Abstract
The modified 2-tier testing algorithm (MTTT) for Lyme disease (LD) has been approved by the US Food and Drug Administration. In this study, we show that the MTTT detected 28% more cases of early infection compared with the standard 2-tier algorithm while retaining high specificity in a region with a high incidence of LD.
Keywords: Borrelia burgdorferi, diagnostics, Lyme disease, modified two-tiered testing, serology
In this study, we show that the modified 2-tier testing algorithm for Lyme disease approved by the US Food and Drug Administration detected 28% more cases of early infection compared with the standard 2-tier algorithm while retaining high specificity.
Lyme disease (LD), or Lyme borreliosis, is the most frequently reported vector-borne disease in Canada [1] caused by the spirochete Borrelia burgdorferi sensu lato species complex that is transmitted to humans by infected blacklegged ticks. An increase in the number of locally acquired Lyme disease cases over the last decade has coincided with the northward geographic expansion of the range of blacklegged tick populations in southeastern and south-central regions of Canada. In 2018, 1487 human cases of Lyme disease were reported in Canada [1]. The province of Nova Scotia is a risk area for Lyme disease with some regions having the highest rates of infection in Canada [1].
The clinical presentation of LD exists on a continuum divided into clinical stages. Early localized infection presents in approximately 80% of patients with erythema migrans (EM) [2]. However, if the rash is absent or left untreated, B burgdorferi can disseminate throughout the body and appear as early disseminated infection, manifesting as multifocal EM rash, nonspecific influenza-like illness, arthralgia, meningitis, neuropathy, or carditis. Ultimately, late disseminated disease occurs if left untreated resulting in oligoarthritis of large joints and rarely neurologic disease.
In Canada, the current method for laboratory diagnosis of LD is detection of antibodies against B burgdorferi using the standard 2-tiered testing algorithm (STTT), which consists of an enzyme immunoassay (EIA) followed by immunoglobulin (Ig)M and/or IgG immunoblots (IBs). The STTT is known to have poor sensitivity to detect early localized infection (<50%) but >99% sensitivity for detecting late infection [3]. Due to the high proportion of false-negative results, early localized LD is usually diagnosed clinically. However, clinical diagnosis of early LD can be challenging, because some patients may not present with an EM rash or may have symptoms confused with other diseases [2]. Therefore, improving test sensitivity for early infection is crucial to permit definitive diagnosis of early LD and prevent consequences of untreated LD.
The first step in the STTT can include EIAs using B burgdorferi whole-cell sonicate (WCS) or conserved synthetic peptides, such as the surface lipoprotein VlsE (variable major protein-like sequence, expressed) or C6 (invariable region 6 of VlsE) or C10 (conserved amino-terminal portion of outer surface protein C) peptide [4]. Although, the specificity improves with conserved synthetic peptides EIAs compared with WCS, IBs are still required for optimal specificity. The IBs are hampered by being time-consuming, having subjective scoring [4], and having limited sensitivity for early localized infection [3]. Moreover, the availability of IBs is geographically restricted in Canada to provincial laboratories in British Columbia and Ontario or the National Microbiology Laboratory (NML) in Manitoba.
The US Food and Drug Administration (FDA) has recently approved an MTTT using 2 EIAs, which has been endorsed by the Centers for Disease Control and Prevention and Infectious Diseases Society of America [5]. We recently showed that an MTTT using a WCS EIA followed by C6 peptide EIA identified 25% more early LD cases than STTT, with a specificity of 99.56% [6]. The C6 EIA has not been approved for use in the MTTT by the FDA, and Immunetics ended its production requiring us to validate 2 new EIAs for the MTTT. The FDA has approved an MTTT using the Zeus C10/VlsE followed by either the Zeus WCS total IgM/IgG or individual IgM and IgG EIAs. The aim of this study is to evaluate whether the new MTTT consisting of the Zeus C10/VlsE EIA followed by the Zeus WCS total IgM/IgG EIA would improve sensitivity to detect early LD without compromising specificity in Nova Scotia, Canada.
METHODS
From March to July 2020, all patient specimens submitted for LD serology that were positive or indeterminate on the Zeus C10/VlsE EIA total antibody (ZEUS ELISA Borrelia VlsE1/pepC10 IgG/IgM) were tested with the Zeus WCS EIA total antibody (ZEUS ELISA Borrelia burgdorferi IgG/IgM) (MTTT) in addition to IB testing at the NML in Winnipeg, Manitoba using the EUROIMMUN Anti-Borrelia burgdorferi US EUROLINE-WB (IgG) and EUROIMMUN Anti-Borrelia EUROLINE-RN-AT-adv (IgM) kits (STTT). The Zeus WCS total IgM/IgG as the second-tier EIA was chosen over separated IgM/and IgG EIAs for the improved laboratory efficiency of using only a 2-step process verses a 3-step process. All samples were submitted by clinicians as part of clinical management and testing was done prospectively.
A retrospective chart review using a standardized data collection tool was used to classify patients as “true LD” and identify the stage of infection. The data obtained included demographics, clinical findings that prompted the serologic test, consultations, and treatment. All data were deidentified, and patients were classified as having “true LD” if they had the following: (1) a positive IgG IB; (2) a negative IgG and positive or negative IgM IB, but with signs or symptoms consistent with early LD (eg, EM rash or influenza-like illness [ILI] defined as fevers, with myalgias, fatigue, or arthralgias); or (3) evidence of seroconversion between consecutive specimens. All patients with positive IgM and negative IgG IBs required compatible clinical syndromes to be categorized as true LD cases because IgM IB can be falsely positive. Patients who did not meet these criteria were considered false-positive cases.
To further assess specificity, a second set of sera including 60 healthy individuals collected as part of a pervious serosurvey [7], and archived residual sera that were positive for antinuclear antibody (ANA), Epstein-Barr virus (EBV) IgM, and syphilis (10 sera for each) where tested using the MTTT. Any positive results were sent to the NML for immunoblot testing.
Patient Consent Statement
The activities described in this manuscript were conducted in fulfillment of ongoing verification of Lyme diagnostic assays used in Nova Scotia and considered a quality assurance initiative by the Nova Scotia Health Research Ethics Board, which did not require full review or patient consent. All clinical specimens tested were obtained from residual samples collected for routine diagnostic testing for Lyme serology, and all data related to clinical specimens were deidentified and used solely with the intent to evaluate the performance of the MTTT algorithm.
RESULTS
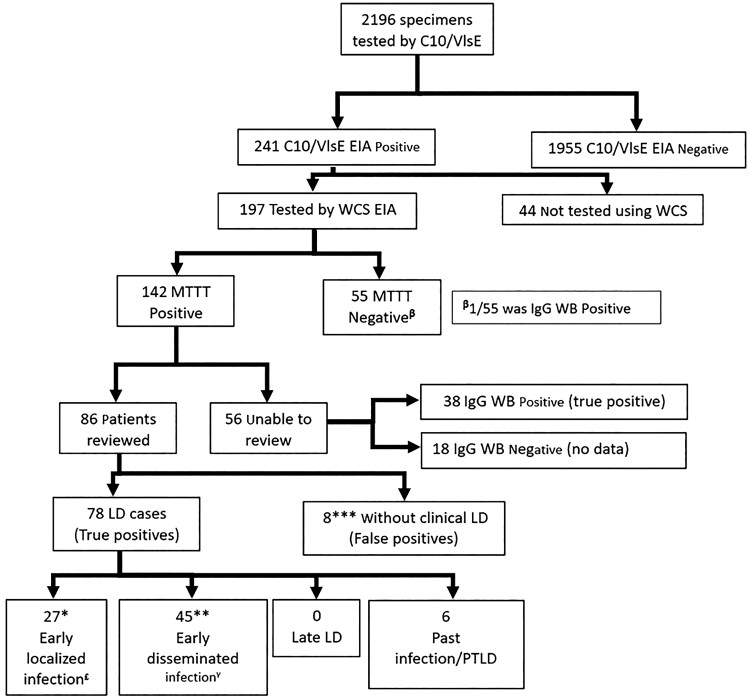
Between March and July 2020, LD serology was performed on 2196 specimens using the Zeus C10/VlsE EIA, producing 1955 negative results (89%) and 241 positive or equivocal results (11%) (Figure 1). Most of the patients in the study were from the south shore region of Nova Scotia, which has the highest incidence of LD in Canada [1].
Figure 1.
Flowchart of serological testing and chart review results. *, 15 immunoglobulin (Ig)M immunoblot (IB) positive (pos)/IgG IB negative (neg); 12 IgM IB neg (1 not tested (NT))/IgG IB neg. **, 38 IgG IB neg (8 IgM IB neg; 28 IgM IB pos; 2 IgM IB NT)/7 IgG IB pos. ***, Of the 8 false positives, all IgG IB neg (2 IgM IB pos/6 IgM IB NT/neg). Five did not have repeat serology, 3 had >4-week follow-ups, and all remained IgG IB neg. £, Of the 27 patients with early localized infection 23 (85%) had erythema migrans (EM) rash; 1 had a rash that was describes as oval but 5 cm in diameter; only 3 patients did not have a rash and presented with fever, myalgias, and fatigue and were diagnosed and treated as Lyme disease (LD) by the clinician. γ, Of the 45 patients with early disseminated infection, 37 had multiple EM; 3 had Bell’s palsy; 2 had symptoms to suggest meningitis; 1 had heart block; and the remaining 2 were diagnosed clinically with LD by the clinician and treated with doxycycline (1 had migratory arthralgia and a borderline IgG WB; 1 had fever, arthralgia, and other neurologic symptoms not defined). EIA, enzyme immunoassay; MTTT, modified 2-tier testing algorithm; PTLD, posttreatment Lyme disease; VlsE, variable major protein-like sequence, expressed; WCS, whole-cell sonicate.
Of the 241 positive or equivocal samples, 197 had Zeus WCS EIA completed, 142 of which were positive. Of the 55 that were negative by MTTT only, 1 had a positive IgG IB (positive bands: p25, 30, 41, 58, 66, and VlsE), suggesting the MTTT was falsely negative. From the 142 LD positive patients, physicians could be contacted for clinical information on 86 patients. Of those charts unavailable for review, 38 of 56 were IgG IB positive, suggesting a past infection.
Of the 86 charts available for review, 78 patients had clinical evidence of LD including 22 who had manifestations of early localized or early disseminated infection but did not have a positive immunoblot (either IgM or IgG, including 3 patients where the IgM was not tested), suggesting the MTTT detected 28% more cases of early infection compared with the STTT. The 8 remaining patients did not have a clinical syndrome compatible with Lyme Disease and were considered false-positive MTTT results. Given only 8 of 2196 patient tests were false positive, the specificity of the MTTT is estimated to be 99.6% (99.2%–99.8%), which is similar to the 99.2% specificity of the STTT previously described [3]. Of the 27 patients with early localized infection, only 3 patients did not have a rash and presented with fever, myalgias, and fatigue and were diagnoses and treated as LD by the local clinician. Although this ILI presentation is more nonspecific, if they are included as false positives, the specificity remains comparable at 99.4% (98.9%–99.7%).
The 60 healthy individuals, 10, sear positive for EBV IgM and ANA were all negative when tested using the MTTT. Of the 10 syphilis sera tested, 2 were positive on MTTT. When tested by STTT, one had a positive IgM immunoblot (positive OspC, p39 and p41 bands) and a negative IgG. The other was negative for both IgM and IgG IBs.
DISCUSSION
The MTTT is now recognized as an acceptable alternative to the STTT in both Canada and the United States [8]. However, laboratories must validate the assay in their populations to ensure its performance is consistent with that reported in the literature. We previously demonstrated that a MTTT using a WCS EIA followed by C6 peptide EIA identified 25% more early LD cases than STTT, with a specificity of 99.56% [6]. However, with the discontinuation of the C6 EIA, we were required to revalidate the MTTT using an alternative. The current study shows that the MTTT using the Zeus C10/VlsE EIA followed by the Zeus WCS EIA detected 28% more early infections while maintaining a specificity of 99.6%, which is consistent with our previous findings and those of other researchers using a variety of other EIA combinations [3, 8]. The MTTT is not without its limitations. Similar to the STTT, the MTTT cannot distinguish active and past infections and cannot diagnose reinfection because antibody responses can persist for years [9]. In our specificity panel, we found that 2 of 10 sera with positive syphilis serology were positive by MTTT, 1 of which had a positive IgM IB. There were no clinical data available on these specimens to determine whether the strong reaction on the IgM WB was a true early infection or a false-positive result. False-positive MTTT and STTT in sera containing syphilis antibodies have been documented in previous studies [10, 11]. Because of the possibility of false positives generated by the IgM component of the assay, STTT can still be helpful in late infections, such as Lyme arthritis, or when false-positive results are suspected in cases in which the serologic result does not fit the clinical picture [12]. Because the charts of patients with a negative first step EIAs in this report were not reviewed, the sensitivity of the MTTT cannot be accurately determined. Although the MTTT has improved sensitivity, literature suggests that it is less than 70% in early localized infections. As such, patients presenting with EM should still receive empiric treatment rather than relying on serologic results [6, 8].
There are several limitations to this study. The LD cases were not evaluated by the authors, and categorization of LD relied on the clinical description provided by the attending clinician using a retrospective chart review. However, these are experienced clinicians because the majority of the cases were in a region with the highest incident of LD in Canada. The ability to confirm early localized infection with culture or polymerase chain reaction would have been informative in determining sensitivity and less susceptible to selection bias, but these techniques were not available. The ILI criteria used for categorization for early localized infection is nonspecific and could be due to other infections; however, only 3 of 27 cases of early localized infection presented with ILI and if these were considered false positives, the specificity of the MTTT was essentially unchanged. In this study, several tests were ordered from emergency departments where follow-up with the ordering clinician was difficult. We were unable to review the clinical information on 55 patients, which could have resulted in an overrepresentation of specificity. Although 38 patients had positive IgG WBs confirming they had been exposed to B burgdorferi, we could not assign 17 patients as true positives or false positives. If all of these are considered false positives, the specificity of the MTTT would drop to 98.7% (98.14%–99.18%), but it is still within the range suggested in the review by Waddell et al [3] 99.2% (98.3%–99.6%). Although strain diversity can affect the performance of diagnostic tests, it is more directly related to Western blots [13]. How it impacts the performance of the MTTT has not been assessed. However, it is possible that potentially unique strains of B burgdorferi could circulate in Nova Scotia limiting its performance in other regions. Therefore, further validations studies will be required to verify the MTTT’s sensitivity for detecting early LD in other locations.
CONCLUSIONS
An MTTT using the Zeus C10/VlsE followed by the Zeus WCS EIA improves the sensitivity for detection of early LD and has equivalent specificity compared with STTT. Since implementation at the Nova Scotia Diagnostic Microbiology Laboratory on April 1, 2021, the MTTT has resulted in shorter turnaround times, expedited management of patients, and cost savings.
Acknowledgments
We thank Colleen Jackson and all the technologists in the Division of Microbiology and all the primary care providers who assisted the study team.
Financial support. This work was unfunded and conducted as part of an internal validation performed at the QEII microbiology laboratory Nova Scotia Health, Central zone .
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Farhan Khan, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada; Department of Pathology, Dalhousie University, Halifax, Nova Scotia, Canada.
Ziyad Allehebi, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada; Department of Pathology, Dalhousie University, Halifax, Nova Scotia, Canada.
Yahya Shabi, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada; Department of Pathology, Dalhousie University, Halifax, Nova Scotia, Canada.
Ian Davis, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada; Department of Pathology, Dalhousie University, Halifax, Nova Scotia, Canada.
Jason LeBlanc, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada; Department of Pathology, Dalhousie University, Halifax, Nova Scotia, Canada.
Robbin Lindsay, Zoonotic Diseases and Special Pathogens Division, National Microbiology Laboratory (NML), Public Health Agency of Canada (PHAC), Winnipeg, Manitoba, Canada.
Todd Hatchette, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Science Centre, Halifax, Nova Scotia, Canada; Department of Pathology, Dalhousie University, Halifax, Nova Scotia, Canada.
References
- 1. Lyme Disease Surveillance Report: Preliminary Annual Edition 2018 . Available at: https://www.canada.ca/content/dam/hc-sc/documents/services/publications/diseases-and-conditions/lyme-disease-surveillance-report-2018/ld-report-eng.pdf. ISBN: 978-0-660-36082-9. Accessed 3 March 2021.
- 2. Hatchette T, Davis I, Johnston B. Lyme disease: clinical diagnosis and treatment. Can Commun Dis Rep 2014; 40:194–208. doi: 10.14745/ccdr.v40i11a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waddell LA, Greig J, Mascarenhas M, et al. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS One 2016; 11:e0168613. doi: 10.1371/journal.pone.0168613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66:1133–9. doi: 10.1093/cid/cix943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep 2019; 68:703. doi: 10.15585/mmwr.mm6832a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis IRC, McNeil SA, Allen W, et al. Performance of a modified two-tiered testing enzyme immunoassay algorithm for serologic diagnosis of Lyme disease in Nova Scotia. J Clin Microbiol 2020; 58:e01841-19. doi: 10.1128/JCM.01841-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatchette TF, Johnston BL, Schleihauf E, et al. Epidemiology of Lyme disease, Nova Scotia, Canada, 2002-2013. Emerg Infect Dis 2015; 21:1751–8. doi: 10.3201/eid2110.141640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatchette T, Lindsay R. Modified two-tiered testing algorithm for Lyme disease serology: the Canadian context. Can Commun Dis Rep 2020; 46:125–31.doi: 10.14745/ccdr.v46i05a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalish RA, McHugh G, Granquist J, et al. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 2001; 33:780–5. doi: 10.1086/322669. [DOI] [PubMed] [Google Scholar]
- 10. Pegalajar-Jurado A, Schriefer ME, Welch RJ, et al. Evaluation of modified two-tiered testing algorithms for Lyme disease laboratory diagnosis using well-characterized serum samples. J Clin Microbiol 2018; 56:e01943-17. doi: 10.1128/JCM.01943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore A, Nelson C, Molins C, et al. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis 2016; 22:1169–77. doi: 10.3201/eid2207.151694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seriburi V, Ndukwe N, Chang Z, et al. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18:1236–40. doi: 10.1111/j.1469-0691.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 13. Ogden NH, Arsenault J, Hatchette TF, et al. Antibody responses to Borrelia burgdorferi detected by Western blot vary geographically in Canada. PLoS One 2017; 12:e0171731. doi: 10.1371/journal.pone.0171731. [DOI] [PMC free article] [PubMed] [Google Scholar]