Abstract
Background:
Chronic Pelvic Pain (CPP) is a debilitating problem that afflicts 15-20% of women in the United States. Although over 200,000 hysterectomies are performed annually for the treatment of CPP, prior studies indicate that one in four women undergo the discomfort and morbidity of hysterectomy without relief of pain. Factors that predict treatment failure remain poorly characterized.
Objectives:
To describe the incidence of persistent pelvic pain in the 6 months following hysterectomy in women with chronic pelvic pain and determine whether a simple, self-report measure of central sensitization is associated with a greater risk of persistent pelvic pain following hysterectomy.
Study Design:
We conducted a prospective, observational cohort study of women undergoing hysterectomy for a benign indication at an academic tertiary care center. Patients with preoperative chronic pelvic pain, defined as average pelvic pain ≥3 on a 0-10 numeric rating scale for >3 months prior to hysterectomy, were included in this analysis. Patients completed validated assessments of pain, anxiety, depression, and centralized pain (using the 2011 Fibromyalgia Survey Criteria, 0-31 points) preoperatively and 6-months after hysterectomy. Demographic information, surgical history, intraoperative findings and surgical pathology were abstracted from the electronic medical record. Multivariate logistic regression was used to identify independent predictors of persistent pelvic pain 6 months following hysterectomy, defined as <50% improvement in pelvic pain severity.
Results:
Among 176 participants with pelvic pain prior to hysterectomy, 126 (71.6%) were retained at 6-months, and 15 (11.9%) reported persistent pelvic pain. There was no difference in age (p=0.46), race (p=0.55), average pain severity during menses (p=0.68), average overall pelvic pain (p=0.10), or pain duration (p=0.80) in those with and without persistent pelvic pain. While intraoperative findings of endometriosis (p=0.05) and uterine fibroids (p=0.03) were associated with a higher incidence of persistent pain on univariate analysis, surgical route (p=0.46), pelvic adhesions (0.51), uterine weight (p=0.66) and adenomyosis on histopathology (p=0.27), were not related to risk of persistent pain. Higher preoperative centralized pain scores (p=0.01), but not depression (p=0.64) or anxiety (p=0.45) were more common in women with persistent pelvic pain. Multivariate logistic regression adjusting for age, preoperative pain severity, anxiety, depression and operative findings of endometriosis and fibroids indicated that every 1-point increase in centralized pain prior to hysterectomy was associated with a 27% increase in odds of persistent pelvic pain (OR 1.27, 95%CI 1.03, 1.57) 6-months after surgery.
Conclusion:
While the majority of women with CPP report considerable improvement in pain following hysterectomy, higher degrees of centralized pain prior to hysterectomy is a robust predictor of persistent pelvic pain.
Keywords: Hysterectomy, pelvic pain, persistent pain, fibromyalgia, nociplastic pain, central sensitization, centralized pain, endometriosis
Condensation:
Higher degrees of centralized pain prior to hysterectomy are a robust predictor of persistent pelvic pain 6 months after surgery.
Introduction:
Chronic pelvic pain (CPP) is a debilitating problem that afflicts 15-20% of reproductive-age women.1,2 The significant suffering it causes often leads to multiple surgeries and long-term medical therapies at a cost of at least $2.8 billion annually.1 Pain and suffering often persist despite these treatments, and leads to decreased productivity, impaired emotional well-being, and reduced quality of life.3-6 Although hysterectomy is often touted as definitive treatment for CPP, prior studies suggest that one in four women undergo the discomfort and morbidity of hysterectomy without adequate relief of pelvic pain.7-9 At present, we know little about who will fail to respond to surgery due to our incomplete understanding of the mechanisms underlying the pathogenesis of pain in this complex condition.
Although the pathogenesis of pelvic pain is not fully understood, current evidence suggests that it is a multifactorial, heterogeneous condition that can result from any combination of the three mechanisms of pain defined by the International Association for the Study of Pain (IASP): nociceptive pain, neuropathic pain, and nociplastic pain.10 Nociceptive pain is pain that arises from activation of peripheral nociceptors due to actual or threatened tissue damage, such as inflammation. Neuropathic pain is defined as pain caused by a lesion or disease of the peripheral nervous system. Nociplastic pain, often termed “centralized pain” or “central sensitization,” is thought to be due to central nervous system alterations in pain processing, has been shown to be strongly associated with pain severity and disability, and appears to predict both acute and chronic post-surgical pain. We use the term “central sensitization” hereafter.
While nociceptive pain has often been considered the primary mechanism of pain in women undergoing hysterectomy due to pelvic pathology such as endometriosis or adenomyosis, numerous studies from our group and others have also identified evidence of central sensitization in women CPP, including those with endometriosis-associated pelvic pain.11-13 The presence of central sensitization prior to hysterectomy may explain why some women experience persistent pain following surgery, as this etiology of pain is less likely to resolve with hysterectomy. Self-report symptoms of central sensitization captured preoperatively have been shown to predict poor outcomes in patients undergoing hip and knee replacement.14 However, no prospective study has explored the relationship between preoperative measures of central sensitization and persistent pain in women with CPP undergoing hysterectomy. Thus, the objectives of this prospective, observational cohort study are to describe the incidence of persistent pelvic pain in the 6 months following hysterectomy in women with CPP and determine whether a simple, self-report measure of central sensitization is associated with an increased risk of persistent pelvic pain following hysterectomy.
Materials and Methods:
This study was approved by the University of Michigan Institutional Review Board. As previously described, this prospective observational study named “Analgesics Outcomes Study (AOS)” was designed to assess the prevalence and predictors of acute and chronic pain after surgery. Reporting of these findings conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. This analysis is limited to adult patients undergoing hysterectomy for benign indications at the University of Michigan from December 2015 to June 2018. Patients were recruited in the 30 days prior to surgery and were eligible to participate if hysterectomy was for benign, non-obstetric indications. Patients were excluded if they did not read English, were unable to provide written informed consent, or were incarcerated. Informed consent was obtained from all patients.
Baseline phenotyping battery:
Patients completed standardized questionnaires and a battery of validated self-report measures of pain and psychological status in the 30 days prior to surgery. Self-report demographic information included race, marital status, level of education, occupational and disability status.
Pain severity:
Participants rated their average severity of “pelvic pain, cramping, or discomfort” in the past month using an 11-point numeric rating scale (NRS, 0-10) with 0 indicating “no pain” and 10 indicating “pain as bad as you can imagine.” They also reported the number of days of any pelvic pain (0-30 days) in the past month, severe pelvic pain (0-30 days) in the past month, and duration of pelvic pain (years). The average severity of pain with urination, full bladder, bowel movements, pain with vaginal intercourse, and pelvic pain in the week before, during, and week after menses (among women with at least one menses in the past 3 months) was recorded using the same 11-point NRS.
Central sensitization:
The degree of centrally enhanced pain processes was measured using the American College of Rheumatology (ACR) 2011 Fibromyalgia Survey Criteria.15 This validated self-report measure is the sum of the total number of painful body areas (0 to 19 points) and the severity of related symptom such as fatigue, trouble thinking, and sleeping difficulties (0 to 12 points). While a score of ≥ 13 has been used as epidemiological diagnostic criteria for fibromyalgia, central sensitization (or nociplastic pain) is thought to be a continuum and the total score (ranging 0-31 points) is frequently used as a proxy index for this pain mechanism, with higher values indicating a greater degree of central sensitization. It has demonstrated good reliability, convergent and discriminant validity, relates strongly to functional neuroimaging findings in central sensitization, and is a robust predictor of both pain and disability. The Fibromyalgia Score has been shown to be highly predictive of acute postsurgical opioid use among patients undergoing hysterectomy or knee or hip arthroplasty16,17, as well as persistent pain 6 months following knee or hip arthroplasty.14
Anxiety and depression:
Anxiety and depression prior to surgery were measured using The Hospital Anxiety and Depression Scale (HADS). The HADS is a validated questionnaire that contains 7 questions about anxiety and 7 questions about depression, with a score range of 0-21 for each measure, with higher scores indicating more depressive and anxiety symptoms.18 Similar to previous studies18, a cut-off score of 11 or higher was defined as clinically significant depression and anxiety.
Medical record review:
The medical records of all participants were abstracted using standardized case report forms for medical and surgical history, operative procedure and findings, histopathology, intraoperative and postoperative complications (SA, ST, and KG abstracted the data and were blinded to the results). Variables included age, body mass index, history of surgically-documented endometriosis, number of prior surgeries for pelvic pain or endometriosis, indication for hysterectomy, mode of hysterectomy (vaginal, laparoscopic, or laparotomy), presence of and severity of endometriosis or adhesions at hysterectomy, specimen weight (grams), presence of endometriosis, fibroids or adenomyosis on histopathology, intraoperative or postoperative complications. Indications for hysterectomy included abnormal uterine bleeding, CPP, dysmenorrhea, fibroids, prolapse; participants may have more than one indication. When identified at the time of hysterectomy, endometriosis stage was scored according to the revised American Fertility Society endometriosis scoring system.19 Adhesions were scored using the validated scoring system developed by the Adhesion Scoring Group.20
Longitudinal assessment:
Patients were evaluated at 1, 3, and 6 months after hysterectomy using the same validated pain measurements used in the baseline phenotyping, using a combination of phone interview or postal mail.
Primary and secondary outcomes:
In order to identify the prevalence and risk factors for persistent pelvic pain among patients reporting clinically impactful pelvic pain at time of hysterectomy, this analysis was limited to patients who reported their average pelvic pain in the month prior to hysterectomy as 3 or more on the 0-10 NRS scale. The primary outcome was failure to achieve 50% or more improvement in average pelvic pain score 6 months after hysterectomy. Secondary outcomes included measures of change in pain over time.
Statistical analysis:
Analysis was performed using R software version 3.6.2. Descriptive statistics (measures of central tendency, variability, statistical hypothesis tests) were run on the response and explanatory variables. The descriptive analysis was used to prescreen covariates that have potential association with the response, which were included in the multivariate models. A series of multivariate models including backward variable selection were used to understand the pain response. These included a logistic model to evaluate the primary outcome of a dichotomized change in pain (the persistent pain response defined as failure to achieve 50% or more improvement in pelvic pain 6 months after hysterectomy). Secondary outcome included linear model regressing change in pain at 6 months vs. the baseline and a mixed longitudinal linear model with Gaussian subject-specific intercept regressing measurements of pain over time. Likelihood ratio tests and Akaike/Bayesian information criteria (AIC/BIC) were used to trim the models and identify significant explanatory variables.
Results:
A total of 359 patients undergoing hysterectomy for a benign indication were enrolled, 338 had complete data on average pelvic pain score at baseline, and 176 reported an average pelvic pain score of ≥ 3 of 10 at baseline. Among these participants who were eligible for this analysis, 126 (71.6%) were retained at 6 months and represent the sample for this analysis.
Six months following hysterectomy, 15 (11.9%) patients reported persistent pelvic pain defined as failure to achieve 50% or more improvement in average pelvic pain. There was no difference in age (p=0.46), race (p=0.55), average pain severity (p=0.59) or pain duration (p=0.80) in those with and without persistent pelvic pain (Table 1). Higher preoperative centralized pain scores, measured using the Fibromyalgia Survey Score, were more common in women with persistent pelvic pain (p=0.01), but there were no significant differences in prevalence of depression (p=0.13) or anxiety (p=0.28). While intraoperative findings of endometriosis (p=0.045) and uterine fibroids (p=0.03) were associated with a higher incidence of persistent pain on univariate analysis, surgical route of hysterectomy (p=0.46), pelvic adhesions (0.51), uterine weight (p=0.66) and adenomyosis on histopathology (p=0.27), were not related to risk of persistent pain (Table 2).
Table 1.
Baseline characteristics and persistent pelvic pain
| Pain improved (n=111) |
Persistent pain (n=15) |
P value | |
|---|---|---|---|
| Age (year) | 45.5 ± 10.0 | 43.4 ± 4.3 | 0.46 |
| BMI (kg/m2) | 32.9 ± 7.4 | 31.7 ± 7.6 | 0.55 |
| Race (%) | |||
| White | 87 (79.1) | 9 (64.3) | |
| Black | 17 (15.5) | 5 (35.7) | 0.14 |
| Other | 6 (5.4) | 0 | |
| Married, yes | 89 (82.2) | 11 (78.6) | 0.84 |
| Education | |||
| No HS diploma | 3 (2.7) | 1 (7.1) | |
| HS or equivalent | 17 (15.5) | 2(14.3) | 0.85 |
| Some college | 32 (29.1) | 4 (28.6) | |
| College graduate+ | 58 (52.7) | 7 (50.0) | |
| Disability Payments, yes | 18 (16.4) | 2 (14.3) | 0.85 |
| Occupational Status | |||
| Full time job or study | 38 (34.6) | 6 (42.9) | |
| Part time | 58 (52.7) | 5 (35.7) | 0.44 |
| Not working | 14 (12.7) | 3 (21.4) | |
| Pain severity (0-10 NRS) | |||
| Week before menses | 4.6 ± 2.7 | 5.0± 4.1 | 0.68 |
| During menses | 7.1 ± 2.3 | 6.5 ± 3.0 | 0.44 |
| Week after menses | 3.1 ± 2.4 | 3.6 ± 3.5 | 0.54 |
| With voiding | 2.1 ± 2.5 | 1.8 ± 2.7 | 0.78 |
| With bowel movements | 1.9 ± 2.6 | 2.7 ± 2.7 | 0.25 |
| Average pelvic pain in past 3-months | 5.3 ± 1.8 | 6.1 ± 2.4 | 0.10 |
| Days of any pelvic pain per month | 16.5 ± 10.6 | 15.1 ± 10.3 | 0.63 |
| Days of severe pelvic pain per month | 7.6 ± 7.7 | 7.5 ± 8.8 | 0.99 |
| Duration of pelvic pain (years) | 9.6 ± 9.8 | 10.6 ± 12.7 | 0.80 |
| History of surgically documented endometriosis prior to hysterectomy | 11 (10.0) | 2 (13.3) | 0.69 |
| Number of prior surgeries for endometriosis or CPP | 92 (84.4) | 11 (78.6) | |
| 0 | 14 (12.8) | 3 (21.4) | 0.58 |
| 1 | 3 (2.8) | 0 | |
| 2 | |||
| Number of all prior surgeries | |||
| 0 | 35 (32.1) | 3 (21.4) | 0.23 |
| 1 | 30 (27.5) | 7 (50.0) | |
| >=2 | 4 (40.4) | 4 (28.6) | |
| Current opioid user | 18 (16.2) | 2 (13.3) | 0.77 |
| Fibromyalgia Survey Score (0-31) | 7.4 ± 4.5 | 10.6 ± 5.2 | 0.01 |
| Depression (HADS depression score ≥11) | 6 (7.7) | 1 (12.5) | 0.64 |
| Anxiety (HADS anxiety score ≥11) | 19 (24.4) | 1 (12.5) | 0.45 |
NRS (numeric rating score); CPP (chronic pelvic pain). All values are reported as n (%) or mean ± standard deviation
Table 2.
Surgery Characteristics and persistent pelvic pain 6 months after hysterectomy
| Pain improved (n=111) |
Persistent pain (n=15) |
P value | |
|---|---|---|---|
| Hysterectomy Indication (may have >1) | |||
| Abnormal uterine bleeding | 65 (59.1) | 10 (66.7) | 0.57 |
| Fibroids | 34 (30.9) | 5 (33.3) | 0.85 |
| Chronic Pelvic Pain | 27 (24.5) | 3 (20.0) | 0.70 |
| Dysmenorrhea | 18 (16.4) | 2 (13.3) | 0.76 |
| Prolapse | 19 (17.3) | 13 (13.3) | 0.70 |
| Mode of Surgery | |||
| Open | 8 (7.3) | 0 | |
| Laparoscopic or Robotic | 74 (67.3) | 12 (13.3) | 0.46 |
| Vaginal | 28 (25.5) | 3 (46.9) | |
| Uterine Weight (grams) | 256.0 ± 337.5 | 216.5 ± 161.9 | 0.66 |
| Surgical Complications | |||
| Intraoperative complication | 2 (1.9) | 0 | 0.89 |
| Postoperative complication | 5 (4.9) | 1 (6.7) | 0.77 |
| Endometriosis identified at time of hysterectomy, yes | 14 (13.2) | 5 (33.3) | 0.05 |
| Stage of Endometriosis at time of hysterectomy | 92 (86.8) | 10 (66.7) | 0.009 |
| 0 | 13 (12.3) | 3 (20.0) | |
| 1-2 | 1 (0.94) | 2 (13.3) | |
| 3-4 | |||
| Presence of Adhesions | |||
| None | 66 (61.1) | 10 (77.4) | 0.51 |
| Moderate | 31 (28.7) | 2 (16.13) | |
| Severe | 11 (10.2) | 2 (6.45) | |
| Surgical menopause at completion of hysterectomy | 9 (8.2) | 0 | 0.12 |
| Adenomyosis on pathology | 46 (44.7) | 9 (60.0) | 0.27 |
| Fibroids on pathology | 52 (50.5) | 12 (80.0) | 0.03 |
All values are reported as n (%) or mean ± standard deviation
Patient characteristics were also compared according to the severity of central sensitization, classified by Fibromyalgia Survey Score tertile. Patients with scores from 0-4 were classified as Low FM, scores from 5-8 as Moderate FM, and 9-31 as High FM, using the same cut-offs as previously reported.14 The groups differed significantly relative to current opioid use, and multiple measures of pain symptoms, including pain severity, pain exacerbating factors, and pain frequency, with greater overall pain burden in patients with more centralized pain (Table 3).
Table 3.
Baseline phenotype by Fibromyalgia Survey Score Tertile
| All | Fibromyalgia Survey Score | ||||
|---|---|---|---|---|---|
| Low (0-4) (n=50) |
Moderate (5- 8) (n=49) |
High (9- 31) (n=74) |
P value |
||
| Age (year) | 44.8 ± 9.8 | 45.9 ± 9.8 | 44.8 ± 11.3 | 43.5 ± 8.2 | 0.38 |
| BMI (kg/m2) | 32.3 ± 7.5 | 31.1 ± 7.1 | 31.1 ± 7.3 | 33.6 ± 7.9 | 0.12 |
| Race | |||||
| White | 133 (76.4) | 37 (74.0) | 39 (79.6) | 55 (76.4) | |
| Black | 30 (17.2) | 10 (20.0) | 9 (18.4) | 11 (15.3) | 0.65 |
| Other | 11 (6.3) | 3 (6.0) | 1 (2.0) | 6 (8.3) | |
| Married, yes | 42 (84.0) | 42 (85.7) | 54 (75.0) | 0.27 | |
| Education | |||||
| No HS diploma | 8 (4.6) | 2 (4.0) | 1 (2.0) | 4 (5.6) | 0.72 |
| HS or equivalent | 28 (16.1) | 7 (14.0) | 8 (16.3) | 13(18.1) | 0.72 |
| Some college | 47 (27.0) | 13 (26.0) | 10 (20.4) | 22 (30.6) | |
| college graduate+ | 91 (52.3) | 28 (56.0) | 30 (61.2) | 33 (45.8) | |
| Disability Payments, yes | 27 (15.5) | 5 (10.0) | 6 (12.3) | 15 (20.8) | 0.21 |
| Occupational Status, employed | 59 (33.9) | 14 (28.0) | 14 (28.6) | 28 (38.9) | |
| Full time job or study | 93 (53.5) | 30 (60.0) | 30 (61.2) | 33 (45.8) | 0.44 |
| Part time | 22 (12.6) | 6 (12.0) | 5 (10.2) | 11 (15.3) | |
| Not working | |||||
| Hysterectomy Indication | |||||
| Abnormal uterine bleeding | 104 (60.0) | 23 (47.9) | 29 (59.2) | 51 (68.9) | 0.07 |
| Fibroids | 56 (32.2) | 15 (31.3) | 19 (38.8) | 21 (28.4) | 0.48 |
| Chronic Pelvic Pain | 46 (26.4) | 7 (15.6) | 13 (26.5) | 24 (32.4) | 0.09 |
| Dysmenorrhea | 25 (14.4) | 10 (20.8) | 3 (6.1) | 12 (16.2) | 0.10 |
| Prolapse | 29 (16.7) | 9 (18.8) | 7 (14.3) | 12 (16.2) | 0.84 |
| Current opioid user | 27 (15.3) | 3 (6.0) | 7 (14.3) | 17 (23.0) | 0.03 |
| Overall Pain severity (0-10 NRS) | 4.0 (2.8) | 2.2 2.4 | 4.1 2.6 | 5.1 2.5 | 0.000 |
| History of surgically documented endometriosis prior to hysterectomy | 18 (10.6) | 4 (8.3) | 3 (6.3) | 11 (14.9) | 0.27 |
| Total number of prior surgeries for pelvic pain or endometriosis | 140 (82.8) | 41 (85.4) | 42 (87.5) | 57 (78.1) | 0.19 |
| 0 | 14 (14.2) | 4 (8.3) | 6 (12.5) | 14 (19.2) | |
| 1 | 5 (3.0) | 3 (6.3) | 0 (0) | 2 (2.7) | |
| 2 | |||||
| Total number or prior abdominal surgeries | 53 (31.5) | 18 (37.5) | 16 (34.0) | 19 (26.0) | 0.74 |
| 0 | 51 (30.4) | 13 (27.1) | 14 (29.8) | 24 (32.9) | |
| 1 | 64 (38.1) | 17 (35.4) | 17 (36.2) | 30 (41.1) | |
| >=2 | |||||
| Pelvic Pain severity (0-10 NRS) | |||||
| Week before menses | 4.8 ± 2.8 | 3.8 ± 3.3 | 4.9 ± 2.1 | 5.5 ± 2.9 | 0.04 |
| During menses | 7.0 ± 2.4 | 6.4 ± 2.6 | 6.8 ± 2.3 | 7.7 ± 2.2 | 0.04 |
| Week after menses | 3.4 ± 2.8 | 2.1 ± 2.8 | 3.8 ± 2.7 | 3.9 ± 2.7 | 0.02 |
| With full bladder | 2.1 ± 2.5 | 1.5 ± 2.3 | 2.3 ± 2.6 | 2.3 ± 2.6 | 0.18 |
| With bowel movements | 2.0 ± 2.6 | 1.2 ± 2.4 | 2.1 ± 2.3 | 2.3 ± 2.7 | 0.06 |
| Average pelvic pain in past 3-months | 5.4 ± 2.0 | 4.9 ± 1.8 | 5.3 ± 1.8 | 5.7 ± 2.1 | 0.07 |
| Days of any pelvic pain per month | 16.5 ± 10.7 | 12.4 ± 10.0 | 16.9 ± 11 | 18.9 ± 10.3 | 0.004 |
| Days of severe pelvic pain per month | 8.0 ± 8.2 | 4.7 ± 6.5 | 8.0 ± 7.6 | 10.1 ± 8.8 | 0.002 |
| Duration of pelvic pain (years) | 10.2 ± 10.0 | 11.6 ± 10.1 | 8.8 ± 9.9 | 10.5 ± 10.4 | 0.69 |
NRS (numeric rating score); All values are reported as n (%) or mean ± standard deviation
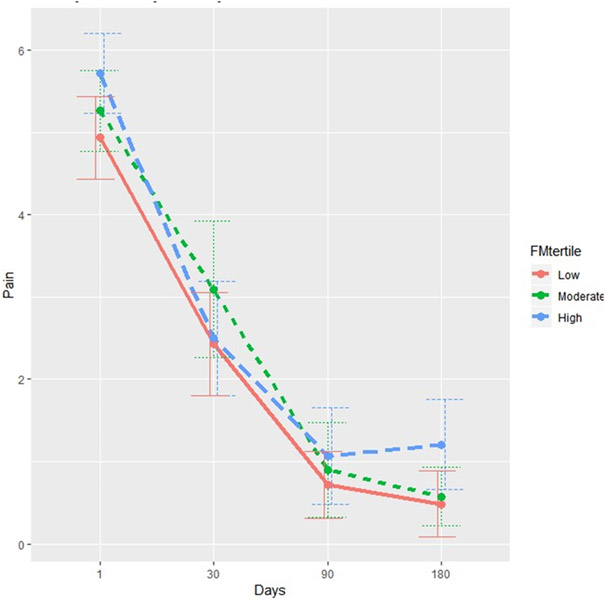
The strong adverse effect of central sensitization on the prevalence, intensity and pattern of persistent pain following hysterectomy was confirmed in several multivariate analyses (Table 4). Multivariate logistic regression controlling for age, preoperative pain severity, anxiety, depression and operative findings of endometriosis and fibroids indicated that every 1-point increase in central sensitization prior to hysterectomy was associated with a 27% increase in odds of persistent pain (OR 1.27, 95%CI 1.03, p=0.026) 6-months after surgery, defined as failure to achieve 50% or more improvement in average pelvic pain (Table 4). When examining the predictors of change in average pelvic pain intensity baseline vs. 6 months (continuous, with values >0 indicating improvement in pain), there was a significant adverse effect of higher central sensitization scores. For every 1-point increase in the preoperative Fibromyalgia Survey Score, patients reported 0.12 points less improvement in pain on the 0-10 NRS (p=0.004). Longitudinal linear mixed model for pain over time showed a trend for the adverse effect of the Fibromyalgia Survey Score, p=0.09. Multivariate analysis of change in pain vs. baseline also exhibited a significant adverse effect of anxiety (p=0.03). Not surprisingly, the longitudinal model has shown a strong improvement in pain over time (p<0.001). The mean pelvic pain intensity (0-10 NRS) in the 6 months after hysterectomy stratified by Fibromyalgia Survey Score tertile is demonstrated in Figure 1.
Table 4.
Multivariate model persistent pelvic pain 6 months after hysterectomy
| Effect and 95% Confidence Interval |
p-value | |
|---|---|---|
| Logistic model; Response: Persistent Pain; Effect: Odds Ratio | ||
| Fibromyalgia Survey Score | 1.27 (1.03, 1.58) | 0.026 |
| Age (years) | .94 (0.83, 1.06) | 0.33 |
| Endometriosis at time of surgery, yes | 1.76 (0.30, 10.51) | 0.54 |
| Fibroids on pathology | 4.48 (0.58, 34.5) | 0.15 |
| Anxiety prior to hysterectomy | 0.34 (0.02, 5.06) | 0.43 |
| Depression prior to hysterectomy | 0.43 (0.01, 12.67) | 0.63 |
| Average pelvic pain in 3-months prior to hysterectomy (0-10 NRS) | 1.12 (0.99, 1.83) | 0.65 |
| Linear model; Response: Change in Pain (continuous); Effect: mean difference per unit change in the explanatory variable | ||
| Fibromyalgia Survey Score | −0.12 (−0.20,−0.04) | 0.004 |
| Age (years) | 0.02 (−0.01,0.05) | 0.25 |
| Endometriosis at time of surgery, yes | −0.34 (−1.10,0.41) | 0.37 |
| Fibroids on pathology | −0.39 (−1.00,0.23) | 0.22 |
| Anxiety prior to hysterectomy | 0.96 (0.10,1.82) | 0.03 |
| Depression prior to hysterectomy | 0.34 (−1.00,1.68) | 0.62 |
| Average pelvic pain in 3-months prior to hysterectomy (0-10 NRS) | 0.89 (0.71,1.07) | <0.001 |
| Linear mixed longitudinal model with Gaussian subject-specific intercept; Response: Longitudinal Pain (continuous); Effect: mean difference per unit change in the respective variable | ||
| Fibromyalgia Survey Score | 0.05 (−0.008,0.10) | 0.099 |
| Age (years) | −0.02 (−0.04,0.008) | 0.20 |
| Endometriosis at time of surgery, yes | 0.096 (−0.45,0.64) | 0.73 |
| Fibroids on pathology | 0.003 (−0.43,0.43) | 0.99 |
| Anxiety prior to hysterectomy | 0.14 (−0.42,0.69) | 0.63 |
| Depression prior to hysterectomy | −0.67 (−1.59,0.25) | 0.16 |
| Average pelvic pain in 3-months prior to hysterectomy (0-10 NRS) | 0.39 (0.27,0.51) | <0.001 |
| Time 1 month vs. baseline | −2.52 (−2.98,−2.06) | <0.001 |
| Time 3 months vs. baseline | −4.29 (−4.77,−3.81) | <0.001 |
| Time 6 month vs. baseline | −4.54 (−5.01,−4.06) | <0.001 |
Figure 1. Mean pelvic pain intensity.
Average Pain (0-10 NRS) by FM Survey Category
Mean pelvic pain intensity (0-10 NRS, 95% confidence interval) in the 6 months after hysterectomy stratified by Fibromyalgia Survey Score terile (low, 0-4 points; medium, 5-8 points; high, 9-31 points).
NRS, numeric rating score
Comment:
Principal Findings:
While the majority of patients with CPP in this cohort reported considerable improvement in pelvic pain following hysterectomy, a higher degree of central sensitization (i.e. nocioplastic pain) prior to hysterectomy, as measured with the Fibromyalgia Survey Score, was strongly associated with less than 50% improvement in pelvic pain six months after hysterectomy. These findings held true across the continuum of the Fibromyalgia Survey Score, even among patients who did not meet the criteria for fibromyalgia diagnosis, defined by a score of 13 or more. Indeed, for every one-point increase in the Fibromyalgia Survey Score prior to hysterectomy, patients had a 27% increase in the odds of failure to improve after hysterectomy. Multiple other factors, which are often assumed to predict pain relief after hysterectomy, were not found to be associated with a higher incidence of pain improvement. For example, patient age, the severity or duration of pelvic pain prior to hysterectomy, history of endometriosis, route of hysterectomy or findings such as adhesions or adenomyosis were not significantly associated with a difference in odds of persistent pain after hysterectomy in this cohort.
Results within the context of existing literature:
Despite the staggering number of hysterectomies performed for CPP, factors that predict who will be among those who experience persistent pain following surgery remain poorly characterized, leaving patients and physicians with little ability to make informed and personalized decisions regarding whether hysterectomy is the appropriate choice for an individual woman with CPP. Existing literature yields an incomplete picture, with factors such as younger age9,21-23, lack of pelvic pathology21, depression24 demonstrating modestly increased risk of persistent pelvic pain. However, clinical factors that often guide the physician’s decision to offer hysterectomy (e.g. dysmenorrhea, uterine tenderness, adenomyosis) have not been shown to predict treatment success.25 Even among women with endometriosis, the incidence of persistent pain is 23% and pain relief does not correlate with the severity of disease.22,23,26
Our study indicates that central sensitization as measured by the Fibromyalgia Survey Score is a strongly associated with the prevalence and severity of persistent pain following hysterectomy and appears to be much more strongly associated than many of the factors explored in the existing literature. In fact, none of the previously explored factors were independently associated with persistent pain in our multivariate model, including presence of endometriosis. Previous studies from our group have demonstrated that central sensitization has been strongly associated with persistent postoperative pain following other surgical procedures14, but this is the first prospective study to confirm similar findings in hysterectomy. These findings are also consistent with prior literature that indicates the presence of pain elsewhere in the body is strongly associated with persistent pain after hysterectomy.8,9,27
Emerging data suggests that CPP, like most chronic pain states, is a heterogeneous condition that results from a complex interaction of ongoing nociceptive input from peripheral tissues (e.g. endometriosis) that can then be amplified and sustained by sensitization of either the peripheral nervous system (peripheral sensitization) and/or central nervous system (central sensitization, now termed nociplastic pain).28,29 The peripheral and central nervous systems play a critical role in determining which afferent signals originating from the periphery will lead to the perception of pain. A critical construct is that within any chronic pain condition individual patients may have markedly different nociceptive, peripheral and central neural contributions to pain, and the balance between these factors determines the intensity of clinical pain and whether a patient responds to a given treatment.
Clinical Implications:
We hypothesize that successful treatment of CPP depends not only on the success of eliminating the relevant peripheral nociceptive input (e.g. hysterectomy), but also an understanding of the degree to which central sensitization independently maintains pelvic pain. We do not intend to imply that patients with high degrees of central sensitization should not have the opportunity to potentially benefit from removal of peripheral pain generators with excision of endometriosis or hysterectomy. Rather, performing a preoperative assessment for the degree of central sensitization may allow gynecologic surgeons and patients have a more informed discussion regarding anticipated postoperative outcomes and risk for persistent pain, as well as opening a conversation about the potential for pharmacologic or behavioral treatments that are believed to target central sensitization. These conversations are relevant to CPP patients with and without endometriosis, as our group and others have demonstrated evidence of central sensitization in CPP regardless of the presence or severity of endometriosis.
Strengths and Limitations:
This is the first prospective study to examine the impact of central sensitization on persistent pelvic pain following hysterectomy. Strengths include prospective use of validated measurement tools encompassing multiple dimensions of pain, incorporation of operative findings and surgical pathology, and 6-month follow up with high participant retention. Despite these strengths, there are limitations. As with all observational data, causal inferences cannot be made. While the retention rate was very high, it is possible that the findings were biased by the loss to follow up. Furthermore, these data are derived from a single, large, academic medical center and may not be generalizable to other populations. For example, it is notable that the incidence of persistent pain in this cohort is considerably less than that described in prior studies. We hypothesize that the clinical practice patterns at our institution may be contributing to this lower rate. Many hysterectomies at our institution are performed by a group of surgeons who also have extensive training in multimodal management of CPP. Therefore, patients with CPP are encouraged to consider non-surgical therapies, including pelvic floor myofascial pain and treatment of overlapping pain conditions, prior to undergoing hysterectomy. This may result in a study sample with lower proportion of patients with untreated conditions such as pelvic floor myofascial pain. This study also contains a small number of patients with endometriosis, therefore limiting the degree of inference in this population. While our hypothesis is consistent with findings in other inflammatory conditions, these findings should be validated in a larger cohort with variable degrees of endometriosis.
Research Implications:
To our knowledge, this is the first study to prospectively describe the association between nociplastic or centralized pain and poorer long-term outcomes in women undergoing hysterectomy. Additional larger, mechanistic studies that simultaneously incorporate validated measures of both peripheral and central factors, such as detailed quantification of pelvic pathology (e.g. endometriosis, adenomyosis), markers of pelvic and systemic inflammation, quantitative sensory testing, and functional brain neuroimaging, are needed to understand the mechanisms of persistent pain, with the goal of developing more effective and personalized treatment options.
Conclusions:
Hysterectomy is likely to result in significant improvement in pain for many patients with CPP, but patients with higher degree of central sensitization are at significantly increased risk for persistent pain following surgery.
AJOG at a Glance:
- Why was the study conducted?
- To describe the incidence of persistent pelvic pain in the 6 months following hysterectomy in women with chronic pelvic pain
- To determine whether a simple, self-report measure of central sensitization was associated with a greater risk of persistent pelvic pain following hysterectomy.
- What are the key findings?
- Approximately 12% of patients report less than 50% improvement in pelvic pain 6 months after hysterectomy.
- Every 1-point increase in centralized pain prior to hysterectomy was associated with a 27% increase in odds of persistent pelvic pain (OR 1.27, 95%CI 1.03, 1.57) 6-months after surgery.
- What does this study add to what is already known?
- While the majority of women with CPP report considerable improvement in pain following hysterectomy, higher degrees of centralized pain prior to hysterectomy is a robust predictor of persistent pelvic pain.
Support:
Dr. As-Sanie is supported by NIH R01 HD088712. Dr. Brummett is supported by NIAMS R01AR060392, NIAMS P50 AR070600, NIDA R01DA038261, NIDA R01DA042859, and Common Fund UM1 NS118922. Dr. Schrepf is supported in part by NIH R43 DA046981. Dr. Till is supported by NICHD 1K23HD09928301A1. Additional support from the Department of Anesthesiology, Michigan Medicine, Ann Arbor, MI.
Footnotes
Disclosure: Dr. As-Sanie is a consultant for Abbvie, Myovant, Bayer, and Eximis, and receives author royalties from UpToDate. Dr. Brummett a consultant for Heron Therapeutics (San Diego, CA), Vertex Pharmaceuticals (Boston, MA), and Alosa Health (Boston, MA), and he provides expert testimony. Dr. Missmer is a consultant for AbbVie and Roche and currently receives grant funding from NIH, DoD, and AbbVie. Remaining authors report no conflicts of interest.
This manuscript is to be presented at the Society for Gynecologic Surgeons Annual Scientific Meeting, June 27-30, 2021, Palm Springs, California.
Contributor Information
Sawsan AS-SANIE, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, Michigan.
Sara R. TILL, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, Michigan.
Andrew SCHREPF, Department of Anesthesiology and Chronic Pain and Fatigue Research Center, University of Michigan, Ann Arbor, Michigan.
Kendall GRIFFITH, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, Michigan.
Alex TSODIKOV, Department of Biostatistics, University of Michigan, Ann Arbor, Michigan.
Stacey A. MISSMER, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University College of Human Medicine, Grand Rapids, Michigan.
Dan CLAUW, Department of Anesthesiology and Chronic Pain and Fatigue Research Center, University of Michigan, Ann Arbor, Michigan.
Chad BRUMMETT, Department of Anesthesiology and Chronic Pain and Fatigue Research Center, University of Michigan, Ann Arbor, Michigan.
REFERENCES:
- 1.Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321–327. [DOI] [PubMed] [Google Scholar]
- 2.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones G, Jenkinson C, Kennedy S. The impact of endometriosis upon quality of life: a qualitative analysis. J Psychosom Obstet Gynaecol. 2004;25(2):123–133. [DOI] [PubMed] [Google Scholar]
- 4.Peveler R, Edwards J, Daddow J, Thomas E. Psychosocial factors and chronic pelvic pain: a comparison of women with endometriosis and with unexplained pain. Journal of psychosomatic research. 1996;40(3):305–315. [DOI] [PubMed] [Google Scholar]
- 5.Della Corte L, Di Filippo C, Gabrielli O, et al. The Burden of Endometriosis on Women's Lifespan: A Narrative Overview on Quality of Life and Psychosocial Wellbeing. Int J Environ Res Public Health. 2020;17(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Missmer SA, Tu FF, Agarwal SK, et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int J Gen Med. 2021;14:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandsborg B, Dueholm M, Nikolajsen L, Kehlet H, Jensen TS. A prospective study of risk factors for pain persisting 4 months after hysterectomy. Clin J Pain. 2009;25(4):263–268. [DOI] [PubMed] [Google Scholar]
- 8.Brandsborg B, Nikolajsen L, Hansen CT, Kehlet H, Jensen TS. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology. 2007;106(5):1003–1012. [DOI] [PubMed] [Google Scholar]
- 9.Montes A, Roca G, Sabate S, et al. Genetic and Clinical Factors Associated with Chronic Postsurgical Pain after Hernia Repair, Hysterectomy, and Thoracotomy: A Two-year Multicenter Cohort Study. Anesthesiology. 2015;122(5):1123–1141. [DOI] [PubMed] [Google Scholar]
- 10.Nicholas M, Vlaeyen JWS, Rief W, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28–37. [DOI] [PubMed] [Google Scholar]
- 11.As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122(5):1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153(5):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional Connectivity is Associated With Altered Brain Chemistry in Women With Endometriosis-Associated Chronic Pelvic Pain. J Pain. 2016;17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser W, Jung E, Erbsloh-Moller B, et al. Validation of the Fibromyalgia Survey Questionnaire within a cross-sectional survey. PLoS One. 2012;7(5):e37504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103–1111. [DOI] [PubMed] [Google Scholar]
- 17.As-Sanie S, Till SR, Mowers EL, et al. Opioid Prescribing Patterns, Patient Use, and Postoperative Pain After Hysterectomy for Benign Indications. Obstet Gynecol. 2017;130(6):1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 19.American Society of Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 20.Improvement of interobserver reproducibility of adhesion scoring systems. Adhesion Scoring Group. Fertil Steril. 1994;62(5):984–988. [PubMed] [Google Scholar]
- 21.Hillis SD, Marchbanks PA, Peterson HB. The effectiveness of hysterectomy for chronic pelvic pain. Obstet Gynecol. 1995;86(6):941–945. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald SR, Klock SC, Milad MP. Long-term outcome of nonconservative surgery (hysterectomy) for endometriosis-associated pain in women <30 years old. Am J Obstet Gynecol. 1999;180(6 Pt 1):1360–1363. [DOI] [PubMed] [Google Scholar]
- 23.Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285–1292. [DOI] [PubMed] [Google Scholar]
- 24.Kjerulff KH, Langenberg PW, Rhodes JC, Harvey LA, Guzinski GM, Stolley PD. Effectiveness of hysterectomy. Obstet Gynecol. 2000;95(3):319–326. [DOI] [PubMed] [Google Scholar]
- 25.Stovall TG, Ling FW, Crawford DA. Hysterectomy for chronic pelvic pain of presumed uterine etiology. Obstet Gynecol. 1990;75(4):676–679. [PubMed] [Google Scholar]
- 26.Namnoum AB, Hickman TN, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64(5):898–902. [DOI] [PubMed] [Google Scholar]
- 27.VanDenKerkhof EG, Hopman WM, Goldstein DH, et al. Impact of perioperative pain intensity, pain qualities, and opioid use on chronic pain after surgery: a prospective cohort study. Reg Anesth Pain Med. 2012;37(1):19–27. [DOI] [PubMed] [Google Scholar]
- 28.Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update. 2014;20(5):737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM. Peripheral changes in endometriosis-associated pain. Hum Reprod Update. 2014;20(5):717–736. [DOI] [PMC free article] [PubMed] [Google Scholar]