Abstract
Objectives
This study investigates the association of daily physical exercise with pain symptoms in endometriosis. We also examined whether an individual’s typical weekly (ie, habitual) exercise frequency influences (ie, moderates) the relationship between their pain symptoms on a given day (day t) and previous-day (day t-1) exercise.
Participants
The sample included 90 382 days of data from 1009 participants (~85% non-Hispanic white) living with endometriosis across 38 countries.
Study design
This was an observational, retrospective study conducted using data from a research mobile app (Phendo) designed for collecting self-reported data on symptoms and self-management of endometriosis.
Primary outcome measures
The two primary outcomes were the composite day-level pain score that includes pain intensity and location, and the change in this score from previous day (Δ-score). We applied generalised linear mixed-level models to examine the effect of previous-day exercise and habitual exercise frequency on these outcomes. We included an interaction term between the two predictors to assess the moderation effect, and adjusted for previous-day pain, menstrual status, education level and body mass index.
Results
The association of previous-day (day t-1) exercise with pain symptoms on day t was moderated by habitual exercise frequency, independent of covariates (rate ratio=0.96, 95% CI=0.95 to 0.98, p=0.0007 for day-level pain score, B=−0.14, 95% CI=−0.26 to −0.016, p=0.026 for Δ-score). Those who regularly engaged in exercise at least three times per week were more likely to experience favourable pain outcomes after having a bout of exercise on the previous day.
Conclusions
Regular exercise might influence the day-level (ie, short-term) association of pain symptoms with exercise. These findings can inform exercise recommendations for endometriosis pain management, especially for those who are at greater risk of lack of regular exercise due to acute exacerbation in their pain after exercise.
Keywords: pain management, health informatics, preventive medicine, epidemiology, complementary medicine
Strengths and limitations of this study.
This study leverages data from a research mobile app (Phendo) designed for collecting self-reported data on symptoms and self-management of endometriosis.
Daily exercise and pain symptom patterns in endometriosis are investigated under ecologically valid conditions.
The participant sample (N=1009) represents 38 countries, ages across the reproductive life span and various person-level characteristics.
The study is limited by self-reported data collection by somewhat consistent trackers and lacks details on duration or intensity of exercise to evaluate as potential moderators.
Participants consisted of mostly non-Hispanic white individuals; therefore, results might not be generalisable to other demographic groups.
Introduction
Exercise, a subset of physical activity (PA) that is planned, structured, repetitive and intended to improve or maintain physical fitness, is an important component of effective pain management (ie, reduction and prevention of pain symptoms).1 2 Both acute (ie, single bout/session) and chronic (ie, repeated bouts/sessions over time) exercise training have been demonstrated to improve numerous pain-related conditions.1 3–7 However, pain-related responses to exercise are variable in populations with chronic pain conditions.8 Similarly, exacerbation of pain with exercise could pose a barrier to regular exercise in such individuals, thus increasing resistance to exercising, which in return can worsen pain, related disability and risk of comorbidities.9–11 Investigation into the naturally occurring pattern of pain symptoms associated with exercise behaviour can help inform the design of exercise-based therapies for targeting disease-related pain symptoms.
Individuals with endometriosis may benefit from such investigations for several reasons.12–14 Endometriosis is a systemic, oestrogen-dependent inflammatory condition characterised primarily by chronic pelvic and abdominal pain, pain with sexual intercourse and infertility.15 16 It significantly impacts daily function and quality of life (QoL),17 18 contributing to a productivity loss of 6.3 hours/week19 and an estimated $69.4 billion in excess health expenditures annually in the USA.18 Existing medical and hormonal therapies have limited efficacy for pain management, often confounded by side effects.20 Opioids and other analgesics are commonly prescribed for long-term use,21 22 despite treatment guidelines recommending use of non-pharmacological therapies including PA.23 Consequently, there is a critical need to identify alternative approaches for endometriosis pain management.
One such approach is exercise, based on various mechanisms proposed in the literature24 that might pertain to endometriosis. These include regulation of the serotonergic and opioid receptors,25 reduction of inflammatory markers associated with pain26 27 and effect of exercise on nerve growth factor expression that is associated with the painful endometriosis lesions.28 29 Exercise can increase pain management self-efficacy, which is associated with improved pain outcomes and QoL, for individuals with chronic pain.30 While the evidence on exercise for pain management is promising,4 31 32 existing data are scarce, cross-sectional and indicate variable effects on pain outcomes.32–36 Despite these limitations, previous reports of exercise-induced adaptations to pain stimuli through increased pain threshold suggest that the regularity with which an individual engages in exercise over the long term (ie, habitual exercise frequency) might influence (ie, moderate) the relationship between their day-level exercise and pain symptoms.37 38 Among regular exercisers, pain-related activation has been demonstrated in the brain’s descending antinociceptive pathway, with corresponding reductions in self-reported pain after acute bouts of at least moderate-intensity exercise.39 Moreover, studies report that habitual exercise frequency moderates a variety of self-reported outcomes (eg, mood, anxiety, fatigue) in response to acute exercise.40–42 While these findings are promising, their generalisability is limited by sample characteristics, laboratory-based experimental pain stimuli and exercise manipulations, and brief measurement duration of up to several hours. Thus, further investigation is needed to examine the relationship between pain symptoms and exercise behaviour with a representative sample, under ecologically valid conditions, while accounting for possible between-individual variability and temporal lags in the outcome that extend beyond several hours.
Accordingly, this study examines the naturally occurring daily patterns of pain symptoms and exercise behaviour in endometriosis. We leverage mobile self-tracking, a particularly useful approach for capturing ecologically valid profiles of the dynamic temporal fluctuations and between-individual variability in pain over time.43 We primarily aim to delineate the degree to which an individual’s typical weekly exercise frequency (ie, habitual exercise) influences (ie, moderates) the association of their pain symptoms on a given day (day t) with their previous-day (day t-1) exercise behaviour (ie, lagged-day effects). Given the previously documented variable course of pain symptomatology in endometriosis,44 we also delineate the variability in day-to-day pain experiences within these analyses.
Materials and methods
Study design
This study was conducted with retrospective data collected through the observational research mobile app ‘Phendo’. Phendo was designed and developed for self-tracking endometriosis symptoms and its management. It is available for iOS (available at https://itunes.apple.com/us/app/phendo/id1145512423) and Android (available at https://play.google.com/store/apps/details?id=com.appliedinformaticsinc.phendo) in App stores for free.
Study sample and inclusion criteria
The study sample comprised Phendo users with a self-reported surgery, clinician or suspected diagnosis of endometriosis and self-tracked exercise and pain data between November 2016 and April 2020. All participants, regardless of diagnosis type, are provided the same set of measures for completion in the app. In a previous study, the endometriosis phenotype (ie, characterisation) obtained using Phendo data was consistent with both the characterisation of the disease in the literature based on standard clinical surveys and clinician (ie, human expert) evaluations.45 We decided a priori to include all participants who selected one of the three affirmative responses in the present analyses, excluding those who indicated that they did not have endometriosis. Out of the initial eligible pool of 9792 Phendo users with reported endometriosis, 7949 had at least 1 day of tracking of the variables of interest for the study. Of these, 1009 users had sufficient amount of data on pain and exercise for analysis (see the Data analysis section) and were included in the study.
Recruitment and informed consent
Study participants were passively recruited through one of the App stores, engagement on study social media sites or word of mouth. Upon downloading Phendo, all potential users went through an informed consent and enrolment process before tracking any data. First, they were provided with an explanation of the app, its overall purpose and link to its website (citizenendo.org) which includes detailed information and instructional videos for using the app. Participants completed a brief ‘verify your understanding’ quiz to ensure their comprehension of how their data might be used for research purposes, anonymity and confidentiality (see online supplemental figures 1 and 2 for example screenshots). This was followed by formal electronic informed consent (and assent for individuals 13–18 years old), a copy of which was sent to the participant. Once enrolled, users were instructed to track daily, but they were free to track as much or as sporadically as they wished, and they did not receive any prompts or requests to track a specific variable from the research team. Findings from a previous study evaluating recruitment and retention patterns within Phendo and seven other similar self-tracking apps indicated that Phendo’s user engagement was similar to standard engagement patterns in research smartphone apps.46 Participants in the current study did not receive financial compensation for their tracking activities.
bmjopen-2021-059280supp001.pdf (20.4KB, pdf)
bmjopen-2021-059280supp002.pdf (485.6KB, pdf)
Study measures
Day-level pain
We assessed day-level pain through multiple items within Phendo: (1) ‘Are you in pain now? Where is the pain?’, (2) ‘Any gastrointestinal or urinary issues?’ (painful urination (dysuria), painful bowel movement (dyschezia)). Phendo pain item response options include all areas of the body (20 available choices, as well as right/left and upper/middle/lower specification), and can be mapped onto a visual, analogous to the McGill Pain Scale.47 Pain severity for each affirmative response was rated on a 3-point categorical scale (mild, moderate or severe), analogous to other commonly used pain rating scales in the literature.48 49 This categorisation has been used for standardisation and comparisons across different pain measures, and demonstrated superior ability to capture the non-linear relationship between reported pain severity and interference with activity than use of numbers.50 51
We computed a heuristic, composite day-level pain score to capture participants’ conceptualisation of their pain experience by summing the severity scores reported for each body area (eg, moderate pain in abdomen, mild pains in chest and leg would yield 2+1+1=4 as the total score).44 This allowed consideration of the multidimensional pain experience in a single outcome. To account for and circumvent any potential pain rumination/catastrophising52 53 and varying tracking habits among participants, the score was computed based on the unique reports of area–severity pairs per day for each participant (eg, if a participant tracked mild abdominal pain three times in a day, this abdomen–mild pair is counted toward the daily pain score only once). This score was the foundation of two study outcome variables: (1) total day-level pain score, and (2) difference in day-level pain score from previous day to the next (ie, t−(t-1)). The latter captures additional nuances in the data, enabling analyses to distinguish between participants with overall high day-level pain scores over time and experience a post-exercise reduction in pain versus those with low pain scores and who do not experience a post-exercise reduction in pain. In the current study sample, the composite pain scores were moderately correlated with scores from other standard pain measures (eg, r=0.36, p<0.0001 with the Pelvic–Abdominal Pain Visual Analog Scale (VAS); r=−0.46, p<0.0001 with Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) Bodily Pain subscale).
Day-level and habitual exercise
Phendo allows tracking of daily exercise through responding to a root question ‘Did you exercise today? (Yes/No)’. Upon selecting a ‘Yes’, users can further customise their entry within this item by adding exercise details through unrestricted free-text responses. We used responses to the root item to compute day-level and mean weekly exercise frequency (ie, habitual exercise) for each participant. We calculated the latter by summing the number of exercise reports tracked per week across the range of days of data and then dividing this number by the total number of weeks of data. We used free-text responses to categorise exercises by modality and to validate that the entries were exercise related. Any non-exercise activity (eg, sleep, meditate, sitting, socialise) was recoded as a no exercise in the analytical data set. This day-level exercise assessment aims to increase ecological validity54 55 and reduce the likelihood of low test–retest reliability and inaccuracy due to recall bias.56 We evaluated the validity of the scores from the Phendo exercise item through a series of analyses with the study sample.57 Results supported its concurrency with other self-reported recall-based measures (ie, Kendall’s τ=0.256, p<0.001 with Exercise Vital Sign58 and τ=0.294, p=0.001 with accelerometers; B=18.73, p=0.039 in association to the Nurses’ Health Study II (NHS-II) Weekly Exercise Scale59 scores).
Standard pain and exercise measures
To allow comparisons of the study sample with others in the literature, we report sample summary scores from the following components of the World Endometriosis Research Foundation Endometriosis Patient Questionnaire60 61 : (1) the two-item Bodily Pain subscale of the SF-36,62 (2) Pelvic–Abdominal Pain VAS (‘Please rate how severe your general pelvic/lower abdominal pain was at its worst in the last 3 months using the pain scale below where 0=no pain and 10=worst imaginable pain.’), and (3) the eight-item NHS-II Weekly Physical Activity Scale.59 It measures self-reported weekly duration of major exercise modalities (ie, walking, running, lap swimming, jogging, bicycling, tennis, callisthenics, other aerobic recreation) in a typical week in the past 12 months. The duration can further be multiplied by their metabolic equivalents based on the Compendium of PA63 and summed to obtain the total weekly exercise-related energy expenditure (EE). We report both the total weekly minutes and EE for the sample.
Patient and public involvement
We developed Phendo measures using patient-centred participatory design, through qualitative (focus groups, interviews) and quantitative research (surveys, coded content analysis) with participants with endometriosis, described in detail elsewhere.64 65 This technique for developing patient-reported outcome measures has been suggested to enhance content validity and relevance of the measure to the target population, thus providing a more comprehensive and accurate representation of the disease under study.54 66–68
Data analysis
Sample characteristics
We characterised the study sample through frequencies (%) and means (SD) of demographics, self-reported pain medication use, and scores on the standard pain and exercise measures for those who completed the surveys. We characterised pain symptomatology in the sample by describing the prevalence of self-tracked pain severities by each body area.
Associations of pain symptoms with exercise behaviour
Using generalised linear mixed models (GLMMs), we separately estimated day-level total pain score and pain score difference as primary outcomes. Both outcomes were regressed on previous-day (day t-1) exercise and mean weekly exercise frequency to estimate the slope of mean pain level on day t and change in pain. We included an interaction term between the two predictors to assess the moderation of the day-level association by each individual’s mean weekly exercise frequency. We included participant as a random effect to account for between-person variability in daily pain by estimating a separate intercept for each participant. Models were further adjusted for menstrual status (binary: yes/no), previous-day (ie, day t-1) pain, body mass index (BMI) and education level. Race/ethnicity and age were not significantly associated with average daily pain reports (F=1.68, p=0.14 for race/ethnicity; r=−0.148, p=0.07 for age), and age was further significantly associated with education level (Kruskal-Wallis X2=64.948, p<0.0001). To avoid redundancy and multicollinearity, race/ethnicity and age were not included as model covariates.
Model specification
We specified a zero-inflated negative binomial distribution when modelling the total pain outcome, as it has been demonstrated to provide the best fit for outcomes with overdispersion and zero inflation (ie, zeros due to both sampling and missingness).69–71 Missing values in the BMI (22%), education level (19%) and menstrual status (22%) were imputed as described in online supplemental file 1 and checked for appropriateness based on convergence and marginal distributions following guidelines72–74 (see online supplemental figures 3–5). Adequacy of imputations for valid statistical inference was verified based on the recommended measures of missing data information of fraction of missing information (λ) and relative increase in variance due to non-response (r)75 76 (see online supplemental table 1). Further details of the model specification are in online supplemental file 1. We included participants who had at least 11 pairs of consecutive days of data in the final analytical sample as this provided sufficient amount of data to (1) ensure model convergence and improve reliability and accuracy of the estimates, particularly the random effects and their variances,77–80 and (2) adequately infer participants’ habitual exercise level by considering at least 3 weeks’ worth of tracking to compute the weekly exercise frequency. Finally, as a post-hoc analysis, we tested the possible influence of type of endometriosis diagnosis by including this categorical variable in the two models described above. We conducted the data analyses using R81 and the glmmTMB package for the GLMMs.70 71 Statistical significance level was set at p<0.05 for all analyses.
bmjopen-2021-059280supp003.pdf (177.2KB, pdf)
bmjopen-2021-059280supp004.pdf (403KB, pdf)
bmjopen-2021-059280supp005.pdf (30.3KB, pdf)
bmjopen-2021-059280supp006.pdf (43.3KB, pdf)
Results
Sample characteristics
Sample characteristics are provided in table 1. Participants (N=1009) had on average 89.6 days of data available for analysis (SD=62.8, range=22–841, IQR=31). Participants collectively represented 38 countries, with a wide age range (14–63 years), and varying education and employment status. Almost 70% (N=702) had laparoscopic confirmation of their diagnosis, 19.8% (N=200) had a clinician diagnosis and 10.6% (N=107) had suspected endometriosis (ie, ‘I think I have endometriosis (know the symptoms, no doctor)’). Scores from the VAS, SF-36 and NHS-II scales are provided in table 2. The overall prevalence rates of non-prescription pain medication use, opioid-based medication use, opioid–paracetamol/acetaminophen combination medication use were 49.35%, 11.19% and 11.39%, respectively (see table 1).
Table 1.
Study sample characteristics
| Characteristic (N) | Mean (SD)/frequency (%) |
| Age (827) | 31.0 (7.26), median=30.6 (MAD=7.41), |
| range=14.3–62.9 | |
| BMI (787) | 25.9 (6.98), median=24.1 (MAD=4.74), range=16.01–72.24 |
| Type of endometriosis diagnosis | |
| Surgery (702) | 69.57 |
| Clinician (200) | 19.82 |
| Self-diagnosis (107) | 10.60 |
| Work environment | |
| Home (218) | 26.42 |
| Outside (570) | 69.09 |
| Unknown (221) | 21.29 |
| Living environment | |
| Rural (129) | 15.27 |
| Suburban (340) | 41.21 |
| Urban (363) | 44.00 |
| Unknown (161) | 19.50 |
| Relationship status | |
| Married/domestic partnership (442) | 53.57 |
| Separated/divorced (28) | 3.39 |
| Single/never married (310) | 37.57 |
| Unknown (229) | 22.69 |
| Education level | |
| College or higher (547) | 66.30 |
| High school graduate or less (74) | 8.96 |
| Some college (209) | 25.33 |
| Unknown (179) | 17.70 |
| Employment status | |
| Employed (541) | 65.57 |
| Not employed (120) | 14.54 |
| Student (129) | 15.63 |
| Unknown (219) | 21.70 |
| Race/ethnicity | |
| White, non-Hispanic (699) | 84.72 |
| Black, non-Hispanic (20) | 2.42 |
| Asian (22) | 2.60 |
| Native American (6) | 0.72 |
| Hispanic (38) | 4.60 |
| Other (51) | 6.18 |
| Unknown (173) | 17.14 |
| Country of residence | |
| USA (444) | 44.00 |
| UK (83) | 8.22 |
| Canada (75) | 7.43 |
| Australia (59) | 5.84 |
| Germany (38) | 3.76 |
| New Zealand (34) | 3.36 |
| Other (69) | 6.83 |
| Unknown (207) | 20.51 |
BMI, body mass index; MAD, Median Absolute Deviation.
Table 2.
Sample study scores on standard measures of pain and exercise
| EPQ-S measures (N) | Mean (SD) |
| SF-36 Bodily Pain (375) | 35.47 (22.33) |
| Pelvic–Abdominal Pain VAS (316) | 7.37 (1.97) |
| NHS-II PA Scale total weekly minutes (359) | 175.2 (280.2) |
| NHS-II PA Scale total weekly EE (359) | 16.13 (30.37) |
EE, energy expenditure; EPQ-S, Endometriosis Patient Questionnaire; NHS-II PA, Nurses’ Health Study II Weekly Physical Activity; SF-36, 36-Item Short-Form Health Survey; VAS, Visual Analog Scale.
Pain symptom patterns
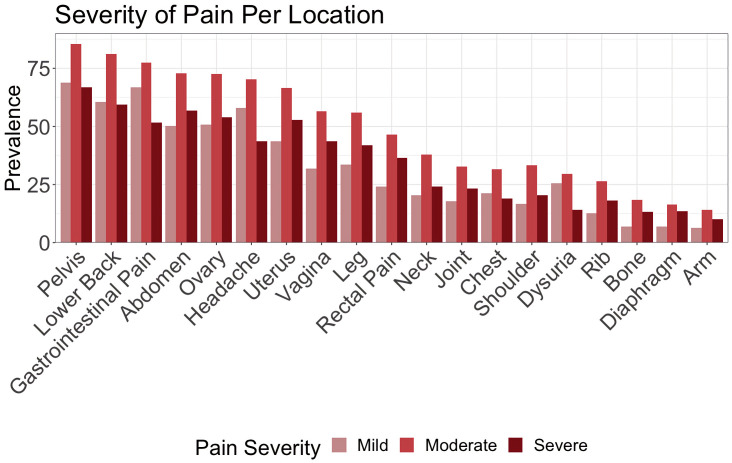
Mean daily pain score was 4.48 (SD=7.11, range=0–79). Mean person-level daily pain score (ie, ‘mean of means’) was 4.82 (SD=4.57, range=0–34). Moderate intensity was the most frequently reported severity across all body areas (mean=49.3%, SD=22.2), and pelvic pain was the most prevalent area, followed by back pain and gastrointestinal pain (see figure 1).
Figure 1.
Prevalence of pain severity by location reported among participants (ie, unique counts of body area–severity per participant). Moderate intensity was the most frequently tracked across all body areas (14.1%–85.4%).
Habitual exercise patterns
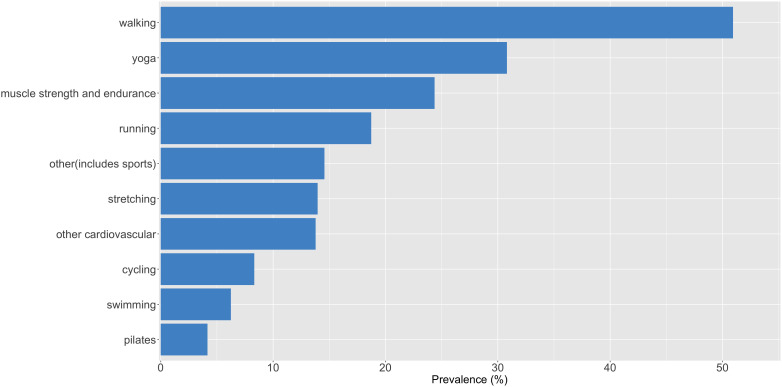
Mean weekly exercise frequency was 1.43/week (SD=1.54, range=0–6.87/week, IQR=2.21). The exercise frequencies were at least three times per week, 21.3% (N=215); one to two times per week, 40.2% (N=406); and no regular exercise, 38.5% (388). Prevalence of the 10 most frequently reported exercise modalities in the sample is depicted in figure 2. Walking was the most common modality, reported by 50.94% of the participants, followed by yoga (30.82%) and muscle strength/endurance training activities (24.38%). Yoga and stretching exercises were collectively reported by almost 45% of the sample.
Figure 2.
Prevalence of self-reported exercise modalities in the study sample. ‘Other cardiovascular’ category includes activities such as dancing, aerobics and using the elliptical machine. ‘Muscle strength and endurance’ category includes activities such as weightlifting and callisthenics. ‘Other exercise’ category includes sports activities such as skiing and soccer, multimodal exercises (eg, high-intensity interval training of both cardiovascular and muscular endurance) or those that did not fit into the other categories (eg, stabilising or balancing exercises, Wii fit or other home-based fitness activities).
Association of day-level pain with exercise
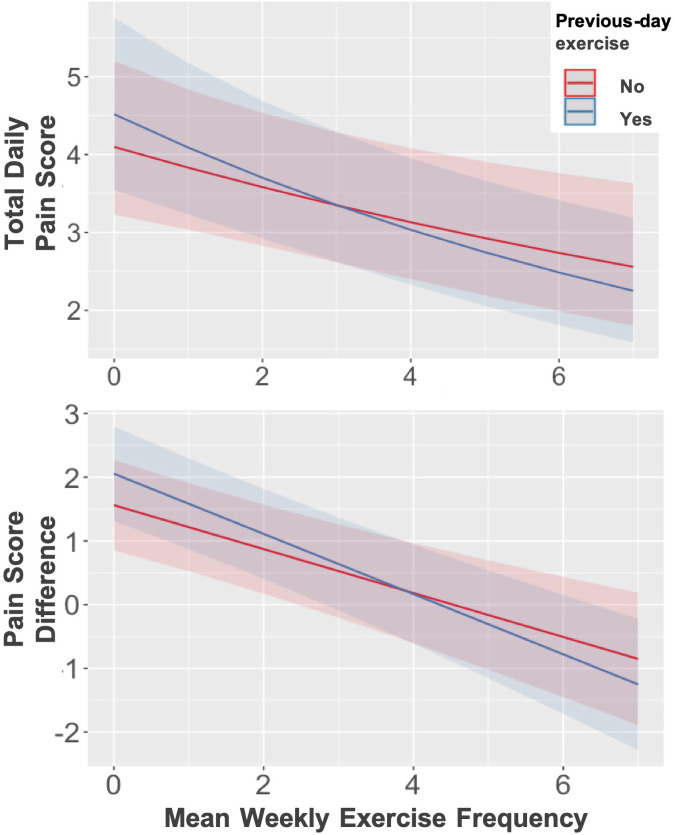
Tables 3 and 4 display results of the GLMMs estimating day-level total pain score and difference. Coefficients for the model interaction terms indicated a small but statistically significant moderation of previous-day exercise by habitual exercise frequency (rate ratio=0.96 for total pain score and −0.14 for pain score difference, p<0.05; see figure 3). Further inspection of this interaction indicated a mean typical exercise frequency of ~3 times/week as the point after which previous-day exercise began to be associated with favourable pain outcomes (eg, a decrease from the predicted mean score) on the following day, adjusted for other day-level and person-level factors (figure 3). On the other hand, those who exercised less frequently or none were more likely to report higher levels of pain and larger increases (or smaller decreases) in pain 1 day after an exercise bout compared with not having exercised the day before.
Table 3.
Results of the regression model estimating day-level total pain score (N=1009)
| Conditional random effects | Variance (95% CI) |
| Participant | 1.09 (0.98 to 1.21) |
| Conditional fixed effects | Rate ratio (95% CI) | Log odds (SE) | z-score |
| Intercept | 4.26*** (3.26 to 5.56) | 1.45*** (0.13) | 10.82 |
| Menstrual status | 1.29*** (1.25 to 1.32) | 0.25*** (0.01) | 20.31 |
| Previous-day pain | 1.02*** (1.02 to 1.03) | 0.02*** (0.00) | 29.69 |
| Body mass index (BMI) | 1.01* (1.00 to 1.02) | 0.01 (0.00) | 2.02 |
| Mean weekly exercise frequency | 0.93* (0.89 to 0.97) | −0.06** (0.02) | −2.96 |
| Previous-day exercise | 1.10* (1.05 to 1.15) | 0.09**(0.15) | 3.88 |
| Some college education level | 0.87 (0.83 to 1.56) | 0.13 (0.15) | 0.86 |
| College or higher education level | 0.93 (0.66 to 1.16) | −0.13 (0.14) | −0.92 |
| Mean weekly exercise frequency×previous-day exercise | 0.96** (0.95 to 0.98) | −0.03** (0.01) | −3.37 |
| Zero inflation terms | Rate ratio (95% CI) | Log odds (SE) | z-score |
| Intercept | 0.17 (0.16 to 0.18) | −1.73***(0.02) | −62.96 |
| Same-day exercise | 5.34 (5.01 to 5.68) | 1.67*** (0.03) | 52.53 |
Previous-day pain and BMI were sample mean centred. BMI and education level were kept as covariates in the model based on their significant associations with mean day-level pain scores (Pearson’s r=0.15 for BMI and Kruskal-Wallis χ2=18.061 for education level, p<0.001).
*P<0.05, **p<0.001, ***p<0.0001.
Table 4.
Results of the regression model estimating pain score difference (N=1009)
| Conditional random effects | Variance (95% CI) |
| Participant (intercept) | 9.16 (8.28 to 10.13) |
| Residual | 26.83 |
| Conditional fixed effects | B coefficient (SE) | 95% CI | z-score |
| Intercept | 2.70*** (0.51) | 1.68 to 3.72 | 5.29 |
| Menstrual status | 1.47*** (0.09) | 1.28 to 1.66 | 15.43 |
| Previous-day pain | −0.86*** (0.01) | −0.87 to 0.85 | −143.43 |
| Body mass index (BMI) | 0.05* (0.01) | 0.01 to 0.10 | 2.86 |
| Mean weekly exercise frequency | −0.27** (0.08) | −0.44 to 0.10 | −3.12 |
| Previous-day exercise | 0.92** (0.18) | 0.56 to 1.27 | 5.08 |
| Some college education level | −0.84 (0.62) | −2.11 to 0.42 | −1.35 |
| College or higher education level | −2.07** (0.52) | −3.10 to 1.03 | −3.96 |
| Mean weekly exercise frequency×previous-day exercise | −0.14* (0.06) | −0.26 to 0.01 | −2.22 |
| Zero inflation terms | B coefficient | 95% CI | z-score |
| Intercept | −0.91*** (0.01) | −0.93 to 0.88 | −63.84 |
| Same-day exercise | 0.70*** (0.02) | 0.66 to 0.75 | 32.09 |
Previous day pain and BMI were sample mean centred. BMI and education level were kept as covariates in the model based on their significant associations with mean day-level pain scores (Pearson’s r=0.15 for BMI and Kruskal-Wallis χ2=18.061 for education level, p<0.001).
*P<0.05, **p<0.001, ***p<0.0001.
Figure 3.
Moderation of effect of previous-day exercise by habitual exercise levels (x-axes). Y axes represent predicted day-level total scores (top) and differences (bottom) in pain. Shaded areas depict 95% CIs. At approximately three times/week of regular exercise, previous-day exercise starts to be associated with more favourable pain outcomes on the following day (ie, decrease from the model predicted mean scores), adjusted for other day-level and person-level factors.
Variability in estimated pain scores
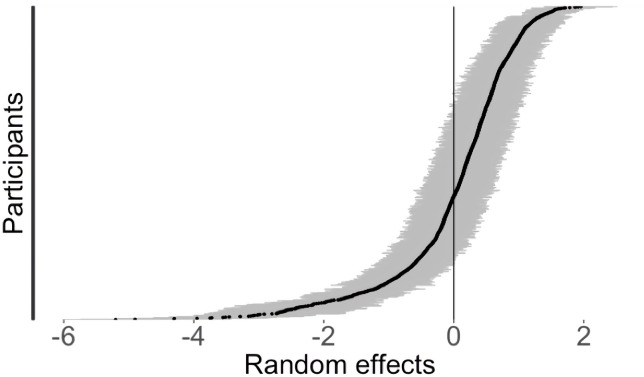
There was substantial between-person variability in average day-level pain scores, based on the statistically significant random effect of participant in the models (see tables 3 and 4, also depicted in figure 4). We quantified the significance of this random effect through a restricted likelihood ratio test (RLRT) based on simulations from the model sample distribution.82 83 This yielded an observed likelihood ratio (RLRT=7183.3, p<0.0001), indicating substantial contribution of the random effect to the total model pain variance.
Figure 4.
Plot of the random effect of the participant on total day pain scores estimated from the multilevel model (N=1009). Y-axis represents the range of estimated average pain scores for each participant. Each black dot represents one participant’s mean (ie, random intercept), grey lines indicate 95% CIs. Distribution of points across the x-axis indicates large variability across individuals (ie, between-group variance), and the grey lines indicate the within-person variability in daily scores over time.
Post-hoc analyses
Inclusion of diagnosis type in the model did not have an influence on the results based on the non-significant B coefficients (p=0.48 and p=0.59 for pain score and p=0.70 and p=0.27 for difference in pain score). There were no differences across the three groups with respect to either daily total pain score or difference (χ2=1415.1, df=1438, p=0.661) (see online supplemental tables 2 and 3 for full results).
Discussion
Summary of findings
We leveraged 90 382 days of mHealth self-tracking data from 1009 women with endometriosis to investigate the association between exercise behaviour and day-level fluctuations in pain. For the average individual, the association between previous-day exercise and pain was moderated by their habitual exercise frequency, that is, the frequency with which they engaged in exercise in a typical week. This effect was consistent across participants and independent of person-level covariates. There was substantial between-person heterogeneity in day-level pain patterns. To our knowledge, this is the first study to quantify the association between day-level pain symptoms and exercise in an international sample of women with endometriosis and to identify habitual weekly exercise frequency as a moderator of this relationship.
Moderation effects
Previous-day exercise was associated with more favourable pain outcomes for participants who engaged in regular exercise at least three times per week in our sample. That is, these participants were more likely to report lower pain score and smaller increases (or larger decreases) in pain the day after an exercise bout, compared with not having exercised the previous day. In contrast, those who engaged in regular exercise less than twice a week were more likely to experience pain symptoms on days after having engaged in exercise. This is in line with the physical activity guidelines,84 85 which recommend aerobic exercise at least three times per week and muscle-strengthening exercise at least twice per week.86 However, there are no specific recommendations for endometriosis in the current guidelines; and systematic reviews recommend ‘overall, general exercise’ without further details due to the lack of adequate research on the optimal dose of exercise for endometriosis pain.4 35 Our findings provide preliminary evidence for informing exercise recommendations for endometriosis pain management (ie, prevention or reduction), specifically for targeting those who are at greater risk of insufficient regular exercise due to acute exacerbation in their pain after exercise. This moderation effect suggests that an individual might need to develop a regular, sustained exercise behaviour (ie, habit) to start experiencing the favourable pain outcomes associated with acute bouts of exercise. Nevertheless, future experimental studies are warranted for a comprehensive investigation of this question.
Patterns of pain symptoms
Our findings of moderate pain in pelvis as the most frequently reported pain are in line with those from others on endometriosis87 and various chronic pain conditions.88 89 The distribution of the total daily pain scores was right skewed (ie, extreme scores on the higher ends of the range) with a mean score that was on the lower end of the range. This could partly be due to the data collection method which includes not just days where the participant experienced pain but also days without pain. Indeed, our participants on average did not report or experience any pain 6.25% of the time. In contrast, traditional study designs typically rely on recall of past pain experience aggregated over a period of time (eg, past week, month) and ask the participant to report their average or highest pain severity over this period.90 91 Such recall-based techniques are prone to peak-and-end effects,92 and catastrophising or other similar biases.91 93 Recruitment from clinical referral points is a common practice and this has been attributed to higher normative scores in the literature,90 as opposed to more even distributions of pain symptomatology among community-based samples.94 Self-tracking facilitates documentation of not only severe pain, but also mild, moderate and no pain instances, therefore enabling a more realistic representation of the pain experience as it dynamically unfolds over time. This can reduce the likelihood of over-representing severe cases, which is a potential limitation attributed to data collected at point of contact in clinical settings.17 However, it is difficult to make direct comparisons with other studies given the different pain measures, warranting further research.
Patterns of exercise behaviour
The mean weekly exercise frequency in the study sample was 1.43/week (SD=1.57, IQR=2.29), with only 24.5% (N=202) engaging in exercise at least three times a week. This suggests that individuals with endometriosis might be at increased risk of physical inactivity,84 86 which is a risk factor for various comorbidities95 and further linked to exacerbation of chronic pain.96 97 These collectively underscore the need to focus efforts on promoting regular exercise in women with endometriosis. Notably, yoga and stretching were reported collectively by almost half of the sample within Phendo. This could indicate that participants use these approaches for pain relief, in line with a previous study reporting efficacy of hatha yoga.32 Nevertheless, participants overall tracked a wide range of exercise modalities across the intensity spectrum (eg, yoga vs running/cycling) as helpful for their symptoms, suggesting between-individual variability in response to a given exercise type or intensity. This can be targeted through individualised exercise prescriptions,24 98 providing precedence for undertaking a precision approach for pain self-management in endometriosis. Various individualisation approaches (eg, adaptive treatment strategies,99 micro-randomised trials,100 just-in-time adaptive interventions101) have been investigated for intervening on health behaviours, including PA.5 100 It would be opportune to implement a similar N-of-1 intervention approach for identifying person-specific optimal ‘dose’ of exercise based on its parameters to target endometriosis pain symptoms.
Consideration of person-level factors
Another novel finding in our study was the similar point estimates for the effect of exercise on pain outcomes between those with clinician/surgical versus suspected diagnosis of endometriosis. Endometriosis is difficult to diagnose, with a 7.6-year delay between symptom onset and its surgical diagnosis.18 102 103 Patients with endometriosis further face insurance-related challenges in accessing healthcare for their condition.15 104 The participants without a formal diagnosis might have sought medical care for their symptoms but not received the needed care (eg, diagnostic testing, referral to a specialist), received false negative diagnostic tests results102 or lacked adequate access to healthcare. This finding underscores the need for further research in endometriosis that considers self-report of endometriosis symptoms, instead of limiting to patients with a physician referral or relying on secondary data sources (eg, electronic health records).
Novel methodological contributions
In contrast to other existing questionnaires in the literature, the self-tracking items in Phendo measure momentary and daily pain symptoms and exercise—a time interval for which there are no standard validated, commonly used measures designed for frequent sampling. Phendo’s pain tracking items are similar in design to other pain measures,47 65 and have been indicated to be reflective of pain documentation in clinical records.44 While mHealth studies have examined the validity, utility and specificity for various pain conditions51 105 106 of their pain measurement approaches, a standard ‘all-in-one’ single outcome that captures the multidimensional pain experience across different populations remains to be established.52 107 Computation of a composite pain has been proposed by others108 as this circumvents numerous limitations in current pain assessment approaches, including lack of a standard single outcome that can be used universally,107 or a validated instrument that captures all the constructs of persistent pain.109 There is furthermore a lack of endometriosis-specific pain measures for repeated assessments, thus the heuristic composite pain measure allowed consideration of two dimensions of pain simultaneously in our analyses. The pain scores in the current study sample were moderately correlated with those from the Pelvic–Abdominal VAS and the SF-36 Bodily Pain measure, which were also similarly correlated with each other (r=0.46, p<0.0001). Nevertheless, future directions include evaluation of this measure in larger samples for its reliability and validity via a nomological network-based analysis.
Limitations
We acknowledge several limitations of this study, including reliance on self-reports for the type of endometriosis diagnosis and exercise behaviour. First, we used a binary measure of exercise in our analyses and did not have sufficient details on duration or intensity for inclusion in the analyses as potential moderators. Of note, similar mHealth measures of daily PA and exercise have been used by others110–112 who reported concordance with accelerometer-based measures,113 and higher correlations than self-report methods with accelerometer measures.110 111 While we provide preliminary evidence toward the validity of Phendo’s exercise tracking item both as a day-level and habitual measure,57 future studies are needed to evaluate it in larger samples and compare against research-grade accelerometers. Similarly, we did not have granular daily data on pain medication use, as it was not investigated as a potential covariate in the analyses. In addition to medications, future studies could consider other pain management approaches for comparison with exercise, given previous research suggesting patients with endometriosis report using a variety of symptom management techniques.44 Next, our sample consisted primarily of white, non-Hispanic women who are relatively consistent mHealth technology users and furthermore can understand English to use the app. Therefore, the results might differ among other groups including non-English speakers or those without an interest in mHealth use for self-management or monitoring.
Conclusion
In this study, we provide evidence that habitual exercise frequency is a potential moderator of the association between pain symptoms and previous-day exercise in endometriosis, indicating that those who regularly exercise at least ~3 times per week are less likely to report pain symptoms after having exercised on the previous day. Individuals with endometriosis are significantly more likely to have higher all-cause healthcare utilisation and direct healthcare costs than those without endometriosis, including twice the prevalence of opioid prescriptions for pain management22 and prolonged duration of prescriptions.21 While guidelines recommend prescribing exercise for management of pain in clinical populations, endometriosis (or general chronic) pain-specific recommendations to guide patients and providers on measurable parameters (time, type, intensity and frequency) are lacking. Future studies are warranted investigating the effects of both acute and chronic exercises on endometriosis pain with a focus on various types, intensities and duration.
Supplementary Material
Acknowledgments
We are grateful to the Phendo participants.
Footnotes
Contributors: IE conceptualised the study, conducted the data analyses, prepared the first draft of the manuscript, and is responsible for the overall content as guarantor. SL-G and ENH were responsible for data acquisition, curation and management. NE acquired the funding and provided the mHealth infrastructure for the study (Phendo app). NE and SB provided guidance on the study design and data analyses. SB provided guidance on the copyediting of the manuscript. SB, NE, SL-G and ENH reviewed and provided feedback on the manuscript.
Funding: Funding for the work is provided by a postdoctoral fellowship from the Data Science Institute at Columbia University and an award from the National Library of Medicine (R01 LM013043).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Study data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Columbia University Irving Medical Center Institutional Review Board (protocol #: AAAQ9812 (M01Y05)).
References
- 1.Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol 2015;29:120–30. 10.1016/j.berh.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain 2019;20:1249–66. 10.1016/j.jpain.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 3.Lemmens J, De Pauw J, Van Soom T, et al. The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: a systematic literature review and meta-analysis. J Headache Pain 2019;20:16. 10.1186/s10194-019-0961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armour M, Ee CC, Naidoo D. Exercise for dysmenorrhoea. Cochrane Database Syst Rev 2019;9:CD004142. 10.1002/14651858.CD004142.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbi M, Aung MS, Gay G, et al. Feasibility and acceptability of mobile phone–based auto-personalized physical activity recommendations for chronic pain self-management: pilot study on adults. J Med Internet Res 2018;20:e10147. 10.2196/10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevel L, Boissoneault J, Alappattu M, et al. Training endogenous pain modulation: a preliminary investigation of neural adaptation following repeated exposure to clinically-relevant pain. Brain Imaging Behav 2020;14:881–96. 10.1007/s11682-018-0033-8 [DOI] [PubMed] [Google Scholar]
- 7.Gordon R, Bloxham S, eds. A systematic review of the effects of exercise and physical activity on non-specific chronic low back pain. Healthcare. Multidisciplinary Digital Publishing Institute, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev 2017;4:CD011279. 10.1002/14651858.CD011279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R, Chomistek AK, Dimitrakoff JD, et al. Physical activity and chronic prostatitis/chronic pelvic pain syndrome. Med Sci Sports Exerc 2015;47:757–64. 10.1249/MSS.0000000000000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto A, Di Raimondo D, Tuttolomondo A, et al. Effects of physical exercise on inflammatory markers of atherosclerosis. Curr Pharm Des 2012;18:4326–49. 10.2174/138161212802481192 [DOI] [PubMed] [Google Scholar]
- 11.Garatachea N, Molinero O, Martínez-García R, et al. Feelings of well being in elderly people: relationship to physical activity and physical function. Arch Gerontol Geriatr 2009;48:306–12. 10.1016/j.archger.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 12.Tennfjord MK, Gabrielsen R, Tellum T. Effect of physical activity and exercise on endometriosis-associated symptoms: a systematic review. BMC Womens Health 2021;21:355. 10.1186/s12905-021-01500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans S, Fernandez S, Olive L, et al. Psychological and mind-body interventions for endometriosis: a systematic review. J Psychosom Res 2019;124:109756. 10.1016/j.jpsychores.2019.109756 [DOI] [PubMed] [Google Scholar]
- 14.Mira TAA, Buen MM, Borges MG, et al. Systematic review and meta-analysis of complementary treatments for women with symptomatic endometriosis. Int J Gynaecol Obstet 2018;143:2–9. 10.1002/ijgo.12576 [DOI] [PubMed] [Google Scholar]
- 15.Fourquet J, Gao X, Zavala D, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril 2010;93:2424–8. 10.1016/j.fertnstert.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schliep KC, Mumford SL, Peterson CM, et al. Pain typology and incident endometriosis. Hum Reprod 2015;30:2427–38. 10.1093/humrep/dev147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Graaff AA, D'Hooghe TM, Dunselman GAJ, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod 2013;28:2677–85. 10.1093/humrep/det284 [DOI] [PubMed] [Google Scholar]
- 18.Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–9. 10.1093/humrep/des073 [DOI] [PubMed] [Google Scholar]
- 19.Soliman AM, Coyne KS, Gries KS, et al. The effect of endometriosis symptoms on absenteeism and presenteeism in the workplace and at home. J Manag Care Spec Pharm 2017;23:745–54. 10.18553/jmcp.2017.23.7.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Practice Committee of the American Society for Reproductive Medicine . Treatment of pelvic pain associated with endometriosis: a committee opinion. 2014 2014/04/01/. Report No.: 0015-0282 contract No.: 4. [DOI] [PubMed]
- 21.Lamvu G, Soliman AM, Manthena SR, et al. Patterns of prescription opioid use in women with endometriosis: evaluating prolonged use, daily dose, and concomitant use with benzodiazepines. Obstet Gynecol 2019;133:1120. 10.1097/AOG.0000000000003267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman AM, Surrey ES, Bonafede M, et al. Health care utilization and costs associated with endometriosis among women with Medicaid insurance. J Manag Care Spec Pharm 2019;25:566–72. 10.18553/jmcp.2019.25.5.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315:1624–45. 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluka KA, Frey-Law L, Hoeger Bement M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018;159:S91–7. 10.1097/j.pain.0000000000001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tour J, Löfgren M, Mannerkorpi K, et al. Gene-to-gene interactions regulate endogenous pain modulation in fibromyalgia patients and healthy controls-antagonistic effects between opioid and serotonin-related genes. Pain 2017;158:1194–203 10.1097/j.pain.0000000000000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobinski F, Teixeira JM, Sluka KA, et al. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018;159:437–50 10.1097/j.pain.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montenegro ML, Bonocher CM, Meola J, et al. Effect of physical exercise on endometriosis experimentally induced in rats. Reprod Sci 2019;26:785–93. 10.1177/1933719118799205 [DOI] [PubMed] [Google Scholar]
- 28.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update 2011;17:327–46. 10.1093/humupd/dmq050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S-J, Yong M-S, Na S-S. Effect of exercise on the expression of nerve growth factor in the spinal cord of rats with induced osteoarthritis. J Phys Ther Sci 2015;27:2551–4. 10.1589/jpts.27.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karasawa Y, Yamada K, Iseki M, et al. Association between change in self-efficacy and reduction in disability among patients with chronic pain. PLoS One 2019;14:e0215404. 10.1371/journal.pone.0215404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armour M, Sinclair J, Chalmers KJ, et al. Self-management strategies amongst Australian women with endometriosis: a national online survey. BMC Complement Altern Med 2019;19:17. 10.1186/s12906-019-2431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves AV, Barros NF, Bahamondes L. The practice of Hatha yoga for the treatment of pain associated with endometriosis. J Altern Complement Med 2017;23:45–52. 10.1089/acm.2015.0343 [DOI] [PubMed] [Google Scholar]
- 33.Ricci E, Viganò P, Cipriani S. Physical activity and endometriosis risk in women with infertility or pain: systematic review and meta-analysis. Medicine 2016;95:e4957-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter SE, Tjaden B, Rock JA, et al. The effect of regular exercise on women receiving danazol for treatment of endometriosis. Int J Gynaecol Obstet 1995;49:299–304. 10.1016/0020-7292(95)02359-k [DOI] [PubMed] [Google Scholar]
- 35.Bonocher CM, Montenegro ML, Rosa E Silva JC, et al. Endometriosis and physical exercises: a systematic review. Reprod Biol Endocrinol 2014;12:4. 10.1186/1477-7827-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naugle KM, Fillingim RB, Riley JL. A meta-analytic review of the hypoalgesic effects of exercise. J Pain 2012;13:1139–50. 10.1016/j.jpain.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janal MN, Colt EWD, Clark CW, et al. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain 1984;19:13–25. 10.1016/0304-3959(84)90061-7 [DOI] [PubMed] [Google Scholar]
- 38.Droste C, Greenlee MW, Schreck M, et al. Experimental pain thresholds and plasma beta-endorphin levels during exercise. Med Sci Sports Exerc 1991;23:334–42. [PubMed] [Google Scholar]
- 39.Scheef L, Jankowski J, Daamen M, et al. An fMRI study on the acute effects of exercise on pain processing in trained athletes. Pain 2012;153:1702–14. 10.1016/j.pain.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 40.Hoffman MD, Hoffman DR. Exercisers achieve greater acute exercise-induced mood enhancement than nonexercisers. Arch Phys Med Rehabil 2008;89:358–63. 10.1016/j.apmr.2007.09.026 [DOI] [PubMed] [Google Scholar]
- 41.Hallgren Mats Å, Moss ND, Gastin P. Regular exercise participation mediates the affective response to acute bouts of vigorous exercise. J Sports Sci Med 2010;9:629–37. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y-C, Chen C, Martínez RM, et al. Habitual physical activity mediates the acute exercise-induced modulation of anxiety-related amygdala functional connectivity. Sci Rep 2019;9:19787. 10.1038/s41598-019-56226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.May M, Junghaenel DU, Ono M, et al. Ecological momentary assessment methodology in chronic pain research: a systematic review. J Pain 2018;19:699–716. 10.1016/j.jpain.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ensari I, Pichon A, Lipsky-Gorman S, et al. Augmenting the clinical data sources for enigmatic diseases: a cross-sectional study of self-tracking data and clinical documentation in endometriosis. Appl Clin Inform 2020;11:769–84. 10.1055/s-0040-1718755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urteaga I, McKillop M, Elhadad N. Learning endometriosis phenotypes from patient-generated data. NPJ Digit Med 2020;3:88. 10.1038/s41746-020-0292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratap A, Neto EC, Snyder P, et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit Med 2020;3:21. 10.1038/s41746-020-0224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain 1975;1:277–99. 10.1016/0304-3959(75)90044-5 [DOI] [PubMed] [Google Scholar]
- 48.Jones KR, Vojir CP, Hutt E, et al. Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J Rehabil Res Dev 2007;44:305–14. 10.1682/jrrd.2006.05.0051 [DOI] [PubMed] [Google Scholar]
- 49.Bestel E, Gotteland J-P, Donnez J. Linzagolix for endometriosis-associated pain: lipid changes after 52 weeks of treatment [25B]. Obstet Gynecol 2020;135:25S. [Google Scholar]
- 50.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277–84. 10.1016/0304-3959(94)00178-H [DOI] [PubMed] [Google Scholar]
- 51.Adams P, Murnane EL, Elfenbein M, et al. Supporting the self-management of chronic pain conditions with tailored Momentary Self-Assessments. Proc SIGCHI Conf Hum Factor Comput Syst 2017;2017:1065–77. 10.1145/3025453.3025832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boonstra AM, Stewart RE, Köke AJA, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol 2016;7:1466. 10.3389/fpsyg.2016.01466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dirks JF, Wunder J, Kinsman R, et al. A pain rating scale and a pain behavior checklist for clinical use: development, norms, and the consistency score. Psychother Psychosom 1993;59:41–9. 10.1159/000288643 [DOI] [PubMed] [Google Scholar]
- 54.Frost MH, Reeve BB, Liepa AM, et al. What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health 2007;10:S94–105. 10.1111/j.1524-4733.2007.00272.x [DOI] [PubMed] [Google Scholar]
- 55.Faurholt-Jepsen M, Munkholm K, Frost M, et al. Electronic self-monitoring of mood using IT platforms in adult patients with bipolar disorder: a systematic review of the validity and evidence. BMC Psychiatry 2016;16:7. 10.1186/s12888-016-0713-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charter RA. Sample size requirements for precise estimates of reliability, generalizability, and validity coefficients. J Clin Exp Neuropsychol 1999;21:559–66. 10.1076/jcen.21.4.559.889 [DOI] [PubMed] [Google Scholar]
- 57.Ensari I, Horan E, Elhadad N, et al. Evaluation of a disease-specific mHealth-based exercise self-tracking measure. MedRXiv 2022. 10.1101/2022.05.16.22275170 [DOI] [Google Scholar]
- 58.Kuntz JL, Young DR, Saelens BE, et al. Validity of the exercise vital sign tool to assess physical activity. Am J Prev Med 2021;60:866–72. 10.1016/j.amepre.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- 60.Vitonis AF, Vincent K, Rahmioglu N, et al. World endometriosis research foundation endometriosis phenome and biobanking harmonization project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil Steril 2014;102:1223–32. 10.1016/j.fertnstert.2014.07.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones G, Kennedy S, Barnard A, et al. Development of an endometriosis quality-of-life instrument: the endometriosis health Profile-30. Obstet Gynecol 2001;98:258–64. 10.1016/s0029-7844(01)01433-8 [DOI] [PubMed] [Google Scholar]
- 62.McHorney CA, Ware JE, Lu JF, et al. The mos 36-item short-form health survey (SF-36): III. tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66. 10.1097/00005650-199401000-00004 [DOI] [PubMed] [Google Scholar]
- 63.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and Met values. Med Sci Sports Exerc 2011;43:1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 64.McKillop M, Voigt N, Schnall R, et al. Exploring self-tracking as a participatory research activity among women with endometriosis. J Participat Med 2016. [Google Scholar]
- 65.McKillop M, Mamykina L, Elhadad N, eds. Designing in the dark: eliciting self-tracking dimensions for understanding enigmatic disease. Proceedings of the 2018 CHI conference on human factors in computing systems. ACM, 2018. [Google Scholar]
- 66.Anthoine E, Moret L, Regnault A, et al. Sample size used to validate a scale: a review of publications on newly-developed patient reported outcomes measures. Health Qual Life Outcomes 2014;12:176. 10.1186/s12955-014-0176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.US Department of Health Human Services . Guidance for industry-Patient-reported outcome measures: use in medical product development to support labeling claims 2009. [DOI] [PMC free article] [PubMed]
- 68.Lomas J, Pickard L, Mohide A. Patient versus clinician item generation for quality-of-life measures: the case of language-disabled adults. Med Care 1987;25:764–9. 10.1097/00005650-198708000-00009 [DOI] [PubMed] [Google Scholar]
- 69.Hu M-C, Pavlicova M, Nunes EV. Zero-inflated and hurdle models of count data with extra zeros: examples from an HIV-risk reduction intervention trial. Am J Drug Alcohol Abuse 2011;37:367–75. 10.3109/00952990.2011.597280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brooks ME, Kristensen K, Benthem KJv, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 2017;9:378–400. 10.32614/RJ-2017-066 [DOI] [Google Scholar]
- 71.Brooks ME, Kristensen K, van Benthem KJ. Modeling zero-inflated count data with glmmTMB. bioRxiv 2017;132753. [Google Scholar]
- 72.Buuren Svan, Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 73.Van Buuren S. Flexible imputation of missing data. CRC press, 2018. [Google Scholar]
- 74.Bondarenko I, Raghunathan T. Graphical and numerical diagnostic tools to assess suitability of multiple imputations and imputation models. Stat Med 2016;35:3007–20. 10.1002/sim.6926 [DOI] [PubMed] [Google Scholar]
- 75.Rubin DB. The calculation of posterior distributions by data augmentation: Comment: a Noniterative Sampling/Importance resampling alternative to the data augmentation algorithm for creating a few Imputations when fractions of missing information are modest: the Sir algorithm. J Am Stat Assoc 1987;82:543–6. 10.2307/2289460 [DOI] [Google Scholar]
- 76.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 2004. [Google Scholar]
- 77.Schunck R. Cluster size and aggregated level 2 variables in multilevel models. A cautionary note 2016;10. [Google Scholar]
- 78.Bell B, Ferron J, Kromrey J, eds. Cluster size in multilevel models: the impact of sparse data structures on point and interval estimates in two-level models, 2008. [Google Scholar]
- 79.Austin PC, Leckie G. The effect of number of clusters and cluster size on statistical power and type I error rates when testing random effects variance components in multilevel linear and logistic regression models. J Stat Comput Simul 2018;88:3151–63. 10.1080/00949655.2018.1504945 [DOI] [Google Scholar]
- 80.Snijders TAB. Power and sample size in multilevel modeling. In: Everitt B, Howell D, eds. Encyclopedia of statistics in behavioral science. Wiley, 2006: 1570–3. [Google Scholar]
- 81.Core Team R . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 1997. [Google Scholar]
- 82.Scheipl F, Greven S, Küchenhoff H. Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models. Comput Stat Data Anal 2008;52:3283–99. 10.1016/j.csda.2007.10.022 [DOI] [Google Scholar]
- 83.Crainiceanu CM, Ruppert D. Likelihood ratio tests in linear mixed models with one variance component. J Royal Statistical Soc B 2004;66:165–85. 10.1111/j.1467-9868.2004.00438.x [DOI] [Google Scholar]
- 84.US Department of Health Human Services Physical activity guidelines advisory committee scientific report. 2018.
- 85.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–8. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warzecha D, Szymusik I, Wielgos M, et al. The impact of endometriosis on the quality of life and the incidence of Depression-A cohort study. Int J Environ Res Public Health 2020;17:17103641. 10.3390/ijerph17103641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Becker N, Thomsen AB, Olsen AK, et al. Pain epidemiology and health related quality of life in chronic non-malignant pain patients referred to a Danish multidisciplinary pain center. Pain 1997;73:393–400. 10.1016/S0304-3959(97)00126-7 [DOI] [PubMed] [Google Scholar]
- 89.Bouhassira D, Lantéri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136:380–7. 10.1016/j.pain.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 90.Nicholas MK, Asghari A, Blyth FM. What do the numbers mean? Normative data in chronic pain measures. Pain 2008;134:158–73. 10.1016/j.pain.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 91.Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth 2013;111:19–25. 10.1093/bja/aet124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider S, Stone AA, Schwartz JE, et al. Peak and end effects in patients' daily recall of pain and fatigue: a within-subjects analysis. J Pain 2011;12:228–35. 10.1016/j.jpain.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Boer MJ, Struys MMRF, Versteegen GJ. Pain‐related catastrophizing in pain patients and people with pain in the general population. Eur J Pain 2012;16:1044–52. 10.1002/j.1532-2149.2012.00136.x [DOI] [PubMed] [Google Scholar]
- 94.Ehde DM, Gibbons LE, Chwastiak L, et al. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler 2003;9:605–11. 10.1191/1352458503ms939oa [DOI] [PubMed] [Google Scholar]
- 95.Katzmarzyk PT, Powell KE, Jakicic JM, et al. Sedentary behavior and health: update from the 2018 physical activity guidelines advisory committee. Med Sci Sports Exerc 2019;51:1227–41. 10.1249/MSS.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basen-Engquist K, Scruggs S, Jhingran A, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol 2009;200:288.e1–288.e8. 10.1016/j.ajog.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dansie EJ, Turk DC, Martin KR, et al. Association of chronic widespread pain with objectively measured physical activity in adults: findings from the National health and nutrition examination survey. J Pain 2014;15:507–15. 10.1016/j.jpain.2014.01.489 [DOI] [PubMed] [Google Scholar]
- 98.Polaski AM, Phelps AL, Kostek MC, et al. Exercise-induced hypoalgesia: a meta-analysis of exercise dosing for the treatment of chronic pain. PLoS One 2019;14:e0210418-e. 10.1371/journal.pone.0210418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Almirall D, Compton SN, Gunlicks-Stoessel M, et al. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat Med 2012;31:1887–902. 10.1002/sim.4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klasnja P, Smith S, Seewald NJ. Efficacy of Contextually tailored suggestions for physical activity: a Micro-randomized optimization trial of HeartSteps. Ann Behav Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med 2018;52:446–62. 10.1007/s12160-016-9830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Falcone T, Mascha E. The elusive diagnostic test for endometriosis. Fertil Steril 2003;80:886–8. 10.1016/S0015-0282(03)01161-0 [DOI] [PubMed] [Google Scholar]
- 103.Marian S, Hermanowicz-Szamatowicz K. Endometriosis - a decade later - still an enigmatic disease. What is the new in the diagnosis and treatment? Gynecol Endocrinol 2020;36:104–8. 10.1080/09513590.2019.1675045 [DOI] [PubMed] [Google Scholar]
- 104.Fourquet J, Zavala DE, Missmer S, et al. Disparities in healthcare services in women with endometriosis with public vs private health insurance. Am J Obstet Gynecol 2019;221:623.e1–623.e11. 10.1016/j.ajog.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 105.Lee RR, Rashid A, Ghio D, et al. "Seeing pain differently": a qualitative investigation into the differences and similarities of pain and rheumatology specialists' interpretation of multidimensional mobile health pain data from children and young people with juvenile idiopathic arthritis. JMIR Mhealth Uhealth 2019;7:e12952. 10.2196/12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jamison RN, Raymond SA, Levine JG, et al. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain 2001;91:277–85. 10.1016/S0304-3959(00)00450-4 [DOI] [PubMed] [Google Scholar]
- 107.Bouhassira D, Attal N. All in one: is it possible to assess all dimensions of any pain with a simple questionnaire? Pain 2009;144:7–8. 10.1016/j.pain.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 108.Pilitsis JG, Fahey M, Custozzo A, et al. Composite score is a better reflection of patient response to chronic pain therapy compared with pain intensity alone. Neuromodulation 2021;24:68–75. 10.1111/ner.13212 [DOI] [PubMed] [Google Scholar]
- 109.Grimmer-Somers K, Vipond N, Kumar S, et al. A review and critique of assessment instruments for patients with persistent pain. J Pain Res 2009;2:21. 10.2147/JPR.S4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Knell G, Gabriel KP, Businelle MS, et al. Ecological momentary assessment of physical activity: validation study. J Med Internet Res 2017;19:e253. 10.2196/jmir.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swendeman D, Comulada WS, Koussa M, et al. Longitudinal validity and reliability of brief smartphone self-monitoring of diet, stress, and physical activity in a diverse sample of mothers. JMIR Mhealth Uhealth 2018;6:e176. 10.2196/mhealth.9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katapally TR, Chu LM. Digital epidemiological and citizen science methodology to capture prospective physical activity in free-living conditions: a SMART platform study. BMJ Open 2020;10:e036787. 10.1136/bmjopen-2020-036787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zink J, Belcher BR, Dzubur E, et al. Association between self-reported and objective activity levels by demographic factors: ecological momentary assessment study in children. JMIR Mhealth Uhealth 2018;6:e150. 10.2196/mhealth.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059280supp001.pdf (20.4KB, pdf)
bmjopen-2021-059280supp002.pdf (485.6KB, pdf)
bmjopen-2021-059280supp003.pdf (177.2KB, pdf)
bmjopen-2021-059280supp004.pdf (403KB, pdf)
bmjopen-2021-059280supp005.pdf (30.3KB, pdf)
bmjopen-2021-059280supp006.pdf (43.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Study data are available upon reasonable request.