Abstract
Objective:
To examine the pathophysiology of ischemic stroke with cancer.
Methods:
We conducted a prospective cross-sectional study from 2016–2020 at two hospitals. We enrolled three groups of 50 adult participants each. The main group included patients with active solid tumor cancer and acute ischemic stroke. The control groups included patients with acute ischemic stroke only or active cancer only. The stroke-only and cancer-only patients were matched to the cancer-plus-stroke patients by age, sex, and cancer type, if applicable. The outcomes were prespecified hematological biomarkers and transcranial Doppler microemboli detection. Hematological biomarkers included markers of coagulation (D-dimer, thrombin-antithrombin), platelet function (P-selectin), and endothelial integrity (thrombomodulin, soluble intercellular adhesion molecule-1 [sICAM-1], soluble vascular cell adhesion molecule-1 [sVCAM-1]). Hematological biomarkers were compared between groups using the Kruskal-Wallis and Wilcoxon Rank-Sum tests. In multivariable linear regression models, we adjusted for race, number of stroke risk factors, smoking, stroke severity, and antithrombotic use. Transcranial Doppler microemboli presence was compared between groups using Chi-square tests.
Results:
Levels of all study biomarkers were different between groups. In univariate between-group comparisons, cancer-plus-stroke participants had higher levels of D-dimer, sICAM-1, sVCAM-1, and thrombomodulin than both control groups; higher levels of thrombin-antithrombin than cancer-only participants; and higher levels of P-selectin than stroke-only participants. Findings were similar in multivariable analyses. Transcranial Doppler microemboli were detected in 32% of cancer-plus-stroke participants, 16% of stroke-only participants, and 6% of cancer-only participants (p=0.005).
Interpretation:
Patients with cancer-related stroke have higher markers of coagulation, platelet, and endothelial dysfunction, and more circulating microemboli, than matched controls.
Introduction
An estimated 4–20% of patients with ischemic stroke have cancer and the coprevalence of these diseases is increasing.1–4 This is presumably due to recent improvements in cancer treatments prolonging patient survival.5, 6 Cancer is an established risk factor for ischemic stroke.7–9 In the first 6 months after cancer diagnosis, patients with cancer face, on average, a two-fold increased risk of ischemic stroke.7 This risk is higher in patients with historically aggressive cancers (e.g., lung and pancreatic) and in those with metastases. Strokes in patients with cancer tend to be more severe and fatal and recur more often than strokes in patients without cancer.10
Approximately 50% of cancer-related strokes are classified as cryptogenic after standard diagnostic evaluation.11–13 Further, the biological factors responsible for the increased risk of stroke with cancer are poorly understood, although recent studies suggest that hypercoagulability may play an important role. For example, the OASIS-Cancer study reported that patients with cancer and cryptogenic ischemic stroke have increased blood levels of extracellular vesicles and neutrophil extracellular trap formation (NETosis) than control patients.14, 15 Although both of these pathophysiological mechanisms are linked to hypercoagulability, their reproducibility and scalability may limit their clinical use. Furthermore, several studies have reported that plasma D-dimer levels are increased in patients with cancer and stroke, but D-dimer is a nonspecific fibrin degradation product and can be increased by stroke itself and in patients with cardiac disease.12, 16, 17 Few other hematological biomarkers have been studied in patients with cancer and stroke, especially markers measuring other aspects of clotting such as platelets and endothelium.
A single-center study reported that 46% of patients with cancer and ischemic stroke had circulating microemboli on transcranial Doppler (TCD) and that the proportion was even higher in those whose stroke mechanism was undetermined.18 This study suggested that embolic disease may be an important contributor to cancer-related stroke. However, it lacked controls; therefore, it could not determine whether cancer-plus-stroke patients have more microemboli than patients with stroke or cancer alone.
To improve our mechanistic understanding of ischemic stroke in patients with cancer, we conducted the Mechanisms of Ischemic Stroke in Cancer Patients (MOST-Cancer) prospective study, which compared prespecified hematological markers of coagulation, platelets, and endothelial activation and TCD markers of embolic disease between patients with active cancer and ischemic stroke, ischemic stroke only, and active cancer only. Our hypothesis was that cancer-plus-stroke patients would have higher markers of hypercoagulability and more circulating microemboli than control patients.
Methods
Design
We conducted a prospective, matched cross-sectional study at NewYork-Presbyterian Hospital/Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center (MSKCC) from January 2016-August 2020 (ClinicalTrials.gov registration: NCT02604667). Both hospitals are academic, urban, and comprehensive cancer centers. NewYork-Presbyterian Hospital/Weill Cornell Medicine is also a comprehensive stroke center.
Population
We enrolled 150 adults (age ≥18 years) into 3 groups of 50 participants each. Participants in each group were enrolled consecutively from among all patients eligible for that group. The main group comprised patients with active solid tumor cancer and subsequent acute ischemic stroke; these patients’ cancer diagnosis always preceded their index stroke. Active cancer was defined per standard criteria as a diagnosis or treatment of any malignant cancer within the past 6 months, or known recurrent or metastatic disease.19, 20 Patients with hematological or primary brain cancers were excluded. Acute ischemic stroke was defined per the American Heart Association’s updated tissue-based definition and required magnetic resonance imaging confirmation.21 The other two groups served as controls and included patients with acute ischemic stroke only and active solid tumor cancer only. Participants in the stroke-only and cancer-only groups were individually matched to participants in the cancer-plus-stroke group by age stratum (≥65 years vs. <65 years), sex, and primary cancer type (if applicable). There were 10 participants in the cancer-plus-stroke group with cancer types for whom we could not find age- and sex-matched controls with the same cancer type; this included two participants with head and neck cancer, two with breast cancer, and one each with sarcoma, melanoma, esophageal, gastric, and primary peritoneal cancer. These participants were matched to patients with lung or pancreatic cancer because these cancer types are associated with high risks of stroke and therefore their selection constituted a conservative bias.7 Data on participants’ cancer types are provided in Table 1. One cancer-plus-stroke participant was inadvertently matched to a stroke-only participant of the opposite sex. To minimize confounding effects on study markers, exclusion criteria were treatment with intravenous/intraarterial thrombolysis, mechanical thrombectomy, platelet count <50,000/mm3, hemodialysis, infection<14 days, and pregnancy.
Table 1.
Cancer Types Among Participants within the Cancer Groups
| Cancer Typea | Cancer-Plus-Stroke (n=50) |
Cancer-Only (n=50) |
|---|---|---|
| Lung | 14 (28) | 23 (46) |
| Pancreatic | 11 (22) | 12 (24) |
| Breast | 8 (16) | 6 (12) |
| Prostate | 5 (10) | 5 (10) |
| Renal | 2 (4) | 1 (2) |
| Head and neck | 2 (2) | 0 (0) |
| Colorectal | 1 (2) | 1 (2) |
| Ovarian | 1 (2) | 1 (2) |
| Bladder | 1 (2) | 1 (2) |
| Esophageal | 1 (1) | 0 (0) |
| Gastric | 1 (1) | 0 (0) |
| Melanoma | 1 (1) | 0 (0) |
| Sarcoma | 1 (1) | 0 (0) |
| Primary peritoneal | 1 (1) | 0 (0) |
Data reported as number (%).
Sample size was determined based on an analysis of pilot data.22 Using unadjusted results, we estimated that 42 participants in each group would provide 80% power to detect differences in the study biomarker that showed the smallest difference between groups in our preliminary analysis (thrombomodulin). To account for multivariable adjustments, we inflated the final sample size by 20% to 50 participants per group. This study was approved by the institutional review boards of participating sites. All patients or their proxies provided written informed consent.
Measurements
Study procedures consisted of a single blood draw and a single TCD microemboli detection study. Trained phlebotomists collected venous blood from study patients, preferably through a peripheral venipuncture, into ethylenediaminetetraacetic acid and sodium citrate tubes. In the cancer-plus-stroke and stroke-only groups, blood was drawn 72–120 hours after stroke onset. In the cancer-only group, blood was drawn immediately after enrollment. Blood samples were immediately centrifuged by at least 1000 g-force/2500 revolutions per minute for 15 minutes, aliquoted into microcentrifuge tubes, and then stored in a −80°C freezer prior to analysis.
Six prespecified hematological biomarkers were evaluated. They were chosen based on pilot data and the expert opinion of our hematology coinvestigators. Hematological biomarkers comprised markers of coagulation (D-dimer, thrombin-antithrombin), platelet function (P-selectin), and endothelial integrity (thrombomodulin, soluble intercellular adhesion molecule-1 [sICAM-1], soluble vascular cell adhesion molecule-1 [sVCAM-1]).
These biomarkers were measured in five batches at MSKCC’s Central Laboratory. D-dimer, a standard-of-care test at MSKCC, was analyzed on its STA R Max system using the STA-Liatest D-Di assay from Diagnostica Stago. P-selectin, thrombomodulin, sICAM-1, and sVCAM-1 were analyzed using commercial ELISA kits purchased from Fisher Scientific Company LLC. Thrombin-antithrombin was analyzed using commercial ELISA kits purchased from Louisville APL Diagnostics, Inc. After internal validation checks, no batch effects were detected.
TCD microemboli detection studies were performed after enrollment using the Spencer ST3 TCD machine or the Natus CareFusion SONARA TCD machine. Experienced technicians used a fixed headframe and two monitoring probes to insonate bilateral middle cerebral artery M1 segments, typically at depths of 45–65 mm, for 30 minutes. Circulating microemboli display a unique sonographic signal on TCD and are characterized as high-intensity transient signals (HITS) (Fig 1). We evaluated for HITS through automated software and manual review of recordings. All potential microemboli were individually reviewed by certified neurosonologists for confirmation. The presence, laterality, and number of HITS were recorded.
Figure 1. An example High Intensity Transient Signal (HITS) on transcranial Doppler.
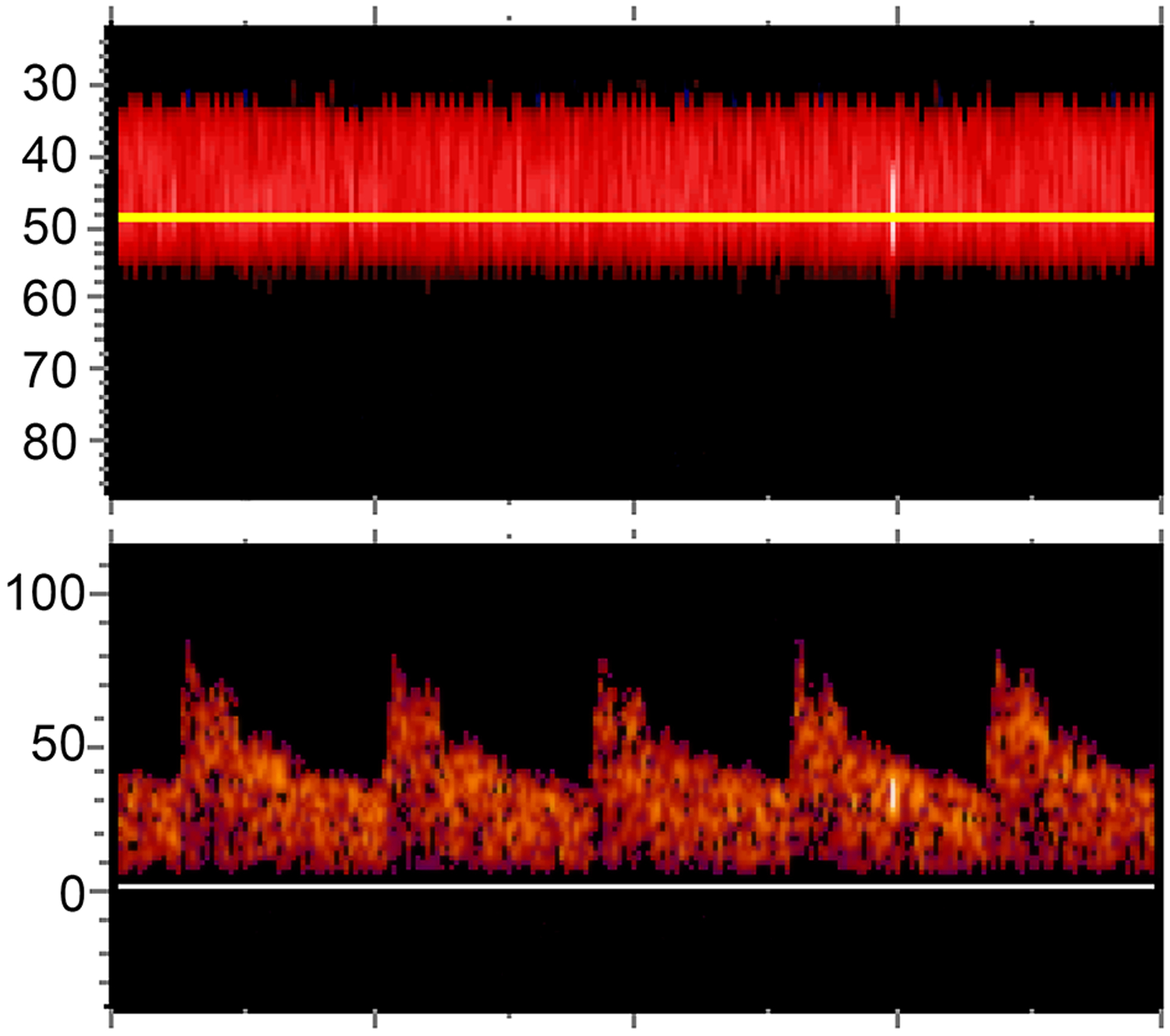
This figure depicts an identified HITS in a patient with active lung cancer and acute ischemic stroke. In the top panel, power M-mode displays the left middle cerebral artery at depths of 33 to 55 mm. The horizontal yellow line denotes the specific depth (48 mm) that has been selected to display the single gate spectral waveform seen in the bottom panel. A HITS (i.e., microemboli) is identified traveling in one direction through the middle cerebral artery. It is characterized visually by a vertical bright streak and acoustically by a “chirping” sound.
We collected information about participants’ demographics; stroke risk factors, including history of hypertension, hyperlipidemia, diabetes, coronary disease, heart failure, atrial fibrillation, peripheral vascular disease, chronic kidney disease, and smoking; cancer type, stage, and treatments; prior venous thromboembolism; NIH stroke scale; antithrombotic medications; stroke diagnostic evaluation; cerebrovascular territories of the index stroke; basic laboratory tests; and stroke mechanism according to the Trial of ORG 10172 (TOAST) classification.23 Stroke mechanisms were adjudicated by B.N. on the day after patients’ hospital discharge using all available information.
Analysis
We report clinical characteristics as median and interquartile range (IQR) if continuous and counts and percentages if categorical. We performed several sets of analysis to compare hematological biomarkers between groups. As this was a matched study, the univariate analyses were considered primary, while the multivariable analyses examined the robustness of findings and were considered secondary. First, as the data were not normally distributed, we performed Kruskal-Wallis tests to determine whether median values were different between the three groups. Second, we performed individual between-group comparisons using Wilcoxon rank-sum tests. Third, we used multiple linear regression to examine the association between study groups and biomarkers while adjusting for race (white vs. nonwhite), number of stroke risk factors (range, 0–10), smoking (any history vs. never), antiplatelet use, treatment-dose anticoagulant use, and NIH stroke scale. These variables were selected as potential confounders based on biological plausibility and expert opinion. We log transformed D-dimer, thrombin-antithrombin, thrombomodulin, sICAM-1, and sVCAM-1 to meet model assumptions.
For the main TCD analysis, we categorized microemboli as a binary variable (present vs. absent). Using the intention-to-treat principle, participants who lacked sufficient transtemporal acoustic windows for TCD monitoring were classified as not having microemboli. In the primary analysis, we used Chi-square tests to compare the presence of microemboli between groups. In the secondary analysis, we used multiple logistic regression to examine the association between study groups and microemboli while adjusting for the number of stroke risk factors, treatment-dose anticoagulant use, and TOAST stroke mechanism (cardioembolic vs. others). These variables were selected as potential confounders based on biological plausibility and expert opinion.
We performed several sensitivity analyses. First, we used Kruskal-Wallis and Wilcoxon rank-sum tests to compare the number of microemboli per participant between groups. Second, we compared hematological biomarker levels and the presence of microemboli between groups when the study cohorts were restricted to cancer-plus-stroke participants and their individually matched controls who had exact matching by cancer type (n=40 in each group). Statistical significance was defined as p<0.05. Analyses were performed using Stata (version 15.1; StataCorp).
Results
Clinical Characteristics
Among 150 total participants, median age was 68 years (IQR, 59–75) and 47% were women (Table 2). In the cancer-plus-stroke group, 88% of cancers were adenocarcinomas, and lung (n=14) and pancreatic (n=11) cancers were the most common primary sites. The median time from cancer diagnosis to study enrollment was 1.0 year (IQR, 0.3–3.5 years) in the cancer-plus-stroke group versus 0.9 years (IQR, 0.3–3.6 years) in the cancer-only group (p=0.78).
Table 2.
Participant Characteristics, Stratified by Study Group
| Characteristica | Cancer-Plus-Stroke (n=50) |
Stroke-Only (n=50) |
Cancer-Only (n=50) |
|---|---|---|---|
| Demographics | |||
| Age, years | 69 (60–76) | 68 (56–74) | 68 (58–74) |
| Female sexb | 24 (48) | 23 (46) | 24 (48) |
| Race | |||
| White | 40 (80) | 41 (82) | 41 (82) |
| Black | 8 (16) | 6 (12) | 9 (18) |
| Other | 2 (4) | 3 (6) | 0 (0) |
| Hispanic ethnicity | 3 (6) | 6 (12) | 6 (12) |
| Vascular Risk Factors | |||
| Hypertension | 30 (60) | 36 (72) | 25 (50) |
| Hyperlipidemia | 22 (44) | 26 (52) | 21 (42) |
| Diabetes | 15 (30) | 13 (26) | 8 (16) |
| Coronary artery disease | 8 (16) | 7 (14) | 5 (10) |
| Congestive heart failure | 5 (10) | 4 (8) | 1 (2) |
| Atrial fibrillation | 5 (10) | 3 (6) | 4 (8) |
| Peripheral vascular disease | 0 (0) | 0 (0) | 0 (0) |
| Chronic kidney disease | 6 (12) | 2 (4) | 2 (4) |
| Prior venous thromboembolism | 14 (28) | 1 (2) | 8 (16) |
| Prior stroke/TIA | 9 (18) | 9 (18) | 1 (2) |
| Smoking (any history) | 28 (56) | 16 (32) | 32 (64) |
| Cancer Characteristics | |||
| Adenocarcinoma | 44 (88) | NA | 39 (78) |
| Systemic metastases | 43 (86) | NA | 35 (70) |
| Chemotherapy within 30 days | 33 (66) | NA | 44 (88) |
| Head, neck, or mediastinal RT | 10 (20) | NA | 17 (34) |
| Basic Laboratory Data | |||
| White blood cell count, 103/uL | 8.3 (5.2–10.3) | 7.6 (6.0–9.9) | 5.5 (3.9–8.1) |
| Platelet count, 103/uL | 198 (149–278) | 201 (178–256) | 218 (185–293) |
| Prothrombin time, sec | 13 (12–15) | 12 (11–13) | 12 (11–13) |
| Partial thromboplastin time, sec | 30 (27–32) | 29 (26–32) | 31 (28–33) |
| Stroke Prevention Medicines c | |||
| Antiplatelet | 32 (64) | 49 (98) | 12 (24) |
| Anticoagulantd | 20 (40) | 6 (12) | 7 (14) |
| Statin | 47 (94) | 49 (98) | 16 (32) |
Abbreviations: TIA, transient ischemic attack; RT, radiotherapy.
Data reported as number (%) for categorical variables and median (interquartile range) for continuous variables.
The discrepancy between groups is because one cancer-plus-stroke participant was inadvertently matched to a stroke-only participant of the opposite sex
Indicates medicines the patient was taking at the time of study enrollment.
Specific to the use of treatment-dose anticoagulation (prophylactic doses are excluded).
Study groups were balanced except that cancer-plus-stroke participants more often had prior venous thromboembolism, metastases, and treatment-dose anticoagulation; while cancer-only participants more often smoked and had lung cancer or treatment with radiotherapy or recent chemotherapy, and less often had prior stroke; and stroke-only participants more often received antiplatelet treatment. Stroke diagnostic evaluations were thorough, with >95% of participants in both stroke groups receiving intra- and extra-cranial vessel imaging, an electrocardiogram, >24 hours of cardiac rhythm monitoring, and an echocardiogram. Comparing cancer-plus-stroke participants to stroke-only participants, adjudicated TOAST stroke mechanisms were more often cardioembolic (36% vs. 16%) and undetermined (48% vs. 42%) and less often small vessel disease (0% vs. 22%) (Table 3). Sixty percent of cancer-plus-stroke participants had multi-territory acute infarcts versus 10% of stroke-only participants.
Table 3.
Stroke Characteristics Among Participants within the Stroke Groups
| Characteristica | Cancer-Plus-Stroke (n=50) |
Stroke-Only (n=50) |
|---|---|---|
| Stroke Prevention Medicines Before Stroke | ||
| Antiplatelet | 15 (30) | 20 (40) |
| Anticoagulant | 23 (46) | 4 (8) |
| Statin | 18 (36) | 22 (44) |
| Stroke Severity/Functional Status | ||
| NIH stroke scale (IQR) | 3 (2–6) | 2 (0–3) |
| Modified Rankin Scale score (IQR) | 3 (2–4) | 2 (1–4) |
| Radiographic Infarct Pattern | ||
| Involves ≥2 vascular territories | 30 (60) | 5 (10) |
| Involves 3 vascular territories | 22 (44) | 2 (4) |
| Stroke Mechanism b | ||
| Cardioembolic | 18 (36) | 8 (16) |
| Large artery atherosclerosis | 5 (10) | 6 (12) |
| Small vessel disease | 0 (0) | 11 (22) |
| Other determined | 3 (6) | 4 (8) |
| Undetermined | 24 (48) | 21 (42) |
Abbreviations: NIH, National Institutes of Health; IQR, interquartile range.
Data reported as number (%) unless otherwise specified.
According to the Trial of Org 10172 Acute Stroke Treatment (TOAST) criteria.
Hematological Biomarkers
Blood samples were collected between the hours of 8:30 AM and 4:55 PM and most were collected in the late morning between 8 AM-11:59 AM (40%) or in the afternoon between noon-3:59 PM (53%). The timing of blood draws differed between the 3 groups (p=0.001); cancer-only participants more often had their blood samples collected in the late morning, while stroke-plus-cancer and stroke-only participants more often had their blood samples collected in the afternoon. The median time from stroke onset to blood sample collection was nearly identical in the cancer-plus-stroke (96 hours [IQR, 85–103]) and stroke-only groups (96 hours [IQR, 86–104]).
The median values of all hematological biomarkers were significantly different among the three study groups (Table 4). Values were highest in the cancer-plus-stroke group, second highest in the cancer-only group (except thrombin-antithrombin), and lowest in the stroke-only group. The biomarker with the largest relative and absolute numeric difference between groups was D-dimer, with median (IQR) values of 2,552 (830–6,640) ng/ml in the cancer-plus-stroke group, 670 (400–1,730) ng/ml in the cancer-only group, and 405 (204–740) ng/ml in the stroke-only group. In direct two-way comparisons, all hematological biomarkers except thrombin-antithrombin were significantly higher in the cancer-plus-stroke group than in the stroke-only group (Fig 2), and all hematological biomarkers except P-selectin were significantly higher in the cancer-plus-stroke group than in the cancer-only group. These findings were similar in sensitivity analyses restricting the study cohorts to the cancer-plus-stroke participants and their individually matched controls who had exact matching by cancer type with the exception that P-selectin was also significantly higher in the cancer-plus-stroke group than in the cancer-only group (p=0.02).
Table 4.
Biomarker Values, Stratified by Study Group
| Biomarkera | Cancer-Plus-Stroke (n=50) |
Stroke-Only (n=50) |
Cancer-Only (n=50) |
P-value Across Groupsb,c |
|---|---|---|---|---|
| Coagulation | ||||
| D-dimer | 2,552 (830–6,640) | 405 (204–740) | 670 (400–1,730) | <0.001 |
| TAT | 20 (4–34) | 6 (2–28) | 4 (1–12) | <0.001 |
| Platelets | ||||
| P-selectin | 40 (28–62) | 26 (21–33) | 34 (27–45) | <0.001 |
| Endothelium | ||||
| Thrombomodulin | 5,310 (4,222–5,117) | 3,712 (3,097–4,371) | 4,081 (3,214–5,117) | <0.001 |
| sICAM-1 | 244 (204–388) | 176 (137–205) | 224 (182–264) | <0.001 |
| sVCAM-1 | 1,075 (803–1,564) | 705 (585–933) | 888 (611–1,053) | <0.001 |
| Embolic disease | ||||
| TCD HITS present | 16 (32) | 8 (16) | 3 (6) | 0.004 |
Abbreviations: TAT, thrombin-antithrombin; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; TCD, transcranial Doppler; HITS, high-intensity transient signals.
Data reported as number (%) for categorical variables and median (interquartile range) for continuous variables. Units of measure for hematological biomarkers are ng/ml, except thrombomodulin which is listed as pg/ml.
Kruskal-Wallis tests were used to determine whether median hematological biomarker levels were different between the three groups, while the Chi-square test was used to determine whether the proportion of participants with TCD HITS were different between the three groups. The listed p-values correspond to these analyses.
Between-group comparisons performed using the Wilcoxon Rank-Sum test for the hematological biomarkers and the Chi-square test for the embolic disease biomarker were also all significant except for TAT when comparing the cancer-plus-stroke group to the stroke-only group (p=0.063) and P-selectin when comparing the cancer-plus-stroke group to the cancer-only group (p=0.062). These analyses’ individual P-values are not provided in the Table.
Figure 2. Box-and-whisker plots comparing hematological biomarker values between the cancer-plus-stroke and stroke-only groups.
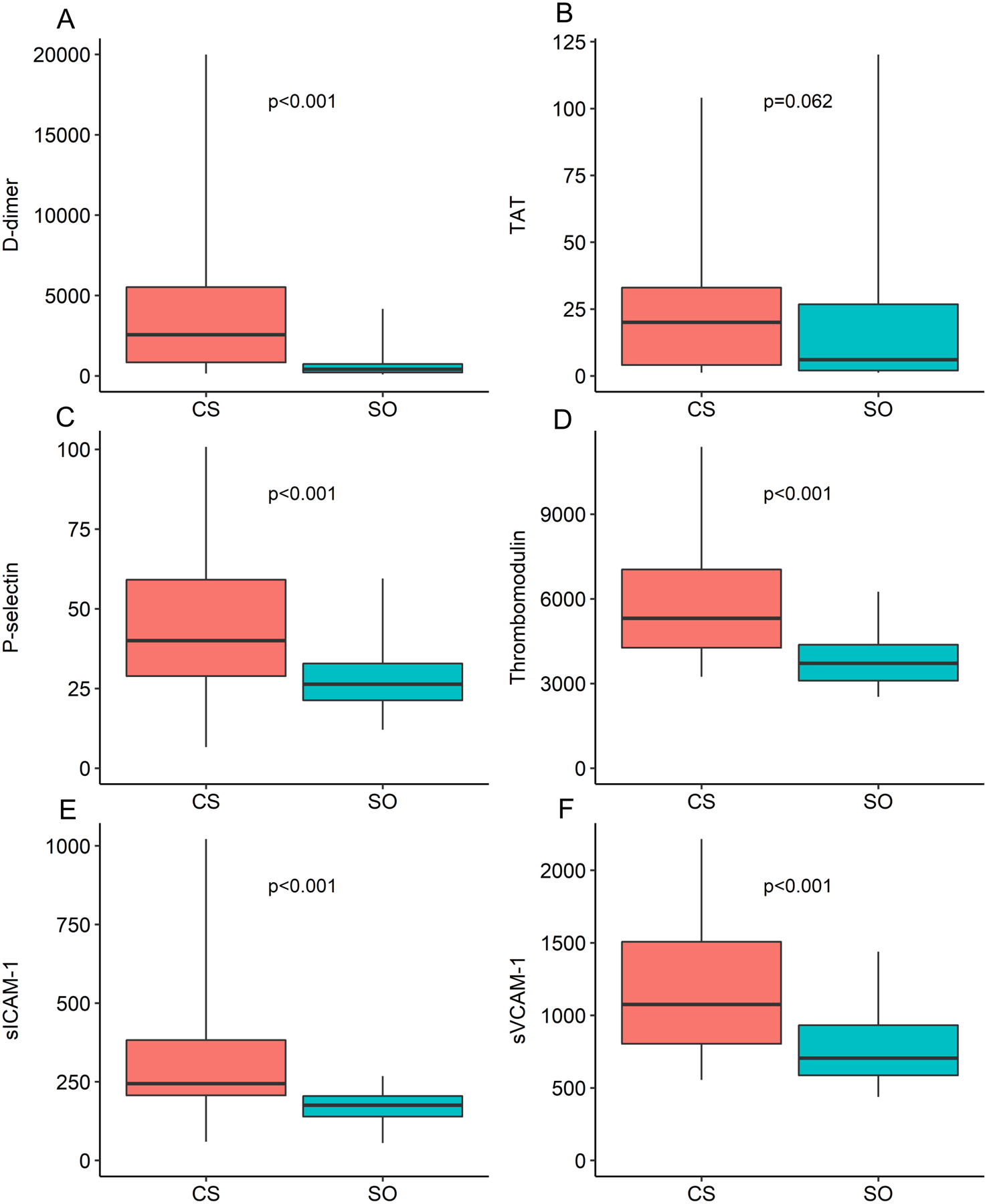
The upper box margins depict the upper quartiles, the lower box margins depict the lower quartiles, and the horizontal black lines depict the median values. The lower whiskers depict the 2.5 percentiles, and the upper whiskers depict the 97.5 percentiles. The Wilcoxon rank-sum test was used to compare biomarker values between groups and generate p-values. D-dimer, thrombin-antithrombin, thrombomodulin, soluble intercellular adhesion molecule-1, and soluble vascular cell adhesion molecule-1 were log-transformed to meet model assumptions. Abbreviations: CS = cancer-plus-stroke, SO = stroke-only, TAT = thrombin-antithrombin, sICAM-1 = soluble intercellular adhesion molecule-1, sVCAM-1 = soluble vascular cell adhesion molecule-1.
In multiple linear regression models adjusting for potential confounders, compared to the stroke-only group, the cancer-plus-stroke group was significantly associated with elevated D-dimer, thrombomodulin, sICAM-1, and sVCAM-1, but not thrombin-antithrombin and P-selectin (Table 5). Meanwhile, compared to the cancer-only group, the cancer-plus-stroke group was significantly associated with elevated D-dimer, thrombin-antithrombin, and thrombomodulin, but not P-selectin, sICAM-1, and sVCAM-1.
Table 5.
Multivariable Regression Analyses Comparing Between-Group Biomarker Values
| Biomarkera,b | Cancer-Plus-Stroke vs. Stroke-Only | Cancer-Plus-Stroke vs. Cancer-Only |
|---|---|---|
| Coagulation | ||
| D-dimer | 4.1 (2.3–72) | 1.9 (1.0–3.7) |
| TAT | 1.9 (0.9–3.8) | 3.0 (1.4–6.4) |
| Platelets | ||
| P-selectin | 7.3 (−1.7–16.4) | 7.2 (−3.9–18.3) |
| Endothelium | ||
| Thrombomodulin | 1.4 (1.2–1.6) | 1.2 (1.0–1.4) |
| sICAM-1 | 1.4 (1.1–1.9) | 1.0 (0.7–1.3) |
| sVCAM-1 | 1.4 (1.1–1.6) | 1.1 (0.9–1.4) |
| Embolic disease | ||
| TCD HITS present | 2.2 (0.7–6.3) | 12.7 (2.9–55.4) |
Abbreviations: TAT, thrombin-antithrombin; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; TCD, transcranial Doppler; HITS, high-intensity transient signals.
Multiple linear regression was used to examine the association between study groups and hematological biomarkers while adjusting for race (white vs. nonwhite), number of stroke risk factors (range, 0–10), smoking (any history vs. never), antiplatelet use, treatment-dose anticoagulant use, and NIH stroke scale. Similarly, multiple logistic regression was used to examine the association between study groups and TCD microemboli while adjusting for the number of stroke risk factors, treatment-dose anticoagulant use, and TOAST stroke mechanism (cardioembolic vs. others).
Data reported as beta-coefficients and their 95% confidence intervals for the hematological biomarkers and odds ratio and their 95% confidence interval for the embolic disease marker.
TCD Microemboli
The median time from stroke onset to TCD performance was 4 days (IQR, 2–4) in the cancer-plus-stroke group versus 3 days (IQR, 2–4) in the stroke-only group (p=0.47). In the cancer-only group, the median time from study enrollment to TCD performance was 14 days (IQR, 7–29).
The proportions of participants with any microemboli (i.e., HITS) were significantly different between the three study groups (p=0.004). Microemboli were detected in 32% of cancer-plus-stroke participants, 16% of stroke-only participants, and 6% of cancer-only participants. Bilateral HITS were detected in 8% of participants in the cancer-plus-stroke and stroke-only groups and 0% of participants in the cancer-only group. Among the cancer-plus-stroke group, 38% of participants with cryptogenic stroke mechanisms had HITS versus 27% of those with determined mechanisms (p=0.423). When restricted to the 85% of participants who had sufficient transtemporal acoustic windows for monitoring, microemboli were detected in 36% of cancer-plus-stroke participants, 19% of stroke-only participants, and 8% of cancer-only participants (p=0.005). In direct two-way comparisons, the proportion of participants with microemboli were not significantly different between the cancer-plus-stroke group and the stroke-only group, while the cancer-plus-stroke group had a significantly higher proportion of participants with microemboli than the cancer-only group.
In multiple logistic regression models adjusting for potential confounders, the cancer-plus-stroke group was associated with increased odds of having microemboli as compared to the cancer-only group (odds ratio, 12.7; 95% confidence interval, 2.9–55.4), but not as compared to the stroke-only group (odds ratio, 2.2; 95% confidence interval, 0.7–6.3).
The primary study findings were grossly unchanged in a sensitivity analysis for which the outcome measure was the number of HITS rather than the presence of HITS. When restricting the study cohorts to the cancer-plus-stroke participants and their individually matched controls who had exact matching by cancer type, the cancer-plus-stroke group had a significantly higher proportion of participants with microemboli than the stroke-only group (p=0.045) and the cancer-only group (p=0.001).
Discussion
In a prospective, multicenter, matched study including three groups of 50 patients each, patients with active cancer and acute ischemic stroke had higher levels of coagulation, platelet, and endothelial activation markers, and more circulating microemboli, than control patients with active cancer only or acute ischemic stroke only. Findings were similar in multivariable analyses adjusting for potential confounders. Among the prespecified study biomarkers, D-dimer, a marker of fibrin degradation, demonstrated the strongest association with cancer-related stroke, as median values were more than tripled that of stroke-only and cancer-only controls. TCD HITS, an ultrasound marker of circulating microemboli, were detected in approximately one-third of the cancer-plus-stroke participants, a prevalence twice that of stroke-only participants. These data implicate coagulation, platelet, and endothelial dysfunction, as well as embolic phenomenon, in the pathogenesis of cancer-related stroke.
Few prospective studies have evaluated mechanistic biomarkers in patients with cancer and stroke. The single-center OASIS-Cancer study, conducted in Korea, reported that among 155 patients with cancer-plus-stroke, 25 with stroke-only, 32 with cancer-only, and 101 healthy volunteers, cancer-derived extracellular vesicles were higher in the cancer-plus-stroke group, particularly among those with cryptogenic mechanisms, and vesicle levels correlated with D-dimer.14 In a subgroup analysis restricted to lung cancer patients, extracellular vesicles from adenocarcinomas were associated with shorter clotting times.24 In a separate analysis of 138 total patients, these investigators demonstrated that circulating DNA levels, a marker for NETosis, were increased in patients with cancer-related stroke versus controls.15 NETosis, an innate immune neutrophil function that traps and engulfs pathogens, is believed to promote thrombosis by activating platelets, complement, coagulation factors, and red blood cells.25 A study from Sweden of 31 patients with ischemic stroke, including 8 with cancer, similarly found that NETosis levels were increased in the cancer subgroup.26
Our study builds on these previous data by (1) including a heterogeneous population enrolled from multiple institutions with carefully-selected matched controls, (2) evaluating a broader group of hematological biomarkers that can be analyzed using commercial kits without special processing and therefore may be more scalable and clinically useful than more specialized measures of coagulation abnormalities, and (3) combining hematological and TCD biomarker data to better inform the mechanistic underpinnings of cancer-related stroke. Our findings indicate that besides abnormal coagulation, activation of endothelium and platelets are also associated with cancer-related stroke. Further, our study validates the findings from two Korean studies that reported a substantial proportion of patients with cancer-related stroke to have circulating microemboli.18, 27 One of these single center studies lacked controls altogether, while the other lacked cancer-only controls. In our study, we were able to show that microemboli were more prevalent in cancer-plus-stroke participants than in matched controls.
Collectively, our data indicate that multifactorial prothrombotic processes and embolic sources contribute to the development of ischemic stroke in patients with solid tumor cancer. One possible stroke mechanism that comprises both factors is nonbacterial thrombotic endocarditis (NBTE). NBTE is characterized by sterile platelet-fibrin vegetations on cardiac valves and is considered a direct manifestation of cancer-mediated hypercoagulability.10 However, these vegetations are generally small and consequently are often undetected during life, even by transesophageal echocardiography.28 Other embolic mechanisms previously implicated in cancer-related stroke including large artery and aortic atherosclerosis, paradoxical embolism, tumor embolism, and atrial cardiopathy.10, 29
Our study has limitations. First, it was conducted at academic cancer centers in New York, and therefore the results may not generalize to other settings. Second, while we matched on age, sex, and cancer type, for recruitment reasons, it was impractical to match on other potentially important factors, including vascular risk factors and time from cancer diagnosis (for the cancer groups). Therefore, some baseline characteristics were imbalanced between groups. To address this, we performed multivariable analyses adjusting for factors that might serve as confounders. Third, although our study included 150 participants and was appropriately powered based on preliminary data, some of our multivariable analyses may have been underpowered, so type 2 error is possible. Fourth, while prospective, our study design was cross-sectional and hematological biomarker samples were collected at a single timepoint thereby preventing an assessment of data reproducibility. Further, in the two stroke groups, the samples were examined after the development of the clinical outcome of interest. Additionally, TCD studies were performed once for 30 minutes. While it is possible that longer or repeat studies would have increased the detection of HITS, we chose to perform a single 30-minute study to align with standard clinical practice. Fifth, technicians and neurosonologists were unblinded to patient data and this could have affected their detection of HITS. While possible, we think this is unlikely because we used automated detection algorithms to screen for HITS and all potential HITS were closely reviewed by interpreting neurosonologists according to standardized criteria. Sixth, hematological biomarkers were analyzed in batches and therefore batch effects are possible. However, we repeated analyses on multiple samples and the results were consistent across different batches (data not presented). Seventh, we restricted our study to patients with solid tumor cancers; consequently, our results may not generalize to hematological or primary brain cancers. Eighth, blood draws more often occurred in the morning in the cancer-only group than in the two stroke groups; therefore, circadian factors could have affected the biomarker analyses.
In summary, we found that in a prospective study, patients with active cancer and acute ischemic stroke have higher markers of coagulation, platelet, and endothelial activation, and more circulating microemboli, than matched controls. These data implicate hypercoagulable and embolic processes in the pathogenesis of ischemic stroke in patients with solid tumor cancer. Future studies should evaluate whether these biomarkers can predict the risk of incident and recurrent stroke and the response to antithrombotic treatment in patients with cancer. Meanwhile, clinicians should explore prothrombotic and embolic pathophysiologies in patients with cancer and stroke, especially when the stroke mechanism is undetermined after standard evaluation.
Acknowledgments
This study was supported by the National Institutes of Health grants K23NS091395, P30CA008748, and KL2TR002385 (Weill Cornell Clinical and Translational Science Center).
Footnotes
Potential Conflicts of Interest
None.
References
- 1.Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis 2013;22:1146–50. [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ, Park JH, Lee MJ, et al. Clues to occult cancer in patients with ischemic stroke. PLoS One 2012;7:e44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilbers J, Sondag L, Mulder S, et al. Cancer prevalence higher in stroke patients than in the general population: the Dutch String-of-Pearls Institute (PSI) Stroke study. Eur J Neurol 2020;27:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiyuna F, Sato N, Matsuo R, et al. Association of Embolic Sources With Cause-Specific Functional Outcomes Among Adults With Cryptogenic Stroke. JAMA Netw Open 2018;1:e182953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch HG, Kramer BS, Black WC. Epidemiologic Signatures in Cancer. N Engl J Med 2019;381:1378–86. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020;383:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navi BB, Reiner AS, Kamel H, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navi BB, Howard G, Howard VJ, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. 2018;90:e2025–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navi BB, Iadecola C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann Neurol 2018;83:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke 2014;45:2292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012;43:3029–34. [DOI] [PubMed] [Google Scholar]
- 13.Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 2010;41:798–801. [DOI] [PubMed] [Google Scholar]
- 14.Bang OY, Chung JW, Lee MJ, et al. Cancer Cell-Derived Extracellular Vesicles Are Associated with Coagulopathy Causing Ischemic Stroke via Tissue Factor-Independent Way: The OASIS-CANCER Study. PLoS One 2016;11:e0159170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang OY, Chung JW, Cho YH, et al. Circulating DNAs, a Marker of Neutrophil Extracellular Traposis and Cancer-Related Stroke: The OASIS-Cancer Study. Stroke 2019;50:2944–7. [DOI] [PubMed] [Google Scholar]
- 16.Stefan O, Vera N, Otto B, et al. Stroke in cancer patients: a risk factor analysis. J Neurooncol 2009;94:221–6. [DOI] [PubMed] [Google Scholar]
- 17.Ohara T, Farhoudi M, Bang OY, et al. The emerging value of serum D-dimer measurement in the work-up and management of ischemic stroke. Int J Stroke 2020;15:122–31. [DOI] [PubMed] [Google Scholar]
- 18.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol 2010;68:213–9. [DOI] [PubMed] [Google Scholar]
- 19.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–53. [DOI] [PubMed] [Google Scholar]
- 20.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 2014;83:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobrow D, Kothadia K, Kamel H, et al. Potential hematological biomarkers of stroke in cancer patients. Stroke 2015;46:AWP205. doi: 10.1161/str.46.suppl_1.wp205. [DOI] [Google Scholar]
- 23.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 24.Chung JW, Cho YH, Ahn MJ, et al. Association of Cancer Cell Type and Extracellular Vesicles With Coagulopathy in Patients With Lung Cancer and Stroke. Stroke 2018;49:1282–5. [DOI] [PubMed] [Google Scholar]
- 25.de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol 2019;16:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thalin C, Demers M, Blomgren B, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res 2016;139:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha J, Lee MJ, Kim SJ, et al. Prevalence and Impact of Venous and Arterial Thromboembolism in Patients With Embolic Stroke of Undetermined Source With or Without Active Cancer. J Am Heart Assoc 2019;8:e013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkler AE, Navi BB, Singer S, et al. Diagnostic yield of echocardiography in cancer patients with ischemic stroke. J Neurooncol 2015;123:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang OY, Chung JW, Lee MJ, et al. Cancer-Related Stroke: An Emerging Subtype of Ischemic Stroke with Unique Pathomechanisms. J Stroke 2020;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


