Abstract
The inoculum size effect in the dimorphic fungus Candida albicans results from production of an extracellular quorum-sensing molecule (QSM). This molecule prevents mycelial development in both a growth morphology assay and a differentiation assay using three chemically distinct triggers for germ tube formation (GTF): l-proline, N-acetylglucosamine, and serum (either pig or fetal bovine). In all cases, the presence of QSM prevents the yeast-to-mycelium conversion, resulting in actively budding yeasts without influencing cellular growth rates. QSM exhibits general cross-reactivity within C. albicans in that supernatants from strain A72 are active on five other strains of C. albicans and vice versa. The QSM excreted by C. albicans is farnesol (C15H26O; molecular weight, 222.37). QSM is extracellular, and is produced continuously during growth and over a temperature range from 23 to 43°C, in amounts roughly proportional to the CFU/milliliter. Production is not dependent on the type of carbon source nor nitrogen source or on the chemical nature of the growth medium. Both commercial mixed isomer and (E,E)-farnesol exhibited QSM activity (the ability to prevent GTF) at a level sufficient to account for all the QSM activity present in C. albicans supernatants, i.e., 50% GTF at ca. 30 to 35 μM. Nerolidol was ca. two times less active than farnesol. Neither geraniol (C10), geranylgeraniol (C20), nor farnesyl pyrophosphate had any QSM activity.
The dimorphic fungus Candida albicans is one of the most important fungi in medicine (26). It is a member of the normal flora residing in the intestinal tract of humans and other animals and is thought to be acquired during passage through the birth canal (26). C. albicans is also the model system for studying the basic biology of dimorphic fungi. Because of its medical importance, molecular tools are available with C. albicans that are unavailable for other dimorphic fungi (3). One unresolved problem in fungal biology is the dependence of cell morphology on initial cell density. For fungi exhibiting yeast-mycelium dimorphism, this phenomenon has been termed the inoculum size effect (19). Under otherwise identical conditions, budding yeasts are produced following inoculation at ≥106 cells/ml, whereas germ tubes and mycelia are produced with inocula of <106 cells/ml. We believe the inoculum size effect is a general phenomenon for all dimorphic fungi. This effect has been especially well documented for C. albicans. Cell density is listed by Odds (26) as 1 of 11 general factors favoring the filamentous form.
In this study we isolate and characterize the extracellular quorum-sensing molecule (QSM) which is responsible for the inoculum size effect in C. albicans. Quorum sensing is a well-known phenomenon in prokaryotes, but it has as yet only been hinted at in eukaryotes (18). Furthermore, since quorum sensing uses extracellular signal molecules, it is poised to mediate interactions of the producing fungus with its chemical and physical environment as well as with other bacteria and fungi. This study is the first to identify an extracellular molecule (farnesol) which mediates a eukaryotic quorum-sensing system.
MATERIALS AND METHODS
Organisms.
C. albicans strains A-72 and SG10261 were obtained from Patrick Sullivan, University of Otago, Dunedin, while strain MEN was from Richard Cannon, University of Otago. Strain SG5314 was from Burk Braun, University of California at San Francisco, and strains LGH 1095 and SG3314 were from Kevin Hazen, Virginia Polytechnic Institute and State University. Homoserine lactone (HSL) indicator strains were obtained from Sue Katz, Arizona College of Osteopathic Medicine, Glendale. (i) Chromobacterium violaceum CV026 was maintained on Luria-Bertani (LB) agar plus kanamycin (25 μg/ml). It detects four to eight carbon N-acyl HSLs. (ii) Agrobacterium tumefaciens A136 (originally from Clay Fuqua) was maintained on LB agar plus tetracycline (4.5 μg/ml) and spectinomycin (50 μg/ml). It detects most C4 to C12 HSLs, regardless of whether their side chains are 3-oxo, 3-hydroxy, or 3-unsubstituted (5, 29). (iii) Escherichia coli strains MG4/pKDT17, DH5α/pECP 615, and DH5α/pQF300I were all maintained on LB agar plus ampicillin (100 μg/ml). They detect C10 to C14 3-oxo and 3-unsubstituted HSLs, PAI-2, and the Vibrio fischeri autoinducer 3-oxo C6 HSL, respectively. QSM from C. albicans was concentrated by extraction into ethyl acetate (6) and then tested for its reactivity toward five bacteria which had been designed as indicator strains for the presence of various HSLs. C. violaceum CV026 produces a purple pigment in response to the appropriate HSL, while the other strains produce β-galactosidase. The CV026 assay was done on LB agar, the three E. coli assays were done on LB agar with ampicillin (100 μg/ml) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 40 μg/ml), and the A136 assay was done on the minimal, defined AT-glucose agar (33) with X-Gal (40 μg/ml).
Growth media and chemicals.
GPP is the defined glucose-phosphate-proline growth-medium described by Kulkarni and Nickerson (19). The modified glucose-salts-biotin (GSB) growth medium contained (per liter of distilled water): 1 g of (NH4)2SO4, 2 g of KH2PO4, 50 mg of MgSO4 · 7H2O, 50 mg of CaCl2 · 2H2O, and 1 g of peptone. After autoclaving, 30 ml of a 50% (wt/vol) glucose stock and 0.4 ml of the GPP/GPR vitamin stock (19) were added aseptically. The final pH was ca. 5.6. The modified GSB was used for preparing C. albicans cell stocks. Cells were harvested after 30 h of growth, washed twice in 50 mM K2HPO4 buffer (pH 6.5), and stored at 4°C in the same buffer at a cell density of ca. 2 × 109 cells/ml. Supernatants containing QSM activity were prepared following 24 h of growth in GPP at 28°C, starting with an initial cell density of 107 cells/ml. For QSM preparation, GPP was always prepared with Kandiyohi distilled water (Kandiyohi Bottled Water Co., Willmar, Minn.). This precaution was taken because several types of distilled water, including the building distilled water for the Beadle Center at the University of Nebraska at Lincoln, permitted ample growth of C. albicans, but the resulting cell-free supernatants did not contain QSM activity. Currently, five of eight distilled waters locally available in Lincoln and central Minnesota permit QSM accumulation. For the experiments to determine the influence of carbon source on QSM production, the glucose in GPP was replaced successively by lactose, sucrose, galactose, fructose, starch, and glycerol. For the experiments to determine the influence of nitrogen source on QSM production, the proline in GPP was replaced successively by l-serine, l-arginine, l-alanine, l-histidine, l-threonine, l-methionine, and l-phenylalanine. The pH values for all of the resulting spent media were very similar (pH 6.1 to 6.4). The sample of mixed isomer farnesol was purchased from Acros Organics (Pittsburgh, Pa.) while (E,E)-farnesol and farnesyl pyrophosphate were purchased from Sigma (St. Louis, Mo.). (E,E)-Farnesol is also called trans-trans farnesol.
QSM bioassay.
Two complementary bioassays were used, both assessing the percent germ tube formation (GTF) at 37°C but differing in whether or not the cells are provided with a complete growth medium during the bioassay. The first, an N-acetylglucosamine (GlcNAc)-triggered differentiation assay, included 0.56 ml of 0.1 M imidazole buffer (pH 6.5), 0.15 ml of 0.1 M MgSO4, 0.13 ml of 0.1 M GlcNAc, and 4.16 ml of either Kandiyohi distilled water or filter-sterilized supernatant.
Bioassays of commercial chemicals were conducted by the addition of the chemical, as a solution in 100% methanol, to the bioassay media; the final concentration of methanol was never greater than 1%. The second, a mycelial growth assay, included 0.5 ml of 0.5 M potassium phosphate buffer (pH 6.5), 0.5 ml of 20% glucose with 30 mM MgSO4, 0.17 ml of 0.3 M l-proline, 0.13 ml of 0.1 M GlcNAc, and 3.7 ml of either Kandiyohi distilled water or filter-sterilized supernatant. In both cases, the assays were conducted in 25-ml Erlenmeyer flasks using C. albicans inoculum which had been stored at 4°C in 50 mM potassium phosphate buffer (pH 6.5). The cells were added in ∼25-μl aliquots to prewarmed (37°C) assay medium to give a final cell density of 107 cells/ml. The flasks were shaken on a New Brunswick Scientific G2 shaker at 37°C and 225 rpm for 4 h and examined for percent GTF by phase-contrast microscopy. At time zero, the inoculated cells are >98% undifferentiated with 0% germ tubes and 0 to 2% budding yeasts.
Purification of QSM.
Five liters GPP was inoculated to a final concentration of 107 cells/ml and incubated at 30°C for 24 h on a New Brunswick Scientific G52 shaker at 125 rpm. After 24 h the cultures were centrifuged at 15,300 × g for 20 min. The supernatant was decanted and filter sterilized by vacuum filtration through Whatman (Maidstone, United Kingdom) 0.45-μm (pore-size) cellulose nitrate filters. The cell-free supernatant was either designated as spent medium for use in a QSM assay or extracted with a one-fifth volume of ethyl acetate. The ethyl acetate was removed under reduced pressure on a rotary evaporator. The residue was suspended in a small volume of 1:4 ethyl acetate-hexane and the soluble portion was subjected to silica flash chromatography with 1:4 ethyl acetate-hexane as the eluant. Individual fractions (1 ml) were examined by thin-layer chromatography (TLC).
TLC.
TLC was conducted on silica gel G plates (Al or glass backed) containing a fluorescent indicator (Alltech, Deerfield, Ill.). Reagents used for staining included 1% aqueous KMnO4, an indicator for alkenes, and a mixture of p-anisaldehyde and sulfuric acid in 1:1 methanol-water, which chars to reveal a variety of organic functional groups. The mobile phase used was 1:4 ethyl acetate-hexane.
HPLC.
High-performance liquid chromatography (HPLC) analysis employed a Waters (Milford, Mass.) pump model 510 and a Waters Tunable Absorbance Detector model 486. Data were analyzed by using Millennium 2010 Chromatography Manager software version 2.00 from Waters. A 5-μm C18 reversed-phase column (4.6 by 250 mm) was used with the following parameters: 210-nm, 1-ml/min flow rate, and 4:1 methanol-H2O eluant.
GC-MS.
Active fractions were resuspended in 100% methanol and analyzed by a Hewlett-Packard 5972 Series II gas chromatography-mass spectroscopy (GC-MS) with a 30-m DB-5 column in both the chemical ionization (methane) and electron ionization (EI) modes. GC used a 1.5-μl sample, injector, and detector temperatures of 250 and 280°C, respectively, and a temperature program of 100°C for 3 min and then 20°C/min until 280°C. MS used a 3-min solvent delay and a scan rate of 1.5 scans/s.
Trimethylsilyl derivatization.
Derivatization of the hydroxyl groups of commercial farnesol and QSM was accomplished by the addition of N,O-bis(trimethyl)trifluoroacetamide (BSTFA; Alltech). The samples were suspended in 500 μl of ethyl acetate, from which 150 μl was removed for analysis as “prior to derivatization.” To the remaining 350 μl, 100 μl of BSTFA was added. The samples were heated for 15 min at 70°C in a water bath and, after cooling, were submitted directly for GC-MS.
RESULTS
QSM from C. albicans.
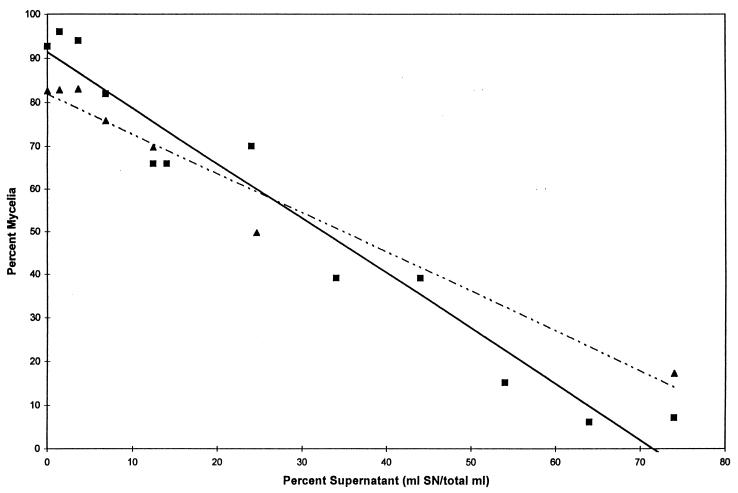
Including spent media from C. albicans as part of the fresh growth media caused a gradual shift in the morphology of C. albicans. At 37°C, with no added culture supernatant, the cells were ca. 90% mycelia. Incorporation of spent growth medium progressively decreased the percent mycelia to only 5 to 10% (Fig. 1). This decrease in the percent mycelia occurred for both a complete growth medium and a GlcNAc-induced differentiation assay for GTF. In both cases, QSM did not just prevent GTF but also caused a morphological shift to actively budding yeast cells.
FIG. 1.
Two bioassays for QSM activity. Symbols: ■ and solid line, GlcNAc-triggered differentiation assay; ▴ and dotted line, mycelial growth assay. The indicated lines are linear regression lines with R2 values of 0.953 and 0.964, respectively. Values represent the average of five or more experiments conducted over the past 4 years.
Both the growth assay and the differentiation assay (Fig. 1) used cell densities of 107 cells/ml with cells that had been washed three times in 50 mM potassium phosphate buffer (pH 6.5). We washed the cells to remove residual QSM activity that had accumulated during their growth in GSB medium. Two washes were sufficient for maximum GTF during the assay even though the first four washes still contained measurable QSM activity (data not shown). For the cell growth bioassay (Fig. 1), varying the initial cell density from 5 × 104 to 5 × 107/ml did not influence the results; QSM-containing spent media shifted the percent mycelia from ca. 80 to 11% (data not shown). For all subsequent assays we chose a cell density of 107/ml so as to avoid the reduced GTF evident at cell densities of >5 × 107/ml, presumably due to QSM produced during the 4-h bioassay.
Generality within C. albicans.
QSM activity was not specific for strain A72; it was general for all strains of C. albicans (Table 1). Supernatant from strain A72 caused a mycelium-to-yeast conversion in cells from strains MEN, SG5314, LGH1095, SG3314, and 10261 (Table 1). Similarly, supernatants from strains MEN, SG5314, LGH1095, SG3314, and 10261 caused a mycelium-to-yeast conversion in A72 cells (Table 1). Thus, all six strains of C. albicans produce QSM activity, and the QSMs for the six strains are either identical or very similar to one another.
TABLE 1.
QSM cross-reactivity of A72 with five other strains of C. albicans
| Cell type | Morphology results (mean % ± SD)a with:
|
||
|---|---|---|---|
| 37°C control | Supernatant from A-72b | A-72 cellsc | |
| A-72 | 16 ± 1 (Y) | 71 ± 4 (Y) | 73 ± 4 (Y) |
| 83 ± 1 (M) | 26 ± 4 (M) | 24 ± 3 (M) | |
| MEN | 49 ± 6 (Y) | 99 ± 1 (Y) | 61 ± 3 (Y) |
| 47 ± 7 (M) | 1 ± 1 (M) | 37 ± 2 (M) | |
| SG 5314 | 8 ± 1 (Y) | 44 ± 6 (Y) | 61 ± 3 (Y) |
| 92 ± 1 (M) | 53 ± 2 (M) | 39 ± 3 (M) | |
| LGH 1095 | 19 ± 2 (Y) | 88 ± 3 (Y) | 73 ± 1 (Y) |
| 78 ± 3 (M) | 10 ± 2 (M) | 27 ± 1 (M) | |
| SG 3314 | 20 ± 9 (Y) | 45 ± 9 (Y) | 74 ± 4 (Y) |
| 80 ± 9 (M) | 50 ± 10 (M) | 21 ± 5 (M) | |
| SG 10261 | 25 ± 7 (Y) | 57 ± 12 (Y) | 44 ± 9 (Y) |
| 72 ± 8 (M) | 34 ± 15 (M) | 55 ± 10 (M) | |
Morphology for the indicated strain in the growth-based bioassay in Fig. 1. For the control, no QSM or spent media were added. Y, percent budding yeasts; M, percent germ tubes for which the length of the germ tube was greater than the diameter of the cell. For all tables, the “Y” and “M” values were obtained as separate measurements so the values may not total 100%.
Same experiment as in column 2 but with spent media from strain A-72.
Morphology of A72 incorporating spent media from the indicated strain of C. albicans.
An exceptionally wide variety of conditions have been reported to favor filamentation in C. albicans (26). Of these, the most widely used methods for inducing germ tubes are serum (15), buffered GlcNAc (30, 31), and l-proline (8). However, QSM activity is not specific for a given method for germ tube induction (Table 2). QSM acts to suppress germ tube induction by GlcNAc, by serum, by l-proline, or by GlcNAc and proline in combination (Fig. 1 and Table 2).
TABLE 2.
Comparison of QSM effectiveness using different methods of inducing GTF
| Germ tube inducera | Morphology results (mean % ± SD)b for expts:
|
|
|---|---|---|
| Without used supernatant | With used supernatant | |
| 10 mM proline | 82 ± 3 (Y) | 100 ± 0 (Y) |
| 18 ± 3 (M) | 0 ± 0 (M) | |
| 2.5 mM GlcNAc | 26 ± 4 (Y) | 81 ± 4 (Y) |
| 74 ± 5 (M) | 19 ± 4 (M) | |
| 10 mM proline + 2.5 mM GlcNAc | 15 ± 1 (Y) | 75 ± 3 (Y) |
| 84 ± 1 (M) | 25 ± 4 (M) | |
| 2% fetal bovine serum | 34 ± 4 (Y) | 97 ± 2 (Y) |
| 66 ± 4 (M) | 3 ± 2 (M) | |
| 5% fetal bovine serum | 7 ± 1 (Y) | 80 ± 6 (Y) |
| 92 ± 1 (M) | 21 ± 5 (M) | |
| 10% fetal bovine serum | 2 ± 0 (Y) | 65 ± 4 (Y) |
| 98 ± 0 (M) | 33 ± 4 (M) | |
| 10% pig serumc | 7 ± 2 (Y) | 32 ± 2 (Y) |
| 93 ± 2 (M) | 68 ± 2 (M) | |
Germ tube inducers present in 50 mM potassium phosphate (pH 6.5).
“Y” and “M” are as defined in Table 1, footnote a. Supernatant was from A-72 cells grown in GPP at 30°C for 24 h at 125 rpm at an inoculum of 107 cells per ml. “Without used supernatant” samples received an equal amount of water or 50 mM potassium phosphate (pH 6.5) instead of supernatant.
The serum was nonpregnant pig serum.
Production of QSM by C. albicans.
QSM was produced by C. albicans cells growing at all temperatures tested from 23 to 43°C (Table 3). From 23 to 37°C, there was a rough correlation between cell number (in CFU/milliliters) and levels of QSM produced, as indicated by the lower percent mycelia when those supernatants were tested in a germ tube bioassay (Table 3). However, on a per-cell basis, significantly more QSM was produced by cells grown at 40°C than at any other temperature (Table 3).
TABLE 3.
Effect of growth temperature on QSM production
| Growth temp (°C)a | Cell density at 24 h (CFU/ml) | Morphology results (mean % ± SD)b obtained with used supernatant |
|---|---|---|
| 23 | (2.4 ± 0.2) × 108 | 55 ± 6 (Y) |
| 44 ± 5 (M) | ||
| 27 | (3.2 ± 0.4) × 108 | 71 ± 3 (Y) |
| 28 ± 2 (M) | ||
| 30 | (3.7 ± 0.2) × 108 | 75 ± 2 (Y) |
| 23 ± 2 (M) | ||
| 33 | (2.8 ± 0.1) × 108 | 84 ± 3 (Y) |
| 16 ± 3 (M) | ||
| 37 | (3.6 ± 0.1) × 108 | 99 ± 2 (Y) |
| 1 ± 2 (M) | ||
| 40 | (4.0 ± 0.1) × 107 | 94 ± 2 (Y) |
| 6 ± 2 (M) | ||
| 43 | (4.0 ± 0.8) × 107 | 72 ± 3 (Y) |
| 27 ± 3 (M) |
Cultures were inoculated to a cell density of 107 per ml in GPP and incubated at the indicated temperature for 24 h on a New Brunswick Scientific G52 shaker at 125 rpm.
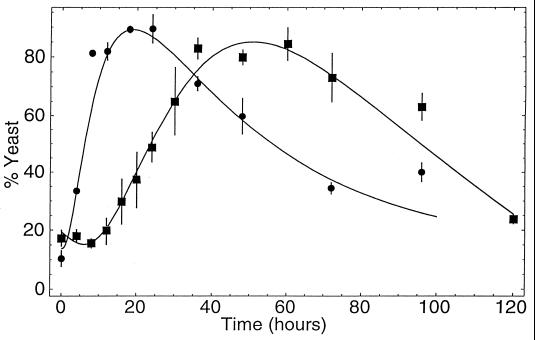
C. albicans cells synthesized QSM continuously during growth, and QSM activity persisted in the broth for at least 2 to 3 days after the onset of stationary phase (Fig. 2). QSM production roughly paralleled cell growth (Fig. 2), and the accumulation of QSM did not alter the growth rate of the producing culture until the normal onset of stationary phase.
FIG. 2.
QSM production versus time. Supernatant samples were removed at the indicated times and used in the mycelial growth assay. Thus, a higher percent yeast indicates more QSM activity. Values are the average of triplicate experiments. Symbols: ●, initial cell density of 107/ml; ■, initial cell density of 105/ml. pH and CFU/milliliter values were also determined at each time point. The two cultures shifted from exponential to stationary phase at ca. 12 and 48 h, respectively, at a cell density of 2.8 × 108/ml in both cases.
The levels of QSM produced were not dependent on the type of growth medium or the specific carbon or nitrogen source provided. Equivalent per-cell QSM production was observed for cells grown (24 h at 25°C) in GPP, GSB (30), modified GSB, Sabouraud-dextose, yeast extract-peptone-dextrose (1), yeast nitrogen base with either glucose or GlcNAc, or Lee's medium (20) (data not shown). Also, with GPP the identity of the carbon source and the nitrogen source did not seem to be important. Equivalent levels of QSM were produced with all seven carbon sources tested, including both fermentable and nonfermentable (glycerol) carbon sources, and all eight amino acid nitrogen sources (data not shown). The small variations in QSM detected correlated well with the cell titers obtained with the respective amino acids (data not shown).
Preliminary evidence on the chemical identity of QSM.
For C. albicans, QSM activity could be extracted into ethyl acetate and then resuspended in either hexane or 90% methanol (6, 21). Following an activity-directed purification, activity was associated with a fraction containing a molecular mass of 222 (6, 21). Cho et al. (6) eliminated the possibilities that QSM was methyl jasmonate or a lactone of jasmonic acid or homojasmonic acid. Active fractions of QSM are stained by dilute aqueous KMnO4 on silica TLC, indicating the presence of carbon-carbon multiple bonds; absorb UV light at 210 to 220 nm but not at wavelengths of ≥240 nm; contain a free hydroxyl, as shown by altered mobility on TLC plates following trimethylsilylation; do not contain a free carboxylic acid, as shown by unaltered TLC mobility following reaction with diazomethane; and do not contain an ester linkage, as shown by the retention of activity after treatment with porcine liver esterase, hydrogen peroxide, basic methanol, or extremes in pH (e.g., pH 7 to 2 to 7 or pH 7 to 12 to 7). Additionally, C. albicans QSM is insensitive to trypsin, pronase, thermolysin, and proteinase K (100 μg/ml for 2 h). Thus, QSM is unlikely to be a conventional peptide. This distinction is important because of recent evidence that the “asexual” C. albicans can, in fact, undergo mating (14, 23). It is also unlikely to be an HSL. The C. albicans QSM was tested versus three bacterial strains which act as reporter strains for a wide range of bacterial HSLs (5, 29). However, in no case did the C. albicans extracts elicit the indicated color development, while in each case the appropriate bacterial positive controls did elicit that response (data not shown).
Heat stability of QSM.
The QSM activity of C. albicans was unchanged by heating at 100°C for 30 min or by autoclaving (data not shown), and activity was retained for at least 3 years at 4°C. It did not matter whether the supernatants were stored in glass or polypropylene vessels. Additionally, QSM activity remained unchanged following 12 freeze-thaw cycles (data not shown).
GC-MS.
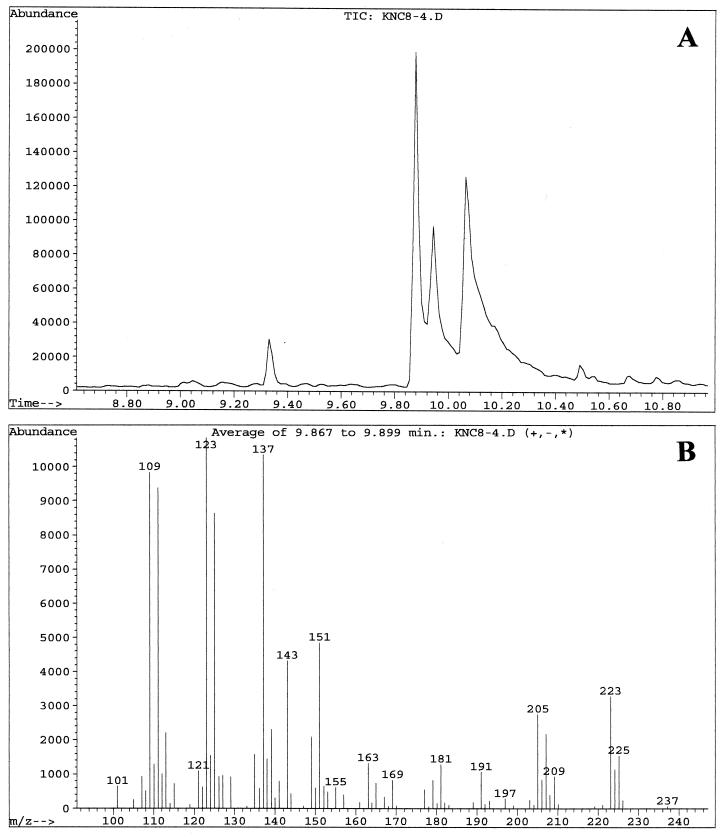
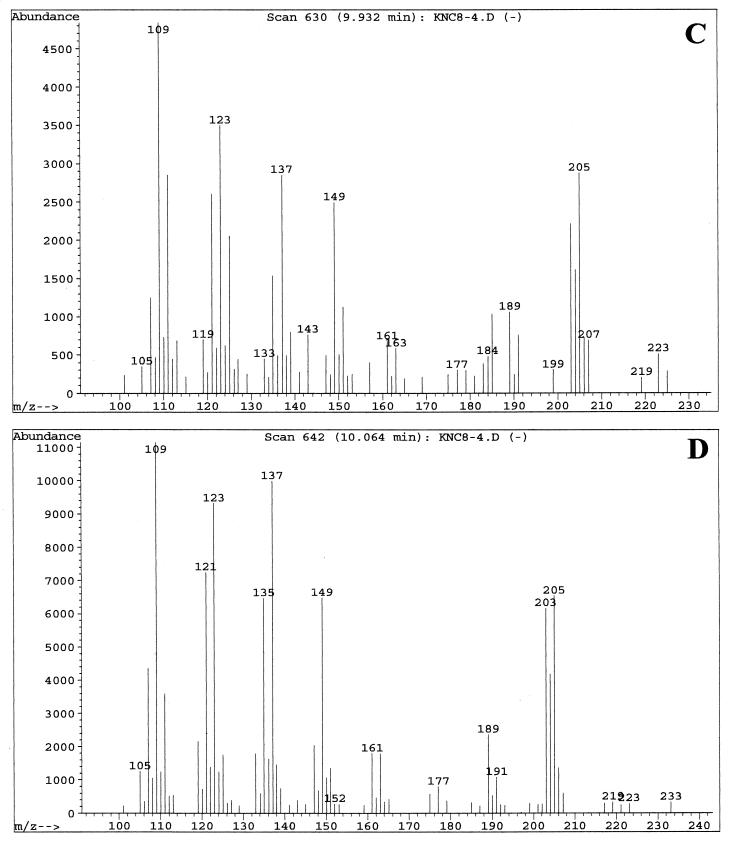
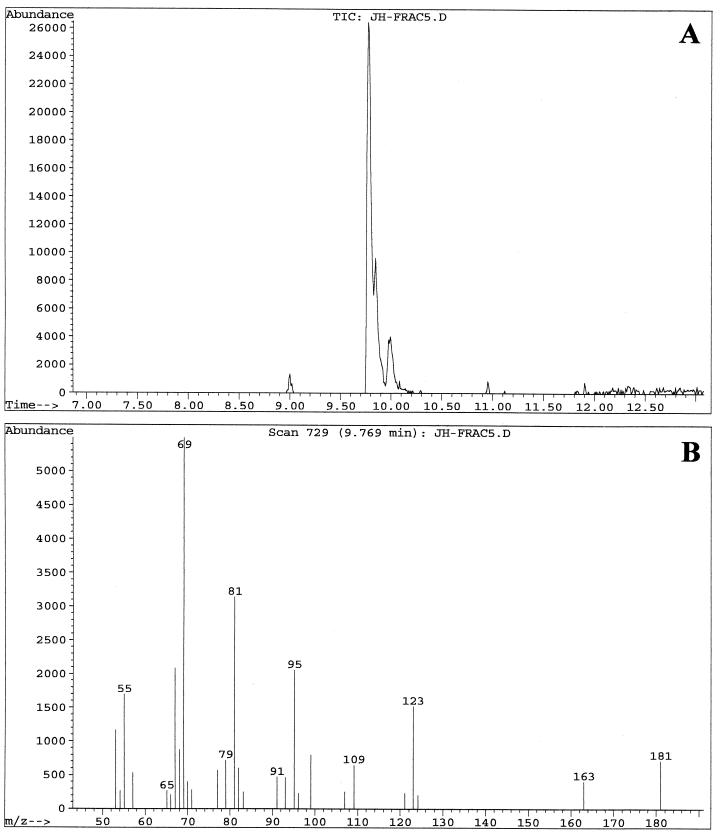
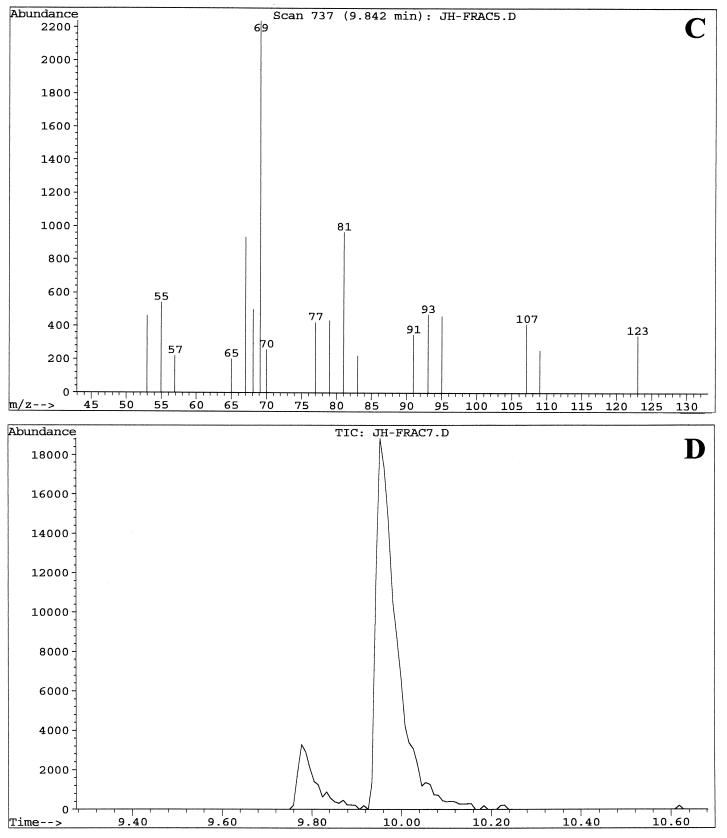
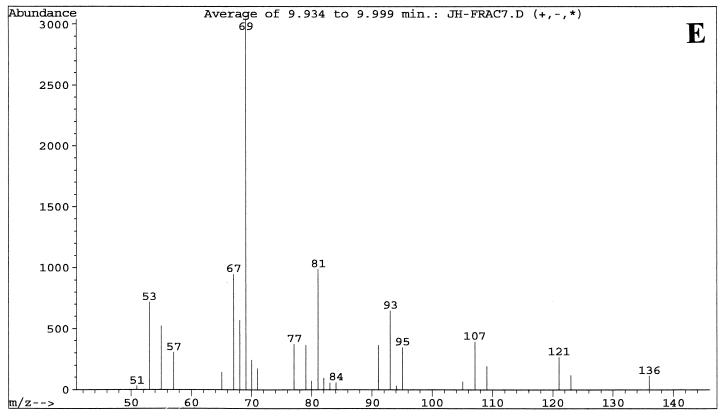
Fractions enriched in the C. albicans QSM showed peaks at 9.8, 9.9, and 10.0 min when examined by GC-MS. These three peaks were analyzed with the MS system in both the chemical ionization (CI) mode (Fig. 3), which emphasizes the parent compound's molecular weight, and the electron induced (EI) ionization mode (Fig. 4), which emphasizes fragmentation data. The three peaks contained the same parent ion (M+H) at m/z 223 in CI mode (Fig. 3) and exhibited the same fragmentation patterns in EI mode (Fig. 4). Presumably, the three peaks represent isomers of one another. Analysis of the results using a library search program (35) suggested 3,7,11-trimethyl-2,6,10-dodecatrien-1-ol (molecular weight = 222.37), better known as the 15-carbon sesquiterpene farnesol. Farnesol can exist as both E and Z geometric isomers at both the 2/3 and 6/7 double bonds, for a total of four possible isomers. When a commercial sample of farnesol, containing a mixture of isomers, was analyzed by GC-MS, three peaks were seen at 9.7, 9.85, and 10.0 min (data not shown) with the relative peak areas of 6:57:37. The commercial sample of (E,E)-farnesol showed only a single peak at 10.0 to 10.1 min (data not shown). In both the CI and the EI modes, the fragmentation patterns of QSM were identical to those of commercial farnesol. At no stage of the QSM analysis did we observe GC-MS peaks containing molecules of 154.2 or 290.5 molecular weight, as would be expected for geraniol (C10) and geranylgeraniol (C20), respectively.
FIG. 3.
GC-MS analysis of QSMs from C. albicans using the CI mode for MS. Samples were run in August 1997. (A) GC showing peak A at 9.88 min, peak B at 9.93 min, and peak C at 10.06 min. (B) MS fragmentation pattern of peak A. (C) MS fragmentation pattern of peak B. (D) MS fragmentation pattern of peak C. The MS patterns only show ions of ≥100 m/z.
FIG. 4.
GC-MS analysis of two QSM-containing column fractions (fractions 5 and 7) using the EI mode for MS. Samples were run in September 2000. (A) GC of fraction 5 showing peak A at 9.77 min, peak B at 9.84 min, and peak C at 9.98 min. (B) MS fragmentation pattern of peak A from fraction 5. (C) MS fragmentation pattern of peak B from fraction 5. (D) GC of fraction 7 showing primarily peak C at 9.98 min. (E) MS fragmentation pattern of peak C from fraction 7. The MS patterns show ions of ≥50 m/z. The strong band at m/z 69 is characteristic of a C5H9 isoprene unit.
Confirmation of farnesol by trimethylsilylation.
Trimethylsilylation is a common method of derivatizing aliphatic alcohols for analysis by GC-MS. In CI mode, the peaks for derivatized mixed isomer farnesol shifted from 9.7, 9.85, and 10.0 min to 10.15, 10.22, and 10.35 min, respectively, while the parent ions shifted from 223 to 293 Da (data not shown). Trimethylsilyl derivatization of the C. albicans QSM also shifted the dominant peak from 10.0 to 10.35 min, and the new parent ion and fragmentation pattern were identical to those of the (E,E)-farnesol standard (data not shown). The addition of a single trimethylsilyl group is consistent with farnesol but inconsistent with two related C15 molecules: the hormone sirenin produced by the water mold Allomyces (11) and the farnesylated portion of the mating pheromones from Tremerogen A-10 and Tremerogen A-9291-I both of which have an extra hydroxyl on the 12 position of their farnesol modification (4).
Farnesol bioassay data.
Commercial preparations of mixed-isomer farnesol (1-hydroxy-3,7,11-trimethyl-2,6,10-dodecatriene), (E,E)-farnesol, and mixed-isomer nerolidol (3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene) were tested for their ability to block GTF. All three showed strong QSM activity in that they reduced GTF by 50% at concentrations of 30 to 35 μM for the farnesol preparations and of ca. 60 μM for the nerolidol.
Two fractions (fractions 5 and 7) from a silica flash column separation of an ethyl acetate extract of the C. albicans supernate were compared by GC-MS. The three isomers of farnesol were separated by GC at 9.7, 9.85, and 10.0 min. For the comparatively inactive fraction 5 (87% GTF by bioassay), the three peaks were 65, 25, and 10% of the total peak area, respectively, whereas for the highly active fraction 7 (only 19% GTF by bioassay), the three peaks were 14, 6, and 80% of the total peak area, respectively.
Confirmation of farnesol by TLC.
The Rf values of commercial mixed isomer and (E,E)-farnesol were compared with those of the QSM-enriched fractions 5 and 7 from C. albicans supernatant. The mixed isomer farnesol ran as a broad band of Rf 0.45 to 0.47, while the (E,E)-farnesol ran as a single band with Rf 0.45, coeluting with the dominant band from the active fraction 7 from C. albicans. TLC analysis of the active chromatographic fractions derived from C. albicans also was conducted using p-anisaldehyde (data not shown). An advantage of this reagent is the ability to produce dramatically different colors depending upon the functionality present in the molecule (7). The two commercial farnesol bands stained blue green with p-anisaldehyde, as did the Rf 0.45 and 0.47 bands from C. albicans supernatant. All of the other bands from C. albicans stained pink or yellow with p-anisaldehyde (data not shown). The apparent shift in QSM's Rf value from 0.50 reported previously (6) to 0.45 merely reflects a switch in the TLC mobile phase from 25% ethyl acetate-hexane to 20% ethyl acetate-hexane.
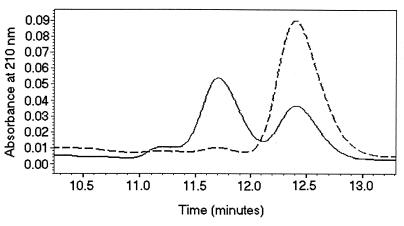
Confirmation of farnesol by HPLC.
The commercial mixed isomer and (E,E)-farnesol were compared with the QSM-enriched fraction 7 from C. albicans by C18-reversed phase high-pressure liquid chromatography (HPLC) monitored at 210 nm (Fig. 5). The mixed-isomer farnesol showed peaks at 11.2, 11.7, and 12.4 min, which were present as the percent area in the ratio 6:56:38. In a separate experiment the (E,E)-farnesol showed a single peak at 12.4 min. This separation of the farnesol isomers duplicates the separation achieved by GC, and in both cases the (E,E)-isomer traveled the furthest. Once again, just as with the GC comparison, the position of the major peak for QSM enriched fraction 7 coincided with the peak for (E,E)-farnesol (Fig. 5).
FIG. 5.
HPLC comparison of mixed-isomer farnesol versus active fraction 7 from C. albicans supernatant. The separation used a C18 reversed phase column eluted with 80% methanol-water monitored at 210 nm. The mixed-isomer farnesol peaks at 11.2, 11.7, and 12.4 min were present (percent area) in the ratio of 6:56:38. The solid line represents 0.4 mM mixed-isomer farnesol.
Farnesol and growth.
In assessing the specificity of farnesol's QSM activity, it is important to determine whether farnesol slows down the overall growth rate for C. albicans. It did not. At concentrations of up to 250 μM, farnesol did not alter the growth rate for C. albicans at 30°C. Actively budding yeasts were observed in both cases.
DISCUSSION
Our studies demonstrate that there is an inoculum size effect in the dimorphic fungus C. albicans that is mediated by extracellular farnesol. Farnesol acts as a QSM in that it prevents the mycelial phase of growth, with a mycelium/yeast threshold at ca. 106 cells/ml in liquid culture. Both QSM purified from C. albicans and commercial farnesol shifted the morphology of C. albicans from mycelia to actively budding yeasts and, at concentrations of up to 250 μM, farnesol did not inhibit the growth rate of C. albicans. We hypothesize that the inoculum size effect is a common phenomenon in the biology of dimorphic fungi for which C. albicans is a useful model system.
We believe this report is the first structural identification of a quorum-sensing system in fungi and the first identification of farnesol production by C. albicans. Farnesol is not mentioned in Odds' (26) comprehensive review of Candida, two recent reviews of the lipids of C. albicans (16, 27), or two standard texts on fungal physiology (10, 12). Farnesol (QSM) is distinct from the morphogenic autoregulatory substance (MARS) isolated from C. albicans by Hazen and Cutler (13). Both QSM and MARS are extracellular molecules produced by C. albicans that act to suppress the yeast-to-mycelium transition. However, MARS differs in that it has a UV maximum at 270 nm, has a nitrogen-containing ring system, reacts with ninhydrin to form a yellow color, has to be bioassayed within 2 days, is inactivated by pH values of <4.5 or >9.0, and has no aroma.
The physical properties of farnesol are appropriate for its role as a QSM; it is lipophilic, with a water solubility of only 1.2 mM (17). In contrast, geranylgeraniol (C20) is not suitable as a QSM because it is not soluble in water, while geraniol (C10) is sufficiently soluble (7.6 mM) but is generally toxic to strains of Candida (2). The properties of farnesol as a QSM are consistent with those expected for a fungal quorum-sensing system. (i) It is extracellular, is diffusible, and can be removed from cells by washing. (ii) It is produced continually during growth in amounts roughly proportional to cell mass. (iii) It is produced by all six strains of C. albicans tested. (iv) It blocks the induction of germ tubes by three chemically distinct triggers: l-proline, GlcNAc, and serum. (v) Its production is dependent on cell growth and not on a particular carbon or nitrogen source. (vi) It alters cell morphology but does not alter growth rate. Thus, its mode of action is likely more specific than just a general inhibition of cell metabolism. (vii) It is produced at all growth temperatures and is itself very heat stable. Even though QSM functions by inhibiting the shift from yeasts to mycelia, C. albicans produces as much or more QSM at temperatures of ≥37°C, where in vitro the cells are growing in the mycelial mode. This continued production of QSM at temperatures of ≥37°C. (i) explains why GTF assays at 37°C have to be conducted at cell densities of ≤107/ml; (ii) means that QSM formation should occur in the human body during candidiasis; and (iii) precludes the simple, discontinuous model wherein GTF and mycelial growth only occur at temperatures of ≥37°C because QSM production does not occur at temperatures above 35 to 36°C.
Farnesol has three carbon-carbon double bonds and exists in four isomers. In this regard, we do not see any inconsistency between the facts that C. albicans excretes primarily the (E,E)-farnesol, while commercial (E,E)-farnesol and mixed-isomer farnesol have equivalent activities and the closely related sesquiterpene nerolidol is also highly active. The fungus Helminthosporium sativum has an enzymatic system for the cis-trans isomerization of the 2,3 double bond of farnesol and epoxy-farnesol using nerolidol as an intermediate (32). The presence of a similar system in C. albicans could allow interconversion of nerolidol and the two farnesols. Also, recognition that C. albican's QSM is slightly volatile explains. (i) why measurements of QSM's heat stability had to be conducted in sealed tubes; (ii) why there was a gradual decline in QSM activity during stationary phase (Fig. 2), even though purified QSM was stable for at least 3 years at 4°C; and (iii) the difficulty we experienced in making quantitative comparisons between QSM activity in the initial supernatants and the amounts of farnesol purified following extraction into ethyl acetate—a high proportion of the farnesol was lost when the ethyl acetate was reduced in volume by rotary evaporation.
The older literature offers ample precedent for the existence of fungal QSMs. Odds (26) cited 13 references that an inoculum size of ≤106/ml favored filamentous growth. A diffusible filament-suppressing molecule was hypothesized based on the distinctive morphology of C. albicans on agar plates following parallel streaking (24) or multiple cross-streaking (25). For instance, Nickerson and Chung (25) noted the “suppression of filamentation in strain 582 (a wild-type dimorphic strain of C. albicans) along the length of the streak facing strain 806 and the appearance of filaments in 582 only at the free ends of lateral streaks,” while with three parallel streaks filamentous cells only arose from the outer borders of the two outer streaks (24). Similarly, Shepherd and Sullivan (30) noted that some C. albicans strains needed to be “activated or conditioned” for GTF by a prolonged aeration under nongrowing conditions. This conditioning, like the successive cell washings described here, would serve to remove excess QSM from the C. albicans cells.
Recognition that farnesol acts to suppress mycelial development in C. albicans in a quorum-sensing manner suggests that farnesol might be the first of a novel class of antifungal compounds. C. albicans normally occurs as a yeast in the human gastrointestinal tract but can form germ tubes in response to an unknown chemical inducer(s). The germ tubes (mycelia) can invade the intestinal wall to enter the blood stream. This morphological transformation from yeast to mycelia is a crucial step in the pathogenesis of C. albicans. Blocking this transition could block the pathogenic nature of this opportunist. These compounds would neither kill the fungi nor prevent their growth. Instead, they would limit their growth to a single morphological form and in so doing restrict pathogenesis. Nonfilamentous mutants of C. albicans are often avirulent (22). We believe this type of single morphology therapy could be especially useful in immunocompromised patients because it would not upset the natural resident flora. Additionally, because it does not kill the fungus, selection for farnesol-tolerant mutants should not be as intense.
Finally, we suggest that the effects of excreted farnesol may be different in vitro and in vivo. All of our results so far have been in vitro; accumulated farnesol (10 to 50 μM) shifted the morphology to budding yeasts. If this suppression of mycelial development also occurs in vivo, the effect of exogenous farnesol should be protective because nonfilamentous mutants of C. albicans are often avirulent (22). However, in vivo farnesol excretion may instead act as a virulence factor. In patients, C. albicans accumulates to very high cell densities and then some of these cells penetrate the tissue. Growth to high cell densities in the restricted microenvironments of the host mucosal foci may produce enough farnesol so that the cells lining the microabscesses no longer constitute an effective barrier to fungal penetration. Free farnesol is not normally present at detectable levels in human plasma (28). The lipophilic properties of free farnesol would favor its membrane localization, and thus it could alter the membrane fluidity of the host cells. Alternatively, according to Voziyan et al. (34), the farnesol could reduce diacylglycerol production. Since diacylglycerol is an activator for protein kinase C, farnesol could inhibit cell growth, leading to apoptosis (34), and thus create an entry site for invading C. albicans. Tests with farnesol and C. albicans in a mouse model system (9) should resolve these competing hypotheses.
ACKNOWLEDGEMENTS
We thank Bill Gerwick, Department of Pharmacy, Oregon State University, for hospitality during K.W.N's recent sabbatical, as well as for the use of GC-MS equipment with a computerized database, Gloria Zeller (Oregon State University) for technical assistance with the bacterial HSL indicator strains, Antonio Romano for pointing out how the early work of W. J. Nickerson indicated a diffusible filament-suppressing molecule, and Sondra Atkins for assistance with manuscript preparation.
REFERENCES
- 1.Atlas R M. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 2.Bard M, Albrecht M R, Gupta N, Guynn C J, Stilwell W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids. 1988;23:534–538. doi: 10.1007/BF02535593. [DOI] [PubMed] [Google Scholar]
- 3.Brown A J P, Gow N A R. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell G A, Naider F, Becker J M. Fungal lipopeptide mating pheromones: a model system for the study of protein prenylation. Microbiol Rev. 1995;59:406–422. doi: 10.1128/mr.59.3.406-422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha C, Gao P, Chen Y-C, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 6.Cho S C, Dussault P H, Lisec A D, Jensen E C, Nickerson K W. Metathesis-based synthesis of jasmonate and homojasmonate lactones, candidates for extracellular quorum sensing molecules in Candida albicans. J Chem Soc Perkin Trans. 1999;1:193–196. [Google Scholar]
- 7.Coscia C J. Handbook of chromatography: terpenoids. Boca Raton, Fla: CRC Press; 1984. [Google Scholar]
- 8.Dabrowa N, Taxer S S S, Howard D H. Germination of Candida albicans induced by proline. Infect Immun. 1976;13:830–835. doi: 10.1128/iai.13.3.830-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Repentigny L, Phaneuf M, Mathieu L-G. Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis in intact immunocompromised mice. Infect Immun. 1992;60:4907–4914. doi: 10.1128/iai.60.11.4907-4914.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garraway M O, Evans R C. Fungal nutrition and physiology. New York, N. Y: John Wiley & Sons; 1984. [Google Scholar]
- 11.Gooday G W, Adams D J. Sex hormones and fungi. Adv Microb Physiol. 1993;34:69–145. doi: 10.1016/s0065-2911(08)60028-4. [DOI] [PubMed] [Google Scholar]
- 12.Griffin D H. Fungal physiology. 2nd ed. New York, N. Y: Wiley-Liss; 1994. [Google Scholar]
- 13.Hazen K C, Cutler J E. Isolation and purification of morphogenic autoregulatory substance produced by Candida albicans. J Biochem. 1983;94:777–783. doi: 10.1093/oxfordjournals.jbchem.a134419. [DOI] [PubMed] [Google Scholar]
- 14.Hull C M, Raisner R M, Johnson A D. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 15.Joshi K R, Bremner D A, Gavin J B, Herdson P B, Parr D N. The formation of germ tubes by Candida albicans in sheep serum and trypticase soya broth. Am J Clin Pathol. 1973;60:839–842. doi: 10.1093/ajcp/60.6.839. [DOI] [PubMed] [Google Scholar]
- 16.Kitajima Y, Nozawa Y. Lipids and dimorphism of Candida albicans and Sporothrix schenckii. In: Prasad R, Ghannoum M A, editors. Lipids of pathogenic fungi. Boca Raton, Fla: CRC Press; 1996. pp. 219–233. [Google Scholar]
- 17.Knobloch K, Pauli A, Iberl B, Weis N, Weigand H. Mode of action of essential oil components on whole cells of bacteria and fungi in plate tests. In: Schreier P, editor. Bioflavour'87. Berlin, Germany: Walter de Gruyter; 1988. pp. 287–299. [Google Scholar]
- 18.Kügler S, Sebghati T S, Eissenberg L G, Goldman W E. Phenotypic variation and intracellular parasitism by Histoplasma capsulatum. Proc Natl Acad Sci USA. 2000;97:8794–8798. doi: 10.1073/pnas.97.16.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni R K, Nickerson K W. Nutritional control of dimorphism in Ceratocystis ulmi. Exp Mycol. 1981;5:148–154. [Google Scholar]
- 20.Lee K L, Buckley H R, Campbell C C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 21.Lisec A D. Quorum sensing in Candida albicans: partial purification of two extracellular candidate molecules. M.S. thesis. Lincoln: University of Nebraska; 1998. [Google Scholar]
- 22.Lo H J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 23.Magee B B, Magee P T. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- 24.Nickerson W J. An enzymatic locus participating in cellular division of a yeast. J Gen Physiol. 1954;37:483–494. doi: 10.1085/jgp.37.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickerson W J, Chung C W. Genetic block in the cellular division mechanism of a morphological mutant of a yeast. Am J Bot. 1954;41:114–120. [Google Scholar]
- 26.Odds F C. Candida and candidosis. 2nd ed. London, England: Bailliere Tindall; 1988. [Google Scholar]
- 27.Prasad R, Koul A, Mukherjee P K, Ghannoum M A. Lipids of Candida albicans. In: Prasad R, Ghannoum M A, editors. Lipids of pathogenic fungi. Boca Raton, Fla: CRC Press; 1996. pp. 105–137. [Google Scholar]
- 28.Saisho Y, Morimoto A, Umeda T. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal Biochem. 1997;252:89–95. doi: 10.1006/abio.1997.2314. [DOI] [PubMed] [Google Scholar]
- 29.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd M G, Sullivan P A. Candida albicans germ-tube formation with immobilized GlcNAc. FEMS Microbiol Lett. 1983;17:167–170. [Google Scholar]
- 31.Simonetti N, Strippoli V, Cassone A. Yeast-mycelial conversion induced by N-acetyl-d-glucosamine in Candida albicans. Nature. 1974;250:344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Marumo S. Trans to cis-2,3 double bond isomerization of epoxyfarnesol and farnesol by fungus. Tetrahedron Lett. 1972;50:5101–5104. [Google Scholar]
- 33.Tempé J, Petit A, Holsters M, van Montagu M, Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc Natl Acad Sci USA. 1977;74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voziyan P A, Haug J S, Melnykovych G. Mechanism of farnesol cytotoxicity: further evidence for the role of PKC-dependent signal transduction in farnesol-induced apoptotic cell death. Biochem Biophys Res Commun. 1995;212:479–486. doi: 10.1006/bbrc.1995.1995. [DOI] [PubMed] [Google Scholar]
- 35.Wise M L, Rossi J, Gerwick W H. Characterization of the substrate binding site of polyenoic fatty acid isomerase, a novel enzyme from the marine alga Ptilota filicina. Biochemistry. 1997;36:2985–2992. doi: 10.1021/bi962158v. [DOI] [PubMed] [Google Scholar]