Abstract
Purpose
Chronic rhinitis (CR) is a common chronic inflammation of the nasal mucosa. Nasal saline irrigation has been demonstrated to be an effective treatment for CR. In this study, we investigated the beneficial effects of hydrogen-rich saline irrigation as an anti-inflammatory irrigation therapy for CR and compared its effectiveness over saline irrigation. Hydrogen-rich saline (HRS) was investigated due to its antioxidant and anti-inflammatory properties.
Methods
A total of 120 patients with CR were randomly divided into two groups, patients irrigated with HR (HRS group) and the control group irrigated with saline (NS group). A randomized, double-blind control study was performed. The main observation index in this study was the total score of nasal symptoms (TNSS). In addition, eosinophilic protein (ECP) of the nasal secretions, nasal nitric oxide (nNO) levels, and levels of regulatory T cells (Treg) and regulatory B cells (Breg) were also compared between the two groups. Furthermore, patients with allergic rhinitis (AR) and non-allergic rhinitis (NAR) were also evaluated based on serum-specific IgE positivity.
Results
After treatment, TNSS and nasal ECP in the two groups decreased significantly (P<0.05), with patients in the HRS group showing significantly lower levels compared to the NS group (P<0.05). There were no significant differences in Treg and Breg levels between the two groups. Subgroup analysis showed that TNSS in the AR-HRS group showed a more significant reduction compared to the AR-NS group (P<0.05); however, there were no significant differences for the other inflammatory biomarkers (P>0.05). ECP levels were reduced significantly in the NAR subgroup compared to NS irrigation (P<0.05). There were no obvious adverse events observed in patients during the entire treatment period.
Conclusion
Compared to saline irrigation, HRS nasal irrigation was found to improve CR clinical symptoms, especially in patients with AR. HRS could effectively be used for the clinical treatment of patients with CR.
Keywords: chronic rhinitis, allergic rhinitis, non-allergic rhinitis, hydrogen-rich, nasal irrigation
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Chronic rhinitis (CR) affects approximately 30% of the global population,1,2 with its incidence increasing every year. CR is a chronic inflammatory disease of the nasal mucosa. Common symptoms include nasal itching, sneezing, anterior or posterior nasal leakage, and nasal congestion.1,3 CR has also been shown to induce secretory otitis media, sinusitis, nasal polyps, and induce or aggravate asthma and other diseases. This seriously affects patients and carries a huge economic burden.4,5
CR can be classified as allergic rhinitis (AR) and non-allergic rhinitis (NAR) based on the presence of a specific allergen.2,6 Approximately 500 million individuals worldwide suffer from AR7 and have become an important chronic inflammatory disease of the respiratory tract. AR is an allergic chronic inflammatory disease of the nose that involves a variety of inflammatory cells, inflammatory factors, and neurotransmitters that are regulated by multiple genes. There are relatively few studies that have been performed on NAR, however, it affects the lives of more than 200 million individuals worldwide.8
Although its etiology is unclear, it involves multiple factors, among which immune factors play an important role in NAR pathogenesis,9 and include local allergic rhinitis.9 Due to the similar symptoms of AR and NAR, the current clinical treatments are similar and mainly include nasal glucocorticoids, antihistamines, and nasal decongestants.6,10,11 However, due to their side effects, the majority of patients prefer nasal saline irrigation that has minimal side effects.12 Nasal irrigation has been recognized as a local treatment for CR. Saline irrigation has been shown to improve the transport function of nasal mucocilia. It removes secretions and foreign bodies, such as dust and bacterial biofilm attached to the nasal mucosa surface.13
Nasal irrigation is simple physiotherapy. Although it may temporarily clear the local inflammatory factors of the nasal mucosa, the clearance function is only temporary. Immune dysfunction is not alleviated by irrigation, hence the beneficial effects usually disappear after one hour.14 Hence, lavage containing a solution of anti-inflammatory agents maybe have better therapeutic effects. In 2007, Ohta et al found that hydrogen (H2), had a selective antioxidant effect as well as an effective anti-inflammatory and anti-allergic effect.15 Soon afterward, hydrogen therapy quickly gained attention in the fields of inflammation and allergy.16–18 Previous studies have demonstrated that H2 dissolved in saline to generate a hydrogen-rich saline (HRS) solution was effective in relieving AR symptoms and reducing inflammatory mediators such as IL-1β, IL-4, IL-5, IL-6, IL-13, and TNFα.19–22 Additional studies have confirmed that HRS could induce an increase in CD4+CD25+ FoxP3+Treg levels in AR guinea pigs and promote IL-10 and TGF-B secretion.23 Chronic inflammation and oxidative stress of the nasal mucosa is one of the main pathogenic mechanisms of CR,24 and one advantage of H2 is its selective antioxidant effects.25 To investigate its role in AR and NAR treatment, we performed a clinical RCT study using HRS nasal irrigation on CR patients, with saline irrigation as a control. Changes in nasal symptom scores were selected as the main observation indicator. Secondary indicators included inflammatory biomarkers, as nasal nitric oxide (nNO), eosinophil cationic protein (ECP) levels in the nasal secretions, and levels of regulatory T cells (Treg) and regulatory T cells (Breg) in the peripheral blood (PBMC). Our study evaluated whether HRS nasal irrigation could effectively improve inflammation of the nasal mucosal in CR patients.
Methods
Study Design and CR Patients
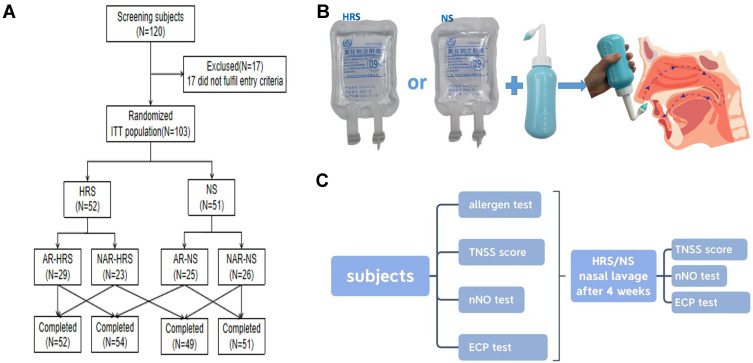
CR patients between 18–65 years were enrolled from the outpatient department of Otorhinolaryngology, Head and Neck Surgery of Shanghai Tongji Hospital between August 2018 and December 2020. Patients were randomly divided into two groups based on a random number table. Patients in the HRS group were nasal cavity irrigated with HRS, while patients in the NS group were nasal cavity irrigated with NS and used as the control for this study (Figure 1A). Inclusion criteria were determined based on relevant publications. These included rhinitis symptoms ie, nasal itching, nasal congestion, sneezing, and runny nose. In addition, the patient should have two or more symptoms that lasted for more than 10 days, and a history of recurrent symptoms for at least 2 years.26–28 Patients with mild rhinitis (ie, symptoms that did not severely affect the quality of life), acute infectious rhinitis; patients diagnosed with chronic sinusitis, severe nasal septum deviation, nasal polyps, nasal tumors, and other nasal diseases by nasal endoscopy and sinus CT were excluded from the study. Patients with severe liver, kidney, and heart diseases and had been treated with medication within 4 weeks of the study were also excluded. To reduce confounding factors, Corticosteroids and antibiotics were not administered during the study. This study was approved by the hospital ethics committee, and all patients signed informed consent. The study was approved by the Research Ethics Committees of Tongji hospital (2018-LYCJ-002) and complied with the declaration of Helsinki. The study was registered in the Chinese Clinical Trial Registry (CHICTR-INR-16009778) on November 8, 2016.
Figure 1.
Method and Study Procedure. (A) Study grouping. (B) Procedure for Nasal irrigation. (C) Study Procedure.
RCT Setting
A randomized double-blind parallel control approach was used in this study. SAS8.2 professional statistical software was used to generate group random numbers, and the subjects were divided into two groups. Based on the group random numbers, the blind classification was accomplished. The random number was the drug number. The number of cases in the trial group and control group was 1:1. Enrolled subjects were randomly assigned based on a random number table and were administered treatment by the clinical study group. Observers and subjects were double-blinded.
Nasal Irrigation
A standard nasal irrigation vessel was used in this study. Irrigated solution for the HRS group consisted of 100 mls of NS dissolved with pure H2 under 0.4MPa pressure to reach saturation levels of 0.6mmol/l.29,30 The prepared HRS was randomly verified by a gas chromatography analyzer to ensure that the H2 concentration of the HRS was stable and consistent. Patients in the control group were treated with irrigation of sterile 100mL NS. The irrigated solution used in both groups had the same appearance and packaging (Figure 1B).
100mL of solution (50mL on each side of the nasal cavity) was used to irrigate both nasal cavities, twice a day continuously for 4 weeks. The patient blew nasal mucus before treatment, then the patient’s head was tilted forward. 100 mls of lavage solution was placed in the nasal washing vessel and then slowly perfused into one side of the nasal cavity. The solution was allowed to flow into the nasopharynx and then expelled from the mouth. Afterward, the other nasal cavity was irrigated in a similar way (Figure 1B).
Evaluation of Nasal Symptoms and Signs
There were five evaluations during the study. Total nasal symptom scores (TNSS) were evaluated at each visit. TNSS included the number of consecutive sneezes, the daily number of runny noses, nasal obstruction, and itching, with 0–3 points for each indicator representing different severities.27,31
nNO Measurements
The levels of nNO were measured before and after treatment using the Sunvou-CA2122 Nacoulomb Respiratory Analyzer (Wuxi Shangwo Biotechnology Co., Ltd., China). The test ambient temperature is 25 °C, and the humidity is 25% - 50%. The patient did not eat, drink or exercise vigorously 2 hours before the test. Let the patient rest for 15 minutes and take the sitting position. After deep inhalation, whistle with the mouth at a constant speed to close the soft palate (to avoid the mixing of gas in the upper and lower airways). At the same time, insert the olive shaped nose probe into one side of the front nostril and continuously suck the gas in the nasal cavity to measure the nNO value. The average value for 6 tests was recorded.
Measurement of IgE and Eosinophil Cationic Protein (ECP) Levels in Nasal Secretions
Serum total IgE and specific IgE levels for 19 common inhalation and ingestion allergens (Euroimmun Medical Diagnostics Co., Ltd. China) were measured using enzyme-linked immunosorbent assays (CAP system). ECP of nasal secretions was also measured (Biolegend, USA) to assess the severity of rhinitis.
Flow Cytometric Analysis for Treg and Breg in PBMCs
To evaluate the effect of treatment on the immune system, the proportion of CD4+CD25+CD127-Treg and CD19+CD24hiCD38hiBreg in PBMCs was measured using flow cytometric analysis before and after treatment. Briefly, PBMCs harvested from the peripheral vein were labeled with anti-CD4-PE, anti-CD25-FITC, and anti-CD127-PC5 monoclonal antibodies (Beckman Coulter, USA) to identify Treg. Breg was identified by labeling PBMCs with anti-CD19-PE, anti-CD24-PC5, and anti-CD38-FITC antibodies (Beckman Coulter, USA). Non-specific fluorescence was determined using an isotype-matched IgG as the control. Data were acquired on a FACSCalibur (Beckman Coulter, USA) and analyzed using the Cell Quest software (BD, USA).
Adverse Events Following Irrigation
To assess safety, changes in blood routine, liver and kidney function, and blood glucose were measured before and after irrigation. In addition, symptoms of nasal discomforts, such as epistaxis, nasal pain, and other adverse reactions were also evaluated.
Statistical Analysis
Sixty patients were required for each treatment group. This was based on data from preliminary studies. The difference between NS and HRS for total mean score of symptoms and signs was 1, SD=1.65, when 1-β (power)=90%, bilateral α=5%. All efficacy assessments were analyzed only for subjects who completed the trial. Quantitative data were expressed as mean ± standard deviation and qualitative data were expressed as a percentage. Student’s t-test was used for comparison between quantitative data groups, and Chi-square or Fisher test was used for qualitative data. A Mixed Model for Repeated Measures (MMRM) was used to correct the score at baseline for the primary efficacy index, and a P-value <0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4.
Results
Baseline Characteristics of Study Participants
A total of 128 patients were recruited for this study, with 120 cases successfully enrolled after meeting the inclusion criteria. There were 17 cases of abscission or early withdrawal, which included 8 cases in the HRS group and 9 cases in the NS group. One patient was dissatisfied with the curative effects and withdrew early, while the remaining patients discontinued early due to personal reasons. In total, 52 patients in the HRS group and 51 patients in the NS group completed the study. The study grouping process and study completion are shown in Figure 1A and C. There were no significant differences between the two groups at baseline, such as gender, age, course of the disease, allergy history, pre-medication symptoms, and TNSS (Table 1) (P>0.05).
Table 1.
Baseline Comparison of the Study Subjects
| HRS (N=52) | NS (N=51) | Statistic | P | Methods | ||
|---|---|---|---|---|---|---|
| Age (years) | Mean(SD) | 42.16±17.16 | 40.80±13.76 | 0.438 | 0.662 | t-test |
| Height(cm) | Mean(SD) | 166.06±9.34 | 165.68±8.37 | 0.215 | 0.830 | t-test |
| Weight(kg) | Mean(SD) | 65.31±11.27 | 63.04±12.18 | 0.976 | 0.331 | t-test |
| BMI | Mean(SD) | 23.56±2.58 | 22.80±2.82 | 1.407 | 0.163 | t-test |
| Gender | ||||||
| Males | n(%) | 24(46.15%) | 18(36.00%) | 1.085 | 0.298 | Chi square test |
| Females | n(%) | 28(53.85%) | 32(64.00%) | |||
| Past medical history | ||||||
| Yes | n(%) | 10(19.23%) | 9(17.65%) | 0.043 | 0.836 | Chi square test |
| No | n(%) | 42(80.77%) | 42(82.35%) | |||
| Allergic history | ||||||
| Yes | n(%) | 3(5.77%) | 3(5.88%) | – | 1.000 | Fisher exact test |
| No | n(%) | 49(94.23%) | 48(94.12%) | |||
| Medical treatment history | ||||||
| Yes | n(%) | 8(15.38%) | 5(9.80%) | 0.727 | 0.394 | Chi square test |
| No | n(%) | 44(84.62%) | 46(90.20%) | |||
| Total nasal symptom scores | ||||||
| Sneeze | 1.47±0.99 | 1.40±0.89 | 0.360 | 0.719 | ||
| Runny nose | 2.45±0.81 | 2.40±0.77 | 0.302 | 0.763 | ||
| Nasal obstruction | 1.96±0.53 | 1.89±0.68 | 0.637 | 0.526 | ||
| Nasal itching | 2.02±0.86 | 1.87±0.69 | 1.007 | 0.317 | ||
| TNSS | 7.80±2.22 | 7.58±1.84 | 0.566 | 0.573 | ||
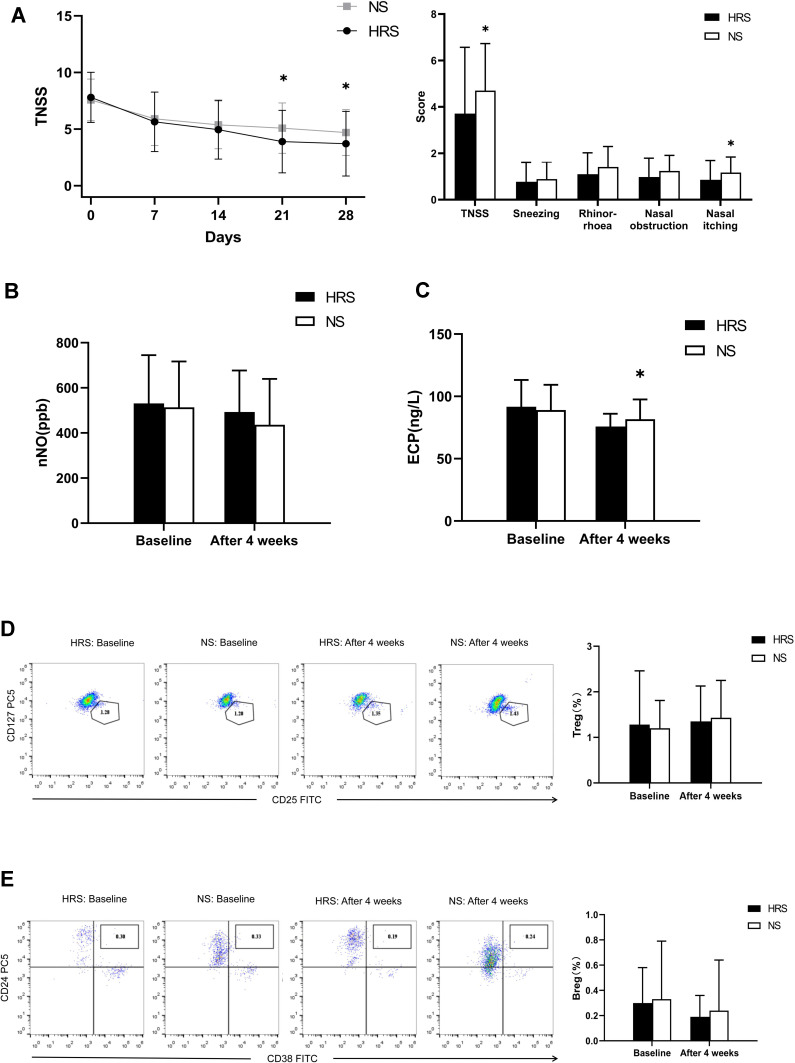
Comparison of the Main Outcome Measures for Total Nasal Symptom Scores (TNSS). HRS Alleviates TNSS of CR Compared to NS
There was no significant difference in TNSS scores between the HRS and NS groups before treatment (P= 0.573>0.05). After 4 weeks of treatment, the TNSS scores for the two groups decreased with statistical significance (P<0.05). The decrease for the HRS group was more obvious compared to the NS group (P=0.043<0.05). In addition, there were no significant differences in the number of sneezes, runny noses, and nasal obstructions between the two groups. Only the score for nasal itching in the HRS group was lower compared to the NS group (P=0.004<0.05) (Figure 2A).
Figure 2.
Comparison of various indicators of CR between the HRS and NS groups. (A) TNSS scores for the HRS group decreased significantly compared to the NS group after four weeks of treatment (P=0.043<0.05), especially in TNSS scores for nasal itching in the AR-HRS group which was higher than the control group (P=0.04<0.05). (B) nNO levels in the two groups decreased significantly after treatment (both P=0.000<0.05), however, no statistical significance was observed (P=0.139>0.05). (C) Both groups showed an obvious decrease in ECP after treatment. ECP levels in the HRS group decreased more significantly compared to the NS group (P=0.029<0.05). (D) After treatment, the proportion of Treg in the two groups increased slightly, but no significant differences were observed between the two groups (P=0.648>0.05). (E) After treatment, the proportion of Breg in the two groups decreased slightly, with no significant difference between the two groups (P=0.493> 0.05). *Significantly different from the HRS group (p<0.05).
HRS Did Not Significantly Improve Secondary Observation Indicators for CR Compared to NS
The average nNO for both groups before treatment was greater than 500 ppb and decreased significantly after treatment (P=0.000<0.05). However, results showed no significant differences between the groups (P= 0.139>0.05) after treatment. HRS could improve nasal mucosal inflammation and reduce nasal NO levels but was not superior to saline irrigation (Figure 2B).
There were no significant differences in the ECP value of nasal secretions between the two groups before treatment (91.71±21.46 vs.81.72±15.89, P>0.05). After treatment, ECP levels for the two groups decreased significantly (P =0.000<0.05). The decrease in the HRS group was more obvious compared to the NS group (75.78±10.27 vs.81.72±15.89, P=0.029<0.05). This indicated that HRS could effectively reduce ECP levels of CR compared to NS treatment (Figure 2C).
After treatment, the number of Treg in both groups increased slightly (P =0.000, 0.000<0.05), accompanied by a significant decrease in the number of Breg (Figure 2D and E) (P=0.000, 0.024<0.05). However, Both Treg and Breg had no significant differences between the two groups (P =0.648,0.493>0.05).
Subgroup Analysis of the Effect of HRS on AR
Based on the presence or absence of serum-specific IgE (allergen), 103 patients who completed the study were further divided into two subgroups, ie, allergen positive group (AR group) and allergen negative group (NAR group).
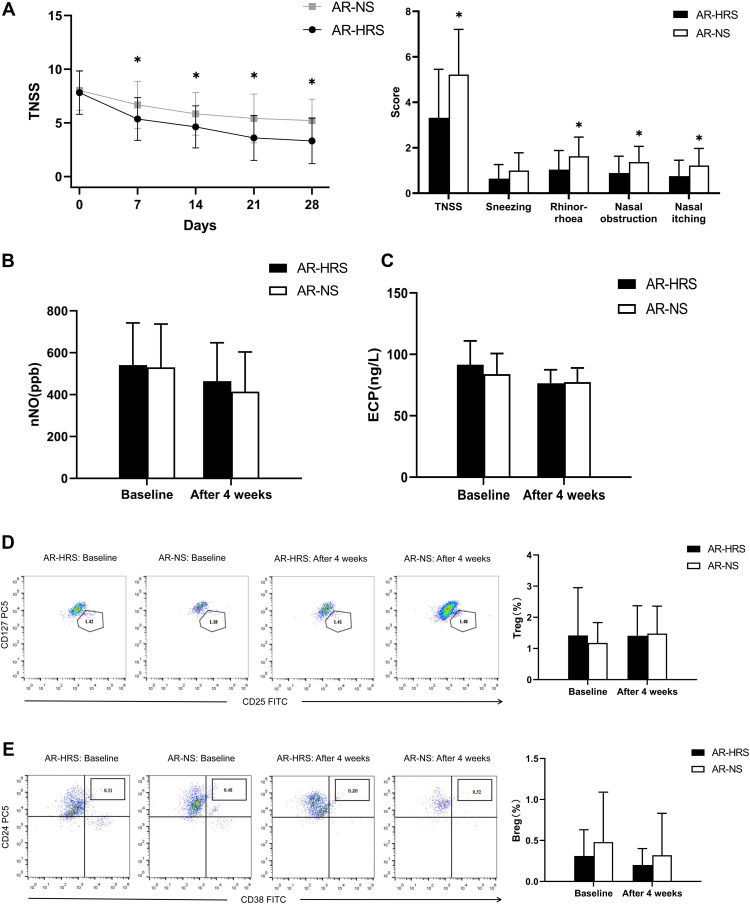
54 patients were allergen positive, of which, 29 patients were administered HRS (AR-HRS group) and 25 patients administered NS (AR-NS group). There were no significant differences in baseline data between the groups (7.82±2.02 vs.8.04±1.86, p>0.05). After treatment, TNSS in the AR-HRS group were significantly better compared to the AR-NS group (3.32±2.13 vs.5.22±1.99, P=0.001<0.05). Scores for runny nose (1.04±0.84 vs.1.63±0.84, P=0.011<0.05), nasal obstruction (0.89±0.74 vs.1.37±0.69, P=0.016<0.05), and nasal itch (0.75±0.70 vs.1.22±0.75, P=0.019<0.05) in the AR-HRS group were significantly lower compared to the AR-NS group (P<0.05) (Figure 3A). This indicated that HRS was superior in alleviating AR symptoms.
Figure 3.
Comparison of various indicators in the AR subgroup. (A) The TNSS scores in the AR-HRS subgroup decreased significantly compared to the NS group after four weeks of treatment (P=0.001<0.05), especially for symptoms of runny nose, nasal obstruction, and nasal itching in the AR-HRS group which had higher TNSS compared to the control group (both P<0.05). (B) nNO levels in the two groups decreased significantly after treatment (both P<0.05), but no statistical significance between the two treatment groups were observed (P=0.401>0.05). (C) ECP for both groups decreased after treatment (both P<0.05), but no significant difference between two groups were observed (P=0.758>0.05). (D) After treatment, Treg proportions decreased in the AR-HRS group (P=0.01<0.05) and increased in the control group (P=0.000<0.05), but no significant difference between the two groups were observed (P=0.803>0.05). (E) After treatment, Breg proportions decreased in the AR-HRS group (P=0.002<0.05), but no significant difference between the groups were observed (P=0.322>0.05).*Significantly different from the AR-HRS group (P <0.05).
After therapy, nNO and ECP levels decreased for both groups (P<0.05) (Figure 3B and C). The number of Treg and Breg in the AR-HRS group reduced significantly after treatment (P<0.05). In the control AR-NS group, the number of Treg increased after treatment (P<0.05 Figure 3D), while the number of Breg had no significant differences after treatment (P=0.063>0.05, Figure 3E). There were no significant differences for all secondary indicators between the two groups (P>0.05). These results suggested that HRS treatment on inflammatory biomarkers was not significantly better than that of saline irrigation.
Subgroup Analysis of the Effect of HRS on NAR
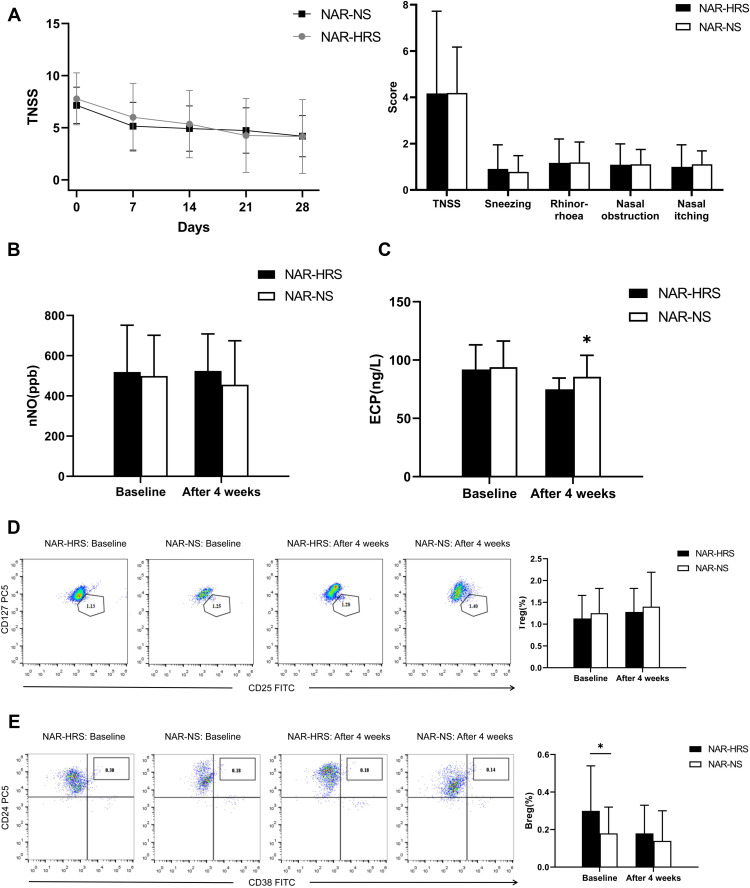
49 patients were allergen negative, of which 23 were administered HRS (NAR-HRS group) and 26 were administered NS (NAR-NS group). There were no differences in baseline data between the two groups. After treatment, TNSS for the two groups decreased. There were no significant differences between the two groups as shown in Figure 4A (4.17±3.55 vs.4.19±1.98, P=0.989>0.05). ECP levels decreased in both groups after treatment (Figure 4C), with a slight statistical decrease in the NAR-HRS group (91.90±24.14 vs.74.95±9.58, P<0.05). nNO levels increased in the NAR-NS group and decreased in the NAR-HRS group. The number of Treg increased, while Breg numbers reduced after treatment in the NAR-HRS group (P<0.05). In the control NAR-NS group, the number of Treg increased after treatment (P<0.05 Figure 4D), while the number of Breg had no significant differences after treatment (P=0.102>0.05, Figure 4E). There were no significant differences between the groups for nNO, Treg, and Breg (Figure 4B, D and E (P>0.05)). In the NAR group, HRS was superior in reducing ECP levels in the nasal cavities compared to NS.
Figure 4.
Comparison of various indicators in the NAR subgroup. (A) There was no significant difference in TNSS between the two treatment groups (P=0.989>0.05). (B) There were no significant differences in nNO levels within and between the two groups after treatment (P=0.291>0.05). (C) After treatment, ECP levels decreased significantly in both groups, while ECP levels in the NAR-HRS group decreased slightly, with a significant difference (P=0.016<0.05). (D) After treatment, Treg proportion in the two groups increased after treatment (both P=0.001<0.05). However, no significant difference between the two groups were observed (P=0.6>0.05); (E) After treatment, Breg proportions decreased in NAR-HRS group (P=0.00<0.05), but no significant difference between the groups were observed (P=0.472>0.05).*Significantly different from the NAR-HRS group (p<0.05).
Safety and Tolerability
There were no significant changes in blood routine, liver and kidney function, and blood glucose levels before and after treatment. No obvious discomfort and adverse reactions were observed in this study. There were 4 cases with mild upper respiratory tract infection (two cases in the HRS group and 2 cases in the NS group) and were found to be not related to irrigation. These patients were not treated with additional therapy. Three cases continued with nasal irrigation, while one patient withdrew early due to lack of curative effect (NS group). The incidence of adverse events was similar between HRS (3.85%) and NS (3.92%) groups. These results indicated that nasal irrigation was safe and tolerable.
Discussion
Our study demonstrated that saline irrigation of the nasal cavity could improve CR symptoms. Compared to saline irrigation, HRS irrigation showed better efficacy in alleviating rhinitis. Saline and HRS irrigation could significantly and similarly reduce inflammatory biomarkers. Subgroup analysis demonstrated that HRS irrigation could improve AR symptoms more obviously. A decrease in ECP levels was the only improvement observed in NAR patients who underwent HRS irrigation compared to the control group. These results indicated that HRS irrigation could improve CR symptoms compared to saline irrigation, especially for AR.
Saline nasal irrigation is an effective treatment for rhinitis. In our study, both TNSS and specific symptoms were significantly improved after saline irrigation. Furthermore, AR and NAR were significantly improved, in addition to a significant reduction of inflammatory biomarkers. This suggested that saline nasal irrigation was an effective treatment for CR. Although numerous studies have shown that saline nasal washing can improve AR symptoms,32 only a few studies have been conducted on its effects on NAR. Saline improves rhinitis symptoms mainly by cleaning nasal inflammatory irritants and secretions to reduce inflammatory stimulation. The main immunological pathogenesis of AR is allergic inflammation. However, NAR is induced in the absence of an allergen, as determined by negative skin prick test and serum sIgE levels.33,34 It has been related to a variety of inflammatory factors secreted by the nasal cavity, such as local production of tryptase, eosinophilic protein, and sIgE. In this study, we observed that NAR and AR had similar levels of ECP, nNO, and other inflammatory biomarkers. Hence, CR inflammatory reaction could be alleviated by clearing these inflammatory factors by nasal irrigation with saline.
We next accessed the therapeutic effects of HRS on CR by measuring TNSS and evaluating specific symptom scores. TNSS of both groups decreased significantly compared to before treatment. HRS group decreased more significantly compared to the NS group. However, of the four specific symptoms measured, only the nasal itch score in the HRS group decreased significantly compared to the NS group. This indicated that HRS could improve CR clinical symptoms, by improving nasal itch symptoms. CR inflammatory biomarkers, such as nNO, Treg, and Breg, also showed significant differences after treatment, but no significant differences were observed between the two groups, except for ECP levels of nasal secretions that were better in the HRS group compared to the control. Subgroup analysis found that compared to saline irrigation, HRS could better improve TNSS in the AR group. The three specific symptoms of runny nose, nasal obstruction, and nasal itching were all shown to be significantly improved. Our study showed that HRS had a better therapeutic effect on AR. However, the inflammatory biomarkers, such as nNO and ECP, Treg, and Breg, did not show significant differences between the two groups. Although TNSS decreased significantly after treatment in the NAR subgroup, there was no significant difference between the two treatment groups. Only ECP levels of nasal secretions showed a significant difference between the groups. These results indicated that HRS had a good therapeutic effect on CR, especially in AR patients.
In recent years, increasing attention has been paid to the anti-inflammatory effects of H2. It is very safe with no side effects either when inhaled or consumed. H2 has also been shown to not affect temperature regulation, blood pressure, physiology, pH, or pO2, and is nontoxic at concentrations even far above the clinically effective dosage.35 H2 has been shown to upregulate Nrf2/ARE-Keap1 signaling pathway by increasing Nrf236 and enhances the antioxidant stress ability of the body by reducing the production of reactive oxygen species.37 The antioxidant and anti-inflammatory effects of hydrogen had been demonstrated in numerous animal studies, especially in allergic inflammation, which reduces pro-inflammatory factor levels.23,26–28 It has even been demonstrated to down-regulate Treg levels to reduce allergic inflammation. Considering the effectiveness of saline irrigation for the treatment of rhinitis, we dissolved hydrogen in saline (HRS) to evaluate its efficacy. Our results demonstrated no adverse reactions in terms of safety. For the AR group, we observed HRS had a better curative effect compared to saline irrigation, suggesting its effectiveness in controlling AR inflammation. This suggested that HRS may exert its curative effect in CR especially for AR.
ECP is released by activated eosinophils38 and may be the main reason for chronic airway inflammation. It is also an objective biomarker for airway allergic inflammation. ECP levels correlate well with the number of eosinophils cells and hence could be useful to evaluate patients with AR or NAR. It is also an effective clinical indicator for evaluating the effectiveness of therapy.39 After HRS treatment, ECP of CR (all patients with chronic rhinitis included in the analysis) decreased significantly. There were significant differences in patients with CR and the NAR subgroup compared to NS treatment. In addition, there was no significant difference between the two groups in the AR subgroup. This indicated that HRS irrigation relative to saline irrigation was not sufficient to reduce ECP levels. However, HRS may achieve anti-inflammatory effects by inhibiting ECP secretion in NAR nasal cavity, and hence may be related to local hypersensitive in NAR.
nNO is a marker for nasal inflammation and could be used to evaluate clinical anti-inflammatory efficacy. We observed nNO was high in both the HRS and NS groups before treatment. After 4 weeks of treatment, nNO decreased significantly in both groups, but no significant differences were observed between the groups. Subgroup analysis also found that nNO decreased after treatment with no significant differences between the groups. This indicated that HRS had no obvious advantages over saline in reducing nasal NO levels.
Treg and Breg cells play an important regulatory role in AR immune regulation.40,41 The levels of Treg and Breg in the peripheral blood of AR patients are lower compared to healthy individuals.42 We observed that Treg levels increased and Breg levels decreased in the CR, AR, and NAR subgroups after treatment. Nasal clearing could change the immune status, however, there were no significant differences in Treg and Breg levels in the HRS and NS groups, in addition to the AR and NAR subgroups. Furthermore, we found that HRS was not superior to saline irrigation with respect to immune regulation after four weeks of treatment. This is not consistent with previous animal studies. An explanation could be due to the short treatment duration of this study.
Conclusion
In conclusion, our results suggested that HRS nasal irrigation could better improve the nasal symptoms and reduce the ECP levels in nasal secretions of CR patients compared to saline irrigation. This was especially observed in AR patients who showed improved efficacy. Considering the safety of this treatment, HRS should be recommended for the clinical treatment of CR, especially in AR patients.
Acknowledgments
We are grateful to all the patients who participated in the study. We would also like to thank the late Ling Du who contributed significantly to this study.
Funding Statement
This work was supported by the National Science Foundation of China (No.81873689), Clinical Research Plan of SHDC (No. SHDC2020CR4090), Clinical Science and Technology Innovation Project of SHDC (No. SHDC12019X07), Health Commission Advanced Technology Promotion Project of Shanghai City (No.2019SY071), Clinical Research Project of Tongji Hospital of Tongji University (Grant No.ITJ(ZD)1801), Clinical Research Program of Shanghai Municipal Health Commission ((No.202140293).
Data Sharing Statement
Related data and materials are available upon request to Ling Jin, Kai Fan, and Shaoqing Yu.
Ethics Approval
The study was approved by the Research Ethics Committees of Tongji hospital (Permit Number: 2018-LYCJ-002).
Author Contributions
Ling Jin: Data collection and curation, formal analysis, investigation, methodology, writing the original draft, and project administration. Kai Fan: Data collection and curation, formal analysis, investigation, methodology, and writing the original draft. Shiwang Tan: Data curation, formal analysis, investigation, and methodology. Shuangxi Liu: methodology. Qin Ge: project management, distribution, and collection of the drugs. Yang Wang: methodology. Zisheng Ai: Statistical analysis, supervision, validation, project administration, and the writing, review, and editing of the manuscript. Shaoqing Yu: conceptualization, supervision, funding acquisition, validation, project administration, and the writing, review, and editing of the manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hellings PW, Klimek L, Cingi C, et al. Non-allergic rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy. 2017;72(11):1657–1665. doi: 10.1111/all.13200 [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Khaltaev N, Cruz AA, et al. GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86:8–160. doi: 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- 3.Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy. 2013;68(9):1102–1116. doi: 10.1111/all.12235 [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos NG, Bernstein JA, Demoly P, et al. Phenotypes and endotypes of rhinitis and their impact on management: a PRACTALL report. Allergy. 2015;70(5):474–494. doi: 10.1111/all.12573 [DOI] [PubMed] [Google Scholar]
- 5.Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69(10):1275–1279. doi: 10.1111/all.12470. [DOI] [PubMed] [Google Scholar]
- 6.Agnihotri NT, McGrath KG. Allergic and nonallergic rhinitis. Allergy Asthma Proc. 2019;40(6):376–379. doi: 10.2500/aap.2019.40.4251 [DOI] [PubMed] [Google Scholar]
- 7.Bukstein D. The Obama dilemma: allergic rhinitis (animal dander allergy)-The great burden of illness. Allergy Asthma Proc. 2009;30(6):567–572. doi: 10.2500/aap.2009.30.3297 [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Fokkens W, Burney P, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy. 2008;63(7):842–853. doi: 10.1111/j.1398-9995.2008.01715.x [DOI] [PubMed] [Google Scholar]
- 9.Meng Y, Wang C, Zhang L. Diagnosis and treatment of non-allergic rhinitis: focus on immunologic mechanisms. Expert Rev Clin Immunol. 2021;17(1):51–62. doi: 10.1080/1744666X.2020.1858804 [DOI] [PubMed] [Google Scholar]
- 10.Sur DKC, Plesa ML. Chronic nonallergic rhinitis. Am Fam Physician. 2018;98(3):171–176. PMID: 30215894. [PubMed] [Google Scholar]
- 11.Sur DK, Plesa ML. Treatment of allergic rhinitis. Am Fam Physician. 2015;92(11):985–992. PMID: 26760413. [PubMed] [Google Scholar]
- 12.Inthavong K, Shang Y, Wong E, Singh N. Characterization of nasal irrigation flow from a squeeze bottle using computational fluid dynamics. Int Forum Allergy Rhinol. 2020;10(1):29–40. doi: 10.1002/alr.22476 [DOI] [PubMed] [Google Scholar]
- 13.Satdhabudha A, Poachanukoon O. Efficacy of buffered hypertonic saline nasal irrigation in children with symptomatic allergic rhinitis: a randomized double-blind study. Int J Pediatr Otorhinolaryngol. 2012;76(4):583–588. doi: 10.1016/j.ijporl.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 14.Wabnitz DA, Wormald PJ. A blinded, randomized, controlled study on the effect of buffered 0.9% and 3% sodium chloride intranasal sprays on ciliary beat frequency. Laryngoscope. 2005;115(5):803–805. doi: 10.1097/01.MLG.0000157284.93280.F5 [DOI] [PubMed] [Google Scholar]
- 15.Ohta S, Nakao A, Ohno K. The 2011 medical molecular hydrogen symposium: an inaugural symposium of the journal medical gas research. Med Gas Res. 2011;1(1):10. doi: 10.1186/2045-9912-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh T, Fujita Y, Ito M, et al. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389(4):651–656. doi: 10.1016/j.bbrc.2009.09.047 [DOI] [PubMed] [Google Scholar]
- 17.Xiao M, Zhu T, Wang T, Wen FQ. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-κB in a murine model of asthma. Eur Rev Med Pharmacol Sci. 2013;17(8):1033–1043. [PubMed] [Google Scholar]
- 18.Liu Z, Geng W, Jiang C, et al. Hydrogen-rich saline inhibits tobacco smoke-induced chronic obstructive pulmonary disease by alleviating airway inflammation and mucus hypersecretion in rats. Exp Biol Med (Maywood). 2017;242(15):1534–1541. doi: 10.1177/1535370217725249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Yu S, Li J, Xu W, Ge R. Changes in IL-4 and IL-13 expression in allergic-rhinitis treated with hydrogen-rich saline in Guinea-pig model. Allergol Immunopathol. 2017;45(4):350–355. doi: 10.1016/j.aller.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 20.Du Z, Jia H, Liu J, Zhao X, Xu W. Effects of three hydrogen-rich liquids on hemorrhagic shock in rats. J Surg Res. 2015;193(1):377–382. doi: 10.1016/j.jss.2014.06.051 [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Guo S, Zhang Y, Xu Y, Zhao P, Zhao X. Effects of hydrogen-rich saline on hepatectomy-induced postoperative cognitive dysfunction in old mice. Mol Neurobiol. 2017;54(4):2579–2584. doi: 10.1007/s12035-016-9825-2 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Chen-Yoshikawa TF, Saito M, et al. Immersing lungs in hydrogen-rich saline attenuates lung ischaemia-reperfusion injury. Eur J Cardiothorac Surg. 2017;51(3):442–448. doi: 10.1093/ejcts/ezw342 [DOI] [PubMed] [Google Scholar]
- 23.Xu F, Yu S, Qin M, et al. Hydrogen-rich saline ameliorates allergic rhinitis by reversing the imbalance of Th1/Th2 and up-regulation of CD4+CD25+Foxp3+Regulatory T cells, interleukin-10, and membrane-bound transforming growth factor-β in Guinea Pigs. Inflammation. 2018;41(1):81–92. doi: 10.1007/s10753-017-0666-6 [DOI] [PubMed] [Google Scholar]
- 24.Chung DH, Lee KH, Kim SW, Shin SY, Cho JS. Comparison of pre- and post-operative stress levels in patients with allergic rhinitis and non-allergic rhinitis. Eur Arch Otorhinolaryngol. 2012;269(11):2355–2359. doi: 10.1007/s00405-012-1946-2 [DOI] [PubMed] [Google Scholar]
- 25.Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038 [DOI] [PubMed] [Google Scholar]
- 26.Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis executive summary. Otolaryngol Head Neck Surg. 2015;152(2):197–206. doi: 10.1177/0194599814562166. [DOI] [PubMed] [Google Scholar]
- 27.Ellis AK, Soliman M, Steacy L, et al. The Allergic Rhinitis - Clinical Investigator Collaborative (AR-CIC): nasal allergen challenge protocol optimization for studying AR pathophysiology and evaluating novel therapies. Allergy Asthma Clin Immunol. 2015;11(1):16. doi: 10.1186/s13223-015-0082-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poddighe D, Gelardi M, Licari A, Del Giudice MM, Marseglia GL. Non-allergic rhinitis in children: epidemiological aspects, pathological features, diagnostic methodology and clinical management. World J Methodol. 2016;6(4):200–213. doi: 10.5662/wjm.v6.i4.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao L, Chen H, Wu Q, Xie K. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med. 2019;44(3):1048–1062. doi: 10.3892/ijmm.2019.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi: 10.1038/nm1577 [DOI] [PubMed] [Google Scholar]
- 31.Hwang PH, Lin B, Weiss R, Atkins J, Johnson J. Cryosurgical posterior nasal tissue ablation for the treatment of rhinitis. Int Forum Allergy Rhinol. 2017;7(10):952–956. doi: 10.1002/alr.21991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Jin L, Liu SX, Fan K, Qin ML, Yu SQ. Role of nasal saline irrigation in the treatment of allergic rhinitis in children and adults: a systematic analysis. Allergol Immunopathol. 2020;48(4):360–367. doi: 10.1016/j.aller.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 33.Wedbäck A, Enbom H, Eriksson NE, Movérare R, Malcus I. Seasonal non-allergic rhinitis (SNAR)–a new disease entity? A clinical and immunological comparison between SNAR, seasonal allergic rhinitis and persistent non-allergic rhinitis. Rhinology. 2005;43(2):86–92. [PubMed] [Google Scholar]
- 34.Rondón C, Canto G, Blanca M. Local allergic rhinitis: a new entity, characterization and further studies. Curr Opin Allergy Clin Immunol. 2010;10(1):1–7. doi: 10.1097/ACI.0b013e328334f5fb [DOI] [PubMed] [Google Scholar]
- 35.Ono H, Nishijima Y, Adachi N, et al. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Med Gas Res. 2012;2(1):21. doi: 10.1186/2045-9912-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu S, Zhao C, Che N, Jing L, Ge R. Hydrogen-rich saline attenuates eosinophil activation in a Guinea pig model of allergic rhinitis via reducing oxidative stress. J Inflamm. 2017;14(1):1. doi: 10.1186/s12950-016-0148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Q, Li XX, Zhang P, et al. Hydrogen gas protects against serum and glucose deprivation-induced myocardial injury in H9c2 cells through activation of the NF-E2-related factor 2/heme oxygenase 1 signaling pathway. Mol Med Rep. 2014;10(2):1143–1149. doi: 10.3892/mmr.2014.2283 [DOI] [PubMed] [Google Scholar]
- 38.Morshed M, Yousefi S, Stöckle C, Simon HU, Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy. 2012;67(9):1127–1137. doi: 10.1111/j.1398-9995.2012.02868.x [DOI] [PubMed] [Google Scholar]
- 39.Nielsen LP, Peterson CG, Dahl R. Serum eosinophil granule proteins predict asthma risk in allergic rhinitis. Allergy. 2009;64(5):733–737. doi: 10.1111/j.1398-9995.2008.01869.x [DOI] [PubMed] [Google Scholar]
- 40.Pierini A, Schneidawind D, Nishikii H, Negrin RS. Regulatory T cell immunotherapy in immune-mediated diseases. Curr Stem Cell Rep. 2015;1(4):177–186. doi: 10.1007/s40778-015-0025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correale J, Farez MF, Ysrraelit MC. Role of prolactin in B cell regulation in multiple sclerosis. J Neuroimmunol. 2014;269(1–2):76–86. doi: 10.1016/j.jneuroim.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita T, Baatjes A, Smith SG, et al. Natural regulatory T cells in isolated early responders compared with dual responders with allergic asthma. J Allergy Clin Immunol. 2014;133(3):696–703. doi: 10.1016/j.jaci.2013.08.025 [DOI] [PubMed] [Google Scholar]