Abstract
The preformed antimicrobial compounds produced by maize, 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one and its desmethoxy derivative 2,4-dihydroxy-2H-1,4-benzoxazin-3-one, are highly reactive benzoxazinoids that quickly degrade to the antimicrobials 6-methoxy-2-benzoxazolinone (MBOA) and 2-benzoxazolinone (BOA), respectively. Fusarium verticillioides (= F. moniliforme) is highly tolerant to MBOA and BOA and can actively transform these compounds to nontoxic metabolites. Eleven of 29 Fusarium species had some level of tolerance to MBOA and BOA; the most tolerant, in decreasing order, were F. verticillioides, F. subglutinans, F. cerealis (= F. crookwellense), and F. graminearum. The difference in tolerance among species was due to their ability to detoxify the antimicrobials. The limited number of species having tolerance suggested the potential utility of these compounds as biologically active agents for inclusion within a semiselective isolation medium. By replacing the pentachloronitrobenzene in Nash-Snyder medium with 1.0 mg of BOA per ml, we developed a medium that resulted in superior frequencies of isolation of F. verticillioides from corn while effectively suppressing competing fungi. Since the BOA medium provided consistent, quantitative results with reduced in vitro and taxonomic efforts, it should prove useful for surveys of F. verticillioides infection in field samples.
Fusarium verticillioides (synonym, F. moniliforme; teleomorph, Gibberella moniliformis; mating population A of the Gibberella fujikuroi species complex) is a cosmopolitan ascomyceteous fungus consistently associated with maize worldwide. F. verticillioides is not host specific and has been reported to exist in association with many plant species in addition to maize (6), even though the exact number may be confounded by historical confusion over fungal species delimitation and taxonomy (24, 29). Host range and plant-fungus interactions are of significant interest in terms of understanding the distribution, biology, and population dynamics of this mycotoxigenic fungus. While a number of mycotoxins are produced by F. verticillioides (3), fumonisin B1 is of greatest concern because of its causal role in equine leukoencephalomalacia (33), porcine pulmonary edema (12), liver cancer in laboratory rats (40), and possibly human esophageal cancer (32). Thus, consistent, quantitative procedures are important for the surveillance and isolation of F. verticillioides and its toxins. Assessment of infection in field samples typically involves use of the Nash-Snyder (36) pentachloronitrobenzene (PCNB) medium, a semiselective medium allowing isolation of many Fusarium species.
While several major diseases of maize, including seed rot, seedling blight, root rot, stalk rot, and ear rot, are attributed to F. verticillioides (27, 53), the fungus is capable of persistent symptomless (endophytic) infections (4), which is of concern because of the impact on control strategies. In an effort to identify host resistance factors, various plant defense responses to F. verticillioides infection of germinating kernels have been studied (9, 14, 21). In addition to these protein-based defenses, maize begins to synthesize and store chemical defenses once germination is initiated. Collectively referred to as benzoxazinoids or cyclic hydroxamic acids, the main compounds produced by maize are 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one (DIMBOA) and its desmethoxy derivative 2,4-dihydroxy-2H-1,4-benzoxazin-3-one (DIBOA), with the former occurring in greater concentrations (19, 59). Wheat also produces both of these compounds, while rye produces just DIBOA (59). Other grains, such as rice or sorghum, do not produce benzoxazinoids. DIMBOA and DIBOA are initially produced and stored as stable, biologically inactive β-glucosides. Upon plant cell disruption, the glucosides are enzymatically converted to the biologically active aglycones by β-glucosidase (22). Because they are preformed compounds that are immediately available upon pathogenic attack or cellular damage, these compounds are referred to as phytoanticipins (49).
Free DIMBOA and DIBOA are highly reactive and spontaneously degrade to the corresponding benzoxazolinones, 6-methoxy-2-benzoxazolinone (MBOA) and 2-benzoxazolinone (BOA), respectively (22). The half-life for DIMBOA or DIBOA is 24 h or less in aqueous solution (pH 5 to 7.5) at 25°C (54). Many insects, fungi, and bacteria are deterred or inhibited by these compounds, resulting in increased plant resistance (1, 13, 15, 26, 39). However, the main fungal inhabitant of maize, F. verticillioides, detoxifies MBOA and BOA within 24 h by actively metabolizing them into N-(2-hydroxy-4-methoxyphenyl)malonamic acid (HMPMA) and N-(2-hydroxyphenyl)malonamic acid (HPMA), respectively (46, 58). Likewise, Fusarium subglutinans, which is also a common pathogen associated with maize, can transform MBOA and BOA into HMPMA and HPMA, but it does so at half the rate of F. verticillioides (51).
The objective of this study was to screen Fusarium species from diverse hosts and geographic locations to identify species that are tolerant to MBOA and BOA. As a result of this survey, we developed a semiselective isolation medium based on the antimicrobial activity of BOA. By utilizing the taxonomically limited capacity to tolerate BOA, this BOA medium, a modification of the PCNB medium of Nash and Snyder (36), significantly enhances the frequency of isolation of F. verticillioides while reducing the frequency of isolation of sensitive fungi. Thus, in vitro and taxonomic efforts are reduced, and the resulting frequencies may more accurately reflect the true level of F. verticillioides infection in field samples.
MATERIALS AND METHODS
Fungal strains assessed for tolerance to BOA and MBOA.
We assessed 125 strains from 29 species of Fusarium for tolerance to the corn antimicrobials, and they represent a broad range of geographical locations and host organisms. The strains examined are listed below, along with their locations and host organisms, and for those isolates deposited in multiple culture collections, the source and strain number used for this study are given first (25, 28, 30, 31, 41, 42, 56). Designations for the sources of the strains examined are as follows: ARSEF, Agricultural Research Service (ARS) Collection of Entomopathogenic Fungi, Ithaca, N.Y.; ATCC, American Type Culture Collection, Manassas, Va.; CBS, Centraalbureau voor Schimmelcultures, Baarn, The Netherlands; FGSC, Fungal Genetics Stock Center, Department of Microbiology, University of Kansas Medical Center, Kansas City; FRC, Fusarium Research Center, Pennsylvania State University, University Park; IMI, International Mycological Institute, Egham, England; JFL, John F. Leslie, Department of Plant Pathology, Kansas State University, Manhattan; MRC, Medical Research Council, Tygerberg, South Africa; NRRL, Northern Regional Research Laboratory (= NCAUR), USDA ARS, Peoria, Ill.; and RRC, Russell Research Center, USDA ARS, Athens, Ga. For long-term storage, conidia were frozen at −80°C in 15% glycerol. For routine culturing, the spores were streaked onto potato dextrose agar (PDA) (Difco, Detroit, Mich.) and incubated at 23°C in the dark.
Strains of F. verticillioides examined.
The following F. verticillioides strains were examined: FGSC 7983, lab strain; FGSC 8067, lab strain; FGSC 8070, lab strain; JFL A00015 (= FGSC 6895), lab strain; JFL A00102 (= FGSC 7598), California, sorghum; JFL A00149 (= FGSC 7600 = FRC M3125 = NRRL 20956), California, maize; JFL A00169, Italy, rice; JFL A00171 (= FGSC 7601), Italy, rice; JFL A00195, Guatemala, maize; JFL A00206, Brazil, sorghum; JFL A00273, Taiwan, sugarcane; JFL A00501, Kansas, maize; JFL A00708, Georgia, rye; JFL A00999 (= FGSC 7603 = ATCC 201261 = FRC M3703 = NRRL 20984), Indiana, maize; JFL A02976 (= FRC M3839), Alabama, soil; JFL A03823 (= FRC M1212), Turkey, banana; JFL A03957, Thailand, maize; JFL A04426 (= FRC M6537), Thailand, banana; JFL A04516 (= FRC M5496 = FGSC 7606 = NRRL 22055), Nepal, maize; JFL A04524 (= FRC M5500), Nepal, maize; JFL A04643, lab strain; JFL A04801 (= MRC 4315), South Africa, maize; JFL A04930 (= FRC M5042), Nigeria, sorghum; JFL A04934 (= FRC M5067), Nigeria, millet; JFL A07203, Costa Rica, maize; JFL A08264, Uruguay, maize; MRC 826 (= FRC M1325 = NRRL 13447), South Africa, maize; MRC 1069, South Africa, maize; NRRL 13563 (= ATCC 52131), North Carolina, Pinus taeda; NRRL 22001 (= JFL A01562 = FRC M5331), China, rice; NRRL 22050 (= JFL A04362), Egypt, sorghum; NRRL 22052 (= JFL A04424 = FRC M6536), Thailand, banana; NRRL 25058 (= CBS 167.87), United States, Pinus sp.; NRRL 25059 (= CBS 624.87), Honduras, banana; NRRL 25087 (= ARSEF 2252), France, Diptera on Solidago sp.; NRRL 25115 (= ARSEF 3677), Mexico, whitefly on cauliflower; NRRL 25116 (= ARSEF 3678), California, whitefly on broccoli; NRRL 25117 (= ARSEF 3679), Mexico, whitefly on kale; NRRL 25228 (= IMI 244440), North Carolina, Homo sapiens; NRRL 25368 (= IMI316823), India, Trigonella feonum-graecum; NRRL 25370 (= IMI 312010), Ghana, Triplochiton scleroxylon; NRRL 25383 (= ATCC 60858), Canada, alligator; RRC PAT, Italy, maize; RRC 38, Italy, maize; RRC 371, Georgia, maize; RRC 373, barley seed; RRC 374, toxic maize feed; RRC 386, Georgia, maize; RRC 387, Georgia, maize; RRC 388, Georgia, maize; RRC 389, Georgia, maize; RRC 408, toxic maize feed; RRC 415, Mississippi, maize; RRC 417, Mississippi, maize; RRC 437, Georgia, maize; and RRC 438, Georgia, maize.
Other Fusarium species examined.
Other Fusarium species examined were as follows: Fusarium acutatum NRRL 13309 (= CBS 402.97 = FRC O1117), India, unknown; F. acutatum NRRL 25118 (= ARSEF 3704), Pakistan, aphid on Triticum sp.; Fusarium annulatum NRRL 13614 (= CBS 258.54 = FRC M1636), Vietnam, rice; Fusarium anthophilum NRRL 25214, Germany, Hippeastrum sp.; F. anthophilum NRRL 25216 (= CBS 222.76), Germany, Euphorbia pulcherrima; Fusarium bactridioides NRRL 20476 (= CBS 177.35), Arizona, Cronartium conigenum on Pinus leiophylla; Fusarium begoniae NRRL 25300 (= CBS 403.97), Germany, Begonia elatior hybrid; Fusarium beomiforme NRRL 13606 (= FRC M1425), Australia, soil; F. beomiforme NRRL 25185 (= FRC M1089), Papua New Guinea, soil; Fusarium brevicatenulatum NRRL 25446 (= CBS 404.97), Madagascar, Striga asiatica; F. brevicatenulatum NRRL 25447 (= CBS 100196), Madagascar, S. asiatica; Fusarium bulbicola NRRL 13618 (= CBS 220.76), The Netherlands, Nerine bowdenii; Fusarium circinatum NRRL 25333, South Africa, Pinus patula; F. circinatum NRRL 26431, Japan, Pinus sp.; Fusarium concolor NRRL 13994 (= CBS 183.34) Uruguay, Hordeum sp.; Fusarium cerealis (=F. crookwellense) FRC R6354, Canada, maize; F. cerealis FRC R7161, Canada, wheat; F. cerealis NRRL 25491, The Netherlands, iris; F. cearealis NRRL 29300, New Zealand, Ipomoea batatas; F. cearealis NRRL 29312, Jalisco, Mexico, maize; F. cearealis NRRL 29317, Toluca, Mexico, maize; F. cearealis NRRL 29331, Poland, wheat; F. cearealis RRC 449, unknown; F. cearealis RRC 450, unknown; Fusarium denticulatum NRRL 25189 (= CBS 406.97), Cuba, I. batas; F. denticulatum NRRL 25311 (= CBS 407.97), Louisiana, I. batas; Fusarium dlaminii NRRL 13164 (= FRC M1637 = ATCC 58097 = CBS 175.88), South Africa, maize field soil; Fusarium fujikuroi ATCC 14164, Taiwan, rice; F. fujikuroi JFL C01993 (= FRC M1148), Taiwan, rice; F. fujikuroi JFL C01995 (= FRC M1150), Taiwan, rice; F. fujikuroi JFL C01996 (= FRC M1151), Taiwan, rice; Fusarium globosum NRRL 25190 (= CBS 741.97), Japan, wheat; F. globosum NRRL 26131 (= CBS 428.97 = MRC 6647), South Africa, maize; F. globosum NRRL 26134 (= CBS 431.97 = MRC 6660), South Africa, maize; Fusarium graminearum NRRL 5885, Ohio, maize; F. graminearum NRRL 26916, South Africa, maize; Fusarium inflexum NRRL 20433 (= CBS 716.74), Germany, Vicia faba; Fusarium napiforme NRRL 25196 (= FRC M3560), South Africa, Pennisetum typhoides; Fusarium nygamai NRRL 13448 (= ATCC 58555 = FRC M1375 = CBS 749.97), Australia, sorghum; F. nygamai NRRL 22106 (= CBS 834.85), India, Cajanus sp.; F. nygamai NRRL 25449, Morocco, rice; F. nygamai NRRL 25596 (= ATCC 15645), Greece, tobacco; Fusarium oxysporum NRRL 13307 (= FRC O1080), Florida, tomato; F. oxysporum NRRL 22539 (= CBS 129.81), Florida, Chrysanthemum sp.; F. oxysporum NRRL 22544 (= CBS 167.30), unknown, tomato; Fusarium proliferatum JFL D00666 (= FRC M5123), Kansas, maize; F. proliferatum JFL D01591 (= FRC M5360 = NRRL 22003), China, maize; F. proliferatum JFL D02877 (= FRC M3685), Missouri, sorghum; F. proliferatum JFL D02937 (= FRC M3785), North Carolina, maize; F. proliferatum JFL D04366, Egypt, maize; F. proliferatum JFL D04375, Egypt, cotton; Fusarium pseudoanthophilum NRRL 25206 (= CBS 745.97), Gweru, Zimbabwe, maize; F. pseudoanthophilum NRRL 25209 (= CBS 415.97), Karoi, Zimbabwe, maize; F. pseudoanthophilum NRRL 25211 (= CBS 414.97), Gambiza, Zimbabwe, maize; Fusarium pseudonygamai NRRL 6022 (= CBS 416.97 = MRC 1412), Nigeria, P. typhoides; F. pseudonygamai NRRL 13592 (= FRC M1166 = CBS 417.97), Nigeria, P. typhoides; Fusarium sacchari JFL B01722, Philippines, sorghum; F. sacchari JFL B03828 (= FRC M1217 = NRRL 22042), Germany, Cattleya sp.; F. sacchari JFL B03852 (= NRRL 22043), lab strain; Fusarium sambucinum FRC R9148, North Dakota, potato; F. sambucinum FRC R9240, Idaho, potato; Fusarium subglutinans JFL E01257, Kansas, maize; F. subglutinans JFL E01583 (= FRC M5352 = NRRL 22002), China, maize; F. subglutinans JFL E02972 (= FRC M3833), North Carolina, maize; F. subglutinans JFL E03809 (= FRC M845 = NRRL 22034), Iran, maize; Fusarium thapsinum JFL F00921 (= FRC M5132 = MRC 5708), Kansas, sorghum; F. thapsinum JFL F01054 (= FRC M5594 = MRC 5709), Kansas, sorghum; F. thapsinum JFL F03869 (= MRC 6002), South Africa, sorghum; and Fusarium sp. strain NRRL 25221, Zimbabwe, maize.
Quantitative tolerance to BOA and MBOA.
The antifungal activities of BOA (product no. 157058 from Aldrich Chemical Co., Milwaukee, Wis.) and MBOA (product no. 5349 from Lancaster Synthesis, Morecambe, Lancashire, England, and product no. M0640 from Sigma Chemical Co., St. Louis, Mo.) were assessed by measuring the radial growth of fungal strains in the presence of these compounds. Stock solutions of BOA (100 mg/ml) and MBOA (40 mg/ml) were prepared in ethanol. Fungi were assessed on 0.25, 0.5, 0.75, and 1.0 mg of BOA or MBOA per ml. All plates, including the controls, contained either 1% ethanol (for BOA experiments) or 2.5% ethanol (for MBOA experiments). Using a no. 2 cork borer, inocula were taken from the advancing margins of strains growing on PDA and were transferred to the centers of PDA plates (100-mm diameter) containing either BOA or MBOA at one of the above-mentioned concentrations. Plates were incubated in the dark at 23°C. At 7 days postinoculation, two perpendicular transects intersecting beneath the inoculum were drawn on the bottom of the plates. Radial measurements were made in the four directions along the lines from the edge of the inoculum to the advancing margin of the colony. Percent tolerance to BOA was calculated based on a strain's radial growth on PDA containing 1.0 mg of BOA per ml compared to its growth on control plates. A strain was considered tolerant if it had any measurable radial growth on PDA amended with 1.0 mg of BOA per ml. Additional observations were made 14 days postinoculation to note any general differences in growth responses. Each fungus was assessed twice, with three replicates each time. Significant differences in mean radial growth were statistically assessed by analysis of variance and t tests (least significant difference) using the SAS System for Windows (version 6.12; SAS Institute Inc., Cary, N.C.).
Qualitative tolerance to BOA.
Inocula were taken as described above and transferred to PDA containing 1.0 mg of BOA per ml in 24-well culture plates. Each strain was placed in three separate wells and incubated in the dark at 23°C. Growth was assessed at 7 days postinoculation. Fungi were scored as tolerant if radial mycelial growth filled the well. Only those strains showing a mixed response were assessed a second time.
Assessment of metabolism of BOA and MBOA by TLC.
We developed a thin-layer chromatography (TLC) procedure to assess in vitro BOA and MBOA metabolism by the various Fusarium species. Tolerant strains were grown on PDA containing a 1.0-mg/ml concentration of either BOA or MBOA, while sensitive strains were grown on PDA containing a 0.5-mg/ml concentration of BOA. Depending on the strain and the BOA or MBOA concentration, incubation periods ranged from 7 to 15 days to allow radial growth to roughly the same extent (10 to 20 mm). Agar plugs were taken from the cultures using a no. 5 cork borer and were spotted onto a TLC sheet (catalog no. 4420222; Whatman Ltd., Maidstone, Kent, England) (20 by 20 cm, silica gel coating, 254-nm UV indicator, aluminum backing) along a marked line of origin. TLC sheets were placed in a 50°C oven for 10 min immediately prior to use to remove any ambient moisture. The agar plugs were sampled at three locations within a culture petri dish (100-mm diameter): from the outermost margin of the plate, from just ahead of the advancing margin of the colony, and from within the colony. A plug from each location was placed on the TLC sheet for approximately 30 s with the lower face of the plug in contact with the silica gel. Absorption of moisture from the plug facilitated transfer of extracellular metabolites to the silica gel. A single plug was sampled from each location for cultures growing on 1.0 mg of BOA or MBOA per ml, whereas two plugs were sampled from each location for cultures growing on 0.5 mg of BOA per ml. In the latter case, the two plugs were spotted on the TLC sheet at the same position, with drying of the spot between applications. Tolerant strains identified during the qualitative screen using 24-well culture plates were assessed for metabolism of BOA by taking a single plug from one well and spotting it on a TLC sheet. After all spots were dry, the TLC sheets were developed in a saturated chamber containing toluene-ethyl acetate-formic acid (50:40:10). Developed sheets were dried to evaporate the solvents and then photographed under UV light (254 nm). Metabolites appeared as dark spots. The presumptive HPMA and HMPMA metabolites were recovered individually by scraping off the silica gel containing the metabolite and eluting it in methanol or by chromatography of extracts through a silica gel column (58). UV-visible light absorption spectroscopy (46) and electron impact mass spectroscopy (58) were performed on the presumptive HPMA in comparison to a standard provided by M. D. Richardson. For the presumptive HMPMA, UV-visible light spectroscopy data were compared to published spectra (20, 46).
Plant material used for medium evaluation.
Seed of sweet corn cultivar Silver Queen were externally and internally sterilized (5). Half of the seed were inoculated by submersion for 3 min in a spore suspension (106 to 109 conidia/ml) of F. verticillioides strain RRC PATgus, which was transformed for hygromycin resistance and β-glucuronidase (GUS) reporter gene expression (57). Uninoculated and inoculated seed were planted the following day at an irrigated site at the Georgia Coastal Plain Experiment Station, Tifton. The uninoculated control plants served as indicators of natural infection by F. verticillioides. Appropriate notification and containment procedures for field release of RRC PATgus were conducted according to the 7 Code of Federal Regulations part 340 and a University of Georgia agreement for recombinant DNA experiments.
Vegetative tissues (roots, stems, and leaves) and kernels were sampled from physiologically mature plants. Matured kernels and all plant tissue samples were stored at 4°C until analyzed. The stems, leaves, and roots were each cut into several small sections (approximately 1 cm3) and surface disinfected, as were the kernels, in an excess volume of 100% commercial bleach (5.25% sodium hypochlorite). Samples were agitated on a rotary shaker for 2 to 5 min (kernels) or for 1 min (vegetative tissues), followed by a rinse in sterile distilled water for 1 min. The bleach-killed ends of the vegetative sections were trimmed off. Sterilized tissues were placed on isolation media. The sterilization procedure did not have an apparent effect on the viability of the endophytic fungi within the seed or tissue.
Isolation media and evaluation procedure.
F. verticillioides strain RRC PATgus was used as an indicator of recovery frequency and for medium evaluation. PDA and the PCNB medium of Nash and Snyder (36), the standard semiselective medium for isolation of Fusarium species, were compared with PDA plus 1.0 mg of BOA per ml and with Nash and Snyder's PCNB formulation in which PCNB was replaced with 1.0 mg of BOA per ml. This new formulation, which is herein referred to as the BOA medium, consisted of the following: peptone (Difco), 15 g; KH2PO4, 1 g; MgSO4 · 7H2O, 0.5 g; streptomycin, 0.3 mg; agar, 20 g; H2O, 1 liter; and BOA, 1 g (10 ml of a 100-mg/ml solution in 100% ethanol, added after autoclaving the other combined ingredients and cooling to 50°C). The potassium chloride (KCl) medium of Nelson et al. (38) also was amended with 1.0 mg of BOA per ml. Modified Czapek's minimal medium (44) containing hygromycin B (150 μg/ml) (MM+Hyg) also was used.
Each medium experiment used either 100 kernels or 100 plant tissue pieces from either an inoculated experimental plot or a naturally infected plot. The surface-sterilized samples were plated onto the media. Each plate contained either five kernels or five tissue pieces, with three to six replica plates per experiment. Two experiments were performed for each medium. All plates were arbitrarily arranged on a laboratory bench and incubated under fluorescent lighting at room temperature (22 to 25°C) for 14 to 21 days. The most prominent fungus growing from each kernel or tissue was scored, subcultured, and identified at least to the genus level. Fusarium isolates were identified to species level (37, 38). The selectivity of each medium was determined as the frequency of F. verticillioides emerging from the plant material compared to the frequency of other fungi based on 100 plant samples per experiment. The reported frequencies are averages from two experiments, and data were evaluated by analysis of variance and Student's t test performed in Microsoft Excel (version 2000; Microsoft Corporation).
Assay for GUS expression.
Confirmation that fungal isolates recovered from plant materials were the same as the transformed strain inoculated onto the seed was based on hygromycin resistance and a positive GUS reaction (57). Expression of GUS in isolated hyphae and infected plant tissue was determined by incubation in a staining solution containing 1 mM X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronide cyclohexylammonium salt) in 96-well microtiter plates. Samples were examined microscopically for blue-stained hyphae after 48 h.
RESULTS
Survey of Fusarium species for tolerance to BOA.
Tolerance of fungi to BOA was assessed across 29 species of Fusarium encompassing a wide range of geographical regions and host organisms or substrates. Most species were sensitive to this antimicrobial compound and were unable to grow on media containing 1.0 mg of BOA per ml. BOA was considered fungistatic, since removal of most inocula to unamended PDA resulted in resumed radial growth (data not shown).
Only 11 of the 29 species had some level of tolerance to 1.0 mg of BOA per ml (Table 1). These ranged from highly tolerant species, such as F. verticillioides, to those such as F. beomiforme, which was minimally tolerant. Of the 56 strains of F. verticillioides screened, only NRRL 25059 was sensitive to BOA (Table 1; Fig. 1A). The identity of NRRL 25059 is supported by molecular phylogenetic analysis (41) and fertile crosses with F. verticillioides strain MRC 826 as part of a genetic examination of tolerance and detoxification (data not shown). NRRL 25059 is from banana in Honduras, but other banana isolates from Turkey and Thailand (i.e., JFL A03823, JFL A04426, and NRRL 22052) were tolerant to BOA. While maize was the most common host for the F. verticillioides isolates examined, other host species included sorghum, rice, rye, millet, pine, insects, alligator, and a human.
TABLE 1.
Quantitative assessment of tolerance among Fusarium species
| Speciesa | Strainb | Radial growth (mm) on BOAd | Tolerance (%) to BOAf | Metabolism of BOAg |
|---|---|---|---|---|
| F. verticillioides | JFL A00999 | 8.1 ± 0.9 | 25 | + |
| JFL A04516 | 15 ± 1.4 | 43 | + | |
| MRC 826 | 21 ± 1.7 | 64 | + | |
| NRRL 25059 | 0 | 0 | − | |
| RRC 408 | 19 ± 0.9 | 53 | + | |
| F. beomiforme | NRRL 13606 | 0.3 ± 0.3 | 0.8 | + |
| NRRL 25185 | 0.3 ± 0.4 | 0.9 | − | |
| F. brevicatenulatum | NRRL 25446 | 1.5 ± 1.1 | 5.0 | + |
| NRRL 25447 | 2.1 ± 1.5 | 7.0 | + | |
| F. cerealis | RRC 449 | 15 ± 4.5 | 39 | + |
| RRC 450 | 12 ± 3.5 | 34 | + | |
| F. circinatum | NRRL 25333 | 2.2 ± 0.9 | 7.0 | + |
| NRRL 26431 | 0.2 ± 0.4 | 0.7 | + | |
| F. graminearum | NRRL 5885 | 0.1 ± 0.3 | 0.3 | + |
| NRRL 26916 | 9.3 ± 1.8 | 27 | + | |
| F. oxysporum | NRRL 13307 | 0.6 ± 0.6 | 2.1 | + |
| NRRL 22539 | 0 | 0 | − | |
| NRRL 22544 | 0 | 0 | − | |
| F. proliferatum | JFL D00666 | 0e | 0 | + |
| JFL D02877 | 0e | 0 | + | |
| JFL D02937 | 0e | 0 | + | |
| F. pseudoanthophilum | NRRL 25206 | 0.3 ± 0.6 | 1.3 | + |
| NRRL 25209 | 0 | 0 | − | |
| NRRL 25211 | 0.6 ± 1.2 | 2.2 | + | |
| F. subglutinans | JFL E01583 | 12 ± 0.4 | 41 | + |
| JFL E03809 | 10 ± 0.9 | 39 | + | |
| Fusarium sp. | NRRL 25221c | 1.0 ± 1.0 | 3.2 | + |
All taxa except F. beomiforme, F. graminearum, and F. oxysporum are members of the G. fujikuroi complex.
See Materials and Methods for more information on the strains.
Despite its unresolved taxonomy, this strain is phylogenetically distinct from the other taxa (41) and is included here because of its tolerance to BOA and association with maize.
Mean radial growth (± standard deviation) of strains at 7 days postinoculation on PDA amended with 1.0 mg of BOA per ml. Results are based on two experiments with three replicates each.
This strain is considered weakly tolerant because after 14 days incubation it produced measurable growth and metabolized BOA to HPMA. This was not observed for truly sensitive strains.
Values represent radial growth on BOA-amended PDA as a percentage of growth on control plates.
+, ability to metabolize BOA; −, lack of metabolism. Transformation of BOA was assessed by TLC of agar plug samples as described in Materials and Methods.
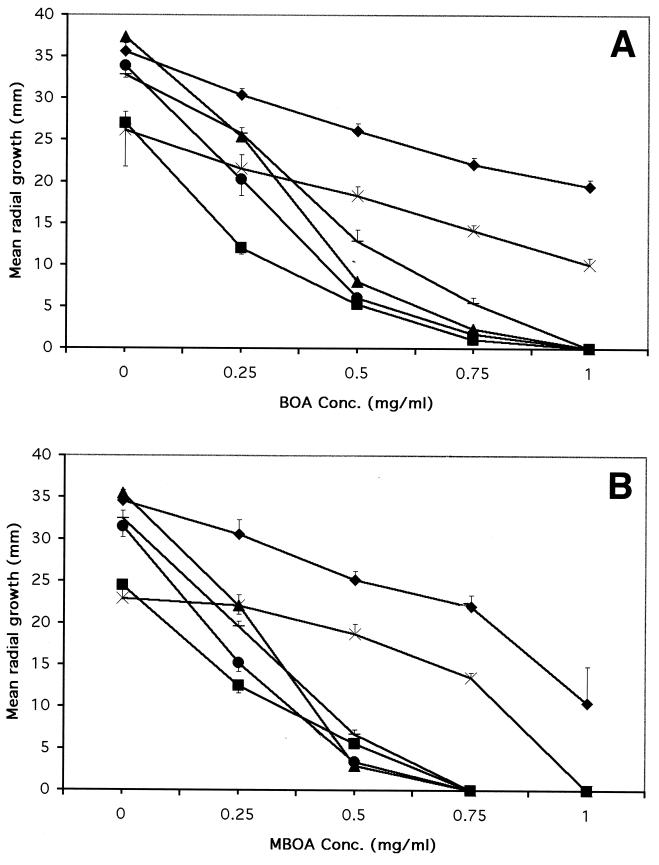
FIG. 1.
Mean radial growth of F. verticillioides RRC 408 (⧫), F. verticillioides NRRL 25059 (■), F. sacchari B01722 (▴), F. proliferatum D02877 (—), F. subglutinans E03809 (×), and F. thapsinum F01054 (●) on BOA-amended PDA (A) and on MBOA-amended PDA (B) at 7 days postinoculation. Mean values are from two experiments with three replicates each. Error bars indicate standard deviations. Extents of growth of F. verticillioides RRC 408 and F. subglutinans E03809 on 1.0 mg of BOA per ml were significantly different from each other and from those of the remaining strains, while the extent of growth of RRC 408 on 1.0 mg of MBOA per ml was significantly different from those of all other strains (P = 0.0001).
Quantification of tolerance.
For the 11 identified tolerant Fusarium species, we measured radial growth of selected strains on PDA amended with BOA and/or MBOA (Table 1 and Fig. 1). The species most tolerant to the antimicrobials were, in decreasing order, F. verticillioides, F. subglutinans, F. cerealis (=F. crookwellense), and F. graminearum (Table 1). The tolerance level varied within some species. For example, F. graminearum NRRL 26916 was moderately tolerant to BOA (27%), but strain NRRL 5885 showed a much weaker tolerance of 0.3%. Most of the tolerant strains having little growth at 7 days postinoculation, e.g., NRRL 5885, had more significant growth after 14 days. If a strain was sensitive to BOA, no growth occurred even after 14 days of incubation. The sensitive species had much reduced radial growth starting at around 0.25 mg of BOA per ml at 7 days postinoculation, with no growth occurring on 1.0 mg of BOA per ml (Fig. 1A).
MBOA was considerably more toxic than BOA (Fig. 1B). For example, F. verticillioides RRC 408 had radial growths of 19 mm on 1.0 mg of BOA per ml but only 11 mm on 1.0 mg of MBOA per ml (Fig. 1). The sensitive species had no radial growth on 0.75 mg of MBOA per ml.
Assessing metabolism by TLC.
Fungi tolerant to BOA could metabolize it to the nontoxic HPMA, while sensitive fungi could not (Table 1 and Fig. 2). Metabolism of MBOA to HMPMA was assessed in the same manner, with the same general results (data not shown). Thus, the basis of tolerance in these strains appears to be the ability to actively metabolize the antimicrobials. Only one strain, F. beomiforme NRRL 25185, exhibited some tolerance but could not metabolize BOA (Table 1). The mechanism underlying this low level of tolerance is unknown.
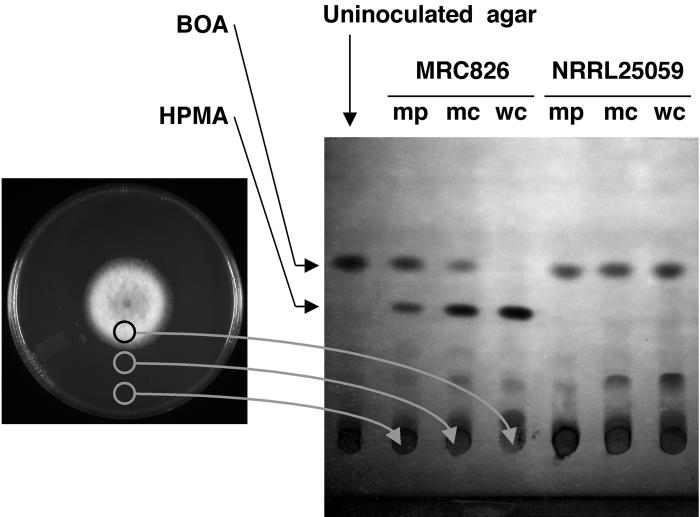
FIG. 2.
Monitoring the decomposition of BOA to HPMA using TLC. TLC profiles for F. verticillioides strains MRC 826 and NRRL 25059 are shown. Cultures were grown on PDA amended with either 0.5 or 1.0 mg of BOA per ml (see Materials and Methods). Agar plugs were taken from three regions of a 7- to 15-day-old culture and spotted onto the TLC sheet as indicated (mp, margin of plate; mc, margin of colony; and wc, within colony). The sheet was developed in toluene-ethyl acetate-formic acid (50:40:10), and the top of the image corresponds to the solvent front.
For tolerant strains such as F. verticillioides MRC 826, the detoxification products HPMA and HMPMA accumulated far ahead of the advancing margin of the fungus (Fig. 2). Their accumulation increased near the colony, while a decrease in BOA and MBOA was observed. Samples taken from within the colony contained only HPMA or HMPMA. TLC profiles for sensitive strains, e.g., F. verticillioides NRRL 25059, showed that the antimicrobials were not metabolized at any point in the plate (Fig. 2).
UV absorbance spectra and electron impact mass spectroscopy data were identical for a standard of HPMA and the metabolite observed by TLC as the BOA detoxification product (data not shown). Only UV absorbance spectra were compared for HMPMA, and these were supportive of the TLC metabolite's identity (data not shown) (20, 58). Thus, the TLC procedure was effective at monitoring the transformation of BOA and MBOA into nontoxic metabolites.
BOA isolation medium.
F. verticillioides was readily isolated from maize kernels on all BOA-amended media, with excellent suppression of contaminating fungi such as other species of Fusarium, Aspergillus flavus, Trichoderma sp., Mucor sp., Penicillium oxalicum, and Penicillium chrysogenum (Table 2). The frequency of isolation of F. verticillioides from kernels produced on inoculated plants was significantly greater on the BOA medium than on the PCNB medium (Table 2). Suppression of competing fungi also was slightly better with the BOA medium. In addition, the frequency of isolation of F. verticillioides from naturally infected kernels was significantly greater on the BOA medium (92%) than on the PCNB medium (65%) (P < 0.002). When PDA was amended with BOA, an insignificant increase in the frequency of isolation of F. verticillioides occurred, but a dramatic decrease in the number of competing fungi was observed (Table 2).
TABLE 2.
Frequency of fungi isolated from surface-disinfected kernels produced on plants infected with F. verticillioides RRC PATgus
| Medium | Isolation frequency (%)a
|
|
|---|---|---|
| F. verticillioides | Fusarium spp. and other fungib | |
| PDA | 46 a | 34 a |
| PDA-BOA | 50 a | 2 b |
| PCNB | 71 b | 9 c |
| BOA medium | 86 c | 5 c |
| MM+Hyg | 37 d | 22 a |
Average recovery of fungi from two experiments, with each experiment having a total of 100 kernels for each medium. Frequencies with in a row do not add to 100% since some kernels were not infected. In each column, numbers followed by the same letter are not significantly different (P < 0.002).
Includes A. flavus, P. oxalicum, P. chrysogenum, and species of Fusarium and Trichoderma.
Comparison of the 37% frequency of isolation of F. verticillioides on MM+Hyg with the 86% isolation frequency on BOA medium (Table 2) suggests that much of the F. verticillioides isolated on BOA medium may not have been the inoculated hygromycin-resistant transformant, RRC PATgus. However, the relatively low frequency of F. verticillioides on MM+Hyg also may have resulted from suppression and overgrowth by a large number of contaminating fungi. GUS reporter gene expression was assessed only for the F. verticillioides isolates recovered on MM+Hyg, and all were positive. The apparent isolation of only the transformed strain RRC PATgus on MM+Hyg, which contains 150 μg of hygromycin B per ml, is consistent with the native level of hygromycin sensitivity among field isolates of F. verticillioides (56).
In addition to kernels, we also assessed vegetative tissues for F. verticillioides infections using the BOA and PCNB media. All vegetative tissues yielded F. verticillioides, and the isolation frequency was higher on BOA medium (83%) than on the PCNB medium (54%). Other Fusarium species that were occasionally isolated from kernels and plant tissues included F. proliferatum, F. subglutinans, F. culmorum, and F. graminearum.
The addition of BOA to the various media reduced the growth rate of tolerant fungi but did not drastically alter fungal morphology, thus allowing for early identification of Fusarium species, especially on the BOA and PDA-BOA media. The PCNB medium altered both sporulation and morphology of Fusarium isolates, which prevented early identification of suspect fungi since they had to be transferred to PDA or other media. Since the PCNB and BOA medium formulations differ only in the respective chemical agents, the morphological aberrations appear to be due to the compound PCNB. Adding BOA to the KCl medium, a sporulation-inducing medium used for identification of some Fusarium species, did not prevent the production of long chains of conidia characteristic of F. verticillioides. However, isolation frequencies based on the KCl-BOA medium were less consistent, because the fungi did not grow as distinct colonies on the plates but rather grew as aerial hyphae over the entire plate, especially the lid, preventing valid counts.
Many of the fungi sensitive to BOA grew only on the plant tissue and not on the agar surface. For example, P. oxalicum grew on the upper surfaces of kernel tips but did not grow on the surfaces of BOA-amended media. In all media amended with BOA, the growth of yeasts, bacteria, and filamentous fungal species such as Rhizopus and Trichoderma was either suppressed or prevented. If fungi sensitive to BOA were present together with F. verticillioides on a BOA-amended medium, they would begin to grow and colonize the medium once F. verticillioides initiated its metabolism of BOA. Thus, the presence of another species on the BOA medium in addition to F. verticillioides need not mean that the other species also was tolerant to BOA. Plant samples placed on the BOA medium must be monitored closely for fast-growing contaminant fungi that may initiate growth after F. verticillioides begins to detoxify BOA. However, we generally observed such problems only if plates were incubated for longer than 14 to 21 days. In most cases, F. verticillioides infections could be assessed at 7 days postinoculation, but the longer incubations were conducted to ensure accurate quantification of frequencies.
DISCUSSION
We found that the ability to detoxify the benzoxazolinones MBOA and BOA varies among species within and outside the G. fujikuroi species complex. We selected for this study Fusarium species that either were phylogenetically related to F. verticillioides (41) or were associated with maize, wheat, or rye, which are producers of the benzoxazinoids (39). Of 29 Fusarium species examined, 11 species had some level of tolerance to BOA (Table 1), with the most tolerant being F. verticillioides, F. subglutinans, F. cerealis, and F. graminearum. The majority of the tolerant strains were associated with maize. Except for one strain (NRRL 25185), tolerance could be attributed to the ability to detoxify BOA, while sensitivity was apparently due to the lack of detoxification.
Fungal detoxification of plant antimicrobial compounds is known to enhance virulence and in some associations is essential for pathogenicity (8, 11, 43, 50, 52). Determining if a correlation exists between benzoxazinoid detoxification and fungal virulence depends on measurement of in planta aspects of infection, colonization, and compatibility. If detoxification of benzoxazinoids enhances fungal infection and virulence, the effect is probably of greatest value at the seedling stage of plant development, because this is when the compounds are in the greatest concentration (1, 19, 26, 39). While these antimicrobials are continuously synthesized throughout plant development, the concentration per unit of biomass is diluted as the plant matures (26). Thus, the potential impact of detoxification on virulence may be greatest in terms of seedling blight and root rot. Also, the impact of antimicrobial detoxification on endophytic colonization must be addressed, because infections are often established at the seedling stage. The discovery of the sensitive strain of F. verticillioides, NRRL 25059, should enable us to address the genetics and physiology of benzoxazinoid detoxification and its effects on virulence towards corn seedlings and endophytic colonization.
We have already addressed one aspect of the genetic basis for benzoxazinoid detoxification. We screened strains of F. verticillioides that have lost chromosome 12, the smallest and apparently dispensable chromosome (55), for their tolerance to and metabolism of BOA. Strains FGSC 7983, FGSC 8067, and FGSC 8070 were identified by Xu and Leslie (55) among meiotic progeny used to generate a genetic map of F. verticillioides, and all could detoxify BOA. This scenario is unlike that in Nectria haematococca, where detoxification genes for the phytoalexins pisatin and maackiain are located on a dispensable chromosome and strains that have lost the chromosome are less virulent in part due to their inability to detoxify the phytoalexins (17, 34, 52).
Both the broad distribution of benzoxazinoid tolerance among fungal species and the high frequency of tolerant strains within certain species such as F. verticillioides suggest that the detoxification of these compounds may be selectively advantageous. The inability of F. verticillioides strain NRRL 25059, which was isolated from banana, to detoxify BOA could be due to a lack of selection pressure, since banana is not known to produce benzoxazinoids. In relation to taxonomic distribution, the same metabolic transformation of MBOA and BOA to HMPMA and HPMA, respectively, is performed by Gaeumannomyces graminis var. graminis and G. graminis var. tritici (20). These two G. graminis varieties are both pathogens of wheat, a producer of benzoxazinoids.
Strains of F. brevicatenulatum isolated from the dicot S. asiatica (i.e., witchweed) also were tolerant to BOA (Table 1). This parasitic, non-benzoxazinoid-producing plant forms haustoria and penetrates roots of corn, sorghum, and other monocots. Here again there is intimate contact between a benzoxazinoid-producing host (corn) and an endophytic fungus, resulting in the potential need for detoxification of the antimicrobials.
Also tolerant to BOA was F. circinatum (teleomorph, Gibberella circinata; synonym, G. fujikuroi mating population H), a pathogen of pine trees (Table 1). This was initially somewhat surprising, because pine is not known to produce benzoxazinoids. However, a mating study of Fusarium isolates from teosinte in Mexico found that there was limited interfertility between one of the isolates and F. circinatum, indicating a possible close relationship (16). Teosinte is the nearest wild relative of maize and a known producer of benzoxazinoids (10, 47). Recent molecular phylogenetic data support a close relationship between F. circinatum, the Fusarium isolate from teosinte, and the maize pathogen F. subglutinans (E. Steenkamp, personal communication). Therefore, the physiological capacity to detoxify BOA may predate speciation of these taxa. Nothing is known regarding the pathogenicity of F. circinatum toward maize or teosinte, but since the fungus has retained some tolerance to BOA, perhaps it has an unobserved association with these plants. We have not assessed the Fusarium isolate from teosinte for tolerance to BOA.
F. globosum is morphologically and phylogenetically related to other species within the G. fujikuroi complex (41, 45). This affiliation is supported by its production of fumonisin mycotoxins (48). Interestingly, all tested strains of F. globosum were very sensitive to BOA even though they were isolated from maize and wheat, which are producers of MBOA and BOA. The nature of the in planta associations between F. globosum and maize, including infection, colonization, and virulence, needs to be investigated.
The TLC assay for assessment of BOA and MBOA metabolism (Fig. 2) has proven valuable for its ease and rapidity. In all cases but one (F. beomiforme NRRL 25185), tolerance to the antimicrobials was shown to be associated with detoxification (Table 1). TLC also confirmed that sensitivity was associated with the inability to detoxify benzoxazolinones (Fig. 2). Extracellular, as opposed to intracellular, localization of the detoxification products, HMPMA and HPMA, was supported by the TLC assay because of the application procedure for agar plugs. Because the bottom, agar side of plugs was laid onto the TLC sheets, only extracellular aqueous metabolites were absorbed into the silica gel. Thus, the actual detoxification process itself may be extracellular, with the functioning enzyme(s) secreted into the medium. The observation that HPMA accumulates at a distance from the fungal colony (Fig. 2) further suggests that the detoxification process may be extracellular. However, this observation is just as easily explained by simple diffusion of HPMA through the agar along a concentration gradient. Therefore, the detoxification process could be localized to the cell surface, or it could even be intracellular, with uptake and excretion mechanisms necessary for BOA and HPMA transport, respectively. In vitro assays are not supportive of extracellular enzymatic activity but have demonstrated diffusion of the metabolites through the agar (data not shown).
As reported here, tolerance to BOA and MBOA is restricted to only a few Fusarium species. This observation, along with the tolerant nature of F. verticillioides, indicated the possible utility of these compounds as selective agents in an isolation medium. Both compounds were very stable in agar media, retaining their integrity and activity for more than a year when stored in the dark at room temperature (data not shown). While BOA is not as toxic to fungi as MBOA (Fig. 1), the lower cost of BOA made it financially less restrictive as an additive. The BOA medium, a modification of the PCNB medium of Nash and Snyder (36), was superior to other media in terms of enhancing the frequency of isolation of F. verticillioides while suppressing the growth of sensitive fungi. The PCNB medium is the traditional standard for isolation of Fusarium species from soil and plant collections (7) and would still be preferred when assessing isolation frequencies of Fusarium species that are sensitive to BOA. However, for those who work with BOA-tolerant species, the BOA medium is an enhancement over the PCNB medium because of the limited taxonomic distribution of BOA tolerance and the production of normal colony morphologies, which should alleviate the necessity for subculturing in order to identify the isolates. As with the PCNB medium, the BOA medium generally results in fungal isolation within 5 to 7 days, even though we extended the duration of the assays to ensure accurate quantification of infection. To enhance isolation of less tolerant species (e.g., F. graminearum) while still suppressing sensitive fungi, the BOA medium formulation can be amended by adding less BOA (e.g., 0.75 mg/ml).
Prior observations (2, 4, 18, 23, 35) of systemic infections and vertical transmission of F. verticillioides via clonal infection of seed are supported by this study. The genetically marked strain RRC PATgus was transmitted from seed to seed. However, the relatively low recovery of RRC PATgus on the MM+Hyg medium suggests that a large proportion of the F. verticillioides isolates recovered on the other media may have infected the kernels through other pathways, such as the silks (35).
ACKNOWLEDGMENTS
We thank Kerry O'Donnell and David M. Geiser for providing many of the fungal strains, Filmore Meredith and Maurice Snook for chemical analysis of metabolites, and Sarah F. Covert for presubmission review of the manuscript.
This study was supported in part by a Training Grant in Molecular and Cellular Mycology (T32-AI-07373) from the National Institutes of Health (NIH) awarded to A. E. Glenn.
REFERENCES
- 1.Argandona V H, Luza J G, Niemeyer H M, Corcuera L J. Role of hydroxamic acids in the resistance of cereals to aphids. Phytochemistry. 1980;19:1665–1668. [Google Scholar]
- 2.Bacon C W, Bennett R M, Hinton D M, Voss K A. Scanning electron microscopy of Fusarium moniliforme within asymptomatic corn kernels and kernels associated with equine leukoencephalomalacia. Plant Dis. 1992;76:144–148. [Google Scholar]
- 3.Bacon C W, Hinton D M. Fusaric acid and pathogenic interactions of corn and non-corn isolates of Fusarium moniliforme, a nonobligate pathogen of corn. Adv Exp Med Biol. 1996;392:175–191. doi: 10.1007/978-1-4899-1379-1_16. [DOI] [PubMed] [Google Scholar]
- 4.Bacon C W, Hinton D M. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can J Bot. 1996;74:1195–1202. [Google Scholar]
- 5.Bacon C W, Hinton D M, Richardson M D. A corn seedling assay for resistance to Fusarium moniliforme. Plant Dis. 1994;78:302–305. [Google Scholar]
- 6.Bacon C W, Porter J K, Norred W P, Leslie J F. Production of fusaric acid by Fusarium species. Appl Environ Microbiol. 1996;62:4039–4043. doi: 10.1128/aem.62.11.4039-4043.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth C. The genus Fusarium. Kew, Surrey, England: Commonwealth Mycological Institute; 1971. [Google Scholar]
- 8.Bowyer P, Clarke B R, Lunness P, Daniels M J, Osbourn A E. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science. 1995;267:371–374. doi: 10.1126/science.7824933. [DOI] [PubMed] [Google Scholar]
- 9.Casacuberta J M, Puigdomenech P, San Segundo B. A gene coding for a basic pathogenesis-related (PR-like) protein from Zea mays. Molecular cloning and induction by a fungus (Fusarium moniliforme) in germinating maize seeds. Plant Mol Biol. 1991;16:527–536. doi: 10.1007/BF00023419. [DOI] [PubMed] [Google Scholar]
- 10.Chang S, Brewbaker J L, Tang C. Benzoxazolinones in teosinte and Tripsacum. Maize Gen Coop Newslett. 1976;50:32. [Google Scholar]
- 11.Ciuffetti L M, VanEtten H D. Virulence of a pisatin demethylase-deficient Nectria haematococca MPVI isolate is increased by transformation with a pisatin demethylase gene. Mol Plant-Microbe Interact. 1996;9:787–792. [Google Scholar]
- 12.Colvin B M, Harrison L R. Fumonisin-induced pulmonary edema and hydrothorax in swine. Mycopathologia. 1992;117:79–82. doi: 10.1007/BF00497282. [DOI] [PubMed] [Google Scholar]
- 13.Corcuera L J, Woodward M D, Helgeson J P, Kelman A, Upper C D. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one, an inhibitor from Zea mays with differential activity against soft rotting Erwinia species. Plant Physiol. 1978;61:791–795. doi: 10.1104/pp.61.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordero M J, Raventos D, San Segundo B. Differential expression and induction of chitinases and β-1,3-glucanases in response to fungal infection during germination of maize seeds. Mol Plant-Microbe Interact. 1994;7:23–31. [Google Scholar]
- 15.Couture R M, Routley D G, Dunn G M. Role of cyclic hydroxamic acids in monogenic resistance of maize to Helminthosporium turcicum. Physiol Plant Pathol. 1971;1:515–521. [Google Scholar]
- 16.Desjardins A E, Plattner R D, Gordon T R. Gibberella fujikuroi mating population A and Fusarium subglutinans from teosinte species and maize from Mexico and Central America. Mycol Res. 2000;104:865–872. [Google Scholar]
- 17.Enkerli J, Bhatt G, Covert S F. Maackiain detoxification contributes to the virulence of Nectria haematococca MP VI on chickpea. Mol Plant-Microbe Interact. 1998;11:317–326. [Google Scholar]
- 18.Foley D C. Systemic infection of corn by Fusarium moniliforme. Phytopathology. 1962;52:870–872. [Google Scholar]
- 19.Frey M, Chomet P, Glawischnig E, Stettner C, Grun S, Winklmair A, Eisenreich W, Bacher A, Meeley R B, Briggs S P, Simcox K, Gierl A. Analysis of a chemical plant defense mechanism in grasses. Science. 1997;277:696–699. doi: 10.1126/science.277.5326.696. [DOI] [PubMed] [Google Scholar]
- 20.Friebe A, Vilich V, Hennig L, Kluge M, Sicker D. Detoxification of benzoxazolinone allelochemicals from wheat by Gaeumannomyces graminis var. tritici, G. graminis var. graminis, G. graminis var. avenae, and Fusarium culmorum. Appl Environ Microbiol. 1998;64:2386–2391. doi: 10.1128/aem.64.7.2386-2391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo B Z, Chen Z Y, Brown R L, Lax A R, Cleveland T E, Russin J S, Mehta A D, Selitrennikoff C P, Widstrom N W. Germination induces accumulation of specific proteins and antifungal activities in corn kernels. Phytopathology. 1997;87:1174–1178. doi: 10.1094/PHYTO.1997.87.11.1174. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto Y, Shudo K. Chemistry of biologically active benzoxazinoids. Phytochemistry. 1996;43:551–559. doi: 10.1016/0031-9422(96)00330-5. [DOI] [PubMed] [Google Scholar]
- 23.Kedera C J, Leslie J F, Claflin L E. Systemic infection of corn by Fusarium moniliforme. Phytopathology. 1992;82:1138. [Google Scholar]
- 24.Kerenyi Z, Zeller K, Hornok L, Leslie J F. Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl Environ Microbiol. 1999;65:4071–4076. doi: 10.1128/aem.65.9.4071-4076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klittich C J R, Leslie J F. Identification of a second mating population within the Fusarium moniliforme anamorph of Gibberella fujikuroi. Mycologia. 1992;84:541–547. [Google Scholar]
- 26.Klun J A, Robinson J F. Concentration of two 1,4-benzoxazinones in dent corn at various stages of development of the plant and its relation to resistance of the host plant to the European corn borer. J Econ Entomol. 1969;62:214–220. [Google Scholar]
- 27.Kommedahl T, Windels C E. Root-, stalk-, and ear-infecting Fusarium species on corn in the USA. In: Nelson P E, Toussoun T A, Cook R J, editors. Fusarium: diseases, biology and taxonomy. University Park: Pennsylvania State University Press; 1981. pp. 94–103. [Google Scholar]
- 28.Leslie J F. Mating populations in Gibberella fujikuroi (Fusarium section Liseola) Phytopathology. 1991;81:1058–1060. [Google Scholar]
- 29.Leslie J F. Gibberella fujikuroi: available populations and variable traits. Can J Bot. 1995;73:S282–S291. [Google Scholar]
- 30.Leslie J F, Pearson C A S, Nelson P E, Toussoun T A. Fusarium spp. from corn, sorghum, and soybean fields in the central and eastern United States. Phytopathology. 1990;80:343–350. [Google Scholar]
- 31.Leslie J F, Plattner R D, Desjardins A E, Klittich C J R. Fumonisin B1 production by strains from different mating populations of Gibberella fujikuroi (Fusarium section Liseola) Phytopathology. 1992;82:341–345. [Google Scholar]
- 32.Marasas W F O. Fumonisins: their implications for human and animal health. Nat Toxins. 1995;3:193–198. doi: 10.1002/nt.2620030405. [DOI] [PubMed] [Google Scholar]
- 33.Marasas W F O, Kellerman T S, Gelderblom W C, Coetzer J A, Thiel P G, van der Lugt J J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res. 1988;55:197–203. [PubMed] [Google Scholar]
- 34.Miao V P, Covert S F, VanEtten H D. A fungal gene for antibiotic resistance on a dispensable (“B”) chromosome. Science. 1991;254:1773–1776. doi: 10.1126/science.1763326. [DOI] [PubMed] [Google Scholar]
- 35.Munkvold G P, McGee D C, Carlton W M. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology. 1997;87:209–217. doi: 10.1094/PHYTO.1997.87.2.209. [DOI] [PubMed] [Google Scholar]
- 36.Nash S M, Snyder W C. Quantitative estimations by plate counts of propagules of the bean root rot Fusarium in field soils. Phytopathology. 1962;52:567–572. [Google Scholar]
- 37.Nelson P E. Taxonomy and biology of Fusarium moniliforme. Mycopathologia. 1992;117:29–36. doi: 10.1007/BF00497276. [DOI] [PubMed] [Google Scholar]
- 38.Nelson P E, Toussoun T A, Marasas W F O. Fusarium species, an illustrated manual for identification. University Park: Pennsylvania State University Press; 1983. [Google Scholar]
- 39.Niemeyer H M. Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defense chemicals in the Gramineae. Phytochemistry. 1988;27:3349–3358. [Google Scholar]
- 40.Norred W P, Voss K A, Riley R T, Plattner R D. Fumonisin toxicity and metabolism studies at the USDA. Adv Exp Med Biol. 1996;392:225–236. doi: 10.1007/978-1-4899-1379-1_20. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell K, Cigelnik E, Nirenberg H I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465–493. [Google Scholar]
- 42.Onyike N B N, Nelson P E. Fusarium species associated with sorghum grain from Nigeria, Lesotho, and Zimbabwe. Mycologia. 1992;84:452–458. [Google Scholar]
- 43.Osbourn A E. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puhalla J E, Spieth P T. Heterokaryosis in Fusarium moniliforme. Exp Mycol. 1983;7:328–335. [Google Scholar]
- 45.Rheeder J P, Marasas W F O, Nelson P E. Fusarium globosum, a new species from corn in southern Africa. Mycologia. 1996;88:509–513. [Google Scholar]
- 46.Richardson M D, Bacon C W. Catabolism of 6-methoxy-benzoxazolinone and 2-benzoxazolinone by Fusarium moniliforme. Mycologia. 1995;87:510–517. [Google Scholar]
- 47.Shahid M, Esen A. DIMBOA-glu concentration in different taxa of teosinte. Maize Gen Coop Newslett. 1998;72:23–24. [Google Scholar]
- 48.Sydenham E W, Shephard G S, Stockenstrom S, Rheeder J P, Marasas W F O, Van Der Merwe M J. Production of fumonisin B analogues and related compounds by Fusarium globosum, a newly described species from corn. J Agric Food Chem. 1997;45:4004–4010. [Google Scholar]
- 49.VanEtten H D, Mansfield J W, Bailey J A, Farmer E E. Two classes of plant antibiotics: phytoalexins versus “phytoanticipins.”. Plant Cell. 1994;6:1191–1192. doi: 10.1105/tpc.6.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanEtten H D, Sandrock R W, Wasmann C C, Soby S D, McCluskey K, Wang P. Detoxification of phytoanticipins and phytoalexins by phytopathogenic fungi. Can J Bot. 1995;73:S518–S525. [Google Scholar]
- 51.Vilich V, Lohndorf B, Sikora R A, Friebe A. Metabolism of benzoxazolinone allelochemicals of Zea mays by Fusarium subglutinans. Mycol Res. 1999;103:1529–1532. [Google Scholar]
- 52.Wasmann C C, VanEtten H D. Transformation-mediated chromosome loss and disruption of a gene for pisatin demethylase decrease the virulence of Nectria haematococca on pea. Mol Plant-Microbe Interact. 1996;9:793–803. [Google Scholar]
- 53.White D G. Compendium of corn diseases. 3rd ed. St. Paul, Minn: APS Press; 1999. [Google Scholar]
- 54.Woodward M D, Corcuera L J, Helgeson J P, Upper C D. Decomposition of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one in aqueous solutions. Plant Physiol. 1978;61:796–802. doi: 10.1104/pp.61.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J-R, Leslie J F. A genetic map of Gibberella fujikuroi mating population A (Fusarium moniliforme) Genetics. 1996;143:175–189. doi: 10.1017/s0016672300034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan K, Dickman M B, Xu J-R, Leslie J F. Sensitivity of field strains of Gibberella fujikuroi (Fusarium section Liseola) to benomyl and hygromycin B. Mycologia. 1993;85:206–213. [Google Scholar]
- 57.Yates I E, Hiett K L, Kapczynski D R, Smart W, Glenn A E, Hinton D M, Bacon C W, Meinersmann R, Liu S, Jaworski A J. GUS transformation of the maize fungal endophyte Fusarium moniliforme. Mycol Res. 1999;103:129–136. [Google Scholar]
- 58.Yue Q, Bacon C W, Richardson M D. Biotransformation of 2-benzoxazolinone and 6-methoxy-benzoxazolinone by Fusarium moniliforme. Phytochemistry. 1998;48:451–454. [Google Scholar]
- 59.Zuniga G E, Argandona V H, Niemeyer H M, Corcuera L J. Hydroxamic acid content in wild and cultivated Gramineae. Phytochemistry. 1983;22:2665–2668. [Google Scholar]