Abstract
Objectives
Dynamics of antibody responses following SARS-CoV-2 infection are controversial in terms of immunity and persistence. We aimed to assess longitudinally the trend of antibody serological titres, their correlation with clinical severity as well as clinical reinfection during a follow-up.
Design
Longitudinal cohort, 12 months follow-up study.
Setting
USL Umbria 2.
Participants
Consecutive subjects aged 15–75 who were discharged with the diagnosis of Sars-Cov-2 from the hospitals of the AUSL Umbria 2, or resulted positive to a PCR test for SARS-CoV-2 infection with or without symptoms were recruited. SARS-CoV-2 serological testing for antibodies targeting the Nucleocapside and Spike proteins were determined.
Results
Of 184 eligible subjects, 149 were available for evaluation: 17 were classified as oligo/asymptomatic, 107 as symptomatic, 25 as hospital admitted. Participants differed in terms of signs and symptoms as well as treatment. Overall there was a significant difference in terms of antibody titres between groups (anti-S: p<0.00; anti-N: p=0.019). Median anti-S titres in the symptomatic and hospital admitted participants were significantly higher compared with the oligo/asymptomatic participants. During follow-up, the median titre of anti-S antibodies did not show significant variations (p=0.500) and the difference within groups remained constant overtime. Subjects that showed an anti-S titre above the threshold of 12 U/mL were 88.7% at first visit and 88.2% at last follow-up. Anti-N values were higher in the hospital admitted participants compared with the other two groups. Anti-N titre reduced constantly overtime (p<0.001) and across the three groups of participants. The percentage of the subjects with serological titre above threshold (<1.4 U/mL) decreased from 74.5%% to 29.2% (p<0.001). None of the participants developed clinically evident reinfection.
Conclusion
Anti-N and anti-S correlate well with clinical severity. While anti-N declines overtime, anti-S antibodies persist for at least 1 year.
Keywords: infectious diseases, COVID-19, virology, immunology
Strengths and limitations of this study.
The key strength of this study is the evaluation of anti-Sars-Cov-2 serology using two types of serological assays and the follow-up that endured for at least 12 months.
In addition to serological evaluation, participants were also followed up clinically.
The study does not have a baseline serological testing since it was conceived in late April 2020 when most of the participants were discharged from hospital or had their symptoms resolved.
The study lacks clinical and serological information regarding those who died during the pandemic event, hence we are unable to conclude whether quantitative serological testing could predict survival.
Background
COVID-19 infection is associated with severe morbidity and mortality, and it presents important challenges in different settings including prevention, treatment as well as diagnostic and prognostic significance based on immune response. The diagnosis of COVID-19 disease depends much on clinical or epidemiological context though it is mainly based on the molecular testing of symptomatic subjects. Since false-negative nasopharyngeal RT-PCR tests results are not infrequent,1–4 the antibody tests allow for better collection of epidemiological data, determination of the immune status of asymptomatic individuals, and screening of previous exposure.5 Hence, COVID-19 serological tests, despite their limitation and somewhat challenging performance characteristics, can be an appropriate tool to better diagnose recent or past infection.4
Serological tests have been introduced to detect antigens namely the spike protein (S), the protein nucleocapside (N) and the virus membrane.6 N and S proteins were found to be the major immunogenic proteins.7 As in the MERS-CoV infection, antibodies against proteins S, 3a, N and 9b were detected in the sera from convalescent-phase SARS patients.7 Though anti-S and anti-N were dominant and could persist in the sera of SARS patients until week 30, only anti-S3 demonstrated significant neutralising activity.7
Current methods available for serological testing include rapid diagnostic test, ELISA, neutralisation assays and chemiluminescent immunoassays (CLIA).6 ELISA and CLIA are considered suitable for first line screening because of the large throughput, short processing time and simple operating procedure.8
A Cochrane review evaluated the diagnostic accuracy of antibody tests—using RT-PCR as reference standard—to determine whether a person presenting in the community or in primary or secondary care has SARS-CoV-2 infection.9 The authors concluded antibody tests are likely to have a useful role for detecting previous SARS-CoV-2 infection if used 15 (sensitivity 91%; 95% CI 87% to 94%) or more days after the onset of symptoms.9
In addition to antibody profile, longitudinal persistence of immunity in convalescent COVID-19 subjects has been another issue of debate for months after the first pandemic. An observational study published during that pandemic10 found in 23 patients a positive correlation between enzyme immunoassay antibodies and neutralising antibody titre but concluded that further investigation is needed on the role of anti-COVID antibodies in immunopathology and/or antiviral treatment.10
We performed a longitudinal cohort study in Umbria of subjects with a confirmed diagnosis of Sars-Cov-2 between February and April 2020 with a follow-up of at least 12 months. Levels of IgG antibodies against SARS-CoV-2 Nucleocapside (N), and neutralising antibodies were determined.
The objectives of our study were (1) to describe differences in clinical and treatment characteristics between clinical categories of subjects who had SARS-CoV-2 (oligo/asymptomatic, symptomatic and hospital admitted); (2) to assess the correlation between serological titres and the clinical categories; (3) to evaluate the trend of anti-Sars-Cov-2 titres among the clinical categories over a follow-up of 12 months. In addition, we performed a clinical and history evaluation of the participants for a possible viral infection at every time follow-up.
Methods
Study design and target population
This study was prospective longitudinal in design. Our cohort of interest was characterised by consecutive subjects aged 15–75 who, from February 2020 to April 2021, (1) were discharged with the diagnosis of SARS-CoV-2 from the hospitals of the AUSL Umbria 2 or (2) resulted positive to a PCR test for Sars-Cov-2 infection. These subjects were invited to undertake serological SARS-CoV-2 testing for antibodies targeting the Nucleocapside (N) protein and S proteins of SARS-CoV-2. All the cohort was clinically and serologically followed-up longitudinally. After enrolment, serology testing was performed every 3–4 months for every participant until the end of follow-up. Clinical signs and symptoms as well as specific COVID-19 treatments were recorded at baseline. During follow-up, at the time of sample collection, participants were evaluated for potential COVID-19 related clinical reinfection or rehospitalised on reinfection.
For our analysis, participants were categorised as follows: (1) oligo/asymptomatic, (2) symptomatic and (3) hospital admitted. Oligosymptomatic were those participants with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia) that may last for more than 3 days. Conversely, symptomatic patients were those with more than one symptom lasting at least 3 days and without any hospital admission.
Laboratory methods
Serum samples were analysed using two commercial serological assays: Abbott SARS-CoV-2 IgG, DiaSorin Liaison SARS-CoV-2 S1/S2 IgG.
The qualitative detection of anti-N IgG was performed using a chemiluminescent microparticle immunoassay (Abbott ARCHITECT SARS-CoV-2 IgG). A signal/cut-off ratio of ≥1.4 was interpreted as reactive according to the manufacturer’s instructions.11 Studies report that clinical sensitivity is time-dependent and after day 14 it ranges between 84.2% and 100% whereas specificity results 99.6%–100%.12 13 Prior to analyses of patient samples, calibration was performed and negative quality control signal/cut-off ratio ≤0.78 and positive quality control signal/cut-off ratio 1.65–8.40 were achieved.
The quantitative detection of anti-S IgG was evaluated using a standardised automated chemiluminescent assay (DiaSorin S.p.A., Saluggia, Italy). A detection of ≥12 AU/mL was interpreted as positive according to the manufacturer’s instructions.14 The test’s sensitivity is time-dependent, that is 25% in the first 5 days after RT-PCR-confirmed diagnosis, 90% from day 5 to day 15, and 97% from day 15 forward.
Statistical analysis
Demographic characteristics of the study participants was described by calculating the frequencies and percentages for categorical variables, and medians and IQRs for continuous variables. The analysis of the normal distribution of the sample was evaluated analytically with the Kolmogorov-Smirnov test and visually with the Q-Q plots.
Trends of anti-SARS-CoV-2 titres among the three groups (ie, hospital admitted, symptomatic, olygo/asymptomatic) have been analysed using a mixed effects model for repeated measurements (MMRM). Logarithm transformations of the anti-SARS-CoV-2 titres were used as dependent variables to meet the normality assumption. The model included the group, follow-up and group by follow-up interaction as fixed effects. The interaction was regardless of significance. Group comparisons at each follow-up were estimated by differences between least squares means from the group by follow-up interaction, with accompanying p values and 95% CIs. An unstructured covariance matrix has been used for random effects. We also modelled the evolution of positivity of serological titres (ie, <12 U/mL for anti-S titre and <1.4 U/mL for anti-N titre) with an MMRM with a binomial logit link. In hospitalised subjects, anti-S titre was above the cut-off, so we weren’t able to include these patients in the model. Furthermore, due to the few negative patients, we fitted only models with titre positivity depending on clinical characteristics or follow-up. For anti-N titre positivity, we were able to fit a full model with the group, follow-up and group by follow-up interaction as fixed effects. The interaction was included in the model regardless of significance. Due to convergence issues, a Toeplitz covariance matrix has been used for the random effects. To test the hypothesis of a different rate of decline of anti-SARS-CoV-2 titres depending of baseline levels, we also performed an MMRM with change from baseline in the values of anti-SARS-CoV-2 titres as dependent variables. The models included baseline, follow-up and baseline by follow-up interaction.
For all the models, parameters have been estimated using Restricted Maximum Likelihood (REML) with the Newton-Raphson algorithm and Kenward-Roger method for calculating the df. Point estimates and 95% CIs were plotted for the MMRM of log anti-SARS-CoV-2 titres.
All other models were fitted using the Proc Mixed and Proc Glimmix procedures from the SAS software V.9.4 (SAS Institute).
Results
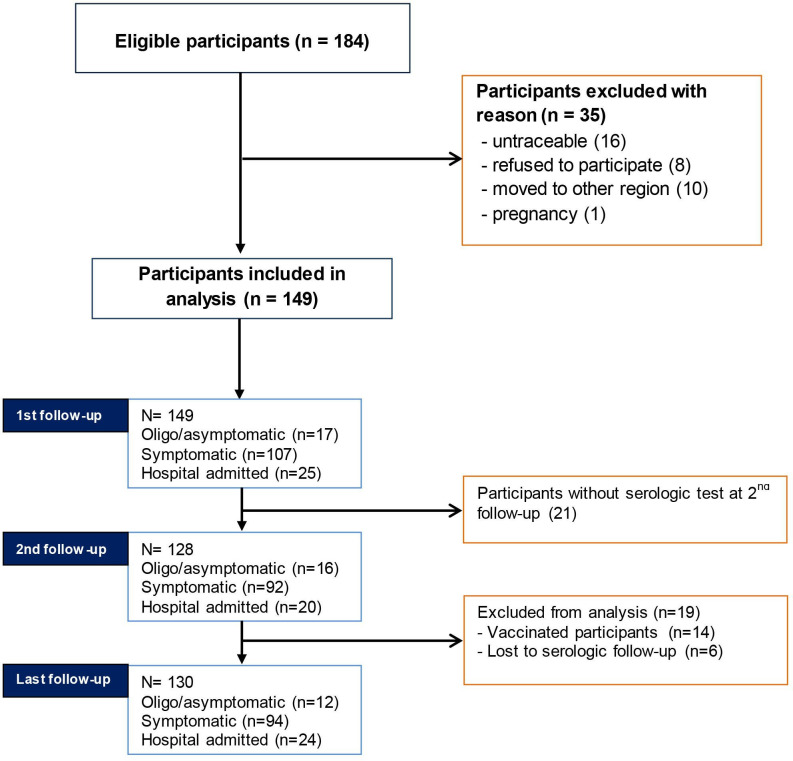
A total of 184 potentially eligible subjects were identified. After excluding 35 subjects with reasons 149 met the inclusion criteria and signed the informed consent. Of this cohort, 21 were not available to perform the serological test at the second follow-up but 19 of these returned for the last follow-up. Subsequently, 14 subjects received anti-COVID-19 vaccination and 6 were unavailable for serological testing and were excluded from analysis. At 12 months follow-up, 130 participants were still available for clinical and serological evaluation. All of the excluded subjects at final follow-up were traceable through telephone contact and were possible to obtain their health status. Figure 1 shows the study screen process.
Figure 1.
Study screening process.
Clinical and treatment difference between oligo/asymptomatic, symptomatic and hospital admitted participants
Of the initial cohort, 17 (11%) were oligo/asymptomatic, 107 (72%) were symptomatic participants (without hospital admission), 25 (17%) were participants who were admitted to hospital. The mean age was 49 years (median 54). While 52% of the cohort were female, men tended to have more severe symptoms reaching 80% of the Hospital admitted participants (table 1).
Table 1.
Basic characteristic of the cohort classified by symptom severity
| Oligo/asymptomatic participants* | Symptomatic participants† | Hospital admitted participants | |
| N (%) | 17 (11) | 107 (71) | 25 (16) |
| Male (%) | 11 (64) | 40 (37) | 20 (80) |
| Age (median; p25, p75) | 42 (33–57) | 53 (39–59) | 56 (54–64) |
| Clinical signs and symptoms | |||
| Fever | 7 (44) | 94 (83) | 23 (92) |
| Headache/musculoskeletal pain | 3 (19) | 58 (51) | 11 (44) |
| Ageusia/anosmia | 7 (43) | 59 (52) | 3 (12) |
| Asthenia | 1 (6) | 59 (52) | 8 (32) |
| Cough | 0 (0) | 43 (38) | 23 (92) |
| Dyspnoea | 0 (0) | 17 (15) | 25 (100) |
| Pneumonia | 0 (0) | 3 (3) | 25 (100) |
| Treatment | |||
| Antibiotic | 0 (0) | 28 (25) | 25 (100) |
| Hydroxychloroquine | 0 (0) | 12 (10) | 25 (75) |
| Heparin | 0 (0) | 1 (1) | 24 (96) |
| Antiviral | 0 (0) | 0 (0) | 8 (32) |
| Monoclonal antibody | 0 (0) | 0 (0) | 4 (16) |
| Steroids | 0 (0) | 14 (13) | 5 (20) |
| NSAIDs | 0 (0) | 7 (6) | 0 (21) |
| Paracetamol | 1 (6) | 20 (18) | 1 (4) |
*Oligosymptomatic patients are those with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia).
†Symptomatic patients are those with one more symptom lasting at least 3 days and without any hospital admission.
NSAIDs, Non-steroidal anti-inflammatory drugs.
Type and duration of symptoms
The most common symptom was fever which resulted common to all the three groups. Headache or musculoskeletal pain was common to symptomatic participants whereas cough and dyspnoea was present in all of the admitted participants indicating the severity of the disease. All hospital admitted participants had radiographically documented pneumonia (table 1).
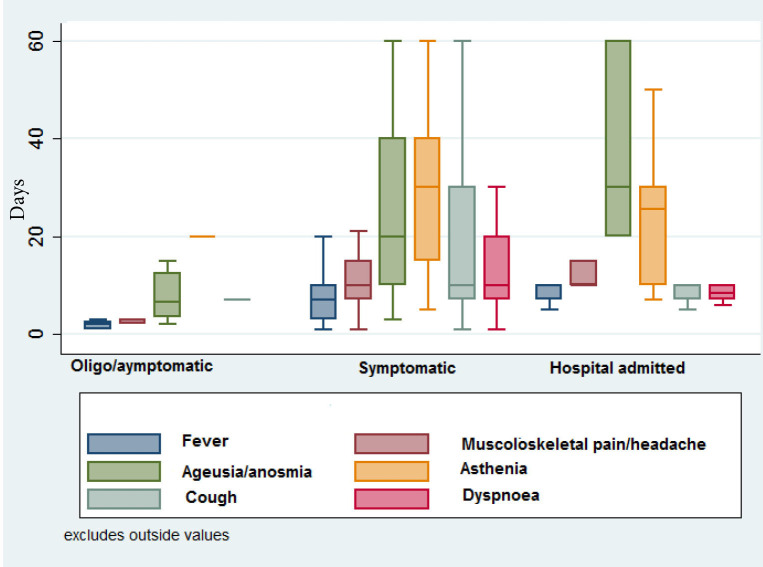
The most persistent symptoms were asthenia (median 30 days) as well as anosmia and/or ageusia (median 30 days). Anosmia/ageusia persisted across the three groups and the median symptoms’ duration increased as severity of symptoms increased (median: 6 days in oligo/asymptomatic participants, 20 days in Symptomatic participants, 30 days in Hospital admitted participants). Similarly, median duration for asthenia was 20 days in the oligo/asymptomatic participants, 30 days in Symptomatic participants and 25 days in hospital admitted participants. In 35 patients, anosmia/ageusia lasted for more than 6 months but resolved completely within 10 months. Duration of symptoms across the three groups of participants are depicted in figure 2. Duration of ageusia/anosmia and asthenia resulted higher in females than in males.
Figure 2.
Duration (in days) of signs and symptoms across the three groups of participants. Numbers in denominators are participants available at follow-up. Oligo-symptomatic patients are those with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia). Symptomatic patients are those with one more symptom lasting at least 3 days and without any hospital admission.
Treatment used
Most of the oligo/asymptomatic participants were not treated or reported the use of paracetamol or anti-inflammatory agents. Anti-inflammatory agents were most used in Symptomatic participants. Twenty-five per cent of symptomatic participants and 100% of hospital-admitted participants used antibiotics. Hydroxychloroquine was used by 10% of the symptomatic participants and by 100% of hospital-admitted participants. Low-dose hepar was almost exclusively used by hospitalised participants. Antivirals and monoclonal antibodies were used in the 32% and 16% of the hospitalised patients, respectively (table 1).
Anti-N and anti-S antibodies: trend and correlation with clinical severity
Anti-spike
The median value of the antibody anti-S (Diasorin) titre at first visit (that is between June and September 2020) across the whole cohort was 71.7 U/mL (IQR 31.0–112.0). The median anti-S titre was 81.0 U/mL, (IQR 31.0–112.0) in males and 65.6 U/mL (IQR 30.0–112.0) in females.
Anti-S antibody values differed significantly across the three groups of participants (p<0.001). At first time follow-up, median titres in the symptomatic (+35.0 U/mL, p=0.001) and hospital-admitted participants (+135.1 U/mL, p<0.001) were significantly higher compared with the oligo/asymptomatic participants; similarly, anti-S titre levels were higher in the Hospital admitted participants compared with symptomatic subjects (+100.0 U/mL, p<0.001) indicating that the more significant were the clinical signs and symptoms the higher was the anti-S antibody response.
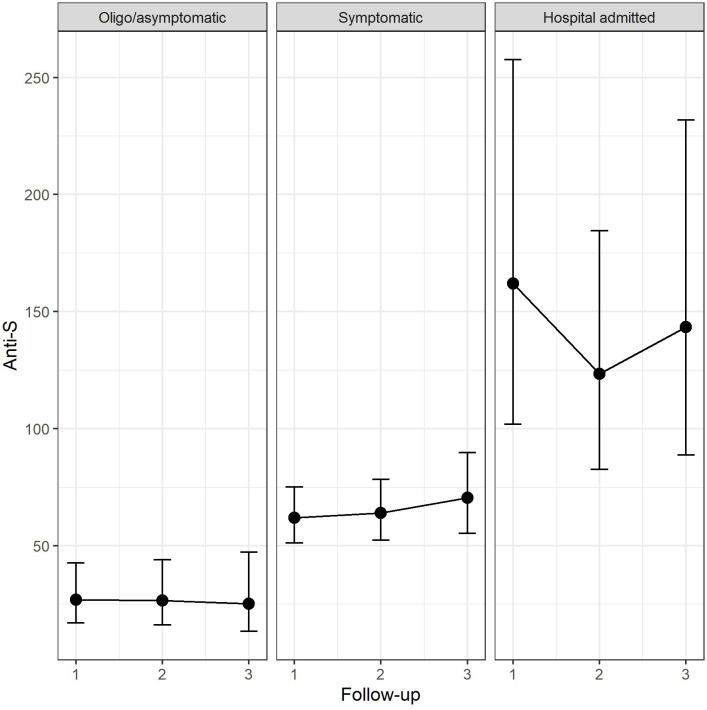
At subsequent follow-up visits the median titre of anti-S antibodies did not show significant variations (p=0.500) and the difference within groups remained constant overtime (table 2). Figure 3 shows estimated values of anti-S antibody titres among groups of participants in each periods of follow-up.
Table 2.
Median (IQR) anti-N and anti-S titres according to clinical classification of participants
| Oligo/asymptomatic* | Symptomatic† | Hospital admitted | Total | |
| Anti-spike serology | ||||
|
First follow-up N=149 |
14.6 (10.0, 85.0) | 69.5 (33.9, 100.0) | 232.0 (112.0, 256.0) | 71.7 (30.0, 112.0) |
|
Second follow-up N=128 |
18.1 (10.0, 86.0) | 66.0 (28.6, 136.0) | 134.5 (75.0, 208.0) | 72.9 (29.1, 144.0) |
|
Last follow-up N=130 |
16.0 (7.2, 92.0) | 72.2 (27.4, 242.0) | 116.0 (82.9, 216.0) | 85.0 (29.1, 190.0) |
| Antinucleocapside serology | ||||
|
First follow-up N=149 |
3.05 (1.19, 4.93) | 3.05 (1.19, 4.93) | 4.55 (2.89, 6.02) | 3.11 (1.39, 5.00) |
|
Second follow-up N=128 |
1.07 (0.36, 4.00) | 1.33 (0.56, 2.40) | 2.44 (1.75,4.22) | 1.7 (0.62, 2.89) |
|
Last follow-up N=130 |
0.34 (0.15, 0.88) | 0.74 (0.31, 1.60) | 1.34 (0.77, 2.38) | 0.8 (0.33, 1.71) |
*Oligosymptomatic patients are those with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia).
†Symptomatic patients are those with one more symptom lasting at least 3 days and without any hospital admission.
Figure 3.
Ab-anti-S titre across the three groups of participants compared across the three periods of follow-up (estimated means and 95% CI). Oligosymptomatic patients are those with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia) Symptomatic patients are those with one more symptom lasting at least 3 days and without any hospital admission.
The value of the antibody anti-S titre at first visit did not influence the rate of change at follow-up.
The subjects that showed an anti-S titre above the threshold of 12 U/mL were 88.7% at first follow-up, 90.4% at the second and 88.2% at the third. Overall there was a significant difference between clinical groups with symptomatic showing a higher probability of positivity across all follow-up visits (p=0.031). The difference across follow-up visits were not significant (p=0.833). None of the hospital admitted subjects had their antibody titre below threshold (table 3).
Table 3.
Persistence of positivity to anti-N and anti-S antibodies and according to clinical classification of participant (n/N (%))
| Oligo/asymptomatic* | Symptomatic† | Hospital admitted | Total | |
| Anti-spike serology | ||||
|
First follow-up N=149 |
5/9 (55.6) | 44/47 (93.6) | 6/6 (100.0) | 55/62 (88.7) |
|
Second follow-up N=128 |
8/11 (72.7) | 59/65 (90.8) | 18/18 (100.0) | 85/94 (90.4) |
|
Last follow-up N=130 |
8/11 (72.7) | 68/78 (87.2) | 21/21 (100.0) | 97/110 (88.2) |
| Antinucleocapside serology | ||||
|
First follow-up N=149 |
10/17 (58.8) | 78/107 (72.9) | 23/25 (92.0) | 111/149 (74.5) |
|
Second follow-up N=128 |
5/11 (45.5) | 39/81 (48.2) | 17/21 (81.0) | 61/113 (54.0) |
|
Last follow-up N=130 |
1/11 (9.1) | 22/80 (27.5) | 10/22 (45.5) | 33/113 (29.2) |
Numbers in denominators are participants available at follow-up.
*Oligosymptomatic patients are those with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia).
†Symptomatic patients are those with one more symptom lasting at least 3 days and without any hospital admission.
Anti-nucleocapside
The median value of the antibody anti-N titre at first visit across the whole cohort was 3.1 U/mL (IQR 1.39–5.00) with non-significant higher values in males (3.84 U/mL, IQR 1.89–5.82) than females (2.50 U/mL, IQR 0.97–4.30). Overall there was a significant difference in terms of antibody titres between groups (p=0.019).
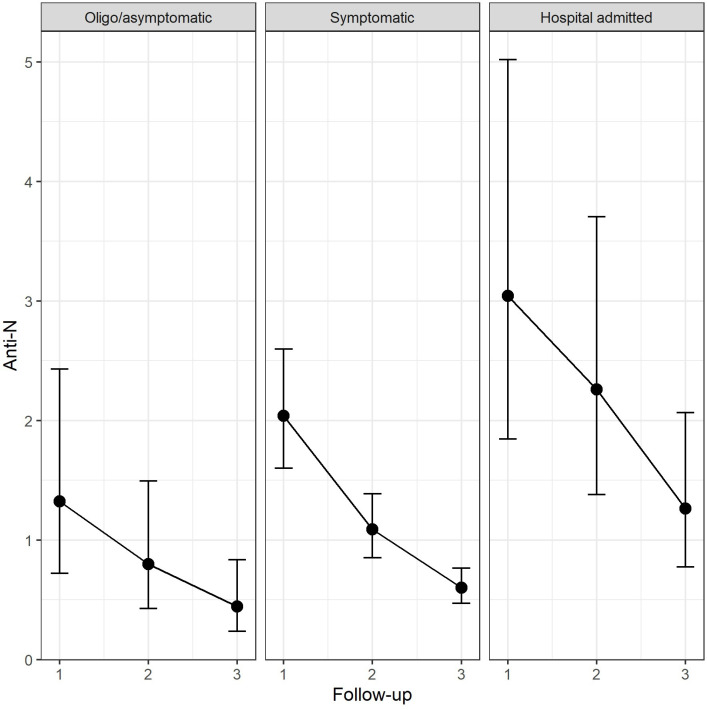
Antibody values were higher in the hospital admitted participants compared with symptomatic participants at follow-up 2 (+1.2 U/mL, p=0.010) and follow-up 3 (+0.7 U/mL, p=0.008). Antibody values were higher in the hospital admitted participants compared with the oligo/asymptomatic participants at follow-up 1 (+0.8 U/mL, p=0.038) follow-up 2 (+1.5 U/mL, p=0.011) and follow-up 3 (+1.7 U/mL, p=0.011). No statistically significant differences were observed between oligo/asymptomatic and symptomatic participants. The antibody titre reduced constantly overtime (p<0.001) (table 2). Figure 4 shows estimated values of anti-N antibody titres among groups of participants in each periods of follow-up.
Figure 4.
Ab-anti-N titre across the three groups of participants compared across the three periods of follow-up (estimated means and 95% CI). Oligosymptomatic patients are those with symptoms enduring for less than 3 days or with only one symptom (anosmia/ageusia or asthenia) symptomatic patients are those with one more symptom lasting at least 3 days and without any hospital admission.
The value of the antibody anti-N titre at first visit did not influence the rate of change at follow-up.
Difference in percentage of anti-N titre between the first and second follow-up and between the first and last follow-up showed a substantial decrease in the serological titre across the three groups of participants (table 3). The percentage of the subjects with serological titre above threshold (<1.4 U/mL) decreased from 74.5% to 29.2% (p<0.001). The percentage of the subjects with serological titre above threshold (<1.4 U/mL) was significantly higher in hospital admitted compared with Oligo/asymptomatic participants (p=0.031).
Clinical follow-up
During serological follow-up participants underwent a history examination and clinical visit. When participants were not available for clinical visit their health status and history examination of recent or past reinfection was ascertained through telephone call. None of the participants in any of the group had any sign or symptom that could be attributed to a possible clinical SARS-CoV-2 reinfection or was hospitalised on reinfection. Since the study did not consider the PCR or antigenic test during follow-up, we cannot exclude that some participants might have developed asymptomatic SARS-CoV-2 reinfection.
Discussion
We enrolled a substantial number of subjects to whom a diagnosis of SARS-CoV-2 was made during the first pandemic episode within the area of local health unit 2 of Umbria where the main hospitals to which participants had access were Foligno, Spoleto and Orvieto.
We first categorised participants according to their clinical status described clinical and therapeutic differences. Subsequently, we performed quantitative determination of anti-S and anti-N antibodies during a 12-month period follow-up and evaluated the trend of both antibodies and their correlation with the severity of disease. Our study showed a positive correlation between severity of symptoms and both anti-N and anti-S titres. Anti-N antibodies titres resulted significantly higher in those participants that had severe symptoms than those who had less significant symptoms, but declined consistently over time though 46% of hospital admitted participants showed anti-N titres persistently above the threshold even after 12 months of follow-up.
Anti-S antibody response was significantly higher in patients who had noteworthy symptoms. In particular, subjects that were admitted to hospital showed significant levels of antibody titre with a median that was higher than 100 U/mL compared with participants who belong to the other two groups and such results persisted consistently during the entire period of follow-up.
Despite initial reports that persistence of antibody against SARS-Cov-2 was limited to a few months,15 in agreement with our conclusion, several subsequent studies reported that antibodies against SARS-CoV-2 persist over time.16–24 In a prospective longitudinal cohort study Harris et al16 found that anti-N antibodies were detected first but declined rapidly and predicted their negativity within 1 year whereas anti-S antibodies persisted for 6 months and the authors predicted stability over 54 weeks which our longitudinal assessment was able to confirm. More recently, He et al25 found that neutralising antibodies developed in approximately 40% of a cohort of 9500 individuals and the titres of neutralising antibodies did not significantly decrease during the 9 months of observation. Similarly, Favresse et al found stable antibody titres over a period of 10 months with the highest positivity rates in patients with clinically significant past SARS-CoV-2 infection.26
Since published data report of possible reinfection with SARS-CoV-2,27–29 we aimed also to follow participants clinically as well as in terms of history examination in order to explore possible recent or current clinical reinfection or hospital admission on infection. Interestingly, none of the participants showed clinically manifested reinfection. We are unsure, however, whether some of the participants might have developed asymptomatic SARS-Cov-2 infection as this is a possible event that has been reported in the literature.29
On the other hand, we find that 32% of serologically positive particlogiipants at the initial visit, showed a significant increase in the antibody titres during the subsequent pandemic infection. Hence, we speculate that this increase could have been due to the spread of a new infection and that these subjects did not develop the disease due to the protective effect of anti-S antibodies. Despite our speculation, a growing number of studies are showing that natural infection does protect against SARS-CoV-2 reinfection and/or symptomatic disease.27 28 30–32
Strength and limitation
Strengths of our study include a follow-up that lasted for at least 1 year and the use of both types of serological assays for the understanding of antibody characteristics.
We acknowledge some limitations of our study. First, our study does not have a baseline serological testing since it was conceived in late April and it was not possible to obtain serological testing when participants had the disease. The time from disease onset and the first clinical and serological testing was 3–6 months. We believe that this could not have biased our results, however, we are unsure whether those that resulted negative at the first visit—who predominately were oligo/asymptomatic participants—could have positive result on the first visit. Second, the study lacks clinical and serological information regarding those who died during the pandemic event, hence we are unable to conclude whether quantitative serological testing could predict survival.
Conclusion
Immunological response from SARS-CoV-2 infection is characterised by both anti N and anti-S antibodies. Anti-N antibodies do not persist overtime but they can be useful for the diagnosis of recent Sars-Cov-2 infection. Anti-S antibody titres correlate with disease severity and persist for at least 1 year.
Supplementary Material
Acknowledgments
We would like to thank all the participants for their dedication. We are indebted to the following personnel who collaborated in recruiting and assisting the participants as well as those who helped collecting blood sample: Luana Falchi, Loris Taschini, Arianna Fringuello, Francesca Malagoli, Luciana Di Cintio, Fabiano Pastorelli, Isabella Giaggiolo, Paolo Cappelletti and Daniela La Manna.
Footnotes
Twitter: @paoloeusebi
Collaborators: Ab-Covid: Alessandra De Masi, Manuela Costantini, Anna Chiara Lombardo, Anna Rita Vecchiarelli, Miria Flaviani, Carla Merigiola.
Contributors: IA and MM conceived the original idea of the study. IA, PE, AG, EP, SP, RA, SA, AF, EM, LA, PM, MLO, MR, MB, EB and MM: participated in the data analysis and interpretation, drafted and critically revised the final version of the manuscript. IA, AG, EP, SP, RA, LA, PM, MR, MB, EB and MM: supervised the laboratory analysis. IA, AG, EP, SP, RA, LA, PM, MR, MB, EB and MM: participated in the data collection. MM is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The planning conduct and reporting was performed in accordance with the Declaration of Helsinki, as revised in 2013. This study was approved by the Comitato Etico Regionale—Umbria. The approval number is CER 3695/20). Written informed consent was obtained. Participants gave informed consent to participate in the study before taking part.
References
- 1.Domenico M, Fortunato F, Mazzilli S, et al. Estimating the proportion of asymptomatic COVID-19 cases in an Italian region with intermediate incidence during the first pandemic wave: an observational retrospective study. BioMed Research International 2022. 10.1155/2022/3401566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection : A Narrative Review. Ann Intern Med 2020;173:362–7. 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond Princess cruise SHIP, Yokohama, Japan, 2020. Eurosurveillance 2020;25:2000180. 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection - challenges and implications. N Engl J Med 2020;383:e38. 10.1056/NEJMp2015897 [DOI] [PubMed] [Google Scholar]
- 5.Sullivan PS, Sailey C, Guest JL, et al. Detection of SARS-CoV-2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider-observed, home-collected blood, saliva, and oropharyngeal samples. JMIR Public Health Surveill 2020;6:e19054. 10.2196/19054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopel J, Goyal H, Perisetti A. Antibody tests for COVID-19. Proc 2020;34:63–72. 10.1080/08998280.2020.1829261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu M, Shi Y, Guo Z, et al. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect 2005;7:882–9. 10.1016/j.micinf.2005.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Y, Chang L, Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): current status, challenges, and countermeasures. Rev Med Virol 2020;30:e2106. 10.1002/rmv.2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2020;6:CD013652. 10.1002/14651858.CD013652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20:S1473-3099(20)30196-1. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diagnostics Division, Abbott Laboratories . SARS-CoV-2 IgG for use with ARCHITECT 2020.
- 12.Chew KL, Tan SS, Saw S, et al. Clinical evaluation of serological IgG antibody response on the Abbott architect for established SARS-CoV-2 infection. Clin Microbiol Infect 2020;26:1256.e9–e11. 10.1016/j.cmi.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020;58:e00941. 10.1128/JCM.00941-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.S.p.A. D. LIAISION®SARS-CoV-2 S1/S2 IgG package insert 2020-04, 2020. [Google Scholar]
- 15.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020;5:1598–607. 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RJ, Whitaker HJ, Andrews NJ, et al. Serological surveillance of SARS-CoV-2: six-month trends and antibody response in a cohort of public health workers. J Infect 2021;82:162–9. 10.1016/j.jinf.2021.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez PT, García PD, Salas MR, et al. SARS-CoV-2 IgG seropositivity in a cohort of 449 non-hospitalized individuals during Spanish COVID-19 lockdown. Sci Rep 2021;11:21612. 10.1038/s41598-021-00990-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol 2021;18:318–27. 10.1038/s41423-020-00588-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffante G, Chandel S, Ferrante D, et al. Persistence of neutralizing antibodies to SARS-CoV-2 in first wave infected individuals at ten months Post-Infection: the UnIRSA cohort study. Viruses 2021;13:2270. 10.3390/v13112270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol 2021;147:545–57. 10.1016/j.jaci.2020.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogan M, Kozhaya L, Placek L, et al. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun Biol 2021;4:129. 10.1038/s42003-021-01649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rijkers G, Murk J-L, Wintermans B, et al. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J Infect Dis 2020;222:1265–9. 10.1093/infdis/jiaa463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021;184:476–88. 10.1016/j.cell.2020.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020;370:1227–30. 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Z, Ren L, Yang J, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet 2021;397:1075–84. 10.1016/S0140-6736(21)00238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favresse J, Eucher C, Elsen M, et al. Persistence of anti-sars-cov-2 antibodies depends on the analytical kit: a report for up to 10 months after infection. Microorganisms 2021;9:556–13. 10.3390/microorganisms9030556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehan MM, Reddy AJ, Rothberg MB. Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis 2021;73:1882–6. 10.1093/cid/ciab234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen CH, Michlmayr D, Gubbels SM, et al. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet 2021;397:1204–12. 10.1016/S0140-6736(21)00575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flacco ME, Acuti Martellucci C, Soldato G, et al. Rate of reinfections after SARS-CoV-2 primary infection in the population of an Italian Province: a cohort study. J Public Health 2021:fdab346. 10.1093/pubmed/fdab346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021;384:533–40. 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilz S, Chakeri A, Ioannidis JP, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest 2021;51:e13520. 10.1111/eci.13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet 2021;397:1421–3. 10.1016/S0140-6736(21)00782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.