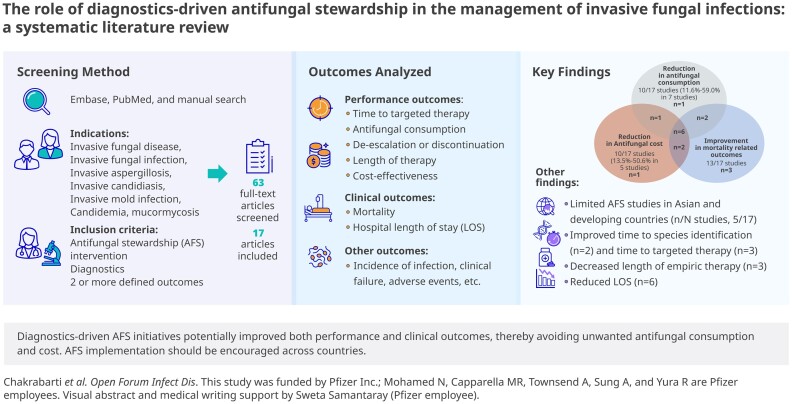
Abstract
Antifungal stewardship (AFS) programs are key to optimizing antifungal use and improving outcomes in patients with invasive fungal infections. Our systematic literature review evaluated the impact of diagnostics in AFS programs by assessing performance and clinical measures. Most eligible studies were from Europe and the United States (n = 12/17). Diagnostic approaches included serum β-1–3-D-glucan test (n/N studies, 7/17), galactomannan test (4/17), computed tomography scan (3/17), magnetic resonance (2/17), matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS; 2/17), polymerase chain reaction (1/17), peptide nucleic acid fluorescent in situ hybridization (PNA-FISH) assay (1/17), and other routine methods (9/17). Time to species identification decreased significantly using MALDI-TOF and PNA-FISH (n = 2). Time to targeted therapy and length of empiric therapy also decreased (n = 3). Antifungal consumption decreased by 11.6%–59.0% (7/13). Cost-savings ranged from 13.5% to 50.6% (5/10). Mortality rate (13/16) and length of stay (6/7) also decreased. No negative impact was reported on patient outcomes. Diagnostics-driven interventions can potentially improve AFS measures (antifungal consumption, cost, mortality, and length of stay); therefore, AFS implementation should be encouraged.
Keywords: antifungal stewardship, diagnostics, invasive fungal infections
Graphical Abstract
Graphical abstract.
Global estimates reveal that ≥3 million individuals are infected by severe fungal infections, and mortality associated with fungal disease is >1.6 million annually [1]. Invasive fungal infections (IFIs), a potential clinical problem particularly in the immunosuppressed population, are associated with significant morbidity, mortality, and increased health care costs [2]. Moreover, patients with severe influenza, coronavirus disease 2019 (COVID-19), liver cirrhosis, chronic obstructive pulmonary disease, long period of intensive care unit (ICU) stay, and those receiving biological therapies and/or corticosteroids are also identified at risk for IFI [3]. Fungal disease poses a considerable economic burden, with $7.2 billion spent in the United States in 2017 alone [4].
Initiation of appropriate antifungal therapy (AFT) is a key factor for the successful management of IFI [5]. Considering that antifungal drug classes are limited compared with antibacterial, judicious use of antifungals is necessary for appropriate treatment of IFIs [6]. Appropriate AFT could be limited by cost, toxicity, availability, and affordability [2]. With the increasing need to optimize AFT, many institutions have now recognized the importance of multidisciplinary antifungal stewardship (AFS) approaches [7]. Although AFS, an emerging component of antimicrobial stewardship (AMS), has similar goals and core elements to optimize and guide therapy, it differs in its unique complexities [2, 8]. These include difficulties in diagnosis, lack of consensus on management and de-escalation methodologies, as well as limited antifungal resistance reporting [2]. However, there are very few AMS programs integrating AFS and even fewer dedicated AFS programs [9].
AFS programs aim to optimize antifungal use, limit antifungal resistance, and improve outcomes for patients [5], which relies on early, accurate diagnosis of IFIs and identification of the causative fungal pathogens [10]. Lack of effective and rapid fungal diagnostics may fail to detect infection and potentially delay targeted treatment, leading to overexposure to potentially toxic antifungal agents and increasing the risk of antifungal resistance, thereby resulting in poor patient outcomes [10]. While conventional approaches including direct microscopy, histopathology, and culture methods remain the gold standard for IFI diagnosis; low sensitivity and long turnaround time (TAT) delay appropriate therapy, resulting in prolonged empiric treatment [10]. Several studies have highlighted the potential of fungal biomarkers such as galactomannan (GM) and (1–3)-β-d-glucan (BDG) and molecular assays such as polymerase chain reaction (PCR) for the diagnosis and management of IFIs [11–13]. Other non-culture-based diagnostic techniques including miniaturized magnetic resonance (MR)–based technology [14], peptide nucleic acid fluorescent in situ hybridization (PNA-FISH) [15], and matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) [16] enable rapid species identification. Additionally, use of artificial intelligence and machine learning algorithms in AFS have the potential to facilitate IFI diagnosis [17]. Timely and accurate diagnosis can aid stewardship efforts and facilitate AFS outcomes to attain maximal impact and save resources [5].
Recommendations to improve AFS include driving clinical knowledge and awareness on fungal infections, access to antifungals, and effective diagnostic approaches and reporting on these parameters intrinsic to AFS [2, 18]. To address these recommendations with supportive evidence, we report the findings of a systematic literature review (SLR) that evaluated the role of diagnostics in AFS programs and its impact on the management of IFIs.
METHODS
Search Strategy
Systematic searches of PubMed and EMBASE were performed to identify studies that utilized both an AFS and a diagnostic approach (either as a test or recommendation) between the period January 2010 and January 2021, during which there was more emphasis on AFS and diagnostic-driven treatments. Search terms included invasive fungal disease, invasive fungal infection, invasive aspergillosis, invasive candidiasis, invasive mold infection, invasive mold disease, candidemia, candiduria, candidosis, mucormycosis, antifungal or antimicrobial stewardship. Additionally, a manual search of the reference lists of relevant articles was conducted.
Inclusion and Exclusion Criteria
Inclusion criteria for studies: study involved an AFS program (independent or part of AMS) and evaluated patients with IFI indication; diagnostic approach was included; studies had ≥2 defined outcomes of interest including time to targeted therapy, antifungal consumption, de-escalation or discontinuation, length of therapy, mortality, hospital length of stay (LOS), cost-effectiveness, and other outcomes such as incidence of infection, clinical failure, and adverse events (AEs).
Review articles, conference abstracts, and non-English-language articles were excluded.
Study Selection and Data Extraction
Relevant studies were identified based on a title and abstract screening done independently by 2 researchers, followed by a full-text review of the shortlisted studies. Data from the eligible studies were extracted in an Excel spreadsheet as follows: study details (year of publication, region, country, patient population, study setting, study design, and study period), organism or condition, performance measures (including diagnostic approach, TAT, time to targeted therapy, antifungal consumption, antifungal cost-savings, de-escalation or discontinuation, and length of therapy), clinical measures (including mortality and LOS), and other miscellaneous outcomes (rate of 60-day clinical failure, overall AE incidence, incidence of hospital-acquired candidemia, and prevalence of Candida species). Any disagreements on inclusion were resolved by discussion with a third researcher.
Synthesis of Results
This SLR followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology [19]. The data from the studies were summarized using a narrative synthesis approach based on a qualitative analysis, and no statistical methods were used.
RESULTS
Data Extraction
A total of 422 articles were identified from literature searches, and only 63 articles were considered for full-text assessment. Of these, 17 articles that fulfilled all eligibility criteria were included in the SLR [20–36]. A PRISMA flowchart of search results summarizing the study selection process is illustrated in Figure 1.
Figure 1.
PRISMA flow diagram of the search process and study selection.
Study Characteristics
The majority of the eligible studies were conducted at a single center, such as tertiary care hospitals and university hospitals, and were from Europe (n = 8) and the United States (n = 4), while a few were identified from Asia (Japan, n = 4; Thailand, n = 1). These studies (n/N, 9/17) evaluated AFS interventions using routine methods including blood culture (BC), manual solid media culture (CHROMagar Candida), manual yeast identification system (Analytical Profile Index [API] 20C), and automated, growth-based platforms (VITEK2 system). Additionally, other diagnostic tools used were BDG test (7/17), GM test (3/17), computed tomography (CT) scan (3/17), magnetic resonance (T2Candida Panel, T2CP, 2/17), MALDI-TOF mass spectrometry (2/17), PCR (1/17), and PNA-FISH assay (Yeast Traffic Light, 1/17). While a total of 11 studies were based only on Candida-related indications [21–23, 25, 27, 28, 30–32, 34, 35], other studies (n = 6) were based on ≥1 indication involving different fungal pathogens (including Aspergillus, Cryptococcus, Histoplasma, etc.) without data stratification for different species [20, 24–26, 29, 33, 36]. Additionally, 11 studies utilized pre- and postintervention methods to evaluate the impact of AFS programs [21, 23–27, 31, 33–36]. Study settings/design and diagnostic approaches are summarized in Tables 1 and 2, respectively. The impact of diagnostics on AFS intervention was qualitatively analyzed by clinical and performance measures, as summarized in Table 2.
Table 1.
Description of Studies Included in the Systematic Review
| Reference | Region (Asia Pacific, Latin America, North America, Europe, AfME) | Country | Patient Population, Size | Study Setting | Study Design & Study Period; AFS Approach | Organism (or Condition) |
|---|---|---|---|---|---|---|
| Machado et al. (2021) [26] | Europe | Spain | Adult, hospitalized patients with solid tumor or solid organ transplantation receiving systemic AFT: PRE-period (initial period of bedside-based AFS, n = 85) and POST-period (AFS complemented with BDG, n = 112) | Tertiary care hospital | PRE (October 2011–August 2014, advice by doctor as per protocol) and POST (September 2014–July 2017, advice complemented with serum BDG); multidisciplinary, bedside AFS team (microbiologists, ID specialists, pharmacists) involving bedside advice provided by ID specialists and pharmacy alerts to prescribers via electronic prescription system and review of antifungal prescriptions | Candidemia, invasive candidiasis, aspergillosis, mucormycosis, PJP, and scedosporiasis |
| Hare et al. (2020) [22] | Europe | Ireland | 60 patients divided into 2 groups (n = 30 each): Probable/proven IC and colonized/no evidence IC, further subdivided into compliant with AFS care pathway and noncompliant | 27-bed CrCU in tertiary left | Observational study (December 1, 2017, to July 31, 2018); diagnostic-driven AFS program that implemented a care pathway (treatment algorithm) utilizing once-weekly BDG testing for antifungal management of suspected IC in CrCU | Invasive candidiasis |
| Martín-Gutiérrez et al. (2020) [27] | Europe | Spain | Adult patients with hospital-acquired candidemia | Tertiary care teaching hospital | Quasi-experimental before (Jan 2009–Dec 2010) and after (Jan 2011–Dec 2017) study of interrupted time series (36 quarters between January 2009 and December 2017); multidisciplinary, educational ASP implemented in Jan 2011 and led by ID physician, mainly comprising educational interviews and training of prescriber by an advisor on appropriate antimicrobial prescription | Hospital-acquired candidemia |
| Samura et al. (2020) [31] | Asia Pacific | Japan | Inpatients who developed candidemia (N = 38) | General hospital | Pre-AFP (April 2008–March 2012) and post-AFP (April 2012–March 2016); pharmacy-led AFP with active consultation between ward and ID pharmacists who recommend guideline-based antifungal treatment and clinical examination to the physician | Hospital-acquired candidemia |
| Steuber et al. (2020) [32] | North America | USA | Patients with a T2CP result during the study period (N = 628) | 971-bed community hospital | Single-left, retrospective, observational study (December 2015–June 2018); AFS activities involved education provided to ID providers on use and benefits of T2CP and peer-to-peer discussions, with pharmacist reviewing negative T2CP results sent via email and contacting prescriber within 24 h (if broad-spectrum AFT continued) to de-escalate therapy | Candidemia |
| Ito-Takeichi et al. (2019) [25] | Asia Pacific | Japan | Patients with Candida bloodstream infection (N = 57) | Tertiary care hospital | Single-left, prospective cohort study; before-intervention group (August 2009–July 2013) and after-intervention group (August 2013–July 2016); AFS team comprising ID physician and pharmacist performed daily review of intravenous antifungal prescriptions and monitoring of measured BDG values in hospitalized patients | Candidemia |
| Kawaguchi et al. (2019) [35] | Asia Pacific | Japan | Patients receiving systemic antifungals (N = 1793) | 980-bed tertiary care teaching hospital | Single-left, observational study; pre-intervention period (January 2011–December 2013) and intervention period (January 2014–December 2016); AST team (ID physicians, pharmacists, medical biologists, and nurses) performed daily monitoring of blood culture results to confirm appropriate empiric therapy and antifungal prescriptions, conducted weekly meetings, and followed candidemia management bundles for patients with positive blood cultures | Candidemia |
| Whitney et al. (2019) [36] | Europe | England | Adult patients at risk of IFI (acute leukemia, autologous and allogeneic stem cell transplantation, renal dialysis and transplantation, inpatients in infectious diseases wards and adult ICUs—general, cardiothoracic, and neurosurgical; N = 432) | 1300-bed teaching hospital | Single-left, observational study; pre-intervention period (April 2009–September 2010) and intervention period (October 2010–September 2016); adult patients receiving AFT were reviewed weekly by an ID consultant and antimicrobial pharmacist, which included monitoring medical notes, drug charts, laboratory tests and imaging, and discussions with clinical team and further recommendations for patient care | IFI (Candida, Aspergillus, Cryptococcus, Histoplasma capsulatum, Lichtheimia corymbifera, Saccharomyces cerevisiae, Trichosporon mucoides, mucormycosis) |
| Murakami et al. (2018) [34] | Asia Pacific | Japan | Adult patients with hospital-acquired candidemia (N = 76) | Rural tertiary hospital | Before and after study of episodes of hospital-acquired candidemia; pre-intervention period (November 2006–October 2009) and intervention period (November 2009–October 2012); multidisciplinary AST wherein attending physician and other members were informed real-time about patients with positive blood culture via email or telephone to implement candidemia care bundle and discuss appropriate management | Hospital-acquired candidemia |
| Patch et al. (2018) [28] | North America | USA | Adult, phase 1 (patients with positive blood cultures or other normally sterile site cultures, n = 19), phase 2 (positive T2CP, n = 20) | Multihospital community health system | Two-phase retrospective analysisa: phase 1 (Sept 2014–Jan 2015) and phase 2 (T2C panel implementation; Sept 2015–May 2016); an active AFS team reviewed diagnostic results and monitored antifungal prescribing in patients | Candidemia and invasive candidiasis |
| Rautemaa-Richardson et al. (2018) [30] | Europe | UK | Patients prescribed for micafungin with suspected or proven invasive candidosis (Candida spp. isolated from blood cultures) and admitted to ICU were audited over a 4-mo period (April–July) in 2014 (n = 39) and 2016 (n = 29) | 71-bed tertiary referral teaching hospital | Single-left study of patients with proven/suspected disease with BDG audited over a 4-mo period: Apr 2014–Jul 2014 and Apr 2016–Jul 2016; local guideline on managing antifungal prescriptions in ICU patients with IC was developed and implemented, with trained AFS champion (ICU physician) working closely with ID team to improve adherence to the guideline and reduce inappropriate use of antifungals | Invasive candidosis |
| Ramos et al. (2015) [29] | Europe | Spain | Patients with hematological and solid organ transplantation and prescribed restricted antifungals (N = 262) | Tertiary university hospital | Prospective study with ASP implementation (Oct 2012–May 2013); AFS team led by 2 ID specialists reviewed restricted antifungals (lipid formulations of amphotericin B, echinocandins, and voriconazole) prescribed to hospitalized patients and included recommendations in electronic medical records to be followed by the prescribing physician within 24 h | Aspergillus spp., Candida spp., Scedosporium spp., Rhodotorula mucilaginosa, other fungal species |
| Valerio et al. (2015) [33] | Europe | Spain | Hospitalized patients receiving systemic antifungals (N = 453) | Tertiary care hospital | Quasi-experimental study with a time-series design: noninterventional period (Oct 2010–Sep 2011) vs diagnostic intervention period (Oct 2011–Sep 2012); noncompulsory, multidisciplinary AFS supported by computerized system to provide real-time alerts on antifungal prescriptions, which involved implementation of bundle of noninterventional measures (including local guidelines for IFI management and audit of antifungal use and cost) during the first year and ID specialists monitoring patients (receiving candins, liposomal amphotericin B, voriconazole, or posaconazole) and recommending appropriate therapy and diagnostic advice following discussion with the attending physician | Invasive fungal infections (Candida spp., Aspergillus spp., Scedosporium spp., Mucor spp., Cunninghamella spp., Cryptococcus neoformans, Rhodotorula rubra, Trichosporon asahii, Pneumocystis jiroveci, Blastoschizomyces capitatus) |
| Alfandari et al. (2014) [20] | Europe | France | Patients on chemotherapy receiving antifungals based on treatment recommendations | Teaching hospital | 2003–2012; AFS implemented in 2002 in close collaboration with hematologists and ID specialists who developed evidence-based local guidelines with treatment algorithm and prescription guidance, and also conducted biweekly meetings and telephone counseling to discuss patient files | Invasive fungal infection (including invasive lung aspergillosis and candidemia) |
| Huang et al. (2013) [24] | North America | USA | Adult patients with yeast infection (N = 35) | University hospital | Single-left, pre–post quasi-experimental study; pre (VITEK-2 + ASP): Sep 2011–Nov 2011; post (MALDI-TOF + ASP + real-time support): Sep 2012–Nov 2012; AFS team (ID physicians and pharmacists) reviewed electronic medical records and provided evidence-based recommendations for positive yeast identification and susceptibility results received via real-time alerts on electronic pages | Candida spp., Cryptococcus spp. |
| Heil et al. (2012) [23] | North America | USA | Adult patients with Candida infections (N = 82) | University hospital | Pre-implementation group (routine methods): Jun 2009–Sep 2010); postimplementation group (PNA-FISH): Sep 2010–Jun 2011; on-call clinical pharmacist was informed about PNA-FISH test results by the laboratory personnel via pager, who then provided recommendation based on treatment protocol | Candidemia |
| Apisarnthanarak et al. (2010) [21] | Asia Pacific | Thailand | Adults (N = 1106) | Tertiary care hospital | Quasi-experimental study comprised 1.5 y before and 1.5 y after the implementation of AFS; before AFS (Jan 2006–Jun 30, 2007) and after AFS (Jul 2007–Dec 2008); AFS committee reviewed antifungal prescription practices, calculated antifungal costs based on dose administered and purchase price, and introduced necessary interventions (education tool on hepatic and/or renal dose adjustments, antifungal prescription forms, and prescription control strategies) | Candidiasis (Candida spp.) |
Abbreviations: AFP, antifungal stewardship program; AFS, antifungal stewardship; AFT, antifungal therapy; ASP, antimicrobial stewardship program, BDG, serum β-1–3-D-glucan; CrCU, critical care unit; ICU, intensive care unit; ID, infectious diseases; IFI, invasive fungal infection; PJP, Pneumocystis jirovecii pneumonia; T2CP, T2Candida Panel.
Phase 1 refers to antifungal therapy without a diagnostic test, and phase 2 refers to antifungal therapy post-T2CP.
Table 2.
Description of Outcomes of Interest Listed in the Studies Included in the Review
| Reference | Diagnostic Approach Used (Test/ Recommendation; Purpose) |
Turn-around Time | Time to Targeted Therapy | Antifungal Consumption | Antifungal Cost Savings | De-escalation/ Discontinuation | Length of Therapy | Mortality | Hospital LOS | Other Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Machado et al. (2021) [26] | Serum BDG and GM (test; diagnosis, determining antifungal use adequacy, and improving antifungal management in patients) | Not reported | Not reported | Use of caspofungin (PRE-period vs POST-period) (21.2% vs. 6.2%) Decreased, P = .002 Use of fluconazole: 18.8% vs. 45.5% Increased, P < .001 No changes in other antifungals |
↓28.1% (cost of AF treatment reduced by €779.6/patient) | Yes | Median (IQR) days of empirical AF treatment (PRE-period vs. POST-period): 9 (4–14) vs. 5 (2–11) d, P = .04 | All-cause mortality (PRE-period vs. POST-period): 44.7% vs. 34.8% Decreased, NS IFI-related mortality: 10.6% vs. 4.5% Decreased, NS |
Not reported | Not reported |
| Hare et al. (2020) [22] | Serum BDG (recommendation; to manage AFT in patients with IC in CrCU) | Median (IQR) TAT for BDG: 4 days (IQR, 2–6) d | Not reported | No sustained reduction in overall antifungal consumption | Not reported | Not reported | Median (IQR) duration of empiric AFT in colonized/no IC in compliant vs non-compliant: 5.5 (4–7) vs 14.5 (9–23) d Decreased, P < .001 | All-cause mortality (compliant proven/probable IC cases vs non-compliant proven/probable IC): 24% (6/25) vs 50% (3/6) Decreased, NS | Median (IQR) ICU LOS in compliant vs non-compliant for colonized/No IC: 10 (6–32) vs 23 (15–60) d Decreased, NS |
Not reported |
| Martín-Gutiérrez et al. (2020) [47] | API ID32C (2009–2010) and MALDI-TOF MS (2011–2017) (recommendation; used for detection of hospital-acquired candidemia) | Not reported | Not reported | ↓38.4% | Not reported | Not reported | Not reported | 14-d mortality rate: 36.1% in 2010 to 19.2% in 2017 Decreased, NS | Not reported | Incidence density of hospital-acquired candidemia significantly decreased during the study period (P = .009) Fluconazole resistance decreased during study period |
| Samura et al. (2020) [31] | BC (Recommendation; diagnosis and management of AFT in inpatients) | Not reported | Not reported | Rate of optimal antifungal dose (pre-AFP group vs post-AFP group): 71.4% vs 100% Increased, P = .028 | ↓36.8% | Not reported | Median (IQR) days of therapy (pre- vs post-AFP groups): 6.0 (0.3–15.7) vs 3.4 (1.9–3.4) Decreased, P < .001 |
30-d mortality rates (pre- vs post-AFP groups): 29.4% (5/17) and 60.0% (12/20) Increased, NS | Not reported | Not reported |
| Steuber et al. (2020) [32] | T2CP (test; overall provider-utilization for appropriate treatment decisions) | Not reported | Not reported | Antifungal therapy was avoided in 60.4% of negative cases | Not reported | Yes | Antifungal duration of therapy (negative vs positive T2CP): 4.9 ± 6.3 vs 10 ± 10 d Decreased, P = .03 | Patients with negative vs positive T2CP result: 27.1% (154/568) vs 26.7% (16/60) Decreased, NS | Patients with negative vs positive T2CP result: 26.3 ± 28.5 vs 32.5 ± 38.4 d Decreased, NS | Not reported |
| Ito-Takeichi et al. (2019) [25] | BDG (test; used for monitoring infection in hospitalized patients to optimize AFT) | Not reported | Not reported | ↓11.6% NS Median antifungal use for intravenous agents (pre- vs post- intervention): 16.7 (2.9–31.9) vs 11.9 (6.4–17.8) DOTs/1000 patient-d Decreased, P = .006 |
Annual cost savings of US$138991 | Not reported | Not reported | 60-d mortality (before-intervention group vs after-intervention group): 42.9% vs 18.2%; cumulative survival (before-intervention group vs after-intervention group): HR, 0.44; 95% CI: 0.18–1.11 Decreased, NS | Median hospital LOS (before-intervention vs after-intervention groups): 67 (15–547) vs 85 (7–220) d Increased, NS | Rate of 60-d clinical failure (before- vs after- intervention): 80.0% (28/35) vs 36.4% (8/22) Decreased, P < .001 Overall incidence of AEs (before- vs after- intervention): (51.4% [18/35] vs 13.6% [3/22], Decreased, P = .004 |
| Kawaguchi et al. (2019) [35] | BC (test; to manage appropriate antifungal utilization and costs) | Not reported | Not reported | Monthly average DDDs/1000 patient-d (pre-intervention group vs intervention group): 23.3 ± 8.0 vs 20.4 ± 10.8 Decreased, NS Monthly average DOTs/1000 patient-d (pre-intervention group vs intervention group): 15.1 ± 3.1 vs 12.7 ± 4.3 Decreased, P = .009 |
↓13.5% | Not reported | Not reported | 30-d mortality (pre-intervention group vs intervention group): 40.9% vs 30.0% Decreased,NS In-hospital mortality (pre-intervention group vs intervention group): 63.6% vs 36.7% Decreased, NS |
Not reported | Prevalence of non-major Candida species (C. rugosa, C. guilliermondii, C. lusitaniae, and other isolates; pre-intervention group vs intervention group): 7.1% vs 8.5%, Increased, NS Achievement rates of candidemia management bundle (pre-intervention group vs intervention group): 13.6% vs 50.0% Increased, P = .006 |
| Whitney et al. (2019) [36] | High-resolution chest CT scan, PCR, BDG and GM (test; to confirm IFI diagnosis as “none”, “proven”, “probabale”, or “possible” and manage antifungal prescribing) | Median (IQR) TAT for GM test: 13 (11–17) d Median (IQR) TAT for BDG: 12 (9–14) d |
Not reported | Overall antifungal consumption decreased by 26% from 2009–2012 followed by a steady increase from 2013–2017. Empiric antifungal therapy (pre-intervention period vs intervention period): 44% (8/18) vs 62% (86/138) Decreased, NS |
No change (antifungal cost reduced by 30% and then increased to 20% above baseline over 5-y period) | Yes | Not reported | Inpatient mortality (pre-intervention period vs intervention period): 38% (19/50) vs 26% (101/383) Decreased, NS |
LOS (proven/probable IFI vs None): 47 vs 30 d Decreased, P < .0001 |
Not reported |
| Murakami et al. (2018) [34] | BC (recommendation; to diagnose patients with hospital-acquired candidemia) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 30-d all-cause mortality (preintervention group vs intervention group): 23.9% (11/46) vs 23.3% (7/30), adjusted HR, 0.68; 95% CI 0.24–1.91 NS | Not reported | Not reported |
| Patch et al. (2018) [28] | T2CP and BC (test; to evaluate impact of rapid diagnostics on time to initiation appropriate therapy and antifungal utilization in candidemia patients) | Not reported | Mean time to appropriate therapy (from the time the first positive BC was drawn) Phase 1 vs Phase 2: 34 (1–92) vs 6 (1–13) h; Decreased, P = .00147 | Empirical antifungal therapy avoided in 58.4% of T2Candida-negative patients | Total savings of US$48400 | Yes | Average duration of therapy (patients receiving empirical micafungin without evidence of infection, Phase 1 vs T2Candida and BC negative, Phase 2): 6.7 vs 2.4 d Decreased | Phase 1 micafungin cohort vs Phase 2 cohort (where antifungal therapy was withheld or stopped based on a negative T2 result): 23% vs 21% Decreased, NS | Median hospital LOS (candidaemia, Phase 1 vs T2Candida positive, Phase 2): 20 (12–33) vs 12 (9–24) d Decreased, NS | Not reported |
| Rautemaa-Richardson et al. (2018) [30] | Serum BDG (test; to rule out suspected invasive candidosis in patients and manage appropriate treatment dicontinuation) | Not reported | Not reported | ↓49% | ↓50.6% | Yes | Not reported | Not reported | Not reported | Not reported |
| Ramos et al. (2015) [29] | Conventional culture methods and special techniques where applicable (recommendation; to manage appropriate antifungal prescribing and make treatment recommendations based on fungal species identification and guidelines) | Not reported | Not reported | ↓42% | Not reported | Not reported | Not reported | Mortality (treatment modified per ASP recommendations vs not modified): 17% vs 30% Decreased, NS | Not reported | Not reported |
| Valerio et al. (2015) [33] | Blood or catheter cultures (35.9%), GM, BDG, and/or antifungal levels (19.7%), and radiological tests ie, chest scan or magnetic resonance (13.9%; (recommendation; to optimize the use of AFT in patients) | Not reported | Not reported | ↓17.5% | ↓23.7% | Yes | Not reported | Candidaemia-related mortality (pre-AFS and during AFS): 28.0% and 16.4% Decreased, NS | Not reported | Not reported |
| Alfandari et al. (2014) [20] | CT scan, serum BDG and GM (recommendation; diagnostic recommendations based on treatment algorithm to optimize AFT) | Not reported | Not reported | ↓40% | Cost of antifungal prescriptions decreased | Not reported | Not reported | Early IFI-related mortality decreased | Not reported | Not reported |
| Huang et al. (2013) [24] | MALDI-TOF MS, conventional method (VITEK®2; test; for diagnosis and identification of yeast isolates) | Time to organism identific-ation (pre-intervention vs post- intervention period): 84.0 vs 55.9 h Decreased, P < .001 |
Overall, time to effective therapy (pre-intervention vs post-intervention period): 30.1 ± 67.7 vs 20.4 ± 20.7 h Decreased, P = .021 Time to optimal therapy: 90.3 ± 75.4 vs 47.3 ± 121.5 h, Decreased, P < .001 For yeasts, time to effective therapy: 68.6 ± 74.4 vs 45.6 ± 32.0 h Decreased, NS For yeasts, time to optimal therapy: 57.1 ± 60.9 vs 50.9 ± 28.2 h Decreased, NS |
Not reported | Not reported | Not reported | Not reported | Overall, 30-d all-cause mortality (pre-intervention vs post intervention period): 20.3% (52/256) vs 12.7% (31/245) Decreased, P = .021 For yeasts, 30-d all-cause mortality (pre-intervention vs post-intervention period): 33.3% (6/18) vs 17.7% (3/17) Decreased, NS Improved overall survival. Increased, P = .02 |
Overall, LOS (pre-intervention vs post- intervention period): 14.2 ± 20.8 vs 11.4 ± 12.9 d Decreased, NS For yeasts, LOS (pre-intervention vs post-intervention period): 20.9 ± 24.0 vs. 22.1 ± 15.5 d Increased, NS |
Not reported |
| Heil et al. (2012) [23] | PNA FISH assay vs routine methods: CHROMagar Candida and API 20C (test; to evaluate utility for rapid identification of Candida species, time to targeted therapy, and other clinical outcomes) | Median time to species identification (PNA FISH vs routine methods): 0.2 vs 4 d Decreased, P < .001 | Mean time to targeted therapy (pre-implementation group vs post-implementation group): 2.3 vs 0.6 d Decreased, P = .0016 |
Not reported | US$415 per patient | Not reported | Not reported | Pre-implementation group vs post-implementation group: 31% (19/61) vs 24% (5/21) Decreased, NS | Median LOS hospital (pre-implementation group vs post-implementation group): 25 (16–33) vs 12 (9–30) d Decreased, NS |
Not reported |
| Apisarnthanarak et al. (2010) [21] | Conventional identification procedures: API 20C yeast identification system) (recommendation; for identification of Candida isolates and evaluate appropriate antifungal use) | Not reported | Not reported | ↓59% | Total cost savings US$31615 | Yes | Not reported | Crude mortality (pre-intervention period vs post-intervention period): 24% vs 21% Decreased, NS | Not reported | Not reported |
↓ indicates reduction.
Abbreviations: AE, adverse events; AF, antifungal; AFP, antifungal stewardship program; AFS, antifungal stewardship; AFT, antifungal Therapy; API, Analytical Profile Index; BC, blood culture; BDG, serum β-1–3-D-glucan; CT, computed tomography; DOT, days of therapy; GM, galactomannan; HR, hazard ratio; IFI, invasive fungal infections; LOS, length of stay; MALDI-TOF MS, matrix-assisted laser desorption and ionization time-off light mass spectrometry; NR, not reported; NS, not significant; PNA FISH, peptide nucleic acid fluorescence in situ hybridization; TAT, turn-around time; T2CP, T2 Candida Panel.
Performance Measures
Turnaround Time
A total of 4 studies evaluated TAT for diagnostic results [22–24, 36]. Hare et al. reported a median TAT (interquartile range [IQR]) of 4 (2–6) days with once-weekly BDG testing implemented for managing antifungal use in patients with IC in a critical care unit. The study did not report any observed impact on antifungal consumption [22]. Whitney et al. reported a median (IQR) TAT of 13 (11–17) days for BDG and 12 (9–14) days for GM with samples sent to the national reference laboratory. Both tests were used for confirming diagnosis in patients at risk of IFI and receiving AFT. The study reported an initial decrease in antifungal consumption and cost during the early years, which steadily increased during the later period [36]. Importantly, Huang et al. reported a significant reduction in time to species identification and, hence, shorter TAT in the intervention group using MALDI-TOF compared with VITEK2 (55.9 vs 84.0 hours; P < .001), which were used for identification of yeasts in patients with bloodstream infections (BSIs). Use of MALDI-TOF combined with real-time AMS intervention resulted in significantly improved overall survival in the intervention group [24]. Likewise, Heil et al. reported significantly reduced time to species identification in the postimplementation group with Yeast Traffic Light PNA-FISH compared with CHROMagar Candida (0.2 vs 4 days; P < .001) in hospitalized patients with candidemia. The tests were used to evaluate utility in species identification, time to therapy, and time to culture clearance. Implementation of PNA-FISH as part of the AMS protocol decreased time to targeted AFT and time to culture clearance (Table 2) [23].
Time to Therapy
Time to targeted or appropriate therapy was reported by 3/17 studies [23, 24, 28]. Patch et al. evaluated the impact of T2CP on appropriate antifungal use and demonstrated a significant reduction in mean time to appropriate therapy (from the time of blood drawn in T2Candida-positive patients; 6 hours) as compared with BC (from the time the first positive BC was drawn; 34 hours; P = .0147). This led to appropriate utilization of antifungals in patients with candidemia [28]. In an AMS study reported by Huang et al., there was a nonsignificant decrease in time to starting effective therapy (ie, time from BC draw to administration of the first antimicrobial per microbiology report; 45.6 ± 32.0 vs 68.6 ± 74.4 hours) and time to completing optimal therapy (ie, time from BC draw to the time to appropriate therapy, which included de-escalation and discontinuation of antimicrobials; 50.9 ± 28.2 vs 57.1 ± 60.9 hours) in the intervention group with the use of MALDI-TOF and real-time clinical decision support software compared with the pre-intervention group using conventional methods (without real-time support) for yeast-specific outcomes in patients with candidemia. These were further associated with a nonsignificant reduction in ICU LOS and 30-day mortality in the intervention group. However, a significant reduction in time to effective and optimal therapy was noted for pooled outcomes (bacteria and yeast) and associated with significant reduction in ICU LOS and mortality [24]. Heil et al. demonstrated that use of the Yeast Traffic Light PNA-FISH assay in the implementation group resulted in a significant mean time to targeted therapy reduction (ie, time from positive BC to administration of targeted AFT) compared with CHROMagar Candida and API 20C (0.6 vs 2.3 days; P = .0016) in the pre-implementation group. This led to a significant decrease in the time to culture clearance in the implementation group (P = .01) for hospitalized patients with candidemia (Table 2) [23].
Antifungal Consumption and Potential Cost-Savings
Ten studies reported a reduction in antifungal consumption as a result of stewardship interventions [20, 21, 25, 27–30, 32, 33, 35]. Of these, 7 studies demonstrated a reduction ranging from 11.6% to 59.0% [20, 21, 25, 27, 29, 30, 33]. Decrease in antifungal cost was reported by 10 studies [20, 21, 23, 25, 26, 28, 30, 31, 33, 35], of which 5 studies demonstrated a cost-savings ranging from 13.5% to 50.6% (Table 2) [26, 30, 31, 33, 35]. Alfandari et al. reported a cost-savings of €2 million within 7 years (2005–2012) [20]. Three other studies reported individual cost-savings of $31 615 over 18 months [21], $48 400 over 8 months [28], and $138 991 annually [25]. Interestingly, Heil et al. demonstrated a cost-saving of US$415 per patient based on switching from a more expensive echinocandin therapy to fluconazole therapy and reduced mean time to targeted therapy in the postimplementation group [23]. Likewise, Machado et al. reported less use of caspofungin (21.2% vs 6.2%; P = .002) and higher use of fluconazole (18.8% vs 45.5%; P < .001) in the postintervention group with AFS, complemented with BDG, which aimed to evaluate antifungal adequacy and management in hospitalized patients with solid tumor or solid organ transplantation and receiving systemic AFT [26]. Both studies highlighted the importance and benefit of early de-escalation to lower-spectrum antifungals.
Length of Antifungal Therapy, De-escalation, and Discontinuation
A decrease in the length of empiric AFT in the postintervention group complemented with diagnostic results was reported in 3 studies [26, 28, 32]. Additionally, Samura et al. reported a significant reduction in the overall median duration of therapy (P < .001) with the AFS approach supported by BC assessment in hospitalized patients with candidemia (Table 2) [31].
Notably, antifungal treatment discontinuation was demonstrated in 7 studies in this review. Apisarnthanarak et al. reported a significant decrease in inappropriate antifungal use in the postintervention period compared with the pre-intervention period (24% [98/412] vs 71% [493/694]; P < .001) in hospitalized patients with candidiasis [21]. Machado et al. demonstrated that BDG testing facilitated treatment discontinuation in 46.7% of patients (35/75) without IFI receiving systemic AFT. Patch et al. reported discontinuation of empirical AFT in 58.4% (101/173) of patients with candidemia [28], and Steuber et al. demonstrated antifungal discontinuation in 46.7% (105/225) of patients with candidemia due to negative T2CP [32]. Rautemaa-Richardson et al. reported treatment discontinuation in 64.1% (25/39) and 31% (9/29) of ICU patients with invasive candidosis in 2014 and 2016, respectively, based on negative BC and/or BDG results [30]. Valerio et al. reported antifungal discontinuation and de-escalation in 7.1% (32/453) and 17.4% (79/453) of patients receiving systemic AFT for IFI, respectively [33]. Whitney et al. revealed a significant rate of de-escalation in the postimplementation group (87%, 26/30) compared with pre-implementation (50%, 2/4; P = .004) based on diagnostic results in patients at risk of IFI receiving inappropriate AFT. Antifungal prescriptions were discontinued for 62% of patients (86/138) without IFI in the postintervention period compared with 44% (8/18) in pre-implementation period (Table 2) [36].
Clinical Measures
Mortality
Twelve studies reported improvement in mortality-associated outcomes in the postintervention groups, but none were statistically significant [20–23, 25–29, 33, 35, 36]. Additionally, Huang et al. demonstrated a nonsignificant decrease in 30-day all-cause mortality using MALDI-TOF for yeast-specific outcomes (17.7% vs 33.3%) in patients with candidemia. However, the reduction was significant for overall mortality (both bacteria and yeasts; P = .021) (Table 2) [24].
Hospital Length of Stay
Six studies reported reduction in hospital LOS outcomes, and 5 of them demonstrated a nonsignificant decrease due to AFS interventions [22–24, 28, 32]. Whitney et al. indicated a significant decrease in LOS in patients without infection compared with those with proven or probable IFI (30 vs 47 days; P < .0001) (Table 2) [36].
Other Outcomes
The rate of 60-day clinical failure (including a switch of antifungal agent due to low efficacy or AEs, persistence of candidemia, and death due to infection within 60 days) significantly decreased in the postintervention group from 80.0% to 36.4% (P < .001), with a significant decrease in the overall incidence of adverse events (51.4% vs 13.6%; P = .004) in patients with Candida BSI as reported by Ito-Takeichi et al. [25]. Machado et al. demonstrated an improved AF adequacy score with serum BDG testing in the postintervention period (mean, 7.75 vs 9.29; P < .001) among patients with solid tumor or solid organ transplantation who were receiving systemic AFT [26]. Additionally, Martín-Gutiérrez et al. reported a significant decrease in hospital-acquired candidemia during the study period, with a low rate of fluconazole resistance [27]. In contrast, Kawaguchi et al. reported a higher prevalence of nonmajor Candida species (C. rugosa, C. guilliermondii, C. lusitaniae, and other isolates) in the intervention period in patients with candidemia (7.1% vs 8.5%; P = .04) (Table 2) [35].
DISCUSSION
Considering that AFS is a relatively new concept [8], studies focused on AFS outcomes are lacking. While most of the implemented AFS models aim to review and limit inappropriate antifungal prescriptions [9, 37], encourage education and bedside interventions [38], and evaluate care bundle implementation [39, 40], there is a paucity of studies on diagnostic-based AFS interventions and their impact on quality of care. This SLR demonstrated that such AFS interventions aid in improving both performance and clinical measures. As identified in this SLR, the majority were single-center studies from Europe and the United States. Serum BDG testing was most frequently used, followed by the GM test. Importantly, a marked reduction in antifungal consumption and increased cost-savings were noted. The time to targeted therapy and length of empiric therapy improved across 3 studies, while treatment discontinuation and/or de-escalation of inappropriate or empiric therapy was observed in 7 studies. Mortality improved in 13 studies, and LOS decreased in 6 studies.
The primary goal of AFS programs is to optimize AF therapy and patient outcomes through monitoring appropriate antifungal prescribing and duration of therapy [41–43]. Inappropriate use of antifungals is often associated with high cost, potential toxicity, drug interaction, and increased drug resistance [44]. Notably, the diagnostics-driven AFS initiatives used in the majority of studies in this SLR demonstrated cost-savings and reduced antifungal consumption. Decreased antifungal consumption due to early targeted therapy, decreased duration of therapy, and discontinuation or de-escalation of empirical treatment were identified as factors attributing to the cost-savings [9]. Even non-diagnostic-driven AFS studies have demonstrated reduced antifungal usage and cost [35–37, 45]. Moreover, one of the studies included in this SLR also reported a low rate of fluconazole resistance and reduced hospital-acquired candidemia during a comprehensive 9-year AMS program, which may be explained by reduced antimicrobial pressure [27]. Overall, these findings highlight the potential of AFS strategies to reduce unnecessary expenditure associated with empiric or preemptive AFT by optimal antifungal use as well as reducing the risk of emerging antifungal resistance.
Although there was no significant impact on mortality, it is worth mentioning that a decrease in mortality was observed in the postintervention groups among most of the studies in this SLR. Notably, Vena et al. demonstrated a significant reduction in 14- and 30-day mortality in patients with candidemia in a well-structured AFS program with a systematic bundle approach [40]. Additionally, no significant impact on hospital LOS was observed in the studies; however, LOS decreased in 6 studies, which is encouraging considering the high health care costs (including AFT and ward cost) associated with IFIs [44]. A potential explanation for most studies not achieving statistical significance in outcomes including mortality and LOS could be low patient numbers (n < 150 in 6/10 studies), crude mortality outcomes being considered rather than AFS-attributable mortality, and unequal size of pre- and postintervention arms. Moreover, clinically relevant outcomes including resistance rates and infection-related or all-cause mortality are considered secondary outcomes because studies are typically underpowered to detect significant changes in these outcomes. Importantly, the majority of AFS studies analyzed achieved the target of optimizing antifungal use without any negative impact such as increasing proven IFIs or mortality. Overall, these results demonstrate the safety of diagnostics-driven AFS approaches.
Integrating rapid diagnostic tests (RDTs) into AFS programs may enable faster TATs of results, leading to earlier initiation of appropriate therapy and improving clinical outcomes [46]. Several techniques such as Candida albicans germ tube–specific antibody (CAGTA; for diagnosing deep-seated candidiasis) [47], multiplex PCR assay [48], and lateral-flow immunoassay [49, 50] have faster TATs and provide rapid diagnosis. Another SLR comprising 3 additional studies (besides Heil et al. and Huang et al., which are included in the current review) assessed the impact of RDTs and real-time clinical decision support on AFS goals for IC [51]. The additional studies employed various RDTs including T2MR (T2CP), rapid multiplex PCR (FilmArray Blood Culture ID Panel), MALDI-TOF, and PNA-FISH (AdvanDx) [52–54]. Overall, that review highlighted real-time AFS efforts combined with RDTs that resulted in optimal antifungal use, improved mortality, and reduced health care costs for IC [51].
The lack of access and availability of rapid diagnostic tools is limiting. This SLR demonstrated wider use of serum BDG and GM tests for diagnosis compared with other RDTs for fungal species identification such as MALDI-TOF, T2MR (T2CP), and PNA-FISH (AdvanDx). Another study evaluating diagnostic capacity in UK laboratories revealed that 49% (33/68) of laboratories performed microscopy from specimens or cultures as the first line of examination. Other diagnostic methods used were MALDI-TOF (83%, 57/69), the VITEK2 system (43%, 30/69), API identification (32%, 22/69), chromogenic agar (65%, 45/69), and molecular sequencing [5]. A recent report from the Fungal Diagnostic Laboratory Consortium in North America identified several gaps in fungal diagnostic capacity across clinical laboratories, including the need for optimal diagnostic approaches [55]. These findings suggest a lack of standardized diagnostic algorithms across clinical laboratories.
Another major limitation associated with commercial assays like the BDG tests includes analysis of multiple samples in batches, as reported by several studies [56, 57]. This impacts timely and effective reporting and communicating test results with physicians. In the current SLR, Hare et al. reported a longer median TAT (IQR) of 4 (2–6) days for once-weekly BDG testing for optimizing antifungal usage in patients with IC in critical care, and which subsequently did not reduce antifungal consumption [22]. Interestingly, another study demonstrated that using twice-weekly in-house BDG testing for IC diagnosis impacted therapeutic decisions in 57% (41/72) of ICU patients. The impact was positive (including AF abstention, interruption, initiation, and continuation) in 73% (30/41) of patients. Notably, a median TAT of 2 working days was observed in the study (TAT might have further prolonged due to weekends) [56]. Diagnostic approaches promoting batch testing of samples for cost-effective measures lead to delayed TAT, thereby limiting potential time-saving benefits of rapid diagnostics for IFIs [57]. Additionally, many laboratories lack in-house/onsite testing facilities and outsource samples to a reference center. The additional time required for transport of samples adds to the TAT, thereby limiting the targeted therapy initiation and prolonging the length of inappropriate therapy, thus leading to poor clinical response. In this SLR, Whitney et al. aimed to optimize antifungal prescribing in patients with IFI with the use of GM and BDG and revealed a longer median TAT for both tests (<2 weeks) as samples were transported to a reference laboratory, without any major change in overall antifungal consumption and cost [36]. A recent UK-based survey revealed that outsourcing was prevalent for non-culture-based diagnostic tests including serum BDG and GM tests [5]. Taken together, these findings suggest the urgent need to close such diagnostic gaps by improving local infrastructure and capabilities.
This SLR identified more prevalent AFS implementation in resource-rich regions such as the United States and Europe and limited or lack of studies in Asia and other developing countries (including from Africa and Latin America), which is corroborated by other studies from the Asia Fungal Working Group [58, 59]. These studies have highlighted the lack of AFS programs and diagnostic challenges associated with appropriate management of IFI [58, 59]. Likewise, a recent survey from Latin America and the Caribbean identified the limited scope for diagnostics across laboratories for fungal identification [60]. The lack of fungal diagnostic capabilities in these regions underscores the need for implementing appropriate AFS strategies and building laboratory capabilities in resource-limited clinical settings.
Despite the increasing focus on management of IFIs, only a few studies on AFS programs have assessed and reported relevant outcomes on quality of care. There is an urgent need for well-designed AFS studies supported by evidence-based recommendations. Adopting a multifaceted, standardized approach and establishing an appropriate core set of metrics to evaluate the impact of AFS on outcomes would be key to a successful stewardship program [8, 43, 44, 61, 62]. A list of proposed metrics or measures as identified across studies is summarized in Table 3.
Table 3.
| Outcome | Proposed Metrics |
|---|---|
| Antifungal consumption |
|
| Antifungal prescribing quality |
|
| Diagnosis |
|
| Microbiological |
|
| Clinical |
|
| Cost |
|
Abbreviations: AFS, antifungal stewardship; DDD, defined daily dose; IFI, invasive fungal infections; LOS, length of stay.
The current SLR has certain limitations. Limited evidence was available to evaluate diagnostic-driven AFS interventions, with few studies with pre- and postintervention approaches. Considering the heterogeneity of studies, direct comparison across studies was not possible due to variability in AFS initiatives, study methodologies, and outcomes analyzed, and hence the data must be interpreted accordingly. Most studies were conducted at single centers with small patient numbers (n < 150). Considering that most of the studies focused on Candida diagnosis, adequate evidence on diagnostic setup and challenges for other fungal pathogens may not have been presented. Additionally, studies with >1 indication or pathogen did not stratify reported outcomes based on included species, and therefore separate analysis of outcomes based on species was not feasible. In the absence of appropriate control groups, it is also difficult to fully ascertain whether the benefits in reported outcomes may be attributed to diagnostic tests used in combination with AFS initiatives compared with the tests alone. Finally, this review is not supported statistically, and therefore, caution is required before drawing definitive conclusions.
CONCLUSIONS
This SLR provides crucial evidence on the potential of AFS initiatives to implement diagnostic approaches that improve clinical and economic outcomes for patients. Implementation of appropriate diagnostic tests yielding results 24 hours every day should be fostered to support timely and appropriate AFT. Additionally, AFS programs must focus on clinical indicators to show improvement in patient outcomes, in addition to achieving the cost-savings associated with decreased antifungal consumption. The current review also identified a gap in implementing and reporting AFS in developing countries. Of note, access to advanced diagnostic techniques is a major challenge in developing countries and remains a potential issue to be addressed. Considering that AFS studies did not demonstrate any negative impact on patient outcomes, AFS initiatives should be encouraged across countries.
Acknowledgments
The authors acknowledge Sweta Samantaray, PhD, an employee of Pfizer, for data analysis and writing support for this manuscript. The authors would also like to thank Sonia Philipose, PhD, CMPP, and Arjun Krishnakumar, PhD, CMPP, both employees of Pfizer, for their support in literature searches and data analysis, as well as editorial assistance.
Financial support . This study was supported by Pfizer Inc.
Potential conflicts of interest . Mohamed N, Capparella MR, Townsend A, Sung A, and Yura R are employees of Pfizer and may hold stock or stock options. Yura R also holds stocks at Novartis, JNJ, and BMS. Chakrabarti A has received honoraria, research grants, and travel grants from WHO, ICMR, DST, DBT, Pfizer, Gilead, and MSD. Muñoz P has received honoraria from Pfizer and Gilead. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions . Arunaloke Chakrabarti: conceptualization, methodology, writing - review & editing; Naglaa Mohamed: conceptualization, methodology, writing - review & editing; Maria Rita Capparella: conceptualization, methodology, writing - review & editing; Anita H. Sung: conceptualization, methodology, writing - review & editing; Renee Yura: conceptualization, methodology, writing - review & editing; Patricia Muñoz: conceptualization, methodology, writing - review & editing; Andy Townsend: conceptualization, methodology, writing - review & editing, funding acquisition.
Patient consent . This manuscript does not include factors necessitating patient consent.
Prior presentation . Some results from this manuscript have been previously presented as a poster presentation at the European Congress of Clinical Microbiology & Infectious Diseases, Vienna, Austria, 2021 (abstract no. 00931).
Contributor Information
Arunaloke Chakrabarti, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Naglaa Mohamed, Pfizer Inc., New York, USA.
Maria Rita Capparella, Pfizer International Operations, Paris, France.
Andy Townsend, Pfizer Hospital Medical Affairs, Pfizer, Congleton, UK.
Anita H Sung, Pfizer Inc., New York, USA.
Renee Yura, WRD & Medical, Pfizer, Cambridge, Massachusetts, USA.
Patricia Muñoz, Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Instituto de Investigación Sanitaria Hospital Gregorio Marañón, Madrid, Spain; Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain; CIBER Enfermedades Respiratorias – CIBERES (CB06/06/0058), Madrid, Spain.
References
- 1. Bongomin F, Gago S, Oladele RO, et al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017; 3(4):1–29. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson MD, Lewis RE, Dodds Ashley ES, et al. Core recommendations for antifungal stewardship: a statement of the Mycoses Study Group Education and Research Consortium. J Infect Dis 2020; 222:S175–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Latgé JP, Chamilos G. Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev 2019; 33:e00140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019; 68:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schelenz S, Owens K, Guy R, et al. National mycology laboratory diagnostic capacity for invasive fungal diseases in 2017: evidence of sub-optimal practice. J Infect 2019; 79:167–73. [DOI] [PubMed] [Google Scholar]
- 6. Chakrabarti A, Meis JF, Cornely OA. International Society for Human and Animal Mycology (ISHAM)—new initiatives. J Fungi (Basel) 2020; 6(3):1–6. doi: 10.3390/jof6030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valerio M, Vena A, Rodríguez-González CG, et al. Repeated antifungal use audits are essential for selecting the targets for intervention in antifungal stewardship. Eur J Clin Microbiol Infect Dis 2018; 37:1993–2000. [DOI] [PubMed] [Google Scholar]
- 8. Hart E, Nguyen M, Allen M, et al. A systematic review of the impact of antifungal stewardship interventions in the United States. Ann Clin Microbiol Antimicrob 2019; 18(1):1–10. doi: 10.1186/s12941-019-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Micallef C, Aliyu SH, Santos R, et al. Introduction of an antifungal stewardship programme targeting high-cost antifungals at a tertiary hospital in Cambridge, England. J Antimicrob Chemother 2015; 70:1908–11. [DOI] [PubMed] [Google Scholar]
- 10. Kidd SE, Chen SC, Meyer W, et al. A new age in molecular diagnostics for invasive fungal disease: are we ready? Front Microbiol 2020; 10:1–20. doi: 10.3389/fmicb.2019.02903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Acosta J, Catalan M, del Palacio-Pérez-Medel A, et al. Prospective study in critically ill non-neutropenic patients: diagnostic potential of (1,3)-β-D-glucan assay and circulating galactomannan for the diagnosis of invasive fungal disease. Eur J Clin Microbiol Infect Dis 2012; 31:721–31. [DOI] [PubMed] [Google Scholar]
- 12. Aquino VR, Nagel F, Andreolla HF, et al. The performance of real-time PCR, galactomannan, and fungal culture in the diagnosis of invasive aspergillosis in ventilated patients with chronic obstructive pulmonary disease (COPD). Mycopathologia 2012; 174:163–9. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen MH, Wissel MC, Shields RK, et al. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin Infect Dis 2012; 54:1240–8. [DOI] [PubMed] [Google Scholar]
- 14. Mylonakis E, Zacharioudakis IM, Clancy CJ, et al. Efficacy of T2 magnetic resonance assay in monitoring candidemia after initiation of antifungal therapy: the Serial Therapeutic and Antifungal Monitoring Protocol (STAMP) trial. J Clin Microbiol 2018; 56:e01756-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stone NR, Gorton RL, Barker K, et al. Evaluation of PNA-FISH yeast traffic light for rapid identification of yeast directly from positive blood cultures and assessment of clinical impact. J Clin Microbiol 2013; 51:1301–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendrickx M. MALDI-TOF MS and filamentous fungal identification: a success story? Curr Fungal Infect Rep 2017; 11:60–5. [Google Scholar]
- 17. Baggio D, Peel T, Peleg AY, et al. Closing the gap in surveillance and audit of invasive mold diseases for antifungal stewardship using machine learning. J Clin Med 2019; 8(9):1–13. doi: 10.3390/jcm8091390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wattal C, Chakrabarti A, Oberoi JK, et al. Issues in antifungal stewardship: an opportunity that should not be lost. J Antimicrob Chemother 2017; 72:969–74. [DOI] [PubMed] [Google Scholar]
- 19. PRISMA Checklist. 2020. Available at: http://www.prisma-statement.org/documents/PRISMA_2020_checklist.pdf. Accessed 23 August 2021.
- 20. Alfandari S, Berthon C, Coiteux V. Antifungal stewardship: implementation in a French teaching hospital. Med Mal Infect 2014; 44:154–8. [DOI] [PubMed] [Google Scholar]
- 21. Apisarnthanarak A, Yatrasert A, Mundy LM. Impact of education and an antifungal stewardship program for candidiasis at a Thai tertiary care center. Infect Control Hosp Epidemiol 2010; 31:722–7. [DOI] [PubMed] [Google Scholar]
- 22. Hare D, Coates C, Kelly M, et al. Antifungal stewardship in critical care: implementing a diagnostics-driven care pathway in the management of invasive candidiasis. Infect Prevent Practice 2020; 2:100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heil EL, Daniels LM, Long DM, et al. Impact of a rapid peptide nucleic acid fluorescence in situ hybridization assay on treatment of Candida infections. Am J Health Syst Pharm 2012; 69:1910–4. [DOI] [PubMed] [Google Scholar]
- 24. Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57:1237–45. [DOI] [PubMed] [Google Scholar]
- 25. Ito-Takeichi S, Niwa T, Fujibayashi A, et al. The impact of implementing an antifungal stewardship with monitoring of 1-3, β-D-glucan values on antifungal consumption and clinical outcomes. J Clin Pharm Ther 2019; 44:454–62. [DOI] [PubMed] [Google Scholar]
- 26. Machado M, Chamorro de Vega E, Martínez-Jiménez MDC, et al. Utility of 1,3 β-d-glucan assay for guidance in antifungal stewardship programs for oncologic patients and solid organ transplant recipients. J Fungi (Basel) 2021; 7(1):2–12. doi: 10.3390/jof7010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martín-Gutiérrez G, Peñalva G, Ruiz-Pérez de Pipaón M, et al. Efficacy and safety of a comprehensive educational antimicrobial stewardship program focused on antifungal use. J Infect 2020; 80:342–9. [DOI] [PubMed] [Google Scholar]
- 28. Patch ME, Weisz E, Cubillos A, et al. Impact of rapid, culture-independent diagnosis of candidaemia and invasive candidiasis in a community health system. J Antimicrob Chemother 2018; 73:iv27–30. [DOI] [PubMed] [Google Scholar]
- 29. Ramos A, Pérez-Velilla C, Asensio A, et al. Antifungal stewardship in a tertiary hospital. Rev Iberoam Micol 2015; 32:209–13. [DOI] [PubMed] [Google Scholar]
- 30. Rautemaa-Richardson R, Rautemaa V, Al-Wathiqi F, et al. Impact of a diagnostics-driven antifungal stewardship programme in a UK tertiary referral teaching hospital. J Antimicrob Chemother 2018; 73:3488–95. [DOI] [PubMed] [Google Scholar]
- 31. Samura M, Hirose N, Kurata T, et al. Support for fungal infection treatment mediated by pharmacist-led antifungal stewardship activities. J Infect Chemother 2020; 26:272–9. [DOI] [PubMed] [Google Scholar]
- 32. Steuber TD, Tucker-Heard G, Edwards J, et al. Utilization and impact of a rapid Candida panel on antifungal stewardship program within a large community hospital. Diagn Microbiol Infect Dis 2020; 97:115086. [DOI] [PubMed] [Google Scholar]
- 33. Valerio M, Muñoz P, Rodríguez CG, et al. Antifungal stewardship in a tertiary-care institution: a bedside intervention. Clin Microbiol Infect 2015; 21:492.e1–9. [DOI] [PubMed] [Google Scholar]
- 34. Murakami M, Komatsu H, Sugiyama M, et al. Antimicrobial stewardship without infectious disease physician for patients with candidemia: a before and after study. J Gen Fam Med 2018; 19:82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawaguchi H, Yamada K, Imoto W, et al. The effects of antifungal stewardship programs at a tertiary-care teaching hospital in Japan. J Infect Chemother 2019; 25:458–62. [DOI] [PubMed] [Google Scholar]
- 36. Whitney L, Al-Ghusein H, Glass S, et al. Effectiveness of an antifungal stewardship programme at a London teaching hospital 2010-16. J Antimicrob Chemother 2019; 74:234–41. [DOI] [PubMed] [Google Scholar]
- 37. López-Medrano F, Juan RS, Lizasoain M, et al. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital. Clin Microbiol Infect 2013; 19:56–61. [DOI] [PubMed] [Google Scholar]
- 38. Santiago-García B, Rincón-López EM, Ponce Salas B, et al. Effect of an intervention to improve the prescription of antifungals in pediatric hematology-oncology. Pediatr Blood Cancer 2020; 67:e27963. [DOI] [PubMed] [Google Scholar]
- 39. Antworth A, Collins CD, Kunapuli A, et al. Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. Pharmacotherapy 2013; 33:137–43. [DOI] [PubMed] [Google Scholar]
- 40. Vena A, Bouza E, Corisco R, et al. Efficacy of a “checklist” intervention bundle on the clinical outcome of patients with Candida bloodstream infections: a quasi-experimental pre-post study. Infect Dis Ther 2020; 9:119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valerio M, Vena A, Bouza E, et al. How much European prescribing physicians know about invasive fungal infections management? BMC Infect Dis 2015; 15:1–8. doi: 10.1186/s12879-015-0809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valerio M, Muñoz P, Rodríguez-González C, et al. Training should be the first step toward an antifungal stewardship program. Enferm Infecc Microbiol Clin 2015; 33:221–7. [DOI] [PubMed] [Google Scholar]
- 43. Khanina A, Urbancic KF, Haeusler GM, et al. Establishing essential metrics for antifungal stewardship in hospitals: the results of an international Delphi survey. J Antimicrob Chemother 2021; 76:253–62. [DOI] [PubMed] [Google Scholar]
- 44. Hamdy RF, Zaoutis TE, Seo SK. Antifungal stewardship considerations for adults and pediatrics. Virulence 2017; 8:658–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morii D, Ichinose N, Yokozawa T, Oda T. Impact of an infectious disease specialist on antifungal use: an interrupted time-series analysis in a tertiary hospital in Tokyo. J Hosp Infect 2018; 99:133–8. [DOI] [PubMed] [Google Scholar]
- 46. Beganovic M, McCreary EK, Mahoney MV, et al. Interplay between rapid diagnostic tests and antimicrobial stewardship programs among patients with bloodstream and other severe infections. J Appl Lab Med 2019; 3:601–16. [DOI] [PubMed] [Google Scholar]
- 47. Martínez-Jiménez MC, Muñoz P, Guinea J, et al. Potential role of Candida albicans germ tube antibody in the diagnosis of deep-seated candidemia. Med Mycol 2014; 52:270–5. [DOI] [PubMed] [Google Scholar]
- 48. Altun O, Almuhayawi M, Ullberg M, Ozenci V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J Clin Microbiol 2013; 51:4130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takazono T, Ito Y, Tashiro M, et al. Evaluation of Aspergillus-specific lateral-flow device test using serum and bronchoalveolar lavage fluid for diagnosis of chronic pulmonary aspergillosis. J Clin Microbiol 2019; 57:e00095-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He Z-X, Shi L-C, Ran X-Y, et al. Development of a lateral flow immunoassay for the rapid diagnosis of invasive candidiasis. Front Microbiol 2016; 7:1–7. doi: 10.3389/fmicb.2016.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sofjan AK, Musgrove RJ, Garey KW. Impact of new diagnostic approaches for invasive candidiasis on antifungal stewardship. Curr Fungal Infect Rep 2016; 10:68–77. [Google Scholar]
- 52. Bilir SP, Ferrufino CP, Pfaller MA, Munakata J. The economic impact of rapid Candida species identification by T2Candida among high-risk patients. Future Microbiol 2015; 10:1133–44. [DOI] [PubMed] [Google Scholar]
- 53. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aitken SL, Beyda ND, Shah DN, et al. Clinical practice patterns in hospitalized patients at risk for invasive candidiasis: role of antifungal stewardship programs in an era of rapid diagnostics. Ann Pharmacother 2014; 48:683–90. [DOI] [PubMed] [Google Scholar]
- 55. Zhang SX, Babady NE, Hanson KE, et al. Recognition of diagnostic gaps for laboratory diagnosis of fungal diseases: expert opinion from the Fungal Diagnostics Laboratories Consortium (FDLC). J Clin Microbiol 2021; 59:e0178420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kritikos A, Poissy J, Croxatto A, et al. Impact of the beta-glucan test on management of intensive care unit patients at risk for invasive candidiasis. J Clin Microbiol 2020; 58:e01996-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friedrich R, Rappold E, Bogdan C, Held J. Comparative analysis of the wako β-glucan test and the Fungitell assay for diagnosis of candidemia and Pneumocystis jirovecii pneumonia. J Clin Microbiol 2018; 56:e00464-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan BH, Chakrabarti A, Patel A, et al. Clinicians’ challenges in managing patients with invasive fungal diseases in seven Asian countries: an Asia Fungal Working Group (AFWG) survey. Int J Infect Dis 2020; 95:471–80. [DOI] [PubMed] [Google Scholar]
- 59. Chindamporn A, Chakrabarti A, Li R, et al. Survey of laboratory practices for diagnosis of fungal infection in seven Asian countries: an Asia Fungal Working Group (AFWG) initiative. Med Mycol 2018; 56:416–25. [DOI] [PubMed] [Google Scholar]
- 60. Falci DR, Pasqualotto AC. Clinical mycology in Latin America and the Caribbean: a snapshot of diagnostic and therapeutic capabilities. Mycoses 2019; 62:368–73. [DOI] [PubMed] [Google Scholar]
- 61. Morris AM. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis 2014; 6:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ananda-Rajah MR, Slavin MA, Thursky KT. The case for antifungal stewardship. Curr Opin Infect Dis 2012; 25:107–15. [DOI] [PubMed] [Google Scholar]