Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a major nonacquired immune deficiency syndrome-defining condition for persons with human immunodeficiency virus (PWH). We aimed to validate noninvasive tests for the diagnosis of NAFLD in PWH.
Methods
This is a cross-sectional study of PWH on stable antiretroviral therapy with persistently elevated transaminases and no known liver disease. The area under the receiver operating characteristic curve (AUROC) was calculated to compare the diagnostic accuracy of liver biopsy with abdominal ultrasound, transient elastography (TE) (including controlled attenuation parameter [CAP]), and noninvasive markers of steatosis (triglyceride and glucose index [TyG], hepatic steatosis index [HSI], fatty liver index [FLI]) and fibrosis ([FIB]-4, aminotransferase-to-platelet ratio index [APRI], NAFLD fibrosis score). We developed a diagnostic algorithm with serial combinations of markers.
Results
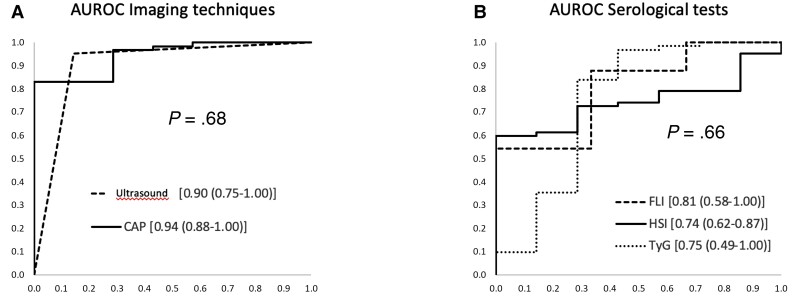
Of 146 patients with increased transaminase levels, 69 underwent liver biopsy (90% steatosis, 61% steatohepatitis, and 4% F ≥3). The AUROC for steatosis was as follows: ultrasound, 0.90 (0.75–1); CAP, 0.94 (0.88–1); FLI, 0.81 (0.58–1); HSI, 0.74 (0.62–0.87); and TyG, 0.75 (0.49–1). For liver fibrosis ≥F3, the AUROC for TE, APRI, FIB-4, and NAFLD fibrosis score was 0.92 (0.82–1), 0.96 (0.90–1), 0.97 (0.93–1), and 0.85 (0.68–1). Optimal diagnostic performance for liver steatosis was for 2 noninvasive combined models of tests with TyG and FLI/HSI as the first tests and ultrasound or CAP as the second tests: AUROC = 0.99 (0.97–1, P < .001) and 0.92 (0.77–1, P < .001).
Conclusions
Ultrasound and CAP performed best in diagnosing liver steatosis, and FLI, TyG, and HSI performed well. We propose an easy-to-implement algorithm with TyG or FLI as the first test and ultrasound or CAP as the second test to accurately diagnose or exclude NAFLD.
Keywords: HIV, liver biopsy, liver fibrosis, liver steatosis, noninvasive markers
An easy-to-implement algorithm diminishes diagnostic uncertainty and the likelihood of diagnostic errors, thus eventually reducing the need for liver biopsy. Ultrasound and controlled attenuation parameter perform very well in diagnosing liver steatosis in persons with HIV.
Acquired immune deficiency syndrome (AIDS)-related mortality in persons with human immunodeficiency virus (PWH) has fallen considerably in the antiretroviral therapy (ART) era, whereas non-AIDS-associated comorbidity is increasing [1]. Liver disease remains prevalent despite the high percentage of patients cured of hepatitis C with direct-acting antivirals and the excellent control of replication of hepatitis B virus with nucleot(s)ide analogs [2]. This continued prevalence is because nonalcoholic fatty liver disease (NAFLD) affects 10%–30% of PWH, often leading to persistent hypertransaminasemia without chronic viral hepatitis or excessive alcohol consumption [3–5].
Nonalcoholic fatty liver disease includes various histopathologic entities: hepatosteatosis, nonalcoholic steatohepatitis (NASH), and liver fibrosis, which can present simultaneously. Nonalcoholic steatohepatitis is characterized by inflammation with(out) hepatocyte ballooning and is associated with fibrosis and cirrhosis [6]. The presence of NAFLD is associated with increased morbidity and mortality and a higher prevalence of cardiovascular risk factors, especially those related to insulin resistance [7]. In addition to cardiovascular disease, NASH and fibrosis result in liver-specific morbidity and mortality resulting from cirrhosis and hepatocellular carcinoma [8].
Liver biopsy is the gold standard diagnostic test for NAFLD, NASH, and fibrosis. However, it is invasive, expensive, and cumbersome in clinical practice. As a result, several noninvasive methods have been developed. Steatosis can be diagnosed using validated imaging techniques, namely, liver ultrasound, the controlled attenuation parameter (CAP) (FibroScan), and magnetic resonance imaging proton-density-fat-fraction (MRI-PDFF). However, these techniques are not always available in routine practice and are poorly validated among PWH [8]. Other proposed serum markers, such as the fatty liver index (FLI), hepatic steatosis index (HSI), and triglyceride and glucose index (TyG), have not been explored in PWH.
Diagnosis and staging of liver fibrosis caused by NAFLD among PWH have received little attention. A recent report from the ECHAM (European Cohort HIV Ageing and Metabolic liver disease) cohort, in which the authors validated the aspartate aminotransferase-to-platelet ratio index (APRI) and transient elastography (TE) for fibrosis, found worse results with TE [8]. Other widely used indexes in non-human immunodeficiency virus (HIV)-infected patients include the Fibrosis 4 score (FIB-4) and the NAFLD fibrosis score (NFS), which have not been explored among PWH with NAFLD.
The objectives of our study were to compare various tests for diagnosis of steatosis and fibrosis with liver biopsy in PWH with increased transaminase levels. We also aimed to develop an algorithm to improve the diagnosis of steatosis in this population using techniques that are readily available in daily clinical practice.
METHODS
Study Design and Setting
Participants and Protocol
The study population included all PWH seen at Hospital Universitario La Paz, Madrid, Spain with increased transaminase levels for ≥6 months (confirmed by ≥2 samples) from January 2017 to June 2018. Other inclusion criteria were being on stable combination ART and having HIV-ribonucleic acid <50 copies/mL for ≥1 year. The exclusion criteria were past or present chronic HBV or active HCV coinfection, high alcohol consumption (>30 g/day in men or ≥20 g/day in women), potential drug hepatotoxicity, and other liver diseases.
Patient Consent Statement
Participants were enrolled in the study after providing their written informed consent. The study was approved by the local ethics committee (Comité Ético de Investigación Clínica del Hospital Universitario La Paz [Ethics Committee of Hospital Universitario La Paz]) and conducted according to the Declaration of Helsinki.
All patients underwent screening for autoimmune, genetic, and metabolic liver disease. Fatty liver index, HSI, TyG, FIB-4, and APRI were calculated for all patients, who also underwent liver ultrasound and measurement of liver stiffness and steatosis by TE and CAP. Liver biopsy was offered to all patients according to the European Association for the Study of the Liver (EASL)-European Association for the Study of Diabetes guidelines [6]. We report data from a cross-sectional analysis of participants who agreed to undergo liver biopsy and the other diagnostic tests.
Variables and Measurements
Demographic, clinical, laboratory, and HIV-related data (eg, time since diagnosis and exposure to ART) were collected [9–12]. Steatosis and fibrosis were diagnosed using each technique based on previously validated cutoffs for the general population (Supplementary material).
Noninvasive Markers of Liver Steatosis
The biochemical indexes calculated were TyG, HSI, and FLI. Steatosis was defined as TyG >8.38, HSI >36, and FLI >60 [13,14] (Supplementary material).
Noninvasive Markers of Liver Fibrosis
The biochemical indexes calculated were APRI, FIB-4, and NFS. Fibrosis was considered significant (F ≥2) if the FIB-4 was >1.3 or APRI was >0.5; advanced liver fibrosis (F ≥3) was defined as FIB-4 ≥2.67 or APRI >1.5 [15]. An NFS lower than −1.45 indicates absence of significant fibrosis (F ≥2); scores from −1.45 to 0.675 are indeterminate; and scores greater than 0.675 indicate the presence of advanced fibrosis (F ≥3) [16] (Supplementary material).
Liver Ultrasound
Fasting real-time ultrasound was performed using a Canon Aplio 500 Platinum device. Ultrasound results were categorized as presence or absence of hepatic steatosis by one of the investigators (A.O.), who was blind to TE findings [17] (Supplementary material).
Transient Elastography
Transient elastography was performed under fasting conditions using a FibroScan® device (Probe M, FS402; Echosens, Paris, France, www.echosens.com) with measurement of CAP. The cutoff value for diagnosis of steatosis was >238 dB/m [18]. An experienced operator, who was blind to the liver ultrasound diagnosis, performed TE according to the manufacturer’s protocol: (F ≥2) if TE ≥7.0 kPa and advanced (F ≥3) if >9.6 kPa [–15,19,21] (Supplementary material).
Liver Biopsy
Ultrasound-guided percutaneous liver biopsy was performed with a 16-gauge needle. Liver histology was interpreted by 2 experienced liver pathologists (L.G. and C.M.), who were blind to clinical data (including noninvasive test results). Only liver biopsies with >11 portal spaces were considered valid for the study [22] (Supplementary material).
Statistical Analysis
Variables were summarized as proportions for categorical variables and median and interquartile range for continuous variables. Continuous variables were analyzed using the t test or Mann-Whitney test, depending on the normality of the distribution (Kolmogorov-Smirnov test with a Lilliefors correction).
Nonparametric receiver operating characteristic curve (ROC) analysis considering the results obtained in the biopsy as “gold standard” was conducted to assess the performance of ultrasound, CAP, TyG, HSI, and FLI for the diagnosis of steatosis; TE, APRI, FIB-4, and NFS were used for the diagnosis of fibrosis. The optimal cutoff for each noninvasive marker was defined. The rule-in and rule-out points of the serological markers were used to build the diagnostic algorithms to confirm or rule out steatosis (Supplementary material).
RESULTS
Study Population
Of the 146 patients with persistently elevated transaminases, 69 underwent liver biopsy, (91% men, 82% white, median age 49 [41–54] years). Table 1 shows the findings for patients who underwent and did not undergo biopsy. These were similar for all the general characteristics, except CD4 lymphocyte count (which was higher in those who underwent biopsy) and for body mass index (BMI), diagnosis of diabetes mellitus (DM)/impaired fasting glucose, and arterial hypertension, which were more prevalent in patients who underwent biopsy.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics of the Study Population | Total Study Population | Patients Who Did Not Undergo LB | Patients Who Underwent LB | P Value |
|---|---|---|---|---|
| n = 146 | n = 77 | n = 69 | ||
| Age (years)a | 49 (41–54) | 46 (39–54) | 50 (44–54) | .13 |
| Female sex, n (%) | 13 (9) | 8 (10) | 5 (7) | .57 |
| Ethnicity, n (%) | .42 | |||
| White | 109 (82) | 54 (77) | 55 (87) | |
| Black | 5 (4) | 3 (4) | 2 (3) | |
| Asian | 1 (1) | 1 (1) | 0 (0) | |
| Hispanic/Latino | 18 (14) | 12 (17) | 6 (10) | |
| Stage C AIDS, n (%) | 20 (16) | 9 (13) | 11 (20) | .65 |
| Transmission route, n (%) | ||||
| MSM | 90 (66) | 46 (61) | 44 (72) | .73 |
| MSW | 36 (27) | 23 (31) | 13 (21) | .13 |
| IDU | 9 (7) | 4 (5) | 5 (8) | .74 |
| Transfusion/Hemophilia | 6 (4) | 3 (4) | 3 (5) | 1.00 |
| Unknown | 3 (2) | 1 (1) | 2 (3) | .60 |
| HIV viral load <50 copies/mL, n (%) | 146 (100) | 77 (100) | 69 (100) | |
| Time with HIV infection (years)a | 11 (7–20) | 11 (7–19) | 14 (7–21) | .23 |
| CD4 cell count (cells/µL)a | 740 (593–930) | 674 (560–850) | 829 (650–980) | .002 |
| Nadir CD4 cell count (cells/µL)a | 265 (178–375) | 260 (157–340) | 270 (178–420) | .21 |
| ART on Treatment, n (%) | ||||
| 2 NRTI + RPV | 45 (31) | 24 (31) | 21 (30) | .30 |
| 2 NRTI + INI | 40 (27) | 21 (27) | 19 (27) | .60 |
| PI-based regimenb | 18 (12) | 8 (10) | 10 (14) | .46 |
| 2 NRTI + EFV | 18 (12) | 12 (16) | 8 (9) | .31 |
| Other | 27 (18) | 12 (16) | 11 (16) | .54 |
| Duration of cumulative exposure to ART (years) | 11 (5.7–18.5) | |||
| Duration of cumulative exposure to current ART (months) | 16 (8–33) | |||
| Duration of Cumulative Exposure to ART by Drug (months) | ||||
| EFV | 56 (21–92) | 64 (21–97) | 48 (26–76) | .50 |
| RAL | 20 (7–52) | 49 (13–61) | 15 (6–36) | .13 |
| RPV | 31 (10–43) | 35 (6–47) | 29 (13–41) | .70 |
| BMI (kg/m2)a | 27 (24–30) | 26 (24–29) | 29 (25–31) | .001 |
| Waist circumference (cm) | 99 (90–108) | 94 (87–100) | 101 (92–110) | <.001 |
| Waist-hip index | 1 (1–1) | 1 (1–1) | 1 (1–1) | .010 |
| AST (IU/L)a | 36 (28–43) | 34 (26–42) | 37 (29–47) | .16 |
| ALT (IU/L)a | 50 (41–77) | 46 (39–67) | 57 (43–83) | .03 |
| GGT (IU/L)a | 47 (31–98) | 45 (30–89) | 50 (31–118) | .57 |
| Glucose (mg/dL)a | 97 (90–105) | 94 (87–98) | 102 (95–109) | <.001 |
| Cholesterol (mg/dL)a | 182 (159–203) | 189 (163–204) | 177 (156–202) | .12 |
| LDL-C (mg/dL)a | 110 (92–126) | 113 (95–130) | 106 (92–124) | .27 |
| HDL-C (mg/dL)a | 39 (33–48) | 41 (34–54) | 38 (33–45) | .05 |
| Alcohol consumptionc, n (%) | 10 (7) | 6 (8.5) | 4 (6) | .75 |
| Triglycerides (mg/dL)a | 147 (97–213) | 131 (94–201) | 158 (110–227) | .16 |
| Diabetes mellitus or abnormal fasting glucose, n (%) | 63 (44) | 18 (23) | 45 (65) | <.001 |
| Arterial hypertension, n (%) | 57 (39) | 18 (23) | 39 (57) | <.001 |
| Dyslipidemia, n (%) | ||||
| Hypercholesterolemia, n (%) | 71 (49) | 38 (49) | 33 (48) | .87 |
| Hypertriglyceridemia, n (%) | 74 (51) | 34 (44) | 40 (58) | .10 |
| Mixed, n (%) | 71 (68) | 38 (72) | 33 (65) | .53 |
| Cardiovascular events, n (%)d | 2 (1) | 1 (1) | 1 (1) | 1.00 |
| Metabolic syndrome, n (%) | 60 (41) | 18 (23) | 42 (61) | <.001 |
| Lipid-lowering drugs, n (%) | 56 (38) | 27 (35) | 29 (42) | .40 |
| Glucose-lowering drugs, n (%) Metformin | 20 (14) | 3 (4) | 17 (25) | <.001 |
| Insulin | 2 (1) | 0 (0) | 2 (3) | .22 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ALT, alanine aminotransferase; ART, antiretroviral therapy; AST, aspartate aminotransferase; BMI, body mass index; EFV, efavirenz; GGT, gamma glutamyl transferase; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; IDU, injection drug user; INI, integrase inhibitor; LDL-C, low-density lipoprotein cholesterol; MSM, men who have sex with men; MSW, male sex worker; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; RAL, raltegravir; RPV, rilpivirine.
Median (interquartile range).
PI-based regimens: cobicistat-boosted darunavir (DRV/c) monotherapy, 5 subjects; lamivudine (3TC) or DTG or RAL+ DRV/c 4 subjects; tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) + DRV/c, 1 subject.
Alcohol consumption (>20 g/d [women], >30 g/d [men]).
Cardiovascular events: ischemic heart disease and ischemic stroke.
All the patients were receiving antiretroviral treatment and had an undetectable viral load. The most frequent antiretroviral regimen was the combination of 2 nonnucleoside reverse-transcriptase inhibitors (NNRTIs) + rilpivirine (31%), followed by 2 NNRTIs + integrase inhibitor (27%); regimens based on protease inhibitors or efavirenz were administered in 12% of patients (Table 1). No differences were found for the distribution of the current antiretroviral combinations between patients who underwent and did not undergo biopsy. The analysis of the history of antiretroviral therapy revealed that patients who underwent biopsy had received zidovudine (AZT) (35% vs 16% [P = .01]) and stavudine (d4T) (35% vs 21% [P = .06]) more frequently than those who did not. No differences were found between the groups for the remaining previously used antiretroviral drugs.
Histopathological Findings
Steatosis affected 62 of the 69 patients who underwent liver biopsy (89.9%) (mild, 33 [53.2%]; moderate, 16 [25.8%]; severe, 13 [21%]). Nonalcoholic steatohepatitis was diagnosed in 38 cases (61.3%). The NAFLD Activity Score ≥5 was found in 20.7% of patients with steatosis and fibrosis in 21 (mild [F1–F2], 18 [26.1%]; severe [ > F3], 3 [4.3%]).
Of the 7 patients without steatosis, one was diagnosed with central veno-occlusive disease (fibrosis grade 1A), and another with cryptogenic hepatitis that was healing. Liver biopsy was normal in the remaining 5 patients (7.2%). Four patients were diagnosed with a concomitant condition (5.8%) (3 hemochromatosis and 1 primary biliary cholangitis) (Table 2).
Table 2.
Histological Findings and Noninvasive Tests Results
| Diagnosis | n; N (%) |
|---|---|
| Histological findings | |
| Steatosis | |
| No | 7; 69 (10.1) |
| Yes (any degree) | 62; 69 (89.9) |
| Mild (<33%) | 33; 62 (53.2) |
| Moderate (33–66%) | 16; 62 (25.8) |
| Severe (>66%) | 13; 62 (21) |
| Steatohepatitis (steatosis only) | 38; 62 (61.3) |
| Ballooning (steatohepatitis only) | 29; 38 (76.3) |
| Kleiner (NAS score)a (steatosis only) | |
| ≤2 | 19; 58 (32.8) |
| 3–4 | 27; 58 (46.6) |
| ≥5 | 12; 58 (20.7) |
| Fibrosis | |
| No | 48; 69 (68.1) |
| F1 | 18; 69 (26.1) |
| F2 | 1; 69 (1.4) |
| F≥3 | 3; 69 (4.3) |
| Other diagnosisb | 6c; 69 (8.7) |
| Noninvasive Markers | |
| Steatosis (Any Degree) | |
| US (100%)c | 60; 69 (87) |
| CAP ≥ 238 (96%)c | 54; 66 (81.8) |
| TyG >8.38(100%)c | 61; 69 (88.4) |
| HSI >36 (100%)c | 62; 69 (89.9) |
| FLI >60 (87%)c | 41; 60 (68.3) |
| Significant Fibrosis (≥F2) | |
| TE ≥7.0 (98%)c | 26; 68 (38.2) |
| FIB-4 >1.3 (100%)c | 17; 69 (24.6) |
| APRI >0.5 (100%)c | 22; 69 (31.9) |
| NAFLD > −1.45 (93%)c | 30; 64 (46.9) |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; CAP, controlled attenuation parameter; FIB-4, Fibrosis 4 score; FLI, fatty liver index; HSI, hepatic steatosis index; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; TE, transient elastography; TyG, triglyceride and glucose index; US, ultrasound.
Kleiner: NAS, 0–8: ≤2: no nonalcoholic steatohepatitis (NASH), 3–4: indeterminate, ≥5: likely or definitive NASH [22]. This score is calculated in patients with liver steatosis (n = 62); the Kleiner score was not available in 4 subjects.
Hemochromatosis, 3; primary biliary cirrhosis, 1; central veno-occlusive disease, 1; cryptogenic hepatitis, 1.
Applicability rate.
Noninvasive Markers of Steatosis
Liver steatosis was observed on ultrasound in 60 patients (87%). Transient elastography with CAP was performed in 66 patients (except 3 owing to poor visualization), 82% of whom had values compatible with steatosis. According to serologic indices, liver steatosis affected 68%–89% of patients (Table 2).
Noninvasive Markers of Liver Fibrosis
Of the 68 patients who underwent TE (except 1 owing to poor visualization), 20 (29%) had significant fibrosis; 13 (19%) of these had advanced fibrosis (F ≥3). The noninvasive serologic markers of liver fibrosis APRI and FIB-4 ruled out significant fibrosis in 68% and 75% of patients, respectively; the NFS ruled out disease in 53%. Four patients had advanced fibrosis (F ≥3) by FIB-4 (4.3%), 2 by APRI (2.9%), and 6 (6.2%) by NFS (Table 2).
Diagnostic Accuracy of Noninvasive Markers of Liver Steatosis
Ultrasound and CAP performed optimally in the diagnosis of liver steatosis and liver stiffness, with an area under the ROC curve (AUROC) of 0.9 (95% confidence interval [CI], 0.75–1; P = .02) and 0.94 (95% CI, 0.88–1; P < .001), respectively (Figure 1A, Table 3). The AUROC for FLI was high (0.81; 95% CI, 0.58–1), although not statistically significant. The AUROC for HSI and TyG was 0.74 (95% CI, 0.62–0.87; P <.03) and 0.75 (95% CI, 0.49–1; P < .05), respectively. We did not find statistically significant differences between the AUROCs (Figure 1B, Table 3).
Figure 1.
Area under the receiver operating characteristic curve (AUROC) of noninvasive tests for liver steatosis. A, AUROC for imaging techniques. B, AUROC for serological tests. CAP, controlled attenuation parameter; FLI, fatty liver index; HSI, hepatic steatosis index; TyG, triglyceride and glucose index.
Table 3.
Accuracy of Noninvasive Markers for the Diagnosis of Steatosis in Comparison With Liver Biopsy
| Tools | AUC (95% CI) | P Value | Cutoff | Sens % | Spec % | PPV % | NPV % | LR+ | LR− | Accuracy % |
|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasound | 0.90 (0.75–1) | .002 | Yes | 95 | 86 | 98 | 67 | 6.66 | 0.06 | 94 |
| CAP | 0.94 (0.88–1) | <.001 | ≥238a | 88 | 71 | 96 | 42 | 3.08 | 0.17 | 86 |
| >254b | 83 | 100 | 100 | 41 | ∞ | 0.17 | 85 | |||
| >218c | 97 | 71 | 97 | 71 | 3.38 | 0.05 | 94 | |||
| FLI | 0.81 (0.58–1) | .075 | >60a | 70 | 67 | 98 | 11 | 2.11 | 0.45 | 70 |
| ≥76.5b | 54 | 100 | 100 | 10 | ∞ | 0.46 | 57 | |||
| ≥30c | 95 | 33 | 96 | 25 | 1.42 | 0.16 | 92 | |||
| ≥41.3d | 88 | 67 | 98 | 22 | 2.63 | 0.18 | 87 | |||
| HSI | 0.74 (0.6–0.87) | .035 | >36a | 90 | 14 | 90 | 14 | 1.05 | 0.68 | 83 |
| ≥41.3b,d | 60 | 100 | 100 | 22 | ∞ | 0.4 | 64 | |||
| ≥ 35c | 94 | 14 | 91 | 20 | 1.09 | 0.45 | 86 | |||
| TyG | 0.75 (0.49–1) | .032 | >8.38a | 94 | 57 | 95 | 50 | 2.18 | 0.11 | 90 |
| ≥9.75b | 10 | 100 | 100 | 11 | ∞ | 0.9 | 19 | |||
| >8.38me | 98 | 57 | 95 | 80 | 2.3 | 0.03 | 94 | |||
| ≥8.58d | 84 | 71 | 96 | 33 | 2.94 | 0.23 | 83 | |||
| Models | ||||||||||
| 1. TyG/FLI/CAP; ECO | 0.99 (0.97–1) | <.001 | (1) | 98 | 100 | 100 | 86 | ∞ | 0.02 | 98 |
| 2. TyG/HSI/CAP; ECO | 0.92 (0.77–1) | <.001 | (2) | 98 | 86 | 98 | 86 | 6.89 | 0.02 | 97 |
Abbreviations: AUC, area under the curve; CAP, controlled attenuation parameter; CI, confidence interval; ECO, ultrasound; FIB-4, Fibrosis 4 score; FLI, fatty liver index; HSI, hepatic steatosis index; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; TyG, triglyceride and glucose index.
Literature reference point.
Rule-in.
Rule-out.
Optimum point established by the Youden index.
8.38m: TyG >8.38 or TyG <8.38 with hypoglycemic and/or lipid-lowering treatment.
(1) FLI ≥76.5 or FLI <76.5 and TyG >8.38 with ultrasound (US) steatosis or CAP >203.
(2) HSI ≥41.3 or HSI <41.3 and TyG >8.38 with US steatosis or CAP >213.
We analyzed the diagnostic yield of all the tests using current cutoffs for each and those obtained using the Youden index. Sensitivity was >0.85 for all except HSI. Specificity was lower overall and improved in all tests, except for ultrasound, after modification of the cutoffs. With a prevalence of steatosis (any grade) of 90%, the values FLI ≥41.3, HSI >41.3, and TyG ≥8.58 had a positive predictive value >95% for predicting steatosis (Table 3).
Diagnostic Accuracy of Noninvasive Markers of Liver Fibrosis
The diagnostic yield of APRI, FIB-4, and TE for any grade of fibrosis (F ≥1) was not very high because the AUROCs were 0.76 (95% CI, 0.63–0.89; P = .001), 0.76 (95% CI, 0.65–0.87; P = .001), and 0.72 (95% CI, 0.59–0.85; P = .005), respectively. Similarly, the AUROC for the NFS was low (AUROC, 0.69 [95% CI, 0.56–0.82; P = .01]). In the case of advanced fibrosis (F ≥3), APRI, FIB-4, and TE yielded very high AUROCs: 0.95 (95% CI, 0.9–1; P < .01), 0.97 (95% CI, 0.93–1; P <.01), and 0.92 (95% CI, 0.82–1; P < .05), whereas that of the NFS was lower (0.85; 95% CI, 0.68–1; P = .09). No further analyses were performed owing to the low percentage of patients with advanced fibrosis in the cohort (4.3%).
Diagnostic Performance of Combined Noninvasive Steatosis Tools
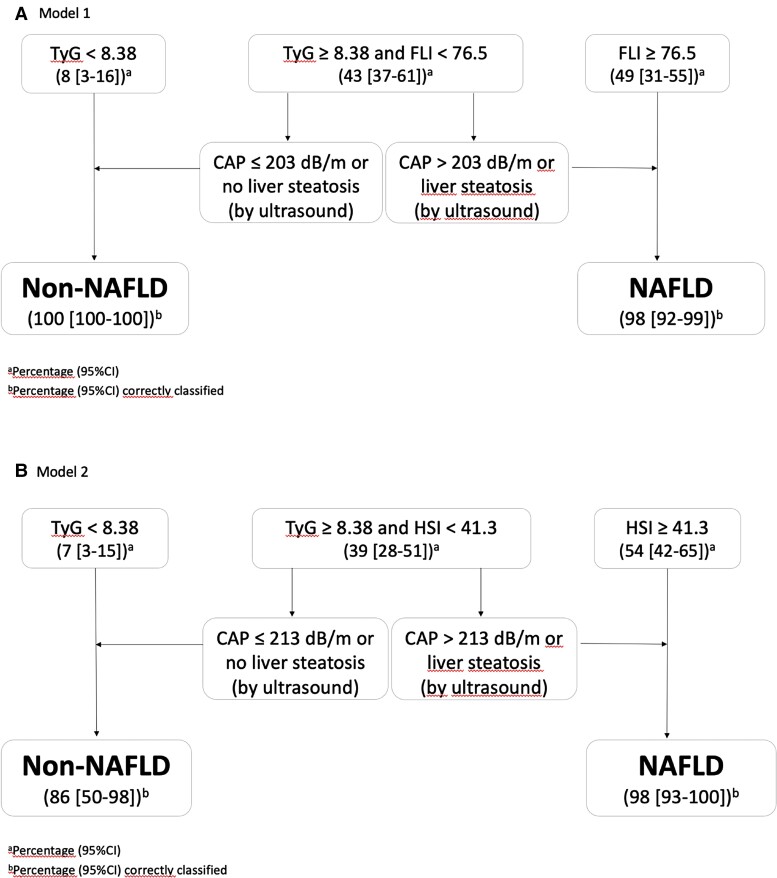
Given that the AUROC of the serologic markers was lower than for ultrasound and CAP, we explored the yield of combining several of these tests sequentially. Two combination models were developed. In the first, we performed TyG or FLI followed by ultrasound or CAP when the previous results were inconclusive. In the second, we replaced FLI with HSI to facilitate applicability, because anthropometric data are not always available (Figure 2).
Figure 2.
Diagnostic algorithm models for the diagnosis of nonalcoholic fatty liver disease (NAFLD). A, Model 1. B, Model 2. CAP, controlled attenuation parameter; CI, confidence interval; HSI, hepatic steatosis index; TyG, triglyceride and glucose index; TyGm, TyG <8.38 without hypoglycemic and/or lipid-lowering treatment.
We selected a TyG cutoff of ≤8.38 in patients who had not received glucose- or lipid-lowering agents, because TyG is calculated based on fasting glucose and triglyceride values. In addition, low values could affect the sensitivity of the test in patients taking glucose- and/or lipid-lowering agents. We adopted this approach to rule out the presence of NAFLD and either HSI ≥41.3 or FLI ≥76.5 (rule-in cutoff) and thus confirm NAFLD. In the case of TyG values >8.38 or ≤8.38 in patients treated with lipid-lowering agents or statins and HSI <41.3 or FLI <76.5, we identified a gray area, where it is necessary to perform a third, nonbiochemical test. We evaluated abdominal ultrasound and CAP, finding no differences between them; therefore, application of one or the other test depends on local availability. In CAP, new optimal cutoff points were defined for diagnosis of steatosis in each model (203 dB/m for FLI; 213 dB/m for HSI) (Table 3, Figure 2).
With the aim of improving the diagnostic yield of the tests different combinations were studied. Combining the serological and image non-invasive tests in sequence, revealed a high diagnostic yield for both models, with 4 AUROCs of 0.99 (P < .001) and 0.92 (P < .001), respectively, and excellent positive and negative 5 likelihood ratios (Table 3). Our models revealed that only 40% of patients would require ultrasound or CAP to rule out NAFLD in 86%–100% of patients and confirm the diagnosis of NAFLD in 100% (Figure 2).
DISCUSSION
We found the performance of ultrasound and CAP to be excellent for diagnosis of liver steatosis (any grade). We also studied, for the first time, 3 highly applicable noninvasive serological tests—FLI, TyG, and HSI—in PWH and found that they performed well for diagnosis of liver steatosis. Finally, because ultrasound and CAP are not as accessible as serological tests in clinical practice, we sought an easy-to-apply and cost-effective approach for diagnosis of steatosis. Therefore, we propose serial combination of 2 or 3 noninvasive tests to facilitate diagnosis of NAFLD in PWH.
Abdominal ultrasound is the most commonly used technique for diagnosis of steatosis in our setting. However, very few studies have compared ultrasound with liver biopsy in PWH. Our results show excellent accuracy, with an AUROC of 0.90 for identification of steatosis (any grade) (sensitivity, 95%; specificity, 86% [P < .05]). Of note, sensitivity for diagnosis of steatosis (any grade) is greater in PWH than in the general population, ranging from 71% to 85% depending on the series. We believe this could be explained by lower BMI values in PWH [23].
Controlled attenuation parameter is a simple, rapid, nonoperator-dependent procedure that quantifies liver steatosis during measurement of liver stiffness. Macías et al [22] and Vuille-Lessard et al [23] were the first authors to study the prevalence of steatosis in PWH. They used TE/CAP with a cutoff of 238 dB/m for diagnosis of steatosis (any grade), which was subsequently validated by Sasso et al [24] using dual-energy x-ray absorptiometry. For the first time, we validated this cutoff of 238 dB/m using liver biopsy as the gold standard and obtained an AUROC of 0.94 for any grade of steatosis. Lemoine et al [9] recently compared TE/CAP and MRI-PDFF with liver biopsy for diagnosis of NAFLD in PWH (median age of 40 years and well controlled infection). The authors found both methods to be highly accurate, with results similar to ours (AUROC 0.87), although the cutoff selected by Lemoine et al [9] (280 dB/m) was for the diagnosis of moderate-to-severe steatosis. In contrast, we selected a cutoff for any grade of steatosis, because our biopsy findings showed that NASH could be found even in mild forms and that patients with NASH have the highest risk of progression of liver disease.
Despite the favorable results reported for ultrasound and CAP in the diagnosis of NAFLD in PWH, we are aware that these tests are not universally available and that saturation of health systems makes it difficult to perform them quickly. We examined 3 easy-to-apply scores that had previously been validated in non-HIV-infected patients: FLI and HSI (recommended in guidelines) and TyG (which does not require anthropometric data) [13]. Compared with liver biopsy, our approach was accurate, although inferior to ultrasound and CAP. We determined new cutoffs that improved the diagnostic characteristics and established rule-in rule-out values to maximize the usefulness of each of the 3 scores. Our models for diagnosis of NAFLD confirm or rule out this diagnosis in more than half of patients. Furthermore, given that our results support the similar and high diagnostic yield of FibroScan/CAP with ultrasound, centers could use their most accessible and/or available method. A recently published diagnostic algorithm for NAFLD and NASH applied 2 simultaneous or sequential noninvasive methods to obtain the optimal diagnostic yield [26]. In contrast with our proposal, which focuses on identifying patients with NAFLD, Altamirano et al [26] prioritize the diagnosis of fibrosis and grade of fibrosis, followed by analysis of the probability of NAFLD and NASH. However, we believe that a diagnosis of NAFLD is very important, even in the absence of fibrosis, because prognosis in persons with NAFLD is determined by cardiovascular morbidity and mortality and not by hepatic morbidity and mortality.
In 2019, Sebastiani et al [27] applied the EASL diagnostic algorithm to 3 cohorts of PWH and found that 1 in 5 patients would benefit from screening for NAFLD and that 1 in 10 fulfilled the criteria for referral to a hepatologist. However, this algorithm was applied to patients who already had obesity and/or diabetes. Our cohort included a large percentage of patients with NAFLD who were not obese and did not have DM, thus highlighting the need for a less restrictive strategy. We recorded a high percentage of impaired fasting glucose in patients who already had NAFLD, thus suggesting the need to screen for NAFLD in PWH, even in the absence of traditional cardiovascular risk factors or obesity, and to tackle the diagnosis of NAFLD as both a cause of liver disease and yet another indicator of cardiovascular risk.
Although our techniques were not as successful for diagnosis of liver fibrosis (any grade), the results are similar to those found for viral hepatitis and significant fibrosis [28]. Accuracy increased considerably for diagnosis of severe fibrosis with APRI, FIB-4, TE, and the NFS (0.95, 0.97, 0.92, and 0.85, respectively). In a recent report, Lemoine et al [9] observed that both APRI and FIB-4 had an AUROC >80% for fibrosis ≥2, although their study revealed poorer results for TE (AUROC 0.61). Our results show high applicability for TE (>95%), probably because PWH had a lower BMI, thus potentially explaining our better results.
Our study is subject to a series of limitations. First, given that patients with worse metabolic profiles were significantly represented, our findings could lack external validity. This overrepresentation could be because patients with metabolic syndrome were more concerned about the health problem of NAFLD and more readily agreed to liver biopsy. Nevertheless, we believe that the population included in the study shows the type of patients most frequently managed in the HIV clinic, ie, with grade 1–2 elevated transaminases and no other obvious causes of liver damage. Second, very few biopsies revealed advanced liver fibrosis, thus preventing us from conclusively validating noninvasive markers of fibrosis in this subgroup. Third, the lack of biopsy specimens meant that the performance of the algorithms proposed could change according to the clinical, biochemical, and metabolic characteristics of patients and the prevalence of steatosis. Our strategies should be validated in other PWH populations where the prevalence of NAFLD is low and in patients with a higher prevalence of advanced fibrosis.
In contrast with imaging-based methods of diagnosis of steatosis, eg, ultrasound, CAP, and magnetic resonance imaging, our study validates for the first time in PWH noninvasive serological biochemical markers of steatosis used previously in the general population. Although the yield was not excellent on an individual basis, these markers are easy to use and useful when combined for diagnosis of NAFLD in PWH.
CONCLUSIONS
In conclusion, ultrasound and CAP performed best for diagnosis of liver steatosis of any grade, whereas FLI, TyG, and HSI had an acceptable AUROC compared with biopsy. We propose an easy-to-implement algorithm with TyG or FLI as the first test and ultrasound or CAP as the second for accurate diagnosis or exclusion of NAFLD. Our approach also diminishes diagnostic uncertainty and the likelihood of diagnostic errors, thus eventually reducing the need for liver biopsy.
Supplementary Material
Acknowledgments
We are grateful to Ana Delgado for data management and Lucía Serrano for the statistical analysis. We acknowledge Mr. Thomas O’Boyle for writing assistance.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by grants from Instituto de Salud Carlos III (ISCIII; Grant Number PI17/01218) as part of the Plan Nacional R + D + I.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
C Busca, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
M Sánchez-Conde, Unidad de VIH, Servicio Enfermedades Infecciosas, Hospital Ramón y Cajal, Madrid, Spain.
M Rico, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
M Rosas, Unidad de VIH, Servicio Enfermedades Infecciosas, Hospital Ramón y Cajal, Madrid, Spain.
E Valencia, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
A Moreno, Unidad de VIH, Servicio Enfermedades Infecciosas, Hospital Ramón y Cajal, Madrid, Spain.
V Moreno, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
L Martín-Carbonero, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
S Moreno, Unidad de VIH, Servicio Enfermedades Infecciosas, Hospital Ramón y Cajal, Madrid, Spain.
I Pérez-Valero, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
J I Bernardino, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
J R Arribas, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
J González, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
A Olveira, Unidad Hepatología, Servicio Gastroenterología, Hospital Universitario La Paz, Madrid, Spain.
P Castillo, Unidad Hepatología, Servicio Gastroenterología, Hospital Universitario La Paz, Madrid, Spain.
M Abadía, Unidad Hepatología, Servicio Gastroenterología, Hospital Universitario La Paz, Madrid, Spain.
L Guerra, Unidad de Hígado, Servicio de Anatomía Patológica, Hospital Universitario La Paz, Madrid, Spain.
C Mendez, Unidad de Hígado, Servicio de Anatomía Patológica, Hospital Universitario La Paz, Madrid, Spain.
M L Montes, Unidad de VIH, Servicio de Medicina Interna, Hospital Universitario La Paz, IdiPAZ, Madrid, Spain.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 2. Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol 2010; 8:1002–12. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maurice JB, Patel A, Scott AJ, et al. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. doi: 10.1097/QAD.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 4. Fourman LT, Stanley TL, Zheng I, et al. Clinical predictors of liver fibrosis presence and progression in human immunodeficiency virus–associated nonalcoholic fatty liver disease. Clin Infect Dis 2020; 72:2087–94. doi: 10.1093/cid/ciaa382/5818260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paik JM, Henry L, Golabi P, et al. Presumed nonalcoholic fatty liver disease among Medicare beneficiaries with HIV, 2006–2016. Open Forum Infect Dis 2020; 7:ofz509. doi: 10.1093/ofid/ofz509/5697470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts 2016; 9:65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishijima T, Gatanaga H, Shimbo T, et al. Traditional but not HIV-related factors are associated with nonalcoholic fatty liver disease in Asian patients with HIV-1 infection. PLoS One 2014; 9:e87596. doi: 10.1371/journal.pone.0087596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Welzen BJ, Mudrikova T, El Idrissi A, Hoepelman AIM, Arends JE. A review of non-alcoholic fatty liver disease in HIV-infected patients: the next big thing? Infect Dis Ther 2019; 8:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lemoine M, Assoumou L, De Wit S, et al. Diagnostic accuracy of noninvasive markers of steatosis, NASH, and liver fibrosis in HIV-monoinfected individuals at risk of nonalcoholic fatty liver disease (NAFLD). J Acquir Immune Defic Syndr 2019; 80:e86–94. doi: 10.1097/QAI.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 10. Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34:3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). Eur Heart J 2013; 34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 12. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 13. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001; 285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14. Fedchuk L, Nascimbeni F, Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2014; 40:1209–22. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 15. Price JC, Dodge JL, Ma Y, et al. Controlled attenuation parameter and magnetic resonance spectroscopy-measured liver steatosis are discordant in obese HIV-infected adults. AIDS 2017; 31:2119–25. doi: 10.1097/QAD.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7:1104–12. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernaez R, Lazo M, Bonekamp M, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–21. http://doi.wiley.com/10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19. Iogna Prat L, Roccarina D, Lever R, et al. Etiology and severity of liver disease in HIV-positive patients with suspected NAFLD. J Acquir Immune Defic Syndr 2019; 80:474–80. [DOI] [PubMed] [Google Scholar]
- 20. Mofrad P. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003; 37:1286–92. [DOI] [PubMed] [Google Scholar]
- 21. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017; 66:1022–30. [DOI] [PubMed] [Google Scholar]
- 22. Macías J, González J, Tural C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS 2014; 28:1279–87. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 23. Vuille-Lessard É, Lebouché B, Lennox L, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016; 30:2635–43. doi: 10.1097/QAD.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 24. Sasso M, Miette V, Sandrin L, et al. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using fibroscan®. Clin Res Hepatol Gastroenterol 2012; 36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 25. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–57. [DOI] [PubMed] [Google Scholar]
- 26. Altamirano J, Qi Q, Choudhry S, et al. Non-invasive diagnosis: non-alcoholic fatty liver disease and alcoholic liver disease. Transl Gastroenterol Hepatol 2020; 5:31. doi: 10.21037/tgh.2019.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sebastiani G, Cocciolillo S, Mazzola G, et al. Application of guidelines for the management of nonalcoholic fatty liver disease in three prospective cohorts of monoinfected patients. HIV Med 2020; 21:96–108. doi: 10.1111/hiv.12799. [DOI] [PubMed] [Google Scholar]
- 28. Boursier J, Konate A, Guilluy M, et al. Learning curve and interobserver reproducibility evaluation of liver stiffness measurement by transient elastography. Eur J Gastroenterol Hepatol 2008; 20:693–701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.