Abstract
Background and Aim
In Ayurveda, ashwagandha is a popular plant for promoting youthful energy, longevity, and overall well‐being. It is also an excellent aphrodisiac herb that aids in the improvement and maintenance of normal sexual health. The present study aims to evaluate the effect of ashwagandha root extract on improving sexual health in adult males.
Methods
In this 8‐week randomized, double‐blind, placebo‐controlled study, we investigated the aphrodisiac property of an ashwagandha root extract in adult males. Fifty participants with lower sexual desire were randomly allocated to take 300 mg of ashwagandha root extract or placebo capsules twice daily. Outcomes were measured using the derogatis interview for sexual functioning‐male (DISF‐M) questionnaire, serum testosterone, serum prolactin, and short‐form survey—36 quality of life questionnaire before and after the intervention.
Results
Compared to placebo, ashwagandha root extract supplementation was associated with a statistically significant increase in the total DISF‐M scores (mean difference −9.8; 95% confidence interval, −10.73 to −8.87; p < 0.0001; t‐test). It was also associated with a statistically significant increase in serum testosterone levels (−66.52; −80.70 to −52.34; p < 0.0001; t‐test). However, the prolactin level did not change after intervention in both the ashwagandha and placebo groups (−1.06; −2.78 to 0.66; p > 0.05).
Conclusion
These findings suggest that ashwagandha demonstrated a significant subjective perception of sexual well‐being and assisted in increasing serum testosterone levels in the participants.
Keywords: aphrodisiac, ashwagandha, DISF‐M, male sexual wellness, prolactin, testosterone
1. INTRODUCTION
Sexual health is a vital component of overall personal wellness in both men and women. The World Health Organization (WHO) defined sexual health as “a state of physical, emotional, mental, and social well‐being concerning sexuality; it is not merely the absence of disease, dysfunction or infirmity…”. 1 Various biological, psychological, and social factors can often impact sexual drive. Anxiety and stress are the primary psychological factors that interfere with sexual arousal. However, with the rapid pace of human development, the number of reported cases of stress and anxiety has seen a sharp uptick year on year, especially in young adults, 2 and an increase in the number of cases of male sexual dysfunction has also been observed. 3 Many researchers studied the substantial impact that such psychological factors could play and yielded empirical evidence for a significant association between stress‐related events and sexuality. 4 , 5 , 6 , 7
Androgens are required to develop primary and secondary sexual traits and normal sexual function. 8 Among all the androgens, testosterone plays a vital role and initiates spermatogenesis via the activation of androgen receptors. Optimal testosterone level improves spermatogenesis, psychological condition, libido, energy, bone density, erectile function, and muscle strength. 9 , 10 Moreover, studies found that males' hypogonadism correlates with other disease conditions such as type 2 diabetes mellitus, 11 cardiovascular disorders, 12 and psychological disorders. 13 Similarly, prolactin also has an impact on male fertility. Researchers have demonstrated that hyperprolactinemia (HPRL) is related to erectile dysfunction, 14 and it can diminish sperm production in males by decreasing the release of gonadotropins, thereby influencing spermatogenesis. 15 This condition also impairs the release of luteinizing hormone, thus, reducing the level of serum testosterone. 16
Alternative medicine has emerged as an efficient therapy for reproductive health issues and helps boost sexual performance in both men and women. The traditional Indian Ayurvedic system of medicine recommends a therapeutic method, “Vajikarana Rasayana,“ for the improvement in sexual activity, improved reproductive functionalities, and general well‐being. 17 , 18 , 19 Herbs like Tribulus terrestris, Withania somnifera, and several others are used regularly and known for their aphrodisiac properties. 20 , 21 Hence, plants with adaptogenic, antioxidant, and aphrodisiac properties effectively have multifaceted sexual health benefits.
W. somnifera is an adaptogen and a well‐known “Rasayana“ having aphrodisiac properties. Previous studies have shown that ashwagandha root extract is safe, tolerable, and efficacious in treating oligospermia and associated issues. 22 It efficiently enhances semen quality by managing the optimum concentration of essential amino acids, citrate, and lactate in seminal plasma. 23 Studies demonstrated that ashwagandha helps reduce oxidative stress, improve hormonal balance, and overall reproductive health improvement in males. 24 Mahdi et al. 25 reported that W. somnifera extract alleviates stress and improves semen quality in males. Other reports also mentioned the significant effect of Withania extract in improving male sexual health. 26 , 27
Thus, considering the probable impact of the ashwagandha supplementation on sexual wellness, this novel clinical trial was conducted to understand the safety and efficacy of the ashwagandha root extract in improving sexual health in men.
2. MATERIALS AND METHODS
2.1. Study design
The efficacy and safety of the ashwagandha root extract on sexual function in healthy adult males were evaluated in this 8‐week, randomized, double‐blind, placebo‐controlled exploratory clinical study. The study followed the Central Drugs Standard Control Organization's good clinical practice (GCP) recommendations, the Declaration of Helsinki (2013 updated edition), and the Indian Council for Medical Research (ICMR) ethical guidelines for biomedical research on human subjects. The Institutional Ethics Committee approved the trial, and this study was prospectively registered with the Clinical Trials Registry of India.
2.2. Recruitment and randomization
Male participants attending the outpatient facility of the site/clinic for general health check‐ups were informed about the study and recruited after screening and obtaining informed consent.
In a 1:1 randomization ratio, participants were randomly assigned to one of two groups (ashwagandha or placebo). The randomization code was computer‐generated through randomly permuted blocks by an independent biostatistician. Each block had an equal number of individuals assigned to each treatment group. The Investigator was provided the randomization codes in separate envelopes for each participant and instructed to assign study numbers to the eligible participants. The ashwagandha and the placebo group products were manufactured, packed in identical containers, and labeled similarly to ensure blinding. Personnel responsible for data collection and statistical analysis were also blinded to the treatment allocation.
2.3. Sample size estimation
G*Power (Version 3.1.9.3) was used to estimate the sample size. It was based on a published pilot study 22 and an authentic Ayurveda textbook, 18 where the ashwagandha is reported to have an excellent aphrodisiac effect. We assumed that the ashwagandha treatment would outperform the placebo in terms of the total score on the derogatis interview for sexual functioning (DISF‐M), with an effect size of 0.85 (large effect) after 8 weeks of treatment. Twenty‐five subjects in each group had 80% power at 5% significance to meet our primary objective of an effect size of 0.85. The sample size was inclusive of the 10% lost to follow‐up. Thus, 50 healthy male subjects were recruited for the study.
2.4. Study participants
Before selection and enrollment in the study, all the participants read and signed the informed consent form. The eligibility criteria for the study are detailed below:
2.5. Eligibility criteria
2.5.1. Inclusion criteria
Adult males (Asian Indians) aged between 21 and 45 years with no significant medical history were eligible to participate. The medical history was based on the clinical interview with subjects and any past clinical examination within 1 year. All recruited participants were required to have low sexual desire as measured by a score of 15 or less on the sexual‐desire domain (range: 0–24, with higher scores indicating greater desire) of the derogatis interview for sexual functioning‐male (DISF‐M) questionnaire. Also, participants agreed to engage in a monogamous relationship for the entire study duration as studies have shown that polygamous behavior can impact hormonal levels, especially serum testosterone levels. 28 , 29
2.5.2. Exclusion criteria
Participants were excluded from the study if they had pre‐existing conditions like cardiovascular disease, diabetes, neurological disorders, and depression (using Hamilton Depression Rating Scale). Participants planning to start a family by IVF or alternative methods were also excluded. Additionally, concurrent use of energy supplements, herbal or pharmaceutical aphrodisiacs, body composition enhancing agents, or other concurrent medication such as beta‐blockers, contraceptives, and psychotropic medications resulted in ineligibility for participation. Another noninclusion criterion was if the Investigator's opinion suggests that the subject would not be compliant with the study protocol.
2.6. Study treatments
The investigational product KSM‐66 Ashwgandha®, 300 mg Capsule (yellowish brown powder), contains a high concentration of full‐spectrum aqueous root extract standardized to more than 5% of total withanolides by high‐performance liquid chromatography. It was manufactured by Ixoreal Biomed Inc. The batch number of the product used was KSM/16/2016, and the chemo profile of the study product was confirmed by a third party, an independent laboratory. The placebo capsules, identical to the ashwagandha in appearance, shape, color, and packaging, were made of maize starch powder. Participants were instructed to take one capsule with milk or water for 8 weeks twice a day after major meals.
2.7. Outcomes
A trained study physician evaluated all the participants at baseline, Week 4, and Week 8. The general health conditions and vital parameters of each participant were measured at each visit. The assessed vital parameters included measuring systolic and diastolic blood pressure, pulse rate, respiratory rate, and body temperature.
2.7.1. Primary outcome
The derogatis interview for sexual functioning‐male (DISF‐M) questionnaire. The DISF‐M is a selfreport questionnaire designed to assess sexual activity and quality of sexual function in males. 30 It comprises a set of 25 questions into five domains: sexual cognition/fantasy, sexual arousal, sexual behavior/experiences, orgasm, and drive/desire.
2.7.2. Secondary outcomes
Hormonal evaluations
Blood samples to assess serum testosterone (reference range: 241–827 ng/dl) and serum prolactin (reference range: 2.1–17.7 ng/ml) were collected by venipuncture at baseline and Week 8. At the end of the trials, the serum concentrations of total testosterone and serum prolactin were measured in serum samples frozen at −80°C. Steroid assays were performed at the Thyrocare (Lucknow) by commercial Human Enzyme‐linked Immunosorbent Assay (ELISA) kit (Prolactin by Cat. No. ab108655, Abcam & Testosterone by Cat. No. ab174569, Abcam). All samples from each participant were measured in the same assay run.
Short‐form survey‐36 (SF‐36)
The SF‐36 questionnaire is a commonly used generic, self‐administered instrument for assessing the quality of life (QoL) worldwide. 31 It consists of 36 items that measure health‐related QoL across diverse domains, including physical functioning, emotional well‐being, pain, energy, and fatigue.
2.8. Safety assessment
Clinical safety was assessed based on the frequency of adverse events reported by the participants. Participants were asked to report and record any adverse events they experienced during the study, along with the severity, duration, and relationship with the interventional material, which the Ethics committee also assessed.
2.9. Statistical analysis
MedCalc® (version 20.011) was utilized to do all the statistical calculations. The intention‐to‐treat (ITT) anonymized data (n = 50) was used to analyze efficacy and safety. Each outcome was prespecified. Quantitative data were presented as mean, standard deviation (SD), and 95% confidence interval, whereas categorical variables were presented as count and percentages. The changes in the scores of primary and secondary variables from baseline were also calculated and illustrated as effect size, mean difference, and percent change from baseline.
Normality and distribution of variables were checked using the Shapiro–Wilk and F‐test for homogeneity of variances, respectively, for the between‐group and within‐group comparisons. All the nonnormal data were transformed using a logarithm with base 10. The repeated‐measures analysis of variance (ANOVA) was used to compare the baseline scores of the DISF‐M, SF‐36, and vital parameters to the post‐intervention scores at Weeks 4 and 8, followed by post‐hoc individual comparisons using an independent two‐sample t‐test. And the serum testosterone and prolactin levels were analyzed using the two‐sample t‐test. The Bonferroni correction, where applicable, was applied to all the p‐values (threshold of 0.05) reported for efficacy and safety parameters.
3. RESULTS
3.1. Demographics
As shown in Figure 1, of 70 men assessed between May 2016 to September 2016, only 50 participants met the eligibility criteria and were enrolled in the study. Participants were randomly divided into the treatment and placebo groups, with 25 participants in each group. However, one participant in the ashwagandha group (due to travel) and two in the placebo group (lost the bottle and forgot to consume) discontinued intervention after the first visit. All the participants completed the study, and total data were collected from all completing participants. The baseline demographic data and vital parameters are illustrated in Table 1, demonstrating that the study population between the two groups was homogenous, with statistically nonsignificant differences between the groups.
Figure 1.
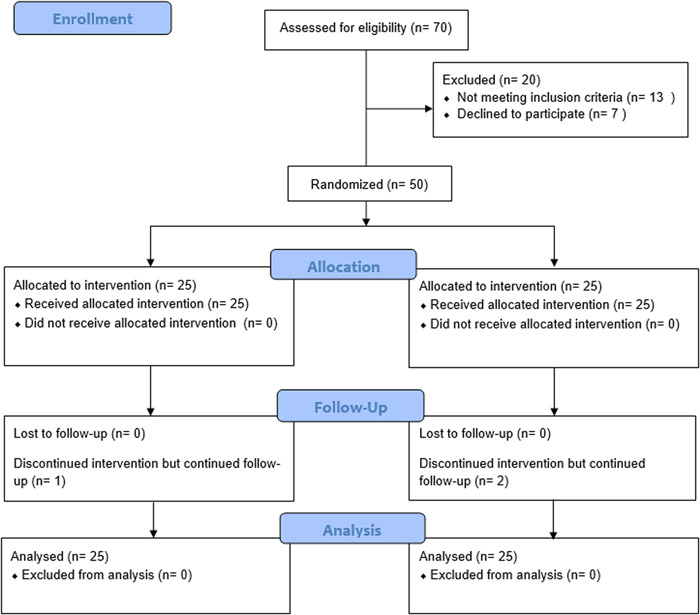
CONSORT flow diagram: participants checklist
Table 1.
Baseline demographic characteristics
| Mean (SD) | |||
|---|---|---|---|
| Variables | Ashwagandha (n = 25) | Placebo (n = 25) | p‐value |
| Age (years) | 34.32 (3.21) | 35.20 (3.66) | 0.40 |
| Height (cm) | 169.52 (2.60) | 168.48 (2.02) | 0.12 |
| Weight (Kg) | 66.06 (4.21) | 65.74 (3.47) | 0.79 |
| Body mass index (kg/m2) | 22.94 (1.61) | 23.16 (1.16) | 0.58 |
| Systolic blood pressure (mmHg) | 122.52 (5.05) | 121.6 (5.06) | 0.52 |
| Diastolic blood pressure (mmHg) | 80.16 (2.70) | 79.48 (3.28) | 0.41 |
3.2. Primary outcome measure
3.2.1. The DISF‐M questionnaire
Changes in the DISF‐M scores for each domain at each time point across both the treatment groups are detailed in Table 2. A statistically significant increase (p < 0.001) in the total DISF‐M score was observed over time in the ashwagandha and placebo groups. When comparing the ashwagandha group to the placebo group, a between‐group analysis using an independent student t‐test demonstrated a statistically significant improvement in the total DISF‐M score in the ashwagandha group (−9.8; −10.73 to −8.87; p < 0.0001) (Figure 2). The ashwagandha group saw a 40% increase in total DISF‐M score compared to a 25% increase in the placebo group. Also, the effect size of changes in the total DISF‐M score of the ashwagandha group compared to the placebo group is 1.7. This difference represents an 88.5% probability of superiority of the ashwagandha root supplement compared to the placebo.
Table 2.
Comparison of derogatis interview for sexual functioning‐male (DISF‐M) scores
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| DISF Domains | Group | Baseline (n = 25) | Week 4 (n = 25) | Week 8 (n = 25) | Mean difference (95% CI)a | p‐value (between group)* |
| Sexual cognition/fantasy | Ashwagandha | 17.48 (2.53) | 22.16 (2.52) | 23.08 (2.36) | −0.92 (−1.19 to −1.64) | <0.0001 |
| Placebo | 18.24 (2.57) | 21.84 (2.57) | 22.92 (2.62) | |||
| Sexual arousal | Ashwagandha | 14.60 (2.27) | 18.56 (2.29) | 19.44 (2.20) | −1.8 (−2.06 to −1.53) | <0.0001 |
| Placebo | 14.12 (1.69) | 16.04 (1.76) | 17.16 (1.72) | |||
| Sexual behavior | Ashwagandha | 13.56 (2.10) | 16.44 (2.16) | 18.40 (1.91) | −1.16 (−1.45 to −0.87) | <0.0001 |
| Placebo | 12.32 (1.84) | 14.52 (1.78) | 16.0 (1.58) | |||
| Orgasm | Ashwagandha | 6.68 (1.57) | 9.52 (1.61) | 11.28 (1.64) | −2.64 (−3.32 to −1.96) | <0.0001 |
| Placebo | 6.08 (1.46) | 7.88 (1.46) | 8.04 (2.44) | |||
| Sexual desire | Ashwagandha | 10.6 (0.91) | 14.4 (1.08) | 16.2 (1.0) | −3.28 (−3.67 to −2.88) | <0.0001 |
| Placebo | 10.32 (1.31) | 11.44 (1.82) | 12.64 (1.18) | |||
| Total score | Ashwagandha | 62.92 (4.75) | 81.08 (4.59) | 88.40 (4.87) | −9.8 (−10.73 to −8.87) | <0.0001 |
| Placebo | 61.08 (4.38) | 71.72 (4.59) | 76.76 (4.54) | |||
p‐value was obtained using independent two‐sample t‐test for mean change within the group (two‐tailed, α = 0.05).
Mean difference was obtained subtracting Week 8 and baseline differences in the ashwagandha and placebo groups.
Figure 2.
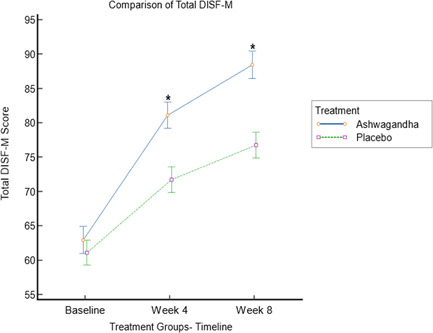
Comparison of total derogatis interview for sexual functioning‐male (DISF‐M) score between ashwagandha and placebo group at baseline, Weeks 4 and 8
Analysis of the subdomains showed a significant difference in sexual fantasy, arousal, orgasm, and drive. A within the group two‐sample t‐test on logarithmically transformed data demonstrated that there were statistically significant improvements in all the domains of DISF‐M, from baseline to Week 8, in ashwagandha treated group (sexual fantasy, p < 0.001; sexual arousal, p < 0.001; sexual behavior, p < 0.001; orgasm, p < 0.001; sexual drive, p < 0.001). There were statistically significant between the group differences in sexual cognition domain score (−0.92; −1.19 to −16.4; p < 0.0001), sexual arousal domain score (−1.8; −2.06 to −1.53; p < 0.0001), sexual behavior domain score (−1.16; −1.45 to −0.87; p < 0.0001), orgasm domain score (−2.64; −3.32 to −1.96; p < 0.0001), and sexual desire domain score (−3.28; −3.67 to −2.88; p < 0.0001).
3.3. Secondary outcome measure
3.3.1. Hormonal assessment
Serum testosterone and serum prolactin levels were evaluated at baseline and Week 8; the results were within the optimal range for all participants in both visits. Changes in the testosterone and prolactin scores are detailed in Table 3. Relative to the value at baseline, the serum testosterone level was statistically significantly increased (p < 0.0001) in the ashwagandha group (Figure 3). A between‐group analysis with a two‐sample independent t‐test showed a statistically significant increase (−66.52; −80.70 to −52.34; p < 0.0001), in the serum testosterone levels in the Ashwagandha group, compared to the placebo group. This rise in the serum testosterone was 17% (Δ = 72 ng/dl; p < 0.0001) in the ashwagandha group compared to 2% changes in the placebo group (Δ = 5.48 ng/dl; p = 0.39). In contrast, both groups observed nonsignificant changes (p > 0.05) in serum prolactin levels.
Table 3.
Hormonal levels for ashwagandha and placebo groups
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Hormone | Group | Baseline (n = 25) | Week 8 (n = 25) | Mean difference (95% CI)a | p‐value (between group)* |
| Testosterone (ng/dl) | Ashwagandha | 402.76 (39.68) | 474.76 (36.25) | −66.52 (−80.70 to −52.34) | <0.0001 |
| Placebo | 415.64 (42.55) | 421.12 (34.09) | |||
| Prolactin (ng/ml) | Ashwagandha | 8.36 (2.75) | 8.03 (3.14) | −1.06 (−2.78 to 0.66) | 0.12 |
| Placebo | 8.79 (3.00) | 9.52 (3.48) | |||
p‐value was obtained using independent two sample t‐test for mean within the group (two‐tailed, α = 0.05).
Mean difference was obtained subtracting Week 8 and baseline differences in the ashwagandha and placebo groups.
Figure 3.
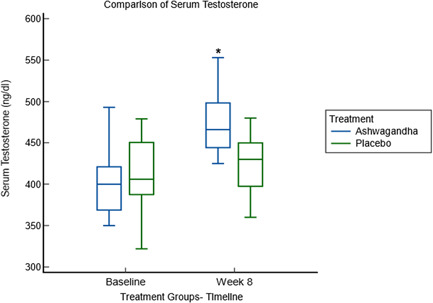
Comparison of serum testosterone between ashwagandha and placebo groups at baseline and Week 8
3.3.2. SF‐36 items questionnaire
A detailed assessment of the QoL parameters was conducted. The parameters considered were physical functioning, energy and vitality, emotional well‐being, social behavior, the existence of any pain, and overall health condition, as presented in Table 4. Slight improvement and stability were observed during the final assessment for almost all the parameters considered in the participants belonging to the experimental group compared to placebo. However, the documented data showed statistically nonsignificant improvement (p > 0.05) in both the groups in the various components of QoL questionnaire scores (Table 4).
Table 4.
Short‐form survey‐36 scores assessing the quality of life
| Mean (SD) | Mean difference (95% CI)a | p‐value (between group)* | ||||
|---|---|---|---|---|---|---|
| Domains | Group | Baseline (n = 25) | Week 4 (n = 25) | Week 8 (n = 25) | ||
| Physical functioning | Ashwagandha | 56.4 (15.51) | 64.2 (16.87) | 74.0 (17.85) | 7.86 (88.85 to −73.13) | 0.25 |
| Placebo | 59.8 (11.31) | 71.20 (25.15) | 69.0 (16.13) | |||
| Energy/fatigue | Ashwagandha | 46.8 (10.29) | 51.8 (10.19) | 55.4 (8.65) | −2 (13.15 to −17.15) | 0.94 |
| Placebo | 45.4 (8.52) | 54.0 (8.41) | 56.0 (11.81) | |||
| Emotional wellbeing | Ashwagandha | 50.72 (6.99) | 57.92 (8.72) | 64.0 (9.52) | 1.36 (13.04 to −10.32) | 0.95 |
| Placebo | 52.24 (5.89) | 60.8 (8.64) | 64.16 (12.08) | |||
| Social functioning | Ashwagandha | 51.0 (6.16) | 54.00 (7.83) | 58.5 (7.83) | −4 (4.64 to −12.64) | 0.56 |
| Placebo | 48.5 (5.49) | 55.5 (8.89) | 60.0 (10.20) | |||
| Pain | Ashwagandha | 46.8 (5.32) | 55.7 (11.62) | 63.3 (13.82) | −0.1 (15.07 to 15.27) | 0.71 |
| Placebo | 45.2 (5.04) | 56.5 (12.09) | 61.8 (15.18) | |||
| General health | Ashwagandha | 54.2 (10.47) | 55.0 (12.41) | 60.2 (13.34) | −3.6 (18.21 to −25.41) | 0.72 |
| Placebo | 51.6 (11.87) | 54.4 (11.93) | 61.2 (11.48) | |||
*p‐value was obtained using independent two‐sample t‐test for mean within the group (two‐tailed, α = 0.05).
Mean difference was obtained subtracting Week 8 and baseline differences in the ashwagandha and placebo groups.
3.4. Safety assessment
The treatment was well‐tolerated among the participants. Overall, seven adverse events (AEs) were recorded, of which four were reported in subjects assigned the ashwagandha capsule (two subjects experienced sleepiness, one developed mild abdominal pain, and one low‐grade joint pain), and three were noted in those allocated the placebo capsule (one subject had abdominal pain, while two subjects had mild diarrhea); only sleepiness may be related to the active intervention. There were no reports of any serious adverse events. The routine physical examinations did not reveal any significant changes during the study and after 8 weeks of treatment. Also, the vital parameters were stable, with no significant differences (p > 0.05) between the study groups.
4. DISCUSSION
A randomized, double‐blind, placebo‐controlled study of 50 healthy male subjects was conducted to test the efficacy of the ashwagandha root extract in improving male sexual health function through the improvement of various sexual domains and an increase of serum testosterone. This clinical study clearly demonstrated that people who took the ashwagandha root extract had an 88.5% greater probability of improving the total DISF‐M sexual health function score. The ashwagandha root extract also increased their abilities to perform better in all the five DISF‐M domains, such as sexual cognition, sexual arousal, sexual behavior, orgasm, and sexual desire. Over time, the ashwagandha and placebo groups demonstrated statistically significant (p < 0.001) increases in total DISF‐M scores. The placebo effect, well‐established in this type of psychosexual research, could explain the increase in the placebo group. A meta‐analysis conducted by Stridh et al. 32 found that among males with erectile dysfunction enrolled in Phosphodiesterase 5 Inhibitor studies, the placebo arm resulted in a small to moderate effect size (Hedges g [SE], 0.35 [0.03]; p < 0.001), which is similar to our study outcome. However, the current study found that ashwagandha root extract was superior to placebo (the difference in overall DISF‐M score between groups was statistically significant [effect size, 1.7; p < 0.0001]).
Standardized herbal medicine could be an affordable and acceptable solution to boost male libido and enhance sexual health. Ashwagandha's use as a traditional Ayurvedic herb in general wellness and various disease conditions is well‐reported. 17 , 33 Literature suggests that ashwagandha was earlier examined for safety, efficacy, and improvement of sexual function in men 22 and women. 33 Both the studies show excellent improvement and maintenance of sexual health, like the current research. It was also shown to improve memory, cognition, and body weight management by optimizing metabolism, sleep, and other factors. 34 , 35 , 36
The increase in serum testosterone using ashwagandha has been reported earlier. 22 , 37 However, the total testosterone levels observed in this study were all within the normal limits, irrespective of group or time. There were nonsignificant changes in serum prolactin levels in both groups. Except for a few instances of general discomfort, ashwagandha was well tolerated, with no serious side effects reported. This finding is consistent with the prior published studies on the ashwagandha (W. somnifera) root extract in healthy volunteers, which was well accepted. 38 , 39 Thus, it could be a safer alternative to improve and maintain normal sexual health in healthy adult males.
The present study has demonstrated that the ashwagandha root extract powder effectively enhances male libido in adult men with normal testosterone and prolactin levels. Physiological alterations were also reported to be positive. The study also supported improving and maintaining the QoL in the participants who took the ashwagandha supplement. One plausible mechanism for the benefit of ashwagandha on sexual performance could be its effect on the GABA receptors, thus facilitating the expression of GnRH expression. 40 The other way ashwagandha can exert its effects on the reproductive system and fecundity is through the action of the withanolides, steroidal lactone triterpenoids, which have chemical structural semblance with testosterone and thus could be hypothesized to impart the benefits of male steroidal hormones. 41 However, further research would be required to verify these observations.
The current trial had certain strengths, including enrollment of men who desired to improve sexual health, appropriate sample sizes, a blinded, placebo‐controlled design, increased serum testosterone to normal reference range, and excellent participant retention. The limitation of the study was that it was only a single‐center study, albeit intentional. Longer‐term follow‐up could have assessed if the effects seen were sustainable. Also, this study is on healthy volunteers, and further studies should be conducted to verify the sexual health improvement in male subjects with clinically low testosterone levels.
5. CONCLUSION
In conclusion, the findings of this study support ashwagandha root extract's aphrodisiac effect on sexual well‐being in adult men. The mechanism by which ashwagandha helps to improve male sexual health could be due to an increase in serum testosterone. Thus, the ashwagandha root extract may be utilized to aid in the enhancement of male sexual function.
AUTHOR CONTRIBUTIONS
Sanjaya Chauhan: Conceptualization; formal analysis; methodology; writing—original draft; writing—review and editing. Manoj K. Srivastava: Data curation; funding acquisition; investigation; project administration; resources; writing—review and editing. Anklesh K. Pathak: Data curation; funding acquisition; investigation; project administration; supervision; writing—review and editing.
CONFLICTS OF INTEREST
The corresponding author declares that he had full access to the data and complete control over the analysis and publication of its results. He declares he did not receive any funding to carry out this project. However, other authors received a financial grant (clinical consultation fee) from Shri Kartikeya Pharma to recruit study volunteers.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee of Om Surgical Centre and Maternity Centre, Varanasi, UP, India (IEC Ref no: ECR/628/Inst/UP/2014; February 28, 2016), and it was prospectively registered with the Clinical Trials Registry of India (Reg. # CTRI/2016/05/006906).
TRANSPARENCY STATEMENT
Sanjaya Chauhan affirms that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
ACKNOWLEDGMENTS
The authors thank Ixoreal BioMed Inc., Los Angeles, California, USA, for supplying the KSM‐66® ashwagandha root extract and the placebo capsules used in this study.
Chauhan S, Srivastava MK, Pathak AK. Effect of standardized root extract of ashwagandha (Withania somnifera) on well‐being and sexual performance in adult males: a randomized controlled trial. Health Sci Rep. 2022;5:e741. 10.1002/hsr2.741
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. World Health Organization, Department of Reproductive Health and Research . Defining Sexual Health: Report of a Technical Consultation on Sexual Health; 2006. https://www.who.int/reproductivehealth/publications/sexual_health/defining_sh/en/
- 2. American Psychological Association . Stress in America™ 2020: a National Mental Health Crisis; 2020. https://www.apa.org/news/press/releases/stress/2020/sia-mental-health-crisis.pdf
- 3. Chen L, Shi GR, Huang DD, et al. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomed Pharmacother. 2019;112:108585. [DOI] [PubMed] [Google Scholar]
- 4. Hale VE, Strassberg DS. The role of anxiety on sexual arousal. Arch Sex Behav. 1990;19(6):569‐581. 10.1007/bf01542466 [DOI] [PubMed] [Google Scholar]
- 5. Rastrelli G, Maggi M. Erectile dysfunction in fit and healthy young men: psychological or pathological. Transl Androl Urol. 2017;6(1):79‐90. 10.21037/tau.2016.09.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosen RC, Fisher WA, Eardley I, et al. The multinational men's attitudes to life events and sexuality (MALES) study: I. prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20(5):607‐617. 10.1185/030079904125003467 [DOI] [PubMed] [Google Scholar]
- 7. Hedon F. Anxiety and erectile dysfunction: a global approach to ED enhances results and quality of life. Int J Impot Res. 2003;15(Suppl 2):S16‐S19. 10.1038/sj.ijir.3900994 [DOI] [PubMed] [Google Scholar]
- 8. Dohle GR, Smit M, Weber RF. Androgens and male fertility. World J Urol. 2003;21(5):341‐345. 10.1007/s00345-003-0365-9 [DOI] [PubMed] [Google Scholar]
- 9. Khera M. Male hormones and men's quality of life. Curr Opin Urol. 2016;26(2):152‐157. 10.1097/MOU.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 10. Bain J. The many faces of testosterone. Clin Interv Aging. 2008;2:567‐576. 10.2147/cia.s1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta‐analysis study. Int J Androl. 2011;34(6 Pt 1):528‐540. 10.1111/j.1365-2605.2010.01117.x [DOI] [PubMed] [Google Scholar]
- 12. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2011;96(10):3007‐3019. 10.1210/jc.2011-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ford AH, Yeap BB, Flicker L, et al. Prospective longitudinal study of testosterone and incident depression in older men: the health in men study. Psychoneuroendocrinology. 2016;64:57‐65. 10.1016/j.psyneuen.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 14. Fiala L, Lenz J, Sajdlova R. Effect of increased prolactin and psychosocial stress on erectile function. Andrologia. 2021;53(4):e14009. [DOI] [PubMed] [Google Scholar]
- 15. Dabbous Z, Atkin SL. Hyperprolactinaemia in male infertility: clinical case scenarios. Arab J Urol. 2018;16(1):44‐52. 10.1016/j.aju.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buvat J. Hyperprolactinemia and sexual function in men: a short review. Int J Impot Res. 2003;15(5):373‐377. 10.1038/sj.ijir.3901043 [DOI] [PubMed] [Google Scholar]
- 17. Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208‐213. 10.4314/ajtcam.v8i5S.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Department of Indian System of Medicine & Homeopathy . The Ayurvedic Pharmacopoeia of India, Part‐I, Volume‐ III, first . Government of India, Ministry of Health and Family Welfare; 2021. http://www.ayurveda.hu/api/API-Vol-1.pdf. [Google Scholar]
- 19. Chauhan NS, Sharma V, Dixit VK, Thakur M. A review on plants used for improvement of sexual performance and virility. BioMed Res Int. 2014;2014:868062. 10.1155/2014/868062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malviya N, Jain S, Gupta VB, Vyas S. Recent studies on aphrodisiac herbs for the management of male sexual dysfunction: a review. Acta Pol Pharm Drug Res. 2011;68(1):3‐8. [PubMed] [Google Scholar]
- 21. Mahajan R, Gajare S. Manifestation of erectile dysfunction with adaptogenic antioxidant aphrodisiac plants. Int J Pharm Biomed Res. 2012;3(1):52‐68. [Google Scholar]
- 22. Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. Clinical evaluation of the spermatogenic activity of the root extract of ashwagandha (Withania somnifera) in oligospermic males: a pilot study. Evid Based Complement Alternat Med. 2013;2013:571420. 10.1155/2013/571420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta A, Mahdi AA, Shukla KK, et al. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: a proton NMR study at 800 MHz. J Ethnopharmacol. 2013;149(1):208‐214. 10.1016/j.jep.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 24. Ahmad MK, Mahdi AA, Shukla KK, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94(3):989‐996. 10.1016/j.fertnstert.2009.04.046 [DOI] [PubMed] [Google Scholar]
- 25. Mahdi AA, Shukla KK, Ahmad MK, et al. Withania somnifera improves semen quality in stress‐related male fertility. Evid Based Complement Alternat Med. 2009;2011:1‐9. 10.1093/ecam/nep138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malviya N, Malviya S, Jain S, Vyas S. A review of the potential of medicinal plants in the management and treatment of male sexual dysfunction. Andrologia. 2016;48(8):880‐893. 10.1111/and.12677 [DOI] [PubMed] [Google Scholar]
- 27. Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R. Role of Withania somnifera (ashwagandha) in the management of male infertility. Reprod Biomed Online. 2018;36(3):311‐326. 10.1016/j.rbmo.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 28. van Anders SM, Hamilton LD, Watson NV. Multiple partners are associated with higher testosterone in North American men and women. Horm Behav. 2007;51(3):454‐459. 10.1016/j.yhbeh.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 29. Farrelly D, Owens R, Elliott HR, Walden HR, Wetherell MA. The effects of being in a “new relationship” on levels of testosterone in men. Evol Psychol. 2015;13(1):250‐261. 10.1177/147470491501300116 [DOI] [PubMed] [Google Scholar]
- 30. Derogatis LR. The derogatis interview for sexual functioning (DISF/DISF‐SR): an introductory report. J Sex Marital Ther. Winter. 1997;23(4):291‐304. 10.1080/00926239708403933 [DOI] [PubMed] [Google Scholar]
- 31. Lins L, Carvalho FM. SF‐36 total score as a single measure of health‐related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725. 10.1177/2050312116671725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stridh A, Pontén M, Arver S, Kirsch I, Abé C, Jensen KB. Placebo responses among men with erectile dysfunction enrolled in phosphodiesterase 5 inhibitor trials. JAMA Network Open. 2020;3(3):e201423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dongre S, Langade D, Bhattacharyya S. Efficacy and safety of ashwagandha (Withania somnifera) root extract in improving sexual function in women: a pilot study. BioMed Res Int. 2015;2015:284154. 10.1155/2015/284154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14(6):599‐612. 10.1080/19390211.2017.1284970 [DOI] [PubMed] [Google Scholar]
- 35. Choudhary D, Bhattacharyya S, Joshi K. Body weight management in adults under chronic stress through treatment with ashwagandha root extract: a double‐blind, randomized, placebo‐controlled trial. J Evid Based Complement Altern Med. 2017;22(1):96‐106. 10.1177/2156587216641830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langade D, Thakare V, Kanchi S, Kelgane S. Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: a double‐blind, randomized, parallel‐group, placebo‐controlled study. J Ethnopharmacol. 2021;264:113276. 10.1016/j.jep.2020.113276 [DOI] [PubMed] [Google Scholar]
- 37. Wankhede S, Langade D, Joshi K, Sinha SR, Bhattacharyya S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;12:43. 10.1186/s12970-015-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raut AA, Rege NN, Tadvi FM, et al. Exploratory study to evaluate tolerability, safety, and activity of ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integr Med. 2012;3(3):111‐114. 10.4103/0975-9476.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verma N, Gupta SK, Tiwari S, Mishra AK. Safety of ashwagandha root extract: a randomized, placebo‐controlled, study in healthy volunteers. Complement Ther Med. 2021;57:102642. 10.1016/j.ctim.2020.102642 [DOI] [PubMed] [Google Scholar]
- 40. Kataria H, Gupta M, Lakhman S, Kaur G. Withania somnifera aqueous extract facilitates the expression and release of GnRH: in vitro and in vivo study. Neurochem Int. 2015;89:111‐119. 10.1016/j.neuint.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 41. Mirjalili M, Moyano E, Bonfill M, Cusido R, Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14(7):2373‐2393. 10.3390/molecules14072373 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study's findings are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


