Abstract
Despite the progressing knowledge in COVID-19 management, remdesivir is the only agent that got approval to inhibit viral replication. However, there are limited data about effective immunomodulatory agents to prevent cytokine release in COVID-19. Cytokine release syndrome in COVID-19 resembles secondary hemophagocytic lymphohistiocytosis, in which interleukin-1 (IL-1) plays a key role. Anakinra is the first recombinant IL-1 receptor antagonist studied for off-label use in COVID-19 treatment.
This study reviews the current clinical evidence on the role of interleukin-1 in COVID-19-related cytokine storm, therapeutic effects, significant clinical concerns, and pros and cons of anakinra administration in the management of COVID-19 patients.
In this review, four items are shown to be important for achieving the optimal therapeutic effects of anakinra in COVID-19 patients. These items include duration of treatment ≥ 10 days, doses ≥ 100 mg, intravenous administration, and early initiation of therapy. Also, anakinra might be more beneficial in the early stages of the disease when higher levels of cytokines are yet to be observed, which could prevent progression to severe illness and mechanical ventilation. Further studies are required to address the SARS-CoV-2 induced cytokine release syndrome and the role of anakinra in identifying ideal treatment approaches for COVID-19 patients based on their clinical status.
Keywords: Acute respiratory distress syndrome, Anakinra, COVID-19, Cytokine release syndrome, Interleukin-1, Multiple organ failure
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects approximately 560 million cases resulting in 6 million deaths worldwide [1]. According to the centers for disease control and prevention (CDC), the clinical symptoms of coronavirus disease 2019 (COVID-19) range from mild to severe disease [2]. Acute respiratory distress syndrome (ARDS) is the most overwhelming complication of COVID-19, with a high mortality rate [3]. Cytokine Release Syndrome (CRS) and interleukin (IL)-1 have a crucial role in the progression of SARS-CoV-2-induced ARDS and multiple organ failure. Remdesivir is the only antiviral that got the U.S food and drug Administration (FDA) approval to block the replication of SARS-CoV-2. Despite the critical role of the proinflammatory phase in COVID-19 pathogenesis, knowledge about immunomodulatory therapy is lacking. Most treatments are under investigation and generally include antivirals, anticoagulants, or conventional medications. The FDA gave Emergency Use Authorization (EUA) to tocilizumab and corticosteroid combination in hospitalized COVID-19 patients. However, other immunomodulators with different mechanisms should be considered in patients not responding to treatment or in any drug shortage situation [4]. The European Medicines Agency (EMA) recently authorized anakinra use in management of COVID-19 [5].
Anakinra is the first recombinant interleukin (IL)-1 receptor antagonist that binds to IL-1α and IL-1β receptors and gets FDA approval to treat rheumatoid arthritis (RA). Moreover, anakinra has been investigated to treat hyperinflammatory states in COVID-19 due to its great safety profile. Considering the beneficial effects of anakinra in CRS management and the pivotal role of IL-1 in SARS-CoV-2-induced CRS, we aimed to review the literature to define which COVID-19 patients benefit most from anakinra.
2. COVID-19-related CRS and the role of IL-1
Cytokine release syndrome has been shown to be crucial in the progression of SARS-CoV-2-induced ARDS. The hyperinflammatory state in COVID-19 resembles the CRS in hemophagocytic lymphohistiocytosis (HLH), macrophage activation syndrome (MAS), or chimeric antigen receptor T-cell therapy [6].
HLH is a life-threatening state of hepatosplenomegaly, cytopenias, tissue hemophagocytosis, and multiorgan failure. The hallmark of HLH pathophysiology relies on the excessive activation of tissue macrophages, causing hyperproduction of IL-1β, IL-18, and ferritin [7], [8], [9].
SARS-CoV-2 can trigger the immune response of infected patients, leading to hyperinflammation, cytokine storm, tissue injury, especially in the lungs, and eventually death. These conditions are associated with high serum proinflammatory cytokines such as IL-1, IL-6, IL-18, and interferon-γ. Furthermore, COVID-19-induced CRS could result in impaired viral clearance, reduced levels of type I interferons, and increased neutrophil extracellular traps (NETs) [10]. The pattern of inflammation and cytokine storm in severe COVID-19 is distinct from other causes of pneumonia in which the balance between anti-inflammatory and proinflammatory cytokines is significantly altered. Notably, a marked increase in IL-1β/IL-10 and IL6/IL-10 ratios is the characteristic feature of inflammation observed in severe COVID-19 [11].
IL-1 is a potent proinflammatory cytokine produced from macrophages in lymphoid and non-lymphoid organs such as the thymus, lymph nodes, lung, liver, and gastrointestinal tract.
IL-1 affects almost all cell types and induces the release of various proinflammatory cytokines, such as tumor necrosis factor-alpha, IL-6, and IL-8. It can also stimulate the thermoregulatory center in the brain and cause fever in patients [12], [13].
The two types of IL-1, IL-1α and IL-1β, are produced by two distinct genes, bind to the same IL-1 receptors, and have the same biologic activities [12]. Dying epithelial and endothelial cells could produce IL-1α, which is fully active. Infiltrating monocytes and macrophages could produce pro-IL-1β, which is cleaved by NLPR3-activated caspase 1 to form active IL-1β. Neutrophils also release pro-IL-1β into the extracellular space, where neutrophil-derived proteases activate them. Active and mature IL-1β is one of the main factors in the acute phase of hyperinflammation and cytokine storm syndrome, which can cause tissue injury and severe COVID-19 [14].
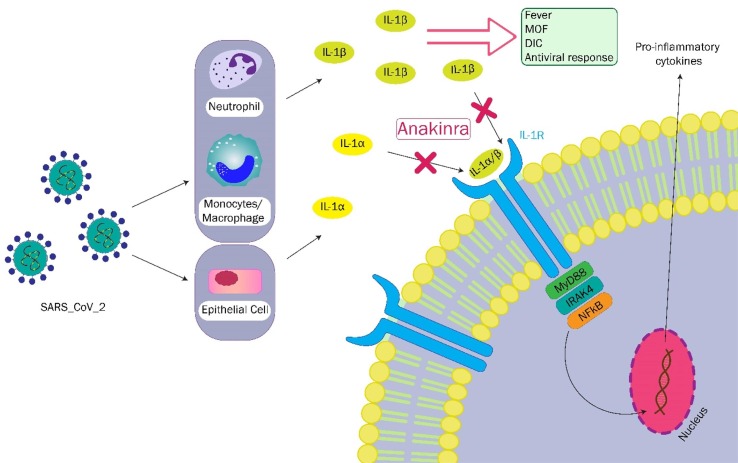
SARS-CoV-2 enters epithelial cells by angiotensin-converting enzyme 2 (ACE2) host receptors and leads to IL-1α release, which results in further cytokine release, such as pro-IL-1β. It also directly stimulates pro-IL-1β release from monocytes, macrophages, and neutrophils. Produced antibodies against the SARS-CoV-2 spike proteins mediate the virus phagocytosis by binding to the Fc-gamma receptors. SARS-CoV-2 accessory protein, ORF3a, stimulates the NLRP3 activation. Virus N protein can also directly activate the NLRP3. Several mechanisms in a host cell, such as reactive oxygen species and C5a production, and adenosine triphosphate (ATP)-release by dying cells, contribute to further activation of NLRP3. Activated NLRP3 leads to caspase 1-related cleavage of pro-IL-1β and pro-IL-18 into mature forms. IL-1β activates macrophages to release various proinflammatory cytokines such as IL-6, proposing a possible role for IL-1 blockers in managing the inflammatory complications caused by SARS-CoV-2 (outlined in Fig. 1 ) [15].
Fig. 1.
The effects and blockade of interleukin-1 (IL-1) signaling by anakinra. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in the epithelial cells of the lungs and destruction of cells leads to IL-1α release, which in turn recruits neutrophils and monocytes and promotes IL-1β release from monocytes/macrophages. Furthermore, SARS-CoV-2 directly leads to IL-1β release from monocytes/macrophages and neutrophils. Both IL-1α and IL-1β bind to IL-1 receptors. Accordingly, MyD88, IL-1 receptor-associated kinase 4 (IRAK4), and nuclear factor kappa B (NFκB) are phosphorylated. NFκB stimulates the transcription of pro-inflammatory genes. Anakinra blocks IL-1 receptors and inhibits the binding of IL-1α and IL-1β, thus preventing the signaling pathway. DIC, disseminated intravascular coagulation; MOF, multiple organ failure.
3. Anakinra, the rationale for use in COVID-19
Recent data revealed that the severe forms of COVID-19 cause a hyperinflammatory condition that resembles HLH or cytokine storm after chimeric antigen receptor T-cell therapy. The predominantly released cytokines in severe COVID-19 are IL-1, IL-6, IL-18, and interferon-γ. Anakinra is the first biologic recombinant drug that acts as an IL-1 receptor antagonist, which can inhibit IL-1α and IL-1β. Anakinra has some unique advantages over other biologics used in COVID-19. Anakinra showed an excellent safety profile in the setting of rheumatologic disorders or other causes [16]. It has a short half-life (4 to 6 h) and can be administered subcutaneously or intravenously. The short half-life of anakinra helps flexible dosing and reduces excessive immunosuppression. Also, the rapid clearance after drug discontinuation contributes to better management of adverse effects, such as hepatotoxicity, especially in critically ill patients [17]. Several studies reported that anakinra with doses of>200 mg daily was safe. Administrations of anakinra subcutaneously might result in injection site reactions that usually are mild and do not require drug discontinuation [14].
4. Anakinra in COVID-19; ongoing clinical trials
At present, studies regarding the use of anakinra in COVID-19 are limited. The summary of ongoing or completed clinical trials on the use of anakinra in COVID-19 patients is mentioned in Table 1 . Overall, 6 ongoing trials were registered in the clinicaltrials.gov database to assess the efficacy and safety of anakinra in COVID-19. Of them, two studies are completed in the United States, and the remaining are being performed in France, Sweden, Spain, and Qatar. Study sample sizes range from 15 to 216, with a cumulative sample size of 780. The route of administration is intravenous (IV) or subcutaneous (SC). The doses ranged from 100 mg every 12 h to 100 mg every 6 h for a duration of 7 to 15 days. The outcomes of trials are: not requiring mechanical ventilation on day 15 or day 28 from inclusion or at hospital discharge, time to recovery, and clinical improvement on day 14, defined by the World Health Organization-Clinical Progression Scale (WHO-CPS) score of<3.
Table 1.
Ongoing clinical trials assessing the use of anakinra to treat COVID-19.
| ID/status | Design | Country | Sample size | Intervention group(s) | Compared group | Outcomes |
|---|---|---|---|---|---|---|
|
NCT04603742/ Not yet recruiting |
Double-blind, placebo controlled, multicenter RCT | USA | 170 | IV anakinra 100 mg 4 times a day for 7 days | Normal saline | The number of patients alive without requiring mechanical ventilation |
| NCT04424056/ Not yet recruiting | Open-label RCT | France | 216 | Anakinra or tocilizumab with or without ruxolitinib (doses were not identified) | Standard of care | Ventilation free days at day 28 |
| NCT04412291/ Recruiting | Open-label, multicenter RCT | Sweden | 120 | IV anakinra 100 mg every 6 h for 7 days or IV tocilizumab 8 mg/kg as a single dose | Standard of care | Time to recovery |
| NCT04443881/ Completed | Open-label, multicenter RCT | Spain | 179 | IV anakinra 100 mg every 6 h for a maximum of 15 days | Standard of care | The number of patients not requiring mechanical ventilation at day 15 and day 28. Time to oxygen saturation normalization. ICU stay and hospitalization up to 28 days |
| NCT04643678/ Recruiting | Open-label RCT | Qatar | 80 | SC anakinra 100 mg twice daily for 3 days followed by 100 mg daily from day 4 to day 7 plus standard of care | Standard of care | WHO-CPS score of ≤ 3 at day 14 |
| NCT04362111/ Active, not recruiting | Triple-blind, placebo-controlled RCT | USA | 15 | SC anakinra 100 mg every 6–12 h for 10 days | Normal saline | Hospital discharge at day 28 without requiring mechanical ventilation |
ICU, intensive care unit; IV, intravenous; RCT, randomized clinical trial; SC, subcutaneous; WHO-CPS, World Health Organization-Clinical Progression Scale
5. Anakinra in COVID-19; published studies
Published studies are mostly observational that investigate the safety and efficacy of anakinra alone or combined with other medications. Several case reports of patients with severe disease and COVID-19-related complications, such as ARDS, pericarditis, or myocarditis, who were successfully treated with anakinra are summarized in Table 2 [18], [19], [20], [21], [22], [23].
Table 2.
Non-randomized trials on the use of anakinra in COVID-19.
| Author, year, country | Design | Population (n = sample size) | Anakinra dose | Starting time | Other treatments | Outcomes |
|---|---|---|---|---|---|---|
| Filocamo et al., 2020, Italy | Case report | 50-year-old man with severe COVID-19 | IV 200 mg followed by SC 100 mg every 6 h for 12 days | 10 days from hospital admission | HCQ, LPV/RTV | Improved inflammatory markers, ferritin, an increase lymphocyte count, liver enzymes, respiratory parameters |
| Karadeniz et al, 2020, Turkey | Case report | 33-year-old man with refractory COVID-19-related pericarditis | SC 100 mg daily for 7 days | 11 days from hospital admission | HCQ, moxifloxacin, colchicine, indomethacin, enoxaparin | Improvement in chest pain, echocardiogram, CRP and D-dimer levels |
| Kaps et al, 2020, Germany | Case report | 53-year-old woman with COVID-19-related ARDS | SC 100 mg daily for 9 days | 7 days from hospital admission | HCQ, antibiotic prophylaxis | Decreased body temperature and inflammatory biomarkers have and improved respiratory function. Sepsis occurred on day 19 |
| Nemchand et al, 2020, UK | Case report | 50-year-old man with COVID-19-related cytokine storm | IV 150 mg twice daily for 7 days | 9 days from hospital admission | Co-amoxiclav | Reduced supplemental oxygen requirement, ferritin, and CRP levels |
| Steinhardt et al, 2020, Germany | Case report | 48-year-old man with severe COVID-19, CRS, and ARDS | 2 mg/kg/day for 5 days | 14 days after symptoms onset | Reduced LDH, IL-6, and ferritin levels | |
| Trpkov et al, 2021, Canada | Case report | 62-year-old woman with multiple sclerosis, and COVID-19-related myocarditis | IV 100 mg twice daily for 5 days | 7 days from positive PCR test | Single dose dexamethasone, ceftriaxone, azithromycin | Reduced oxygen requirements, improved blood pressure and ejection fraction |
| Pontali et al, 2020, Italy | Case series | Moderate to severe COVID-19 (n = 5) | IV 300 mg daily for 24 to 48 h | 1.4 days from admission or 7.4 days from disease onset | HCQ, azithromycin, enoxaparin, DRV/RTV, and methylprednisolone in 1 patient | Reduced systemic inflammation and the need for oxygen support |
| Aouba et al, 2020, France | Case series | Moderate to severe COVID-19 with median Hscore of 96 (n = 9) | SC 100 mg twice daily day 1 to 3, followed by 100 mg daily for 7 days | 8 days from symptoms onset | HCQ and azithromycin in 2 patients | Decreased oxygen requirement, Hscore, and CRP levels |
| Navarro-Millan et al, 2020, USA | Case series | Severe COVID-19 with fever, ferritin levels > 1,000 ng/ml, one hyper-inflammatory marker, and acute hypoxemic respiratory failure requiring NC or HFNC (n = 11) | SC 100 mg every 6 h tapered gradually up to 20 days | 16 h to 7 days after initiation of supplemental oxygen | Methylprednisolone, empiric antibiotics, HCQ | Patients received anakinra before 36 h oxygen supplementation did not need MV and all were discharged. Four patients who received anakinra ≥ 4 days required MV, 2 discharged, 1 remains hospitalized, and 1 died |
| Pontali et al, 2021, Italy | Retrospective cohort | Severe COVID-19 with PaO2/FiO2 ≤ 300 mmHg, CRP or ferritin level > 3 times the ULN, or lymphocyte count < 1000/mm3 and D-dimer or LDH level > 3 times the ULN (n = 128) | IV 100 mg every 8 h for 3 days, tapered for maximum 9 days | Within 4 days from hospital admission | IV methylprednisolone, 1–2 mg/kg once or twice daily, with tapering, enoxaparin | Decreased mortality rate, no effect on the rate of ICU admission |
| Huet et al, 2020, France | Prospective cohort | Severe COVID-19 with SaO2 ≤ 93% on 6 L/min oxygen or more or SaO2 ≤ 93% on 3 L/min decreasing by 3% on ambient air, not on IMV (n = 96) | SC 100 mg twice daily for 72 h, followed by 100 mg daily for 7 days | Mean of 8.4 days from symptoms onset | HCQ, azithromycin, B-lactam antibiotics(ceftriaxone or amoxicillin), thromboembolic prophylaxis, methylprednisolone in 2 patients receiving anakinra | Decreased CRP levels, IMV, or death compared to the SOC |
| Balkhair et al, 2021, Oman | Prospective cohort | Severe COVID-19 with RR > 30 breaths/min, SpO2 < 90% on room air, SpO2 ≤ 93% on oxygen ≥ 6 L/min, or ARDS not on IMV (n = 69) | SC 100 mg twice daily for 3 days, followed by 100 mg daily for 7 days | Median of 10 days from symptoms onset | HCQ, tocilizumab, IFN, LPV/RTV, ribavirin, corticosteroids, B-lactam antibiotics (ceftriaxone or piperacillin–tazobactam), azithromycin, and prophylactic dose enoxaparin | Decreased the need for invasive mechanical ventilation except for patents on NIV, no effects on mortality rate, Decreased CRP, LDH, D-dimer, IL-6 levels compared to the control group |
| Cavalli et al, 2020, Italy | Retrospective cohort | Moderate or severe ARDS defined by PaO2/FiO2 100–200 or < 100 mmHg on PEEP ≥ 5 cm H2O, respectively with hyper-inflammation defined by CRP ≥ 100 mg/L or ferritin ≥ 900 ng/ml, requiring NIV out of ICU (n = 52) | SC 200 mg/day (low-dose) for 7 days or IV 10 mg/kg/day (high-dose) for median of 9 days followed by SC 200 mg/day for 3 days | HCQ, LPV/RTV, azithromycin or ceftriaxone | No beneficial effects with low-dose anakinra. Higher survival rate, decreased CRP levels, and similar mechanical ventilation-free survival with high-dose anakinra compared to SOC | |
| Della-Torre et al., 2021, Italy | Prospective cohort | Severe COVID-19 defined by PaO2/FiO2 ≤ 300 mmHg on high flow oxygen and a hyperinflammation defined by high LDH and at least one of the following: CRP ≥ 100 mg/L; IL-6 ≥ 40 pg/ml; or ferritin ≥ 900 ng/ml, not on IMV and not admitted to ICU (n = 210) | IV 10 mg/kg/day until improvement in respiratory parameters | HCQ, LPV/RTV, azithromycin, ceftriaxone, tocilizumab or sarilumab | Decreased mortality in patients with moderate or severe ARDS before development severe respiratory failure. | |
| Clark et al, 2020, UK | Case series | Immunosuppressed ICU patients with severe COVID-19 and high CRP and ferritin levels (4000–30,000 µg/l) (n = 4) | IV 200 mg daily or twice daily in one patient | After clinical deterioration, such as rise in oxygen requirement, CRP, or ferritin levels | Antibiotics and antifungals | Decreased oxygen support and inflammatory markers |
| Villegas et al, 2020, Spain | Case series | Patients with severe COVID-19, tocilizumab-refractory CRS/MAS, and hematological malignancies (n = 5) | SC 100 mg twice daily on day 1, 100 mg daily days 2 to 5 | Between days 9 to 29 after symptoms onset (mean:20 days) | HCQ, LPV/RTV, tocilizumab | Increased C-reactive protein, lactate dehydrogenase, ferritin, D-dimer, fibrinogen, and IL-10. All patients died from severe respiratory failure at median 6 days of anakinra administration |
| Dimopoulos et al, 2020, Greece/Netherlands | Case series | Severe COVID-19 with sHLH and HScores ≥ 169 (n = 8) | IV 200 mg three times daily for 7 days | After initiation of mechanical ventilation | Hydrocortisone, HCQ, azithromycin and other antibiotics | Reduced vasopressors requirement and Hscore, improved respiratory function |
| de la Calle et al, 2021, Spain | Prospective cohort | Tocilizumab-refractory severe COVID-19 (n = 40) | SC 100 mg twice daily on day 0, followed by 100 mg daily from day 1 to 5 | Median of 16 days from symptoms onset | HCQ ± azithromycin, LPV/RTV, interferon-β, antibiotics | No effects on clinical improvement defined by hospital discharge or decreasing ≥ 1 point on a 6-point ordinal scale, and in-hospital mortality rate |
| Kooistra et al, 2020, Netherlands | Prospective cohort | Critically ill COVID-19 on mechanical ventilation with hyper-inflammation defined by persistent high fever, high ferritin levels, or progressive organ dysfunction (n = 60) | IV 300 mg followed by 100 mg every 6 h | Median of 22 days from symptoms onset | Remdesivir, chloroquine, corticosteroids | Decrease in pro-inflammatory markers, BT, and WBC. Similar duration of mechanical ventilation or ICU stay between anakinra and SOC |
| Iglesias-Julian et al, 2020, Spain | Retrospective cohort | ARDS with PaO2/FiO2 < 300 and hyper-inflammation defined by ferritin > 1000 ng/mL or D-dimer > 1.5 μg/mL, and IL-6 < 40 mg/mL (n = 27) | SC 100 mg every 6 h for minimum 3 days, followed by 100 mg daily for 7 days | Median of 14 days from symptoms onset | HCQ, azithromycin, tocilizumab (prior to anakinra), methylprednisolone | Improvement in PaO2/FiO2 ratio, oxygen requirement, and inflammatory biomarkers, similar with patients who received tocilizumab |
| Langer-Gould et al, 2020, USA | Retrospective cohort | Cytokine storm defined by ferritin > 2000 ng/mL and one other abnormal marker or ≥ 4 abnormal inflammatory biomarkers including CRP > 70 mg/L, ferritin > 700 ng/ml, D-dimer > 1000 ng/mL, triglycerides > 265 mg/dL, AST > 59 IU/L, LDH > 300 IU/L, lymphocyte count < 800 cells/µL, and neutrophil count > 8000 cells/µL (n = 93) | Any dose of anakinra for ≥ 5 days or SC anakinra 100 mg every 6 h for one day | Median of 13 days from symptoms onset | Remdesivir, HCQ, or corticosteroids | Decreased mortality and intubation rate similar with the tocilizumab group |
| Franzetti et al, 2021, Italy | Retrospective cohort | ARDS with CRP ≥ 10.0 mg/dl, or ferritin ≥ 900 ng/ml, or both, PaO2/FiO2 < 250 mmHg on room air requiring either CPAP or orotracheal intubation (n = 112) | SC 100 mg 4 times daily for patients in a regular ward, or IV 200 mg 3 times daily for patients in ICU for 7 days | HCQ, LPV/RTV, azithromycin or ceftriaxone, enoxaparin | Improved overall survival and invasive ventilation-free survival, decreased CRP and ferritin levels. No beneficial effects on mortality rate after adjusting for confounding factors. Increased 28-day survival in patients on CPAP. No significant differences in 28-day survival in intubated patients |
ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; CPAP, continuous positive airway pressure; CRP, C-reactive protein; DRV, darunavir; HCQ, hydroxychloroquine; ICU, intensive care unit; IV, intravenous; LDH, lactate dehydrogenase; LMWH, low-molecular weight heparin; LPV, lopinavir; MV, mechanical ventilation; NIV, non-invasive ventilation; PaO2/FiO2, arterial oxygen partial pressure to fractional inspired oxygen ratio; PEEP, positive end-expiratory pressure; RCT, randomized clinical trial; RTV, ritonavir; SC, subcutaneous; suPAR, soluble urokinase plasminogen activator receptor; ULN, upper limit of normal
We divided trials per time point of COVID-19, either early or late, and discussed when is the most appropriate time point for anakinra treatment. Also, the studies were divided according to their designs to help the readers understand the topic better and to make it easier for them to access all kinds of studies.
5.1. Early initiation of anakinra
5.1.1. Case series:
As can be observed in Table 2 , several case series have shown that administration of anakinra within 8 days of symptoms onset or<36 h after developing acute respiratory failure prevented mechanical ventilation and facilitated discharge from the hospital [24], [25], [26].
5.1.2. Non-randomized trials:
The importance of early anti-inflammatory treatment was also addressed in a retrospective cohort study that assessed the effects of IV anakinra administration with or without methylprednisolone within 4 days after admission [27]. After adjustment for confounding factors, early anti-inflammatory therapy decreased the mortality rate by 74% (adjusted hazard ratio [HR], 0.26; P < 0.001). Although prescribing glucocorticoids in combination with anakinra might be a reliable approach to decrease inflammatory markers, the death rate did not significantly differ from patients receiving anakinra alone (15% vs. 13%, respectively).
Candidemia was reported in 3 patients in the anakinra group and 1 patient in the anakinra plus glucocorticoid group. Patients who received early anti-inflammatory therapy were younger and had significantly higher baseline levels of inflammatory markers. In contrast, more patients with comorbidities, mainly cardiovascular disorders, were involved in the control group, contributing to higher mortality rates [28], [29]. Moreover, the proportion of patients who received enoxaparin was higher in the intervention group (95.2% vs. 76.9%; P = 0.004). The key role of anticoagulant therapy in decreased mortality rates of COVID-19 patients might bias the results in favor of the intervention group [30].
A prospective cohort study by Huet et al. evaluated the role of anakinra in severe COVID-19 patients not admitted to the intensive care unit (ICU). The mean age of patients was 71 years, most of whom were men. The results revealed that mechanical ventilation requirement and death were significantly decreased in the anakinra group compared to the control group (25% vs. 73%; HR 0.22, 95% confidence interval [CI] 0.11–0.41; P < 0.0001) [31].
The longer duration of COVID-19 symptoms (mean difference, 2.2 days; P = 0.0088), higher platelet count (P = 0.0071), and patients’ lower mean body mass index (BMI) in the anakinra group might bias the study results. Also, the authors did not mention the reasons for anakinra discontinuation in patients, and the levels of IL-1 were not measured. Notably, more patients in the anakinra group had increased liver enzymes (13% vs. 9%) and thromboembolic events (19% vs. 11%). Although previous studies have demonstrated IL-1 role in increasing the tissue factor, evidence about the effects of IL-1 inhibition on coagulation is conflicting [31], [32], [33]. Further studies are required to determine whether IL-1 blocker anakinra is favorable or detrimental in COVID-19-induced coagulopathy.
In another prospective study by Balkhair et al., hospitalized patients with severe COVID-19 who were not on invasive mechanical ventilation received anakinra to compare with the historical cohort group [34]. The need for invasive mechanical ventilation was significantly higher in the control group (75% vs. 31%; P < 0.001), which remained persistent after adjustment for multiple variables and in both patients with or without hyperinflammation. However, the results were not satisfactory in the subgroup of patients on non-invasive mechanical ventilation, showing that low-dose SC anakinra might not be effective in patients with severe respiratory failure. The in-hospital death rate was not statistically different between the two groups (29% of the anakinra group vs. 46% of the control group; P = 0.08) [34]. It should be noted that the cut off values used for inflammatory biomarkers might be set too high in this study, and some patients in the early phases of hyperinflammation might be missed. No injection site reactions occurred in either group. Brevibacterium spp. was found in the blood culture of one patient in the anakinra group. However, the bloodstream infection rate caused by staphylococcus epidermidis was similar in both groups (P = 0.461). Of note, 21% of the patients in the historical group and no patient in the anakinra group received hydroxychloroquine plus azithromycin (P = 0.004), which might lead to an increased mortality rate [35]. Also, the baseline D-dimer level was higher in the control group. These between-group differences might increase the risk of bias. Besides, two groups were not evaluated simultaneously and were compared over a 10-weeks period, leading to reporting errors.
A retrospective cohort study that showed beneficial effects of high-dose anakinra on COVID-19 mortality enrolled patients with moderate to severe ARDS and hyperinflammation who did not need invasive mechanical ventilation or ICU admission [36]. The proportion of patients categorized as severe ARDS at baseline was higher in the high dose anakinra group (86% vs. 56%). Low-dose anakinra was discontinued after seven days because of no additional beneficial effects. The mortality rate of patients in the high-dose anakinra group was significantly lower than the standard care group on day 21 (10% vs. 44%). There were no significant differences between the two groups in cumulative mechanical ventilation free survival (anakinra 72% vs. standard of care 50%, P = 0.15) [36]. Of note, the results of this study were limited to the special population of COVID-19 patients who did not receive concomitant corticosteroids.
Della-Torre et al. conducted a cohort study to identify which patients with severe COVID-19 and hyperinflammatory features benefit most from IL to 6 and IL-1 blocking agents [37]. Patients admitted to the ICU, hospitalized for>4 days, and patients on mechanical ventilation or immunosuppressant were excluded from the study. The comparison of biologic agents with the matched control group showed a significantly lower mortality risk with anakinra (HR, 0.47; 95% CI, 0.26–0.87; P = 0.01) but not with sarilumab and tocilizumab. In a subset of patients with moderate ARDS defined as PaO2/FiO2 ratio ≥ 100 mmHg, biologic treatment was associated with a lower mortality rate than the usual care (4.7% vs. 30.5%, P = 0.001). However, the difference was insignificant in patients with severe ARDS who had PaO2/FiO2 ratio < 100 mmHg (P = 0.21), except for anakinra-treated patients who had lower mortality rates than the usual care group (HR 0.46; 95% CI 0.22–0.94; P = 0.04). Notably, this study reported the importance of the early administration of IL-1 and IL-6 inhibitors before severe ARDS. Accordingly, in administering IL blockers or other anti-inflammatory agents, such as glucocorticoids, the degree of respiratory impairment should be considered to combat COVID-19-associated inflammation effectively.
An open-label, non-randomized trial measured the level of soluble urokinase plasminogen activator receptor (suPAR) as a predicting factor for early initiation of anakinra to prevent severe respiratory failure in COVID-19 patients with lower respiratory tract infection [38]. Previous investigations demonstrated that the high level of suPAR (6 µg/l) is one of the indicators of developing severe respiratory failure in COVID-19 patients [39], [40]. Patients with suPAR ≥ 6 ng/ml received 100 mg/day of anakinra subcutaneously for 10 days. Of 179 cases in the standard care group, 139 patients were selected by propensity score-matching to compare with the anakinra group (n = 139). The concentration of suPAR was measured by collecting the blood of all patients at the baseline and on day 7.
Exclusion criteria of the study were PaO2/FiO2 ≤ 150 mmHg, stage 4 malignancy, requiring mechanical or non-invasive ventilation under positive pressure, primary immunodeficiency, and corticosteroid use at baseline with a dose of ≥ 0.4 mg/kg prednisolone or equivalent in the last 15 days, receiving any anti-cytokine biological treatment in the last month, neutropenia, pregnancy, or lactation.
The between-group comparison showed that incidence of severe respiratory failure during 14 days was significantly higher in the matched control group (59.2% vs. 22.3%; P < 0.0001). Anakinra was the only independent variable that protects patients from severe respiratory failure (HR, 0.28; 95% CI, 0.18–0.44; P < 0.0001). Also, anakinra significantly reduced 30-day (11.5 vs. 22.3%; HR, 0.49; 95% CI, 0.25–0.97; P = 0.041) and 90-day mortality (16.9% vs. 30.8%; HR, 0.5; 95% CI, 0.3–0.84; P = 0.007). The mean days without ventilator were higher in the anakinra group than the control group (18 vs. 25, P < 0.0001), which significantly decreased hospitalization costs.
This study also showed the inverse association between the IL-10/IL-6 ratio and the SOFA score of patients in the intervention group. In addition, anakinra could decrease the soluble IL-2 receptor and CD-163 levels, which were previously shown to be increased before macrophage activation and deterioration of the condition [41].
However, the authors of this study reported that patients in the anakinra group were enrolled from different health centers, which might raise the risk of bias. Also, the unclear recruitment time of patients in the control group is another limitation of the study. Notably, the measurement of suPAR levels used to identify patients most benefiting from anakinra is unavailable in many centers [38].
5.1.3. Randomized trials:
Although the results of randomized studies are more valuable, the number of published randomized trials related to administration of anakinra in COVID-19 patients, are limited.
A randomized controlled clinical trial by the CORIMUNO-19 Collaborative group assessed the role of anakinra in patients with mild to moderate COVID-19[42]. Inclusion criteria of this study were: requiring oxygen > 3 L/min through a nasal cannula or mask without ventilation support, ≥ 5 points on the WHO-CPS, CRP levels > 25 mg/L, and not requiring ICU admission at the time of enrollment. Patients with ANC ≤ 1.0 × 109 /L, platelet < 50 × 103/L, ALT/AST 5 times more than the upper limit of normal, and estimated GFR < 30 mL/min were excluded from the study.
Overall, 114 patients were randomly assigned to the control (n = 55) or intervention (n = 59) groups. Two primary outcomes were: 1) percentage of patients who died or received non-invasive or mechanical ventilation on day 4 (WHO-CPS > 5) and 2) patients who remained alive without non-invasive or mechanical ventilation on day 14.
Patients in the intervention group received IV anakinra 400 mg for the first 3 days, 200 mg on day 4, and 100 mg on day 5. Patients whose oxygen requirement was not reduced by>50% on day 4 received 400 mg/day of anakinra for 3 additional days. Both groups received standard treatment, such as antibiotics, lopinavir-ritonavir or lopinavir alone, anticoagulants, corticosteroids, hydroxychloroquine, and vasopressor support. One patient (2%) received tocilizumab in the intervention group. The median age of the patients was 66 years (interquartile range [IQR] 59–76), and 70% were men. At baseline, patients in the anakinra group had lower levels of D-dimer (991 vs. 1280) and higher levels of ferritin (1479 vs. 1151) than the control group; however, the significance was not reported. Both groups found that the median time between symptom onset and randomization was 10 days.
There were no significant differences in primary outcomes between the anakinra and control groups. On day 4, 36% of anakinra-treated patients and 38% in the control group had WHO-CPS>5 (90% credible interval, −17.1 to 12.0, posterior benefit probability of 61.2%). On day 14, 47% of anakinra-treated patients and 51% in the control group died or required non-invasive or mechanical ventilation (median HR 0.97; 90% credible interval, 0.62 to 1.52; posterior benefit probability of 54.5%).
Although the mortality rate of patients in the anakinra group was lower on day 14 (15% vs. 24%), post hoc analysis in patients with CRP levels > 150 mg/L or patients already on corticosteroids showed no significant differences. Adverse events were significantly higher in the anakinra-treated patients (113 vs. 60; P = 0.0004). More fungal and bacterial sepsis was observed in the anakinra group (11 vs. 4); however, the difference was insignificant [42].
Low doses of anakinra used in this study may not accurately represent its anti-inflammatory effects. Also, the multicenter nature of this study might lead to different standard treatments in various centers. Furthermore, the strict inclusion criteria used for this study limit the generalizability of the results.
Declercq et al. carried out a multicenter randomized clinical trial to evaluate the role of IL-1 and 6 blockers in COVID-19 patients with CRS, defined as ferritin > 2000 μg/L or ferritin > 1000 μg/L which was increasing within the last 24 h, or lymphocytes < 800/mL with two of the following criteria: LDH > 300 international units (IU)/L, ferritin > 700 μg/L, D-dimers > 1000 ng/mL, or CRP > 70 mg/L [43]. Patients who were mechanically ventilated for>24 h, patients with a clinical frailty scale of>3 prior to COVID-19, less probability to survive within 48 h, patients with active infection, platelets < 50,000/μL or neutrophil count < 1500/μL, a history of diverticulitis or bowel perforation, or patients on immunosuppressant drugs for COVID19-unrelated purposes were excluded from the study. Patients treated with IL-1 blocker (n = 112) received SC anakinra 100 mg daily for 28 days or until hospital discharge, and their outcomes were compared to the non-IL-1 blockade group. Patients in the IL-6 blockade group received either a single dose of IV tocilizumab 8 mg/kg (n = 114) or siltuximab 11 mg/kg (n = 113). The majority of patients were men (77%), and the median age was 65 years. The median time to clinical improvement, defined as a hospital discharge or increase of more than two points on a 6-category ordinal scale, was 12 days in the anakinra-treated and non-IL-1 blockade group (HR, 0.94; 95% CI, 0.73 to 1.21). The mortality rate was also similar between the two groups. The outcomes of patients treated with IL-6 blockers also did not differ from the non-IL-6 blockade group. Although this study did not support the use of anakinra early in the disease course, selection bias might limit the study's interpretation. For example, the decision to exclude patients with a high probability of death was according to the clinician’s judgment without objective criteria. Also, 68 out of 112 patients in the IL-1 blockade group received tocilizumab or siltuximab, making it difficult to discern the pure effects of anakinra.
In the SAVE-MORE trial, 594 hospitalized patients with moderate or severe COVID-19 and suPAR levels ≥ 6 ng/mL were enrolled. Patients with neutrophil count < 1500/mm3, stage IV malignancy, severe hepatic or renal failure, PaO2/FiO2 < 150 mmHg, or primary immunodeficiency, patients requiring non-invasive or mechanical ventilation, receiving corticosteroids with a dose ≥ 0.4 mg/kg/day of prednisone at least for 15 days, receiving anti-cytokine medications within the last 1 month did not enter the study.
Patients were assigned to receive either 100 mg/day of SC anakinra for 10 days (n = 405) or a placebo (n = 189). Anakinra was started at a median of 9 days from symptom onset (IQR 7–11) and 2 days after hospitalization (IQR 2–3).
The mean age of patients was 61.9 years, and 57.9% were men. According to the WHO classification, 91.6% of patients had severe COVID-19, defined by signs of respiratory distress, SpO2 < 90%, or RR > 30 breaths/min.
On day 28, the results revealed that the rate of COVID-19 progression on the WHO-CPS was considerably lower in the anakinra group compared to the placebo group (OR, 0.36; 95% CI, 0.26–0.50; P < 0.0001). Also, compared with the placebo group, lower rate of secondary infections (15.9% vs. 8.4%; P = 0.01) and 28-day mortality (6.9% vs. 3.2%; HR, 0.45; 95% CI, 0.21–0.98; P = 0.04) were observed in patients treated with anakinra [44]. Based on the results of this study, EMA recommended approval for the use of anakinra in COVID-19 patients who require low or high flow oxygen and are at risk of developing severe respiratory failure, defined as serum suPAR levels above 6 ng/ml. Since measuring suPAR level is not routinely available, data from the SAVE-MORE trial were used to validate other biomarkers correlated with developing severe respiratory failure. The Severe COVID-19 Prediction Estimate (SCOPE) score, which includes D-dimer, CRP, ferritin, and IL-6 levels, showed comparable results with suPAR in predicting severe respiratory failure [45]. However, the SCOPE score only included 63% of patients with suPAR ≥ 6 ng/ml. Accordingly, the EMA did not recommend it as an accurate score to recognize COVID-19 patients that might benefit from anakinra [46].
5.2. Late initiation of anakinra
5.2.1. Case series:
Case series of COVID-19 patients who received anakinra after clinical deterioration, mechanical ventilation, and late after symptoms onset showed controversial results as is shown in Table 2 [47], [48], [49]. In a case series by Dimopoulos et al., anakinra administration in severe COVID-19 patients who were mostly intubated improved respiratory function. However, by day 28, 3 patients died due to refractory shock or developing a resistant infection (Acinetobacter baumannii) of the central vein catheter, raising safety concerns of anakinra use in patients on mechanical ventilation [49]. It is noteworthy that immunomodulatory agents like anakinra could be an effective therapeutic option in COVID-19 patients with hyperinflammation and ARDS. However, immune system dysfunction might postpone viral clearance [50].
5.2.2. Non-randomized trials:
In a cohort of patients with severe and tocilizumab-refractory COVID-19, in which the median time between symptoms onset and anakinra initiation was 16 days (IQR 12–25), anakinra did not result in favorable outcomes [4]. The rate of clinical improvement on days 7, 14, and 21 after the intervention was similar between the two groups. Although the in-hospital mortality rate was reduced in the anakinra group, it did not reach the statistically significant level (55% vs. 45%, P = 0.527). The dose of anakinra used in this study was significantly lower than the previous studies. Also, IV anakinra results in drug concentration 24 to 29 times higher than SC administration, and the half-life is shorter when used intravenously [51], [52]. The lack of favorable results in this study might also result from the dependence of the anakinra mechanism of action on downstream suppression of IL-6 synthesis, which might not be beneficial in patients previously treated with tocilizumab as an IL-6 receptor blocker [4].
Kooistra et al. compared COVID-19 patients’ outcomes who received anakinra 22 days from symptoms onset with the standard of care group. Duration of mechanical ventilation (23 days vs. 17 days, P = 0.79) and ICU stay (24 days vs. 17 days, P = 0.54) was higher in the anakinra group; however, it did not reach statistical significance. Moreover, 28-day mortality was not significantly changed with anakinra administration (P = 0.87). Secondary infection was reported in 33% and 23% of patients in the anakinra and control groups, respectively (P = 0.54) [53].
In contrast with studies supporting the beneficial effects of anakinra on mortality rate, this study enrolled only critically ill COVID-19 patients who required mechanical ventilation. Hence, anakinra might be more beneficial in the early stages of the diseases when higher levels of cytokines are yet to be observed, which could prevent progression to severe illness, ICU admission, or mechanical ventilation [8], [27], [31], [34], [54], [55].
Timely administration of anakinra could also be interpreted from a retrospective cohort study by Iglesias-Julián et al. In this study, the median time between COVID-19 symptoms onset and anakinra initiation was 14 days, which was significantly longer than the historical propensity score-matched cohort of patients who received tocilizumab (10 days; P = 0.03). Favorable clinical outcomes were observed in 55.6% of patients in the anakinra group and 88.9% of patients in the tocilizumab group (P = 0.28).
On day 7, the reduction in IL-6 levels was significantly higher in anakinra recipients (P = 0.02) since the tocilizumab mechanism of action, which is an IL-6 receptor blocker. The PaO2/FiO2 ratio was significantly improved 7 days after administration of tocilizumab (P = 0.004), while the improvement was not significant in the anakinra group (P = 0.1) [56]. However, a definite conclusion might not be reasonable due to the presence of confounders. For example, five patients in the anakinra group had baseline CRP levels of>50 mg/L, which is associated with a poor prognosis.
In a retrospective cohort study, 93 patients with COVID-19-related cytokine storm were enrolled to receive either tocilizumab (initiated at a median of 14 days after symptoms onset) or anakinra (initiated at a median of 13 days after symptoms onset) [57]. In the anakinra group, fewer patients died (22% vs. 46.2%), and more were extubated/never intubated compared to the tocilizumab group (63.4% vs. 42.3%). However, the adjusted analysis failed to show any significant differences between the mortality rates of the two groups (HR, 0.46; 95% CI, 0.18–1.20; P = 0.11). Significant differences in baseline characteristics, such as lower intubation rate and obesity in the anakinra group, should be taken into account while interpreting the results. In this study, neutrophilia, acute kidney injury, and hypotension were associated with treatment failure, which had a higher rate in the tocilizumab group and might be the reason for poor response to tocilizumab.
A retrospective observational study included patients with ARDS and high levels of inflammatory biomarkers to compare the anakinra group with the matched cohort of control patients. Although anakinra was associated with a lower death rate (HR, 0.50; 95% CI, 0.28–0.89), it was not persistent after adjusting for all significant confounders.
Notably, anakinra administration in patients on continuous positive airway pressure (CPAP) showed a higher cumulative invasive ventilation-free survival rate than those on higher ventilator support levels (P = 0.048), showing the importance of early initiation of anakinra before clinical deterioration [58].
6. Anakinra use in children, pregnant or, lactating women
Multisystem inflammatory syndrome in children (MIS-C) is recognized as a late complication of COVID-19 in pediatric patients. The treatment strategy for this condition is similar to Kawasaki disease, streptococcal /staphylococcal toxic shock syndrome, and sepsis [59]. The syndrome has similar features to classical Kawasaki disease and Kawasaki shock syndrome, including respiratory and gastrointestinal symptoms and cardiac complications, such as myocarditis and shock, resulting in severe COVID-19 [60]. The immune system's response to the virus cause hyperinflammation and eventually cytokine storm, followed by MIS-C in pediatric patients. Although it is not a common complication of COVID-19, there are several reported cases [61].
3 years old girl was admitted to the hospital with COVID-19-related fever, abdominal pain, bulbar non-exudative conjunctivitis, skin rash, cheilitis, and palmar hands. Her laboratory results showed: thrombocytopenia, severe lymphopenia, hypofibrinogenemia, high levels of CRP, and IL-1Ra. After not responding to IVIG (2 g/kg) and IV methylprednisolone (2 mg/kg/day), she was treated with continuous infusion of anakinra (12 mg/kg/day) [62]. Two days after receiving anakinra, the CRP level was decreased, and after eight days, the patient was discharged with a normal clinical condition.
Another case was a 10-year-old girl with COVID-19-related fever, headache, abdominal pain, vomiting, bilateral cervical lymphadenopathy, and maculopapular skin rash for 5 days. She received antibiotics as empiric treatment, and no improvement was seen. Laboratory results showed lymphopenia, thrombocytopenia, and high inflammatory markers and cytokines (CRP, erythrocyte sedimentation rate, ferritin, IL-1Ra, and IL-6). The immunological workup showed no abnormalities. After failing to respond to methylprednisolone and IVIG, 7 mg/kg/day of SC anakinra was initiated. Due to the beneficial effects of corticosteroids on COVID-19, methylprednisolone was not interrupted after anakinra initiation. A few hours after receiving anakinra, the patient’s clinical condition improved, and anakinra was tapered within ten days without any adverse events [62].
Based on the evidence, COVID-19-induced MIS-C is an inflammatory condition caused by a previous SARS-CoV-2 infection [63]. So, the virus assay could be negative. This hypothesis can justify starting anakinra in case two without a positive RT-PCR test.
Another two-week-old girl with COVID-19-associated MIS-C received anakinra with a loading dose of 2 mg/kg followed by a 0.0.2 mL/kg/hour infusion after not responding to IVIG (2 mg/kg/dose), high-dose aspirin (75 mg/dose every 6 h), a single dose of dexamethasone, and methylprednisolone pulse (30 mg/kg/day for 3 days). Anakinra was administered 17 days after disease onset for 9 days. Her laboratory data showed lymphocytic leukocytosis, normocytic normochromic anemia, thrombocytopenia, hyponatremia, hypoalbuminemia, and high inflammatory markers, such as CRP, troponin, and ferritin. During treatment with anakinra, antibiotics (piperacillin/tazobactam) were continued. The baby was re-spiked with a low-grade fever on day 34 of admission. The second dose of IVIG was administered 24 h after discontinuation of anakinra. Five weeks later, she was discharged from the hospital [64].
Although anakinra showed promising effects in COVID-19-associated MIS-C, it is used as a second-line agent in poor response to the glucocorticoid and IVIG combination. Of note, due to the important contribution of IL-1 to inflammatory cardiac diseases and pericarditis, anakinra can be more effective in these conditions [61], [65], [66].
The evidence on the administration of anakinra to pregnant women with COVID-19 is limited. The American College of Rheumatology does not recommend anakinra use during pregnancy. Due to the low placental transfer level of monoclonal antibodies early in pregnancy, unintentional exposure to anakinra during the first trimester might not be dangerous [67], [68].
In a retrospective study, 14 pregnant patients with severe COVID-19 received SC anakinra with median doses of 2–10 mg/kg/day for a median of 6 days (3 to 15 days). The median duration of pregnancy at diagnosis was 24.5 weeks (16 to 37 weeks), and the median age of patients was 30 years. At the end of the follow-up, one patient died, two gave birth prematurely, and one had a healthy delivery [69]. It is not clear whether anakinra does pass into breast milk; however, it is expected to be minimal due to its large molecular weight. Moreover, the absorption of anakinra from the infant’s gastrointestinal tract is limited after exposure to breast milk. Studies did not show adverse reactions in infants breastfed by mothers who received anakinra [67], [70]. Accordingly, anakinra might be considered in breastfeeding women. However, the decision should be made according to the risk of infant exposure.
7. Possible effects of anakinra on the humoral response to SARS-CoV2
A prospective cohort study assessed the variables affecting the humoral response to COVID-19 in 518 patients with confirmed or suspected SARS-COV-2 infection [71]. The WHO diagnostic criteria were used for enrolling patients with the suspected disease [72].
The RT-PCR results in 60% of the patients were positive. The mean duration between hospitalization and antibody analysis was 82 days. The results revealed that 83% of patients developed IgG antibodies against SARS-COV-2, which was higher among patients with positive RT-PCR tests than those with negative tests (90% vs. 72%). Most patients with positive PCR and without response had no (52%) or minimal (42%) CT involvement.
Multivariate analysis showed that positive PCR, the degree of CT involvement, and the duration between hospitalization and antibody analysis were independent predictors of the positive antibody test. Analysis of patients with confirmed RT-PCR showed that older age (P = 0.04), fever (P = 0.01), hospitalization (P < 0.001), and high serum CRP and ferritin (P < 0.001), low WBC (P = 0.001), oxygen requirement (P = 0.002), receiving favipiravir (P = 0.001), tocilizumab, or anakinra (P = 0.01) were significantly associated with positive antibody response. The only independent factor that was associated with positive antibody results in confirmed patients was moderate to severe thoracic CT involvement (OR 10.95, 95% CI 1.20–99.81, P = 0.034).
Severe COVID-19 led to a higher antibody titer than the milder disease (P < 0.001). Also, anakinra, tocilizumab, or prednisolone administration was associated with higher antibody titers (P < 0.05). However, anakinra, either alone or combined with tocilizumab, was not associated with antibody response in COVID-19 patients independently after multivariate analysis. Because the cumulative duration of use and average dose of these drugs are the important factors that affect the adverse events, and in COVID-19, they are used for short periods. Despite previous findings, these drugs did not deteriorate antibody response.
8. Discussion
SARS-CoV-2 infection can cause multiple organ dysfunction in severe forms of the disease [73]. Currently, vaccines are the most influential tool to combat the virus. However, the new variant of the virus called omicron (B.1.1.529) might reduce the efficacy of designed vaccines and some monoclonal antibody treatments. Remdesivir is the only antiviral that got the U.S FDA approval for the treatment of COVID-19 in viral phases. Among immunomodulators, corticosteroids and tocilizumab are the most studied medications for patients experiencing severe forms of the disease. The adverse effects of these agents, such as secondary severe infections and hyperglycemia, might limit their use in the outpatient setting and raise safety concerns. Anakinra did not result in any critical safety issues when administered in clinical trials for sepsis management. Reports of secondary infections were related to the long-term anakinra use combined with tumor necrosis factor-alpha blockers, but not with a short period of administration.
Anakinra has been considered as an off-label medicine for early treatment of HLH-like syndrome in COVID-19 patients. However, investigational medications without enough scientific evidence can possess some concerning matters. Based on the council of Australian therapeutic advisory groups’ statement in defining the applicability of off-label drug use, there should be adequate evidence to confirm medications’ efficacy and safety and risk to benefit ratio for intentional clinical use. Conducting evidence-based reviews and being conscious of the rationale for using potential treatment candidates extrapolated from other disorders would help use the off-label agents more accurately.
Recently, EMA has authorized the administration of anakinra to treat adult COVID-19 patients who require supplemental oxygen and are at risk of developing severe respiratory failure [5]. The risk of progression to respiratory failure is governed by minimum blood levels of 6 ng/ml for suPAR. Although the higher SCOPE score was also associated with the development of severe respiratory failure, it only included 63% of patients with suPAR ≥ 6 ng/ml and was not recommended as an indicator to identify patients most benefiting from anakinra [45], [46].
Considering the protective role of the immune system against SARS-CoV-2, it is critical to decide what immunomodulatory medication should be used in which clinical status. For example, Wang et al. revealed that early treatment with interferon-α 2b decreased in-hospital mortality, while the late initiation of interferon therapy led to a higher mortality rate [74].
Therefore, the administration time and treatment type are crucial factors affecting immunomodulatory therapy outputs. Understanding the COVID-19 pathophysiology, particularly in the immune response impairment, and defining the relationship between the clinical and paraclinical features and the type of immune system dysregulation, would be appreciated in this regard.
According to our review, anakinra might be beneficial in patients at risk for progression to respiratory failure. However, its effectiveness in patients already suffering from respiratory failure showed controversial results and is not suggested. Also, four items were shown to be important in achieving the optimal therapeutic effects of anakinra in patients with COVID-19 who experience a cytokine storm. These items include duration of treatment of>10 days, doses>100 mg, IV route of administration, and early initiation of therapy.
Corticosteroids combined with anakinra appear to improve clinical outcomes compared to monotherapy with anakinra. Regarding the administration route, the IV anakinra might be desirable in COVID-19 patients with hemodynamic instability. Also, anakinra administration might be reserved for COVID-19 patients with pericarditis and MIS-C, in which anakinra can be more effective [75]. The combination of anakinra with IVIG and corticosteroids could exert a synergistic effect in improving the recovery rate in MIS-C. Anakinra is occasionally administered to manage children with severe COVID-19, and it has been administered in about 10% of MIS-C patients. It can be considered as an alternative second-line treatment in refractory cases of MIS-C.
Studies comparing the effectiveness of anakinra and other immunomodulators are lacking. Moreover, different doses and administration routes of anakinra in COVID-19 were not compared head-to-head in randomized clinical trials, this provides a further rationale for conducting well-designed studies on the SARS-CoV-2 induced CRS and various biologic agents to identify the ideal treatment approaches for COVID-19 patients based on their clinical status.
9. Conclusion
Based on the reviewed studies, four items are important for achieving a positive effect from anakinra in patients with high inflammatory markers and other signs of hyperinflammation, including duration of treatment, anakinra dose, and route and time of drug administration. Administration of high-dose anakinra (>100 mg) in the early phase of inflammation, around the first week of symptom onset, when the patient requires oxygen supplementation and is not on invasive mechanical ventilation for about 10 days might be effective and improve outcomes. Corticosteroids in combination with anakinra might significantly add further beneficial effects. The lack of blinded studies and the presence of interfering factors in the published research prevents the authors of this review from achieving a clear conclusion about the rule of anakinra in treating COVID-19 patients.
CRediT authorship contribution statement
Elnaz Khani: Writing – original draft. Marzieh Shahrabi: Writing – original draft. Haleh Rezaei: Supervision. Fariba Pourkarim: Writing – original draft. Hoda Afsharirad: Investigation. Mohammad Solduzian: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.WHO Coronavirus (COVID-19) Dashboard, World Heal. Organ. (n.d.). https://covid19.who.int.
- 2.Symptoms of COVID-19, 2021, Centers Dis. Control Prev. (n.d.). https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- 3.Hasan S.S., Capstick T., Ahmed R., Kow C.S., Mazhar F., Merchant H.a., Zaidi S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev. Respir. Med. 2020;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Calle C., López-Medrano F., Pablos J.L., Lora-Tamayo J., Maestro-de la Calle G., Sánchez-Fernández M., Fernández-Ruiz M., Pérez-Jacoiste Asín M.A., Caro-Teller J.M., García-García R., Catalán M., Martínez-López J., Sevillano Á., Origüen J., Ripoll M., Juan R.S., Lalueza A., de Miguel B., Carretero O., Aguilar F., Gómez C., Paz-Artal E., Bueno H., Lumbreras C., Aguado J.M. Effectiveness of anakinra for tocilizumab-refractory severe COVID-19: a single-centre retrospective comparative study. Int. J. Infect. Dis. 2021;105:319–325. doi: 10.1016/j.ijid.2021.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EMA recommends approval for use of Kineret in adults with COVID-19, Eur. Med. Agency. (n.d.).
- 6.Que Y., Hu C., Wan K., Hu P., Wang R., Luo J., Li T., Ping R., Hu Q., Sun Y.u., Wu X., Tu L., Du Y., Chang C., Xu G. Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int. Rev. Immunol. 2022;41(2):217–230. doi: 10.1080/08830185.2021.1884248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henter J.-I., Horne AnnaCarin, Aricó M., Egeler R.M., Filipovich A.H., Imashuku S., Ladisch S., McClain K., Webb D., Winiarski J., Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis, Pediatr. Blood. Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 8.Kyriazopoulou E., Leventogiannis K., Norrby-Teglund A., Dimopoulos G., Pantazi A., Orfanos S.E., Rovina N., Tsangaris I., Gkavogianni T., Botsa E., Chassiou E., Kotanidou A., Kontouli C., Chaloulis P., Velissaris D., Savva A., Cullberg J.S., Akinosoglou K., Gogos C., Armaganidis A., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15:1–10. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazodier K., Marin V., Novick D., Farnarier C., Robitail S., Schleinitz N., Veit V., Paul P., Rubinstein M., Dinarello C.A., Harlé J.R., Kaplanski G. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangalmurti N., Hunter C.A. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., Ní Choileáin O., Clarke J., O’Connor E., Hogan G., Ryan D., Sulaiman I., Gunaratnam C., Branagan P., O’Brien M.E., Morgan R.K., Costello R.W., Hurley K., Walsh S., de Barra E., McNally C., McConkey S., Boland F., Galvin S., Kiernan F., O’Rourke J., Dwyer R., Power M., Geoghegan P., Larkin C., O’Leary R.A., Freeman J., Gaffney A., Marsh B., Curley G.F., McElvaney N.G. Characterization of the inflammatory response to severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39:1–16. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethea J.R., Il Yup C., Sparacio S.M., Yancey Gillespie G., Benveniste E.N. Interleukin-1β induction of tumor necrosis factor-alpha gene expression in human astroglioma cells. J. Neuroimmunol. 1992;36(2-3):179–191. doi: 10.1016/0165-5728(92)90049-Q. [DOI] [PubMed] [Google Scholar]
- 14.Cavalli G., Dinarello C.A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 2018;9:1–21. doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrivastava G., León-Juárez M., García-Cordero J., Meza-Sánchez D.E., Cedillo-Barrón L. Inflammasomes and its importance in viral infections. Immunol. Res. 2016;64(5-6):1101–1117. doi: 10.1007/s12026-016-8873-z. [DOI] [PubMed] [Google Scholar]
- 16.Mehta P., Cron R.Q., Hartwell J., Manson J.J., Tattersall R.S. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome, Lancet. Rheumatol. 2020;2(6):e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giancane G., Minoia F., Davì S., Bracciolini G., Consolaro A., Ravelli A. IL-1 inhibition in systemic juvenile idiopathic arthritis. Front. Pharmacol. 2016;7:1–8. doi: 10.3389/fphar.2016.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filocamo G., Mangioni D., Tagliabue P., Aliberti S., Costantino G., Minoia F., Bandera A. Use of anakinra in severe COVID-19: a case report. Int. J. Infect. Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karadeniz H., Yamak B.A., Özger H.S., Sezenöz B., Tufan A., Emmi G. Anakinra for the Treatment of COVID-19-Associated Pericarditis: a Case Report. Cardiovasc. Drugs Ther. 2020;34(6):883–885. doi: 10.1007/s10557-020-07044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaps L., Labenz C., Grimm D., Schwarting A., Galle P.R., Schreiner O. Treatment of cytokine storm syndrome with IL-1 receptor antagonist anakinra in a patient with ARDS caused by COVID-19 infection: a case report. Clin. Case Reports. 2020;8(12):2989–2993. doi: 10.1002/ccr3.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemchand P., Tahir H., Mediwake R., Lee J. Cytokine storm and use of anakinra in a patient with COVID-19. BMJ Case Rep. 2020;13:1–3. doi: 10.1136/bcr-2020-237525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinhardt M.J., Wiebecke S., Weismann D., Frantz S., Tony H.P., Klinker H., Schmalzing M. Biomarker-guided application of low-dose anakinra in an acute respiratory distress syndrome patient with severe COVID-19 and cytokine release syndrome. Scand. J. Rheumatol. 2020;49(5):414–416. doi: 10.1080/03009742.2020.1789734. [DOI] [PubMed] [Google Scholar]
- 23.Trpkov C., MacMullan P., Feuchter P., Kachra R., Heydari B., Merchant N., Bristow M.S., White J.A. Rapid Response to Cytokine Storm Inhibition Using Anakinra in a Patient With COVID-19 Myocarditis. CJC Open. 2021;3(2):210–213. doi: 10.1016/j.cjco.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., Angelelli A., Caorsi R., Feasi M., Calautti F., Castagnola E., Rollandi G.A., Ravelli A., Cassola G., Gattorno M. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J. Allergy Clin. Immunol. 2020;146(1):213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: Case series. Ann. Rheum. Dis. 2020;79(10):1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 26.Navarro‐Millán I., Sattui S.E., Lakhanpal A., Zisa D., Siegel C.H., Crow M.K. Use of Anakinra to Prevent Mechanical Ventilation in Severe COVID-19: a Case Series, Arthritis. Rheumatol. 2020;72(12):1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pontali E., Volpi S., Signori A., Antonucci G., Castellaneta M., Buzzi D., Montale A., Bustaffa M., Angelelli A., Caorsi R., Giambruno E., Bobbio N., Feasi M., Gueli I., Tricerri F., Calautti F., Castagnola E., Moscatelli A., Rollandi G.A., Ravelli A., Cassola G., Sormani M.P., Gattorno M. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J. Allergy Clin. Immunol. 2021;147(4):1217–1225. doi: 10.1016/j.jaci.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng G., Yin M., Chen X., Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020;24:1–3. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khani E., Khiali S., Entezari‐Maleki T. Potential COVID-19 Therapeutic Agents and Vaccines: an Evidence-Based Review. J. Clin. Pharmacol. 2021;61(4):429–460. doi: 10.1002/jcph.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huet G.H.T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J.M., Bézie Y., Laplanche S., Le Berre A., Le Pavec J., Salmeron S., Emmerich J., Mourad J.J., Chatellier G. Anakinra for severe forms of COVID-19, Lancet. Rheumatol. 2020;2:e586–e587. doi: 10.1016/S2665-9913(20)30273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osnes L.T.N., Westvik Å.B., Joø G.-B., Okkenhaug C., Kierulf P. Inhibition of IL-1 induced tissue factor (TF) synthesis and procoagulant activity (PCA) in purified human monocytes by IL-4, IL-10 and IL-13. Cytokine. 1996;8(11):822–827. doi: 10.1006/cyto.1996.0110. [DOI] [PubMed] [Google Scholar]
- 33.Liberale L., Holy E.W., Akhmedov A., Bonetti N.R., Nietlispach F., Matter C.M., Mach F., Montecucco F., Beer J.H., Paneni F., Ruschitzka F., Libby P., Lüscher T.F., Camici G.G. Interleukin-1β Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor. J. Clin. Med. 2019;8:2072. doi: 10.3390/jcm8122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkhair A., Al-Zakwani I., Al Busaidi M., Al-Khirbash A., Al Mubaihsi S., BaTaher H., Al Aghbari J., Al Busaidi I., Al Kindi M., Baawain S., Al Alawi A., Al Lawati A., Al Rawahi B., Al-Baimani K., Al Zidi K., Elfatih N., Dawud B., John B., Rehman F., Yousif F., Al Khadouri G., Saber I., Lal J., Gargouri M., Al-Ward M., AbuDraz N., Al Ruqeishi S., Kumar S., Abdelmottaleb W., Al-Naamani Z., Bin Nazar Z., Balkhair O. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: Results of a prospective, open-label, interventional study. Int. J. Infect. Dis. 2021;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: authors’ response. Clin. Microbiol. Infect. 2021;27(1):138–140. doi: 10.1016/j.cmi.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.K. Ramanathan, D. Antognini, A. Combes, M. Paden, B. Zakhary, M. Ogino, G. Maclaren, D. Brodie, Interleukin-1blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation:, Lancet Rheumatol. 2 (2020) 19–21. https://doi.org/https://doi.org/10.1016/ S2665-9913(20)30127-2.
- 37.Della-Torre E., Lanzillotta M., Campochiaro C., Cavalli G., De Luca G., Tomelleri A., Boffini N., De Lorenzo R., Ruggeri A., Rovere-Querini P., Castagna A., Landoni G., Tresoldi M., Ciceri F., Zangrillo A., Dagna L. Respiratory Impairment Predicts Response to IL-1 and IL-6 Blockade in COVID-19 Patients With Severe Pneumonia and Hyper-Inflammation. Front. Immunol. 2021;12:1–10. doi: 10.3389/fimmu.2021.675678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyriazopoulou E., Panagopoulos P., Metallidis S., Dalekos G.N., Poulakou G., Gatselis N., Karakike E., Saridaki M., Loli G., Stefos A., Prasianaki D., Georgiadou S., Tsachouridou O., Petrakis V., Tsiakos K., Kosmidou M., Lygoura V., Dareioti M., Milionis H., Papanikolaou I.C., Akinosoglou K., Myrodia D.M., Gravvani A., Stamou A., Gkavogianni T., Katrini K., Marantos T., Trontzas I.P., Syrigos K., Chatzis L., Chatzis S., Vechlidis N., Avgoustou C., Chalvatzis S., Kyprianou M., van der Meer J.W.M., Eugen-Olsen J., Netea M.G., Giamarellos-Bourboulis E.J. An open label trial of anakinra to prevent respiratory failure in covid-19. Elife. 2021;10:1–21. doi: 10.7554/eLife.66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rovina N., Akinosoglou K., Eugen-Olsen J., Hayek S., Reiser J., Giamarellos-Bourboulis E.J. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit. Care. 2020;24:4–6. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azam T.U., Shadid H.R., Blakely P., O’Hayer P., Berlin H., Pan M., Zhao P., Zhao L., Pennathur S., Pop-Busui R., Altintas I., Tingleff J., Stauning M.A., Andersen O., Adami M.E., Solomonidi N., Tsilika M., Tober-Lau P., Arnaoutoglou E., Keitel V., Tacke F., Chalkias A., Loosen S.H., Giamarellos-Bourboulis E.J., Eugen-Olsen J., Reiser J., Hayek S.S., Hayek S.S., Blakely P., Berlin H., Azam T.U., Shadid H., Pan M., O’Hayer P., Meloche C., Feroze R., Padalia K.J., Anderson E., Perry D., Bitar A., Kaakati R., Zhao L., Zhao P., Eugen-Olsen J., Altintas I., Tingleff J., Stauning M., Houlind M.B., Lindstrøm M.B., Andersen O., Gamst-Jensen H., Rasmussen L.J.H., Rasmussen C., Nehlin J.O., Kallemose T., Parvaiz I., Giamarellos-Bourboulis E.J., Adami M.E., Solomonidi N., Tsilika M., Saridaki M., Lekakis V., Loosen S., Luedde T., Keitel V., Chalkias A., Arnaoutoglou E., Pantazopoulos I., Laou E., Kolonia K., Skoulakis A., Tacke F., Tober-Lau P., Mohr R., Kurth F., Sander L.E., Jochum C. Soluble urokinase receptor (SuPAR) in COVID-19-Related AKI. J. Am. Soc. Nephrol. 2020;31:2725–2735. doi: 10.1681/ASN.2020060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bleesing J., Prada A., Siegel D.M., Villanueva J., Olson J., Ilowite N.T., Brunner H.I., Griffin T., Graham T.B., Sherry D.D., Passo M.H., Ramanan A.V., Filipovich A., Grom A.A. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56(3):965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 42.Respir Med. 2020;9:19–21. [Google Scholar]
- 43.Declercq J., Van Damme K.F.A., De Leeuw E., Maes B., Bosteels C., Tavernier S.J., De Buyser S., Colman R., Hites M., Verschelden G., Fivez T., Moerman F., Demedts I.K., Dauby N., De Schryver N., Govaerts E., Vandecasteele S.J., Van Laethem J., Anguille S., van der Hilst J., Misset B., Slabbynck H., Wittebole X., Liénart F., Legrand C., Buyse M., Stevens D., Bauters F., Seys L.J.M., Aegerter H., Smole U., Bosteels V., Hoste L., Naesens L., Haerynck F., Vandekerckhove L., Depuydt P., van Braeckel E., Rottey S., Peene I., Van Der Straeten C., Hulstaert F., Lambrecht B.N. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet Respir. Med. 2021;9(12):1427–1438. doi: 10.1016/S2213-2600(21)00377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyriazopoulou E., Poulakou G., Milionis H., Metallidis S., Adamis G., Tsiakos K., Fragkou A., Rapti A., Damoulari C., Fantoni M., Kalomenidis I., Chrysos G., Angheben A., Kainis I., Alexiou Z., Castelli F., Serino F.S., Tsilika M., Bakakos P., Nicastri E., Tzavara V., Kostis E., Dagna L., Koufargyris P., Dimakou K., Savvanis S., Tzatzagou G., Chini M., Cavalli G., Bassetti M., Katrini K., Kotsis V., Tsoukalas G., Selmi C., Bliziotis I., Samarkos M., Doumas M., Ktena S., Masgala A., Papanikolaou I., Kosmidou M., Myrodia D.-M., Argyraki A., Cardellino C.S., Koliakou K., Katsigianni E.-I., Rapti V., Giannitsioti E., Cingolani A., Micha S., Akinosoglou K., Liatsis-Douvitsas O., Symbardi S., Gatselis N., Mouktaroudi M., Ippolito G., Florou E., Kotsaki A., Netea M.G., Eugen-Olsen J., Kyprianou M., Panagopoulos P., Dalekos G.N., Giamarellos-Bourboulis E.J. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat. Med. 2021;27(10):1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giamarellos-Bourboulis E.J., Poulakou G., de Nooijer A., Milionis H., Metallidis S., Ploumidis M., Grigoropoulou P., Rapti A., Segala F.V., Balis E., Giannitsioti E., Rodari P., Kainis I., Alexiou Z., Focà E., Lucio B., Rovina N., Scorzolini L., Dafni M., Ioannou S., Tomelleri A., Dimakou K., Tzatzagou G., Chini M., Bassetti M., Trakatelli C., Tsoukalas G., Selmi C., Samaras C., Saridaki M., Pyrpasopoulou A., Kaldara E., Papanikolaou I., Argyraki A., Akinosoglou K., Koupetori M., Panagopoulos P., Dalekos G.N., Netea M.G. Development and validation of SCOPE score: a clinical score to predict COVID-19 pneumonia progression to severe respiratory failure. Cell Reports Med. 2022;3(3):100560. doi: 10.1016/j.xcrm.2022.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kineret Assessment Report, Eur. Med. Agency. (n.d.). https://www.ema.europa.eu/en/documents/variation-report/kineret-h-c-000363-ii-0086-epar-assessment-report-variation_en.pdf.
- 47.Clark K.E.N., Collas O., Lachmann H., Singh A., Buckley J., Bhagani S. Safety of intravenous anakinra in COVID-19 with evidence of hyperinflammation, a case series. Rheumatol. Adv. Pract. 2020;4:1–6. doi: 10.1093/rap/rkaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villegas C., Poza M., Talayero P., Teller J.M.C., Zafra D., Garcia C., Vera E., Hidalgo M., Lopez N., Cuellar C., Zamanillo I., Íñiguez R., Paz-Artal E., Aguado J.M., Martinez-Lopez J. IL-1R blockade is not effective in patients with hematological malignancies and severe SARS-CoV-2 infection. Ann. Hematol. 2020;99(12):2953–2956. doi: 10.1007/s00277-020-04160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., Netea M.G., Spyridopoulos T., Verheggen R.J., Hoogerwerf J., Lachana A., van de Veerdonk F.L., Giamarellos-Bourboulis E.J. Favorable anakinra responses in severe Covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28(1):117–123.e1. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang L., Yin Z., Hu Y., Mei H. Controlling cytokine storm is vital in COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang B.B., Baughman S., Sullivan J.T. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin. Pharmacol. Ther. 2003;74:85–94. doi: 10.1016/S0009-9236(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 52.Yang B.-B., Gozzi P., Sullivan J.T. Pharmacokinetics of anakinra in subjects of heavier vs. lighter body weights. Clin. Transl. Sci. 2019;12(4):371–378. doi: 10.1111/cts.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kooistra E.J., Waalders N.J.B., Grondman I., Janssen N.A.F., de Nooijer A.H., Netea M.G., van de Veerdonk F.L., Ewalds E., van der Hoeven J.G., Kox M., Pickkers P., Kooistra E.J., Waalders N.J.B., Grondman I., Janssen N.A.F., de Nooijer A.H., Netea M.G., van de Veerdonk F.L., Ewalds E., van der Hoeven J.G., Kox M., Pickkers P., Hemelaar P., Beunders R., Bruse N., Frenzel T., Schouten J., Touw H., van der Velde S., van der Eng H., Roovers N., Klop-Riehl M., Gerretsen J., Claassen W., Heesakkers H., van Schaik T., Buijsse L., Joosten L., de Mast Q., Jaeger M., Kouijzer I., Dijkstra H., Lemmers H., van Crevel R., van de Maat J., Nijman G., Moorlag S., Taks E., Debisarun P., Wertheim H., Hopman J., Rahamat-Langendoen J., Bleeker-Rovers C., Koenen H., Fasse E., van Rijssen E., Kolkman M., van Cranenbroek B., Smeets R., Joosten I. Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit. Care. 2020;24:1–12. doi: 10.1186/s13054-020-03364-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D. B. Shakoory, M.D., George Washington University, Washington, P. J.A. Carcillo, M.D., University of Pittsburgh Medical Center, Pittsburgh, A. W. W. Chatham, M.D., University of Alabama at Birmingham, Birmingham, D. R. L. Amdur, Ph.D., George Washington University, Washington, P. H. Zhao, Ph.D., Temple University, Philadelphia, C. C.A. Dinarello, M.D., University of Colorado Denver, Aurora, A. R.Q. Cron, M.D. Ph.D., and University of Alabama at Birmingham, Birmingham, R. S.M. Opal, M.D. Brown University, Providence, Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial, Crit. Care Med. 44 (2016) 275–281. https://doi.org/10.1097/CCM.0000000000001402.Interleukin-1. [DOI] [PMC free article] [PubMed]
- 56.Iglesias-Julián E., López-Veloso M., de-la-Torre-Ferrera N., Barraza-Vengoechea J.C., Delgado-López P.D., Colazo-Burlato M., Ubeira-Iglesias M., Montero-Baladía M., Lorenzo-Martín A., Minguito-de-la-Iglesia J., García-Muñoz J.P., Sanllorente-Sebastián R., Vicente-González B., Alemán-Alemán A., Buzón-Martín L. High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID-19 patients. J. Autoimmun. 2020;115:102537. doi: 10.1016/j.jaut.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langer-Gould A., Smith J.B., Gonzales E.G., Castillo R.D., Figueroa J.G., Ramanathan A., Li B.H., Gould M.K. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int. J. Infect. Dis. 2020;99:291–297. doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franzetti M., Forastieri A., Borsa N., Pandolfo A., Molteni C., Borghesi L., Pontiggia S., Evasi G., Guiotto L., Erba M., Pozzetti U., Ronchetti A., Valsecchi L., Castaldo G., Longoni E., Colombo D., Soncini M., Crespi S., Maggiolini S., Guzzon D., Piconi S. IL-1 Receptor antagonist anakinra in the treatment of COVID-19 acute respiratory distress syndrome: a retrospective, observational study. J. Immunol. 2021;206(7):1569–1575. doi: 10.4049/jimmunol.2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-Sarmiento J., De Souza D., Jabornisky R., Gonzalez G.A., Arias López M.D.P., Palacio G. Paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): a narrative review and the viewpoint of the latin american society of pediatric intensive care (SLACIP) sepsis committee, BMJ paediatr. Open. 2021;5(1):e000894. doi: 10.1136/bmjpo-2020-000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Bradley Segal J., Nguyen E.L., Barsh G.R., Maskatia S., Mathew R. COVID-19 and kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 61.Tanner T., Wahezi D.M. Hyperinflammation and the utility of immunomodulatory medications in children with COVID-19. Paediatr. Respir. Rev. 2020;35:81–87. doi: 10.1016/j.prrv.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Della Paolera S., Valencic E., Piscianz E., Moressa V., Tommasini A., Sagredini R., Kiren V., Comar M., Taddio A. Case Report: use of anakinra in multisystem inflammatory syndrome during COVID-19 pandemic. Front. Pediatr. 2021;8:1–6. doi: 10.3389/fped.2020.624248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., Barranco M.A., Maxted A.M., Rosenberg E.S., Easton D., Udo T., Kumar J., Pulver W., Smith L., Hutton B., Blog D., Zucker H. Multisystem inflammatory syndrome in children in new york state. N. Engl. J. Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M. S, Refractory Multi-Inflammatory Syndrome in a Two Weeks Old Neonate with COVID-19 Treated Successfully with Intravenous Immunoglobulin, Steroids and Anakinra, Open Access J. Microbiol. Biotechnol. 5 (2020) 1–9. https://doi.org/10.23880/oajmb-16000176.
- 65.Brucato A., Imazio M., Gattorno M., Lazaros G., Maestroni S., Carraro M., Finetti M., Cumetti D., Carobbio A., Ruperto N., Marcolongo R., Lorini M., Rimini A., Valenti A., Erre G.L., Sormani M.P., Belli R., Gaita F., Martini A. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA - J. Am. Med. Assoc. 2016;316:1906–1912. doi: 10.1001/jama.2016.15826. [DOI] [PubMed] [Google Scholar]
- 66.Rajasekaran S., Kruse K., Kovey K., Davis A.T., Hassan N.E., Ndika A.N., Zuiderveen S., Birmingham J. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically Ill children. Pediatr. Crit. Care Med. 2014;15(5):401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 67.Berger C.T., Recher M., Steiner U., Hauser T.M. A patient’s wish: anakinra in pregnancy. Ann. Rheum. Dis. 2009;68(11):1794–1795. doi: 10.1136/ard.2008.105833. [DOI] [PubMed] [Google Scholar]
- 68.N.I, o, Health, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, (2022). [PubMed]
- 69.Karakaş Ö., Erden A., Ünlü S., Erol S.A., Goncu Ayhan Ş., Özdemir B., Tanacan A., Ozden Tokalioglu E., Ateş İ., Moraloğlu Tekin Ö., Omma A., Şahin D., Küçükşahin O. Can Anakinra and corticosteroid treatment be an effective option in pregnant women with severe Covid-19? Women Heal. 2021;61(9):872–879. doi: 10.1080/03630242.2021.1981517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Youngstein T., Hoffmann P., Gül A., Lane T., Williams R., Rowczenio D.M., Ozdogan H., Ugurlu S., Ryan J., Harty L., Riminton S., Headley A.P., Roesler J., Blank N., Kuemmerle-Deschner J.B., Simon A., Woolf A.S., Hawkins P.N., Lachmann H.J. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology. 2017;56:2102–2108. doi: 10.1093/rheumatology/kex305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Başaran S., Şimşek-Yavuz S., Meşe S., Çağatay A., Medetalibeyoğlu A., Öncül O., Özsüt H., Ağaçfidan A., Gül A., Eraksoy H. The effect of tocilizumab, anakinra and prednisolone on antibody response to SARS-CoV-2 in patients with COVID-19: a prospective cohort study with multivariate analysis of factors affecting the antibody response. Int. J. Infect. Dis. 2021;105:756–762. doi: 10.1016/j.ijid.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.World Health Organization. WHO COVID-19 Case definition, Accessed December 16 2020. (n.d.). https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2.
- 73.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020;14(9):865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang N., Zhan Y., Zhu L., Hou Z., Liu F., Song P., Qiu F., Wang X., Zou X., Wan D., Qian X., Wang S., Guo Y., Yu H., Cui M., Tong G., Xu Y., Zheng Z., Lu Y., Hong P. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28(3):455–464.e2. doi: 10.1016/j.chom.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mastrolia M.V., Marrani E., Maccora I., Pagnini I., Simonini G. The role of anti-IL-1 treatment in MIS-C patients. Expert Opin. Biol. Ther. 2022;22(1):1–5. doi: 10.1080/14712598.2022.2006631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.