Summary
Background
Pressurized intraperitoneal aerosolized chemotherapy (PIPAC) is a novel method to treat patients with peritoneal metastases (PM). We aimed to study the tolerability, safety, pharmacokinetics, and tumour response of nanoparticle albumin bound paclitaxel (NAB-PTX) during PIPAC in a Phase I study.
Methods
Eligible patients with biopsy-proven PM from ovarian, breast, gastric, hepatobiliary, or pancreatic origin underwent three PIPAC treatments using NAB-PTX with a four-week interval. The dose of NAB-PTX was escalated from 35 to 140 mg/m2 using a Bayesian design to estimate the maximum tolerated dose (MTD).
Findings
Twenty-three patients were included; thirteen (65%) patients combined PIPAC therapy with continued systemic chemotherapy. The most frequent toxicities were liver toxicity and anaemia. Treatment resulted in seven (35%) responders, six (30%) non-responders and seven (35%) patients with stable PM. Systemic absorption of NAB-PTX was slow, with median peak plasma concentrations reached after 3 to 4 h. Median NAB-PTX tumour tissue concentrations suggested accumulation: 14.6 ng/mg, 19.2 ng/mg and 40.8 ng/mg after the first, second and third PIPAC procedure respectively. EORTC QoL and VAS scores remained stable. Overall survival after one year was 57%.
Interpretation
PIPAC with NAB-PTX results in a favourable PK profile and promising anticancer activity in patients with unresectable PM. The MTD and recommended Phase II clinical trial dose are 140 mg/m2. In patients with impaired hepatobiliary function, a dose of 112.5 mg/m2 is recommended.
Funding
Kom op tegen Kanker (Flemish League against Cancer).
Keywords: PIPAC, Nanoparticle, Paclitaxel, Peritoneal carcinomatosis, Peritoneal metastases
Research in context.
Evidence before this study
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel and promising treatment method for patients with advanced peritoneal metastases (PM). The method exploits the physical properties of the capnoperitoneum to enhance tissue penetration and spatial distribution upon intraperitoneal (IP) drug delivery. The use of nanosized medicine such as nanoparticle albumin bound paclitaxel (NAB-PTX) is hypothesized to prolong IP drug retention, leading to enhanced efficacy and reduced systemic toxicity. We have interrogated PubMed and Web of Science using the search strings ‘PIPAC’, ‘intraperitoneal’, ‘aerosol’, ‘paclitaxel’, and ‘nanoparticle’ in Boolean combinations, until 21/9/2021. No previous clinical studies were retrieved that reported the use of NAB-PTX during PIPAC.
Added value of this study
We performed a phase I dose escalating trial using repeated PIPAC with NAB-PTX in patients with advanced, unresectable PM. We found that NAB-PTX was well tolerated, and the maximum tolerated dose (MTD) was 140 mg/m2 in patients without pre-existing hepatobiliary impairment. Pharmacokinetic analysis conformed slow clearance from the peritoneal cavity, and accumulating tumour tissue concentrations of NAB-PTX upon repeated PIPAC treatments. The quality of life of the patients remained stable, and promising anticancer response was observed.
Implications of all the available evidence
The results of this phase I trial show the safety and preliminary efficacy of IP aerosolized nanoparticle albumin bound paclitaxel in patients with advanced, unresectable PM.
Alt-text: Unlabelled box
Introduction
Peritoneal metastases (PM) are a common manifestation of gastro-intestinal (GI) and gynaecological cancers.1 Compared to other metastatic locations such as the liver, systemic chemotherapy is less active against PM, with a survival typically less than 10 months.1 In addition, the quality of life of these patients is often poor due to debilitating symptoms such as obstruction or ascites formation.2, 3, 4
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) was introduced in 2012 as an innovative drug delivery method to treat PM.5,6 Diagnostic and staging laparoscopy is combined with intraperitoneal (IP) administration of aerosolized drug using a high pressure injector and atomizer.7 The procedure can be repeated at regular (usually 4–6 weeks) intervals, and allows accurate staging and response assessment in patients in whom imaging usually does not show measurable lesions. Also, PIPAC allows to serially obtain tumour histology, and thereby to measure therapeutic response and the dynamics of treatment resistance. Furthermore, the elevated IP pressure enhances drug penetration in tumour tissue.8 Data from feasibility and pilot studies suggest that PIPAC is safe and shows promising anticancer activity in a variety of cancer types.9
The pharmacological advantage of IP drug delivery may be improved using nanoparticles (NPs), prolonging the drug's residence time in the peritoneal cavity.10 Nanoparticle albumin bound paclitaxel (NAB-PTX, ABI-007, Abraxane™) is a 130 nm albumin bound NP approved for the treatment of breast, prostate, and lung cancer. The uptake of NAB-PTX into tumour cells is mediated by the binding of albumin ligands with over-expressed receptors in tumour or endothelial cells, including albondin (gp60), osteonectin, low density lipoprotein receptors, scavenger receptor class-B type1, and transferrin receptors.11 Cristea et al. demonstrated in a Phase I study that IP administration of NAB-PTX is associated with a favourable toxicity profile, a significant pharmacologic advantage and promising clinical activity.12
Aerosolized delivery of NPs using PIPAC could further enhance their clinical benefit, by improving spatial distribution and convection (pressure) driven tissue penetration.7 We report here the results of a Phase I clinical study using IP nebulized NAB-PTX in patients with advanced PM from ovarian, breast, or upper gastrointestinal origin.
Methods
Stability and anticancer effect of NAB-PTX
Nanoparticle albumin bound paclitaxel (NAB-PTX, Abraxane) was purchased from Celgene (Eigenbrakel, Belgium). The delivery of NAB-PTX using PIPAC requires the drug to be diluted in saline to a total volume of 200 mL. Also, the nebulization process exposes the drug to a high pressure inside the nebulizer (maximum of 20 bar, 2000 kPa). Therefore, we first determined the stability and the anticancer efficacy of nebulized, diluted NAB-PTX. The ratio of free and albumin-bound PTX was determined after dilution using ultra-performance liquid chromatography (UPLC) coupled with ultraviolet detection. The anticancer efficacy of nebulized NAB-PTX was measured on human ovarian carcinoma cells (SKOV-3) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Detailed methods are available in the Data Supplement.
Study design and treatment
This was a multicentre, open label, first-in-human Phase I dose escalation trial to explore the safety of NAB-PTX using PIPAC in patients with irresectable PM. The primary objectives were to determine the maximum tolerated dose (MTD) and to assess dose limiting toxicities (DLTs), pharmacokinetics (PK), safety, and tolerability. The MTD was defined as the highest dose of aerosolized NAB-PTX, administered three times using PIPAC, that does not cause unacceptable side effects. The recommended Phase II dose was defined as the MTD. Secondary objectives were assessment of pathological response, longitudinal measurement of quality of life (QoL), and overall survival.
In order to optimize the balance between safety and efficacy, we used a time-to-event continual reassessment model (TITE-CRM), where an initial design was followed until the first DLT occurred. Conservative a priori estimates of DLT were used to calculate the original dose escalation scheme, resulting in a moderate pace of escalation of NAB-PTX dose levels of 35, 70, 90, 112.5, and 140 mg/m². The same dose was used for all three treatments in the same patient. Concurrent systemic anticancer treatment was allowed, with the exception of taxanes (PTX and docetaxel).
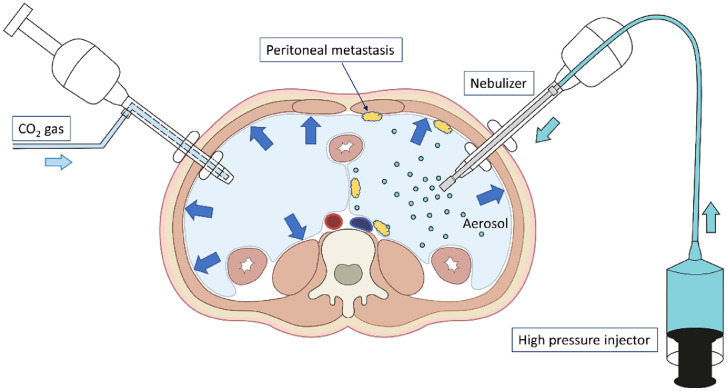
Patients underwent a maximum of three PIPAC procedures with an interval of four weeks as per local procedural protocol. After creation of a CO2 pneumoperitoneum, the peritoneal cavity was explored, the peritoneal cancer index (PCI) was calculated, digital images were obtained, and punch biopsies were taken at each of the four abdominal quadrants. The PCI is based on the size and distribution of peritoneal metastases, and ranges from 0 to 39.13 Next, NAB-PTX was aerosolized in the abdominal cavity under a maximal upstream injection pressure of 20 bar and a flow rate of 0.5 mL/s using a high-pressure injector and a nebulizer (Capnopen®, Capnomed, Zimmern, Germany). The aerosol was left in the peritoneal cavity for 30 minutes and subsequently evacuated together with the CO2 gas using a closed system (Figure 1). The CO2 pneumoperitoneum was then re-established and four additional punch biopsies were obtained, adjacent to the previous location.
Figure 1.
Illustration of pressurized intraperitoneal aerosol chemotherapy (PIPAC). After creation of a CO2 pneumoperitoneum, the peritoneal cavity is explored, the extent of peritoneal disease is scored, biopsies are taken, and anticancer drug is nebulized using a high-pressure injector and nebulizer. The drug containing aerosol is left in situ for 30 minutes.
Eligibility
Adult patients (18 years or older) were included with advanced, biopsy-proven PM from ovarian, breast, gastric, hepatobiliary or pancreatic origin. Detailed inclusion and exclusion criteria are provided in Supplementary Table 1.
Safety assessments
Chemotherapy toxicity and surgical morbidity were scored separately until 30 days after the last PIPAC treatment. Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Surgical complications were scored using the Clavien Dindo classification and the comprehensive complication index (CCI).14 Serious adverse events (SAE) were defined according to the International Conference on Harmonisation – Good Clinical Practice (ICH-GCP) guidelines as any untoward medical occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect. Dose limiting toxicity was recorded in a 14 week-window starting from the first PIPAC and defined a priori as any of the following: 1. any Grade 3 or 4 non-hematologic toxicity excluding fatigue and controllable nausea, vomiting, abdominal pain, and diarrhoea; 2. grade 4 thrombocytopenia; 3. grade 4 neutropenia lasting more than 7 days or associated with fever; 4. failure to perform more than one PIPAC due to toxicity; 5. surgical complication Dindo-Clavien grade IIIB or higher.
Histological response
Punch biopsies were taken at the same location, which was marked with a stainless-steel surgical clip during each PIPAC procedure. Samples were fixed in 4% paraformaldehyde in PBS for 72 hours and embedded in paraffin. Tissues were serially sectioned and stained with haematoxylin & eosin; immunohistochemical staining was performed for epithelial cellular adhesion molecule (EpCAM). The peritoneal regression grading score (PRGS) was determined by a GI pathologist (AH) who was masked for the treatment given.15 The mean score of all samples was calculated per treatment, and percentage changes in mean PRGS between successive PIPAC treatments were calculated and visualized in a waterfall plot.
Pharmacokinetic analysis
Plasma and tissue samples were obtained at predefined intervals. Additional details about sampling design, sample processing and concentration determination are available in the Supplementary Data. Samples were quantified for PTX (parent), 7-epi-PTX (thermodynamic impurity), 3-OH-PTX, 6-OH-PTX (primary metabolites, products from CYP3A and CYP2C8 biotransformation, respectively) and 3,6-diOH-PTX (secondary metabolite).16,17 Non-compartmental PK analysis (NCA) was performed using PKanalix version 2020R1 (Lixoft SAS, Antony, France). Data processing was done using R version 3.6.2 (R Core Team; R-project.org, Vienna, Austria). The following PK parameters were derived for each dosing regimen: maximum concentration (Cmax); time to reach maximum concentration (tmax); area under the plasma concentration-time curve (AUC0–24h); volume of distribution (Vd); clearance (CL) and elimination half-life (t1/2).
Quality of life
QoL was measured with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30.18 The questionnaires were completed one day before each PIPAC, two weeks after each PIPAC, and two and six months after the third PIPAC. Scores of the functional scales, symptom scales and global health status were calculated based on the formulas described in the EORTC QLQ-C30 scoring manual.3 Pre- and postoperative pain was scored on a visual analogue scale (VAS) one day before each PIPAC and at 8, 12, and 24 hours and one week after each PIPAC.
Statistical analysis
Dose escalation was performed using two stage TITE-CRM using conservative priors and with a target probability of DLT set at 50%.19 In a two-stage TITE-CRM, an initial design is followed until the first DLT occurs. From that moment, TITE-CRM updates the initial prior estimate of the probabilities of DLT based on all available information. Patients are assigned to the dose associated with an estimated probability of DLT that is closest to the target probability. This method allows for continuous, staggered accrual of patients.
For different settings of priors and sample sizes, a range of scenarios of ‘real’ probabilities of DLT were simulated, to examine the probability of selecting (near-) optimal doses and avoiding the selection of overly toxic doses. The combination of the conservative prior with a sample size of 20 was deemed to offer a correct balance of cautiousness and avoiding overly toxic doses, with a reasonable probability of selecting near-optimal doses.
Data are presented as mean ± one standard deviation, or median (interquartile range). Data distribution was tested for normality with the Shapiro-Wilk test. Comparisons of means of two groups were performed with the Student t-test or Mann-Whitney U-test, while comparisons between three or more groups were performed with one-way ANOVA. Overall survival was estimated using the Kaplan-Meier method. We tested differences in QoL and VAS scores between treatment groups and between different time points with linear mixed models, which allow for missing data under the assumption of missing at random.
Ethics
The study protocol (ref. AGO/2017/003) was approved by the Ethical Committee of Ghent University Hospital, the sponsor of the study. The trial was registered with EudraCT (2017-001688-20) and Clinicaltrials.gov (NCT03304210). All patients provided written informed consent. The trial was conducted in accordance with the protocol and current guidelines of the International Council for Harmonization (ICH) and Good Clinical Practice (GCP).
Role of the Funding Source
This clinical trial was supported by a grant from the Flemish League against Cancer (Kom op tegen Kanker). The Funder did not have any role in the study design, data collection, data analyses, interpretation, or writing of report. Nanoparticle albumin bound paclitaxel was purchased from the manufacturer (Celgene), who did not provide any material, intellectual, or financial support for this study, and did not have any role in the design, execution, analysis, or reporting of this trial.
Results
Stability and anticancer efficacy of diluted and nebulized NAB-PTX
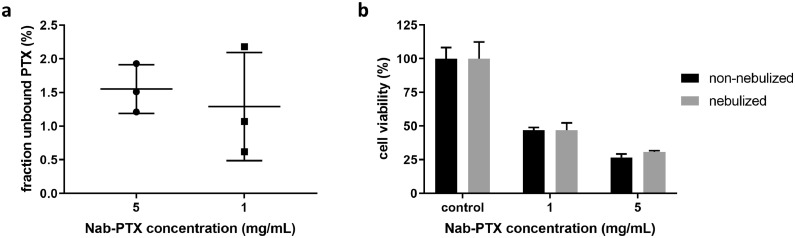
The stability of NAB-PTX diluted in 0.9% NaCl under conditions required for IV administration (5 mg/mL) was compared to the conditions required for IP administration in this clinical Phase I trial (1 mg/mL). This was evaluated by examining the ratio of unbound to total PTX. Figure 2a shows that the fraction of unbound PTX is not significantly increased when NAB-PTX is further diluted to 1 mg/mL (p = 0.636). MTT assays were conducted to study the influence of high-pressure nebulization on the anti-tumour activity of NAB-PTX dilutions. MTT assays (Figure 2b) indicated that high-pressure nebulization of 1 mg/mL and 5 mg/mL dilutions of NAB-PTX did not significantly affect the cytotoxic efficacy (p = 0.99 and 0.06, respectively, Student t test). In addition, cell viability decreased in a dose-dependent manner. These results demonstrate that neither dilutions up to 1 mg/mL nor high-pressure nebulization affect the stability and functionality of NAB-PTX.
Figure 2.
a: Box plots represent the fraction of free PTX of 1 mg/mL (circular dots) and 5 mg/mL (square dots) NAB-PTX concentrations. b: Relative cell viability of human ovarian cancer cells (SKOV-3) following 2 h incubation of non-nebulized (black bars) and nebulized (grey bars) NAB-PTX concentrations. Experiments were performed in triplicate; error bars represent one standard deviation. All differences were non-significant (p > 0.05, Student t test). PTX, paclitaxel; NAB-PTX, nanoparticle albumin bound paclitaxel.
Patient characteristics
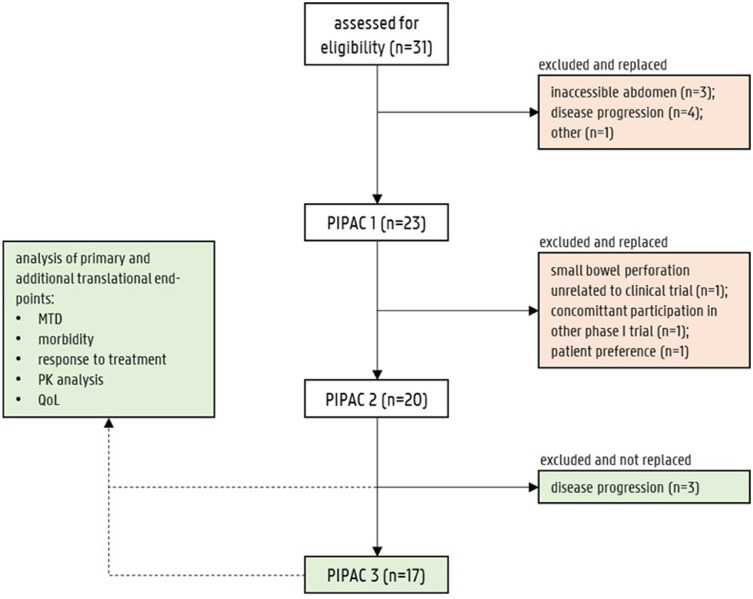
Thirty-one patients were assessed for eligibility and signed the informed consent form. Between September 2017 and March 2020, treatment was initiated in 23 eligible patients at Ghent University Hospital (Ghent, Belgium; n = 22) and at Odense University Hospital (Odense, Denmark; n = 1). Twenty patients underwent at least two consecutive PIPAC treatments. Twenty-one patients were included in the TITE-CRM analysis for the MTD. The starting dose level was 35 mg/m² and was escalated to 140 mg/m². Most patients were pre-treated: 16 (70%) had previous chemotherapy, and nine (45%) patients were undergoing 3rd line or 4th line systemic treatment. Six (30%) patients had a previous debulking with hyperthermic intraperitoneal chemoperfusion (HIPEC), one (5%) patient had a subtotal pancreatectomy, three (15%) patients had an ovariectomy and two (10%) patients had a gastrectomy. Moreover, 13 (65%) patients received concurrent systemic chemotherapy (i.e. FOLFOX, EOX, trastuzumab-pertuzumab, cisplatin-5FU, cisplatin-gemcitabine, ramucirumab, paclitaxel-carboplatin or bevacizumab). Figure 3 illustrates the patient flow and the reasons for dropping out of treatment. Demographic and clinical details of the patients are summarized in Table 1.
Figure 3.
Patient flow diagram. Red boxes represent patients that were excluded and replaced. Green boxes represent patients who completed the trial, or dropped out after two PIPACs but were considered to be eligible for study analysis. The need for replacement of patients was calculated based on the time-to-event continual reassessment method. The results of 20 patients (green boxes) were used for the analysis of this clinical trial.
Table 1.
Patient characteristics and cancer origin. Median (IQR) for age, body mass index (BMI) and peritoneal cancer index (PCI) at first PIPAC procedure. Overall PCI scoring is based on 16 patients. In four patients, the PCI could not be assessed due to extensive adhesions.
| Overall (n = 20) |
35 mg/m² (n = 2) | 70 mg/m² (n = 2) | 90 mg/m² (n = 3) | 112.5 mg/m² (n = 3) | 140 mg/m² (n = 10) | |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age (years) | 57 (49–65) | 64 | 68 | 52 | 59 | 51 |
| Gender (male, %) | 40 | 50 | - | 100 | 33 | 30 |
| BMI (kg/m²) | 23 (19–26) | 23 | 25 | 27 | 21 | 21 |
| PCI | 22 (12–31) | 35 | 24 | 17 | 20 | 21 |
| Concomitant systemic chemotherapy (%) |
65 | 50 | 50 | 67 | 67 | 70 |
| Cancer origin | ||||||
| Ovarian (%) | 20 | - | 50 | - | - | 30 |
| Gastric (%) | 55 | 50 | - | 33 | 100 | 60 |
| Pancreatic (%) | 5 | - | - | 33 | - | - |
| Breast (%) | 5 | 50 | - | - | - | - |
| Gallbladder (%) | 5 | - | - | 33 | - | - |
| Bile duct (%) | 5 | - | - | - | - | 10 |
| Unspecified upper GI (%) | 5 | - | 50 | - | - | - |
Toxicity and tolerability, DLT, and recommended Phase II dose
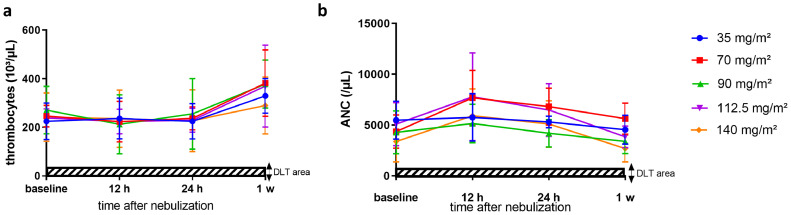
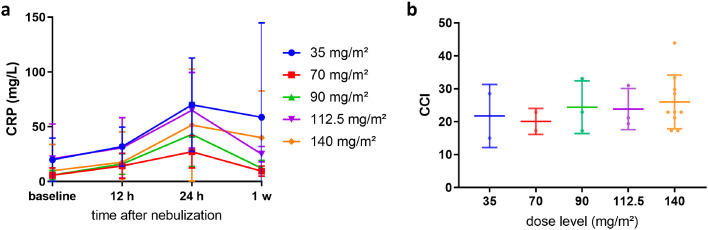
Repeated PIPAC treatment was well tolerated. No major surgical complications or mortality were observed. Grade 1 or 2 thrombopenia was observed at 70 mg/m² (n = 1), 90 mg/m² (n = 1), 112.5 mg/m² (n = 2) and 140 mg/m² (n = 3), and grade 3 thrombopenia was observed in one patient receiving 90 mg/m². Neutropenia was not detected at the lower dose levels. Grade 1 or 2 neutropenia was seen at dose levels of 90 mg/m² (n = 1), 112.5 mg/m² (n = 1) and 140 mg/m² (n = 4). However, one patient allocated to the highest dose experienced grade 3 neutropenia one week after each PIPAC. Thrombopenia and neutropenia recovered spontaneously. Figure 4 shows the time course of thrombocyte and absolute neutrophil counts (ANC) before each PIPAC and 12, 24 hours and one week after each PIPAC. The most frequent treatment-related toxicities were liver toxicity (grade 1 to 3, n = 15 [75%]) and anaemia (grade 1 to 3, n = 14 [70%]). Eight patients (40%; n = 1 at 35 mg/m², n = 1 at 90 mg/m², n = 1 at 112.5 mg/m², and n = 5 at 140 mg/m²) showed surgical (trocar) site complications including one wound infection and wound dehiscence (grade 1 to 3). In four of these patients, antibiotic therapy was required. There was no grade 4 or 5 morbidity. Seven serious adverse events (SAEs) were reported during the study period, including wound infection (n = 1), nausea (n = 1), thrombopenia (n = 1), neutropenia (n = 1), local peritonitis (n = 1), anaphylactic shock due to anaesthesia (n = 1) and paralytic ileus (n = 1). No patient needed a surgical reintervention. The time-course of postoperative C-reactive protein (CRP) is displayed in Figure 5a. The highest CRP-values were noted 24 hours after NAB-PTX administration and showed a downward trend afterwards. The CCI scores of each dose level are illustrated in Figure 5b. No significant difference (p = 0.86, Student t test) was found between the dose levels. An overview of all adverse events related to PIPAC or NAB-PTX is provided in Table 2.
Figure 4.
Thrombocytes (panel a) and absolute neutrophil count (ANC, panel b) after PIPAC with escalating doses of NAB-PTX. Bars represent one standard deviation.
Figure 5.
Evolution of C-reactive protein (CRP) (a) and comprehensive complication index (CCI) (b) according to dose level.
Table 2.
Drug and procedure related adverse events (CTCAE v5.0) until 30 days after the last PIPAC. Adverse events of any grade that occurred in at least one patient are shown; grade 3 adverse events are indicated in bold face. Adverse events that qualify as dose limited toxicities according to the prespecified definition are indicated with shaded areas. No grade 4 or 5 adverse events were observed. AST: aspartate aminotransferase – ALT: alanine aminotransferase – ALP: alkaline phosphatase – GGT: gamma-glutamyltransferase – AEs: adverse events.
| Adverse event | 35 mg/m² (n = 2) |
70 mg/m² (n = 2) |
90 mg/m² (n = 3) |
112.5 mg/m² (n = 3) |
140 mg/m² (n = 10) |
Total N patients (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | ||
| Hematological toxicity | |||||||||||
| Thrombopenia | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 3 | 0 | 8 (40) |
| Leucopenia | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 4 (20) |
| Neutropenia | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | 1 | 7 (35) |
| Anemia | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 7 | 2 | 14 (70) |
| Liver toxicity | |||||||||||
| Elevated AST | 2 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 4 | 2 | 15 (75) |
| Elevated ALT | 1 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 3 | 1 | 12 (60) |
| Elevated ALP | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 4 | 0 | 9 (45) |
| Elevated bilirubin | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 3 (15) |
| Elevated GGT | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 6 | 1 | 11 (55) |
| Glucose and electrolyte disorders | |||||||||||
| Hyperglycemia (> 115 mg/dL) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 8 | 0 | 11 (55) |
| Hyperkalemia (> 4.8 mmol/L) | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 3 | 0 | 8 (40) |
| Hypokalemia (< 3.6 mmol/L) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 (15) |
| hypernatremia (> 144 mmol/L) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5) |
| Hyponatremia (< 135 mmol/L) | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 7 (35) |
| Surgical complications | |||||||||||
| Wound dehiscence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (5) |
| Bleeding at incision | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5) |
| Gastrointestinal disorders | |||||||||||
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 (15) |
| Vomiting | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 (15) |
| Adhesions | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 (10) |
| Diarrhea | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5) |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 (15) |
| Ileus | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 (10) |
| Cardiovascular disorders | |||||||||||
| Hypertension | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 10 (50) |
| Electrocardiogram T wave abnormal | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5) |
| Adverse event | 35 mg/m² (n = 2) |
70 mg/m² (n = 2) |
90 mg/m² (n = 3) |
112.5 mg/m² (n = 3) |
140 mg/m² (n = 10) |
Total patients, N (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | grade ≤ 2 | grade 3 | ||
| Infectious complications | |||||||||||
| Bacterial pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (5) |
| Skin infection (cellulitis) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 (5) |
| Skin infection (abscess) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (5) |
| Wound infection | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 1 | 7 (35) |
| Peritoneal infection (peritonitis) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (5) |
| Skin and subcutaneous tissue disorders | |||||||||||
| Alopecia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 (10) |
| Hyperhidrosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (5) |
| General disorders and administration site conditions | |||||||||||
| Edema lower limbs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (5) |
| Nervous system disorders | |||||||||||
| Peripheral sensory neuropathy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 (15) |
| Immune system disorders | |||||||||||
| Allergic reaction to anesthesia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 (5) |
| Total AEs per dose level, N | 11 | 0 | 14 | 1 | 21 | 1 | 24 | 1 | 72 | 14 | |
| Mean AEs per patient per dose level, N | 5.5 | 0 | 7 | 0.5 | 7 | 0.3 | 8 | 0.3 | 7.2 | 1.4 | |
In total, eight grade 3 toxicities were observed in five patients at the highest dose level. However, we did not consider these toxicities to be dose limiting, since after careful consideration (detailed in the Supplementary Toxicity document) they were highly unlikely to be related to NAB-paclitaxel and/or resolved within a period of 24 hours. We therefore deemed it unethical to declare these as DLTs and deny patients the possibility of further study treatment. Specifically, only one patient developed marked elevation of plasma levels of the hepatocellular enzymes (AST and ALT), but this patient had known malignant infiltration of the hepatoduodenal ligament and dilated bile ducts on pre-trial imaging. The posterior probability of DLT for 140 mg/m2 was 15% (90% Credible Interval 1.6–41%). For the lower doses, the posterior probabilities were below 1%. The MTD and recommended Phase II dose were therefore defined as 140 mg/m2, unless patients have known hepatobiliary functional impairment, in which case the MTD and RP2D is 112.5 mg/m2.
Histological response
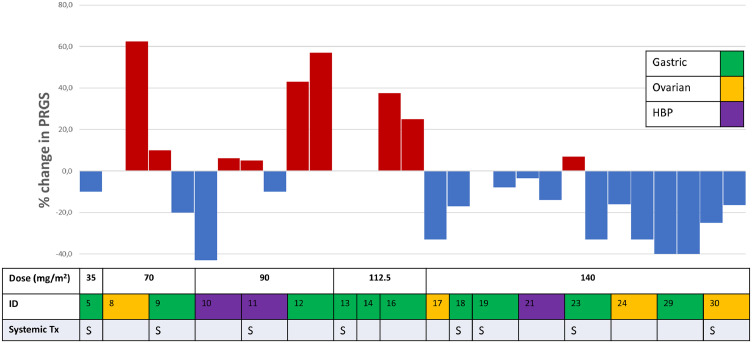
Tumour tissue samples were taken from each abdominal quadrant (n = 4) before aerosol delivery. In 30% of all procedures, less than four biopsies were taken due to technical reasons (adhesions) or because no visible tumour was present. The change in mean PRGS score between PIPAC treatments in patients who received two or three PIPAC treatments is illustrated in Figure 6. The data suggest a dose-response relationship: tumour regression was observed in 1/2, 1/3, 0/3, and 7/8 patients treated with 70, 90, 112.5, and 140 mg/m2, respectively.
Figure 6.
Waterfall plot of the change in mean peritoneal regression grading score (PRGS) of all sampled biopsies according to pathology, concomitant systemic treatment, and dose level. Every bar represents a single PIPAC treatment; if no bar is shown the change was zero. ID, patient identifier; Tx, treatment.
In 13 patients, measurable disease was present on CT scan. In nine patients (69%), CT findings correlated with the change in mean PRGS. After PIPAC with NAB-PTX, seven (35%) responders, six (30%) non-responders and seven (35%) patients with stable PM were found.
Pharmacokinetic Analysis
The final analysis dataset included a total of 514 plasma PK samples and 394 tumour samples from 20 patients who underwent a maximum of three PIPAC procedures. The plasma and tumour samples were analyzed for PTX, 7-epi-PTX, 3-OH-PTX, 6-OH-PTX and 3,6-diOH-PTX.
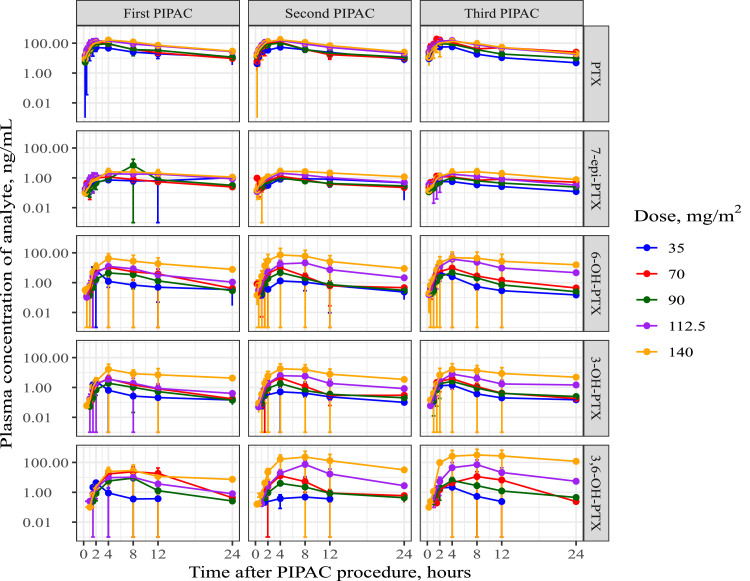
The plasma concentration-time profiles of PTX were subjected to NCA, and the results are presented in Table 3. A graphical representation of the data of PTX and its metabolites is provided in Figure 7. The plasma concentrations of PTX were substantially higher than those of its metabolites. Plasma concentrations were lowest for 7-epi-PTX, and similar for both 3-OH-PTX and 6-OH-PTX, while the plasma concentrations of diOH-PTX were between those of 7-epi-PTX and 3-OH-PTX/6-OH-PTX. Peak plasma concentrations of PTX were reached between 3h and 4h across the different doses, i.e. much later than the end of the PIPAC procedure. Peak plasma concentrations of the metabolites were shifted to later time points for the primary metabolites 3-OH-PTX and 6-OH-PTX, and to even later time points for the secondary metabolite 3,6-diOH-PTX. Peak plasma concentrations (Cmax) of PTX increased less than dose-proportionally, whereas in general, total exposure (AUC) increased dose-proportionally, even though the 90 mg/m2 appeared to deviate. The median PTX terminal half-life across dose cohorts ranged between 7.08 and 8.82 h.
Table 3.
Median (min-max range) pharmacokinetic parameters of different doses of NAB-PTX following PIPAC administration.
| Tmax (h) |
Cmax (ng/mL) |
AUC0-24h (h*ng/mL) |
Vd (L) |
CL (L/h) |
T1/2 (h) |
|
|---|---|---|---|---|---|---|
| 35 mg/m² (n = 2, 5 PIPACs) | 4 (1.5-4) | 57.84 (38.54-77.13) | 541.58 (497.66-733.93) | 1074.4 (883.5-1933.9) | 84.44 (73.07-90.29) | 8.82 (7.25-18.34) |
| 70 mg/m² (n = 2, 6 PIPACs) | 3 (2-4) | 128.95 (101.81-218.91) | 910.23 (727.07-1381.78) | 1141.7 (350.5-1460.2) | 111.77 (77.48-154.41) | 7.08 (2.30-10.65) |
| 90 mg/m² (n = 3, 9 PIPACs) | 4 (2-4) | 106.02 (78.50-114.71) | 806.21 (710.45-936.25) | 2216.5 (1421.6-2851.8) | 190.90 (151.89-239.69) | 8.05 (5.72-12.56) |
| 112.5 mg/m² (n = 3, 9 PIPACs) | 4 (1.5-4) | 151.18 (94.55-220.75) | 1747.44 (1162.14-2448.41) | 961.4 (659.5-1968.6) | 85.20 (63.75-137.69) | 7.82 (6.74-16.02) |
| 140 mg/m² (n = 10, 29 PIPACs) | 4 (2-12) | 158.4 (4.33-415.87) | 1904.83 (82.17-3879.67) | 1330.9 (561.3-42405.0) | 112.02 (49.17-934.73) | 8.23 (4.01-31.44) |
Tmax = time to reach maximum concentration; Cmax = maximum concentration; AUC0-24h = area under the curve, Vd = volume of distribution, CL = clearance; T1/2 = elimination half-life.
Figure 7.
Mean (±SD) plasma concentration vs. time profiles of paclitaxel and its respective metabolites across doses of nab-paclitaxel following PIPAC administration. PIPAC = pressurized intraperitoneal aerosol chemotherapy, PTX = paclitaxel.
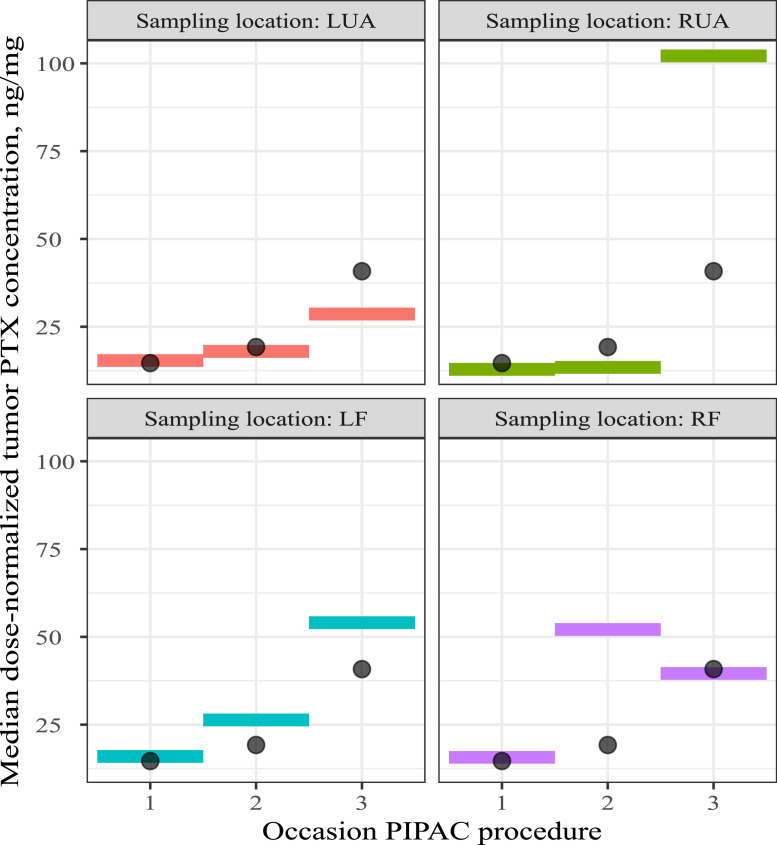
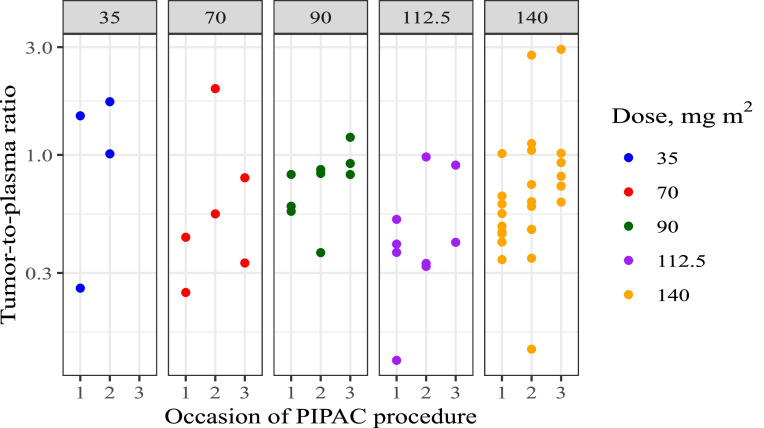
Figure 8 depicts the median tumour PTX concentrations per quadrant across the different PIPAC procedures, while Figure 9 provides the tumour-to-plasma concentration ratios for the different administered doses of NAB-PTX. This ratio was calculated as C_tumour/C_(plasma (TSLD=30min)). Since tumour tissues were sampled at the end of each PIPAC procedure (time since last dose (TSLD) of 30 min), the TSLD 30 min PTX plasma concentrations were used to generate this ratio. In the excised tumour nodules, only PTX and 7-epi-PTX were detected. 7-epi-PTX levels were, similar to plasma, much lower than PTX levels. Tumour concentrations did not show pronounced differences between the different quadrants and increased generally dose-proportionally, even though between-subject variability was high. Median dose-normalized (for 140 mg/m2) PTX tumour concentrations across doses after consecutive PIPAC procedures suggested accumulation: 14.6 ng/mg, 19.2 ng/mg and 40.8 ng/mg for the first, second and third PIPAC procedure respectively. Tissue-to-plasma ratios were comparable between doses, and ranged between 0.3 and 1, supporting dose-proportionality.
Figure 8.
Median dose-normalized tumour paclitaxel concentrations in each of the abdominal quadrants, sampled 0.5 h after initiation of the PIPAC procedure. All concentrations were dose-normalized to 140 mg/m2. Colored boxes: median dose-normalized tumour concentrations stratified per sampling location; Black dots: median dose-normalized tumour PTX concentrations across sampling location; PTX = paclitaxel, PIPAC = pressurized intraperitoneal aerosol chemotherapy, LUA = left upper abdomen, RUA = right upper abdomen, LF = left iliac fossa, RF = right iliac fossa.
Figure 9.
Individual tumour-to-plasma concentration ratios at different doses of nab-paclitaxel following PIPAC administration. PIPAC = pressurized intraperitoneal aerosol chemotherapy.
Quality of life
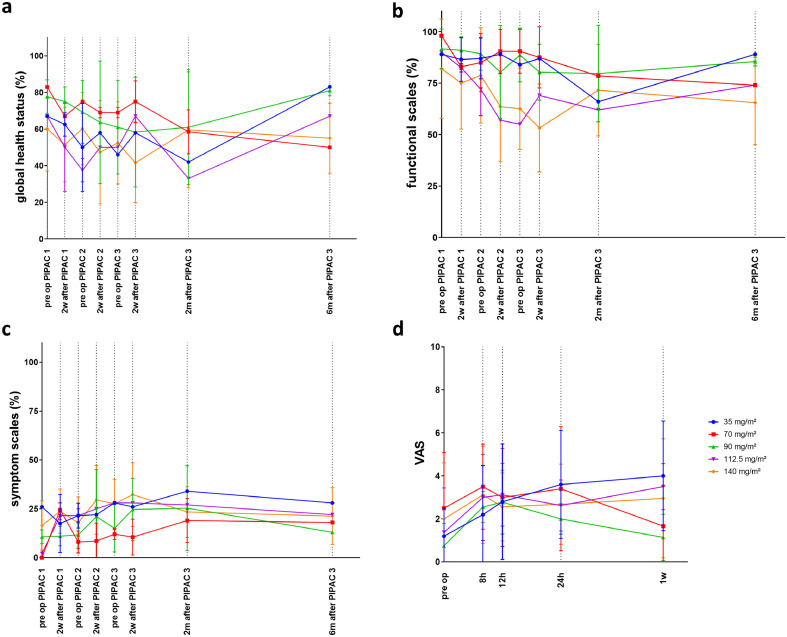
EORTC QLQ-30 global health, functional and symptom scores were determined one day before each PIPAC, two weeks after each PIPAC, and two and six months after the third PIPAC. Using linear mixed models, no significant effects were found of either time (p = 0.73) or dose (p = 0.11) on the global health scores (Figure 10). A significant effect was found of treatment dose (p = 0.037), but not of time (p = 0.66) on the functional scores. Symptom scores were not significantly affected by time (p = 0.43) or drug dose (p = 0.098). For the pain scores (VAS), a significant effect of time was found (p = 0.021), but not of treatment dose (0.19).
Figure 10.
EORTC QLQ-30 global health (a), functional scale (b), symptom scale (c), and visual analog pain score (d) over time in patients undergoing PIPAC with NAB-PTX.
Survival
After a median follow up of 12 months, median overall survival was 10 months (95% confidence interval, 0-21). The actual survival was 50% after one year and 20% after two years.
Discussion
In patients with advanced unresectable PM, very few effective treatment options are available. The use of repeated PIPAC procedures enables locoregional delivery of anticancer therapy, and at the same time allows accurate staging and response monitoring. In this first clinical study using IP aerosolized nanoparticles, we found that PIPAC with NAB-PTX is well tolerated. Haematological toxicity was moderate, with only one patient developing grade 3 neutropenia at the highest dose level. The fact that 65% of patients received concomitant systemic treatment may confound the interpretation of systemic toxicity. However, allowing concomitant anticancer treatment was the only ethical option, since it would not be acceptable to deny patients with metastatic disease a potentially active systemic treatment.
According to the prespecified definition, eight events in five patients formally qualified as a dose limiting toxicity. However, as outlined in the Results, we have carefully addressed these toxicities and decided not to qualify them as ‘dose limiting’ since they were evidently not related to the drug administered, or mild and transient. Of note, there are no standard criteria of what constitutes a DLT (other than indicating the highest dose that does not result in unacceptable toxicity). Often, the definition of DLT excludes grade III-IV non-haematological toxicities that are transient or manageable with adequate supportive care. Furthermore, the pharmacokinetic data support our choice for the highest dose level as MTD (cfr. infra).
In the patient with known malignant infiltration of the hepatoduodenal ligament, dilated bile ducts, and mildly elevated pre-trial liver enzymes, significantly elevated liver enzymes were observed. Hepatic toxicity is a known side effect of NAB-PTX, and most patients had elevated liver enzymes. Paclitaxel and albumin bound PTX are both hepatically metabolized via the cytochrome P450 system, which may explain the hepatocellular toxicity.20 It is prudent, therefore, to suggest using a lower dose in patients with known hepatic and/or biliary functional impairment.
Controllable nausea, vomiting, and pain were excluded from the prespecified DLT criteria, if they occurred early, since they are likely to be the result of the abdominal surgery and general anaesthesia, rather than due to toxicity of paclitaxel in these patients. Surgical complications were more common at the highest dose level; one of the patients in this cohort received concomitant systemic treatment with Bevacizumab.
In patients with extensive metastatic disease, the need for repeated general anaesthesia and surgery could represent a burden. However, we observed that the QoL in these patients remains stable, and outside of clinical trials PIPAC procedures are commonly performed as outpatient procedures.21
Administration of NAB-PTX via PIPAC resulted in a dose-proportional increase in AUC, and a less than dose-proportional increase in Cmax of PTX and its downstream metabolites. This may be an advantage from a safety perspective since peak plasma levels of drugs are often linked to side-effects. In the case of PTX, mainly PTX will contribute to the activity since PTX reaches by far the highest concentrations as compared to its metabolites, combined with having the highest potency. Furthermore, not all metabolites are active.22,23 Peak plasma concentrations of the metabolites are clearly reached at later time points, which is thought to be the result of the downstream metabolism taking time to develop. At the defined MTD (140 mg/m²), the median total PTX plasma Cmax and AUC0–24h were 158 ng/mL and 1905 h*ng/mL, respectively. These results were 2.5-fold lower compared to the catheter-based NAB-PTX IP delivery method performed by Cristea et al. (Cmax: 500 ng/mL, AUC0–24h: 4300 h*ng/mL).24 The latter could be attributed to differences in analytical methods, carrier volume, treatment frequency, and patient population. However, it may also suggest a more complete tissue drug uptake, as shown by the tumour concentration data (cfr infra). As expected, systemic PTX exposure was significantly lower compared to IV treatment: in a Phase I study with NAB-PTX administered at a dose of 135 mg/m2 as a 30 min IV infusion, Cmax and AUCinf were 6100 ng/mL and 6427 h*ng/mL, respectively.25
There was a tendency for an increase in tumour nodule concentrations of both PTX and 7-epi-PTX upon subsequent NAB-PTX PIPAC administrations, which might positively contribute to the efficacy outcome. However, between-subject variability in tumour exposure was high. 7-Epi-PTX is not expected to contribute to the efficacy, and the fact that it could be detected in the tumour nodules indicates that it likely was already present in the administered nebulized solution.26 This is supported by the lack of detectable levels of 3-OH- and 6-OH-PTX in the tumour nodules, which indicates that no metabolism of PTX takes place in the peritoneal cavity or in the tumours. In addition, the blood circulation also does not seem to reach the tumour nodules because in that case one would expect to measure both metabolites also in tumour tissue. This further indicates that absorption of PTX and 7-epi-PTX takes place directly from the peritoneal cavity and/or from the tumour surface. It is striking that the peak plasma concentrations of PTX are reached only hours after the end of the PIPAC procedure, indicating that the tumour concentrations at 30 min were very likely not yet at their maximum. Continued absorption likely takes place from the tissue surfaces covered with PTX at the end of the nebulization procedure. Tumour-to-plasma concentration ratios of PTX ranged between 0.3–1 indicating that PTX does reach the systemic circulation to a roughly similar extent as the tumour.
In agreement with the PK data showing increasing PTX accumulation in tumour tissue during subsequent treatments, histological responses were observed in 35% of patients who received two or more PIPAC procedures. The objective measurement of treatment response is not straightforward in patients with extensive PM. Due to the nature of the study and the inclusion criteria, all patents had extensive, unresectable disease with a high PCI. However, we did observe clinical and histological responses without noticeable changes in PCI, and therefore we decided that a change in PCI is not an adequate metric of treatment response in this particular setting. Also, most patients had disease that was not amenable to size measurements according to the RECIST criteria. Therefore, we have focused on the histological response, as evaluated with H&E staining supplemented with EpCAM immune histochemistry, as the optimal response parameter, although we recognize its limitation in terms of potential heterogeneity and susceptibility to sampling errors. Despite the fact that the PRGS has not yet been demonstrated to correlate with survival in large clinical trials, it is at present the most frequently cited response marker in PIPAC clinical studies, and can be reproducibly assessed, as shown by a high interobserver agreement among reading pathologists.27 Also, a decrease in PRGS was shown to correlate with chemotherapy response in animal PM models.28
Although the study was performed in heavily pre-treated patients with extensive PM, survival results were encouraging, with 50% surviving longer than one year.
In conclusion, IP aerosolized NAB-PTX is well tolerated and shows promising anticancer activity. In patients without hepatobiliary functional impairment, the MTD and recommended dose for a Phase II clinical trial are 140 mg/m2.
Data supplement
Stability and anticancer activity of NAB-PTX
NAB-PTX (Abraxane®; Celgene, New Jersey, United States) was diluted in 0.9% sodium chloride to a concentration of 5 mg/mL to reach the conditions required for IV administration (5 mg/mL) and a concentration of 1 mg/mL to reach the conditions required for IP injection as planned in this clinical Phase I trial. The NAB-PTX solutions were kept at room temperature for one hour, mimicking the time between preparation in the pharmacy of the hospital and administration to the patient. A volume of 500 µL of both NAB-PTX dilutions were transferred to the sample chambers of a single-use rapid equilibrium dialysis (RED) plate (Thermo Fisher Scientific, Merelbeke, Belgium) and 750 µL 0.9% NaCl was added to the corresponding buffer chambers. Equilibrium dialysis was performed in triplicate for both NAB-PTX solutions. The RED plate was covered with sealing tape and was incubated at 37°C for six hours on an orbital shaker at 250 rpm. After dialysis, resulting solutions of the sample chamber and buffer chamber were subjected to UPLC coupled with ultraviolet detection (UPLC-UV) to determine the ratio of free and albumin-bound PTX.
The antitumour activity of NAB-PTX before and after nebulization was determined using the MTT cell viability assay. This way, the functionality of the nebulized formulation could be compared with the native formulation before nebulization. Human ovarian carcinoma cells (SKOV-3) were seeded as monolayers in 24-well plates at a density of 4×104 cells/well. After 24 hours of incubation at 37°C in a 5% CO2-containing humidified atmosphere, cells were exposed for two hours with nebulized or non-nebulized NAB-PTX at 1 and 5 mg/mL. Cells in the control group were exposed to non-nebulized and nebulized PBS (Fischer Scientific, Merelbeke, Belgium). Subsequently, NAB-PTX dilutions were discarded and cells were kept in the incubator for another 72 hours. After incubation, 20 µL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma Aldrich, Overijse, Belgium; 5 mg/mL) was administered to each well for two hours. MTT solutions were then removed and 100 µL of dimethyl sulfoxide (DMSO; Sigma Aldrich, Overijse, Belgium) was added to each well to solubilize formazan crystals. The plate was then covered with aluminium foil and placed on the orbital shaker for 30 minutes at 100 rpm. The absorbance of solubilized formazan was determined at 570 nm in a Paradigm Detection platform and analyzed with the Soft Max Pro 6.1 software (BIO-RAD laboratories, Hemel Hempstead, United Kingdom). Analyses were performed in triplicate.
Pharmacokinetic analysis
Blood samples (4 mL) were collected in heparin-coated blood tubes at the start of nebulization,15, 30, and 60 minutes and 1.5, 2, 4, 8, 12, and 24 hours after each PIPAC. The blood samples were centrifuged at 1,500 g for 10 minutes at 4°C. One mL of plasma was transferred to polypropylene cryovials, and stored in a -80°C freezer until UPLC-MS/MS analysis. Frozen plasma samples were allowed to thaw at room temperature. A volume of 50 µL plasma was then transferred to Eppendorf tubes. After adding 200 µL of internal standard solution mix (13C-PTX, 2 ng/mL), the samples were continuously shaken at 500 rpm for 20 minutes at 37°C by a thermoshaker. Thereafter, 100 µL of the resulting solution was diluted with 150 µL of water (UPLC-MS water, Biosolve, Valkenswaard, The Netherlands) and injected onto the Waters Acquity UPLC system to measure PTX and metabolite concentrations in plasma.29
Tumour tissue samples in each abdominal quadrant (n = 4; punch biopsies of approximately 8–10 mm³) were taken at the end of aerosol delivery for tissue concentration analysis. All samples were immediately snap-frozen in liquid nitrogen and stored at -80°C until analysis. The biopsies were then thawed at room temperature and weighed. Subsequently, internal standard solution mix was added. Tumour tissue was then enzymatically digested using a proteinase-K and a lipase solution (Sigma-Aldrich, Diegem, Belgium). Tissue suspensions were left to incubate overnight at 55°C whilst continuously being shaken (IKA® Werke, Staufen, Germany). After centrifugation of the digested tissue suspension at 10,000 g for 20 minutes, supernatans was collected and subjected to the Waters Acquity UPLC system to measure PTX and metabolite concentration.29
Contributors
Wim Ceelen: study design, data analysis, data interpretation, funding acquisition, writing Louis Sandra: data analysis, data interpretation, writing, review & editing Leen Van de Sande: data collection, data analysis, data interpretation, writing, review & editing Martin Graversen: study design, data collection, data interpretation, writing, review & editing Michael Bau Mortensen: study design, study collection, data interpretation, writing, review & editing An Vermeulen: data analysis, data interpretation, writing, review & editing Elke Gasthuys: data analysis, data interpretation, writing, review & editing Dries Reynders: study design, data analysis, data interpretation, writing, review & editing Sarah Cosyns: study design, data analysis, data interpretation, writing & editing Anne Hoorens: data analysis, data interpretation, writing & editing Wouter Willaert: study design, data analysis, data interpretation, writing, review & editing.
All authors have seen and approved the final version of the manuscript. The source clinical data were verified by Wim Ceelen, Dries Reynders, and Leen Van de Sande; the pharmacokinetic data were verified by An Vermeulen and Elke Gasthuys.
Data sharing statement
The anonymized source clinical and laboratory data from this study are available in full from the first author (wim.ceelen@ugent.be) upon request.
Declaration of interests
None of the authors have a conflict of interest to declare.
Acknowledgments
The authors thank the patients and their families for their willingness to participate in this clinical trial. We thank the study nurses (Soumaya Akhayad and Karen De Meuleneir) and the Clinical Trial Unit (HIRUZ) at Ghent University Hospital for their dedication and support.
Footnotes
This work was presented as an abstract at the 2021 meeting of ASCO (abstract nr 4065).
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104151.
Appendix B. Supplementary materials
References
- 1.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Lambert LA. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65(4):283–298. doi: 10.3322/caac.21277. [DOI] [PubMed] [Google Scholar]
- 3.Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19(41):6979. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonemura Y, Endou Y, Sasaki T, et al. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol. 2010;36(12):1131–1138. doi: 10.1016/j.ejso.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc. 2012;26(7):1849–1855. doi: 10.1007/s00464-012-2148-0. [DOI] [PubMed] [Google Scholar]
- 6.Solass W, Herbette A, Schwarz T, et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc. 2012;26(3):847–852. doi: 10.1007/s00464-011-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi-Gorji M, Van de Sande L, Debbaut C, et al. Intraperitoneal aerosolized drug delivery: Technology, recent developments, and future outlook. Adv Drug Deliv Rev. 2020;160:105–114. doi: 10.1016/j.addr.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Nadiradze G, Horvath P, Sautkin Y, et al. Overcoming drug resistance by taking advantage of physical principles: pressurized intraperitoneal aerosol chemotherapy (PIPAC) Cancers. 2020;12(1):34. doi: 10.3390/cancers12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alyami M, Hubner M, Grass F, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20(7):e368–e377. doi: 10.1016/S1470-2045(19)30318-3. [DOI] [PubMed] [Google Scholar]
- 10.Dakwar GR, Shariati M, Willaert W, Ceelen W, De Smedt SC, Remaut K. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis - mission possible? Adv Drug Deliv Rev. 2017;108:13–24. doi: 10.1016/j.addr.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal H, Yang T, Li T, et al. Serum protein-based nanoparticles for cancer diagnosis and treatment. J Controll Rel. 2021;329:997–1022. doi: 10.1016/j.jconrel.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Cristea MC, Frankel P, Synold T, et al. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother Pharmacol. 2019;83(3):589–598. doi: 10.1007/s00280-019-03767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt A, Rousset P, Benzerdjeb N, et al. Prospective correlation of the radiological, surgical and pathological findings in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: implications for the preoperative estimation of the peritoneal cancer index. Colorectal Dis. 2020;22(12):2123–2132. doi: 10.1111/codi.15368. [DOI] [PubMed] [Google Scholar]
- 14.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 15.Solass W, Sempoux C, Carr NJ, et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology. 2019;74(7):1014–1024. doi: 10.1111/his.13829. [DOI] [PubMed] [Google Scholar]
- 16.Fransson MN, Gréen H, Litton JE, Friberg LE. Influence of Cremophor EL and genetic polymorphisms on the pharmacokinetics of paclitaxel and its metabolites using a mechanism-based model. Drug Metab Dispos. 2011;39(2):247–255. doi: 10.1124/dmd.110.035394. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YY, Liu Y, Zhang JW, et al. Characterization of human cytochrome P450 isoforms involved in the metabolism of 7-epi-paclitaxel. Xenobiotica. 2009;39(4):283–292. doi: 10.1080/00498250802714907. [DOI] [PubMed] [Google Scholar]
- 18.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19.Cheung YK. Chapman & Hall /CRC Press; New York: 2011. Dose Finding by the Continual Reassessment Method. [Google Scholar]
- 20.van Schaik RH. Implications of cytochrome P450 genetic polymorphisms on the toxicity of antitumor agents. Ther Drug Monit. 2004;26(2):236–240. doi: 10.1097/00007691-200404000-00027. [DOI] [PubMed] [Google Scholar]
- 21.Graversen M, Lundell L, Fristrup C, Pfeiffer P, Mortensen MB. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure. Pleura Peritoneum. 2018;3(4) doi: 10.1515/pp-2018-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spratlin J, Sawyer MB. Pharmacogenetics of paclitaxel metabolism. Crit Rev Oncol Hematol. 2007;61(3):222–229. doi: 10.1016/j.critrevonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Cresteil T, Monsarrat B, Alvinerie P, Tréluyer JM, Vieira I, Wright M. Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res. 1994;54(2):386–392. [PubMed] [Google Scholar]
- 24.Cristea MC, Frankel P, Synold T. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother Pharmacol. 2019;83(3):589–598. doi: 10.1007/s00280-019-03767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 26.Yang J, Li K, He D, et al. Toward a better understanding of metabolic and pharmacokinetic characteristics of low-solubility, low-permeability natural medicines. Drug Metab Rev. 2020;52(1):19–43. doi: 10.1080/03602532.2020.1714646. [DOI] [PubMed] [Google Scholar]
- 27.Detlefsen S, Windedal T, Bibeau F, et al. Role of immunohistochemistry for interobserver agreement of Peritoneal Regression Grading Score (PRGS) in peritoneal metastasis. Hum Pathol. 2022;120:77–87. doi: 10.1016/j.humpath.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Taibi A, Lo Dico R, Kaci R, et al. Evaluation of a new histological grading system for assessing the response to chemotherapy of peritoneal metastases from colorectal cancer: a mouse model study. Eur J Surg Oncol. 2020;46(1):160–165. doi: 10.1016/j.ejso.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Xie F, De Thaye E, Vermeulen A, Van Bocxlaer J, Colin P. A dried blood spot assay for paclitaxel and its metabolites. J Pharm Biomed Anal. 2018;148:307–315. doi: 10.1016/j.jpba.2017.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.