Abstract
The ability to decide adaptively between immediate vs. delayed gratification (intertemporal choice) is critical for well-being and is associated with a range of factors that influence quality of life. In contrast to young adults, many older adults show enhanced preference for delayed gratification; however, the neural mechanisms underlying this age difference in intertemporal choice are largely un-studied. Changes in signaling through GABAB receptors (GABABRs) mediate several age-associated differences in cognitive processes linked to intertemporal choice. The current study used a rat model to determine how GABABRs in two brain regions known to regulate intertemporal choice (prelimbic cortex; PrL and basolateral amygdala; BLA) contribute to age differences in this form of decision making in male rats. As in humans, aged rats showed enhanced preference for large, delayed over small, immediate rewards during performance in an intertemporal choice task in operant test chambers. Activation of PrL GABABRs via microinfusion of the agonist baclofen increased choice of large, delayed rewards in young adult rats but did not influence choice in aged rats. Conversely, infusion of baclofen into the BLA strongly reduced choice of large, delayed rewards in both young adult and aged rats. Aged rats further showed a significant reduction in expression of GABABR1 subunit isoforms in the prefrontal cortex, a discovery that is consonant with the null effect of intra-PrL baclofen on intertemporal choice in aged rats. In contrast, expression of GABABR subunits was generally conserved with age in the BLA. Jointly, these findings elucidate a role for GABABRs in intertemporal choice and identify fundamental features of brain maturation and aging that mediate an improved ability to delay gratification.
Keywords: Decision making, Delay discounting, Impulsive choice, Prefrontal cortex, Basolateral amygdala
1. Introduction
The ability to choose adaptively among options that vary in time to arrival (i.e., intertemporal choice) is critical for navigating many aspects of everyday life. Most individuals will choose a large over a small reward in the absence of delays, but reliably “discount” the subjective value of the large reward (i.e., switch their preference to the small, immediate reward) if there is a delay imposed between the choice and its delivery (Green et al., 1994). Greater discounting of large, delayed rewards (i.e., greater impulsive choice) is a hallmark of several neuropsychiatric disorders including schizophrenia, ADHD, and substance use disorders, which generally afflict young adults (Heerey et al., 2007; Perry and Carroll, 2008; Wilbertz et al., 2013). In contrast, older adults often exhibit less discounting of delayed rewards compared to young adults (i. e., decreased impulsive choice; Green et al., 1999). Although such bias can be beneficial in some circumstances, there is competing evidence showing that excessive bias toward large, delayed rewards associates with maladaptive behavior (Steinglass et al., 2012, 2017; Steward et al., 2017). Moreover, age-related alterations in decision making associate with cognitive impairments (Hernandez et al., 2017; Isella et al., 2008; Marschner et al., 2005). As such, decision-making strategies employed by older individuals may materialize as an impairment in adaptability to changes in cost-benefit contingencies. In fact, higher intertemporal discounting rates associate with poor healthcare choices and outcomes in older adults (65+ years of age; Axon et al., 2009; Bradford, 2010; James et al., 2012).
Intertemporal choice is mediated by a network of cortico-striatal-amygdalar circuitry (Berlin et al., 2004; Feja et al., 2014; Gourley et al., 2010; Hosking et al., 2014; Jo et al., 2013; Mar et al., 2011; Massar et al., 2015; Mobini et al., 2002; Walton et al., 2009; Winstanley et al., 2004) that includes the medial prefrontal cortex (mPFC; rodent homo-logue of the human DLPFC; Brown and Bowman, 2002; Uylings et al., 2003; Kesner and Churchwell, 2011; Bizon et al., 2012) and basolateral amygdala (Churchwell et al., 2009; Winstanley et al., 2004), in addition to several neuromodulatory systems (Anderberg et al., 2016; Aurelian et al., 2016; Joutsa et al., 2015; Smith et al., 2016; Winstanley et al., 2006). Functions supported by the mPFC (and particularly the prelimbic subregion of mPFC: PrL) such as working memory, behavioral flexibility, and appetitive behaviors (Floresco et al., 2008b; Ishikawa et al., 2008; Sloan et al., 2006; Zeeb et al., 2015) are foundational to time-based decision making (Hernandez et al., 2017; Hinson et al., 2003; Huckans et al., 2011). Additionally, the BLA plays an important role in associating the actions and outcomes (both positive and negative; Cardinal et al., 2002; Everitt et al., 2003; Yacubian et al., 2006; Tye et al., 2010; Johansen et al., 2012; Canessa et al., 2013; Tremblay et al., 2014; Wassum and Izquierdo, 2015) critical to the decision-making process. Indeed, several studies have shown the supports intertemporal choice (Churchwell et al., 2009; Hernandez et al., 2019; Winstanley et al., 2004). Finally, data from inactivation studies in rodents indicate that connections between the mPFC and BLA are particularly critical for intertemporal choice in young adults (Churchwell et al., 2009).
Aging is associated with perturbations in the balance between excitation and inhibition within the mPFC. Specifically, prior studies show that dysregulated mPFC inhibition contributes to impaired working memory and cognitive flexibility in aging (Bañuelos et al., 2014; Beas et al., 2017; McQuail et al., 2015). While less is known about the aged BLA, there is evidence to suggest alterations in neuronal activity (Roesch et al., 2012). In addition, prior work from our laboratory shows that contributions of the BLA to intertemporal choice change across the lifespan (Hernandez et al., 2019). In young adult rats, the BLA plays distinct roles during the decision-making process such that activity prior to choices biases toward selection of immediate gratification, whereas activity during outcome receipt biases subsequent choices toward delaying gratification. In contrast, in aged rats, BLA activity during outcome delivery does not bias decision making (Hernandez et al., 2019). This body of work suggests that, at least within the context of decision making, brain aging is non-uniform and that regionally distinct changes in neural activity may result in the underlying phenotypic differences associated with intertemporal choice that arise with age. Critical to elucidating the mechanisms by which changes in regional activity contribute to age-related alterations in decision making is a better understanding of the underlying molecular changes that occur in mPFC vs. BLA.
The GABAB receptor (GABABR) is a metabotropic receptor localized to both the presynaptic (Sakaba and Neher, 2003) and extrasynaptic membranes (Cruz et al., 2004; Cryan and Kaupmann, 2005; Labouèbe et al., 2007; Lüscher et al., 1997) where it regulates neurotransmitter release and tonic inhibition, respectively. As such, it is well-positioned to regulate neuronal activity, specifically excitatory and inhibitory signaling. Indeed, it has been shown that altered GABABR signaling in the mPFC is mechanistically linked to cognitive deficits that emerge at advanced ages (Bañuelos et al., 2014; Beas et al., 2017). Baclofen (a GABABR agonist) has been approved for treatment of several clinical conditions (Baker et al., 2014; Overgård et al., 2015; Rekand and Grønning, 2011) some of which are strongly associated with maladaptive decision making (Erickson et al., 2014; Kahn et al., 2009; Muzyk et al., 2012). Notably, the GABABR agonist baclofen has shown treatment potential in a preclinical model of executive dysfunction in aging (Beas et al., 2017), although nothing is known about its effects on age-related alterations in decision making.
Considering the importance of mPFC GABABR signaling in age-related cognitive changes, as well as the critical contributions of the PrL and BLA to intertemporal choice, the current study sought to determine how GABABRs contribute to changes in intertemporal choice at advanced ages. In a first set of experiments, a behavioral pharmacological approach was used to test the effects of targeting GABABRs in PrL on intertemporal choice in young and aged rats. In a second set of experiments, the same approach was used to assess the role of GABABRs within BLA on intertemporal choice in young and aged rats. In a third set of experiments, Western blot analyses were used to evaluate age changes in GABABR expression in a behaviorally naïve cohort of young and aged rats.
2. Materials and methods
2.1. Subjects
Young adult (6 months, n = 40) and aged (26 months, n = 36) male Fischer 344 × Brown-Norway F1 hybrid rats were obtained from the National Institute on Aging aged rodent colony maintained by Charles River Laboratories (at the time at which these experiments were conducted, age-matched females were not available for study). Rats were housed in the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited vivarium facility in the McKnight Brain Institute Building at the University of Florida. The facility was maintained at a consistent temperature of 25 °C with a 12-h light/dark cycle (lights on at 0700) with free access to food and water except as otherwise noted. All animal procedures were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines. One cohort of young adult (n = 15) and aged (n = 12) rats was used to determine how modulation of PrL GABABRs influenced performance in an intertemporal choice task. After cannula placement verification, final group sizes were n = 9 young and n = 9 aged rats. A second cohort of young adult (n = 15) and aged (n = 12) rats was used to determine how modulation of BLA GABABRs influenced performance in the same task. After cannula placement verification, final group sizes were n = 12 young and n = 8 aged rats. A third cohort of behaviorally naïve young adult (n = 10) and aged (n = 12) rats was used to evaluate age differences in GABABR subunit expression in both PrL and BLA.
2.2. Surgical procedures
Rats were anesthetized with isoflurane gas and secured in a stereotaxic frame. Following a midline incision, skin was retracted, and holes were drilled in the skull for placement of guide cannulae and stainless-steel anchoring screws. Bilateral guide cannulae (22-gauge, Plastics One) were implanted to target the PrL (AP: +2.7 from bregma, ML: ± 0.7 from bregma, DV: −3.8 from the skull surface) or the BLA (AP: −3.25 mm from bregma, ML: ±4.95 mm from bregma, DV: −7.3 mm from the skull surface) and secured to the skull and anchoring screws using dental cement. Stainless steel obdurators were placed into the cannulae to minimize the risk of cannula occlusion. Immediately after surgery, rats received subcutaneous injections of buprenorphine (1 mg/kg/day) and meloxicam (2 mg/kg/day). Buprenorphine was also administered 24 h post-operation, and meloxicam 48–72 h post-operation. A topical ointment was applied to the incision to facilitate wound healing. Prior to behavioral procedures, rats received at least 2 weeks post-surgical recovery, with sutures removed after 10–14 days.
2.3. Intertemporal choice task
Behavioral testing was conducted in 8 identical standard rat behavioral test chambers (Coulbourn Instruments). Each test chamber was equipped with a recessed food pellet delivery trough that was fitted with a photobeam to detect head entries. Two retractable levers were positioned to the left and right of the food trough. A computer running Graphic State 4.0 software (Coulbourn Instruments) was used to control the operant chambers and collect data. Before the start of testing, rats were food-restricted to 85% of their free-feeding weights. Food rewards consisted of 45-mg grain-based food pellets (PJAI; Test Diet, Richmond, IN, USA). Behavioral shaping and testing procedures were similar to those used in our previous studies of age-related changes to intertemporal choice (Hernandez et al., 2017; Simon et al., 2010). Each 80 min session consisted of 5 blocks of 12 trials each (Fig. 1). Each 80 s trial began with a 10 s illumination of the food trough and house lights. A nosepoke into the food trough during this time extinguished the food trough light and triggered extension of either a single lever (forced choice trials) or of both levers simultaneously (free choice trials). Each block consisted of 2 forced choice trials (one for each lever) followed by 10 free choice trials. The forced choice trials were designed to remind rats of the delay contingencies in effect for that block. A press on one lever (either left or right, counterbalanced across age groups) resulted in one food pellet (the small reward) delivered immediately. A press on the other lever resulted in 4 food pellets (the large reward) delivered after a variable delay. The identities of the levers (small or large reward) remained consistent throughout testing. Failure to press either lever within 10 s of their extension resulted in the levers being retracted and lights extinguished, and the trial was scored as an omission. Once either lever was pressed, both levers were retracted for the remainder of the trial (i.e., the inter-trial interval). The duration of the delay preceding large reward delivery increased between each block of trials (0, 10, 20, 40, 60 s), but remained constant within each block.
Fig. 1.
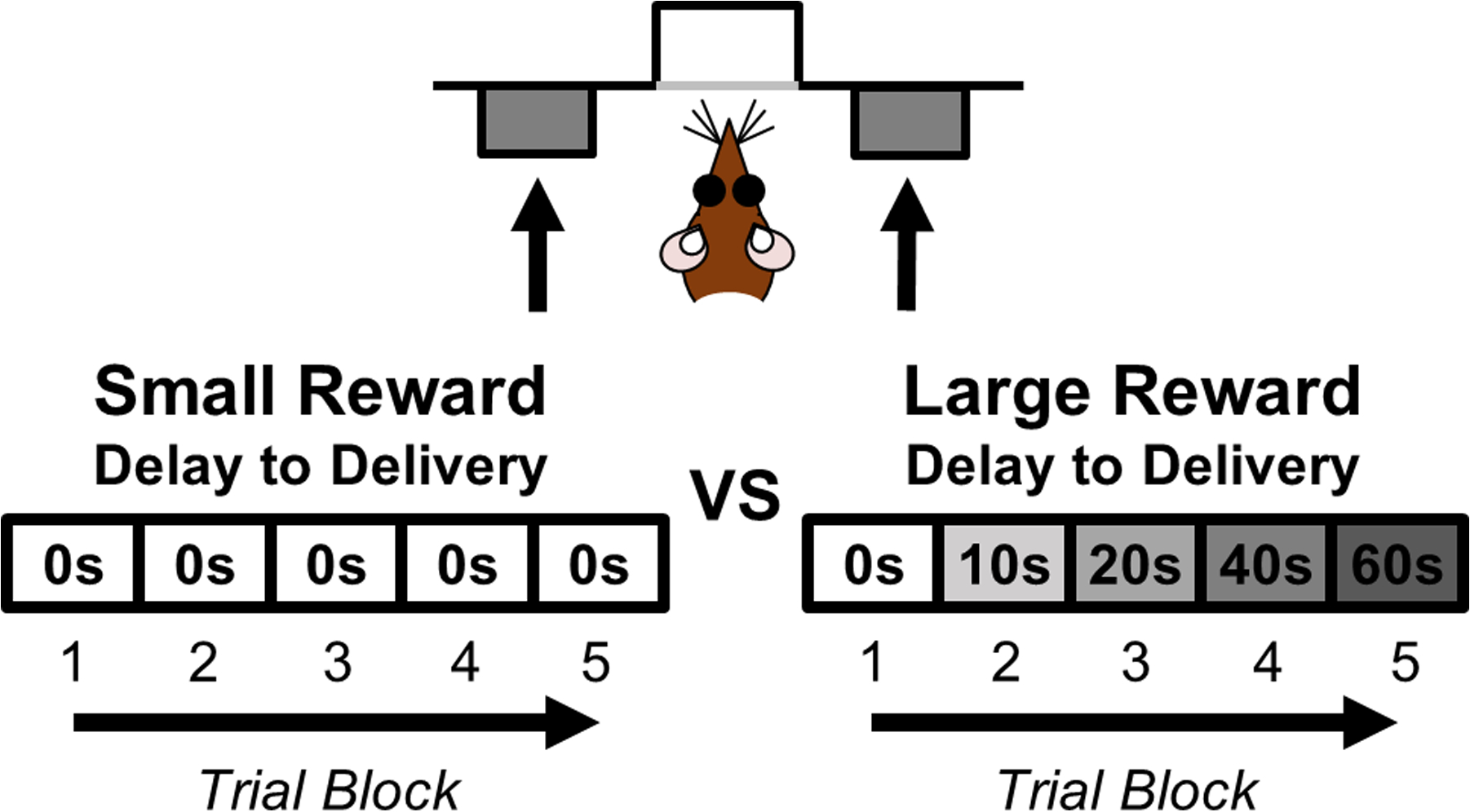
Schematic of the intertemporal choice task illustrating the choices and trial blocks across which the duration of the delay to the large reward increased. On each trial, rats were presented with two response levers that differed with respect to the magnitude and timing of associated reward delivery. Presses on one lever delivered a small (one food pellet), immediate reward, whereas presses on the other lever delivered a large (three food pellets), delayed reward. Levers associated with a small or large reward were counterbalanced across rats. Trials were presented in a blocked design, such that the delay to the large reward increased across successive blocks of trials in a session.
2.4. Drug preparation and intracerebral micro-infusion
The selective GABABR agonist (R)-baclofen (baclofen; Cat. No. 0796, Tocris, Ellison, MO, USA) was dissolved in aCSF at concentrations of 0.03, 0.1, and 0.3 μg per 0.5 μL. The selective GABABR antagonist, CGP55845 (Cat. No. 1248, Tocris), was dissolved in an aCSF solution containing less than 0.01% DMSO at concentrations of 0.05, 0.15, and 0.5 μg per 0.5 μL. These drug doses bracket those previously shown to modulate cognitive performance when infused into the mPFC (Bañuelos et al., 2014; Beas et al., 2017).
For each drug (baclofen and CGP55845), doses were administered using a randomized, within-subjects design such that each rat received each dose of both drugs as well as their respective vehicles, with a 48-h washout period between successive infusions. Baclofen was tested first followed by CGP55845, with 4 days of drug-free testing between drug regimens to re-establish baseline performance. Each infusion was administered by an experimenter who was blinded to the treatment conditions. Drugs were administered using 10 μL Hamilton syringes mounted on an infusion pump (Pump 11 Elite, Harvard Apparatus, Holliston, MA, USA) and connected via PE-20 tubing to micro-injectors (Plastics One) that extended 1 mm past the end of the guide cannulae. Each dose was delivered in a volume of 0.5 μL/hemisphere over a duration of 1 min, and injectors were left in place for one additional minute to allow for diffusion. Behavioral testing began 5 min post-infusion. After completion of behavioral testing, rats were euthanized and cannula placements were verified using histological methods as described in our previous studies (C. M. Hernandez et al., 2018; McQuail et al., 2016a).
2.5. Tissue micro-punching and protein isolation
A separate cohort of behaviorally naive young and aged rats was used for regionally specific Western blot analysis of GABABR expression in mPFC and BLA. Rats were sacrificed by rapid decapitation and whole brains were quickly extracted, frozen on dry ice, and stored at −80 °C. Brains were equilibrated to −10 °C in a cryostat and 360 μm sections were cut through the rostral-caudal extent of the frontal cortex and BLA. A 1 mm tissue biopsy punch tool was used to obtain samples from PrL and BLA. Tissue punches were immediately transferred to homogenization buffer and membrane fractions were isolated according to previously published procedures (A. R. Hernandez et al., 2018b, 2018a; C. M. Hernandez et al., 2018; McQuail et al., 2012). A total of 5 μg of protein for each independent biological sample was loaded in triplicate and separated on 4–15% TGX gels (Bio-Rad Laboratories, Hercules, CA, USA) at 200 V for 45 min in tris-glycine running buffer (Bio-Rad). Total protein was transferred to a 0.45 μm pore nitrocellulose membrane at 20 V for 7 min using iBlot Gel Transfer Nitrocellulose Stacks (NR13046–01, Invitrogen, Waltham, MA, USA) and an iBlot machine (Invitrogen, Waltham, MA, USA). All experiments were conducted in triplicate, and the loading order of samples was counterbalanced between gels and experiments to control for systematic variation in the electrophoresis and electroblotting procedures.
Immediately after transfer, membranes were stained for total protein using LiCor’s Revert total protein stain for 5 min (Li-Cor, 926–11011) and scanned using a 685 nm laser on an Odyssey IR Scanner (Li-Cor, Lincoln, Nebraska USA) to detect total protein per lane. Membranes were then placed into Rockland blocking buffer (Rockland Blocking Buffer, Rockland, Gilbertsville, PA, USA) for 1 h at room temperature. After blocking, membranes were incubated at 4 °C overnight with antibodies raised against target proteins (Table 2). Membranes were washed in tris buffered saline before incubation in donkey anti-rabbit secondary antibodies conjugated to IRDye800 (diluted 1:15000; LI-COR). Blots were scanned using a 785 nm laser on an Odyssey IR Scanner. Total protein within each lane was quantified using Image Lab (BioRad) and intensity of immunoreactive bands were quantified with Image Studio v5.0 (Li-Cor).
Table 2.
Antibody information.
| Antibody | Host | Dilution | Company | Part number |
|---|---|---|---|---|
| GABABR1a | Rabbit | 1:1000 | Cell Signaling Technology | 3835 |
| GABABR1b | Rabbit | 1:1000 | Cell Signaling Technology | 3835 |
| GABABR2 | Rabbit | 1:1000 | Cell Signaling Technology | 3839 |
| vGaT | Rabbit | 1:2000 | Millipore | AB5062P |
2.6. Statistical analyses
In behavioral experiments, the percentage of large reward choices at each delay was the primary measure of interest (Hernandez et al., 2017; Simon et al., 2010). After reaching stable baseline performance, defined as less than 20% variability across a 5-session window (after a mean of 33.7 ± 1.5 sessions for all rats), baseline effects of age and cohort on intertemporal choice performance were tested using a mixed-factor (cohort × age × delay) ANOVA. Rats were then moved on to the counterbalanced drug schedule. The effects of intracerebral infusions on choice behavior were analyzed using a mixed-factor ANOVA, with dose (4 levels) and delay (5 levels) as within-subjects factors and age (2 levels) as a between-subjects factor (within-subject factor p-values were corrected with the Huynh-Feldt procedure whenever sphericity was violated). Main effects of dose were re-tested by means of paired-samples t-tests that were limited to comparisons between vehicle and each dose of drug. Response latency data (the time between lever extension and a lever press) were collected on forced choice trials. Previous work shows that response latencies on forced choice trials can differ for large and small reward levers (Hernandez et al., 2017) and may reflect differences in motivation to obtain differently-preferred rewards (Crespi, 1942; Mai et al., 2012; Setlow et al., 2003; Shimp et al., 2015). As such, we operationally defined incentive/motivation as the latency to respond on forced choice trials. At doses for which choice performance reliably differed from vehicle, latencies to choose the large reward on forced choice trials were also compared using a mixed-factor ANOVA with drug (dose vs vehicle) as a within-subjects factor and age as a between-subjects factor. Additionally, the number of free choice trial omissions was analyzed using a drug dose by age mixed-factor ANOVA. Finally, mixed-factor ANOVAs were used to assess for possible effects of injection number or lingering effects of drug doses on subsequent doses with injection number (injection number × age × delay) or prior day’s dose (prior day’s dose × age × delay) as within-subjects factors.
For analyses of protein expression in PrL and BLA, immunoreactivity for GABABR subunits was normalized to total protein per lane and technical replicates were averaged for each independent biological sample. All protein expression data were then transformed and expressed as a percentage of young values. Independent-samples t-tests were used to compare expression of each subunit between young and aged groups.
3. Results
3.1. Effect of age on intertemporal choice performance under baseline conditions
Two separate cohorts of rats were used to assess the effects of GABABR modulation in PrL (cohort 1) and BLA (cohort 2). Performance of each cohort prior to drug infusions is shown separately in Fig. 2. Both young and aged rats showed equivalent preference for the large reward in the absence of delays, but decreased their choice of the large reward as the delay to its delivery increased (Cohort 1 (PrL), main effect of delay: F(4, 100) = 43.005, p < 0.001; Cohort 2 (BLA), main effect of delay: F(4, 100) = 31.333, p < 0.001). Aged rats in both cohorts, however, reliably preferred the large, delayed reward compared to young, and this age-associated preference was more robust at long delays (Cohort 1 (mPFC): main effect of age: F(1, 25) = 4.501, p = 0.044; age × delay interaction: F(4, 100) = 5.695, p = 0.001; Cohort 2 (BLA): main effect of age: F(1, 25) = 5.677, p = 0.025; p < 0.001; age × delay interaction: F(4, 100) = 6.704, p < 0.001). Choice performance did not differ between the two cohorts (cohort × delay × age) prior to drug infusions (no main effects or interactions involving cohort; main effect of cohort: F(1, 50) = 0.129, p = 0.721; main effect of age: F(1, 50) = 10.163, p = 0.002; main effect of delay: F(4, 200) = 73.576, p < 0.001; cohort × age: F(1, 50) = 0.058, p = 0.811; cohort × delay: F(4, 200) = 0.501, p = 0.735; age by delay: F(4, 200) = 12.324, p < 0.001; cohort × delay × age: F(4, 200) = 0.099, p = 0.754).
Fig. 2.
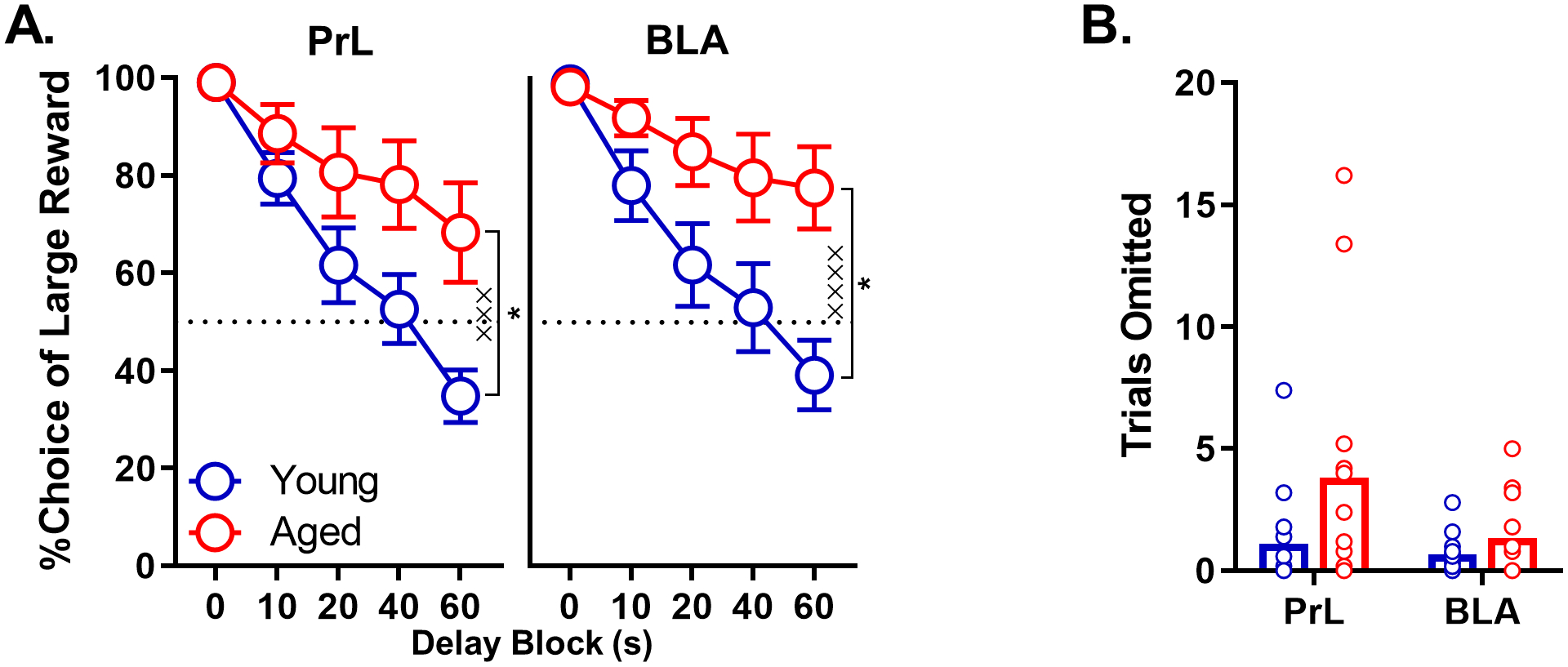
Choice performance under baseline conditions in a group of young and aged rats with PrL cannula and another group of young and aged rats with BLA cannula. A) Aged rats with cannula targeting either the PrL or BLA showed a disproportionately greater choice of the large reward at longer delays. B) The number of trial omissions did not differ between young and aged rats in either cannula cohort. C) Latency to choose the large and small rewards in young (C) and aged (D) rats plotted as a function of the delay to large reward delivery. See text for statistical details. In A, percent choice of the large rewarded (y-axis) is plotted as a function of delay (x-axis) in young (blue lines, blue circles) and aged (red lines, red circles) rats. In B, mean trials omitted (y-axis) is plotted as a function of age (x-axis) for young (blue bars) and aged (red bars) rats. In C, mean response latency (y-axis) is plotted as a function of delay block (x-axis) in young (blue lines, triangles for small reward, squares for large reward) and aged (red lines, triangles for small reward, squares for large reward) rats. Error bars represent the standard error of the mean (SEM).
3.2. GABABRs and prelimbic cortex
Fig. 3 shows the locations of cannula tips in the PrL for rats included in behavioral pharmacology experiments. A total of n = 5 young and n = 4 aged rats were excluded from the analyses due to misplaced cannulae (see Table 1 for final group sizes).
Fig. 3.
Cannula placements targeting the PrL in both young (left) and aged (right) rats. Filled black circles represent verified and on-target cannula placements in both groups. Cannula placements are mapped to standardized coronal sections corresponding to +2.70 mm through +3.20 mm from bregma according to the atlas of Paxinos and Watson (2005).
Table 1.
Initial and final group numbers.
| Experiment | Region | Age | ni | nf |
|---|---|---|---|---|
| Pharmacology | PrL | Young | 14 | 9 |
| Aged | 13 | 9 | ||
| BLA | Young | 15 | 12 | |
| Aged | 15 | 8 | ||
| vGaT | PrL | Young | 12 | 11 |
| Aged | 12 | 12 | ||
| BLA | Young | 12 | 11 | |
| Aged | 12 | 12 | ||
| GABABR1A | PrL | Young | 12 | 11 |
| Aged | 12 | 10 | ||
| BLA | Young | 12 | 11 | |
| Aged | 12 | 12 | ||
| GABABR1B | PrL | Young | 12 | 11 |
| Aged | 12 | 10 | ||
| BLA | Young | 12 | 11 | |
| Aged | 12 | 10 | ||
| GABABR2 | PrL | Young | 12 | 12 |
| Aged | 12 | 12 | ||
| BLA | Young | 12 | 12 | |
| Aged | 12 | 12 |
Effects of Intra-PrL infusions of baclofen on intertemporal choice.
After achieving stable baseline performance, drug doses were tested using a randomized, within-subjects design. Fig. 4A shows mean choice performance of young and aged rats under vehicle and baclofen conditions. A multifactor ANOVA (dose × delay × age) indicated that infusion of baclofen into the PrL increased choice of the large reward (main effect of dose: F(3,48) = 4.009, p = 0.028), particularly at long delays (dose × delay interaction: F(12,192) = 3.863; p < 0.01). A trend toward a dose × delay × age interaction was also noted (F(12,192) = 1.913, p = 0.055). To further explore the main effect of baclofen on choice behavior, follow-up comparisons were used to compare performance following each dose of drug to performance under the vehicle condition. These comparisons indicated that the 0.30 μg baclofen dose reliably increased choice of the large, delayed reward relative to vehicle (11 ± 4%; t(17) = −2.472, p = 0.024), while performance after infusion of 0.03 μg or 0.10 μg doses did not differ from vehicle (ts(17) = −0.291−0.691, ps = 0.499–0.744). Additional analyses were conducted to further elucidate the effect of 0.30 μg baclofen on rats’ performance. Fig. 4B shows choice of the large reward lever (averaged across delays) for each rat under both vehicle and 0.30 μg baclofen conditions. In young rats, 0.30 μg baclofen produced a consistent (in all but one rat) increase in choice of the large, delayed reward (t(8) = 2.917; p = 0.019), whereas the effect of this dose in aged rats was less reliable (t(8) = 0.783; p = 0.456), likely due to a ceiling effect in vehicle performance such that several aged rats were already maximizing choice of the large, delayed reward. Consistent with the patterns of choice behavior at this dose, baclofen also reliably reduced rats’ latency to choose the large reward on forced choice trials relative to vehicle conditions (Table 3; young paired t-test: t(8) = −2.447, p = 0.040; aged paired t-test: t(8) = −2.788, p = 0.024). These data are consistent with the idea that baclofen increased the incentive motivation to obtain the large reward (Amsel, 1950; Crespi, 1942; Hernandez et al., 2017; Mai et al., 2012; Orsini et al., 2015; Setlow et al., 2003; Shimp et al., 2015). To test nonspecific effects of 0.30 μg baclofen on behavior, the total number of omitted trials per session was evaluated using an age × drug (vehicle vs 0.30 μg) ANOVA. There were no effects of baclofen on trial omissions (age: F(1,16) = 2.246, p = 0.153; dose: F(1,16) = 3.333, p = 0.087; age × dose: F(1,16) = 0.117, p = 0.737; see Table 4).
Fig. 4.
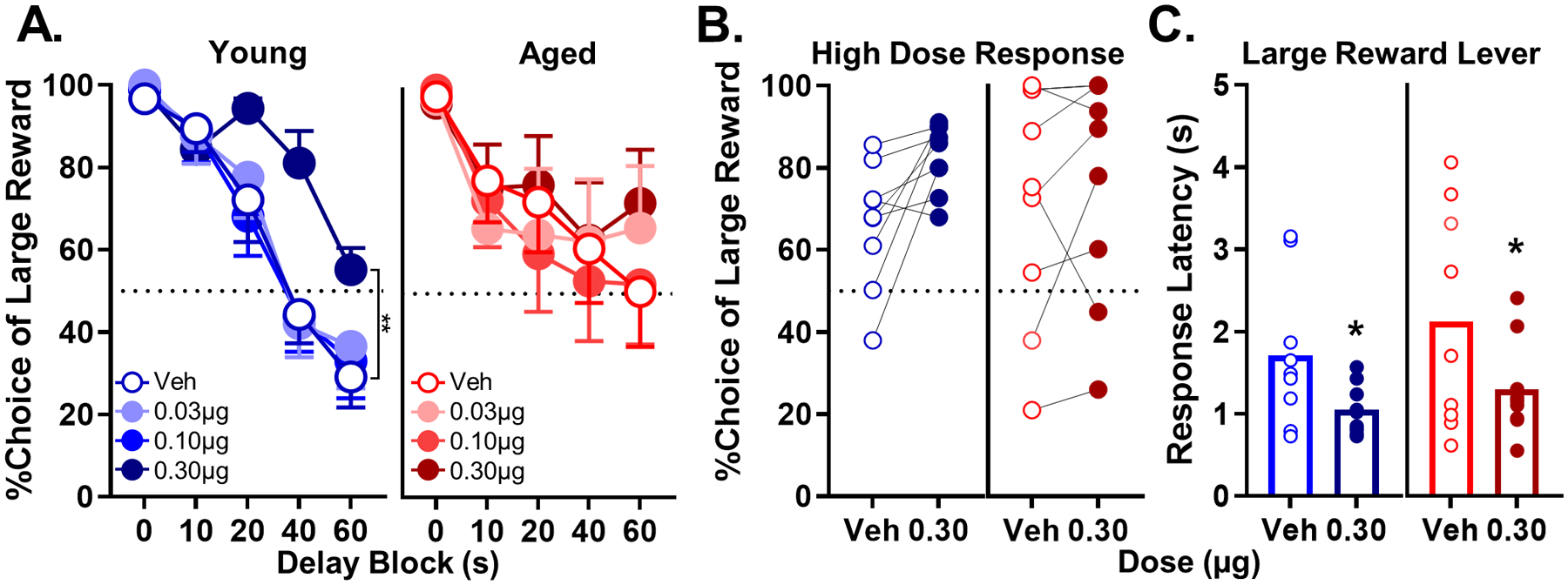
Effects of intra-PrL microinfusions of baclofen in young and aged rats. A) The effect of baclofen on choice performance in young rats. Baclofen impacted choice performance in a dose-dependent manner such that 0.30 μg dose significantly increased choice of the large, delayed reward (n = 9 young; **p < 0.01). Baclofen did not significantly affect choice performance in aged rats (n = 9 aged). B) The effect of 0.30 μg dose of baclofen on individual performance in young and aged rats. C) Response latency to the lever associated with the large, delayed reward during forced choice trials. The 0.30 μg dose of baclofen significantly decreased response latency in young and aged rats (*p < 0.05). In A, percent choice of the large reward (y-axis) is plotted as a function of delay (x-axis) comparing vehicle (blue lines, open circles) to different baclofen doses (blue lines, filled circles) in young rats. In aged rats, percent choice of the large reward (y-axis) is plotted as a function of delay (x-axis) comparing vehicle (red lines, open circles) to different baclofen doses (red lines, filled circles). In B, vehicle (blue, open circles) is compared to the 0.30 μg baclofen dose (blue, filled circles) in young rats, whereas vehicle (red, open circles) is compared to the 0.30 μg baclofen dose (red, filled circles) in aged rats. In C, mean response latency to the large reward lever in seconds (y-axis) is plotted as a function of baclofen dose for young (x-axis; blue open bars represent vehicle and blue, filled bars represent 0.3 μg baclofen) and aged (x-axis; red open bars represent vehicle and red, filled bars represent each baclofen dose) rats. Error bars represent the standard error of the mean (SEM).
Table 3.
Lever response latencies in rats that received intra-PrL baclofen infusions.
| Latency PrL baclofen | Small reward lever | Large reward lever | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veh | 0.03 | 0.1 | 0.3 | Veh | 0.03 | 0.1 | 0.3 | |||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Young | 1.963 | 0.354 | 1.523 | 0.180 | 1.708 | 0.284 | 1.594 | 0.210 | 1.713 | 0.296 | 1.649 | 0.296 | 1.670 | 0.225 | 1.050* | 0.102 |
| Aged | 2.918 | 0.324 | 2.386 | 0.205 | 2.509 | 0.140 | 2.267 | 0.252 | 2.121 | 0.444 | 1.700 | 0.370 | 2.492 | 0.727 | 1.295* | 0.194 |
Table 4.
Trial omissions in rats that received intra-PrL baclofen infusions.
| Omissions PrL baclofen | Veh | 0.03 | 0.1 | 0.3 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Young | 2.333 | 1.397 | 0.556 | 0.176 | 1.000 | 0.527 | 0.889 | 0.564 |
| Aged | 4.556 | 1.526 | 6.778 | 2.385 | 2.667 | 0.882 | 2.444 | 0.689 |
To evaluate potential carry-over effects of the drug micro-infusions, performance on intervening days of the drug schedule (i.e. wash-out days) was also evaluated using a multi-factor (prior day’s dose × delay × age) ANOVA. Besides the expected main effect of delay (F(4, 60) = 27.437, p < 0.001), there was no residual effect of baclofen on task performance on the wash-out days following drug infusion (main effect of prior day’s dose: F(3, 45) = 1.625, p = 0.197; main effect of age: F(1, 15) = 0.364, p = 0.556; main effect of prior day’s dose × age: F(3, 45) = 0.879, p = 0.459; dose × delay: F(12, 180) = 1.747, p = 0.066; delay × age: F(4, 60) = 2.573, p = 0.073; dose × delay × age: F(12, 180) = 0.367, p = 0.973; data not shown). The effect of injection number was also tested using a multi-factor (injection number × delay × age) ANOVA. Besides the expected main effect of delay (F(4, 64) = 25.978, p < 0.001) and delay × age interaction (F(4, 64) = 2.998, p = 0.025), the cumulative number of micro-infusions did not influence performance (main effect of injection day: F(3, 48) = 1.665, p = 0.187; main effect of age: F(1, 16) = 0.025, p = 0.876; injection number × delay: F(12, 192) = 1.730, p = 0.112; injection number × age: F(3, 48) = 2.050, p = 0.119; injection number × delay × age: F(12, 192) = 1.543, p = 0.112; data not shown).
Effects of Intra-PrL infusions of CGP on intertemporal choice.
To determine whether endogenous, tonic activation of GABABRs contributes to age-dependent differences in choice behavior, the effects of administering the selective GABABR antagonist (CGP55845) into PrL were evaluated. Fig. 5 shows mean choice performance of young and aged rats under vehicle and CGP55845 conditions. No main effects or interactions were observed with a multifactor ANOVA (dose × delay block × age, Fs < 1.07, ps < 0.39), indicating that the antagonist did not reliably influence choice behavior in either age group. Additionally, no dose of CGP affected trial omissions (dose: F(3,48)=0.388, p=0.688; dose × age: F(3,48)=0.592, p=0.564; Table 5).
Fig. 5.
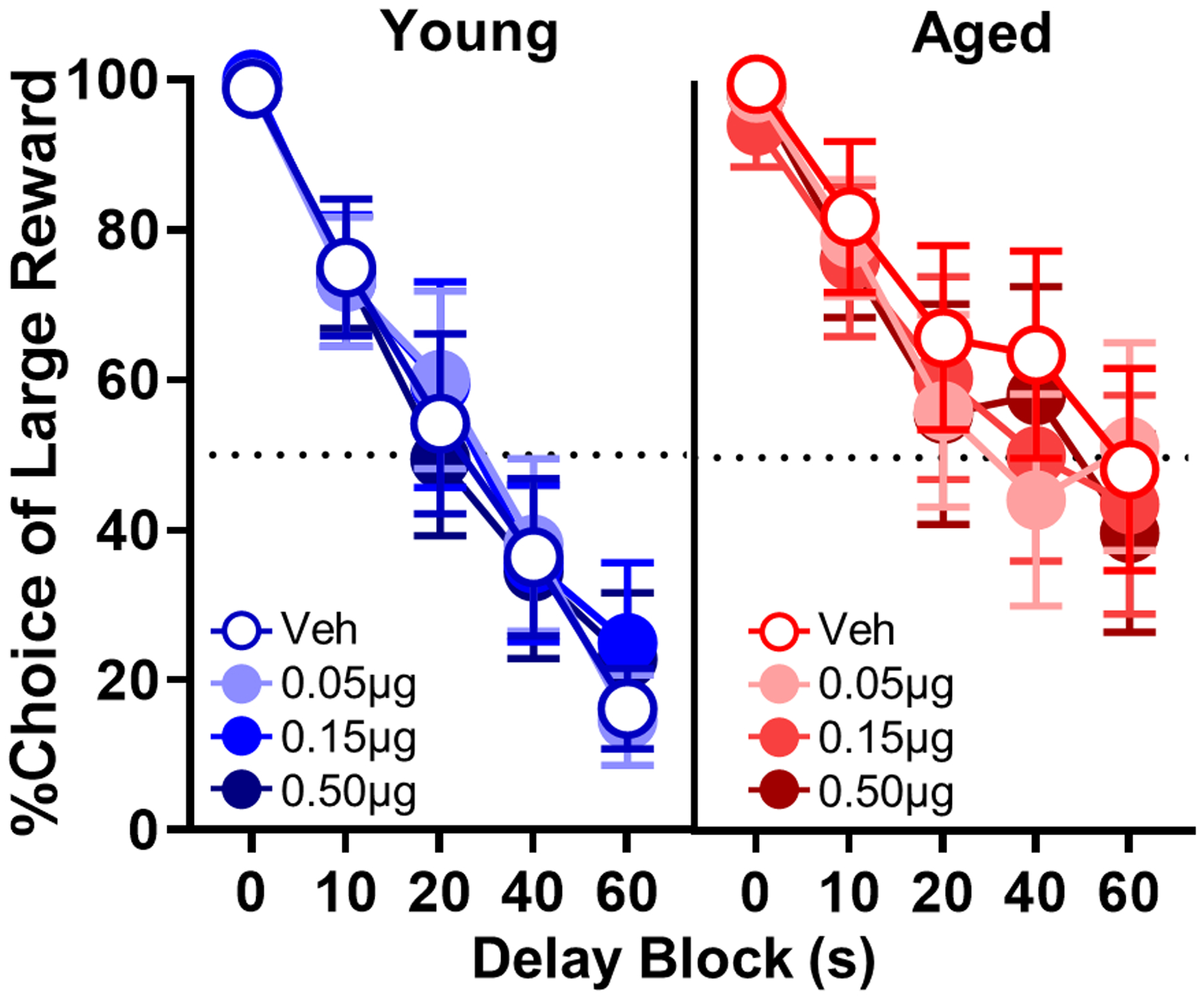
Effects of intra-PrL microinfusions of CGP55845 in young and aged rats. CGP55845 did not alter choice performance in young rats (n = 9) or aged (n = 8) rats. Percent choice of the large rewarded (y-axis) is plotted as a function of delay (x-axis) comparing vehicle (blue lines, open circles) to different CGP55845 doses (blue lines, filled circles) in young and comparing vehicle (red lines, open circles) to different CGP55845 doses (red lines, filled circles) in aged rats. Error bars represent the standard error of the mean (SEM).
Table 5.
Trial omissions in rats that received intra-PrL CGP infusions.
| Omissions PrL CGP | Veh | 0.05 | 0.15 | 0.5 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Young | 1.556 | 1.200 | 2.556 | 2.082 | 2.778 | 2.532 | 2.111 | 1.867 |
| Aged | 8.278 | 5.296 | 4.444 | 1.676 | 6.444 | 2.604 | 3.556 | 1.676 |
GABABR expression is attenuated in aged PrL.
Collectively, the behavioral pharmacological data show that although activation of GABABRs in PrL is not critical for maintenance of normal intertemporal choice behavior in either age group, exogenous activation of these receptors can increase rats’ preference for large, delayed over small, immediate rewards (decreased impulsive choice). To determine the status of GABABR availability in both young and aged rats, expression of GABABR subunits in the PrL was assessed in a separate behaviorally naïve cohort of young and aged rats (Fig. 6). In aged PrL, expression of both the R1a (t(20) = 2.453, p = 0.023) and R1b (t(19) = 2.497, p = 0.022) subunits was attenuated, whereas the R2 subunit was not reliably changed (t(22) = 1.259, p = 0.221). Expression of the vesicular GABA transporter (vGaT) was also unchanged with age (t(22) = −0.243, p = 0.910), consistent with the interpretation that the age-associated reductions in the R1 subunit were not secondary to a more global reduction in GABAergic synapses.
Fig. 6.
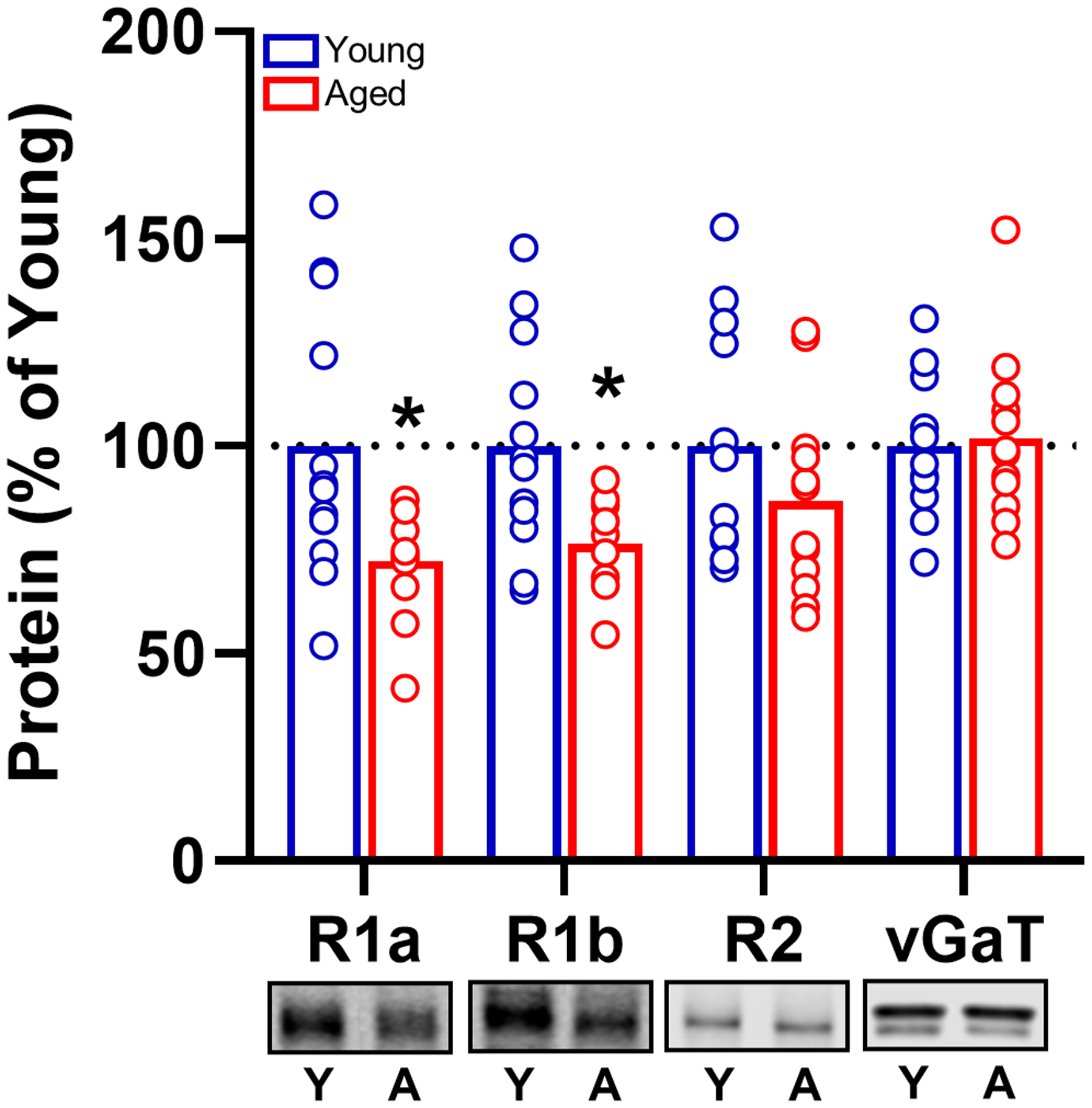
Effect of age on GABAB receptor protein in the PrL. GABABR1a and R1b protein levels within the aged medial prefrontal cortex are reduced compared to young. In contrast, there is no significant effect of age on the R2 isoform. Similarly, there is no age effect on vGaT expression. Open, blue bars represent relative intensity normalized total protein in young, whereas red bars represent relative intensity normalized to total protein in aged rats. Data are expressed as percent of young. Error bars represent standard error of the mean (SEM).
3.3. GABABRs and basolateral amygdala
Fig. 7 shows the placements of cannula in the BLA for rats included in behavioral pharmacology experiments. A total of n = 3 young and n = 7 aged rats were excluded from the analyses due to misplaced cannulae (see Table 1 for final group sizes).
Fig. 7.
Cannula placements targeting the BLA in both young (left) and aged (right) rats. Filled black circles represent verified and on-target cannula placements in both groups. Cannula placements are mapped to standardized coronal sections corresponding to −2.80 mm through −3.30 mm from bregma according to the atlas of Paxinos and Watson (2005).
Effects of Intra-BLA infusions of baclofen on intertemporal choice.
Fig. 8A shows mean choice performance of young and aged rats under vehicle and baclofen conditions. A multi-factor ANOVA (dose × delay × age) revealed a significant main effect of baclofen dose (F(3,54) = 8.362, p < 0.001), which did not differ between age groups (dose × age: F(3,54) = 0.925, p = 0.435) or across delays (dose × delay: F(12,216) = 1.328, p = 0.204; dose × delay × age: F(12,216) = 0.818, p = 0.632). Follow-up comparisons determined that both 0.1 μg (−13 ± 3%; t(19) = 3.860, p = 0.001) and 0.3 μg baclofen (−17 ± 3%; t(19) = 5.038, p < 0.001) reliably decreased choice of the large, delayed reward relative to vehicle, while infusion of 0.03 μg did not significantly influence choice behavior. Follow-up analyses focused on the dose of baclofen (0.3 μg) that produced the largest effect on choice behavior. Consistent with the patterns of choice behavior at this dose, baclofen also reliably increased rats’ latency to choose the large reward on forced choice trials relative to vehicle conditions (Table 6; young paired t-test: t(11) = −2.351, p = 0.038; aged paired t-test: t(7) = −5.393, p = 0.001). These data are consistent with baclofen reducing the incentive motivation for the large reward (Amsel, 1950; Crespi, 1942; Hernandez et al., 2017; Mai et al., 2012; Orsini et al., 2015; Setlow et al., 2003; Shimp et al., 2015). To test nonspecific effects of 0.30 μg baclofen on behavior, the total number of omitted trials per session was evaluated using an age × drug (vehicle vs 0.30 μg) ANOVA. A main effect of age (F(1,18) = 5.575, p = 0.030) indicated that aged rats omitted reliably more trials than young, and a main effect of dose (F(1,18) = 17.106, p = 0.001) indicated 0.3 μg of baclofen increased trial omissions, whereas age and dose did not interact (F(1,18) = 2.873, p = 0.107; see Table 7 for details).
Fig. 8.
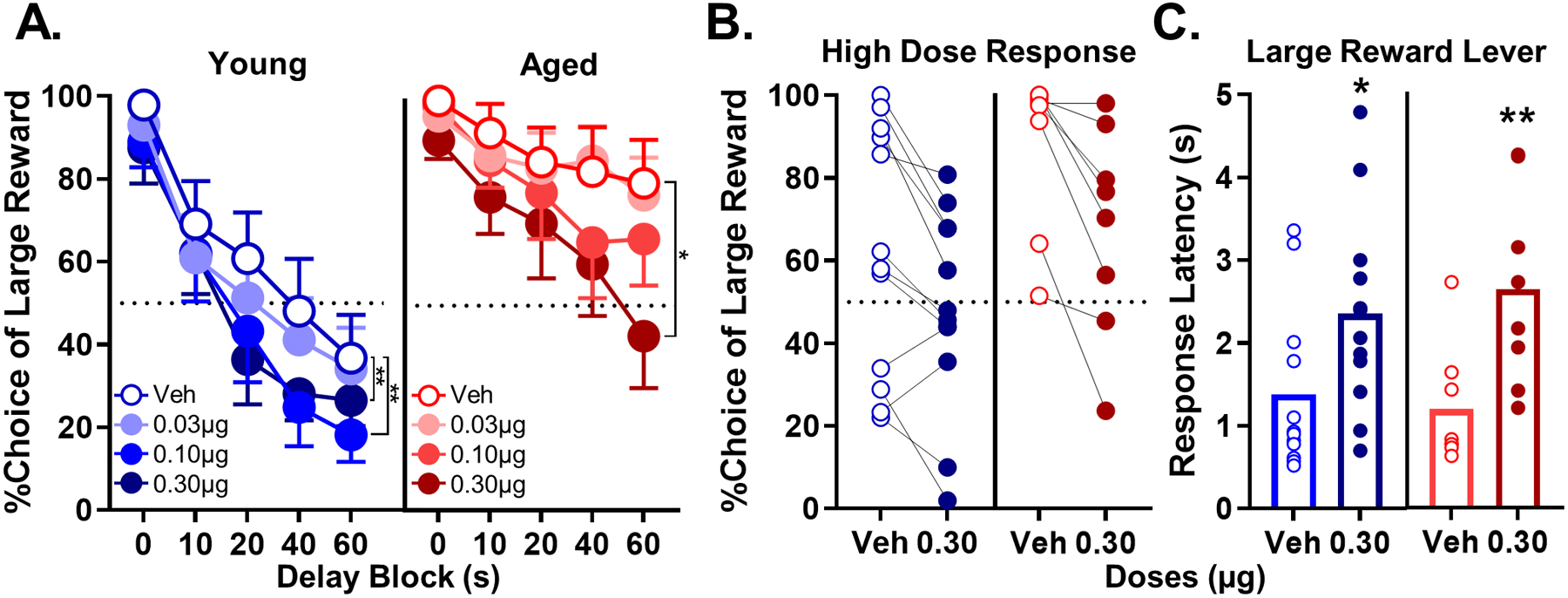
Effects of intra-BLA microinfusions of baclofen in young and aged rats. A) The effect of baclofen on choice performance in young rats. Baclofen impacted choice performance in a dose-dependent manner such that 0.1 and 0.3 μg doses significantly decreased choice of the large, delayed reward (n = 12 young; **p < 0.01). Baclofen also impacted choice performance in a dose-dependent manner such that the 0.3 μg dose significantly decreased choice of the large, delayed reward (n = 8 aged; *p < 0.05). B) The effect of 0.30 μg dose of baclofen on individual performance in young and aged rats. C) Response latency to the lever associated with the large, delayed reward during forced choice trials. The 0.30 μg dose of baclofen significantly increased response latency in young and aged rats (*p < 0.05). In A, percent choice of the large reward (y-axis) is plotted as a function of delay (x-axis) comparing vehicle (blue lines, open circles) to different baclofen doses (blue lines, filled circles) in young rats. In aged rats, percent choice of the large reward (y-axis) is plotted as a function of delay (x-axis) comparing vehicle (red lines, open circles) to different baclofen doses (red lines, filled circles). In B, vehicle (blue, open circles) is compared to the 0.30 μg baclofen dose (blue, filled circles) in young rats, whereas vehicle (red, open circles) is compared to the 0.30 μg baclofen dose (red, filled circles) in aged rats. In C, mean response latency to the large reward lever in seconds (y-axis) is plotted as a function of baclofen dose for young (x-axis; blue open bars represent vehicle and blue, filled bars represent 0.3 μg baclofen) and aged (x-axis; red open bars represent vehicle and red, filled bars represent each baclofen dose) rats. Error bars represent the standard error of the mean (SEM).
Table 6.
Lever response latencies in rats that received intra-BLA baclofen infusions.
| Latency BLA baclofen | Small reward lever | Large reward lever | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veh | 0.03 | 0.1 | 0.3 | Veh | 0.03 | 0.1 | 0.3 | |||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Young | 1.849 | 0.328 | 1.936 | 0.274 | 1.888 | 0.195 | 2.302 | 0.195 | 1.382 | 0.288 | 1.693 | 0.196 | 1.609 | 0.274 | 2.353* | 0.346 |
| Aged | 2.198 | 0.258 | 2.410 | 0.407 | 3.24* | 0.296 | 3.411 | 0.574 | 1.191 | 0.255 | 1.508 | 0.368 | 1.614 | 0.192 | 2.643** | 0.418 |
Table 7.
Trial omissions in rats that received intra-BLA baclofen infusions.
| Omissions BLA baclofen | Veh | 0.03 | 0.1 | 0.3 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Young | 1.333 | 0.445 | 4.083 | 1.530 | 3.583 | 1.221 | 4.333 | 1.798 |
| Aged | 3.125 | 1.493 | 3.625 | 1.772 | 3.750 | 1.677 | 10.292** | 1.630 |
To evaluate potential carry-over effects of the drug micro-infusions, performance on intervening days of the drug schedule (i.e., wash-out days) was also evaluated using a multi-factor (prior day’s dose × delay × age) ANOVA. Besides the expected main effect of delay (F(4, 68) = 17.788, p < 0.001) and delay × age interaction (F(4, 68) = 3.383, p = 0.014), there was no residual effect of baclofen on task performance on the wash-out days following drug infusion (main effect prior day’s dose: F(3, 51) = 0.355, p = 0.720; main effect of age: F(1, 17) = 3.340, p = 0.085; main effect prior day’s dose × age: F(3, 51) = 0.369, p = 0.710; dose × delay: F(12, 204) = 0.801, p = 0.625; dose × delay × age: F(12, 204) = 0.847, p = 0.582; data not shown). The effect of injection number was also tested using a multi-factor (injection number × delay × age) ANOVA. Besides the expected main effect of delay (F(4, 72) = 31.435, p = 3.83E-15) and delay × age interaction (F(4, 72) = 7.002, p = 0.005), the cumulative number of micro-infusions did not influence performance (main effect of injection day: F(3, 54) = 2.691, p = 0.055; main effect of age: F(1, 18) = 4.616, p = 0.046; injection number × delay: F(12, 216) = 0.795, p = 0.624; injection number × age: F(3, 54) = 1.170, p = 0.330; injection number × delay × age: F(12, 216) = 0.590, p = 0.849; data not shown). As there was a main effect of age and a trending effect of injection number, a follow up repeated-measures (injection number × delay) ANOVA was done in young and aged rats separately. The cumulative number of micro-infusions did not influence performance in either young (main effect of injection number: F(3, 33) = 2.100, p = 0.119; data not shown) or aged (main effect of injection number: F(3, 21) = 1.476, p = 0.250; data not shown) rats. Jointly, these findings indicate that stimulating GABABRs in the BLA promotes impulsive choice in rats, regardless of age-associated differences in discounting behavior.
Effects of Intra-BLA infusions of CGP on intertemporal choice.
To determine whether endogenous, tonic activation of GABABRs contributes to age-dependent differences in choice behavior, intra-BLA infusions of the GABABR antagonist, CGP55845, were administered using the same within-subjects design. CGP55845 did not produce a main effect of dose (F(3,54) = 1.150, p = 0.335; Fig. 9) or interact with age (F(3,54) = 0.742, p = 0.531) or delay (F(3,54) = 0.742, p = 0.531). A dose × age ANOVA revealed that CGP did increase trial omissions (F(3,54) = 3.437, p = 0.038; Table 8) in a manner that did not interact with age (F(3,54) = 0.539, p = 0.606). Follow-up tests comparing vehicle to the 0.15 μg dose revealed a trend towards greater omissions (+2.5 ± 0.7; t(19) = 2.064, p = 0.053). The dose effect on trial omissions suggests that the null effects of CGP on choice performance were not due to administration of a behaviorally inert dose range.
Fig. 9.
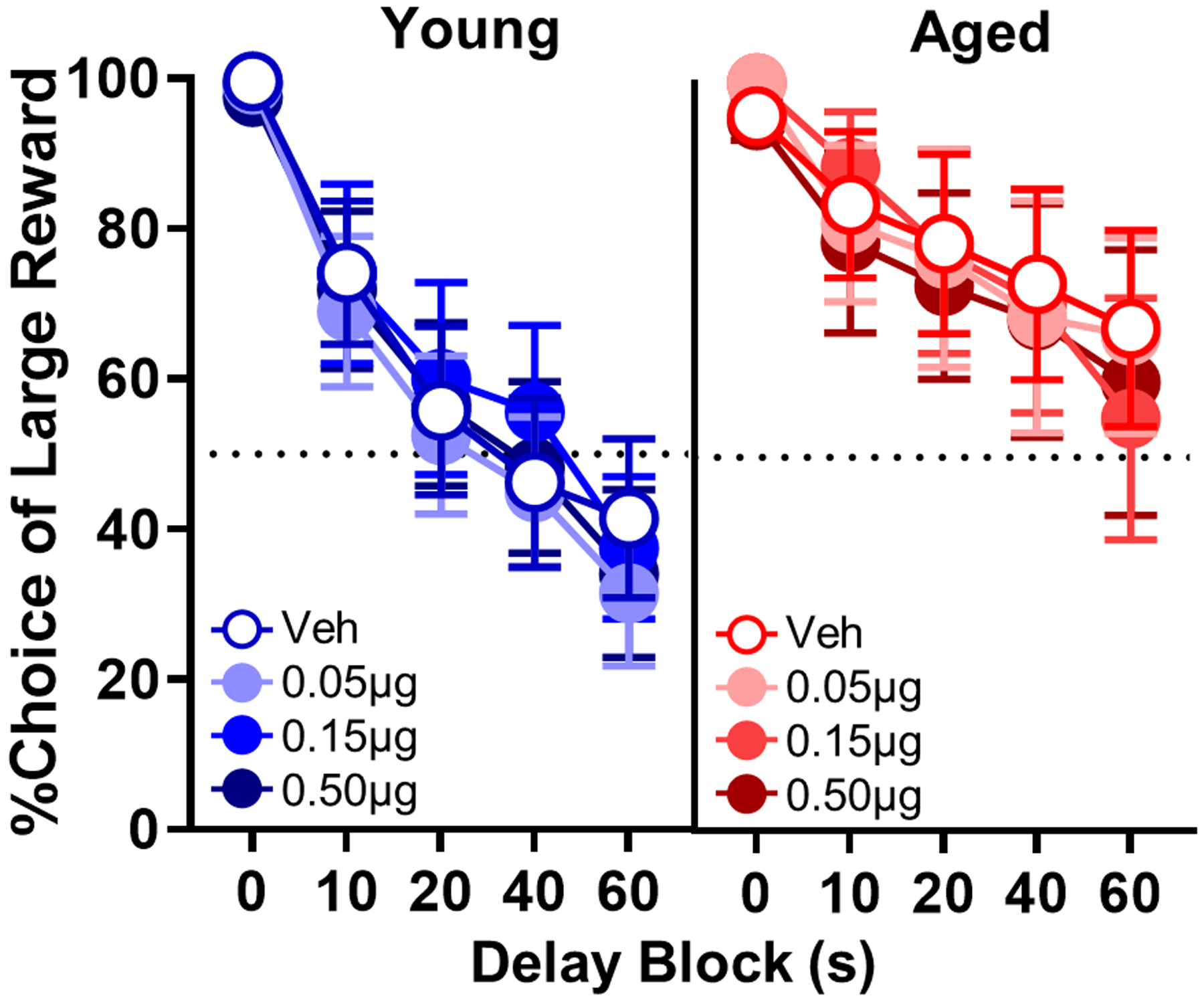
Effects of intra-BLA microinfusions of CGP55845 in young and aged rats. CGP55845 did not alter choice performance in young (n = 12) or aged (n = 8) rats. Percent choice of the large reward (y-axis) is plotted as a function of delay (x-axis) comparing vehicle (blue lines, open circles) to different CGP55845 doses (blue lines, filled circles) in young and comparing vehicle (red lines, open circles) to different CGP55845 doses (red lines, filled circles) in aged rats. Error bars represent the standard error of the mean (SEM).
Table 8.
Trial omissions in rats that received intra-BLA CGP infusions.
| Omissions BLA CGP | Veh | 0.05 | 0.15 | 0.5 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Young | 1.17 | 0.40 | 2.00 | 0.74 | 3.67 | 1.67 | 1.33 | 0.43 |
| Aged | 3.44 | 1.60 | 2.00 | 0.65 | 5.88 | 2.19 | 2.75 | 1.52 |
GABABR expression is not attenuated in aged BLA.
Collectively, the behavioral pharmacological data show that although endogenous activation of GABABRs in BLA is not critical for maintenance of normal intertemporal choice behavior in either age groups, exogenous activation of these receptors can decrease rats’ preference for large, delayed over small, immediate rewards (increased impulsive choice). To determine the status of GABABR availability in both young and aged rats, expression of GABABR subunits in the BLA was assessed in a separate behaviorally naïve cohort of young and aged rats (Fig. 10). Although there were numerical reductions in R1a and R2 subunit expression, none of these reductions reached statistical significance (Fig. 10; R1a: t(19) = 1.450, p = 0.163; R1b: t(20) = −0.409, p = 0.687; R2: t(22) = 1.866, p = 0.075). Additionally, expression of vGaT was not changed with age (t(22) = 0.141, p = 0.889). Taken together, these data suggest that aging selectively attenuates expression of GABABR1 isoforms in the PrL, but not the BLA.
Fig. 10.
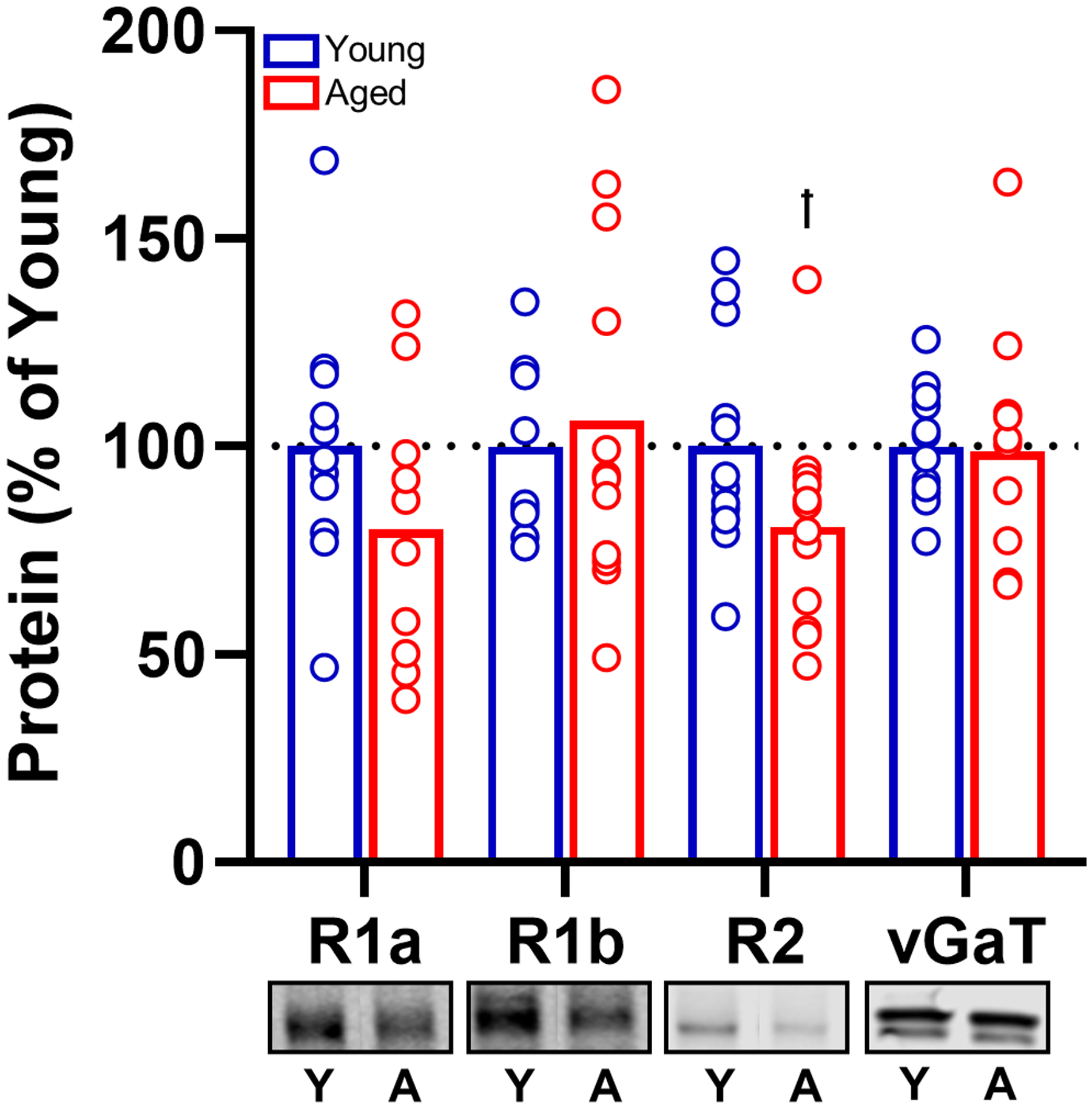
Effect of age on GABAB receptor protein in the BLA. GABABR1a and R1b protein levels within the aged BLA were unchanged relative to young. In contrast, there was a trending age-related reduction in the R2 isoform (†p = 0.075). There was no age effect on vGaT expression. Open, blue bars represent relative intensity normalized total protein in young, whereas red bars represent relative intensity normalized to total protein in aged rats. Data are expressed as percent of young. Error bars represent standard error of the mean (SEM).
4. Discussion
The current study sought to determine how GABABRs in PrL and BLA contribute to intertemporal choice and to examine how naturally occurring differences in choice preference between young adult and aged rats interact with GABABR signaling in these brain regions. We found that GABABR signaling in PrL attenuates impulsive choice while GABABR signaling in the BLA increases impulsive choice. Further, aging nullifies contributions of GABABRs in PrL to impulsive choice whereas the role of GABABRs in BLA is preserved with age. These effects appear to relate to differential expression of GABABR and susceptibility to decline with aging across these two brain structures.
4.1. Opposing effects of GABABRs in PrL and BLA on intertemporal choice
The first major finding of this study was that intra-PrL infusion of the GABABR agonist baclofen attenuates impulsive choice whereas intra-BLA infusion enhances impulsive choice. Elevated impulsivity is a core symptom of neuropsychiatric disease and substance use disorders, conditions that associate with abnormal PFC GABAergic signaling (de Jonge et al., 2017; Li et al., 2019; Tyacke et al., 2010). Even in the absence of disease states, lower GABA levels in the PFC associate with greater trait impulsivity in healthy human males (Boy et al., 2011). To our knowledge, our finding is the first to reveal that selective activation of GABABRs in mPFC increases the ability to delay gratification. This effect may be specific to GABABRs (rather than GABA signaling more broadly), as, on the one hand, stimulating GABAARs in mPFC with the selective agonist, muscimol, increases impulsive actions (Feja and Koch, 2014; Murphy et al., 2012; Paine et al., 2011) and impulsive choice (Churchwell et al., 2009; but see Feja and Koch, 2014), while on the other hand, inhibiting GABAARs in mPFC with the selective antagonist, bicuculline, reduces choice of large rewards in other forms of cost-benefit decision making (Piantadosi et al., 2016). The precise mechanism whereby intra-PrL baclofen promotes willingness to delay gratification is unclear, but the present results differ from previously reported consequences of co-infusing baclofen and muscimol into mPFC during probability-based decision-making tasks that pair choice of a large reward associated with escalating risk of reward omission or footshock punishment. In such studies, co-infusing baclofen and muscimol into mPFC increases choice of large, risky, rewards, resulting in sub-optimal choice strategies (de Visser et al., 2011; Orsini et al., 2018; St Onge and Floresco, 2010; Zeeb et al., 2015). However, conclusions across studies may pertain to the common need for mPFC activity to support flexible modification of choice strategies in relation to shifting time- or risk-based reinforcement contingencies. Indeed, combined baclofen and muscimol induces a strong bias to retain initial response strategies, regardless of whether risk is experimentally increased or decreased during the testing session (Orsini et al., 2018; St Onge and Floresco, 2010). Experimentally inactivating the mPFC produces perseverative responses in set-shifting tasks that formally evaluate cognitive flexibility (Birrell and Brown, 2000; Floresco et al., 2008a). Consequently, escalating doses of baclofen infused into PrL may inhibit adjustments to neural activity needed to shift choice strategies toward the smaller, immediate reward as progressively longer delays are interposed between choice and receipt of the large reward. Collectively, these data accentuate an important role for mPFC-dependent cognitive flexibility in optimizing choice strategies.
In contrast to our findings in PrL, activation of GABABRs in BLA dose-dependently increased impulsive choice without impairing reward magnitude discrimination. Contemporaneous with increasing impulsive choice were longer latencies to press the large reward lever during forced-choice trials, reflective of diminished incentive for the large reward. While the role of the BLA in assigning incentive value to rewards is well documented (Baxter and Murray, 2002; Parkes and Balleine, 2013; Shiflett and Balleine, 2010), the data presented here unmask a specific role for BLA GABABRs in impulsive choice, ostensibly by altering the cost-benefit incentive structure of rewards. Although few studies have explicitly investigated the role of GABABRs in intertemporal choice, one study used the GABAAR agonist muscimol in a T-maze version of an intertemporal choice task to show that enhancing GABAAR activity in the BLA increases impulsive choice (Churchwell et al., 2009). In the current study, specific activation of BLA GABABRs also increased impulsive choice. Jointly, these studies agree with others that use combinations of muscimol and baclofen to inhibit BLA and broadly shift choice behavior away from high-value/high-cost rewards towards low-value/low-cost rewards (i.e. smaller/less palatable food rewards obtained after shorter delays, lower effort, or with greater probability; (Ghods-Sharifi et al., 2009) (Hosking et al., 2014) (Hart and Izquierdo, 2017) (Janak and Tye, 2015). In contrast to baclofen, CGP55845 did not affect impulsive choice, suggesting that tonic inhibition mediated by GABABRs does not contribute to the role of the BLA in intertemporal choice, although it is unlikely that these null effects were due to administration of non-effective doses, as positive evidence for drug activity was evident in the increase in trial omissions.
4.2. Contributions of GABABRs in decision-making in aging
The second major finding of this study was that sensitivity to effects of baclofen on intertemporal choice in aged rats was absent, or greatly reduced, when infusions were directed to PrL, whereas the effects of BLA infusions were similar between young adult and aged rats. While prefrontal GABABRs are susceptible to decline with aging, as demonstrated in this study and in our prior work (Bañuelos et al., 2014; Beas et al., 2017; McQuail et al., 2012), the null effects of baclofen are not conclusively ascribed to differences in the abundance or composition of GABABRs in the aged mPFC, as we have previously determined that GABABR ligands can significantly influence other forms of mPFC-dependent cognition, namely working memory and cognitive flexibility, in aged rats (Bañuelos et al., 2014; Beas et al., 2017). Electrophysiological studies strongly suggest that loss of GABABRs from aged mPFC increases tonic inhibition of layer 2/3 pyramidal neurons (Carpenter et al., 2016; Luebke et al., 2004). However, the present findings would indicate that enhanced tonic inhibition in the aged mPFC does not mediate the increased willingness of aged rats to delay gratification, as GABABR blockade with CGP55845 failed to affect intertemporal choice. Therefore, it will be important for future work to determine whether aging merely leads to a diminished contribution of mPFC GABABRs to time-based decision making and to what degree other mechanisms within the aged mPFC guide decision-making. Indeed, our lab and others have documented age-related loss of NMDARs from mPFC (Dyall et al., 2007; McQuail et al., 2016b, 2021; Mitchell and Anderson, 1998; Wenk et al., 1991) that normally contribute to intertemporal choice in young adult rats (Isherwood et al., 2015; Yates et al., 2015, 2017, 2018a, 2018b). The present findings and existing data suggest that further study of the age-related imbalance of excitatory-inhibitory signaling is needed to fully understand how mPFC aging contributes to changes in choice behavior across the lifespan.
In contrast to our findings in the mPFC, intra-BLA baclofen significantly increased impulsive choice in both young adult and aged rats, and GABABR subunit expression was not changed by age in this brain region. These findings suggest that aging does not alter the role of BLA GABABRs in intertemporal choice. As such, decision making alterations arising across the lifespan may be better explained by changes to the functional circuitry in the BLA rather than a specific receptor. Although BLA neuron number seems to remain largely stable, it does undergo a number of morphological and functional alterations with age (Lolova and Davidoff, 1991; Rubinow and Juraska, 2009; Rubinow et al., 2009; Roesch et al., 2012; Burke et al., 2014; Prager et al., 2016). Although these changes do not appear to extend to altered GABABR protein expression or function, age differences in intertemporal choice could arise from a reliance on compensatory cognitive strategies or differential processing of rewards and costs (Hernandez et al., 2019; Löckenhoff, 2011; Mather et al., 2012; Pachur et al., 2017; Samanez-Larkin and Knutson, 2015) independent of GABABRs.
4.3. Behavioral phenotypes of aging align with behavioral phenotypes of neuropsychiatric disease
The neural circuitry mediating decision making is complex, and it has been shown that communication between the mPFC and BLA is critical to intertemporal choice (Churchwell et al., 2009). The age-related alterations to intertemporal choice reported in this study and others (Eppinger et al., 2012; Green et al., 1994; Hernandez et al., 2017; Jimura et al., 2011; Simon et al., 2010) may be accompanied by impairments in inhibitory signaling in PrL and BLA circuitry (Lolova and Davidoff, 1991; Roesch et al., 2012). This would be consistent with evidence showing that age-related impairments to several cognitive domains manifest from perturbations in inhibitory signaling (Bañuelos et al., 2014; Beas et al., 2017; Carpenter et al., 2016; McQuail et al., 2012, 2021; Patrylo et al., 2007; Potier et al., 1992, 2006; Spiegel et al., 2013; Stanley and Shetty, 2004; Stranahan et al., 2012; Yates et al., 2008). Indeed, recently published data support the idea that decision making in aging is strongly associated with impairments in other cognitive domains. For example, performance among aged rats on the intertemporal choice task was negatively correlated with performance on a test of cognitive flexibility (set shifting), such that rats showing a greater preference for large, delayed rewards also showed greater impairments in cognitive flexibility (Hernandez et al., 2017). Aged rats with a greater preference for large, delayed rewards also showed reduced motivation to earn rewards during instrumental responding for food on a progressive ratio schedule (Hernandez et al., 2017). Both cognitive and motivational impairments could influence the intertemporal choice phenotype in aging.
Sustained preference for large, delayed rewards and impairments in cognitive flexibility are found in individuals diagnosed with anorexia nervosa and trait anxiety disorders (Steinglass et al., 2012, 2017; Steward et al., 2017). These extremes in the ability to delay gratification are considered highly maladaptive and potentially stem from pathological increases in inhibitory control over incentive drives (Kaye et al., 2013). The emergent intertemporal choice phenotype in older adults may arise from similar cognitive inhibitory perturbations, and as such, the aged phenotype of decision making may be maladaptive. Baclofen has been approved for treatment of several clinical conditions (Baker et al., 2014; Overgård et al., 2015; Rekand and Grønning, 2011), some of which are strongly associated with maladaptive decision making (Erickson et al., 2014; Kahn et al., 2009; Muzyk et al., 2012). The effects of baclofen on intertemporal choice in aged rats reported here show promise as a therapeutic intervention in a preclinical model of age-related maladaptive decision making. Notably, baclofen did not excessively alter choice performance in aged rats such that they surpassed the parametric space in which adaptive decisions were made (i. e., baclofen altered aged rats’ choice performance to the same degree as young rats’ choice performance). On the heels of clinical studies already using baclofen to treat maladaptive behaviors (Berry-Kravis et al., 2012; Erickson et al., 2014; Kahn et al., 2009; Muzyk et al., 2012), a next step in the same direction for the current study will be to follow up the current findings with experiments designed to test the effects of systemic routes of baclofen administration, specifically in this preclinical model of maladaptive decision making in aging. In addition, given that there can be considerable sex differences in intertemporal choice as well as other forms of cost-benefit decision making (Hernandez et al., 2020; Orsini and Setlow, 2017), it will be extremely important to determine whether the effects of both age and GABABR manipulations differ in females and males.
Supplementary Material
Acknowledgements
We thank Vicky S. Kelly, Sahil Ghay, Brandon M. Hellbusch, Matt M. Bruner, Shannon C. Wall, and Bonnie I. McLaurin for technical assistance. Supported by a McKnight Predoctoral Fellowship, the Pat Tillman Foundation, and NIH/NICHD 2T32HD071866-06 (CMH), F32AG051371, K01AG061263, P20GM109091, P20GM103641 (JAM), R01AG029421 and the McKnight Brain Research Foundation (JLB), RF1AG060778 (JLB, BS, CJF).
Abbreviations:
- FBN
Fischer 344 × Brown Norway hybrid
- ITI
intertrial interval
- mPFC
medial prefrontal cortex
- PrL
prelimbic cortex
- BLA
basolateral amygdala
Footnotes
CRediT authorship contribution statement
Caesar M. Hernandez: Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Joseph A. McQuail: Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Tyler W. Ten Eyck: Investigation, Writing – review & editing. Alexa-Rae Wheeler: Investigation, Writing – review & editing. Chase C. Labiste: Investigation, Writing – review & editing. Barry Setlow: Writing – review & editing, Project administration, Funding acquisition. Jennifer L. Bizon: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2022.109001.
References
- Amsel A, 1950. The combination of a primary appetitional need with primary and secondary emotionally derived needs. J. Exp. Psychol 40, 1–14. 10.1037/h0063415. [DOI] [Google Scholar]
- Anderberg RH, Hansson C, Fenander M, Richard JE, Dickson SL, Nissbrandt H, Bergquist F, Skibicka KP, 2016. The stomach-derived hormone ghrelin increases impulsive behavior. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 1199–1209. 10.1038/npp.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelian L, Warnock KT, Balan I, Puche A, June H, 2016. TLR4 signaling in VTA dopaminergic neurons regulates impulsivity through tyrosine hydroxylase modulation. Transl. Psychiatry 6, e815. 10.1038/tp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axon RN, Bradford WD, Egan BM, 2009. The role of individual time preferences in health behaviors among hypertensive adults: a pilot study. J. Am. Soc. Hypertens 3, 35–41. 10.1016/j.jash.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Baker KW, Tann B, Mutlu A, Gaebler-Spira D, 2014. Improvements in children with cerebral palsy following intrathecal baclofen: use of the rehabilitation Institute of Chicago Care and comfort caregiver questionnaire (RIC CareQ). J. Child Neurol 29, 312–317. 10.1177/0883073812475156. [DOI] [PubMed] [Google Scholar]
- Bañuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL, 2014. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J. Neurosci. Off. J. Soc. Neurosci 34, 3457–3466. 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA, 2002. The amygdala and reward. Nat. Rev. Neurosci 3, 563–573. 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Bañuelos C, Setlow B, Bizon JL, 2017. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neurosci. Cognit. Flexibility: Dev. Dis. Treat 345, 274–286. 10.1016/j.neuroscience.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U, 2004. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain J. Neurol 127, 1108–1126. 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV, Gonzalez-Heydrich J, Wang PP, Carpenter RL, Bear MF, Hagerman RJ, 2012. Effects of STX209 (Arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl. Med 4, 152ra127. 10.1126/scitranslmed.3004214, 152ra127. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ, 2000. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. Off. J. Soc. Neurosci 20, 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL, 2012. Characterizing cognitive aging of working memory and executive function in animal models. Front. Aging Neurosci 4, 19. 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Lawrence AD, Singh KD, Husain M, Sumner P, 2011. Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol. Psychiatr 70, 866–872. 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford WD, 2010. The association between individual time preferences and health maintenance habits. Med. Decis. Making 30, 99–112. 10.1177/0272989X09342276. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM, 2002. Rodent models of prefrontal cortical function. Trends Neurosci. 25, 340–343. [DOI] [PubMed] [Google Scholar]
- Burke SN, Thome A, Plange K, Engle JR, Trouard TP, Gothard KM, Barnes CA, 2014. Orbitofrontal cortex volume in area 11/13 predicts reward devaluation, but not reversal learning performance, in young and aged monkeys. J. Neurosci 34, 9905–9916. 10.1523/JNEUROSCI.3918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa N, Crespi C, Motterlini M, Baud-Bovy G, Chierchia G, Pantaleo G, Tettamanti M, Cappa SF, 2013. The functional and structural neural basis of individual differences in loss aversion. J. Neurosci. Off. J. Soc. Neurosci 33, 14307–14317. 10.1523/JNEUROSCI.0497-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ, 2002. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Carpenter HE, Kelly KB, Bizon JL, Frazier CJ, 2016. Age-related changes in tonic activation of presynaptic versus extrasynaptic γ-amniobutyric acid type B receptors in rat medial prefrontal cortex. Neurobiol. Aging 45, 88–97. 10.1016/j.neurobiolaging.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP, 2009. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav. Neurosci 123, 1185–1196. 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi LP, 1942. Quantitative variation of incentive and performance in the white rat. Am. J. Psychol 55, 467–517. 10.2307/1417120. [DOI] [Google Scholar]
- Cruz HG, Ivanova T, Lunn M-L, Stoffel M, Slesinger PA, Lüscher C, 2004. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat. Neurosci 7, 153–159. 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K, 2005. Don’t worry ‘B’ happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol. Sci 26, 36–43. 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- de Jonge JC, Vinkers CH, Hulshoff Pol HE, Marsman A, 2017. GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Front. Psychiatr 8 10.3389/fpsyt.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, Homberg JR, Mitsogiannis M, Zeeb FD, Rivalan M, Fitoussi A, Galhardo V, van den Bos R, Winstanley CA, Dellu-Hagedorn F, 2011. Rodent versions of the Iowa gambling task: opportunities and challenges for the understanding of decision-making. Front. Neurosci 5, 109. 10.3389/fnins.2011.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT, 2007. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol. Aging 28, 424–439. 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD, 2012. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One 7, e36953. 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Veenstra-Vanderweele JM, Melmed RD, McCracken JT, Ginsberg LD, Sikich L, Scahill L, Cherubini M, Zarevics P, Walton-Bowen K, Carpenter RL, Bear MF, Wang PP, King BH, 2014. STX209 (Arbaclofen) for autism spectrum disorders: an 8-week open-label study. J. Autism Dev. Disord 44, 958–964. 10.1007/s10803-013-1963-z. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW, 2003. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann. N. Y. Acad. Sci 985, 233–250. [PubMed] [Google Scholar]
- Feja M, Hayn L, Koch M, 2014. Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 54, 31–42. 10.1016/j.pnpbp.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Feja M, Koch M, 2014. Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav. Brain Res 264, 230–239. 10.1016/j.bbr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL, 2008a. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav. Brain Res 190, 85–96. 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA, 2008b. Cortico-limbicstriatal circuits subserving different forms of cost-benefit decision making. Cognit. Affect Behav. Neurosci 8, 375–389. 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB, 2009. Fundamental contribution by the basolateral amygdala to different forms of decision making. J. Neurosci. Off. J. Soc. Neurosci 29, 5251–5259. 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR, 2010. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur. J. Neurosci 32, 1726–1734. 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J, 1994. Discounting of delayed rewards: a life-span comparison. Psychol. Sci 5, 33–36. 10.1111/j.1467-9280.1994.tb00610.x. [DOI] [Google Scholar]
- Green L, Myerson J, Ostaszewski P, 1999. Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behav. Process 46, 89–96. 10.1016/S0376-6357(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Hart EE, Izquierdo A, 2017. Basolateral amygdala supports the maintenance of value and effortful choice of a preferred option. Eur. J. Neurosci 45, 388–397. 10.1111/ejn.13497. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM, 2007. Delay discounting in schizophrenia. Cognit. Neuropsychiatry 12, 213–221. 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Hernandez CM, Campos K, Truckenbrod L, Federico Q, Moon B, McQuail JA, Maurer AP, Bizon JL, Burke SN, 2018a. A ketogenic Diet improves cognition and has biochemical effects in prefrontal cortex that are dissociable from Hippocampus. Front. Aging Neurosci 10 10.3389/fnagi.2018.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Hernandez CM, Campos KT, Truckenbrod LM, Sakarya Y, McQuail JA, Carter CS, Bizon JL, Maurer AP, Burke SN, de Cabo R, 2018b. The antiepileptic ketogenic Diet alters hippocampal transporter levels and reduces adiposity in aged rats. J. Gerontol. Ser 73, 450–458. 10.1093/gerona/glx193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, McQuail JA, Schwabe MR, Burke SN, Setlow B, Bizon JL, 2018. Age-related declines in prefrontal cortical expression of metabotropic glutamate receptors that support working memory. eNeuro 5. 10.1523/ENEURO.0164-18.2018. ENEURO.0164–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Orsini C, Wheeler A-R, Ten Eyck TW, Betzhold SM, Labiste CC, Wright NG, Setlow B, Bizon JL, 2020. Testicular hormones mediate robust sex differences in impulsive choice in rats. eLife 9, e58604. 10.7554/eLife.58604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Orsini CA, Labiste CC, Wheeler A-R, Ten Eyck TW, Bruner MM, Sahagian TJ, Harden SW, Frazier CJ, Setlow B, Bizon JL, 2019. Optogenetic dissection of basolateral amygdala contributions to intertemporal choice in young and aged rats. eLife 8. 10.7554/eLife.46174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Vetere LM, Orsini CA, McQuail JA, Maurer AP, Burke SN, Setlow B, Bizon JL, 2017. Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344 × brown Norway hybrid rats. Neurobiol. Aging 60, 141–152. 10.1016/j.neurobiolaging.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P, 2003. Impulsive decision making and working memory. J. Exp. Psychol. Learn. Mem. Cogn 29, 298–306. 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA, 2014. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 39, 1558–1567. 10.1038/npp.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Woodhouse J, Parcel T, Mull L, Schwartz D, Mitchell A, Lahna D, Johnson A, Loftis J, Woods SP, Mitchell SH, Hoffman W, 2011. Discounting of delayed rewards and executive dysfunction in individuals infected with hepatitis C. J. Clin. Exp. Neuropsychol 33, 176–186. 10.1080/13803395.2010.499355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isella V, Mapelli C, Morielli N, Pelati O, Franceschi M, Appollonio IM, 2008. Age-related quantitative and qualitative changes in decision making ability. Behav. Neurol 19, 59–63. 10.1155/2008/893727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isherwood SN, Pekcec A, Nicholson JR, Robbins TW, Dalley JW, 2015. Dissociable effects of mGluR5 allosteric modulation on distinct forms of impulsivity in rats: interaction with NMDA receptor antagonism. Psychopharmacology 232, 3327–3344. 10.1007/s00213-015-3984-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL, 2008. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience 155, 573–584. 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Boyle PA, Bennett JS, Bennett DA, 2012. The impact of health and financial literacy on decision making in community-based older adults. Gerontology 58, 531–539. 10.1159/000339094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L, 2011. Domain independence and stability in young and older adults’ discounting of delayed rewards. Behav. Process 87, 253–259. 10.1016/j.beproc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Kim K-U, Lee D, Jung MW, 2013. Effect of orbitofrontal cortex lesions on temporal discounting in rats. Behav. Brain Res 245, 22–28. 10.1016/j.bbr.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Wolff SBE, Lüthi A, LeDoux JE, 2012. Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biol. Psychiatr. Optogenetics Transl. Neurosci. Psychiatr 71, 1053–1060. 10.1016/j.biopsych.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Voon V, Johansson J, Niemelä S, Bergman J, Kaasinen V, 2015. Dopaminergic function and intertemporal choice. Transl. Psychiatry 5, e491. 10.1038/tp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R, Biswas K, Childress A-R, Shoptaw S, Fudala PJ, Gorgon L, Montoya I, Collins J, McSherry F, Li S-H, Chiang N, Alathari H, Watson D, Liberto J, Beresford T, Stock C, Wallace C, Gruber V, Elkashef A, 2009. Multi-center trial of baclofen for abstinence initiation in severe cocaine-dependent individuals. Drug Alcohol Depend. 103, 59–64. 10.1016/j.drugalcdep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A, 2013. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 36, 110–120. 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC, 2011. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol. Learn. Mem 96, 417–431. 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Labouèbe G, Lomazzi M, Cruz HG, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Lüscher C, 2007. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci 10, 1559–1568. 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang CC, Weidacker Kathrin, null, Zhang Y, He N, Jin H, Chen W, Voon V, Edden RAE, Yan F, 2019. Investigation of anterior cingulate cortex gamma-aminobutyric acid and glutamate-glutamine levels in obsessive-compulsive disorder using magnetic resonance spectroscopy. BMC Psychiatr. 19, 164. 10.1186/s12888-019-2160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff CE, 2011. Age, time, and decision making: from processing speed to global time horizons. Ann. N. Y. Acad. Sci 1235, 44–56. 10.1111/j.1749-6632.2011.06209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolova I, Davidoff M, 1991. Changes in GABA-immunoreactivity and GABA-transaminase activity in rat amygdaloid complex in aging. J. Hirnforsch 32, 231–238. [PubMed] [Google Scholar]
- Luebke JI, Chang Y-M, Moore TL, Rosene DL, 2004. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 125, 277–288. 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA, 1997. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19, 687–695. 10.1016/S0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W, 2012. Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cognit. Affect Behav. Neurosci 12, 74–84. 10.3758/s13415-011-0068-4. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW, 2011. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J. Neurosci. Off. J. Soc. Neurosci 31, 6398–6404. 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR, 2005. Reward-based decision-making and aging. Brain Res. Bull In: 2nd Conference on NeuroEconomics - ConNEcs, pp. 382–390. 10.1016/j.brainresbull.2005.06.010, 67, 2004. [DOI] [PubMed] [Google Scholar]
- Massar SAA, Libedinsky C, Weiyan C, Huettel SA, Chee MWL, 2015. Separate and overlapping brain areas encode subjective value during delay and effort discounting. Neuroimage 120, 104–113. 10.1016/j.neuroimage.2015.06.080. [DOI] [PubMed] [Google Scholar]
- Mather M, Mazar N, Gorlick MA, Lighthall NR, Burgeno J, Schoeke A, Ariely D, 2012. Risk preferences and aging: the “certainty effect” in older adults’ decision making. Psychol. Aging 27, 801–816. 10.1037/a0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Bañuelos C, LaSarge CL, Nicolle MM, Bizon JL, 2012. GABA(B) receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol. Aging 33, 1124. 10.1016/j.neurobiolaging.2011.11.011 e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Hernandez CM, Bizon JL, Frazier CJ, 2021. Attenuated NMDAR signaling on fast-spiking interneurons in prefrontal cortex contributes to age-related decline of cognitive flexibility. Neuropharmacology 197, 108720. 10.1016/j.neuropharm.2021.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Simpson KL, Frazier CJ, Setlow B, Bizon JL, 2016a. NR2A-Containing NMDARs in the prefrontal cortex are required for working memory and associated with age-related cognitive decline. J. Neurosci. Off. J. Soc. Neurosci 36, 12537–12548. 10.1523/JNEUROSCI.2332-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Simpson KL, Frazier CJ, Setlow B, Bizon JL, 2016b. NR2A-Containing NMDARs in the prefrontal cortex are required for working memory and associated with age-related cognitive decline. J. Neurosci. Off. J. Soc. Neurosci 36, 12537–12548. 10.1523/JNEUROSCI.2332-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Frazier CJ, Bizon JL, 2015. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol. Med 21, 450–460. 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Anderson KJ, 1998. Age-related changes in [3H]MK-801 binding in the Fischer 344 rat brain. Neurobiol. Aging 19, 259–265. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM, 2002. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 160, 290–298. 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando ABP, Urcelay GP, Robinson ESJ, Mar AC, Theobald DEH, Dalley JW, Robbins TW, 2012. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology 219, 401–410. 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyk AJ, Rivelli SK, Gagliardi JP, 2012. Defining the role of baclofen for the treatment of alcohol dependence. CNS Drugs 26, 69–78. 10.2165/11597320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Heshmati SC, Garman TS, Wall SC, Bizon JL, Setlow B, 2018. Contributions of medial prefrontal cortex to decision making involving risk of punishment. Neuropharmacology 139, 205–216. 10.1016/j.neuropharm.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Setlow B, 2017. Sex differences in animal models of decision making. J. Neurosci. Res 95, 260–269. 10.1002/jnr.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B, 2015. Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment. J. Neurosci. Off. J. Soc. Neurosci 35, 1368–1379. 10.1523/JNEUROSCI.3586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgård TM, Kjærsgaard-Hansen L, Søe M, Illum NO, 2015. Positive experience with intrathecal baclofen treatment in children with severe cerebral palsy. Dan. Med. J 62, A4999. [PubMed] [Google Scholar]
- Pachur T, Mata R, Hertwig R, 2017. Who dares, who errs? Disentangling cognitive and motivational roots of age differences in decisions under risk. Psychol. Sci 28, 504–518. 10.1177/0956797616687729. [DOI] [PubMed] [Google Scholar]
- Paine TA, Slipp LE, Carlezon WA, 2011. Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 36, 1703–1713. 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Balleine BW, 2013. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J. Neurosci 33, 8753–8763. 10.1523/JNEUROSCI.5071-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Tyagi I, Willingham AL, Lee S, Williamson A, 2007. Dentate filter function is altered in a proepileptic fashion during aging. Epilepsia 48, 1964–1978. 10.1111/j.1528-1167.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2005. The rat brain in stereotaxic coordinates, 5th ed. Academic Press, San Diego (CA. [Google Scholar]
- Perry JL, Carroll ME, 2008. The role of impulsive behavior in drug abuse. Psychopharmacology 200, 1–26. 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Piantadosi PT, Khayambashi S, Schluter MG, Kutarna A, Floresco SB, 2016. Perturbations in reward-related decision-making induced by reduced prefrontal cortical GABA transmission: relevance for psychiatric disorders. Neuropharmacology 101, 279–290. 10.1016/j.neuropharm.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Potier B, Jouvenceau A, Epelbaum J, Dutar P, 2006. Age-related alterations of GABAergic input to CA1 Pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience 142, 187–201. 10.1016/j.neuroscience.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Potier B, Rascol O, Jazat F, Lamour Y, Dutar P, 1992. Alterations in the properties of hippocampal pyramidal neurons in the aged rat. Neuroscience 48, 793–806. 10.1016/0306-4522(92)90267-6. [DOI] [PubMed] [Google Scholar]
- Prager EM, Bergstrom HC, Wynn GH, Braga MFM, 2016. The basolateral amygdala γ-aminobutyric acidergic system in health and disease. J. Neurosci. Res 94, 548–567. 10.1002/jnr.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekand T, Grønning M, 2011. Treatment of spasticity related to multiple sclerosis with intrathecal baclofen: a long-term follow-up. J. Rehabil. Med 43, 511–514. 10.2340/16501977-0811. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Esber GR, Bryden DW, Cerri DH, Haney ZR, Schoenbaum G, 2012. Normal aging alters learning and attention-related teaching signals in basolateral amygdala. J. Neurosci. Off. J. Soc. Neurosci 32, 13137–13144. 10.1523/JNEUROSCI.2393-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow MJ, Drogos LL, Juraska JM, 2009. Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiol. Aging 30, 137–146. 10.1016/j.neurobiolaging.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM, 2009. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J. Comp. Neurol 512, 717–725. 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E, 2003. Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature 424, 775–778. 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Knutson B, 2015. Decision making in the ageing brain: changes in affective and motivational circuits. Nat. Rev. Neurosci 16, 278–289. 10.1038/nrn3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M, 2003. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron 38, 625–636. 10.1016/S0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW, 2010. At the limbic–motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur. J. Neurosci 32, 1735–1743. 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp KG, Mitchell MR, Beas BS, Bizon JL, Setlow B, 2015. Affective and cognitive mechanisms of risky decision making. Neurobiol. Learn. Mem 117, 60–70. 10.1016/j.nlm.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL, 2010. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol. Aging 31, 853–862. 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB, 2006. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav. Brain Res 171, 116–126. 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Smith CT, Wallace DL, Dang LC, Aarts E, Jagust WJ, D’Esposito M, Boettiger CA, 2016. Modulation of impulsivity and reward sensitivity in intertemporal choice by striatal and midbrain dopamine synthesis in healthy adults. J. Neurophysiol 115, 1146–1156. 10.1152/jn.00261.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M, 2013. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J. Comp. Neurol 521, 3508–3523. 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB, 2010. Prefrontal cortical contribution to risk-based decision making. Cereb. Cortex N. Y. N 20, 1816–1828. 10.1093/cercor/bhp250, 1991. [DOI] [PubMed] [Google Scholar]
- Stanley DP, Shetty AK, 2004. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem 89, 204–216. 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- Steinglass JE, Figner B, Berkowitz S, Simpson HB, Weber EU, Walsh BT, 2012. Increased capacity to delay reward in anorexia nervosa. J. Int. Neuropsychol. Soc 18, 773–780. 10.1017/S1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinglass JE, Lempert KM, Choo T-H, Kimeldorf MB, Wall M, Walsh BT, Fyer AJ, Schneier FR, Simpson HB, 2017. Temporal discounting across three psychiatric disorders: anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depress. Anxiety 34, 463–470. 10.1002/da.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward T, Mestre-Bach G, Vintró-Alcaraz C, Agüera Z, Jiménez-Murcia S, Granero R, Fernández-Aranda F, 2017. Delay discounting of reward and impulsivity in eating disorders: from anorexia nervosa to binge eating disorder. Eur. Eat Disord. Rev 25, 601–606. 10.1002/erv.2543. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M, 2012. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J. Comp. Neurol 520, 1318–1326. 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Cocker PJ, Hosking JG, Zeeb FD, Rogers RD, Winstanley CA, 2014. Dissociable effects of basolateral amygdala lesions on decision making biases in rats when loss or gain is emphasized. Cognit. Affect Behav. Neurosci 14, 1184–1195. 10.3758/s13415-014-0271-1. [DOI] [PubMed] [Google Scholar]
- Tyacke RJ, Lingford-Hughes A, Reed LJ, Nutt DJ, 2010. GABAB receptors in addiction and its treatment. Adv. Pharmacol. San Diego Calif 58, 373–396. 10.1016/S1054-3589(10)58014-1. [DOI] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH, 2010. Amygdala neural encoding of the absence of reward during extinction. J. Neurosci. Off. J. Soc. Neurosci 30, 116–125. 10.1523/JNEUROSCI.4240-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B, 2003. Do rats have a prefrontal cortex? Behav. Brain Res 146, 3–17. [DOI] [PubMed] [Google Scholar]
- Walton ME, Groves J, Jennings KA, Croxson PL, Sharp T, Rushworth MFS, Bannerman DM, 2009. Comparing the role of the anterior cingulate cortex and 6-hydroxydopamine nucleus accumbens lesions on operant effort-based decision making. Eur. J. Neurosci 29, 1678–1691. 10.1111/j.1460-9568.2009.06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A, 2015. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev 57, 271–283. 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC, 1991. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol. Aging 12, 93–98. 10.1016/0197-4580(91)90047-N. [DOI] [PubMed] [Google Scholar]
- Wilbertz G, Trueg A, Sonuga-Barke EJS, Blechert J, Philipsen A, Tebartz van Elst L, 2013. Neural and psychophysiological markers of delay aversion in attention-deficit hyperactivity disorder. J. Abnorm. Psychol 122, 566–572. 10.1037/a0031924. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW, 2004. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J. Neurosci. Off. J. Soc. Neurosci 24, 4718–4722. 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Cardinal RN, Robbins TW, 2006. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb. Cortex N. Y. N 16, 106–114. 10.1093/cercor/bhi088, 1991. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C, 2006. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J. Neurosci. Off. J. Soc. Neurosci 26, 9530–9537. 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Batten SR, Bardo MT, Beckmann JS, 2015. Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology 232, 1187–1196. 10.1007/s00213-014-3747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Gunkel BT, Rogers KK, Breitenstein KA, Hughes MN, Johnson AB, Sharpe SM, 2018a. Effects of N-methyl-d-aspartate receptor (NMDAr) uncompetitive antagonists in a delay discounting paradigm using a concurrent-chains procedure. Behav. Brain Res 349, 125–129. 10.1016/j.bbr.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Gunkel BT, Rogers KK, Hughes MN, Prior NA, 2017. Effects of N-methyl-D-aspartate receptor ligands on sensitivity to reinforcer magnitude and delayed reinforcement in a delay-discounting procedure. Psychopharmacology 234, 461–473. 10.1007/s00213-016-4469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Prior NA, Chitwood MR, Day HA, Heidel JR, Hopkins SE, Muncie BT, Paradella-Bradley TA, Sestito AP, Vecchiola AN, Wells EE, 2018b. Effects of GluN2B-selective antagonists on delay and probability discounting in male rats: modulation by delay/probability presentation order. Exp. Clin. Psychopharmacol 26, 525–540. 10.1037/pha0000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM, 2008. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 1218, 1–12. 10.1016/j.brainres.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Baarendse PJJ, Vanderschuren LJMJ, Winstanley CA, 2015. Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology 232, 4481–4491. 10.1007/s00213-015-4075-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


