Abstract
Analysis of the expression of inflammatory markers before starting treatment in human patients with cancer helps to predict outcomes and prognosis; however, there have been few studies on this topic in veterinary medicine. The present study aimed to evaluate inflammatory indices before treatment with autologous antitumor vaccine alone or this vaccine plus metronomic chemotherapy (MC) to predict response and prognosis. The indices included the neutrophil–lymphocyte ratio (NRL), platelet–lymphocyte ratio (PLR), monocyte–lymphocyte ratio (MLR), systemic immune-inflammation index (SII), C-reactive-protein–albumin ratio (CRP/ALB), lactate dehydrogenase level (LDH), frequency of blood lymphocyte subsets (CD4+, CD8+, Treg, and CD4/CD8 ratio) and frequency of blood myeloid-derived suppressor cells (MDSCs: monocytic [M]- MDSCs, and granulocytic [PMN]-MDSCs). Blood samples were collected from 25 dogs with oral melanoma treated with the autologous antitumor vaccine and from nine dogs that received MC plus vaccine before surgery. There were no statistically significant differences in the progression-free survival (PFS) or overall survival (OS) between the groups. In addition to the clinical stage, the CRP/ALB ratio and blood circulating Tregs in the univariate analysis showed an association with PFS and OS, and thus were selected for multivariable analysis. The CRP/ALB ratio was associated with PFS [hazard ratio (HR), 1.1; 95% confidence interval (CI), 1.0–1.1; p = 0.017] and OS [HR, 1; 95%CI, 1.0–1.1; p = 0.023]. Similarly, Treg was associated with PFS (HR, 1.6; 95% CI, 1.2–2.1; p = 0.001) and OS (HR, 1.6; 95% CI, 1.2–2.1; p = 0.001). Furthermore, canine patients with a CRP/ALB ratio above the cut-off point of 1.9 (established by receiver operating characteristic curve analysis) had worse PFS and OS, indicating the impact of the preoperative CRP/ALB ratio on the PFS and OS of dogs with oral melanoma. The CRP/ALB ratio and frequency of circulating Tregs are potential prognostic markers in dogs with oral melanoma.
Keywords: neoplasm, cancer vaccines, inflammation, immunosuppression, immunomodulation
Introduction
Prognostic and predictive markers for companion animal neoplasias are gaining increasing interest in veterinary medicine, particularly for heterogeneous neoplasias, such as canine oral melanoma. Assessing the tumor microenvironment characteristics and the immune/inflammatory profile are known to improve prognostic precision and refine the predicted response for personalized therapy approaches.
Currently, immunotherapy in veterinary patients has attracted interest in experimental and clinical trials. The growing use of immunotherapy for the treatment of different types of cancer in human medicine and the marked variation in response to this therapeutic approach have motivated the study of blood markers that can predict the patient's response to therapy, not only in the search for prognostic markers, but also to select patients appropriately, because this therapeutic modality is costly (1–3).
Canine oral melanoma is the most frequent neoplasm of the oral cavity in dogs and has an invasive and highly metastatic nature (4, 5). Treatment is based on surgical removal of the neoplasm, with or without radiotherapy and electrochemotherapy. After local control, the use of adjuvant therapy is recommended for metastasis control (4, 5). This neoplasm is difficult to control, and consequently there have been numerous studies using different modalities of immunotherapy, and studies attempting to predict responses to such treatment. In addition to predicting treatment response, determining prognostic factors could add parameters and improve the accuracy of the clinical staging in patients. The possibility of genetically engineering immunogenic antigens for melanoma vaccines (6), which could be financially limited for clinical use in domestic animal data from animal models, supports the potential use of active immunotherapy using tumor lysate vaccines with pathogen adjuvants, such as Bacillus Calmette–Guerin (BCG), to improve treatment for melanoma (7, 8).
Inflammation contributes to cancer progression, since the continuous presence of inflammatory cells creates the conditions for tumor development, e.g., apoptosis evasion, sustained angiogenesis, self-sufficiency in growth signaling, insensitivity to anti-growth signaling, and tissue invasion/metastasis. Infiltration of immune system cells into the tumor microenvironment contributes to cancer progression (9, 10). The chronic systemic inflammatory response in human patients with cancer (hepatocellular carcinoma, colorectal carcinoma, and gastric carcinoma) is believed to be directly related to the degree of disease progression (11–13). Considering the cost/benefit ratio in a veterinary setting, some inflammatory parameters, calculated based on the blood cell count, serum lactate dehydrogenase (LDH) measurement, and blood immunophenotyping are not costly, and thus are applicable in clinical practice.
Some useful indices are calculated based on blood cell count and inflammatory proteins, such as the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), monocyte–lymphocyte ratio (MLR), systemic immune-inflammation index (SII), C-reactive-protein–albumin ratio (CRP/ALB ratio), and frequency of circulating immune cells (subsets of T lymphocytes and myeloid-derived suppressor cells). Inflammatory indices, widely studied in human cancer patients with melanoma and different types of carcinomas (colorectal, cervical, urothelial, pancreatic, and nasopharyngeal), are useful in indicating prognosis and predicting responses to different therapeutic modalities, such as surgery, chemotherapy, immunotherapy, and specific antitumor drugs (1–3, 12, 14–19).
High values of these indices or a high frequency of immunosuppressive cells are found in human patients with more aggressive cancers and more advanced stages of the disease (20–22). However, there have been few relevant studies in veterinary medicine (23–30). For human patients with melanoma, in addition to the immune profile, the serum level of LDH is considered an important prognostic factor (31), particularly in patients with metastases; however, in veterinary medicine, there have been no studies in dogs with oral melanoma, although there are some reports on other neoplasms (32–34).
This study aimed to assess whether the values of inflammatory indices, serum LDH levels, and frequency of immune circulating cells could predict the response to treatment and prognosis that correlate with overall survival (OS) and progression-free survival (PFS) in dogs with oral melanoma that received tumor lysate vaccine with BCG [alone, or in combination with metronomic chemotherapy (MC)].
Materials and Methods
Patient Enrollment
Blood, tumor samples, and clinical data were prospectively collected from 90 canine patients with oral melanoma attending several Brazilian private clinics and teaching veterinary hospitals. Of these, 25 patients were included in this study. Sixteen received only the tumor lysate vaccine as adjuvant treatment, and nine received the tumor lysate vaccine plus MC. Surgery [complete or incomplete excision alone or plus electrochemotherapy (ECT)], adjuvant treatment (tumor lysate vaccine alone or plus MC), and follow-up were performed in the respective veterinary clinics (Table 1). The clinical study was neither randomized nor blinded, and the treatment was chosen by a clinical veterinarian in conjuntion with the patient's owner.
Table 1.
Characterization and treatment of 25 dogs with oral melanoma.
| ID | Breed | Sex | Age (years) | Clinical Stage | Surgery | ECT | MC |
|---|---|---|---|---|---|---|---|
| 1 | Golden Retriever | Male | 12 | III | Incomplete | Yes | Not |
| 2 | Dachshund | Female | 14 | IV | Incomplete | Yes | Not |
| 3 | Golden Retriever | Male | 9 | IV | Incomplete | Yes | Yes |
| 4 | Mixed | Female | 16 | IV | Incomplete | Yes | Not |
| 5 | Schnauzer | Male | 11 | II | Complete | Not | Not |
| 6 | Shih Tzu | Male | 15 | II | Incomplete | Yes | Yes |
| 7 | Mixed | Male | 15 | III | Incomplete | Yes | Not |
| 8 | Mixed | Female | 7 | III | Complete | Not | Yes |
| 9 | Mixed | Female | 13 | III | Incomplete | Not | Not |
| 10 | Mixed | Female | 16 | III | Incomplete | Yes | Not |
| 11 | Poodle | Male | 17 | II | Incomplete | Not | Not |
| 12 | Boxer | Male | 11 | II | Incomplete | Yes | Not |
| 13 | Rottweiler | Male | 8 | III | Incomplete | Yes | Not |
| 14 | Mixed | Female | 12 | II | Complete | Not | Not |
| 15 | Pinscher | Male | 13 | II | Complete | Yes | Not |
| 16 | Beagle | Male | 13 | IV | Incomplete | Yes | Yes |
| 17 | Dachshund | Female | 17 | II | Incomplete | Yes | Not |
| 18 | Cocker Spainel | Male | 14 | III | Incomplete | Yes | Yes |
| 19 | Dachshund | Male | 13 | III | Complete | Yes | Yes |
| 20 | Pinscher | Female | 11 | III | Complete | Yes | Not |
| 21 | Yorkshire | Female | 15 | III | Incomplete | Not | Yes |
| 22 | Dachshund | Male | 14 | III | Incomplete | Yes | Not |
| 23 | Mixed | Male | 15 | III | Incomplete | Yes | Not |
| 24 | Chow Chow | Male | 13 | III | Incomplete | Not | Yes |
| 25 | Mixed | Male | 16 | III | Incomplete | Yes | Yes |
ECT, electrochemotherapy; MC, metronomic chemotherapy.
The vaccine was produced in the laboratory of the Department of Pathology (School of Veterinary Medicine and Animal Science, University of São Paulo) and delivered to veterinarians. The study was approved by the Ethics Committee on Animal Use (CUSA/FMVZ-USP) under protocol number 3716290817, and all animals were included in the study after obtaining their owners' consent.
Inclusion and Exclusion Criteria
Canine patients with oral melanoma diagnosed by histopathological and immunohistochemical analyses of amelanotic samples were included. Immunohistochemical antibodies used to confirm melanoma were directed against HMB-45, tyrosinase, and Melan-A present in the melanoma cocktail (Bio SB, Santa Barbara, CA, USA).
Dogs without previous treatment or who had completed previous treatment 1 month earlier were included. Patients with concomitant diseases, such as advanced heart disease, advanced chronic kidney disease, endocrine disorders, infectious diseases, concomitant neoplasms, missing data, or death from melanoma-unrelated causes were excluded (35, 36).
Treatment Strategies, Evaluation, and Disease Assessment
All patients underwent surgical removal of the oral melanoma; the excision was complete in some and incomplete in others. Additionally, ECT was performed in some patients to expand the surgical bed. ECT was applied to the surgical bed immediately after incomplete tumor excision and was performed by trained professionals. It involved the application of 15,000 UI/m2 intravenous bleomycin, followed 5 min later by eight square-wave electric pulses of 1,220 V/cm at an interval of 100 mHz to 1 Hz. The electroporators used by different oncology veterinary services were a Vetpulser BK 100 (Brunner, São Paulo, Brazil), VetCP 125 (Veterinary Cell Porator, São Paulo, Brazil), and ELECTROvet EZ (Leroy Biotech, Saint-Orens-de-Gameville, France).
The World Health Organization classification of tumors in domestic animals was used for clinical staging, and examinations were performed before surgery and every 3 months thereafter, or earlier if necessary (37). The examinations included physical examination, complete blood count, complete serum biochemistry, triple thoracic radiography, abdominal ultrasound examination, and echocardiography. Bone invasion data were unavailable. MC consisted of oral cyclophosphamide 15 mg/m2 daily or every other day.
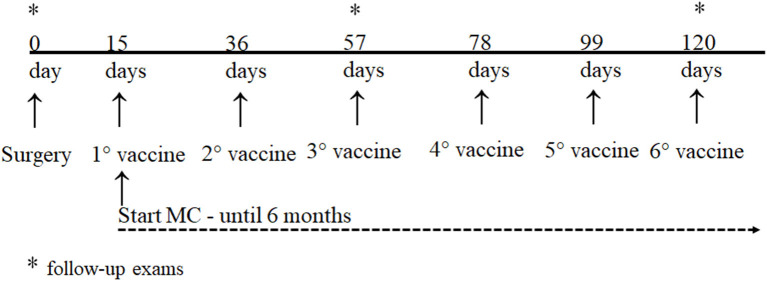
The tumor lysate vaccine with BCG is an autologous vaccine, as it is prepared with the patient's tumor (each dose contained 5 × 106 to 9 × 106 tumor cells). The complete vaccine protocol is presented in the Supplementary Material. The first dose was administered 15 days after surgery, and the following doses were administered at intervals of 21–28 days. A minimum of 3–6 doses is recommended. The vaccine was administered intradermally near the popliteal lymph node (distant from the tumor). Patients undergoing concomitant use of MC started the first dose of the vaccine for a minimum period of 6 months (Figure 1).
Figure 1.
Treatment and follow-up protocol for dogs with oral melanoma.
The primary outcome was PFS, which was defined as the time interval between the surgical removal of the tumor (date of inclusion in the study) and detection of metastasis and/or local recurrence (38). The patients were examined every 21 or 28 days after they were vaccinated, and every 3 months after the end of the vaccine treatment. At the same time, imaging and blood tests were performed. The secondary outcome evaluated was OS, which was defined as the duration in days between the surgical removal of the tumor and death or the last follow-up.
Blood Harvest and Inflammatory Indices Analysis
Blood samples (5 ml) were collected from the cephalic or jugular vein and were stored in EDTA anticoagulant tubes. Absolute red blood cell, leukocyte, and platelet counts were determined using a Poch-100Iv Diff (Sysmex, São Paulo, Brazil) veterinary automatic cell counter, whereas differential leukocyte counts and the morphological analysis of cells were obtained and performed, respectively, by two different and experienced pathologist veterinarians, under a microscope, using a freshly stained Rosenfeld slide.
An average of blood count data was used to calculate the inflammatory indices: NRL (n° neutrophils/n° lymphocytes), PLR (n° platelets/n° lymphocytes), MLR (n° monocytes/n° lymphocytes), and SII (n° platelets × NRL).
Blood was stored in a tube (with or without clot activator), and serum was separated up to 24 h after collection and stored at −80°C until use. LDH (kinetic determination) and albumin (bromocresol green) were measured using an automated device (Chem Well T; Labtest Interteck, Minas Gerais, Brazil) with reagents supplied by the manufacturer. CRP levels were determined using a chemistry immunoassay technique (Catalyst One, Idexx, São Paulo, Brazil). The CRP/ALB ratio was calculated by dividing the CRP by the albumin value.
Peripheral Blood Mononuclear Cells Isolation and Immunophenotyping by Flow Cytometry
The remaining blood used for the inflammatory index was used for PBMC separation using the Ficoll-Paque density gradient centrifugation method (at 900 g for 25 min, break-off, 22 °C; Sigma cat# 15120), and cells obtained after separation were cryopreserved and stored in liquid nitrogen until analysis. PBMCs were thawed at 37°C, and nonspecific antibody binding was blocked by the pretreatment of cells with Fc-block reagent (eBiosciences, Thermo Fisher Scientific, Waltham, MA, USA, mixed by vortexing, and incubated for 15 min at 25°C.
For the frequency of lymphocyte subsets, aliquots of 1 × 105 cells in a final volume of 100 μl were first incubated with an FcR blocking reagent (eBiosciences, Thermo Fisher Scientific, Waltham, MA, USA), vortexed, and incubated for 15 min at 25 °C. Different antibodies were used for the detection of the T cell population, and the following combinations of monoclonal antibodies (MAb) were used: anti-CD3-FITC/anti-CD4-APC/anti-CD8-Alexa Fluor 647 (cat #TC014 Bio-Rad, Hercules, CA, USA; CA clone 17.2a12; clone YKIX 302.9; and clone YCATE 55.9, respectively) for CD4 and CD8 T lymphocyte labeling, and a combination of anti-CD4-APC antibody (cat# MCA1038GA Bio-Rad; clone YKIX 302.9), anti-CD25-FITC (cat# MCA5916 Bio-Rad, clone BC96), and anti-Foxp3-RPE (cat# 72-5774-40 eBiosciences, Thermo Fisher Scientific, clone 236A/E7) antibody for Treg lymphocytes detection. Incubation was performed for 30 min at 4°C and protected from light. For lymphocyte staining, after following the surface staining protocol with CD4 and CD25 antibodies, lymphocytes cells were incubated with fixation/permeabilization buffer (cat# 00-5123-43 eBioscience, Thermo Fisher Scientific) for 20 min at 4°C in the dark. After fixation, cells were washed with 2 ml of permeabilization buffer, and the anti-Foxp3 antibody was added (1:50) in 100 μl of permeabilization buffer to stain intracellular markers, incubated for 20 min at 25°C, and protected from light. After incubation, cells were washed twice with 2 ml of permeabilization buffer and then washed once with 1 ml of phosphate-buffered saline containing 2% fetal calf serum (cat# ES-009-B, Merck).
MDSCs represent a heterogeneous cell population consisting of two major subsets: monocytic (M) and granulocytic (PMN) MDSCs, which are identified by a combination of multiple lineage markers. Another aliquot of PBMCs was thawed and diluted to 1 × 105 cells in a final volume of 100 μl and was incubated with Fc blocking as previously described. The (M)-MDSCs population was identified as CD11b+CD14-MHCII-, while the (PMN)-MDSCs were CD11b+CD14+MHCII- (PE/Cy5 anti-mouse CD11b, cat# MCA177S Abcam, Waltham, MA, USA, clone CA16.3E10), CD14 (mouse anti-human CD14 Alexa Fluor 647, cat# MCA1568A647 Bio-Rad/, and MHCII (rat anti-dog MHC CLASS II FITC, cat# MCA1044F Bio-Rad, clone YKIX334.2). After washing, all sets of cells were fixed with phosphate-buffered saline containing 1% paraformaldehyde, acquired using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and analyzed for cell surface phenotypes using FlowJo software (Tree Star, Bioz, Los Altos, CA, USA). For each sample, multiparametric data were acquired for 10,000 events. The percentage of MDSCs was calculated based on the percentage of cells labeled within the overall PBMC population. Appropriate isotype control was also used (data not shown). During data acquisition, a gate was drawn to exclude debris cells and lymphocytes, and myeloid cells were identified based on the cell characteristic properties in the forward (FSC) and side (SSC) scatter.
Statistical Analysis
The log-rank test was used to compare PFS and OS between dogs that received only the tumor lysate vaccine and those that received the lysate vaccine in combination with MC. Cox regression was performed to calculate the hazard ratio (HR) with a 95% confidence interval (CI) for clinical stage, inflammatory indices (NRL, MLR, PLR, SII, and CRP/ALB ratio), LDH dosage, and the frequency of circulating cells (lymphocytes CD4+, CD8+, CD4/CD8 ratio, Tregs, (M)-MDSCs, and (PMN)-MDSCs) to select variables for inclusion in multivariate analysis. Consequently, another Cox regression analysis with the selected variables was performed to generate multiple models. The CRP/ALB ratio was used to construct a receiver operating characteristic (ROC) curve and establish a cut-off value that was then submitted to the log-rank test to compare PFS and OS between groups. Statistical analyses were performed with SPSS version 25.0 (IBM Corp., Armonk, NY, USA), and significance was set at 5%.
Results
Of the 25 included patients, 16 (64%) were male and 9 (36%) were female, with a mean age of 13 years (standard deviation, 2.6). The most affected breeds were mixed breeds [8 (32%)], Dachshund [4 (16%)], Pinscher [2 (8%)], Golden Retriever [2 (8%)], Poodle [1 (4%)], Cocker Spaniel [1 (4%)], Yorkshire [1 (4%)], Rottweiler [1 (4%)], Schnauzer [1 (4%)], Boxer [1 (4%)], Shih Tzu [1 (4%)], Chow Chow [1 (4%)], and Beagle [1 (4%)]. Regarding clinical staging, 7 (28%), 14 (56%), and 4 (16%) patients were diagnosed with stage II, III, and IV cancers, respectively; all stage IV cancer patients had lung metastasis. Of the 18 patients with stage III and IV cancer, 12 (67%) showed lymph node metastasis at the time of inclusion. Furthermore, 21 (84%) and 4 (16%) patients had melanotic and amelanotic melanomas, respectively.
Complete and incomplete surgical excision of the tumor was performed in six (24%) and 19 (76%) patients, respectively. ECT was performed in 18 (72%) patients immediately after surgical excision and was not performed in seven (28%) patients. Furthermore, nine (36%) patients received MC.
First, a log-rank test was conducted to evaluate patients who received MC in addition to the vaccine for PFS and OS. There were no statistically significant differences between the two groups in terms of PFS (p = 0.294) or OS (p = 0.553). The median, minimum, and maximum values of inflammatory indices, LDH level, CRP, albumin, and frequency of circulating cells according to the clinical stage are summarized in Table 2.
Table 2.
Median, minimum, and maximum value of inflammatory indices (NRL, PLR, MLR, SII, and CRP/ALB ratio), CRP, albumin, LDH, and frequency of circulating cells (lymphocytes CD4+, CD8+, CD4/CD8 ratio, Tregs, (M)-MDSCs, and (PMN)-MDSCs) according to clinical stage.
| Clinical stage | All dogs (n = 25) | II (n = 7) | III (n = 14) | IV (n = 4) |
|---|---|---|---|---|
| NRL | 5.3 (1.6–20.3) | 5 (1.6–6.2) | 5.1 (1.7–20.3) | 9.3 (5.1–12.8) |
| PLR | 250 (67–581) | 267 (136–481) | 249 (66–581) | 254 (93–467) |
| MLR | 0.3 (0.1–1.0) | 0.3 (0.1–0.7) | 0.3 (0.1–0.7) | 0.5 (0.2–1.0) |
| SII (× 109/L) | 2,430 (488–7,117) | 1,960 (1,096–4,648) | 2,160 (488–7,117) | 5,438 (3,164–6,284) |
| CRP (mg/L) | 18 (0.4–86) | 7 (0.5–65) | 18 (3–62) | 40 (2–8.6) |
| Albumin (g/dl) | 3.1 (1.8–4.9) | 3 (2.7–4.9) | 3.1 (2.2–3.8) | 3.2 (1.8–3.8) |
| CRP/ALB ratio | 5.3 (0.1–42.2) | 2.1 (0.1–24.1) | 5.5 (0.9–21.4) | 14 (0.6–42.2) |
| LDH (U/L) | 762 (95–2,982) | 666 (514–2,982) | 865 (95–2,273) | 791 (400–1,582) |
| CD4 (%) | 43.5 (15–65) | 43.5 (15–57) | 45.7 (29.5–65) | 37.5 (29–47) |
| CD8 (%) | 26.7 (6–45) | 28.7 (6–45) | 28 (10–44) | 21.5 (14–26.7) |
| CD4/CD8 ratio | 1.5 (0.34–9.17) | 1.2 (0.3–9.1) | 1.5 (1.0–5.4) | 0.3 (1.4–2.7) |
| T reg (%) | 2.7 (0.3–9.7) | 2.7 (0.3–3) | 3 (0.8–9.7) | 3 (0.5–4) |
| (M)-MDSCs (%) | 10.8 (2–29.2) | 10.7 (4.8–29.2) | 14.9 (8–28) | 5.5 (2–25) |
| (PMN)-MDSCs (%) | 12 (3.9–34) | 7.5 (3.9–34) | 12.2 (4–34) | 12.5 (9–19) |
| Total MDSCs (%) | 22.8 (2–34) | 18.2 (3.9–34) | 27.1 (4–34) | 18 (2–25) |
NRL, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; MLR, monocyte–lymphocyte ratio; SII, systemic immune–inflammation index; CRP, C-reactive protein; CRP/ALB ratio, C-reactive protein–albumin ratio; LDH, lactate dehydrogenase; Treg, T regulatory cells; (M)-MDSCs, monocytic myeloid-derived suppressors cells; (PMN)-MDSCs, granulocytic myeloid-derived suppressors cells.
Univariate analysis (Cox regression model) was performed for each variable in relation to PFS and OS. In addition to the previously noted variables, the clinical stage and type of surgery performed (complete or incomplete) were identified, which have previously been recognized as prognostic factors in dogs with oral melanoma (Table 3).
Table 3.
Effect of clinical stage, surgical margins, adjuvant treatment (tumor lysate vaccine alone or plus MC), inflammatory indices (NRL, PLR, MLR, SII, and CRP/ALB ratio), albumin, LDH, and frequency of circulating cells (lymphocytes CD4, CD8, CD4/CD8 ratio, Tregs, (M)-MDSCs, and (PMN)-MDSCs) on the PFS and OS of 25 canine patients with oral melanoma.
| Variable | PFSa | HR (95% CI) | p–value | OSa | HR (95% CI) | p–value |
|---|---|---|---|---|---|---|
| All dogs (n = 25) | 68 | 115 | ||||
| Stage | ||||||
| II (n = 7) | 181 | 1 | 0.015* | 265 | 1 | 0.043* |
| III (n = 14) | 40 | 4.0 (1.4–11.6) | 0.011* | 101 | 2.9 (0.9–8.4) | 0.051* |
| IV (n = 4) | 38 | 7.1 (1.7–30.3) | 0.008* | 68 | 5.8 (1.4–24.4) | 0.015* |
| Surgery | ||||||
| Incomplete (n = 19) | 56 | 1 | 94 | 1 | ||
| Complete (n = 6) | 121 | 1.6 (0.6–4.4) | 0.346 | 153 | 1.3 (0.5–3.5) | 0.635 |
| Adjuvant | ||||||
| LyVaccine + MC (n = 9) | 56 | 1 | 115 | 1 | ||
| LyVaccine only (n = 16) | 78 | 1.6 (0.7–3.7) | 0.298 | 134 | 1.4 (0.6–3.3) | 0.459 |
| NRL | 1.0 (0.9–1.1) | 0.333 | 1.0 (0.9–1.1) | 0.369 | ||
| PLR | 0.9 (0.9–1.0) | 0.412 | 0.9 (0.9–1.0) | 0.621 | ||
| MLR | 2.6 (0.4–17.9) | 0.329 | 4.4 (0.6–30.4) | 0.127 | ||
| SII | 1.0 (1.0–1.0) | 0.365 | 1.0 (1.0–1.0) | 0.301 | ||
| CRP | 1.0 (0.99–1.0 | 0.064 | 1.0 (0.9–1.0) | 0.094 | ||
| Albumin | 0.5 (0.2–1.1) | 0.072 | 0.4 (0.1–1.0) | 0.058 | ||
| CRP/ALB ratio | 1.0 (1.0–1.1) | 0.053* | 1.0 (0.9–1.1) | 0.060 | ||
| LDH | 1.0 (0.9–1.0) | 0.138 | 1.0 (0.9–1.0) | 0.249 | ||
| CD4 | 0.9 (0.9–1.0) | 0.248 | 0.9 (0.9–1.0) | 0.153 | ||
| CD8 | 1.0 (0.9–1.0) | 0.846 | 1.1 (0.9–1.0) | 0.596 | ||
| CD4/CD8 ratio | 0.9 (0.7–1.2) | 0.580 | 0.9 (0.7–1.2) | 0.441 | ||
| Treg | 1.5 (1.2–1.9) | 0.001* | 1.4 (1.1–1.7) | 0.001* | ||
| (M)–MDSCs | 1.0 (0.9–1.1) | 0.311 | 1.0 (0.9–1.1) | 0.490 | ||
| (PMN)–MDSCs | 1.0 (0.9–1.1) | 0.666 | 0.9 (0.9–1.0) | 0.793 |
aMedian in days; *Statistical significance.
95%CI, 95% confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression–free survival; LyVaccine, lysate vaccine plus BCG; MC, metronomic chemotherapy; NRL, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; MLR, monocyte–lymphocyte ratio; SII, systemic immune-inflammation index; CRP, C-reactive-protein; CRP/ALB ratio, C-reactive-protein-albumin ratio; LDH, lactate dehydrogenase; Treg, T regulatory cells; (M)-MDSCs, monocytic myeloid-derived suppressors cells; (PMN)- MDSCs, granulocytic myeloid-derived suppressors cells.
Based on the results of the univariate analysis, the clinical stage, CRP/ALB ratio, and Treg frequency showed a statistically significant difference in relation to PFS and OS and were selected for multivariable analysis (Table 4).
Table 4.
Effect of clinical stage, surgical margins, inflammatory indices (NRL, PLR, MLR, SII, and CRP/ALB ratio), albumin, LDH, and frequency of circulating cells (lymphocytes CD4, CD8, CD4/CD8 ratio, Tregs, (M)-MDSCs, and (PMN)-MDSCs) on the PFS and OS of 25 canine patients with oral melanoma.
| Variable | PFSa | HR (95% CI) | p–value | OSa | HR (95% CI) | p–value |
|---|---|---|---|---|---|---|
| All dogs | 68 | 115 | ||||
| Stage | ||||||
| II | 181 | 1 | 0.010* | 265 | 1 | 0.015* |
| III | 40 | 4.0 (1.3–12.6) | 0.017* | 101 | 3.1 (0.9–10.1) | 0.054* |
| IV | 38 | 10.2 (2.1–50.5) | 0.004* | 68 | 12.2 (2.2–67.5) | 0.004* |
| CRP/ALB ratio | 1.1 (1.0–1.1) | 0.017* | 1.0 (1.0–1.1) | 0.023* | ||
| Treg | 1.6 (1.2–2.1) | 0.001* | 1.6 (1.2–2.1) | 0.001* |
aMedian in days; *Statistical significance.
95%CI, 95% confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; CRP/ALB ratio, C-reactive protein/Albumin ratio; Treg, T regulatory cells.
After the multivariable analysis showed a statistically significant difference in clinical staging, the CRP/ALB ratio and Treg frequency were subjected to ROC curve analysis, in relation to PFS and OS. Analysis of the ROC curve for Treg showed an AUC of 0.424 for PFS and 0.523 for OS; thus, it was not possible to establish a cut-off point. The analysis of the ROC curve for the CRP/ALB ratio showed an AUC of 0.891 for PFS and 0.886 for OS, and a cut-off score of 1.9 was selected. This cut-off score had a sensitivity of 78.3% and 81.8%, a specificity of 100% and 100% for predicting PFS and OS, respectively.
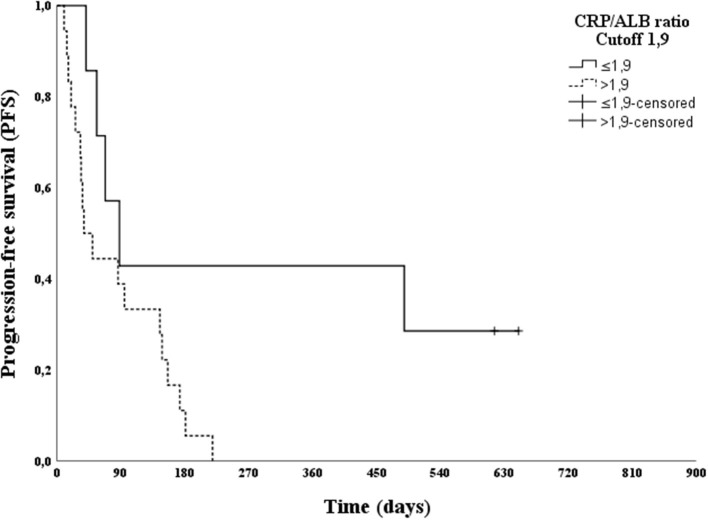
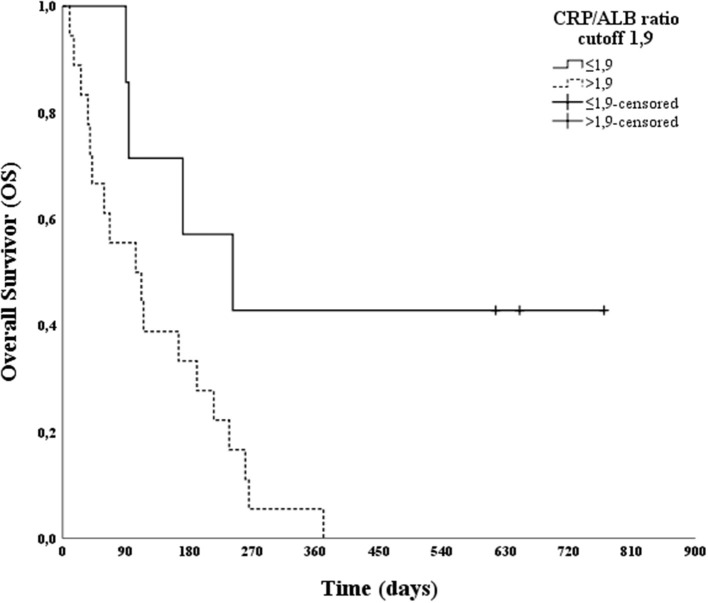
The effect of a CRP/ALB ratio cut-off of 1.9 on PFS and OS was evaluated using a log-rank test. A statistically significant difference was found between patients with CRP/ALB ratios greater than or less than 1.9 (p = 0.036 for PFS and p = 0.023 for OS). Moreover, Kaplan–Meier curves were plotted (Figures 2, 3).
Figure 2.
Kaplan–Meier progression-free survival (PFS) curve according to the C-reactive protein/Albumin (CRP/ALB) ratio cut-off point (1.9) in 25 canine patients with oral melanoma.
Figure 3.
Kaplan–Meier overall survival (OS) curve according to the C-reactive protein/Albumin (CRP/ALB) ratio cut-off point (1.9) in 25 canine patients with oral melanoma.
After treatment, 23 of the 25 patients experienced tumor recurrence, with 14 (61%) having recurrence at the tumor site and nine (39%) having metastases. Of the nine patients with metastasis, 6 (67%) had metastases to the lungs, while three (33%) had metastases to the regional lymph nodes.
Discussion
The tumor microenvironment is characterized by persistent inflammation. In patients with solid tumors, inflammatory indices, LDH, CRP, albumin, and the frequency of immune circulating cells (lymphocytes and myeloid subsets) are inflammation-based prognostic scoring systems that reflect cell-mediated immunity and systemic inflammation (39). However, the utility of these reliable markers of the immune profile and systemic inflammation in preoperative evaluation for estimating prognosis and assessing combinatorial therapy outcomes in dogs with oral melanoma has not been verified. In the present study, all of these factors were subjected to univariate analyses, which revealed that the CRP/ALB ratio and Treg lymphocyte frequency were associated with PFS and OS. Therefore, these were added to a second, multivariable analysis, which showed that, in addition to the clinical stage, the CRP/ALB ratio and Treg frequency were associated with PFS and OS. The ROC curve showed that both parameters were accurate; however, only the CRP/ALB ratio showed an adequate AUC that made it possible to establish a cut-off value. Although the sensitivity was equal for different specificities, the derived cut-off point of 1.9 was the lowest value capable of distinguishing between patients with better and worse prognoses.
Our results showed that patients with CRP/ALB > 1.9 before tumor removal had shorter PFS and OS. Therefore, even with the small number of patients and the heterogeneity of our study group, the results showed that the CRP/ALB ratio is a promising prognostic tool for clinical staging in dogs with oral melanoma. In addition to being easily applicable in veterinary clinical routine, the levels of CRP and albumin are easily accessible outside the scope of research. In human medicine, several studies have recently shown the prognostic potential of the CRP/ALB ratio as a prognostic factor in cancer patients with different types of solid tumors (14, 16, 17, 19). However, in veterinary medicine, only one study has evaluated the importance of high values of this ratio in terms of prognosis in dogs with acute pancreatitis (40). The CRP/ALB ratio effectively reflects the patient's inflammatory status, as it assesses the increase in CRP (a positive acute-phase protein) and the concomitant decrease in albumin (a negative acute-phase protein).
Peripheral blood is a non-invasive source for exploring potential biomarkers. The other NLR, PLR, MLR, and SII indices showed no association with PFS and OS. The number of patients and group heterogeneity probably influenced these results, because, in human cancer patients, high values of these indices were associated with a lower survival rate and a lower treatment response rate in several types of tumors. In human patients, NRL ranges from 2 to 5 for different types of tumors, while in veterinary medicine, the NRL in patients with high-grade mast cell tumors, high-grade sarcoma, and oral cavity neoplasms was > 5.6, > 4.5, and > 8.56, respectively (24–26). The median NRL in the study group was 5.3, which is close to the NRL of dogs with mast cell tumors and high-grade sarcomas, and slightly lower than that of dogs with tumors in the oral cavity. The NRL of 5.3 was higher than that reported in the study by Rejec et al., which reported that healthy dogs and dogs with periodontal disease had an NRL of 2.7 and 4, respectively, and showed an increasing trend in more advanced stage patients, as the median NRL in patients with stages II, III, and IV was 5, 5.1, and 9.3, respectively (26). Rejec et al. also found a PLR of 145 and 290 in healthy dogs and those with oral cavity neoplasia, respectively. Even though the difference did not reach statistical significance, there was a tendency for PLR to be increased in dogs with cancer, a result similar to that of our study, which found a PLR of 250 (26). MLR and SII have not been studied in dogs, although high values have been associated with a worse prognosis in human patients with cancer (15). An important issue that was not evaluated in the present study is the influence of age, periodontal disease, and obesity on these inflammatory indices. Patients with obesity or those with concomitant periodontal disease were not excluded, and the median age of the patients in the group was 13 years. This study aimed to evaluate these indices considering their clinical applicability, since real-life patients with oral melanoma are older, obese, and have some degree of periodontal disease.
Measurement of LDH is part of the clinical staging of cancer in human patients with cutaneous melanoma. In particular, LDH levels are high among those with stage IV disease and who have a shorter OS (31). In our study, we found no evidence of association of LDH with PFS and OS, and the median LDH level was 762 IU/L. This value was higher than that reported by other studies, in which dogs with neoplasms had higher LDH levels than did healthy dogs. Marconato et al. studied dogs with different types of neoplasia and reported that the level of LDH was 341 UI/L in dogs with cancer, as compared to 142 UI/L in healthy dogs. Campos et al. studied dogs with mammary tumors and reported that the median LDH level was 414 UI/L in dogs with tumors, as compared to 201 UI/L in healthy dogs (33, 34). Zanatta et al. found a median LDH of 342 UI/L in healthy dogs and a value between 893 and 1,433 UI/L in dogs with multicentric lymphoma at different stages of therapy (32).
We also evaluated the frequency of circulating CD4+, CD8+, the CD4/CD8 ratio, Tregs, (M)-MDSCs, and (PMN)-MDSCs. There was no correlation of PFS and OS with the frequency of CD4+, CD8+, the CD4/CD8 ratio, and MDSCs. The frequency of CD4+ lymphocytes in dogs with melanoma in the present study was 43%, which was consistent with the prevalence in published studies of dogs with various types of cancer, in that the frequency of CD4+ ranged from 32 to 51% and did not differ markedly from the frequency in healthy dogs in these studies, which ranged from 35 to 38% (27–30). The same trend was observed with the frequency of CD8+ lymphocytes, which was 26% in our study (within the values found for cancer patients, which varied from 13 to 31%), but was not markedly different from the values found for healthy dogs, which varied between 20 and 26% in other studies (27–30). In our study, the CD4/CD8 ratio found was 1.5 in dogs with melanoma, close to the value of 1.6 found by Estrela-Lima et al. in dogs with metastatic malignant breast cancer and that of 1.43 found by Mucha et al. in dogs with various neoplasms (29, 30).
A higher frequency of Treg lymphocytes was associated with worse PFS and OS. Other studies have shown a trend toward an increase in Treg lymphocytes in dogs with different neoplasms and a more accentuated increase in dogs with metastatic disease (41–43). Tominaga et al. studied dogs with oral melanoma and found a 10% higher frequency of circulating Tregs than the 2.7% value found in our study (43). Other studies in dogs with different types of neoplasms found that Treg lymphocyte frequencies ranged from 5.2 to 13% in dogs with neoplasms and from 0.64 to 4.3% in healthy dogs. The differences across studies can be explained by the different methodologies used and the low number of patients. There may be a need for studies with a larger number of healthy dogs and dogs with cancer to establish a reliable reference value. Despite the increased frequency of Treg lymphocytes in our study, which may be used as a prognostic tool in future, this parameter is not yet accessible in clinical practice, but is used in research.
We found a total MDSC frequency of 22% in patients with melanoma, which is consistent with the findings of other studies that used similar methodologies to evaluate MSDCs, although there was no correlation of MDSC with PFS and OS (30, 44). Goulart et al. found a frequency of 36% in dogs with malignant neoplasms with metastases and 10% in healthy dogs (44). Mucha et al. reported an MDSC frequency of 18% in dogs with neoplasms with metastases, and 0.25% in healthy dogs (30). Other studies that characterized the subpopulations of (M)-MDSCs and (PMN)-MDSCs used different methodologies, such as different antibodies and types of samples, including whole blood or PMBCs, and found different proportions of these cell populations in the peripheral blood of dogs with cancer (45, 46). As there are no standardized methodologies, we cannot compare the findings of the current study with those of previously published studies. Our study showed that the frequency of the MSDC population found in dogs with oral melanoma was higher than that found in healthy dogs, following the same trend seen in human patients with cancer, who have a high frequency of these immunosuppressive cells in the peripheral blood (20, 30, 44).
In this study, we also sought to assess MC and/or immune lysate vaccine plus BCG could improve PFS and OS, but no statistically significant differences were observed between the groups with or without MC added to the tumor lysate vaccine. In addition to its antiangiogenic effect, MC has an immunomodulatory effect by reducing local and circulating immunosuppressive cells, such as Tregs, (M)-MDSCs, and (PMN)-MDSCs (47, 48). This effect was not observed in the study group, and we believe that the sample size studied was small, especially in the more advanced stage patients (only four patients with stage IV). Furthermore, the increased frequency of Tregs in our study showed that the immune profile of our patients reflected dysfunction or progressive loss of function of T cells in the tumor microenvironment.
We emphasize that in our clinical routine, most dogs diagnosed with oral melanoma were in advanced stages of the disease, given that this condition is typically diagnosed late. Another important consideration for the patients included in the study was the type of surgery performed, as most patients underwent surgery in which the tumor was not completely removed. This is due to the refusal of owners to perform major or mutilating surgeries on older dogs and refusal of using conventional chemotherapy due to the higher incidence of side effects. These patients were included in the study to receive the tumor lysate vaccine plus BCG, with or without MC, which has a lower incidence of side effects. In Brazil, electrochemotherapy has been widely used to eliminate tumor cells in the surgical bed. Radiotherapy is another modality available for local melanoma control; however, it is rarely used in Brazil due to the high cost of the procedure. These characteristics of our study group, i.e., local control of the disease (incomplete surgery with electrochemotherapy) and advanced stage (no patients in stage I and 56% patients in stage III), explain the shorter PFS and OS time in our study than in other studies conducted among dogs with oral melanoma. In our study, the median PFS and OS were 181 and 265 days for stage II, 40 and 101 days for stage III, and 38 and 68 days for stage IV, respectively, which are lower than those of other studies that only performed surgery in patients and obtained OS times of 511–874 days for stage I; 160–818 days for stage II, and 160–818 days for stage III disease (49, 50).
Studies in dogs with oral melanoma have determined that the type of surgery performed, and clinical stage are prognostic factors, since surgeries with wide surgical margins and patients with less advanced stages have a longer OS (50). However, there were no statistically significant differences in the type of surgery performed, as most patients (76%) underwent incomplete surgery, explaining the large local recurrence rate of 61%. There was a statistically significant difference in PFS and OS according to disease stage: 181 and 265 days for stage II, 40 and 101 days for stage III, and 38 and 68 days for stage IV. In the multivariable analysis, the clinical staging was added to the CRP/ALB ratio and Treg frequency as an adjustment as a well-established prognostic factor (5, 37, 50).
Surgery remains a key therapy in veterinary medical oncology for canine oral melanoma; however, new approaches have been proposed. The search for combinatorial therapies, such as debulking the tumor mass (complete or incomplete surgery), treatments with antineovasculogenic effects (MC), and potentiated immune responses (cancer vaccine strategies), expands the potential for more effective therapy for canine oral melanoma, a neoplasm of which the growth and invasion is difficult to control.
In conclusion, we did not find a better response to the vaccine in canine oral melanoma patients who received MC. In addition, we studied several inflammatory indices and the frequency of circulating cells to evaluate the inflammatory and immunological status of dogs with oral melanoma to predict the response to treatment and prognosis. The purpose of obtaining this information was to improve treatment planning. In addition, some of these parameters can be easily applied in routine clinical practice. The most promising of these are the CRP/ALB ratio and Tregs frequency. Nevertheless, the study had limitations due to the low number of animals and heterogeneity of the group in terms of clinical staging and forms of local control.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Ethics Committee on Animal Use (CEUA/FMVZ-USP) #3716290817. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
JSG divulgated the study, received samples, and vaccine production, trained veterinarians, collected and compiled clinical data, performed blood analyses, analyzed the results, and wrote and edited the manuscript. VN received samples, vaccine production, and performed blood analyses. RL curated the data, performed the statistical analysis, and reviewed the results. CM supervised the study and acted as the study coordinator, acquired funding, and wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), process n° 2020/01004-3, and PhD scholarship granted by Brazilian Funding Institution CAPES.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the clinical veterinarians who provided the samples and collected data for this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.888411/full#supplementary-material
References
- 1.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab treated metastatic melanoma patients. Br J Cancer. (2015) 112:1904–10. 10.1038/bjc.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martens A, Wistuba-Hamprecht K, Yuan J, Postow MA, Wong P, Capone M, et al. Increases in absolute lymphocytes and circulating CD4 and CD8 T cells are associated with positive clinical outcome of melanoma patients treated with Ipilimumab. Clin Cancer Res. (2016) 22:4848–58. 10.1158/1078-0432.CCR-16-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Wu X, Yu H, Li S, Mao L, Chi Z, et al. Systemic immune-inflammation index and circulating t-cell immune index predict outcomes in high-risk acral melanoma patients treated with high-dose interferon. Transl Oncol. (2017) 10:719–25. 10.1016/j.tranon.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SH, Goldschmidt MH, Macmanus PM. A comparative review of melanocytic neoplasms. Vet Pathol. (2002) 39:651–78. 10.1354/vp.39-6-651 [DOI] [PubMed] [Google Scholar]
- 5.Splanger WL, Kass PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. (2006) 43:136–49. 10.1354/vp.43-2-136 [DOI] [PubMed] [Google Scholar]
- 6.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. (2017) 547:222–6. 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- 7.Laucius JF, Bodurtha AJ, Mastrangelo JM, Bellet RE. A phase II study of autologous irradiated tumor cells plus BCG in patients with metastatic malignant melanoma. Cancer. (1977) 40:2091–3. [DOI] [PubMed] [Google Scholar]
- 8.Faries MB, Mozzillo N, Kashani-Sabet M, Thompson JF, Kelley MC, DeConti RC. Long-term survival after complete surgical resection and adjuvant immunotherapy for distant melanoma metastases. Ann Surg Oncol. (2017) 24:3991–4000. 10.1245/s10434-017-6072-3 [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 11.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. (2009) 12:223–6. 10.1097/MCO.0b013e32832a7902 [DOI] [PubMed] [Google Scholar]
- 12.Guthrie GJK, Charles KA, Roxburgh CSD, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. (2013) 88:218–30. 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 13.Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. (2017) 116:134–46. 10.1016/j.critrevonc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Gao N, Yang R, Meng Z, Wang W. The prognostic value of C-reactive protein/albumin ratio in nasopharyngeal carcinoma: a meta-analysis. Biosci Rep. (2018) 38:BSR20180686. 10.1042/BSR20180686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. (2019) 9:3284. 10.1038/s41598-019-39150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto T, Fujitani M, Fukuyama H, Hatanaka S, Koizumi Y, Kawabata A. The C-reactive protein/albumin ratio is useful for predicting short-term survival in cancer and noncancer patients. J Palliat Med. (2019) 22:532–7. 10.1089/jpm.2018.0404 [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Li X. C-reactive protein to albumin ratio in colorectal cancer: a meta-analysis of prognostic value. Dose Response. (2019) 17:1559325819889814. 10.1177/1559325819889814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Li W, Wang D, Wu B. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surg Oncol. (2020) 18:273. 10.1186/s12957-020-02048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zang Y, Fan Y, Gao Z. Pretreatment C-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: a meta-analysis. Medicine. (2020) 99:e20595. 10.1097/MD.0000000000020595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. (2012) 35:107–15. 10.1097/CJI.0b013e318242169f [DOI] [PubMed] [Google Scholar]
- 21.Idorn M, Køllgaard T, Kongsted P, Sengeløv L, Straten PT. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. (2014) 63:1177–87. 10.1007/s00262-014-1591-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isherwood J, Arshad A, Chung WY, Runau F, Cooke J, Pollard C, et al. Myeloid derived suppressor cells are reduced and T regulatory cells stabilised in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega 3. Ann Transl Med. (2020) 8:172. 10.21037/atm.2020.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutz M, Boudreaux B, Kearney M, Stroda K, Gaunt S, Shiomitsu K. Prognostic value of baseline absolute lymphocyte concentration and neutrophil/lymphocyte ratio in dogs with newly diagnosed multi-centric lymphoma. Vet Comp Oncol. (2013) 13:337–7. 10.1111/vco.12045 [DOI] [PubMed] [Google Scholar]
- 24.Macfarlane MJ, Macfarlane LL, Scase T, Parkin T, Morris JS. Use of neutrophil to lymphocyte ratio for predicting histopathological grade of canine mast cell tumours. Vet Rec. (2016) 179:491–6. 10.1136/vr.103650 [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane L, Morris J, Pratschke K, Mellor D, Scase T, Macfarlane M, et al. Diagnostic value of neutrophil–lymphocyte and albumin–globulin ratios in canine soft tissue sarcoma. J Small Anim Pract. (2016) 57:135–41. 10.1111/jsap.12435 [DOI] [PubMed] [Google Scholar]
- 26.Rejec A, Butinar J, Gawoe J, Petelin M. Evaluation of complete blood count índices (NRL, PLR, MPV/PLT, and PLCRi) in healthy dogs, dogs with periodontitis, and dogs with oropharyngeal tumors as potential biomarkers of systemic inflammatory response. J Vet Dent. (2017) 34:231–40. 10.1177/0898756417731775 [DOI] [PubMed] [Google Scholar]
- 27.O'Neill K, Guth A, Biller B, Elmslie R, Dow S. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med. (2009) 23:875–81. 10.1111/j.1939-1676.2009.0333.x [DOI] [PubMed] [Google Scholar]
- 28.Itoh I, Horiuchi Y, Nagasaki T, Sakonju I, Kakuta T, Fukushima U, et al. Evaluation of immunological status in tumor-bearing dogs. Vet Immunol Immunopathol. (2009) 132:85–90. 10.1016/j.vetimm.2009.04.020 [DOI] [PubMed] [Google Scholar]
- 29.Estrela-lima A, Araújo MSS, Costa-Neto JM, Ribeiro LGR, Damasceno KA, D'Assis MJMH, et al. Understanding of the immunological heterogeneity of canine mammary carcinomas to provide immunophenotypic features of circulating leukocytes as clinically relevant prognostic biomarker. Breast Cancer Res Treat. (2012) 131:751–63. 10.1007/s10549-011-1452-z [DOI] [PubMed] [Google Scholar]
- 30.Mucha J, Rybicka A, Dolka I, Szymańska J, Manuali E, Parzeniecka-Jaworska M, et al. Immunosuppression in dogs during mammary cancer development. Vet Pathol. (2016) 53:1147–53. 10.1177/0300985816634808 [DOI] [PubMed] [Google Scholar]
- 31.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. (2009) 27:6199–206. 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanatta R, Abate O, D'Angelo A, Miniscalco B, Mannelli A. Diagnostic and prognostic value of serum lactate dehydrogenase (LDH) and LDH isoenzymes in canine lymphoma. Vet Res Commun. (2003) 27(Suppl 1):449–52. 10.1023/B:VERC.0000014201.82393.67 [DOI] [PubMed] [Google Scholar]
- 33.Marconato L, Crispino G, Finotello R, Mazzotti S, Salerni F, Zini E. Serum lactate dehydrogenase activity in canine malignancies. Vet Comp Oncol. (2009) 7:236–43. 10.1111/j.1476-5829.2009.00196.x [DOI] [PubMed] [Google Scholar]
- 34.Campos LC, Lavalle GE, Estrela-Lima A, Melgaço de Faria JC, Guimarães JE, Dutra AP. CA153, CEA, and LDH in dogs with malignant mammary tumors. J Vet Intern Med. (2012) 26:1383–8. 10.1111/j.1939-1676.2012.01014.x [DOI] [PubMed] [Google Scholar]
- 35.Atkins CJ, Bonagura J, Ettinger S, Fox P, Gordon S, Haggstrom J, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. (2009) 33:1127–40. [DOI] [PubMed] [Google Scholar]
- 36.International Renal Interest Society . IRIS Staging of CKD (modified 2019). Available online at: http://www.iris-kidney.com/guidelines/staging.html (accessed January 25, 2022).
- 37.Owen LN. TNM Classification of Tumours in Domestic Animals. Geneva: World Health Organization; (1980). p. 16–20. [Google Scholar]
- 38.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v10): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. (2015) 13:176–83. 10.1111/vco.12032 [DOI] [PubMed] [Google Scholar]
- 39.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 40.Gori E, Pierini A, Lippi I, Ceccherini G, Perondi F, Marchetti V. Evaluation of C-reactive protein/albumin ratio and its relationship with survival in dogs with acute pancreatitis. N Z Vet J. (2020) 68:345–8. 10.1080/00480169.2020.1780995 [DOI] [PubMed] [Google Scholar]
- 41.Biller BJ, Elmslie RE, Burnett R C, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. (2007) 116:69–78. 10.1016/j.vetimm.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 42.Horiuchi Y, Tominaga M, Ichikawa M, Yamashita M, Jikumaru Y, Nariai Y, et al. Increase of regulatory T cells in the peripheral blood of dogs with metastatic tumors. Microbiol Immunol. (2009) 53:468–74. 10.1111/j.1348-0421.2009.00144.x [DOI] [PubMed] [Google Scholar]
- 43.Tominaga M, Horiuchi Y, Ichikawa M, Yamashita M, Okano K, Jikumaru Y. Flow cytometric analysis of peripheral blood and tumor-infiltrating regulatory T cells in dogs with oral malignant melanoma. J Vet Diagn Invest. (2010) 22:438–41. 10.1177/104063871002200317 [DOI] [PubMed] [Google Scholar]
- 44.Goulart MR, Pluhar GE, Ohlfest JR. Identification of myeloid derived suppressor cells in dogs with naturally occurring cancer. PLoS ONE. (2012) 7:e33274. 10.1371/journal.pone.0033274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutchison S, Sahay B, Ch de Mello S, Sayour EJ, Lejeune A, Szivek A, et al. Characterization of myeloid-derived suppressor cells and cytokines GM-CSF, IL-10 and MCP-1 in dogs with malignant melanoma receiving a GD3-based immunotherapy. Vet Immunol Immunopathol. (2019) 216:109912. 10.1016/j.vetimm.2019.109912 [DOI] [PubMed] [Google Scholar]
- 46.Goulart MR, Hlavaty SI, Chang Y, Polton G, Stell A, Perry J, et al. Phenotypic and transcriptomic characterization of canine myeloid-derived suppressor cells. Sci Rep. (2019) 9:3574. 10.1038/s41598-019-40285-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutsaers AJ. Chemotherapy: new uses for old drugs. Vet Clin North Am Small Anim Pract. (2007) 37:1079–90. 10.1016/j.cvsm.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 48.Gaspar TB, Henriques J, Marconato L, Queiroga FL. The use of low-dose metronomic chemotherapy in dogs-insight into a modern cancer field. Vet Comp Oncol. (2018) 16:2–11. 10.1111/vco.12309 [DOI] [PubMed] [Google Scholar]
- 49.Macewen EG, Patnaik AK, Harvey HJ, Hayes AA, Matus R. Canine oral melanoma: Comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Invest. (1986) 4:397–402. 10.3109/07357908609017520 [DOI] [PubMed] [Google Scholar]
- 50.Boston SE, Lu X, Culp WTN, Montinaro V, Romanelli G, Dudley RM, et al. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001-2012). J Am Vet Med Assoc. (2014) 245:401–7. 10.2460/javma.245.4.401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.