SUMMARY
Mycobacteria are intrinsically resistant to a variety of stresses including many antibiotics. Although a number of pathways have been described to account for the observed resistances, the mechanisms that control the expression of genes required in these processes remain poorly defined. Here we report the role of a predicted anti-sigma factor, MSMEG_6129 and a predicted eukaryotic like serine/threonine protein kinase, MSMEG_5437, in the intrinsic resistance of Mycobacterium smegmatis to a variety of stresses including the genotoxic agent mitomycin C, hydrogen peroxide and at least four different antibiotics - isoniazid, chloramphenicol, erythromycin and tetracycline. We show that MSMEG_5437 influences the phosphorylation state of MSMEG_6129. Further, MSMEG_6129 controls the expression of a plethora of genes including efflux pumps, ABC transporters, catalases and transcription factors, either directly or via regulators like WhiB7, which account for the observed multi-drug resistance phenotypes. MSMEG_6129 in turn phosphorylates a contiguously located putative anti-anti-sigma factor, MSMEG_6127. We therefore propose that MSMEG_5437, MSMEG_6129 and MSMEG_6127 are components of a master regulatory network, upstream of whiB7, that controls the activity of one or more of the 28 sigma factors in M. smegmatis. Together, this network controls the expression of a regulon required for resistance to several unrelated antibiotics.
Keywords: Anti-sigma factor, intrinsic drug resistance, whiB7
INTRODUCTION
The genus Mycobacterium includes both pathogenic and saprophytic species that are able to survive exposure to environmental stresses, including oxidative and genotoxic stress, hypoxia, nutrient starvation, and exposure to multiple antibiotics (Hingley-Wilson et al., 2003, Zahrt and Deretic, 2002). Mycobacterial responses to stress are of particular interest for understanding the pathogenesis of Mycobacterium tuberculosis and its sensitivity and reaction to antibiotics (McKinney, 2000). A variety of such pathways and regulators have been described (Zahrt and Deretic, 2002, Rustad et al., 2009, McKinney et al., 2000, Rodrigue et al., 2006, Missiakas and Raina, 1998). The ability of M. tuberculosis to persist for many years in its human host, and the requirement for lengthy antibiotic regimens to eliminate drug sensitive strains reflects the effectiveness of the responses to stressful environments. Many of the processes are likely to be adapted from stress responses that are common to mycobacteria and related strains, including saprophytes such as Mycobacterium smegmatis. The detailed pathways, signals, regulatory responses and molecular interactions are not yet well understood; however, it is clear that mycobacteria have very complex and overlapping regulatory pathways reflected in the M. tuberculosis proteome of about 190 transcription regulators including sigma factors, two component systems, protein kinases as well as over a hundred transcription activators and repressors (Bishai, 1998).
Transcriptional reprogramming in bacteria is mediated by a complex array of regulatory factors including a repertoire of sigma factors that reversibly associate with RNA polymerase to enable transcription initiation at specific subsets of promoters (Gruber and Gross, 2003). Alternate sigma factors are often regulated post-translationally by anti-sigma factors that function by binding to and sequestering their cognate sigma factors thus preventing promoter recognition by RNA polymerase (Helmann, 1999). Anti-anti-sigma factors relieve this repression by binding to the anti-sigma factor (Hughes and Mathee, 1998). This complex set of three-partner-switching interactions ultimately determines the concentrations of active sigma factors and consequently the gene expression profile, and has been best studied in Bacillus spp (Kroos et al., 1999).
Transcription regulation in mycobacteria appears more intricate and presents significant departures from the less complex systems of Bacillus and Escherichia coli. Mycobacterium tuberculosis encodes 11 alternate sigma factors and M. smegmatis encodes at least 26 (Rodrigue et al., 2006), each of which is presumably regulated by its cognate set of regulators. To date regulators of only four mycobacterial sigma factors - σH, σF, σE,σL- have been experimentally demonstrated and highlight the intricasies of regulation (Barik et al., 2010, Beaucher et al., 2002, Greenstein et al., 2007, Malik et al., 2009, Park et al., 2008, Raman et al., 2001, Song et al., 2003, Dainese et al., 2006). The oxidative stress responsive alternate sigma factor, σH, is controlled by the anti-sigma factor RshA (Park et al., 2008, Raman et al., 2001, Song et al., 2003). RshA is a redox sensor and sequesters σH in a reducing environment; under oxidizing conditions disulfde bond formation between cysteines in RshA releases σH repression. The RshA-σH interaction has also been shown to be under further control of phosphorylation by the eukaryotic like STPK, PknB, such that phosphorylation of RshA leads to dissociation of the complex(Raman et al., 2001, Park et al., 2008, Song et al., 2003). The stress responsive σF is also under a complex set of regulatory mechanism involving the anti-sigma factor UsfX which is in turn controlled by two anti-anti-sigma factors RsfA and RsfB(Beaucher et al., 2002). RsfA is redox sensor and regulates UsfX under reducing conditions while RsfB is controlled by phosphorylation. Furthermore, unlike other studied anti-sigma factors, UsfX does not phosphorylate RsfB and the kinase that phosphorylates RsfB is hitherto unknown (Beaucher et al., 2002, Malik et al., 2009, Greenstein et al., 2007). σE is regulated by RseA in a redox dependent manner; pknB dependent phosphorylation of RseA is further required for cleavage of RseA thereby activating the σE regulon (Barik et al., 2010).
Mycobacteria are intrinsically resistant to several commonly used antibiotics (da Silva et al., 2011). This has been attributed to the highly impermeable mycolic acid containing cell wall as well as to the constitutive and inducible expression of several active drug efflux mechanisms. Three transcriptional regulators, LfrR, MarR and WhiB7, are known to affect drug susceptibility(Morris et al., 2005, Buroni et al., 2006, McDermott et al., 1998) of which M. tuberculosis whiB7 is best studied and has been shown to influence sensitivity to five unrelated antibiotics (Morris et al., 2005). Further, it is known that levels of drug resistance are influenced by the degrees of expression of the drug targets and their regulators – including InhA for isoniazid (Larsen et al., 2002), EthA that catalyzes the activation of ethionamide (Engohang-Ndong et al., 2004) and LfrA the fluoroquinolone efflux pump (Buroni et al., 2006) and are thus likely to be influenced by the complex regulatory transcription pathways. A recent demonstration of the involvement of SigI in isoniazid resistance in M. tuberculosis(Lee et al., 2012b) as well as SigA in multi-drug resistance in M. smegmatis(Burian et al., 2013) further underscores the significance of global transcription modulation in intrinsic resistance of mycobacteria to antibiotics.
Here we present data that suggests the existence of a master regulatory network upstream of whiB7, comprised of an anti-sigma factor (MSMEG_6129) and an anti-anti sigma factor (MSMEG_6127), that influences resistance in M. smegmatis to mitomycin C, hydrogen peroxide (H2O2) and several unrelated antibiotics by controlling a large regulon including ABC transporters and drug efflux pumps, catalases, WhiB7 and other transcription factors. Conceivably this circuit is itself responsive to regulatory proteins and signals that fine-tune its activity, one of which we demonstrate is MSMEG_5437, a eukaryotic like serine/threonine protein kinase.
RESULTS
MSMEG_5437 is required for M. smegmatis tolerance to mitomycin C and antibiotics
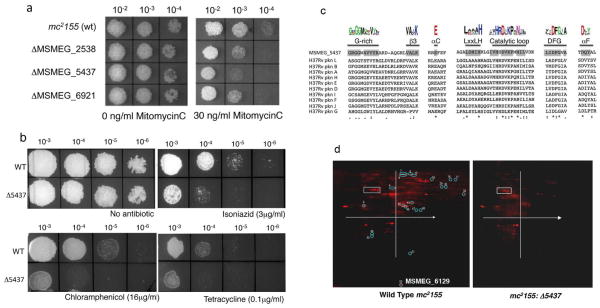
In a Transposon Site Hybridization (TraSH)-based search (Murry et al., 2008, Sassetti et al., 2001) for genes responsive to mitomycin C (MMC) in M. smegmatis, we identified 28 novel genes corresponding to a variety of different pathways or processes that are at least five-fold underrepresented under MMC exposure (Table S1). Three putative regulatory proteins, MSMEG_5437, MSMEG_6921 and MSMEG_2538 – encoding a predicted serine-threonine kinase, a conserved hypothetic regulator, and a MarA-like transcription factor respectively – are of particular interest as potential components of a genotoxic stress-responsive pathway (Table S1). Isogenic deletion strains of the three genes constructed by recombineering (van Kessel and Hatfull, 2008) are hypersensitive to 30 ng/ml MMC by at least one order of magnitude compared to wild-type M. smegmatis (Fig. 1a). Of these, mc2155:Δ5437 shows the greatest sensitivity towards MMC. However the MSMEG_5437-dependent response is unlikely to be within the DNA repair pathway per se, because the mutant is unaltered in its response to either ultraviolet light or hydrogen peroxide (H2O2) (Fig. S1). Surprisingly, the mutant has a pronounced increase in sensitivity to the unrelated antibiotics isoniazid, tetracycline and chloramphenicol, suggesting a central involvement of MSMEG_5437 in response to environmental stresses (Fig. 1b). The antibiotic susceptibility profile is, however, quite specific and no change in resistance was observed with rifampicin, ethionamide, ethambutol, or ciprofloxacin (Fig. S1).
Figure 1. MSMEG_5437, a predicted STPK, is required for M. smegmatis tolerance to MitomycinC and antibiotics.
(a) Ten fold dilutions of gene replacement mutants in MSMEG_2538, MSMEG_5437 and MSMEG_6921 were spotted on Middlebrook 7H10 containing 30ng/ml of MMC with ΔMSMEG_5437 being the most sensitive of the mutants. (b) Ten fold serial dilutions of wild type and mc2155:Δ5437 were spotted on Middlebrook 7H10 containing indicated concentrations of isoniazid, chloramphenicol and tetracycline as well as a control lacking antibiotic. The mutant is hypersensitive to all three antibiotics tested. (c) Multiple sequence alignment of MSMEG_5437 with the eukaryotic like STPKs in M. tuberculosis showing the conserved signature motifs of bacterial ser/thr kinases and the conserved kinase domain in MSMEG_5437 shaded in grey. Deviations in the sequence of MSMEG_5437 are observed in the glycine rich motif and the catalytic loop and are marked. (d) Total cell lysate was prepared from wild type mc2155 and mc2155: Δ5437 and separated by 2-D gel electrophoresis. The gels were fixed and stained with a fluorescent phosphoprotein that stains all phosphorylated proteins. Differentially phosphorylated proteins were located using a combination of ImageQuant and DeCyder softwares. The white box and x and y_axis on each slide are for orientation purposes. Fifty one differentially phosphorylated proteins were identified and are circled and numbered. Twenty one protein spots were completely absent in mc2155: Δ5437 and were excised from the gel and identified by MALDI TOF/TOF (tandem mass spectrometry MS/MS). Identity of these spots is presented in Table 1.
BLASTP searches of MSMEG_5437 against the NCBI protein database revealed similarity to the eukaryotic-like serine/threonine protein kinases (STPKs) found in bacteria, and MSMEG_5437 is one of 18 putative STPKs (pknA through pknL) identified in the genome sequence of M. smegmatis. MSMEG_5437 shows sequence conservation to the catalytic kinase domain that is well conserved in all mycobacterial kinases and it is difficult to discern which class of kinases it is functionally related to. However, MSMEG_5437 contains almost all of the conserved signature motifs of the catalytic domains of eukaryotic STPKs that is also present in mycobacterial kinases (Av-Gay and Everett, 2000) including the critical catalytic lysine residue at position 126 (Fig. 1c). To determine the in vivo targets of MSMEG_5437 we performed 2-DIGE analysis of total protein from wild type and mc2155: Δ5437 bacteria followed by phosphoprotein staining. A prominent decrease in the in vivo phosphoprotein levels in the mutant is consistent with it either acting directly as a kinase, or being required for the activity of other M. smegmatis kinases (Fig. 1d). We could identify a total of 52 protein species that were differentially phosphorylated in the mutant; of these 18 were completely undetectable in mc2155: Δ5437 and their identity was determined using mass-spectrometry. These proteins represent a broad array of diverse predicted functions (Table 1).
Table 1. Identification of phosphoproteins absent in mc2155: Δ5437 using MALDI-TOF.
Total cell lysate was prepared from wild type mc2155 and mc2155: Δ5437 were separated by 2-D gel electrophoresis and the phosphorylated proteins identified by fluorescent staining. Differentially phosphorylated proteins were located using a combination of ImageQuant and DeCyder softwares. Of the 51 differentially phosphorylated proteins 21 protein spots were completely absent in mc2155: Δ5437 and were excised from the gel and identified by MALDITOF/TOF (tandem mass spectrometry MS/MS).
| Spot # | M. smegmatis ID | M. tuberculosis ID | Protein ID |
|---|---|---|---|
| 4 | MSMEG_0059 | Rv3868 | ATPase, AAA family protein |
| 9 | MSMEG_1881 | Rv3240c | preprotein translocase subunit SecA |
| 10 | MSMEG_6091 | Rv3596c | negative regulator of genetic competence ClpC/mecB |
| 16, 17 | MSMEG_0456 | Rv0006 | DNA gyrase subunit A |
| 15 | MSMEG_0005 | Rv0005 | DNA gyrase subunit B |
| 30 | MSMEG_1670 | Rv3318 | succinate dehydrogenase flavoprotein subunit |
| 33 | MSMEG_6761 | Rv3302c | glycerol-3-phosphate dehydrogenase 2 |
| 8 | MSMEG_3642 | Rv1832 | glycine dehydrogenase |
| 45, 46, 47 | MSMEG_2080 | Rv3140 | putative acyl-CoA dehydrogenase |
| 29 | MSMEG_1807 | Rv3285 | acetyl-/propionyl-coenzyme A carboxylase alpha chain |
| 7 | MSMEG_2412 | Rv2967c | pyruvate carboxylase |
| 48 | MSMEG_3046 | Rv1383 | carbamoyl phosphate synthase small subunit |
| 49 | MSMEG_0702 | Rv0791c | monooxygenase |
| 50 | MSMEG_2351 | Rv3029c | electron transfer flavoprotein, beta subunit |
| 51 | MSMEG_6427 | Rv3846 | [Mn] superoxide dismutase |
| 52 | MSMEG_6129 | - | Putative anti-sigma factor |
| 43 | MSMEG_1401 | Rv0685 | elongation factor Tu |
| 6 | MSMEG_3102 | Rv1448c | transaldolase |
MSMEG_6129 is responsive to MSMEG_5437 and is required for sensitivity to antibiotics and Mitomycin C (MMC)
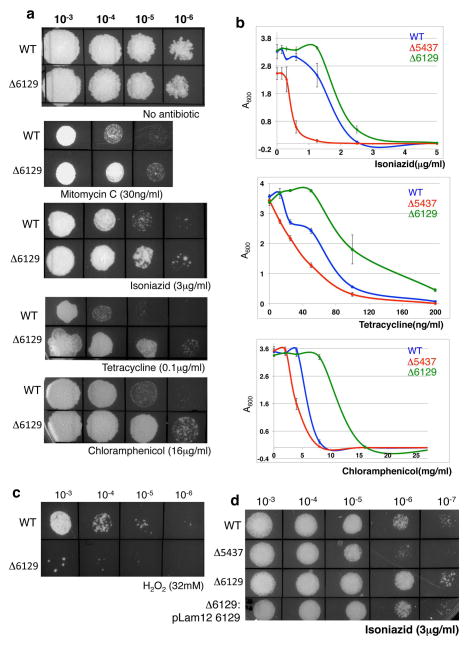
It is plausible that at least a subset of the proteins whose phosphorylation is reduced in the mc2155:Δ5437 mutant is involved in pathways contributing to increased antibiotic susceptibility. We initially chose to construct isogenic deletion mutations in MSMEG_0059, MSMEG_6091, MSMEG_0456, MSMEG_6427 and MSMEG_6129 as representatives of different functional categories; of these only the phenotype of mc2155:Δ6129 was striking. When compared to wild type mc2155, mc2155:Δ6129 showed increased resistance to MMC, INH, tetracycline and chloramphenicol, rather than increased sensitivity (Fig. 2a). The two mutant strains were different from each other and the wild type in their sensitivities to antibiotics when grown in agar as well as in their minimum inhibitory concentrations in liquid media (Fig. 2b and Table 2). This is in contrast to mc2155:Δ5437, which showed increased susceptibility to the same drugs. Interestingly, mc2155:Δ6129 also showed strongly increased sensitivity to hydrogen peroxide (Fig. 2c), a phenotype not observed with mc2155:Δ5437. Genetic complementation of mc2155:Δ6129 with an exogenous copy of MSMEG_6129 restores the phenotype to that of wild type bacteria (Fig. 2d) confirming that the MSMEG_6129 is responsible for the phenotype.
Figure 2. M. smegmatis mc2155:Δ6129 is resistant to multiple antibiotics but hypersensitive to H2O2.
(a) Ten fold serial dilutions of wild type and mc2155:Δ6129 were spotted on Middlebrook 7H10 containing indicated concentrations of antibiotics. (b) Survival curves of wild type, mc2155:Δ5437 and mc2155:Δ6129 grown for 36 hours in liquid media containing the indicated antibiotics is shown in panels on the right. mc2155:Δ6129 is more resistant to all the drugs tested as compared to the wildtype parent strain. Error bars represent standard deviation of means of three independent experiments. (c) mc2155:Δ6129 shows almost 100% killing when exposed to 32mM H2O2 for 30 mins, a concentration where the viability of wildtype is unchanged. (d) Overexpression of MSMEG_6129 in wildtype cells makes the bacteria hypersensitive to isoniazid similar to mc2155:Δ5437 whereas exogenous expression of MSMEG_6129 in mc2155:Δ6129 restores isoniazid sensitivity to wild type levels.
Table 2. Survival of wild type mc2155:Δ5437 and mc2155:Δ6129 in antibiotic containing liquid media.
The Minimum Inhibitory Concentrations (μg/ml) of Isoniazid, Tetracycline and Chloramphenicol are shown for wild type, mc2155:Δ5437 and mc2155:Δ6129.
| WT | ΔMSMEG_5437 | ΔMSMEG_6129 | |
|---|---|---|---|
| Isoniazid | 5 | 2.5 | 10 |
| Tetracycline | 100 | 75 | 200 |
| Chloramphenicol | 16 | 8 | 32 |
Proposed model for the function of MSMEG_6129 in antibiotic resistance
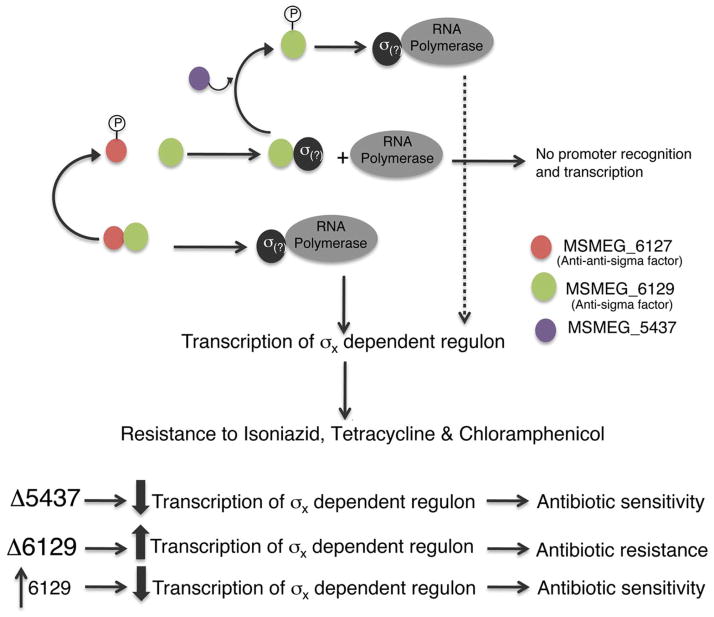
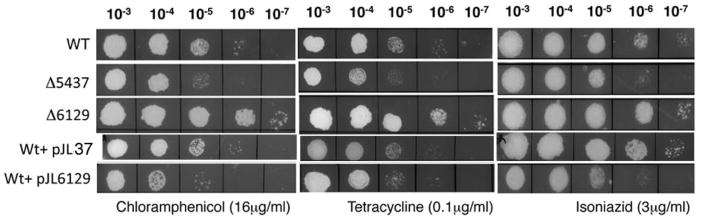
Bioinformatic analyses using BLAST and HHPRED suggest that MSMEG_6129 is an anti-sigma factor. Furthermore, the adjacent MSMEG_6127 is a predicted anti-anti sigma factor. This was immediately striking as the phenotypes of mc2155:Δ5437 and mc2155:Δ6129 can be accounted for by assuming that the unphosphorylated form of MSMEG_6129 binds to a cognate sigma factor, and prevents it from associating with RNA Polymerase to transcribe a regulon influencing drug sensitivities. The proposed model is shown in Figure 3. Although MSMEG_6129 was identified as a phosphorylation substrate of MSMEG_5437 in the 2-DIGE analysis, we have been unable to demonstrate phosphorylation of MSMEG_6129 by MSMEG_5437 in vitro using purified proteins. While it is likely that MSMEG_5437 has an indirect influence on the phosphorylation status of MSMEG_6129, it is possible that phosphorylation may require activation of MSMEG_5437 or additional cofactors and it is too early to dismiss a direct interaction without further biochemical analyses. In the absence of MSMEG_5437, as in the mc2155:Δ5437 strain, MSMEG_6129 is constitutively unphosphorylated resulting in the sequestration of its cognate sigma factor and down regulation of the antibiotic resistance regulon and consequent antibiotic sensitivity. Deletion of MSMEG_6129 would therefore be expected to increase expression of the regulon with resulting antibiotic resistance. The model also predicts that an overexpression of MSMEG_6129 would shift the balance towards increased sequestration of the sigma factor and mimic the phenotype of a mc2155:Δ5437 mutant. Indeed we find that overexpression of MSMEG_6129 from an acetamide inducible promoter renders the bacteria sensitive to isoniazid, chloramphenicol and tetracycline to a level exhibited by mc2155:Δ5437 (Fig. 4).
Figure 3. Proposed model showing the interaction of transcription regulatory factors in antibiotic resistance in M. smegmatis.
We propose that MSMEG_6129 (green) regulates a hitherto unknown sigma factor required for transcription of antibiotic resistance genes. MSMEG_6129 is presumably under negative regulation by MSMEG_6127 (red) and MSMEG_5437 (purple).
Figure 4. Overexpression of MSMEG_6129 in wild type mc2155 results in antibiotic sensitivity.
Ten fold serial dilutions of wild type, mc2155:Δ5437, mc2155:Δ6129 and mc2155+ pJL37MSMEG_6129 (a strain overexpressing MSMEG_6129) were spotted on Middlebrook 7H10 containing indicated concentrations of antibiotics. Overexpression of MSMEG_6129 results in antibiotic sensitivity to levels observed for mc2155:Δ5437.
MSMEG_6127, a predicted anti-anti-sigma, is a phosphorylation substrate of MSMEG_6129
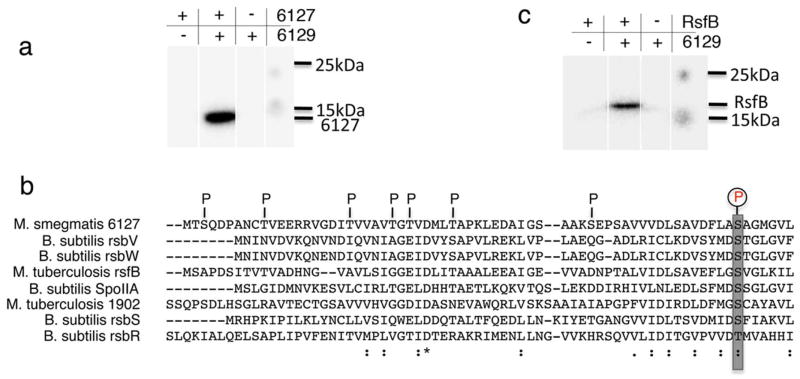
The adjacent organization of MSMEG_6129 and the anti-anti sigma factor (MSMEG_6127) raises the possibility that the two interact with each other and that MSMEG_6127 is the cognate negative regulator of MSMEG_6129. In most systems studied to date the tripartite interactions between sigma, anti-sigma and anti-anti-sigma factors is regulated by phosphorelay signals in which the anti-sigma factor typically phosphorylates the anti-anti-sigma factor. Figure 5a shows robust in vitro phosphorylation of MSMEG_6127 by MSMEG_6129 using overexpressed proteins. Analysis of the sites phosphorylated in MSMEG_6127 by MSMEG_6129 in vitro using LC-MS/MS shows eight ser/thr residues that are phosphorylated in MSMEG_6127 and includes Ser63 which coincides with the consensus phosphorylation site in the anti-anti sigma factor family (Fig. 5b) consistent with the idea that MSMEG_6129 and MSMEG_6127 constitute a cognate anti-sigma: anti-anti-sigma pair thereby lending support to the model proposed in Figure 3.
Figure 5. MSMEG_6129 phosphorylates MSMEG_6127 in vitro.
(a) MSMEG_6127 and (b) M. tuberculosis RsfB purified from E. coli were incubated with purified MSMEG_6129 in kinase buffer containing 1μCi of γ-P32 ATP at 25°C for 30 mins and separated on a 15% SDS-PAGE followed by autoradiography. The positions of MSMEG_6127 and RsfB are marked as well as the 15 kDa and 25 kDa standards. (c) The position of residues phosphorylated in MSMEG_6127 by MSMEG_6129 identified by MALDI TOF/TOF are indicated and include the consensus anti-sigma factor phosphorylation site at position S63.
Although a homologue of MSMEG_6129 could not be identified in M. tuberculosis, MSMEG_6127 shows 62% similarity (37% identity) to the M. tuberculosis RsfB protein, which is the cognate anti-anti-sigma factor of UsfX, the negative regulator of σF in M. tuberculosis. Because MSMEG_6129 can phosphorylate MSMEG_6127 in vitro, we therefore tested the ability of MSMEG_6129 to phosphorylate the M. tuberculosis homologue of MSMEG_6127, RsfB. We find that indeed RsfB can function as a substrate of MSMEG_6129 in vitro (Fig. 5c).
MSMEG_6129 controls a diverse array of genes
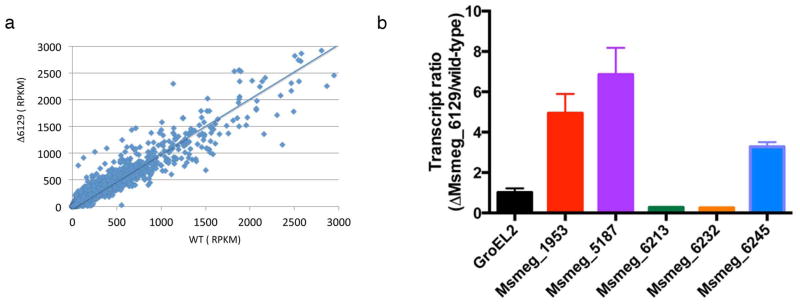
While most anti-sigma factors are located in the vicinity of their cognate σ factors, the identity of the σ factor(s) regulated by MSMEG_6129 is not immediately obvious from the genome organization. However, the genes under its control can be identified by comparing the transcriptome of mc2155:Δ6129 with wild-type M. smegmatis using high throughput cDNA sequencing (RNAseq). A total of 11342404 reads aligning to ORFs were obtained for wild type mc2155 and 11927188 reads for mc2155: Δ6129 of which 74% and 81% respectively correspond to mRNA. The number of transcripts of each gene in mc2155:Δ6129 was normalized to a million reads for both the strains and the number of reads per ORF were compared in both strains for each ORF. In order to facilitate our analysis we chose to examine those transcripts whose levels changed at least 2.5 fold in the mc2155:Δ6129 strain as compared to the wild-type. While the expression of most genes remain unchanged, we found 181 genes with induced expression and 40 genes with reduced expression in mc2155:Δ6129 (Figure 6a and Table S2). The disproportionately higher number of induced genes is consistent with the suggested anti-sigma factor function of MSMEG_6129, a deletion of which would result in dysregulated availability of its cognate sigma factors and subsequently increased transcription of downstream genes.
Figure 6. Deletion of MSMEG_6129 results in induction of antibiotic resistance genes.
(a) Scatter plot of mRNA levels of 6716 ORFs in mc2155 and an isogenic mutant of MSMEG_6129. (b) The amount of transcript of MSMEG_1953, MSMEG_5187, MSMEG_6213, MSMEG_6232 and MSMEG_6245 in the wild type as well as mc2155:Δ6129 was quantified using qPCR and expressed as a fold overexpression in mc2155:Δ6129 over wild type. GroEL1 was used as an endogenous control and is shown to remain unchanged in the 2 strains. Error bars represent standard deviation of means of three independent experiments
Among the 181 upregulated genes, 84 are hypothetical proteins of unknown function. Of the remaining, two classes of proteins efflux pumps and transcription regulators are most relevant to the observed phenotype of mc2155:Δ6129 and are discussed below (Table 3). The downregulation of catalase genes is intriguing and their significance is also discussed. The results of RNAseq were also verified using qPCR for the pertinent genes from the above three categories (Fig. 6b).
Table 3. Deletion of MSMEG_6129 results in induction of antibiotic resistance genes.
Total RNA was prepared from wild type and mc2155: Δ6129 strains followed by high throughput cDNA sequencing. The data is normalized to a million reads as well as for gene length for both strains (Reads per million bases per kilobase of ORF= RPKM) and expressed as a ratio of RPKM values of the wild typestrain and the mutant. The 3 categories of proteins where the ratio of RPKM values of Δ6129 to the wild type strain is >2.5 and are immediately relevant to the observed drug resistance phenotype of mc2155: Δ6129 are shown. The results of RNA seq were verified using qPCR for the pertinent genes from the above three categories highlighted in grey.
| Category | Gene ID | Fold change | Gene description |
|---|---|---|---|
| in Δ6129 | |||
| Drug Resistance & ABC Transporters | MSMEG_5187 | 9 | tetracycline-resistance determinant TetV |
| MSMEG_5102 | 7.5 | ABC transporter ATP-binding protein | |
| MSMEG_3203 | 4.3 | transporter, LysE family | |
| MSMEG_5047 | 3.7 | drug resistance transporter (Bcr/CfIA) | |
| MSMEG_0422 | 3.5 | Transferase. Toxin production & resistance | |
| MSMEG_5659 | 3.1 | ABC transporter, ATP-binding protein | |
| MSMEG_5660 | 3 | ABC transporter ATP-binding protein | |
| MSMEG_6245 | 2.9 | chloramphenicol resistance protein | |
| MSMEG_6595 | 2.6 | ABC transporter complex | |
| Transcription Regulators | MSMEG_1953 | 6.5 | transcription factor WhiB7 |
| MSMEG_0051 | 6 | transcription factor WhiB6 family | |
| MSMEG_1025 | 37 | TetR-family transcriptional regulator | |
| MSMEG_1420 | 3.7 | probable transcriptional regulatory protein | |
| MSMEG_5610 | 3.6 | putative transcriptional regulator family | |
| MSMEG_2305 | 3.4 | TetR-family transcriptional regulator | |
| MSMEG_6441 | 2.9 | transcriptional regulator, TetR family | |
| MSMEG_0473 | 2.8 | transcriptional regulator, LuxR family | |
| MSMEG_1117 | 2.8 | transcriptional regulator | |
| MSMEG_1970 | 2.7 | sigma factor | |
| MSMEG_5872 | 2.7 | DNA-binding response regulator PhoP | |
| MSMEG_0545 | 2.6 | transcriptional regulator, LuxR family | |
| MSMEG_6199 | 2.6 | Transcription factor WhiB2 | |
| MSMEG_2905 | 2.5 | DNA binding transcription factor | |
| Catalases | MSMEG_6213 | −2.5 | Manganese containing catalase |
| MSMEG_6232 | −2.6 | catalase KatA |
Upregulation of known efflux pumps in mc2155:Δ6129 may account for resistance to Tetracycline and Chloramphenicol
Nine efflux pumps and transporters are seen to be upregulated in mc2155:Δ6129 (Table 3). This is of immediate attention as intrinsic resistance of M. tuberculosis to several antibiotics has been partially linked to the activities of efflux pumps in the cell envelope(da Silva et al., 2011). Transcript levels of MSMEG_5187 which encodes the TetV shows a 9 fold increase in mc2155:Δ6129 as compared to wildtype; TetV is a well-characterized effux pump that is known to confer resistance to tetracycline in mycobacteria and could account for the observed tolerance of the mutant to tetracycline. MSMEG_5047 and MSMEG_6245 encode a drug resistance transporter of the Bcr/CflA subfamily and a chloramphenicol resistance protein respectively both of which belong to the major facilitator superfamily (MFS) of transporters that extrude a variety of chemically unrelated compounds including chloramphenicol. The increased transcription of these could account for the observed tolerance of the mutant to chloramphenicol. In addition to the above efflux proteins, four ABC family of transporters are also up-regulated in mc2155:Δ6129 which could contribute to the efflux of either these or other drugs not yet tested.
Downregulation of catalase genes may account for INH resistance and H2O2 sensitivity in mc2155:Δ6129
While the resistance to tetracycline and chloramphenicol can be accounted for by the overexpression of efflux genes from the RNAseq data, it is not immediately obvious why mc2155:Δ6129 is resistant to isoniazid or sensitive to H2O2. It is noteworthy that a deletion in MSMEG_6129 results in a 2–3 fold decrease in expression of two catalase encoding genes, MSMEG_6213 and MSMEG_6232 (Table 3). Catalase (encoded by katG in M. tuberculosis) is known to activate the prodrug isoniazid which then inhibits InhA, involved in mycolic acid biosynthesis (Chouchane et al., 2000, Baulard et al., 2000). The M. smegmatis genome encodes eight catalases, although the specific role of each of these in the activation of INH or detoxification of H2O2 is unknown. The present transcriptomics data however suggests the involvement of MSMEG_6213 and MSMEG_6232 in the above functions. Moreover, although mc2155:Δ6129 is resistant to isoniazid, its sensitivity to ethionamide, a structural analogue of INH not activated by catalase (Baulard et al., 2000), remains unchanged which further supports the idea that the observed resistance to isoniazid occurs at the stage of drug activation and not at a downstream stage common to both isoniazid and ethionamide. This decrease in catalase expression could also offer an explanation for the observed increase in sensitivity of the mutant to H2O2.
Upregulation of M. smegmatis whiB7 and other transcription regulators in mc2155:Δ6129
The expression of fourteen transcription regulators are seen to be upregulated in mc2155:Δ6129 (Table 3). Of these, three are WhiB-family of transcription factors-MSMEG_0051 (whiB6), MSMEG_1953 (whiB7), and MSMEG_6199(whiB2). Interestingly, M. tuberculosis whiB7 has previously been shown to be involved in the intrinsic resistance of M. tuberculosis to multiple antibiotics by regulating the expression of known drug resistance genes including tap (Rv1258c), Rv1473 and Rv1988 (Morris et al., 2005, Ainsa et al., 1998). A knock out of whiB7 renders M. tuberculosis more sensitive to several unrelated antibiotics including chloramphenicol, tetracycline and erythomycin whereas overexpression of whiB7 results in increased resistance to these antibiotics (Morris et al., 2005). A gene replacement mutant of MSMEG_1953 was constructed using recombineering and tested for its sensitivity to isoniazid, chloramphenicol, tetracycline and erythromycin. As with an M. tuberculosis ΔwhiB7 mutant, ΔMSMEG_1953 was sensitive to chloramphenicol, tetracycline and erythromycin suggesting overlaps in the regulatory mechanisms of antibiotic resistance in M. smegmatis and M. tuberculosis (Table 4). Similar results were also observed by Burian et al who showed that M. smegmatis whiB7 can be induced with erythromycin (Burian et al., 2012).
Table 4. Survival of wild type mc2155 and mc2155:Δ1953 in antibiotic containing liquid media.
The Minimum Inhibitory Concentration (μg/ml) of Isoniazid, Tetracycline and Chloramphenicol is shown for wild type, mc2155:Δ1953 and compared to known literature values for M. tuberculosis.
| M. smegmatis | M. tuberculosis | |||
|---|---|---|---|---|
| WT | ΔMSMEG_1953 | WT | ΔwhiB7 | |
| Isoniazid | 5.0 | 5.8 | n/a | n/a |
| Tetracycline | 100 | 25 | 20 | 20 |
| Chloramphenicol | 16 | 5 | 8 | 4 |
| Erythromycin | 5 | 0.6 | 80 | 40 |
| Spectinomycin | 100 | 25 | 128 | 16 |
In addition to the WhiB family of transcription regulators, we also observe the upregulation of three transcription regulators, MSMEG_1025, MSMEG_2305 and MSMEG_6441, that belong to the TetR family, members of which are known to encode proteins involved in multidrug resistance and pathogenecity of gram-positive and gram-negative bacteria(Engohang-Ndong et al., 2004, Ramos et al., 2005). Upregulation of MSMEG_1025, MSMEG_2305 and MSMEG_6441 in mc2155: Δ6129 could contribute directly or indirectly to the observed multi-drug resistance phenotype of mc2155: Δ6129.
MSMEG_6129 functions upstream of M. smegmatis whiB7 controlling both whiB7 dependent and independent pathways
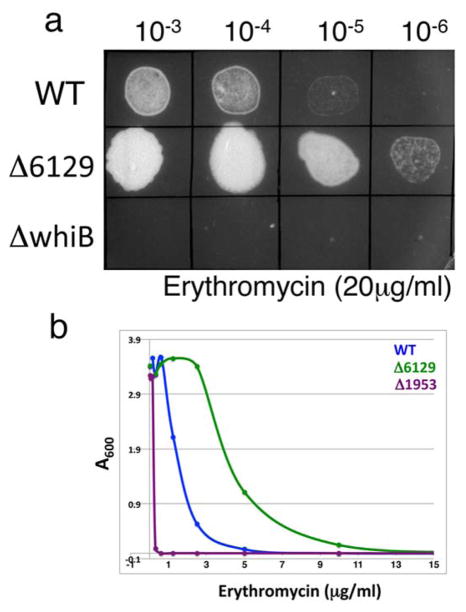
Gene expression profiling of M. smegmatis mc2155:Δ6129 shows striking overlap with the M. tuberculosis strain overexpressing WhiB7 (Morris et al., 2005). We observe that MSMEG_5187 and MSMEG_5102, homologues of Rv1258c (the tetracycline efflux pump) and Rv1473 (a putative macrolide transporter) respectively, as well as homologues of acetyl transferases, cutinases and ureases are upregulated in mc2155:Δ6129 (Table S2) similar to that in the M. tuberculosis strain overexpressing WhiB7 (Burian et al., 2012). Furthermore, M. smegmatis MSMEG_1953 (whiB7) is upregulated >7 fold in mc2155:Δ6129. Taken together, the data suggest that MSMEG_6129 is an upstream regulator controlling the expression of MSMEG_1953 (whiB7), which in turn activates a regulon including antibiotic efflux pumps (MSMEG_5187 and MSMEG_5102). The model therefore predicts mc2155:Δ6129 to display resistance to the other antibiotics that an mc2155:Δ1953 strain is sensitive to and is found to be indeed more tolerant to erythromycin as compared to wild type bacteria (Fig. 7).
Figure 7. Drug sensitivity of mc2155:Δ6129 can be predicted from the known phenotype of mc2155:Δ1953.
(a) Ten fold serial dilutions of wild type, mc2155:Δ6129 and mc2155:ΔwhiB7 were spotted on Middlebrook 7H10 containing 20μg/ml erythromycin. (b) Survival curves of wild type, mc2155:Δ5437 and mc2155:Δ6129 in liquid media containing the indicated concentration of erythromycin. mc2155:Δ6129 shows increased resistance to erythromycin when compared to the wildtype parent strain as predicted.
mc2155:Δ1953 however does not display sensitivity to isoniazid suggesting that the observed resistance of mc2155:Δ6129 to isoniazid occurs via a MSMEG_1953 independent pathway (Table 4). This is consistent with previous observations of Burian et al. who demonstrated that transcription of MSMEG_1953 is induced by several antibiotics but not by isoniazid(Burian et al., 2012). The entire spectrum of phenotypes exhibited by mc2155:Δ6129 is therefore not entirely mediated by MSMEG_1953. We envisage a scenario in which MSMEG_6129 controls the expression of several genes, directly or indirectly through regulators like MSMEG_1953 (whiB7), which finally determines its behavior in the presence of antibiotics and oxidative stresses.
DISCUSSION
The extreme resistance of Mycobacterium spp. to a variety of antibiotics has traditionally been attributed to their waxy and highly impermeable cell envelope that forms a physical barrier against external threats. However, the contribution of metabolic plasticity that minimizes the effect of antibiotics and other chemicals that do permeate this barrier has been less appreciated thus far but is beginning to gain prominence from recent studies that highlight the role of transcriptional regulators like whiB7 and sigI (Lee et al., 2012a, Morris et al., 2005). This opens up broader questions as to how a complex and intertwined transcription regulatory circuit, central to which is the large pool of sigma factors and their upstream regulators, facilitates intrinsic drug resistance in mycobacteria. The results presented here identify new factors and provide novel insights into a mechanism of global transcriptional regulation that directly influences the sensitivity of mycobacteria to antibiotics.
We envisage a scenario in which the anti-sigma factor, MSMEG_6129, controls the expression of a regulon, either directly or indirectly through transcriptional regulators like WhiB7, by negatively regulating an unidentified sigma factor that controls several cellular responses including the response to multiple unrelated antibiotics and oxidative stresses. Moreover, the interaction between MSMEG_6129 and MSMEG_6127 suggest that MSMEG_6129 (the anti-sigma factor) is itself under the control of the MSMEG_6127 (its cognate anti-anti sigma factor) via a phosphorylation dependent mechanism such that phosphorylation of MSMEG_6127 by MSMEG_6129 could release the anti-sigma factor for negative regulation of sigma factor and its downstream genes. This circuit is presumably under further control of an upstream signaling pathway involving MSMEG_5437 that affects the phosphorylation status of MSMEG_6129. The inverse phenotypes of ΔMSMEG_5437 and ΔMSMEG_6129 suggests that the phosphorylated form of the anti-sigma factor is functionally inactive and makes the sigma factor available to transcribe downstream genes involved in antibiotic resistance. It is interesting that MSMEG_5437 shows homology to receptor eukaryotic-like (Hanks-type) ser/thr kinases; 9 of 11 M. tuberculosis Hanks-type kinases are transmembrane kinases and presumably transduce extracellular signals. This makes MSMEG_5437 an attractive target to sense the external milieu and relay the signal to the downstream sigma factors. Although MSMEG_5437 shows homology primarily to the catalytic domains of STPKs along its entire length of 287 amino acids, we note the presence of a significant transmembrane domain (residues 201–227) identified using TMpred (Hofmann, 1993). Further experiments will be required to demonstrate the role of MSMEG_5437 as a membrane sensor. Although at present we do not have compelling evidence for MSMEG_5437 to be a membrane associated kinase, it is of interest that another cytoplasmic mycobacterial STPK, pknG, has been previously implicated in intrinsic drug resistance in M. smegmatis such that a deletion in pknG rendered the bacteria sensitive to erythromycin, vancomycin, rifampin and ethambutol(Wolff et al., 2009).
The identity of the sigma factor regulated by MSMEG_6129 however remains elusive. We explored MSMEG_1804 and MSMEG_1970 as highly likely candidates-MSMEG_1804 is a SigF homologue that has been shown to be under RsfB regulation and MSMEG_1970 is a putative sigma factor with induced expression in mc2155:Δ6129. However, isogenic deletions of MSMEG_1804 and MSMEG_1970 were indistinguishable from wild type in their resistance to isoniazid, chloramphenicol and tetracycline consistent with previous reports from Gebhard et al (Gebhard et al., 2008) who showed that the isoniazid sensitivity of ΔsigF remains unchanged. Interestingly, a recent study identified a direct physical interaction between Whib7 and σA (Burian et al., 2013), implying that expression of WhiB7 and its regulon are dependent on σA activity. This raises the possibility that MSMEG_6129 exerts its effect via interaction with σA and offers a mechanism in which the abundance and promoter occupancy of σA could be regulated by MSMEG_6129 over a dynamic range to facilitate mycobacterial adaptation to its changing environment.
While MSMEG_6129 plays a pivotal role in regulating intrinsic resistance to multiple antibiotics in M. smegmatis, a homologue in M. tuberculosis is conspicuously absent even though the two species show similar drug tolerance behaviors. At least two lines of evidence presented here suggest that transcription pathways that regulate antibiotic resistance, while not identical, show substantial overlap between the two species. First, M. smegmatis ΔwhiB7 (MSMEG_1953) is highly sensitive to tetracycline, chloramphenicol and erythromycin similar to that of an M. tuberculosis ΔwhiB7 strain (Fig. 7 and Table 4). In addition, the genes regulated by WhiB7 in both species show significant overlap (Table S2 and (Morris et al., 2005)). Therefore, it is highly likely that a functional homologue of MSMEG_6129 is present in M. tuberculosis that regulates intrinsic drug resistance pathways in the pathogen. This relationship between sigma factors and intrinsic drug tolerance through a phosphorelay mechanism offers a means to render mycobacteria more sensitive to the currently available antibiotic regime.
However it can’t be ruled out that M. smegmatis has diverged from M. tuberculosis, with the involvement of additional factors. This is supported by the presence of 26 alternate sigma factors in M. smegmatis, as against 11 in M. tuberculosis. The requirement for an additional regulatory network in M. smegmatis to control intrinsic drug resistance may not be entirely unexpected considering the competitive habitat of this saprophyte that is shared with other soil bacteria capable of synthesizing a plethora of antibiotics.
EXPERIMENTAL PROCEDURES
Media and Strains
Mycobacterium smegmatis was grown at 37°C in Middlebrook 7H9 (DIFCO) supplemented with 10% ADC and 0.05% Tween 20. Antibiotics were added as required to indicated amounts. M. smegmatis overexpressing MSMEG_6129 and MSMEG_6127 were grown in Middlebrook 7H9 containing 0.2% succinate to an OD of 0.4 and induced with 0.02% acetamide for an additional 18h wherever indicated. Gene replacement mutants were constructed using recombineering as described previously(van Kessel and Hatfull, 2007) using recombineering functions encoded on pJV53. The recombineering construct for each gene was generated by cloning in the multiple cloning sites flanking the hygromycin cassette of pYUB854. The left arm and right arms were generated using the following primer pairs respectively: MSMEG_5437 – ctcgttgagcatctagagcgccacca/gttgcgcgcgaggcgtgtggcgagg and cacgcggtggatgctagcctcgcga/gaggagcagcccacagatctggtgg, MSMEG_6129-cgagcgtgaaatgcgtctagagccat/ggttgagccaaaagcttaaggggtg and gttgatgcacgcctagccgaccgt/cgcgcgatcgacatcctcgaggtgg, MSMEG_1953-ttctcggcgaatctagagatccgggt/ccgccacgattcttaaggatcgtgcc and ctcgaccgcggaagctttgtcgcgc/ggaagtgtcgatcgaagatctcgag, MSMEG_1804-ccggcagcggaacttaaggagtggcgcg/gcggaaggcacctctagacgatcttctcgcgc and gagcgggtcggctagctcgcagatgc/cttggccccctcgagaccgtggaccac, MSMEG_1970-gccgcggaggcctcgtccatcgc/cgttgccaggagtctagagcacgctcgaagc and ctggaggcaatggagctagcgcgatcgtgc/ggtgaactcgctcgaggcacgggcgtcag. Upon confirmation of the mutant by PCR, the recombineering plasmid was cured by repeated passage through antibiotic free medium.
Protein overexpression and purification
MSMEG_6127 and MSMEG_6129 were amplified from M. smegmatis genomic DNA and using the primer pairs caaacaccgtcggaggtgcatatgac/ctacccggttgcgcacactcgagtgtgtagccaccaggtccatatgacagac/gtgcagcaggtggtgaagcttccgcagtt, cloned in pET21a (Novagen) and transformed into BL21(DE3) Star, grown to an A600 of 0.4 and induced with 1mM IPTG. The cells were lysed in a buffer containing 50mM Tris-HCl (pH 8.0), 300mM NaCl and 5% glycerol and the clarified lysate was loaded on a Ni-NTA column (Qiagen). Non-specifically bound proteins were removed by washing with lysis buffer containing 20mM imidazole and the histidine tagged protein was eluted with 100mM imidazole.
Identification of mitomycin C responsive genes in M. smegmatis
For construction of transposon libraries, M. smegmatis cultures were grown to OD600 of 0.6 in Middlebrook 7H9 + ADC, washed several times with with MP buffer (50 mM Tris, pH 7.5/150 mM NaCl/10 mM MgSO4/2 mM CaCl2) and resuspended in 1/10 of the original culture volume of MP buffer. Cells were infected with 1010 phage MycoMarT7/ml of original culture for 3 h at 37°C. Transduced cells were plated on 7H10+ADC + kanamycin (40μg/ml) at 37°C. Libraries were prepared by scraping approximately 50,000 colonies off plates and freezing at −80°C. The mutant library was grown in liquid media to an OD of 0.3 and exposed to 30ng/ml of mitomycin C for 12 hours, a concentration that was previously determined to be sublethal to wild type bacteria. An equal volume of culture was set aside without mitomycin C exposure and served as the control. Genomic DNA was isolated from each sample and conditional lethal genes identified using hybridization on microarrays obtained from The Institute of Genomic research (TIGR) as described previously(Murry et al., 2008).
Antibiotic and H2O2 Sensitivity Assays
Wild type mc2155 and mutant strains were grown to an A600 of 0.6–0.7 tested for their susceptibility to antibiotics by spotting a 10 fold serial dilution initially on Middlebrook 7H10 (DIFCO) plates containing a range of each drug: mitomycin C (20–40ng/ml), isoniazid (1–16μg/ml), rifampicin (1–16μg/ml), ethionamide (8–128μg/ml), tetracycline (0.05–1μg/ml), chloramplenicol (8–128μg/ml) and erythromycin (20–60μg/ml). The concentration of antibiotic showing the most prominent difference in each case was then used in subsequent experiments. Antibiotic susceptibility on liquid media was assayed by inoculating the desired strain in a two fold dilution series of each antibiotic at an initial A600of 0.0004. The cultures were incubated at 37°C and the A600 was measured after 36hs. Wild type mc2155 and mutant strains were grown to an A600 of 0.3 and exposed to varying concentrations of H2O2 (0, 4, 6, 16, 32, 64 and 128mM) at 37°C for 30minutes. Following exposure each sample was rapidly diluted in Middlebrook 7H9 and 10 fold serial dilutions were spotted on Middlebrook 7H10 agar plates.
In vitro kinase assays and determination of phosphorylation sites
5 μM of purified anti-anti sigma factor (MSMEG_6127 or RsfB) was mixed with increasing amounts of MSMEG_6129 to molar ratios of 1:1, 1:2, 1:4, 1:8 in a buffer containing 50mM TrisHCl, (pH 8.0), 50mM NaCl, 10mM MgCl2 and 10mM MnCl2. Reactions were started by the addition of 1μCi of [γ-P32] ATP and 15μM ATP, incubated at 25°C for 30 mins and terminated by the addition of 20mM EDTA. The proteins were separated on a 15% SDS PAGE gel and visualized by autoradiography.
5 μM of MSMEG_6127 purified from E. coli was mixed with 10μM of MSMEG_6129 in a buffer containing 50mM TrisHCl, (pH 8.0), 50mM NaCl, 10mM MgCl2 and 10mM MnCl2.and 15μM ATP at 25°C for 30 mins. The samples were separated on a 15% SDS/PAGE, stained with coomassie blue followed by excision of MSMEG_6127 from the gel. The phosphorylated residues were identified using MALDI tandem MS/MS (Applied Biomics, Inc.).
2D gel electrophoresis and phosphoprotein staining
Phosphoprotein analysis was performed by Applied Biomics, Inc. CA. In brief, the wild type and mc2155:Δ5437 strains were grown to exponential phase and the protein extracts are differentially labeled with Cy-3 and Cy-5 and separated by 2-dimensional gel electrophoresis. Phosphorylated proteins are then stained with a fluorescent phospho-protein staining solution followed by destaining. The changes in abundance of phosphorylated proteins between the two samples was then identified using the ImageQuant and DeCyder softwares. 18 protein spots were completely absent in mc2155: Δ5437 and were excised from the gel and identified by MALDI TOF/TOF (tandem mass spectrometry MS/MS).
RNA preparation and RNA-seq analysis and qPCR
Total RNA was prepared from wild type and mc2155:Δ6129 strains grown to exponential phase in Middlebrook 7H9-ADC using the Qiagen RNA preparation kit followed by two DNAse I treatments. Approximately 5μg total RNA samples were treated with the Ribo-Zero™ rRNA removal procedure (Epicentre) to enrich for mRNA. Approximately 500ng of each sample was then used to synthesize cDNA by reverse transcription using the Ovation® RNA-Seq System (Nugen) followed by library preparation using the Encore NGS Multiplex System I (Nugen) and high throughput sequencing on the Illumina platform.
The sequence data were demultiplexed using FASTQ Groomer and Barcode Splitter on the Galaxy platform. Each read was trimmed by 6bp at the left end and 2bp at the right end, then aligned to the annotated M. smegmatis genome using Bowtie with standard settings (Galaxy). The number of reads corresponding to each ORF was calculated, and the data were normalized to a million reads as well as to the size of each ORF, and expressed as Reads per Kilobase per million mapped reads (RPKM) and are available as Excel files.
Primers for qRT-PCR were generated using PrimerExpress software (Applied Biosystems). cDNA was generated using random hexamers and Maxima reverse transcriptase (Fermentas), and qRT-PCR performed using the Maxima SYBR Green qPCR Master Mix (Fermentas). Applied Biosystems 7300 Real-Time PCR System was used with cycling conditions of: 50°C for 2′, 95°C for 10′, and 40 cycles of 95°C for 15″, 60°C for 1′.
Supplementary Material
Acknowledgments
We would like to thank Eric Rubin for providing φMycoMarT7 and Graham Hatful for critical reading of the manuscript. We also thank Carlos A. Guerrero for excellent technical support and Rebecca Brown for help constructing mutants in M. smegmatis. This work was supported by NIH grant 1R21AI090178.
References
- AINSA JA, BLOKPOEL MC, OTAL I, YOUNG DB, DE SMET KA, MARTIN C. Molecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosis. J Bacteriol. 1998;180:5836–43. doi: 10.1128/jb.180.22.5836-5843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AV-GAY Y, EVERETT M. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 2000;8:238–44. doi: 10.1016/s0966-842x(00)01734-0. [DOI] [PubMed] [Google Scholar]
- BARIK S, SUREKA K, MUKHERJEE P, BASU J, KUNDU M. RseA, the SigE specific anti-sigma factor of Mycobacterium tuberculosis, is inactivated by phosphorylation-dependent ClpC1P2 proteolysis. Mol Microbiol. 2010;75:592–606. doi: 10.1111/j.1365-2958.2009.07008.x. [DOI] [PubMed] [Google Scholar]
- BAULARD AR, BETTS JC, ENGOHANG-NDONG J, QUAN S, MCADAM RA, BRENNAN PJ, LOCHT C, BESRA GS. Activation of the pro-drug ethionamide is regulated in mycobacteria. J Biol Chem. 2000;275:28326–31. doi: 10.1074/jbc.M003744200. [DOI] [PubMed] [Google Scholar]
- BEAUCHER J, RODRIGUE S, JACQUES PE, SMITH I, BRZEZINSKI R, GAUDREAU L. Novel Mycobacterium tuberculosis anti-sigma factor antagonists control sigmaF activity by distinct mechanisms. Molecular microbiology. 2002;45:1527–40. doi: 10.1046/j.1365-2958.2002.03135.x. [DOI] [PubMed] [Google Scholar]
- BISHAI W. The Mycobacterium tuberculosis genomic sequence: anatomy of a master adaptor. Trends Microbiol. 1998;6:464–5. doi: 10.1016/s0966-842x(98)01414-0. [DOI] [PubMed] [Google Scholar]
- BURIAN J, RAMON-GARCIA S, HOWES CG, THOMPSON CJ. WhiB7, a transcriptional activator that coordinates physiology with intrinsic drug resistance in Mycobacterium tuberculosis. Expert review of anti-infective therapy. 2012;10:1037–47. doi: 10.1586/eri.12.90. [DOI] [PubMed] [Google Scholar]
- BURIAN J, YIM G, HSING M, AXERIO-CILIES P, CHERKASOV A, SPIEGELMAN GB, THOMPSON CJ. The mycobacterial antibiotic resistance determinant WhiB7 acts as a transcriptional activator by binding the primary sigma factor SigA (RpoV) Nucleic acids research. 2013 doi: 10.1093/nar/gkt751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURONI S, MANINA G, GUGLIERAME P, PASCA MR, RICCARDI G, DE ROSSI E. LfrR is a repressor that regulates expression of the efflux pump LfrA in Mycobacterium smegmatis. Antimicrob Agents Chemother. 2006;50:4044–52. doi: 10.1128/AAC.00656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUCHANE S, LIPPAI I, MAGLIOZZO RS. Catalase-peroxidase (Mycobacterium tuberculosis KatG) catalysis and isoniazid activation. Biochemistry. 2000;39:9975–83. doi: 10.1021/bi0005815. [DOI] [PubMed] [Google Scholar]
- DA SILVA PE, VON GROLL A, MARTIN A, PALOMINO JC. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol. 2011;63:1–9. doi: 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- DAINESE E, RODRIGUE S, DELOGU G, PROVVEDI R, LAFLAMME L, BRZEZINSKI R, FADDA G, SMITH I, GAUDREAU L, PALU G, MANGANELLI R. Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic-function sigma factor sigma L and roles in virulence and in global regulation of gene expression. Infect Immun. 2006;74:2457–61. doi: 10.1128/IAI.74.4.2457-2461.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGOHANG-NDONG J, BAILLAT D, AUMERCIER M, BELLEFONTAINE F, BESRA GS, LOCHT C, BAULARD AR. EthR, a repressor of the TetR/CamR family implicated in ethionamide resistance in mycobacteria, octamerizes cooperatively on its operator. Mol Microbiol. 2004;51:175–88. doi: 10.1046/j.1365-2958.2003.03809.x. [DOI] [PubMed] [Google Scholar]
- GEBHARD S, HUMPEL A, MCLELLAN AD, COOK GM. The alternative sigma factor SigF of Mycobacterium smegmatis is required for survival of heat shock, acidic pH and oxidative stress. Microbiology. 2008;154:2786–95. doi: 10.1099/mic.0.2008/018044-0. [DOI] [PubMed] [Google Scholar]
- GREENSTEIN AE, MACGURN JA, BAER CE, FALICK AM, COX JS, ALBER T. M. tuberculosis Ser/Thr protein kinase D phosphorylates an anti-anti-sigma factor homolog. PLoS pathogens. 2007;3:e49. doi: 10.1371/journal.ppat.0030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUBER TM, GROSS CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–66. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- HELMANN JD. Anti-sigma factors. Curr Opin Microbiol. 1999;2:135–41. doi: 10.1016/S1369-5274(99)80024-1. [DOI] [PubMed] [Google Scholar]
- HINGLEY-WILSON SM, SAMBANDAMURTHY VK, JACOBS WR., JR Survival perspectives from the world’s most successful pathogen, Mycobacterium tuberculosis. Nat Immunol. 2003;4:949–55. doi: 10.1038/ni981. [DOI] [PubMed] [Google Scholar]
- HOFMANN KASW. TMbase-A database of membrane spanning proteins segment. Biol Chem Hoppe-Seyler. 1993;374 [Google Scholar]
- HUGHES KT, MATHEE K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–86. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- KROOS L, ZHANG B, ICHIKAWA H, YU YT. Control of sigma factor activity during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1285–94. doi: 10.1046/j.1365-2958.1999.01214.x. [DOI] [PubMed] [Google Scholar]
- LARSEN MH, VILCHEZE C, KREMER L, BESRA GS, PARSONS L, SALFINGER M, HEIFETS L, HAZBON MH, ALLAND D, SACCHETTINI JC, JACOBS WR., JR Overexpression of inhA, but not kasA, confers resistance to isoniazid and ethionamide in Mycobacterium smegmatis, M. bovis BCG and M. tuberculosis. Mol Microbiol. 2002;46:453–66. doi: 10.1046/j.1365-2958.2002.03162.x. [DOI] [PubMed] [Google Scholar]
- LEE JH, AMMERMAN NC, NOLAN S, GEIMAN DE, LUN S, GUO H, BISHAI WR. Isoniazid resistance without a loss of fitness in Mycobacterium tuberculosis. Nat Commun. 2012a;3:753. doi: 10.1038/ncomms1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE JH, AMMERMAN NC, NOLAN S, GEIMAN DE, LUN S, GUO H, BISHAI WR. Isoniazid resistance without a loss of fitness in Mycobacterium tuberculosis. Nature communications. 2012b;3:753. doi: 10.1038/ncomms1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALIK SS, LUTHRA A, RAMACHANDRAN R. Interactions of the M. tuberculosis UsfX with the cognate sigma factor SigF and the anti-anti sigma factor RsfA. Biochim Biophys Acta. 2009;1794:541–53. doi: 10.1016/j.bbapap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- MCDERMOTT PF, WHITE DG, PODGLAJEN I, ALEKSHUN MN, LEVY SB. Multidrug resistance following expression of the Escherichia coli marA gene in Mycobacterium smegmatis. J Bacteriol. 1998;180:2995–8. doi: 10.1128/jb.180.11.2995-2998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKINNEY JD. In vivo veritas: the search for TB drug targets goes live. Nat Med. 2000;6:1330–3. doi: 10.1038/82142. [DOI] [PubMed] [Google Scholar]
- MCKINNEY JD, HONER ZU BENTRUP K, MUNOZ-ELIAS EJ, MICZAK A, CHEN B, CHAN WT, SWENSON D, SACCHETTINI JC, JACOBS WR, JR, RUSSELL DG. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–8. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- MISSIAKAS D, RAINA S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–66. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- MORRIS RP, NGUYEN L, GATFIELD J, VISCONTI K, NGUYEN K, SCHNAPPINGER D, EHRT S, LIU Y, HEIFETS L, PIETERS J, SCHOOLNIK G, THOMPSON CJ. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102:12200–5. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRY JP, SASSETTI CM, LANE JM, XIE Z, RUBIN EJ. Transposon site hybridization in Mycobacterium tuberculosis. Methods Mol Biol. 2008;416:45–59. doi: 10.1007/978-1-59745-321-9_4. [DOI] [PubMed] [Google Scholar]
- PARK ST, KANG CM, HUSSON RN. Regulation of the SigH stress response regulon by an essential protein kinase in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2008;105:13105–10. doi: 10.1073/pnas.0801143105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAN S, SONG T, PUYANG X, BARDAROV S, JACOBS WR, JR, HUSSON RN. The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J Bacteriol. 2001;183:6119–25. doi: 10.1128/JB.183.20.6119-6125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMOS JL, MARTINEZ-BUENO M, MOLINA-HENARES AJ, TERAN W, WATANABE K, ZHANG X, GALLEGOS MT, BRENNAN R, TOBES R. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–56. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUE S, PROVVEDI R, JACQUES PE, GAUDREAU L, MANGANELLI R. The sigma factors of Mycobacterium tuberculosis. FEMS Microbiol Rev. 2006;30:926–41. doi: 10.1111/j.1574-6976.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- RUSTAD TR, SHERRID AM, MINCH KJ, SHERMAN DR. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol. 2009;11:1151–9. doi: 10.1111/j.1462-5822.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- SASSETTI CM, BOYD DH, RUBIN EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci U S A. 2001;98:12712–7. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG T, DOVE SL, LEE KH, HUSSON RN. RshA, an anti-sigma factor that regulates the activity of the mycobacterial stress response sigma factor SigH. Mol Microbiol. 2003;50:949–59. doi: 10.1046/j.1365-2958.2003.03739.x. [DOI] [PubMed] [Google Scholar]
- VAN KESSEL JC, HATFULL GF. Recombineering in Mycobacterium tuberculosis. Nat Methods. 2007;4:147–52. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- VAN KESSEL JC, HATFULL GF. Mycobacterial recombineering. Methods Mol Biol. 2008;435:203–15. doi: 10.1007/978-1-59745-232-8_15. [DOI] [PubMed] [Google Scholar]
- WOLFF KA, NGUYEN HT, CARTABUKE RH, SINGH A, OGWANG S, NGUYEN L. Protein kinase G is required for intrinsic antibiotic resistance in mycobacteria. Antimicrobial agents and chemotherapy. 2009;53:3515–9. doi: 10.1128/AAC.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAHRT TC, DERETIC V. Reactive nitrogen and oxygen intermediates and bacterial defenses: unusual adaptations in Mycobacterium tuberculosis. Antioxid Redox Signal. 2002;4:141–59. doi: 10.1089/152308602753625924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.