Abstract
We present a case of a late preterm infant placed on extracorporeal life support in the first day of life for persistent pulmonary hypertension of the newborn. Developmental arrest, pulmonary vascular hypertensive changes, and pulmonary interstitial glycogenosis were present on lung biopsy at 7 weeks of age. Pulmonary hypertension has persisted through childhood. Genetic testing at 8 years identified a novel mutation in TBX4.
Keywords: genetics, neonatal lung disease, pediatrics, pulmonary arterial hypertension
CASE DESCRIPTION
Following a course of antenatal steroids, a preterm female was born at 34 6/7 weeks' gestation with cyanosis and respiratory distress; surfactant was administered for bilateral hazy opacities on chest X‐ray. Bilateral pneumothoraces prompted chest tube placement. Inhaled nitric oxide (iNO) was initiated after echocardiography showed suprasystemic pulmonary hypertension (PH), with exclusive right‐to‐left flow via the ductus arteriosus, without major structural heart disease. She was cannulated onto venovenous extracorporeal life support at 1d for persistent pulmonary hypertension of the newborn (PPHN). Prostaglandin E1 infusion maintained ductal patency as ductal flow remained right‐to‐left on echocardiography. She was decannulated at 9d. At 27d, she was extubated to nasal CPAP and echocardiography showed modest improvement in PPHN.
Due to atypical, prolonged cardiopulmonary course, further work‐up was undertaken. Chest CT demonstrated diffuse ground glass opacities, most prominent in central and perihilar regions without cysts or air trapping. Surfactant protein genetic analysis and chromosomal microarray were unrevealing. Cardiac catheterization at 37d (FiO2 40%, iNO) demonstrated pulmonary arterial hypertension (PAH), with mean pulmonary artery pressure (mPAP) 30 mmHg, indexed pulmonary vascular resistance (PVRi) 7.2 Woods units/m2, pulmonary‐to‐systemic blood flow (Qp:Qs) 1.4:1 due to patent foramen ovale shunt, and normal pulmonary veins. Angiography showed abnormal distal vascular bed with pruning and decreased capillary density (blush). Lung biopsy at 38d revealed delayed maturation (late saccular stage of development), pulmonary interstitial glycogenosis (PIG), and pulmonary vascular remodeling with medial hypertrophy and focal intimal proliferation (Figure 1).
Figure 1.
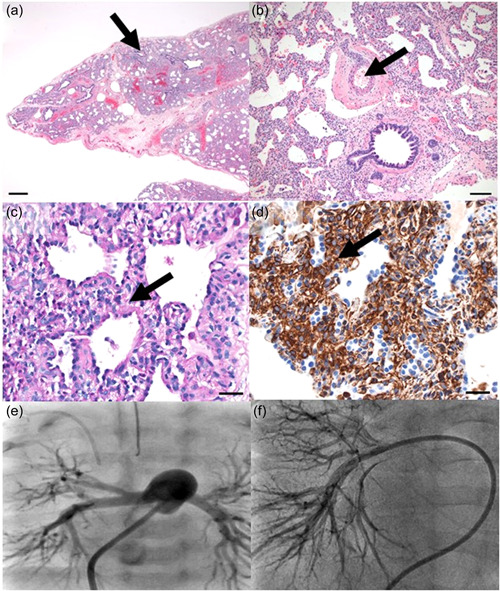
Lung biopsy at 5 weeks of life (a–d) showing: (a) Lung immaturity and moderate alveolar simplification (late saccular stage of development) with alveolar septal widening by oval mesenchymal cells. Bronchiole and alveolar duct closely approaching the pleural surface. Hematoxylin and eosin stain. Scale bar = 500 microns. (b) Pulmonary artery with moderate medial thickening and periarterial adventitial thickening. Hematoxylin and eosin stain. Scale bar = 100 microns. (c–d) Alveolar septal thickening by oval mesenchymal cells with increase in (c) cytoplasmic glycogen by Periodic acid Schiff stain, and (d) vimentin expression by immunohistochemical stain. Scale bar = 30 microns for both images. Angiography at cardiac catheterization (e, f) showing: (e) Contrast in the main pulmonary artery with markedly increased size of distal vessels and pruning with modest capillary blush at 5 weeks of life. (f) Contrast in the right pulmonary artery with relatively normal central and peripheral vessels without significant pruning and decreased capillary blush at 15 months of life, improved from prior
We initiated sildenafil for PAH and prednisolone for PIG; respiratory support was weaned to nasal cannula 1 LPM. She was discharged at 67d on prednisolone, sildenafil, gastrostomy tube feeds, and supplemental oxygen. Bosentan was started after hospital discharge after awaiting insurance authorization. She continued pulmonary vasodilator therapy, but was unable to wean off steroids and oxygen. Repeat cardiac catheterization at 7.5 months (FiO2 28%) showed no improvement with mPAP 38 mmHg and PVRi 8.9 Woods units/m2; she was reactive to iNO (mPAP 26 mmHg, PVRi 5.3 Woods units/m2). With slow clinical improvement, steroids were weaned off at 1 year; she remained on dual pulmonary vasodilator therapy and oxygen. Cardiac catheterization at 15 months (FiO2 26%) showed modest improvement (mPAP 23 mmHg, PVRi 6.3 Woods units/m2). Angiography demonstrated improved, but still decreased, capillary blush (Figure 1). Concurrent chest CT showed decreased ground glass opacities. Sildenafil was discontinued for severe reflux. She continued bosentan and weaned off daytime supplemental oxygen at 18 months. Follow‐up cardiac catheterizations showed slowly worsening PAH; dual therapy with tadalafil was initiated at 3.5 years and she was weaned off supplemental oxygen at 6 years.
At 7 years, her mother reported increasing frequency of cyanosis/desaturation with activity. She had an unremarkable stress echocardiogram, but 6‐min walk distance was only 70% predicted with respiratory distress and decreased oxygen saturations to 85–94% (baseline 98–100%). Spirometry showed restrictive lung disease (FVC 58%). Cardiac catheterization demonstrated improvement (mPAP 20 mmHg, PVRi 4.3 Woods units/m2). Chest CT showed stable lung parenchymal changes. PH genetic panel (Blueprint Genetics, Seattle WA) identified a likely pathogenic novel heterozygous truncating variant in t‐box transcription factor 4 (TBX4c.576C>A(p.Tyr192*)). Parental testing was negative suggesting a de novo variant. Small patella syndrome was diagnosed by physical exam noting a bilateral gap between first and second toes with skeletal survey pending.
DISCUSSION
Described in 2013 (this child's birth year), TBX4 deletions and variants may cause pediatric‐onset PH, as TBX4 plays a key role in lung development. 1 This may manifest as severe, biphasic PH, first as a neonate, similar to this case, or only later in childhood. 2 Lung morphometric abnormalities seen with TBX4 variants include congenital acinar and alveolar dysplasia, alveolar simplification, and pulmonary vascular remodeling. The TBX4 variant we identified has not been previously described. However, the phenotype of persistence of PH with lung immaturity and concurrent PIG is consistent with other published cases.
PIG has been described as a cause of neonatal respiratory distress, diagnosed by lung biopsy within the first days to weeks of life. Diagnostic histologic characteristics include abnormal proliferation of interstitial mesenchymal cells with intracellular glycogen accumulation which can be patchy or diffuse in distribution. 3 , 4 PIG has been seen in cases of abnormal lung growth associated with prematurity, pulmonary hypoplasia, Trisomy 21, and severe congenital heart disease. 3 , 4 PPHN and histologic pulmonary hypertensive changes have been described in association with PIG. 5 , 6 In one series, the majority of infants with PIG also had pulmonary vascular remodeling, and all infants had either or both alveolar simplification or pulmonary hypertensive changes. 5 The relationship between abnormalities in lung growth, PIG, and pulmonary vascular remodeling remains unclear. PIG may be a nonspecific reactive process in infants with other developmental lung disorders or a byproduct of abnormal differentiation of mesenchymal cells. 3 , 5 , 6
PIG was recently described in a subset of patients with PPHN later identified to have TBX4 gene mutations. 2 , 7 In one case series of 19 children with PAH (median age at diagnosis 1.5 years) attributed to a TBX4 mutation, 10 presented with PPHN, 4 had pneumothoraces, and 8 required ECLS. All lung biopsies (n = 7) showed diffusely abnormal alveolarization with pulmonary arterial hypertensive changes; three infant biopsies showed PIG, similar to our patient. 2
TBX4 mutations are thought to cause developmental lung abnormalities due to its role in mesoderm induction and specification during fetal lung development. 2 , 8 Given these structural manifestations, the role of glucocorticoids in these patients is unclear, even in the setting of concurrent PIG. As glucocorticoids promote lung maturity and decrease parenchymal and airway inflammation, they may play a role in improving respiratory status in children with various etiologies of lung disease. 4 , 5 Greater awareness of both clinical and histopathologic findings associated with TBX4 variants can prompt earlier genetic testing, potentially avoiding additional diagnostic procedures, and aiding in management of both interstitial lung disease and PH.
In summary, we describe a novel TBX4 variant associated with severe PPHN, evidence of PIG by histopathology, and persistent childhood PH. The relationship of TBX4, PIG, and early pulmonary arterial remodeling cannot be disentangled in this case, but TBX4 is likely the etiology of our patient's persistence of PPHN.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Tsoi SM, Jones K, Colglazier E, Parker C, Nawaytou H, Teitel D, Fineman JR, Keller RL. Persistence of persistent pulmonary hypertension of the newborn: a case of de novo TBX4 variant. Pulmonary Circulation. 2022;12:e12108. 10.1002/pul2.12108
REFERENCES
- 1. Kerstjens‐Frederikse WS, Bongers EMHF, Roofthooft MTR, Leter EM, Douwes JM, Van Dijk A, Vonk‐Noordegraaf A, Dijk‐Bos KK, Hoefsloot LH, Hoendermis ES, Gille JJP, Sikkema‐Raddatz B, Hofstra RMW, Berger RMF. TBX4 mutations (small patella syndrome) are associated with childhood‐onset pulmonary arterial hypertension. J Med Genet. 2013;50(8):500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galambos C, Mullen MP, Shieh JT. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. European Respiratory Journal [Internet]. 2019. Aug 1; [cited 2021 Sep 2] 54(2): Available from https://erj.ersjournals.com/content/54/2/1801965 [DOI] [PubMed] [Google Scholar]
- 3. Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, Pathology Cooperative Group, Albright EA, Askin FB, Baker P, Chou PM, Cool CM, Coventry SC, Cutz E, Davis MM, Dishop MK, Galambos C, Patterson K, Travis WD, Wert SE, White FV, ChILD Research Co‐operative. Diffuse lung disease in young children. Am J Respir Crit Care Med. 2007;176(11):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deutsch GH, Young LR. Pulmonary interstitial glycogenosis: words of caution. Pediatr Radiol. 2010;40(9):1471–5. [DOI] [PubMed] [Google Scholar]
- 5. Liptzin DR, Baker CD, Darst JR, Weinman JP, Dishop MK, Galambos C, Brinton JT, Deterding RR. Pulmonary interstitial glycogenosis: diagnostic evaluation and clinical course. Pediatr Pulmonol. 2018;53(12):1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Still GG, Li S, Wilson M, Sammut P. Persistent pulmonary hypertension without underlying cardiac disease as a presentation of pulmonary interstitial glycogenosis. Fetal Pediatr Pathol. 2018;37(1):22–6. [DOI] [PubMed] [Google Scholar]
- 7. Suhrie K, Pajor NM, Ahlfeld SK, Dawson DB, Dufendach KR, Kitzmiller JA, Leino D, Lombardo RC, Smolarek TA, Rathbun PA, Whitsett JA, Towe C, Wikenheiser‐Brokamp KA. Neonatal lung disease associated with TBX4 mutations. J Pediatr. 2019;206:286–292.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karolak JA, Szafranski P, Kilner D, Patel C, Scurry B, Kinning E, Chandler K, Jhangiani SN, Coban Akdemir ZH, Lupski JR, Popek E, Stankiewicz P. Heterozygous CTNNB1 and TBX4 variants in a patient with abnormal lung growth, pulmonary hypertension, microcephaly, and spasticity. Clin Genet. 2019;96(4):366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


