was never localized in leads V5 or V6, even though these leads have been among those selected for Tp‐e measurement in several studies (Shimizu et al., 2002; Panikkath et al., 2011; Lubinski et al., 1998; Bachmann et al., 2016), including studies that did not find an association between Tp‐e length and risk of ventricular arrhythmias or sudden cardiac death (Michalek et al., 2020; Porthan et al., 2013).
The explanation for the increased sensitivity of the precordial leads, especially leads V2, V3, and V4, to detect the greatest heterogeneity of ventricular repolarization remains uncertain. Our findings in healthy adults are similar to those reported in healthy children and adolescents, in whom the Tp‐e was also found to vary significantly among leads, and the Tp‐e was also longest in V3 (Bieganowska et al., 2013). It is recognized that repolarization varies significantly across the ventricular wall, termed transmural dispersion of ventricular repolarization (Antzelevitch, 2001). In experimental canine wedge preparations, the end of repolarization of the epicardium, which has the shortest action potentials, corresponds to the peak of the T wave, and end of repolarization of the M cells localized deep within the ventricular wall, and which have the longest action potentials, corresponds to the end of the T wave (Antzelevitch, 2001). It has been hypothesized that the Tp‐e reflects transmural dispersion of ventricular repolarization, which, when prolonged pharmacologically, increases vulnerability to ventricular arrhythmias (Yan & Antzelevitch, 1998). Others have presented strong experimental evidence that the Tp‐e reflects global (rather than transmural) dispersion of ventricular repolarization (Xia et al., 2005). In either case, our findings support the notion that unipolar ECG leads placed anatomically directly over the ventricles, that is, the precordial leads, perhaps simply due to proximity, have the greatest sensitivity to detect this measure of dispersion of ventricular repolarization (Antzelevitch, 2001; Bieganowska et al., 2013).
In patients with suspected congenital long QT syndrome, the QT on the resting ECG may be within the normal limits, and provocative maneuvers are necessary to unmask QT prolongation and confirm the diagnosis (Takenaka et al., 2003; Viskin et al., 2010). Maneuvers that increase heart rate are typically utilized, since abrupt increases in heart rate may paradoxically prolong repolarization in certain forms of congenital long QT syndrome (Takenaka et al., 2003). Interestingly, in a porcine model, direct sympathetic nerve stimulation was found to increase global dispersion of repolarization, which was accompanied by an increase in Tp‐e (Yagishita et al., 2015). It has since been hypothesized that prolongation of the Tp‐e interval may reflect increased sympathetic nerve activity, a widely recognized risk factor for lethal ventricular arrhythmias (Bachmann et al., 2016). Provocative maneuvers have been utilized to detect abnormal Tp‐e intervals (Takenaka et al., 2003), but once again, the ideal leads for recording Tp‐e prolongation during abrupt increases in heart rate have not been established. In our study, we found that the location of the maximal Tp‐e interval during abrupt standing was more widely spread across the precordium. The selection of more leads, specifically leads V2‐V6, was necessary to capture the maximal values to a similar degree of accuracy as during supine‐rest. We speculate that this wider distribution of maximum Tp‐e values may reflect a shift in the relationship of the heart's position to the recording electrodes on the chest wall while standing compared to supine‐rest. Alternatively, the increase in the heart rate, independent of body position, may lead to the wider distribution of lead location for determination of the maximal depolarization; this question warrants further study.
4.1. Limitations
This study was conducted in healthy young people without known cardiac disease. It is possible, but unlikely, that leads other than the precordial leads may be more sensitive to detect Tp‐e prolongation in an older population, in obesity, or in the setting of cardiac pathology. This study could be repeated in these settings to address this question. However, a strength of the study is the validation of our findings in an independent cohort, and the extension of our findings in an additional cohort that smokes or vapes. The results were highly consistent among the cohorts. We have only addressed one aspect of the methodology to measure the ventricular repolarization – that is, which leads are moist sensitive to detect the maximal Tp‐e interval. Other aspects of the methodology for Tp‐e recording and analysis must also be optimized, such as determination of the minimal number of complexes necessary for analysis, and the most accurate analytical software. Although our purpose was not to evaluate the ECG analysis software, only rarely were corrections by the over‐reader necessary. A systematic comparison of available software programs would be of interest. Nonetheless, to our knowledge, this is the first study in adults to address a basic, fundamental, methodological question, that is, which ECG lead(s) should be recorded and analyzed to increase the likelihood of detection of the longest Tp‐e interval.
5. CONCLUSIONS
In summary, in healthy young adults, the Tp‐e interval length is not uniform among the 12 ECG leads. The precordial leads, especially leads V2‐V4 at supine‐rest and leads V2‐V6 during the provocative maneuver of abrupt standing, are most likely to detect the maximal Tp‐e interval. If only a subset of ECG leads is available for recording or analysis, selection of the precordial leads is desirable.
ETHICAL APPROVAL
The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written informed consent was obtained from each participant. The research reported in this paper adhered to the guidelines set forth by the Declaration of Helsinki.
AUTHOR CONTRIBUTIONS
IR involved in drafting the manuscript (with HRM), data collection and analysis, approval of final draft. JM involved in data collection and analysis, manuscript revision, and approval of the final draft. RN and KL involved in data collection, manuscript revision, and approval of the final draft. JG involved in data analysis including statistical analysis, manuscript revision, approval of the final draft. HRM involved in conception and funding of the project, data interpretation, drafting the manuscript (with IR) approval of the final manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Ruedisueli, I. , Ma, J. , Nguyen, R. , Lakhani, K. , Gornbein, J. & Middlekauff, H. R. (2022). Optimizing ECG lead selection for detection of prolongation of ventricular repolarization as measured by the Tpeak‐end interval. Annals of Noninvasive Electrocardiology, 27, e12958. doi: 10.1111/anec.12958
Funding information
This work was supported by Tobacco Related Diseases Research Program (T29IP0319) and by the NIH National Center for Advancing Translational Science (UCLA CTSI Grant Number L1TR001881)
DATA AVAILABILITY STATEMENT
Data will be made available upon request by qualified investigators.
REFERENCES
- Ahnve, S. (1985). QT interval prolongation in acute myocardial infarction. European Heart Journal, 6 Suppl D, 85–95. [DOI] [PubMed] [Google Scholar]
- Antzelevitch, C. (2001). T peak‐tend interval as an index of transmural dispersion of repolarization. European Journal of Clinical Investigation, 31, 555–557. [DOI] [PubMed] [Google Scholar]
- Antzelevitch, C. (2007). Heterogeneity and cardiac arrhythmias: An overview. Heart Rhythm, 4, 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch, C. , Di Diego, J. M. , & Argenziano, M. (2017). Tpeak‐tend as a predictor of ventricular arrhythmogenesis. International Journal of Cardiology, 249, 75–76. [DOI] [PubMed] [Google Scholar]
- Bachmann, T. N. , Skov, M. W. , Rasmussen, P. V. , Graff, C. , Pietersen, A. , Lind, B. , Struijk, J. J. , Olesen, M. S. , Haunsø, S. , Køber, L. , Svendsen, J. H. , Holst, A. G. , & Nielsen, J. B. (2016). Electrocardiographic Tpeak‐tend interval and risk of cardiovascular morbidity and mortality: Results from the Copenhagen ECG study. Heart Rhythm, 13, 915–924. [DOI] [PubMed] [Google Scholar]
- Bieganowska, K. , Sawicka‐Parobczyk, M. , Bieganowski, M. , & Piskorski, J. (2013). Tpeak ‐tend interval in 12‐lead electrocardiogram of healthy children and adolescents tpeak ‐tend interval in childhood. Annals of Noninvasive Electrocardiology, 18, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Hevia, J. , Antzelevitch, C. , Tornes Barzaga, F. , et al. (2006). Tpeak‐tend and Tpeak‐tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. Journal of the American College of Cardiology, 47(9), 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyell, M. W. , Krahn, A. D. , & Goldberger, J. J. (2015). Sudden cardiac death risk stratification. Circulation Research, 116, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C. P. , Kim, A. , & Haigney, M. (2013). QT Variability Index. Progress in Cardiovascular Diseases, 56, 186–194. [DOI] [PubMed] [Google Scholar]
- Haarmark, C. , Graff, C. , Andersen, M. P. , Hardahl, T. , Struijk, J. J. , Toft, E. , Xue, J. , Rowlandson, G. I. , Hansen, P. R. , & Kanters, J. K. (2010). Reference values of electrocardiogram repolarization variables in a healthy population. Journal of Electrocardiology, 43, 31–39. [DOI] [PubMed] [Google Scholar]
- Ip, M. , Diamantakos, E. , Haptonstall, K. , Choroomi, Y. , Moheimani, R. S. , Nguyen, K. H. , Tran, E. , Gornbein, J. , & Middlekauff, H. R. (2020). Tobacco and electronic cigarettes adversely impact ECG indexes of ventricular repolarization: Implication for sudden death risk. American Journal of Physiology. Heart and Circulatory Physiology, 318, H1176–H1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse, M. J. , Coronel, R. , Opthof, T. , Sosunov, E. A. , Anyukhovsky, E. P. , & Rosen, M. R. (2012). Repolarization gradients in the intact heart: Transmural or apico‐basal? Progress in Biophysics and Molecular Biology, 109, 6–15. [DOI] [PubMed] [Google Scholar]
- Lubinski, A. , Lewicka‐Nowak, E. , Kempa, M. , Baczynska, A. M. , Romanowska, I. , & Swiatecka, G. (1998). New insight into repolarization abnormalities in patients with congenital long QT syndrome: The increased transmural dispersion of repolarization. Pacing and Clinical Electrophysiology, 21, 172–175. [DOI] [PubMed] [Google Scholar]
- Malik, M. , Huikuri, H. V. , Lombardi, F. , Schmidt, G. , Verrier, R. L. , Zabel, M. , & e‐Rhythm Group of EHRA . (2019). Is the Tpeak‐tend interval as a measure of repolarization heterogeneity dead or just seriously wounded? Heart Rhythm, 16, 952–953. [DOI] [PubMed] [Google Scholar]
- Malik, M. , Huikuri, H. , Lombardi, F. , Schmidt, G. , Zabel, M. , & e‐Rhythm Study Group of EHRA . (2018). Conundrum of the Tpeak‐tend interval. Journal of Cardiovascular Electrophysiology, 29, 767–770. [DOI] [PubMed] [Google Scholar]
- Markiewicz‐Loskot, G. , Kolarczyk, E. , Mazurek, B. , Loskot, M. , & Szydlowski, L. (2020). Prolongation of electrocardiographic T wave parameters recorded during the head‐up tilt table test as independent markers of syncope severity in children. International Journal of Environmental Research and Public Health, 17(18), 6441–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury, P. , Sacher, F. , Gourraud, J. B. , Pasquié, J. L. , Raczka, F. , Bongard, V. , Duparc, A. , Mondoly, P. , Sadron, M. , Chatel, S. , Derval, N. , Denis, A. , Cardin, C. , Davy, J. M. , Hocini, M. , Jaïs, P. , Jesel, L. , Carrié, D. , Galinier, M. , … Rollin, A. (2015). Increased Tpeak‐tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm, 12, 2469–2476. [DOI] [PubMed] [Google Scholar]
- Michalek, P. , Hatahet, S. B. , Svetlosak, M. , Margitfalvi, P. , Waczulikova, I. , Trnovec, S. , Böhm, A. , Benacka, O. , & Hatala, R. (2020). No association between T‐peak to T‐end interval on the resting ECG and long‐term incidence of ventricular arrhythmias triggering ICD interventions. Frontiers in Physiology, 11, 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikkath, R. , Reinier, K. , Uy‐Evanado, A. , Teodorescu, C. , Hattenhauer, J. , Mariani, R. , Gunson, K. , Jui, J. , & Chugh, S. S. (2011). Prolonged Tpeak‐to‐tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circulation. Arrhythmia and Electrophysiology, 4, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, C. , Burke, J. F. , Patel, H. , Gupta, P. , Kowey, P. R. , Antzelevitch, C. , & Yan, G. X. (2009). Is there a significant transmural gradient in repolarization time in the intact heart? Cellular basis of the T wave: A century of controversy. Circulation. Arrhythmia and Electrophysiology, 2, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porthan, K. , Viitasalo, M. , Toivonen, L. , Havulinna, A. S. , Jula, A. , Tikkanen, J. T. , Väänänen, H. , Nieminen, M. S. , Huikuri, H. V. , Newton‐Cheh, C. , Salomaa, V. , & Oikarinen, L. (2013). Predictive value of electrocardiographic T‐wave morphology parameters and T‐wave peak to T‐wave end interval for sudden cardiac death in the general population. Circulation. Arrhythmia and Electrophysiology, 6, 690–696. [DOI] [PubMed] [Google Scholar]
- Rautaharju, P. M. , Surawicz, B. , Gettes, L. S. , Bailey, J. J. , Childers, R. , Deal, B. J. , Gorgels, A. , Hancock, E. W. , Josephson, M. , Kligfield, P. , Kors, J. A. , Macfarlane, P. , Mason, J. W. , Mirvis, D. M. , Okin, P. , Pahlm, O. , van Herpen, G. , Wagner, G. S. , Wellens, H. , & American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation, Heart Rhythm Society . (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association electrocardiography and arrhythmias committee, council on clinical cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology, 53, 982–991. [DOI] [PubMed] [Google Scholar]
- Shimizu, M. , Ino, H. , Okeie, K. , Yamaguchi, M. , Nagata, M. , Hayashi, K. , Itoh, H. , Iwaki, T. , Oe, K. , Konno, T. , & Mabuchi, H. (2002). T‐peak to T‐end interval may be a better predictor of high‐risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clinical Cardiology, 25, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, N. T. , Orini, M. , Providencia, R. , Simon, R. , Lowe, M. , Segal, O. R. , Chow, A. W. , Schilling, R. J. , Hunter, R. J. , Taggart, P. , & Lambiase, P. D. (2019). Differences in the upslope of the precordial body surface ECG T wave reflect right to left dispersion of repolarization in the intact human heart. Heart Rhythm, 16, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, K. , Ai, T. , Shimizu, W. , Kobori, A. , Ninomiya, T. , Otani, H. , Kubota, T. , Takaki, H. , Kamakura, S. , & Horie, M. (2003). Exercise stress test amplifies genotype‐phenotype correlation in the LQT1 and LQT2 forms of the long‐QT syndrome. Circulation, 107, 838–844. [DOI] [PubMed] [Google Scholar]
- Tse, G. , Gong, M. , Wong, W. T. , Georgopoulos, S. , Letsas, K. P. , Vassiliou, V. S. , Chan, Y. S. , Yan, B. P. , Wong, S. H. , Wu, W. K. K. , Ciobanu, A. , Li, G. , Shenthar, J. , Saguner, A. M. , Ali‐Hasan‐al‐Saegh, S. , Bhardwaj, A. , Sawant, A. C. , Whittaker, P. , Xia, Y. , … Liu, T. (2017). The Tpeak ‐ tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: A systematic review and meta‐analysis. Heart Rhythm, 14, 1131–1137. [DOI] [PubMed] [Google Scholar]
- Viskin, S. , Postema, P. G. , Bhuiyan, Z. A. , Rosso, R. , Kalman, J. M. , Vohra, J. K. , Guevara‐Valdivia, M. E. , Marquez, M. F. , Kogan, E. , Belhassen, B. , Glikson, M. , Strasberg, B. , Antzelevitch, C. , & Wilde, A. A. M. (2010). The response of the QT interval to the brief tachycardia provoked by standing: A bedside test for diagnosing long QT syndrome. Journal of the American College of Cardiology, 55, 1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, J. A. , Gula, L. J. , Klein, G. J. , Yee, R. , Skanes, A. C. , & Krahn, A. D. (2010). Utility of treadmill testing in identification and genotype prediction in long‐QT syndrome. Circulation. Arrhythmia and Electrophysiology, 3, 120–125. [DOI] [PubMed] [Google Scholar]
- Xia, Y. , Liang, Y. , Kongstad, O. , Holm, M. , Olsson, B. , & Yuan, S. (2005). Tpeak‐tend interval as an index of global dispersion of ventricular repolarization: Evaluations using monophasic action potential mapping of the epi‐ and endocardium in swine. Journal of Interventional Cardiac Electrophysiology, 14, 79–87. [DOI] [PubMed] [Google Scholar]
- Yagishita, D. , Chui, R. W. , Yamakawa, K. , Rajendran, P. S. , Ajijola, O. A. , Nakamura, K. , So, E. L. , Mahajan, A. , Shivkumar, K. , & Vaseghi, M. (2015). Sympathetic nerve stimulation, not circulating norepinephrine, modulates T‐peak to T‐end interval by increasing global dispersion of repolarization. Circulation. Arrhythmia and Electrophysiology, 8, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, G. X. , & Antzelevitch, C. (1998). Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT syndrome. Circulation, 98, 1928–1936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request by qualified investigators.


