Abstract
Reoviruses are a common class of enteric viruses capable of infecting a broad range of mammalian species, typically with low pathogenicity. Previous studies have shown that reoviruses are common in raw water sources and are often found along with other animal viruses. This suggests that in addition to the commonly monitored enteroviruses, reoviruses might serve as an informative target for monitoring fecal contamination of drinking water sources. Mammalian reoviruses were detected and identified by a combined cell culture–reverse transcription-PCR (RT-PCR) assay with novel primers targeting the L3 gene that encodes the λ3 major core protein. Five of 26 (19.2%) cytopathic effect-positive cell culture lysates inoculated with surface water were positive for reoviruses by RT-PCR. DNA sequence analysis of RT-PCR products revealed significant sequence diversity among isolates, which is consistent with the sequence diversity among previously characterized mammalian reoviruses. Sequence analysis revealed persistence of a reovirus genotype at a single sampling site, while a sample from another site contained two different reovirus genotypes.
Groundwater and surface water can be subject to fecal contamination from a variety of sources, including humans. This contamination can contain enteric viruses, among other potential pathogens, excreted in the stools of infected individuals. Previous studies have documented the presence of enteric viruses in a variety of water types, including groundwater, surface water, drinking water, and recreational seawater (1, 11, 17, 22). Several waterborne outbreaks of viral gastrointestinal illness have been documented (4, 10). Besides gastrointestinal illnesses, enteric viruses have been linked to more acute conditions, including meningitis and paralysis (14).
Respiratory enteric orphan viruses (which infect the human respiratory and intestinal tracts) belong to the family Reoviridae and the genus Orthoreovirus. Reoviruses are comprised of 10 to 12 double-stranded RNA genomic segments that can reassort both in nature and in laboratory settings. The most common mammalian isolates are type 1 (Lang), type 2 (Jones), and type 3 (Dearing). Reoviruses have a high endemic infection rate in humans and many other mammals (24), and more than 70% of 4-year-old children have seroconverted (25). Reoviruses typically cause only asymptomatic or mild respiratory infections in individuals. However, research suggests that reoviruses may be associated with potentially more severe illnesses. Reoviruses have been linked to neonatal hepatitis, extrahepatic biliary atresia, meningitis, and myocarditis (9, 16, 25, 28, 29). Also, immunocompromised, young, and elderly individuals may become susceptible to severe bacterial respiratory disease due to an initial reovirus infection (5).
There is a paucity of studies on the detection of reoviruses in environmental water samples due to the moderate clinical significance of these viruses. However, the few ecological studies that have monitored the occurrence of reoviruses in water sources have found that they occur quite commonly (8, 12, 18, 27). One study that examined secondary sewage treatment plant effluents showed that reoviruses were present in 84% of the samples and that enteroviruses were present in only 46% of the samples (8). Another 2-year study showed that reoviruses were the most abundant type of viruses isolated from raw river water; 207 of 445 (46.5%) of the strains of viruses isolated were identified as reoviruses (12). Reoviruses kept in agricultural water streams have been shown to survive for 6 months (13).
Most previous studies have used either seroepidemiology or classical cell culture techniques to identify viruses in water samples. Both of these methods are labor-intensive and time-consuming (3), and antibody neutralization tests have been known to fail due to antigenic drift or recombination after a virus has passed through a host (20). Several recent studies have used molecular techniques, such as PCR and integrated cell culture-PCR (23), for detection of viruses. Direct detection of viruses in environmental samples is often hampered by the presence of PCR inhibitors and an inability to assay large equivalent volumes. Integrated cell culture–reverse transcription-PCR (RT-PCR) methods overcome most of these limitations. In addition, direct RT-PCR detection cannot determine the infectivity of viruses, and therefore integrated cell culture–RT-PCR detection has more meaningful implications for public health risk assessments. The advantages of these molecular approaches include a shorter assay time, greater sensitivity, and the ability to genotype and identify the viruses present. Most viral monitoring studies target enteroviruses to determine fecal contamination of water sources. We propose that reoviruses may also be a valuable target for monitoring fecal and viral contamination of water. Previous findings that reoviruses can be present more often than other enteroviruses, including poliovirus, suggest that monitoring for reoviruses may provide a useful indicator of viral contamination. Zoonotic transmission of reoviruses is probable (19), and therefore all occurrences of contamination, whether due to animals or due to humans, are of concern. The objectives of this study were (i) to design primers and develop a combined cell culture–RT-PCR assay for detection of mammalian reoviruses and (ii) to field test the assay with lysates of cytopathic effect (CPE)-positive cell cultures from environmental water samples.
MATERIALS AND METHODS
Viruses.
Reovirus types 1 (Lang), 2 (Jones), and 3 (Dearing) were obtained from the American Type Culture Collection, (ATCC), Manassas, Va. (catalog no. VR-230, VR-231, and VR-824 respectively). The titers of diluted virus stocks were calculated based on the original titers provided by the ATCC. As provided by the ATCC, the viral titers of reovirus types 1, 2, and 3 were 106.5, 107.5, and 105.5 50% tissue culture infective doses (TCID50)/0.2 ml, respectively. TCID50 were converted to estimated PFU as recommended by the ATCC (TCID50 × 0.7).
RT-PCR primers for virus detection.
Primers for detection of reovirus types 1, 2, and 3 were designed by using GenBank sequences. The primer pair REOL3F (5′-CAG TCG ACA CAT TTG TGG TC-3′; positions 3164 to 3183) and REOL3R (5′-GCG TAC TGA CGT GGA TCA TA-3′; positions 3464 to 3483) yielded a 320-bp product. The sequence of the RT-PCR product was unique for each of the three types.
Sampling, cell culture, and nucleic acid extraction.
Water samples (1,000 liters) were collected from 12 sites in six states and examined for enteroviruses by using the U.S. Environmental Protection Agency (EPA) Information Collection Rule (ICR) method (30). Environmental water samples were cultured on Buffalo green monkey (BGM) cells to detect the presence of enteroviruses. To detect reoviruses, cell culture lysates were subsequently examined by RT-PCR with the primers described above. Cell cultures (with medium) from flasks exhibiting viral CPE were lysed by three −80°C-37°C freeze-thaw cycles. Nucleic acids were extracted from the lysates by using a QIAamp viral RNA kit (Qiagen, Valencia, Calif.) according to the manufacturer's directions.
RT-PCR detection of viruses.
Each one-tube 50-μl RT-PCR cocktail contained 10 μl of purified nucleic acid sample; 10 mM Tris (pH 8.3); 50 mM KCl; 2 mM MgCl2; 200 μM dATP, 200 μM dTTP, 200 μM dCTP, and 200 μM dGTP (all from Pharmacia BioTech, Arlington Heights, Ill.); 300 nM forward primer and 300 nM reverse primer (both from Gibco BRL, Grand Island, N.Y.); 2.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, Calif.); 5 U of murine leukemia virus reverse transcriptase (Applied Biosystems); and 20 U of RNase inhibitor (Applied Biosystems). The RT-PCR cocktails were overlaid with mineral oil and amplified with a Stratagene (La Jolla, Calif.) Robocycler 96 thermal cycler. RT was carried out at 42°C for 30 min; this was followed by an initial denaturation step at 95°C for 10 min, heat inactivation of the marine leukemia virus reverse transcriptase, and activation of the AmpliTaq Gold DNA polymerase. The amplification reaction consisted of 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 1 min, and extension at 72°C for 30 s, followed by a single final extension at 72°C for 7 min and a 4°C hold.
Amplification products were separated by horizontal gel electrophoresis on 3.0% agarose gels (Amresco, Solon, Ohio) containing 0.5 μg of ethidium bromide per ml. The gels were electrophoresed for 1.5 h at a constant voltage (100 V) and were visualized under UV light. Gel images were captured with a gel documentation system (UVP, Inc., Upland, Calif.).
Cloning and DNA sequence analysis of RT-PCR products.
RT-PCR products were cloned and sequenced for genotyping and identification. The products were cloned with a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.) used according to the manufacturer's instructions. The cloned products were purified with a QIAquick PCR purification kit (Qiagen). The purified products were sequenced commercially (DNA Sequencing Services, University of Arizona, Tucson). Splits of purified products obtained from duplicate clones of each sample were sequenced and analyzed to identify potential sequencing errors and PCR artifacts. Sequence identities were confirmed by using Gene Runner, version 3.0 (Hastings Software, Inc., Hastings, N.Y.). Sequences were also compared with GenBank sequences by using the BLAST program. A similarity dendrogram and sequence homologies were generated by using GeneBase, version 1 (Applied Maths, Kortrijk, Belgium) and the unweighted pair group method using arithmetic averages.
Nucleotide sequence accession numbers.
The accession numbers were AF129822, AF129821, and AF129820 for the reovirus type 3 (Dearing), reovirus type 2 (Jones), and reovirus type 1 (Lang) L3 gene sequences, respectively (7). Sequences obtained from environmental reoviruses described here have been deposited in the GenBank database under the following accession numbers: RV-A, AF325764; RV-C, AF325765; RV-E, AF325766; RV-G, AF325767; and RV-H, AF325768.
RESULTS
Specificities of the primers.
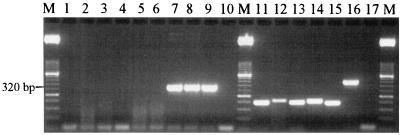
To evaluate the specificities of the reovirus primers, a group of enteric viruses, including reovirus types 1, 2, and 3, were subjected to RT-PCR. Amplification produced the correct 320-bp fragment with reovirus types 1, 2, and 3. No amplification occurred with poliovirus type 3, rotavirus SA-11, echovirus 24, Norwalk-like virus, hepatitis A virus, or adenovirus 41. To verify that the lack of amplification of the nontarget viruses was due to the specificity of the REOL3 primer set and not to PCR inhibition by the samples, each of the virus nucleic acid samples was successfully amplified with the correct corresponding primers (Fig. 1 and Table 1).
FIG. 1.
Specificity of RT-PCR with the REOL3 primer set. Lanes 1 to 9, RT-PCR products obtained by using the REOL3 primer set and poliovirus 3, rotavirus SA-11, echovirus 24, Norwalk-like virus, hepatitis A virus, adenovirus 41, reovirus type 1, reovirus type 2, and reovirus type 3, respectively; lanes 11 to 16, RT-PCR products obtained with appropriate primer sets for poliovirus 3, rotavirus SA-11, echovirus 24, Norwalk-like virus, hepatitis A virus, and adenovirus 41 nucleic acid samples, respectively (to verify amplifiability); lanes 10 and 17, no-template controls; lanes M, 2,000- to 50-bp DNA molecular weight marker XIII (Roche Molecular, Branchburg, N.J.).
TABLE 1.
Specificity of RT-PCR primers
| Primer set | Reference | RT-PCR amplification
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reovirus type 1 | Reovirus type 2 | Reovirus type 3 | Poliovirus 3 | Rotavirus Sa-11 | Echovirus 24 | Norwalk-like virus | Hepatitis A virus | Adenovirus 41 | ||
| ReoL3F/ReoL3R | This study | + | + | + | − | − | − | − | − | − |
| Pan1/Pan2 | 2 | − | − | − | + | − | + | − | − | − |
| Rota1/Rota2 | 2 | − | − | − | − | + | − | − | − | − |
| Nv3/Nv51 | 15 | − | − | − | − | − | − | + | − | − |
| HavC1/HavC2 | 2 | − | − | − | − | − | − | − | + | − |
| Ad40/Ad41 | 22 | − | − | − | − | − | − | − | − | + |
Primer sensitivity.
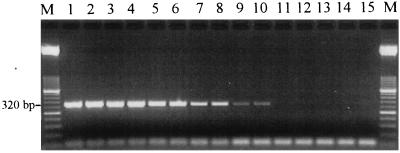
To determine the sensitivity of the reovirus primers for each type of reovirus, a RT-PCR was performed with serially diluted purified nucleic acid from the original virus stock. Reovirus types 1, 2, and 3 all were amplified up to a dilution of a 10−6, which was equivalent to approximately 3, 30, and 0.3 PFU per reaction mixture, respectively (Fig. 2; data for reovirus types 2 and 3 are not shown). These detection sensitivities are comparable to the 7- to 70-PFU/reaction mixture RT-PCR detection sensitivities recently reported for reovirus types 1, 2, and 3 (18).
FIG. 2.
Determination of REOL3 primer set RT-PCR detection sensitivity for reovirus type 1. Lanes 1 to 14, RT-PCR products obtained from 10−2 to 10−8 dilutions (in duplicate), respectively, of purified RNA of the reovirus type 1 stock; lane 15, no-template control; lanes M, 2,000- to 50-bp DNA molecular weight marker XIII. The lowest dilution at which virus was detected was also the 10−6 dilution for reovirus types 2 and 3 (data not shown). This was equivalent to approximately 3, 30, and 0.3 PFU of reovirus types 1, 2, and 3 per reaction mixture, respectively.
Detection of reoviruses.
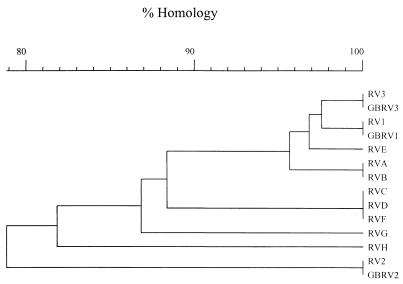
During a previous study in our laboratory, 26 of 251 surface water samples assayed during an 18-month period exhibited viral cell culture CPE. In this study, the CPE-positive samples were examined for the presence of reovirus by using RT-PCR and the REOL3 primer set. For 12 positive samples multiple cell culture flasks (subsamples) exhibited CPE, while for the remaining 14 samples there was only a single positive flask. Thus, the 26 CPE-positive samples produced a total of 50 CPE-positive cell culture flasks. Eight of the 50 flasks, containing cultures corresponding to five different water samples, yielded the expected 320-bp reovirus RT-PCR product. In some instances, multiple CPE-positive flasks for a sample were reovirus RT-PCR positive, while in other instances only one of several CPE-positive flasks for a sample was reovirus RT-PCR positive (Table 2). All eight RT-PCR products were cloned and sequenced along with all three reovirus reference strains. The sequence results are presented in a similarity dendrogram in Fig. 3 and in a table of homologies in Table 3. The sequences of reovirus type 1, 2, and 3 ATCC reference strains were 100% homologous to the corresponding GenBank sequences. These results verified the integrity of both the reference strains and the GenBank sequences.
TABLE 2.
Virus CPE-positive cell culture and combined cell culture–RT-PCR reovirus-positive environmental water samples
| Sample | Location | No. of CPE-positive flasks | No. of flasks RT-PCR positive for reovirus (isolate[s]) |
|---|---|---|---|
| V165A | Tennessee | 2 | 2 (RV-A, RV-B) |
| V169A | Iowa | 1 | 1 (RV-G) |
| V176A | Kentucky | 2 | 2 (RV-C, RV-D) |
| V194A | Iowa | 7 | 2 (RV-E, RV-H) |
| V200A | Kentucky | 1 | 1 (RV-F) |
FIG. 3.
Similarity dendrogram for environmental water sample combined cell culture–RT-PCR reovirus genotypes. The GenBank L3 gene sequences for reovirus types 1, 2, and 3 (GBRV1, GBRV2, and GBRV3, respectively) and sequences obtained in this study from ATCC reovirus types 1, 2 and 3 (RV1, RV2, and RV3, respectively) are included.
TABLE 3.
Levels of L3 RT-PCR amplicon homology for reference reovirus strains and environmental isolates
| Isolate | % Homology
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RV3 | GBRV3 | RV1 | GBRV1 | RV-E | RV-A | RV-B | RV-C | RV-D | RV-F | RV-G | RV-H | RV2 | GBRV2 | |
| RV3 | 100 | |||||||||||||
| GBRV3 | 100 | 100 | ||||||||||||
| RV1 | 97.5 | 97.5 | 100 | |||||||||||
| GBRV1 | 97.5 | 97.8 | 100 | 100 | ||||||||||
| RV-E | 97.2 | 97.2 | 96.8 | 96.8 | 100 | |||||||||
| RV-A | 95.8 | 95.2 | 96.2 | 95.5 | 95.2 | 100 | ||||||||
| RV-B | 95.6 | 95.6 | 96.0 | 96.0 | 95.2 | 100 | 100 | |||||||
| RV-C | 87.9 | 88.3 | 88.7 | 89.1 | 88.3 | 87.9 | 87.6 | 100 | ||||||
| RV-D | 88.1 | 87.5 | 88.8 | 88.2 | 88.3 | 88.3 | 87.6 | 100 | 100 | |||||
| RV-F | 87.6 | 87.6 | 88.3 | 88.3 | 88.3 | 87.6 | 87.6 | 100 | 100 | 100 | ||||
| RV-G | 85.9 | 85.9 | 86.3 | 86.3 | 87.2 | 86.8 | 86.8 | 85.1 | 85.1 | 85.1 | 100 | |||
| RV-H | 80.7 | 80.7 | 80.7 | 80.7 | 81.5 | 81.1 | 81.1 | 81.5 | 81.5 | 81.5 | 82.7 | 100 | ||
| RV2 | 79.2 | 79.2 | 78.8 | 78.8 | 79.1 | 79.6 | 78.3 | 78.4 | 78.4 | 77.1 | 75.5 | 77.1 | 100 | |
| GBRV2 | 79.1 | 80.3 | 78.7 | 80.0 | 79.1 | 79.1 | 78.7 | 78.5 | 78.6 | 77.1 | 75.9 | 77.5 | 100 | 100 |
There were nucleotide differences between each environmental reovirus RT-PCR product and the sequences of the three reference reovirus strains. Sequences RV-A and RV-B were obtained from two flasks prepared from the same sample and were 100% homologous to one another. Similarly, sequences RV-C and RV-D were also obtained from two flasks prepared from a single sample and were identical to each other. However, the sequences obtained from multiple flasks prepared from the same sample were not always homologous, as seen with sequences RV-E and RV-H, which represented two different reovirus genotypes (Fig. 3). Interestingly, the RV-F sample was collected from the same site as the RV-C and RV-D samples was collected but 1 month later, and the RV-F sequence was 100% homologous to the RV-C and RV-D sequences. Conversely, three different reovirus genotypes (RV-E, RV-G, and RV-H) were obtained from a single site.
DISCUSSION
Viral contamination of river water and groundwater, which are potential sources of drinking water, is a pressing issue for both the water industry and the EPA. Therefore, great effort has been put forth to monitor the levels of viruses present in raw water sources. The recent EPA-mandated ICR includes monitoring for enteroviruses in water using cell culture. While the ICR and most other virus-monitoring methods have targeted enteroviruses, we propose that reoviruses may also be a valuable target for monitoring viral water contamination. Here we describe a combined cell culture–RT-PCR assay for detecting and genotyping reoviruses in environmental water samples.
Previous studies have shown that reoviruses are common in raw water sources (8, 12, 27). While both humans and animals may serve as hosts for reoviruses (24), a significant human input into environmental waters may be wastewater treatment plant effluents. In one study, reoviruses remained present at a mean concentration of 1,550 infectious units liter−1, compared to 100 infectious units liter−1 for enteroviruses, in chlorinated secondary wastewater treatment plant effluents (8). In another study, the authors concluded that while animals (swine, cattle, and field mice) may have contributed to reovirus contamination of the watersheds examined, human waste was probably a more significant source of contamination (13). While watershed management practices are different for watersheds polluted by animal sources and watersheds polluted by human sources, both types of waste may contain other pathogens, such as Cryptosporidium, Giardia, Escherichia coli O157:H7, Salmonella, and enteric viruses. Monitoring for many of these organisms in water is often difficult due to their sporadic occurrence, but reovirus detection may help determine the vulnerability of a watershed to fecal pollution.
In the present study, RT-PCR detected reoviruses in 5 of 26 (19.2%) virus CPE-positive environmental water samples. The CPE in other CPE-positive samples were likely due to infection by other viruses, such as vaccine strain polioviruses, coxsackieviruses, echoviruses, or other enteroviruses, and these viruses were not detected by the reovirus RT-PCR method. A previous study reported that reoviruses were present in 31 of 73 (42%) CPE-positive water samples (12). The same study reported that reoviruses were the most commonly isolated viruses in CPE-positive samples; 207 (46.5%) of the 445 strains of viruses isolated were identified as reoviruses. In addition to the different watersheds examined in the different studies, the differences in detection frequency may have been due to the different cell culture methods used. In the previous study each sample was assayed with five different cell lines, while in the present study only BGM cell cultures were used. A study that evaluated the sensitivity of various cell lines to reovirus infection reported that the Madin-Darby bovine kidney (MDBK) cell line was the cell line that was most sensitive to reovirus types 1, 2, and 3 (24), and the Vero and BGM cell lines were found to be 20 and 35% less sensitive than the MDBK cell line, respectively. In addition to the several different cell lines, the replication rates of different viruses in cell culture may have affected the reovirus detection frequency. Reovirus replication in BGM cells is much slower than replication of other enteric viruses (27). We observed that BGM cell cultures inoculated with 10 PFU of virus exhibited CPE between 4 and 5 days postinoculation with poliovirus Sabin strain type 1, while CPE occurred after 9 to 14 days with reovirus type 1, 2, or 3 (unpublished data). Therefore, BGM cell cultures may allow reoviruses to be outcompeted by faster replicating enteroviruses that are also present in a sample. Thus, the choice of cell line may be especially important for analysis of environmental water samples contaminated with very low levels of reoviruses. The BGM cell line has been used to detect reoviruses (18, 24) and is a reasonable choice of a single cell line for simultaneous monitoring of enteroviruses and reoviruses. However, if enterovirus detection is not a concern, the MDBK cell line is a better choice for monitoring reoviruses. Additional research is needed to evaluate the use of the MDBK cell line for detection of reoviruses in water samples.
Sequence analysis of the reovirus RT-PCR products showed that there was substantial sequence diversity among the isolates and that none of the isolates was identical to the ATCC reovirus type 1, 2, or 3 reference strain. These results are not surprising given the high mutation rates of RNA viruses that are due to the lower stability of RNA than of DNA, the high replication rates, and the error-prone nature of RNA polymerases (26). Viruses are also known to undergo genetic reassortment when they pass through a host (21). Goral et al. (6) studied the sequence diversity of the mammalian reovirus S3 gene, which encodes an outer capsid protein, and observed a high degree of variability.
The sequence analysis of the L3 gene products in this study augmented previous work on reovirus sequence diversity. There is great sequence diversity among reovirus isolates, and detection of isolates with low sequence homology is not surprising. For example, ATCC reovirus type 2 and 3 reference strains are only 80.3% homologous (Table 3). It is important to note that despite the sequence diversity at this locus, the primer sites are conserved for each of the reovirus types. Genotypes RV-E and RV-H were obtained from two separate flasks prepared from the same sample, but they exhibited only 81.5% identity (Table 3). This suggests that virions of two different reovirus genotypes were present in the same sample. Conversely, RV-A and RV-B were identical and were from different flasks prepared from the same sample; the same was true of RV-C and RV-D. This suggests that there were multiple reovirus virions having the same genotype in each of the samples. Even more interesting is the observed persistence of the reovirus genotype represented by RV-C, RV-D, and RV-F at the same sampling site for two consecutive months. The possible explanations for this include a common source of reovirus contamination or survival of the reovirus genotype over time under environmental conditions. In contrast, three different reovirus genotypes (RV-E, RV-G, and RV-H) were obtained from a single site. This site also yielded a sample (V194A) (Table 2) that produced viral CPE in multiple cell culture flasks, but only two of the flasks were positive for reovirus as determined by RT-PCR. This suggests that other viruses, probably enteroviruses, were present and that there was a diverse contamination source or multiple contamination sources.
This study is significant because it is the first study to detect and genotype reoviruses in surface water sources used for potable water. Use of reoviruses as an indicator of fecal and viral contamination of water, in addition to the commonly monitored enteroviruses, may lead to more useful monitoring data and more accurate health risk assessments. Monitoring of recreational seawater for reoviruses as an indicator of fecal pollution has recently been proposed by another team of researchers (18). Furthermore, due to the resistance of reoviruses to chlorination and their potential to lead to serious illness in immunocompromised individuals, the occurrence of reoviruses in finished water should also be investigated in the future.
ACKNOWLEDGMENTS
This research was supported by the American Water Works Service Company, Inc. and by the EPA.
We gratefully acknowledge the efforts of Dale Young for sample processing and Mark Denhart for stimulating discussions. We also thank Mark LeChevallier and Morteza Abbaszadegan.
REFERENCES
- 1.Abbaszadegan M, Huber M S, Gerba C P, Pepper I L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993;59:1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbaszadegan M, Stewart P, LeChevallier M. A strategy for detection of viruses in groundwater by PCR. Appl Environ Microbiol. 1999;65:444–449. doi: 10.1128/aem.65.2.444-449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitton G. Introduction to environmental virology. New York, N.Y: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- 4.Fang Z Y, Ye Q, Ho M S, Dong H, Qing S, Penaranda M E, Hung T, Wen L, Glass R I. Investigation of an outbreak of adult diarrhea rotavirus in China. J Infect Dis. 1989;160:948–953. doi: 10.1093/infdis/160.6.948. [DOI] [PubMed] [Google Scholar]
- 5.Fraenkel-Conrat H, Kimball P, Levy J. Virology. 2nd ed. Englewood Cliffs, N.J: Prentice Hall; 1988. [Google Scholar]
- 6.Goral M I, Mochow-Grundy M, Dermody T S. Sequence diversity within the reovirus S3 gene: reoviruses evolve independently of host species, geographic locale, and date of isolation. Virology. 1996;216:265–271. doi: 10.1006/viro.1996.0059. [DOI] [PubMed] [Google Scholar]
- 7.Harrison S J, Farsetta D L, Kim J, Noble S, Broering T J, Nibert M L. Mammalian reovirus L3 gene sequences and evidence for a distinct amino-terminal region of the lambda1 protein. Virology. 1999;258:54–64. doi: 10.1006/viro.1999.9707. [DOI] [PubMed] [Google Scholar]
- 8.Irving L G, Smith F A. One-year survey of enteroviruses, adenoviruses, and reoviruses isolated from effluent at an activated-sludge purification plant. Appl Environ Microbiol. 1981;41:51–59. doi: 10.1128/aem.41.1.51-59.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson P J, Sveger T, Ahlfors K, Ekstrand J, Svensson L. Reovirus type 1 associated with meningitis. Scand J Infect Dis. 1996;28:117–120. doi: 10.3109/00365549609049060. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan J E, Goodman R A, Schonberger L B, Lippy E C, Gary G W. Gastroenteritis due to Norwalk virus: an outbreak associated with a municipal water system. J Infect Dis. 1982;146:190–197. doi: 10.1093/infdis/146.2.190. [DOI] [PubMed] [Google Scholar]
- 11.Keswick B H, Gerba C P, DuPont H L, Rose J B. Detection of enteric viruses in treated drinking water. Appl Environ Microbiol. 1984;47:1290–1294. doi: 10.1128/aem.47.6.1290-1294.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuura K, Hasegawa S, Nakayama T, Morita O, Uetake H. Viral pollution of the rivers in Toyama City. Microbiol Immunol. 1984;28:575–588. doi: 10.1111/j.1348-0421.1984.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura K, Ishikura M, Nakayama T, Hasegawa S, Morita O, Uetake H. Ecological studies on reovirus pollution of rivers in Toyama Prefecture. Microbiol Immunol. 1988;32:1221–1234. doi: 10.1111/j.1348-0421.1988.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 14.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Fields B N, editor. Virology. New York, N.Y: Raven Press; 1990. pp. 549–605. [Google Scholar]
- 15.Moe C L, Gentsch J, Ando T, Grohmann G, Monroe S S, Jiang X, Wang J, Estes M K, Seto Y, Humphrey C. Application of PCR to detect Norwalk virus in fecal specimens from outbreaks of gastroenteritis. J Clin Microbiol. 1994;32:642–648. doi: 10.1128/jcm.32.3.642-648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morecki R, Glaser J H, Johnson A B, Kress Y. Detection of reovirus type 3 in the porta hepatis of an infant with extrahepatic biliary atresia: ultrastructural and immunocytochemical study. Hepatology. 1984;4:1137–1142. doi: 10.1002/hep.1840040608. [DOI] [PubMed] [Google Scholar]
- 17.Muscillo M, Carducci A, La Rosa G, Cantiani L, Marianelli C. Enteric virus detection in Adriatic seawater by cell culture, polymerase chain reaction, and polyacrylamide gel electrophoresis. Water Res. 1997;31:1980–1984. [Google Scholar]
- 18.Muscillo M, La Rosa G, Marianelli C, Zaniratti S, Capobianchi M R, Cantiani L, Carducci A. A new RT-PCR method for the identification of reoviruses in seawater samples. Water Res. 2001;35:548–556. doi: 10.1016/s0043-1354(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 19.Nibert M L, Furlong D B, Fields B N. Mechanisms of viral pathogenesis. Distinct forms of reoviruses and their roles during replication in cells and host. J Clin Invest. 1991;88:727–734. doi: 10.1172/JCI115369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberste M S, Maher K, Flemister M R, Marchetti G, Kilpatrick D R, Pallansch M A. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J Clin Microbiol. 2000;38:1170–1174. doi: 10.1128/jcm.38.3.1170-1174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otelea D, Guillot S, Furione M, Combiescu A A, Balanant J, Candrea A, Crainic R. Genomic modifications in naturally occurring neurovirulent revertants of Sabin 1 polioviruses. Dev Biol Stand. 1993;78:33–38. [PubMed] [Google Scholar]
- 22.Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60:2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds K A, Gerba C P, Pepper I L. Detection of infectious enteroviruses by an integrated cell culture-PCR procedure. Appl Environ Microbiol. 1996;62:1424–1427. doi: 10.1128/aem.62.4.1424-1427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridinger D N, Spendlove R S, Barnett B B, George D B, Roth J C. Evaluation of cell lines and immunofluorescence and plaque assay procedures for quantifying reoviruses in sewage. Appl Environ Microbiol. 1982;43:740–746. doi: 10.1128/aem.43.4.740-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherry B. Pathogenesis of reovirus myocarditis. In: Michael M B, Oldstone A, Tyler K L, editors. Reoviruses: current topics in microbiology and immunology. Heidelberg, Germany: Springer-Verlag; 1998. pp. 51–66. [DOI] [PubMed] [Google Scholar]
- 26.Steinhauer D A, Holland J J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- 27.Tani N, Dohi Y, Kurumatani N, Yonemasu K. Seasonal distribution of adenoviruses, enteroviruses and reoviruses in urban river water. Microbiol Immunol. 1995;39:577–580. doi: 10.1111/j.1348-0421.1995.tb02245.x. [DOI] [PubMed] [Google Scholar]
- 28.Tyler K L. Pathogenesis of reovirus infections of the central nervous system. In: Michael M B, Oldstone A, Tyler K L, editors. Reoviruses: current topics in microbiology and immunology. Heidelberg, Germany: Springer-Verlag; 1998. pp. 93–124. [DOI] [PubMed] [Google Scholar]
- 29.Tyler K L, Sokol R J, Oberhaus S M, Le M, Karrer F M, Narkewicz M R, Tyson R W, Murphy J R, Low R, Brown W R. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 30.U. S. Environmental Protection Agency. ICR microbial laboratory manual. Publication EPA/600/R-95/178. Washington, D.C.: Office of Research and Development, Government Printing Office; 1996. [Google Scholar]